Abstract
Canine hemangiosarcoma is a highly malignant tumor associated with short survival times due to early and widespread metastasis. In humans and rodents, monocytes play key roles in promoting tumor metastasis through stimulating tumor cell extravasation, seeding, growth, and angiogenesis. Therefore, we investigated the potential association between monocyte infiltration and tumor metastasis in hemangiosarcoma and other common canine tumors. Immunohistochemistry was used to quantify CD18+ monocytes within metastases. We found that hemangiosarcoma metastases had significantly greater numbers of CD18+ monocytes compared to metastases from other tumor types. Hemangiosarcoma cells were the highest producers of the monocyte chemokine CCL2, and stimulated canine monocyte migration in a CCL2 dependent manner. These results are consistent with the hypothesis that overexpression of CCL2 and recruitment of large numbers of monocytes may explain in part the aggressive metastatic nature of canine hemangiosarcoma. Thus, therapies designed to block monocyte recruitment may be an effective adjuvant strategy for suppressing hemangiosarcoma metastasis in dogs.
Keywords: cancer, dog, hemangiosarcoma, metastasis, monocytes, CCL2
Introduction
Hemangiosarcoma (HSA) is a malignant vascular neoplasm to which dogs seemed predisposed relative to other species 1, 2. For example, hemangiosarcoma comprises 5-7% of all non-cutaneous malignant canine neoplasms, with the most common sites including the right atrium/auricle, spleen, and the skin 3-10. Based on its histomorphological appearance, the tumor is presumed to arise from transformed endothelial cells; however, the tumors’ origins are still under debate, and more recent investigations suggest that hemangiosarcoma likely originates from hematopoietic endothelial progenitor cells 11-13.
In the dog, hemangiosarcoma is characterized by very aggressive biological behavior and a high rate of rapid and widespread metastasis, with 1-year survival rates following surgery and/or adjuvant chemotherapy reported to be less than 10% 14-17. Current standard of care for canine hemangiosarcoma includes surgical removal of the primary tumor, followed by adjuvant chemotherapy 14-16, 18, 19. However, the benefits of adjuvant chemotherapy are modest at best with median survival times reported to be 6 months or less in multiple, independent studies 14, 15, 18-20. Alternative treatment modalities for canine hemangiosarcoma have been evaluated, including addition of the anti-angiogenic drug minocycline or the non-specific immune stimulant L-MTP-PE to DOX therapy, doxorubicin dose intensification, and combination metronomic therapy with etoposide, cyclophosphamide, and piroxicam, but with little evidence of improved survival times 14, 16, 20-22. Our critical knowledge gaps in understanding the highly malignant nature of hemangiosarcoma currently limit our ability to devise new therapeutic approaches.
Inflammatory monocytes have been shown to play key roles in promoting tumor metastasis in both humans and pre-clinical rodent models 23, 24. In contrast to conventional or steady state monocytes, the numbers of inflammatory monocytes, which are defined in part by their high surface expression of the chemokine receptor CCR2, vary widely in the bloodstream in response to inflammatory stimuli 24. Both tumor cells and stromal cells at sites of metastases have been shown to produce abundant amounts of the monocyte chemokine CCL2, the primary ligand for the CCR2 molecule 24.
Thus, the CCL2-CCR2 chemotactic axis serves to stimulate recruitment of inflammatory monocytes to metastatic sites, where the monocytes can then differentiate into metastasis-associated macrophages (MAMs) 23-26. These MAMs in turn function to prepare the metastatic microenvironment for the arrival of tumor cells by producing key cytokines and growth factors, including vascular endothelial growth factor (VEGF) 23-26. These macrophage-derived soluble factors are critical to the multi-step processes of tumor cell extravasation, survival, growth, and angiogenesis, which are required for the efficient colonization and subsequent outgrowth of tumor cells at the metastatic site. Multiple studies have demonstrated that high serum CCL2 concentrations and elevated circulating monocyte counts are both negative prognostic factors for a variety of cancers in humans, including melanoma, lymphoma, and carcinomas of the prostate, colon, and kidney, among others 27-33. Moreover, we have also demonstrated that increased numbers of circulating monocytes are also negative prognostic factors for canine lymphoma and osteosarcoma 34, 35. Elevated serum concentrations of CCL2 were also associated with shorter disease-free intervals in canine B cell lymphoma 34.
Previous studies have evaluated macrophage infiltrates in canine melanoma, mammary carcinoma, seminoma, glioma, and nasal carcinoma 36-40. In canine mammary tumors, an association between a high density of tumor-associated macrophages (TAMs) and significantly decreased overall survival times was reported 39. However, to our knowledge monocytes have not been quantitated in canine tumors, especially in tumor metastases. Nor have mechanisms regulating monocyte recruitment by tumors been investigated. Therefore, the purpose of this study was to characterize the density of tumor-associated monocytes within pulmonary metastases of several common canine tumors, and to investigate tumor-produced factors that mediate monocyte migration.
In the studies reported here, we found that hemangiosarcoma in dogs was uniquely associated with a high density of infiltrating CD18+ monocytes. Potential mechanisms of monocyte recruitment were also evaluated by means of in vitro and in vivo assays. The findings from these studies demonstrated an important role for the CCL2-CCR2 axis in regulating monocyte recruitment and promoting hemangiosarcoma metastasis in dogs, and suggest that monocyte recruitment may explain in part the highly aggressive metastatic nature of canine hemangiosarcoma. The findings also provide a rationale for immunotherapeutic interventions to interrupt monocyte and macrophage recruitment to sites of hemangiosarcoma metastasis in dogs.
Materials and Methods
Tumor tissues
Formalin-fixed, paraffin-embedded (FFPE) tissues of pulmonary metastases of various canine tumor types were obtained from archived cases submitted to the Colorado State University Veterinary Diagnostic Laboratory (CSU-VDL) for post-mortem evaluation between the years of 2007-2014. Evaluated tumor types included hemangiosarcoma (n=18), osteosarcoma (n=11), transitional cell carcinoma (n=4), melanoma (n=5), and soft tissue sarcoma (n=4). Necropsy reports from the CSU-VDL database were reviewed, and sections were cut and hematoxylin-eosin stained to confirm the previous diagnoses.
Immunohistochemistry
Tissue blocks were sectioned at 5 μm, mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and immunolabeling for the β-2 integrin, pan-leukocyte marker CD18, and chemokine CCL2, was performed using standard methods. Briefly, tissue slides were de-paraffinized in xylenes and re-hydrated using a series of graded-alcohols. Antigen retrieval was performed using either: 1.) A proprietary Leica Bond enzyme-1 (Buffalo Grove, IL) enzymatic retrieval for 10 min (CD18), or 2.) 10mM sodium citrate buffer, pH 6.0, for 20 min at 125 °C in a pressurized chamber (CCL2). Immunolabeling was performed using either a Leica Bond Max autostainer (CD18), or Dako autostainer link 48 (CCL2). Tissues were blocked for endogenous peroxidase by incubation in 3% H202 for 5 minutes. Subsequently, sections were then incubated with the following primary antibodies for 1 hr at room temperature (RT): mouse α canine CD18 (clone CA16.3C10), or rabbit α human CCL2 (Abcam, ab9669, 2.5 μg/ml). For CCL2, detection was performed using the universal labeled streptavidin-biotin2 system (Dako, Carpinteria, CA), which consists of incubation with a mixture of biotinylated goat anti- mouse and rabbit IgG secondary antibodies followed by horseradish peroxidase-labeled streptavidin. For CD18, detection was performed using the Leica Bond Polymer Red Refine Detection (Buffalo Grove, IL) system, which consists of an alkaline phosphatase-linked rabbit anti-mouse IgG. Positive staining was visualized using either refine red (CD18) or DAB (CCL2) chromogen substrates.
CCL2 immunofluorescence
For intra-cellular CCL2 immunofluorescent staining, canine tumor cells (100,000 cells) in complete MEM media were grown on sterilized glass coverslips (Fisher Scientific, Waltham, MA USA) in 24-well plates for 24 h +\− a protein transport inhibitor (Brefeldin A, 10 ug\ml, BioLegend, San Diego, CA USA) for the last 4 hours of culture. After 24 h, cells were fixed in 1% paraformaldehyde for 10 minutes on ice, and then permeabilized via incubation with 0.1% Triton-X100 in PBS containing 0.5% Tween20 (PBST) for 15 minutes at room temperature. Non-specific binding was blocked by 30 min incubation with 5% donkey serum in 1% bovine serum albumin (BSA) (Calbiochem, San Diego, CA USA). Coverslips were incubated with the primary antibody (rabbit α human CCL2 (Abcam, ab9669, 2.5 μg/ml) diluted in 1% BSA containing 0.1% Triton-X100 for 1 hr at RT. Positive CCL2 labeling was visualized using a FITC-labeled donkey α rabbit IgG secondary antibody (Jackson ImmunoResearch Inc., West Grove, PA USA). Nuclei were counterstained with DAPI, and coverslips mounted onto glass slides using Fluoromount aqueous mounting media (Sigma-Aldrich, St. Louis, MO USA).
Western blot for cross-species validation of the anti-human CCL2 antibody
1 μg of recombinant canine CCL2 (R&D systems Inc., Minneapolis, MN USA) was mixed 1:1 with 2x Laemelli sample buffer containing 5% 2-Mercaptoethanol (BioRad Laboratories, Hercules, CA USA), boiled for 5 minutes, cooled on ice, and then loaded in a 20 μL volume into a Mini-Protean TGX 4-20% pre-cast polyacrylamide gel (BioRad Laboratories, Hercules, CA USA) for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE was performed at 150 V for approximately 1 h. Protein was then wet transferred to nitrocellulose membranes (95 V, 50 min, at 4 °C), and membranes were blocked for 1 h at RT in a 5% non-fat dry milk in Tris-buffered saline Tween 20 solution (TBST). After washing in TBST, membranes were incubated with the primary antibody (1.25 μg /mL rabbit anti-human CCL2, abcam9669) diluted in 5% non-fat dry milk in TBST, overnight at 4 °C. The following day membranes were rinsed (x3 with TBST), incubated with the secondary antibody (HRP-linked goat anti-rabbit IgG; Thermo Scientific, Waltham, MA USA) diluted 1: 20,000 in 5% milk-TBST for 1 h at RT. Lastly, membranes were imaged with chemiluminescent substrate (Clarity Western ECL, BioRad) using a Chemi Doc XES + system (BioRad, Hercules, CA, USA).
Image analysis and quantification
For quantification of CD18+ immunoreactivity, (5-8) 40x magnification, intra-tumoral independent fields of multiple pulmonary metastases of each tumor were captured using standardized exposure times and either a Nikon 80i microscope and Olympus DP70 camera, or an Olympus IX83 microscope and Olympus SC30 camera. In order to ensure accurate quantitative assessment of CD18+ cells, we used the color deconvolution algorithm developed for the NIH open-source image analysis software, ImageJ. Using the FastRed and FastBlue vectors for this algorithm, digitized intra-tumoral images of tumor metastases were separated in into 8-bit gray scale images representative of the chromogen color (red) only. A lower threshold limit was then set at a value corresponding to the mean of the isotype control, and universally applied to every single image. Any pixel value falling above this lower threshold value was measured as positive for CD18, and used to determine % area positive within the field. For quality control, image masks of the “thresholded”, positive counted area were also generated and directly visually compared to the originally captured photomicrographs by a board-certified pathologist, to ensure accuracy in representation of CD18+ immunoreactivity (Figure S3). This method was chosen as the most accurate representation of CD18+ immune cell infiltrates within tumor regions, as numerical quantification of single positive cells within tumor fields was impossible due to the marked density and overlap of CD18+ cells within tumor fields.
Tumor cell culture
The DEN-HSA hemangiosarcoma cell line was used for all in vitro monocyte migration and CCL2 immunofluorescence and ELISA assays. The DEN-HSA cell line was provided by Dr. Doug Thamm (Flint Animal Cancer Center), and was originally derived at the University of Wisconsin, Madison, WI from a spontaneous renal hemangiosarcoma of a Golden Retriever 41. Cells were maintained in MEM culture media (Gibco, Grand Island, NY USA) supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO USA), penicillin (100 U/mL), streptomycin (100 μg/mL), L-glutamine (2 mM), and non-essential amino acids (0.1 mM) (All obtained from Gibco). Cells were grown on standard plastic tissue culture flasks (Cell Treat, Shirley, MA USA), incubated under standard conditions of 37 °C, 5% CO2, and humidified air.
In vitro monocyte migration assays and CCL2 neutralization
Peripheral Blood Mononuclear Cells (PBMCs) were isolated from fresh, EDTA-treated canine blood and used for in vitro trans-well migration assays to assess the degree to which HSA tumor-conditioned media elicited canine monocyte migration. For generation of tumor conditioned media, 100,000 DEN-HSA tumor cells were plated in a 24-well plate (Falcon) in 1 mL of complete MEM media and grown for approximately 24 hours prior to harvest of the culture supernatant. 600 μL of HSA-conditioned media was placed in the 24-well plate below each migration chamber to serve as the chemoattractant for the PBMCs. The negative control consisted of 600 μL of complete MEM media alone. The positive control consisted of 600 μL of complete MEM media containing 100 ng/mL of recombinant human CCL2 (Peprotech Inc. Rocky Hill, NJ). For CCL2 neutralization experiments, rabbit polyclonal anti-human CCL2 antibody (Abcam, ab9669) or rabbit IgG (Jackson ImmunoReserach, West Grove, PA) was added to the tumor conditioned media at 5 μg/mL immediately prior to addition of PBMCs. 250,000 PBMCs in 100 μL complete MEM were plated in the top well of the migration chamber insert. Subsequently, cells were allowed to migrate for 4 hours under standard conditions of 37 °C, 5% CO2, and humidified air. Following migration, the non-migrated cells were removed, and membranes were fixed with ice-cold methanol for 10 min on ice, stained with 3% crystal violet (Sigma-Aldrich, St. Louis, MO USA), rinsed with dH20, and air-dried overnight. The following day, membranes were cut from the cell culture inserts, and mounted “migrated-side” up on superfrost plus glass slides using immersion oil. A total of (5) 40x fields per membrane were counted to determine the Mean # of monocytes/40x field for each membrane. Only cells displaying the appropriate nuclear and cytoplasmic characteristics consistent with monocytes were counted and included in the analysis. Neutrophils and lymphocytes were rarely observed on the migrated side of the membrane, but if present were excluded based on their segmented nuclear morphology, and nuclear:cytoplasmic ratio, respectively. Each migration assay was run in technical replicates at minimum, and each experiment was repeated at least once.
Serum and cell culture supernatant CCL2 analysis
A commercially available canine CCL2 ELISA kit (R&D Systems Inc., Minneapolis, MN USA) was used to measure the concentration of CCL2 in tumor-conditioned cell culture media and in the serum of healthy control and hemangiosarcoma-bearing dogs. For in vitro assessment of CCL2 production by HSA tumor cells, 200,000 were plated in a 24-well plate in 1 mL of complete MEM media, and grown for approximately 24 hr prior to harvesting the culture supernatants for ELISA assay. Due to the abundant amount of CCL2 produced by tumor cells, culture supernatants were diluted 1:20 (1:10 with MEM media, and 1:1 with the reagent diluent) prior to ELISA measurement, and a 7-point standard curve with a high standard of 4000 pg/mL was used to determine the concentration of CCL2 within the supernatant. Appropriate controls included cell culture media alone, diluted on a 1:1 ratio with reagent diluent, as well as other canine tumor cell lines (data not shown).
For analysis of serum CCL2 levels in hemangiosarcoma-bearing dogs, archived, frozen, serum samples from dogs with a histologically confirmed diagnosis of hemangiosarcoma (n=24) were obtained from the tissue archive of the Flint Animal Cancer Center. For comparison, serum from “healthy” control dogs (n=6; median age=10 yrs., range 3-12 yrs.) was obtained from personal pets of laboratory personnel or from the Clinical Pathology Laboratory at Colorado State University. These animals were deemed healthy based on their history, medical records, and physical examination, and were not currently receiving any medications at the time of serum sampling.
Statistical analyses
For the comparison of mean values between three or more groups (% CD18+ area analysis, in vitro monocyte migration assays with CCL2 neutralization), a One-way ANOVA with Tukey’s post-test was performed. For comparison of means between two groups (in vitro monocyte migration assays, CCL2 ELISA assays of serum samples and tumor-conditioned media) a two-tailed, unpaired t test was used. All statistical analyses were performed using Graph Pad Prism software (La Jolla, CA, USA).
Results
Immunohistochemical characterization of CD18+ cell infiltrates in pulmonary metastases of various canine tumor types
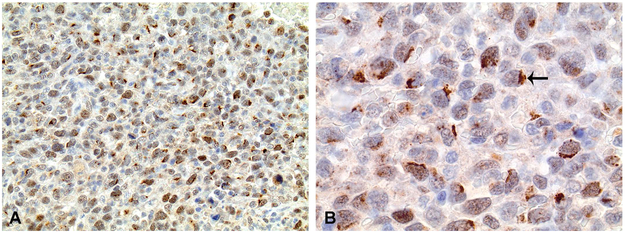
Positive immunostaining for CD18+ cells within pulmonary metastases was characterized by moderate to intense membranous to cytoplasmic labeling of individualized, round to sometimes slightly polygonal to elongate infiltrating immune cells (Figure 1A-D). These cells were easily distinguishable from tumor cells by their lack of cellular and nuclear pleomorphism. The CD18+ cells within metastases predominately consisted primarily of monocytes, along with a smaller population of CD18+ macrophages). The cellular features of the CD18+ cells included the following: 1) diameter larger than typical lymphocytes, containing a larger nucleus, increased cytoplasm, and a lower nuclear:cytoplasmic ratio, and 2) typical mononuclear morphology, characterized by a round to U-shaped nucleus, lacking the nuclear segmentation typical of neutrophils (Figure 2).
Figure 1.
Representative photomicrographs of CD18+ myeloid cell infiltrates within pulmonary metastases of various canine tumor types. (A) The greatest density of infiltrating CD18+ cells were typically present within hemangiosarcoma metastases, and was frequently characterized by a very uniform, diffuse, distribution of CD18+ cells infiltrating throughout the tumor stroma. CD18+ cellular infiltrates within osteosarcoma (B), soft tissue sarcoma (C), and transitional cell carcinoma (D) metastases were typically less dense, and more frequently composed of multifocal, small to medium sized nodular clusters present throughout the tumor stroma. All images 40x magnification. Fast red chromogen. Hematoxylin counterstain.
Figure 2.
Higher magnification image of CD18+ myeloid cell infiltrates within a hemangiosarcoma metastasis. CD18+ cells were typically characterized by a U-shaped to round, mononuclear morphology, consistent with either monocyte (thin arrows) or macrophage (thick arrows) morphology. Importantly, CD18+ cellular infiltrates were localized to the tumor stroma and thin fibrovascular septa forming the vascular spaces, and were not present within the lumens of neoplastic vessels. 100x magnification. Fast red chromogen. Hematoxylin counterstain.
Notably, despite the highly vascular nature of hemangiosarcoma, CD18+ cells within hemangiosarcoma metastases were predominately localized to the fibrous connective tissue stroma and connective tissue bundles forming the vascular spaces, and not within vascular spaces (see Figure 2). Additionally, hemangiosarcoma pulmonary metastases were often comprised of tumor cells arranged in sheets that resulted in an “epithelioid” appearance, which allowed for easier image capture and analysis of CD18+ cell density, as opposed to the highly vascular morphological appearance associated with hemangiosarcoma of the spleen, liver, right atrium, or elsewhere.
The micro-anatomical distribution and density of CD18+ cellular infiltrates within pulmonary metastases varied between tumor types. For hemangiosarcoma metastases, the CD18+ cells had the greatest density and degree of uniformity in cellular infiltrates (Figure 1A). For example, CD18+ cells were typically uniformly distributed, dense infiltrates throughout the tumor core, as well as forming dense rims 3-5 cell layers thick, which circumferentially surrounded the periphery of metastatic nodules (Figure S1). In contrast, CD18+ cells within soft tissue sarcoma and osteosarcoma metastases were more frequently arranged in small to medium sized nodular clusters, randomly scattered throughout the tumor periphery and within the tumor core. Within melanoma and transitional cell carcinoma metastases, CD18+ cells were typically localized to within thin bands of reactive fibrous connective tissue stroma surrounding packets and nests of tumor cells, and were significantly less dense, individualized, and frequently more polygonal to elongate in appearance (Fig. 1 B-D). Lastly, the density of CD18+ cells varied within each tumor type, with some tumors exhibiting markedly high degrees of CD18+ cellular infiltrates, while others cases were almost completely devoid of CD18+ cells.
CD18+ cellular infiltrates are greatest within hemangiosarcoma pulmonary metastases
For accurate quantitative assessment of CD18+ cells, we used the ImageJ color deconvolution algorithm as described in Materials and Methods. For each image, a mask of the positive area as determined by ImageJ was generated and directly compared to its “parent” photomicrograph to ensure accuracy in automated assessment of CD18 immunoreactivity. As shown in Figure S3, the color deconvolution algorithm was accurate in identifying CD18+ immunoreactivity, accurately selecting the Fast red chromogen signal localized to cellular membranes and cytoplasm, but at the same time excluding pigment, hyperchromatic nuclei, or other artifacts. Importantly, this algorithm was also effective in discriminating the Fast red positive chromogen from the red hue of erythrocytes, thus preventing false positives resulting from the vascular nature of the hemangiosarcoma (black arrow, Figure S3). The percentage of CD18+ area within tumor metastases was significantly greater within hemangiosarcoma, which had a mean of 11.75 % (± 2.913 % positive area), as compared to transitional carcinoma metastases (6.229 ± 2.746 %), and melanoma metastases (6.623 ± 3.245 %) (Figure 3 A; One-way ANOVA, Tukey’s post-test, *p<0.05). While the mean % CD18+ area within hemangiosarcoma metastases was numerically greater than for osteosarcoma (9.164 ± 4.168 %) and soft tissue sarcoma metastases (8.924 ± 3.788 %), but did not reach the level of statistical significance (p = 0.28, and 0.56, respectively). Importantly, the mean % CD18+ area within hemangiosarcoma metastases was significantly greater than the mean of the other tumor types combined (11.75 ± 0.69 % vs. 8.11 ± 0.76 %, respectively) (p=0.001).
Figure 3.
Quantification of CD18+ cell density within pulmonary metastases using ImageJ color deconvolution. (A) The density of C18+ cells, expressed as CD18+ area as percentage of tumor area, was greatest within hemangiosarcoma metastases, and was significantly greater than that observed in transitional cell carcinoma and melanoma metastases. (B) Furthermore, the percentage of CD18+ cells were even more significantly greater in hemangiosarcoma metastases, when compared to all other evaluated tumor types combined. HSA=hemangiosarcoma, OSA=osteosarcoma, STS=soft tissue sarcoma, and TCC=transitional cell carcinoma. Data representative of Mean ± SD. *p=0.03 (HSA vs. melanoma) and *p=0.04 (HSA vs TCC); One-way ANOVA, Tukey’s post-test; **p=0.001; unpaired, two-tailed t test.
CCL2 secreted by canine hemangiosarcoma cell line elicits strong monocyte migration.
A canine hemangiosarcoma cell line (DEN-HSA) was used to investigate the immunological mechanisms responsible for monocyte infiltrates observed in hemangiosarcoma metastases. A monocyte migration assay was used to evaluate the effects of DEN-HSA conditioned medium on monocyte recruitment, using canine peripheral blood mononuclear cells (PBMC) from healthy control dogs and transwell plates (Fig. 4, A-C). Following exposure of PBMC to HSA-conditioned medium, the mean number of migrated monocytes per HPF (24.7 ± 10.02) was significantly increased compared to control medium (1.6 ± 2.054) (p<0.05 (Figure 4). In addition, CCL2 secreted by DEN-HSA cells was quantified using a canine CCL2 ELISA, as described previously 42. DEN-HSA conditioned media contained very high concentrations of CCL2 (Fig. 5C), with a mean concentration of 22,830 pg/mL (Fig. 4). Furthermore, a second canine hemangiosarcoma cell line (SB-HSA) was evaluated for CCL2 production by ELISA assay, and was found to also produce significant amounts of the chemokine (Fig. S4).
Figure 4.
Canine hemangiosarcoma tumor-conditioned media stimulates strong monocyte migration in vitro. Representative photomicrographs of crystal violet stained membranes of the cell culture inserts used for quantification of in vitro monocyte migration. Complete MEM media stimulated little to no monocyte migration (A); however, conditioned-media from canine DEN-HSA cells stimulated strong monocyte migration (C), which was significantly greater than the negative control, and at minimum equivalent to the positive control of 100 ng/mL recombinant human rCCL2 (B). Only migrated cells displaying a mononuclear morphology consistent with monocytes were counted and included in the analysis. (D) Quantitative assessment of monocyte migration to media alone (negative control), 100 ng/mL recombinant human CCL2 (positive control), or HSA-conditioned media. Each data point represents the mean (± SD) number of monocytes per 40x field for duplicate membranes as determined by counting (5) independent 40x fields per membrane. *p<0.05, repeated measures One-way ANOVA, Tukey’s post-test. All images are 40x magnification and crystal violet stained.
Figure 5.
Canine hemangiosarcoma cells produce abundant amounts of CCL2 in vitro and elicit canine monocyte migration in a CCL2-CCR2 dependent manner. Immunolabeling of DEN-HSA cells for CCL2 required pre-treatment with a protein transport inhibitor (Brefeldin A). Un-treated cells (A) displayed no immunoreactivity for CCL2, whereas cells pre-treated with Brefeldin A (B), demonstrated, strong, punctate, peri-nuclear immunoreactivity for CCL2, which is more clearly demonstrated in the enlarged single cell image inset. 40x magnification. CCL2=FITC (green). Nuclei=DAPI (blue) (C) Significant amounts of CCL2 was also detected via ELISA in conditioned media from DEN-HSA cells. Tumor cells were seeded in 24-well plates and allowed to grow for 24h prior to harvesting the supernatant for CCL2 measurement via ELISA assay. (D) Neutralization of CCL2 in HSA-conditioned media using an anti-human CCL2 antibody (5 μg/mL) resulted in a significant (~60-80%) reduction in in vitro monocyte migration as compare to the positive control of HSA-conditioned media only. Data representative of Mean ± SD. **p=0.006 unpaired one-tailed t test; ***p=0.0002, ****p<0.0001. One-way ANOVA, Tukey’s post-test.
Next, immunofluorescence staining was used to localize tumor cell production of CCL2. Cross-reactivity of a polyclonal CCL2 antibody for canine CCL2 was confirmed via western blotting against recombinant canine CCL2 (Fig. S2). The antibody detected a band of approximately 15 kDa, consistent with the predicted molecular weight of canine CCL2 (8-12 kDa). DEN-HSA cells were pre-incubated with the protein transport inhibitor Brefeldin A (10 μg/mL) for 4 hours prior to staining. DEN-HSA cells exhibited strong CCL2 positive immunoreactivity, characterized by punctate to granular staining which discretely localized to the peri-nuclear golgi zone of the cytoplasm (Fig. 5B).
To elucidate the factor produced by DEN-HSA cells that stimulated monocyte migration, the DEN-HSA conditioned medium was incubated with a neutralizing CCL2 antibody, and the effects on monocyte migration assessed. Addition of the CCL2 neutralizing antibody to the tumor-conditioned media significantly reduced the number of migrated monocytes to approximately 60-80% of that observed for HSA-conditioned media alone (***p=0.0002) (Fig. 5D). These findings indicated that DEN-HSA in vitro elicited monocyte migration was mediated primarily by CCL2 secretion.
Immunohistochemical localization of CCL2 in hemangiosarcoma pulmonary metastases
Expression of CCL2 in tumor biopsy specimens was almost entirely restricted to tumor cells, with between 50% and 100% of tumor cells within metastatic nodules demonstrating moderate to strong labeling intensity for CCL2 (Figure 6A and S5A). Within tumor cells, the localization of CCL2 immunoreactivity varied from diffuse and intra-cytoplasmic, to focal, intense, and peri-nuclear (Figure 6B). Additionally, most osteosarcoma pulmonary metastases also displayed positive CCL2 labeling, though the pattern of immunoreactivity was typically characterized by weak cytoplasmic labeling for CCL2. Metastatic transitional cell carcinoma tumors demonstrated diffuse, intense, cytoplasmic labeling for CCL2, which was present in 75-100% of tumor cells. Lastly, only one of three metastatic melanoma biopsies demonstrated positive immunolabeling of tumor cells for CCL2. Thus, while there was a wide range of CCL2 expression by different types of tumor cells, hemangiosarcoma metastases were the most consistently strongly CCL2 positive.
Figure 6.
Tumor cells within hemangiosarcoma metastases demonstrate positive immunolabeling for CCL2. All evaluated cases (n=5) demonstrated strong, positive immunolabeling for CCL2. (A) In all cases, greater than 50-75% of tumor cells within hemangiosarcoma pulmonary metastases demonstrated moderate to strong immunoreactivity for CCL2. 20x magnification. DAB chromogen. Hematoxylin counterstain. (B) Higher magnification image of the same case in (A), which demonstrates the strongly positive, intra-cytoplasmic, peri-nuclear (golgi zone) localization for CCL2 within tumor cells. 50x magnification.
Serum CCL2 levels elevated in the blood of dogs with splenic hemangiosarcoma
CCL2 concentrations in the pre-treatment serum of 24 dogs with a histopathologically confirmed diagnosis of hemangiosarcoma, were measured using an ELISA assay as described above. For comparison, CCL2 concentrations were also measured in the serum of 6 age-matched, healthy control dogs. The median age of hemangiosarcoma-bearing dogs was 9.8 years (range of 7-15 years), and consisted of 12 males and 12 females. The median age of the healthy control dogs was 10 years (range of 3-12 years), and consisted of 3 males and 3 females. The mean concentration of CCL2 in the serum of hemangiosarcoma-bearing dogs (267.4 ± 56.64 pg/mL) was significantly elevated (Figure 7) compared to healthy control dogs (123.9 ± 50.68 pg/mL). Values for serum CCL2 concentration were significantly elevated compared to control dogs, whether evaluating dogs with hemangiosarcoma of any primary location, or only dogs with primary splenic hemangiosarcoma (data not shown).
Figure 7.
Serum CCL2 levels are elevated in dogs with hemangiosarcoma vs. “healthy” controls. Serum samples from dogs with HSA (n=24) were obtained at the time of surgical removal of the primary tumor, performed at the CSU Veterinary Teaching Hospital. Samples were frozen and maintained in the tissue archive of the Flint Animal Cancer Center until analysis. Serum CCL2 concentrations were measured using a commercially available canine CCL2 ELISA. Data representative of Mean ± SD. *p=0.03. unpaired, one-tailed t test with Welch’s correction.
Discussion
Experimental and clinical evidence strongly suggests that monocytes promote tumor metastasis in humans and rodent tumor models, but the role of monocytes in canine tumor metastasis has received relatively little attention. Therefore, a primary goal of the current study was to characterize the degree of monocyte infiltration within pulmonary metastases of common and highly metastatic canine tumors of dogs. One of the most important findings that emerged from these studies was that hemangiosarcoma pulmonary metastases appear to have a unique propensity in their ability to recruit monocytes, as these tumors demonstrated a significantly greater degree of CD18+ monocyte infiltration as compared to all other evaluated canine tumor metastases (Figures 1-3). These findings were bolstered by also demonstrating strong CCL2 immunostaining of tumor metastases (Figure 6). In addition, in vitro assays were used to show that hemangiosarcoma cells secreted high concentrations of CCL2 which strongly stimulated monocyte migration (Figures 6 and 7). Thus, it is apparent from these results that the CCL2-CCR2 axis plays an important role in monocyte recruitment in hemangiosarcoma in dogs.
Given that hemangiosarcoma is an endothelial-derived tumor, and monocytes are known to be a rich source of the endothelial growth factor VEGF 24, our findings suggest one important role for monocytes in hemangiosarcoma, namely stimulation of tumor angiogenesis. Interestingly, hematopoietic stem/progenitor cells of a CD11b+ myelo-monocytic phenotype are also one of cell types that preferentially accumulate at metastatic sites even before the arrival of tumor cells, a phenomenon known as the pre-metastatic niche 43-45. This phenomenon has been demonstrated experimentally in syngeneic mouse models of lung carcinoma and melanoma, as well as a human xenograft breast cancer model 43-45. In addition, increased numbers of these cells have been detected in human cancer patients and correlate with increased risk for metastatic progression 43. Once present at metastatic sites, monocytes function to establish a permissive niche for incoming tumor cells through mechanisms involving immune suppression, up-regulation of tumor cell chemoattractants, and promotion of tumor cell survival, both directly via secretion of molecules such as S100A8 and A9, and matrix metalloproteinase-mediated release of VEGF and c-KIT43-45. Furthermore, the functional impact of monocytes on metastatic progression has been confirmed experimentally, as antibody-mediated depletion of these cells completely prevented metastasis in mice bearing well-established tumors 45.
The role of inflammatory monocytes and macrophages in the regulation of tumor responses to chemotherapeutic drugs is also beginning to be defined. Importantly, the functional role of monocytes and macrophages in mediating chemoresistance has been demonstrated via pre-clinical studies in which concurrent macrophage depletion with chemotherapy significantly improved overall survival in mammary tumor bearing mice 46, 47. In addition, clinical evidence supporting a role for macrophages in chemoresistance comes from human breast cancer patients, in which patients having a high tumor gene expression ratio of macrophages to T cells had a significantly lower rate of response to neoadjuvant chemotherapy, and significantly reduced overall survival 46. Thus, the results of our study and the significant presence of monocytes in hemangiosarcoma metastases may be related to the tumors apparent lack of chemotherapy responsiveness. For example, our findings suggest that in canine hemangiosarcoma, the typical poor response to adjuvant chemotherapy may in fact be mediated by the strong monocyte infiltrate present in tumor metastases.
In conclusion, we found that canine hemangiosarcoma, as compared to other common and highly metastatic canine tumors types, was unique in its ability to recruit large numbers of monocytes to sites of tumor metastasis. Moreover, our results suggest that the CCL2-CCR2 chemotactic axis may be the primary driver of this monocyte response. These observations provide important insights into the biology and immunopathogenesis of hemangiosarcoma, and are consistent with the hypothesis that overexpression of CCL2 and recruitment of large numbers of monocytes may explain in part the aggressive metastatic nature of canine hemangiosarcoma. Thus, treatment strategies designed to exploit this monocyte driven response, such as treatment with monocyte depleting agents (eg, liposomal clodronate; 48) or migration inhibitors (eg, CCL2 depleting antibodies or CCR2 antagonists) could be explored to improve the effectiveness of conventional adjuvant chemotherapy for prevention of hemangiosarcoma metastases.
Supplementary Material
Figure S1. Overview of distribution of CD18+ cells within hemangiosarcoma metastases. Low-magnification image demonstrating CD18+ cells localized both circumferentially around the periphery of metastatic nodules (black arrows), as well as diffusely infiltrating through the tumor stroma and core of metastatic nodules. 10x magnification. Fast red chromogen. Hematoxylin counterstain.
Figure S2. Cross species validation of the anti-human CCL2 antibody and isotype control staining of pulmonary metastases for CCL2. (A) Cross-reactivity of the anti-human CCL2 antibody was confirmed via western blot against canine recombinant CCL2 (R&D systems Inc. Minneapolis, MN), which detected a band of ≈ 15 kDa (predicted M.W. of 8-12 kDa). (B) Concentration-matched, rabbit IgG negative control for CCL2 staining of FFPE metastatic tumor tissues demonstrating a complete lack of immunoreactivity within tumor tissues. 20x magnification. DAB chromogen, hematoxylin counterstain.
Figure S3. Validation of the ImageJ color deconvolution algorithm for quantitative assessment of CD18+ cell density. (A) Original 40x image of CD18+ immunolabeling, and (B) corresponding quality control, thresholded image mask of CD18 positive area as determined in ImageJ using the color deconvolution algorithm. Color deconvolution with the Fast red and Fast blue vectors followed by setting a lower threshold limit at the mean gray value of negative control images generated effective masks for accurate quantitative evaluation of CD18+ cellular infiltrates.
Figure S4. Significant amounts of CCL2 was also detected via ELISA in conditioned media from a second canine hemangiosarcoma cell line (SB-HSA). As described for the DEN-HSA cells, tumor cells were seeded in 24-well plates and allowed to grow for 24h prior to harvesting the supernatant for CCL2 measurement via ELISA assay. Data representative of Mean ± SD. **p=0.001 unpaired two-tailed t test.
Figure S5. CCL2 positive immunolabeling within other metastatic canine tumors. (A) Another case of hemangiosarcoma demonstrating strong CCL2 cytoplasmic immunoreactivity in the majority of tumor cells. (B) A transitional cell carcinoma metastasis demonstrating multifocal, strong CCL2 cytoplasmic immunoreactivity in tumor cells. (C) Weak to moderate positive immunolabeling for CCL2 within an osteosarcoma metastasis. (D) A melanoma metastasis that was negative for CCL2. 40x magnification. DAB chromogen. Hematoxylin counterstain.
Acknowledgements
These studies were supported by the AKC Clinician Scientist Fellowship, the Shipley Foundation, and by an NIH T32 training grant (5T32OD010437-13).
References
- 1.Priester WA and McKay FW. The occurrence of tumors in domestic animals. Natl Cancer Inst Monogr. 1980; (54): 1–210. [PubMed] [Google Scholar]
- 2.Spangler WL and Culbertson MR. Prevalence, type, and importance of splenic diseases in dogs: 1,480 cases (1985-1989). J Am Vet Med Assoc. 1992; 200(6): 829–34. [PubMed] [Google Scholar]
- 3.Bastianello SS. A survey on neoplasia in domestic species over a 40-year period from 1935 to 1974 in the Republic of South Africa. VI. Tumours occurring in dogs. Onderstepoort J Vet Res. 1983; 50(3): 199–220. [PubMed] [Google Scholar]
- 4.Brown NO, Patnaik AK and MacEwen EG. Canine hemangiosarcoma: retrospective analysis of 104 cases. J Am Vet Med Assoc. 1985; 186(1): 56–8. [PubMed] [Google Scholar]
- 5.Kleine LJ, Zook BC and Munson TO. Primary cardiac hemangiosarcomas in dogs. J Am Vet Med Assoc. 1970; 157(3): 326–37. [PubMed] [Google Scholar]
- 6.MacVean DW, Monlux AW, Anderson PS Jr., Silberg SL and Roszel JF. Frequency of canine and feline tumors in a defined population. Vet Pathol. 1978; 15(6): 700–15. [DOI] [PubMed] [Google Scholar]
- 7.Pearson GR and Head KW. Malignant haemangioendothelioma (angiosarcoma) in the dog. J Small Anim Pract. 1976; 17(11): 737–45. [DOI] [PubMed] [Google Scholar]
- 8.Schultheiss PC. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J Vet Diagn Invest. 2004; 16(6): 522–6. [DOI] [PubMed] [Google Scholar]
- 9.Srebernik N and Appleby EC. Breed prevalence and sites of haemangioma and haemangiosarcoma in dogs. Vet Rec. 1991; 129(18): 408–9. [DOI] [PubMed] [Google Scholar]
- 10.Vail DM and MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000; 18(8): 781–92. [DOI] [PubMed] [Google Scholar]
- 11.Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GC and Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol. 2006; 34(7): 870–8. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Kakiuchi-Kiyota S, Arnold LL, Johansson SL, Wert D and Cohen SM. Pathogenesis of human hemangiosarcomas and hemangiomas. Hum Pathol. 2013; 44(10): 2302–11. [DOI] [PubMed] [Google Scholar]
- 13.Tamburini BA, Phang TL, Fosmire SP, Scott MC, Trapp SC, Duckett MM, Robinson SR, Slansky JE, Sharkey LC, Cutter GR, Wojcieszyn JW, Bellgrau D, Gemmill RM, Hunter LE and Modiano JF. Gene expression profiling identifies inflammation and angiogenesis as distinguishing features of canine hemangiosarcoma. BMC Cancer. 2010; 10: 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorenmo K, Duda L, Barber L, Cronin K, Sammarco C, Usborne A, Goldschmidt M and Shofer F. Canine hemangiosarcoma treated with standard chemotherapy and minocycline. J Vet Intern Med. 2000; 14(4): 395–8. [DOI] [PubMed] [Google Scholar]
- 15.Sorenmo KU, Jeglum KA and Helfand SC. Chemotherapy of canine hemangiosarcoma with doxorubicin and cyclophosphamide. J Vet Intern Med. 1993; 7(6): 370–6. [DOI] [PubMed] [Google Scholar]
- 16.Vail DM, MacEwen EG, Kurzman ID, Dubielzig RR, Helfand SC, Kisseberth WC, London CA, Obradovich JE, Madewell BR, Rodriguez CO Jr., and et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcoma in the dog: a randomized multi-institutional clinical trial. Clin Cancer Res. 1995; 1(10): 1165–70. [PubMed] [Google Scholar]
- 17.Wendelburg KM, Price LL, Burgess KE, Lyons JA, Lew FH and Berg J. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001-2012). J Am Vet Med Assoc. 2015; 247(4): 393–403. [DOI] [PubMed] [Google Scholar]
- 18.Hammer AS, Couto CG, Filppi J, Getzy D and Shank K. Efficacy and toxicity of VAC chemotherapy (vincristine, doxorubicin, and cyclophosphamide) in dogs with hemangiosarcoma. J Vet Intern Med. 1991; 5(3): 160–6. [DOI] [PubMed] [Google Scholar]
- 19.Ogilvie GK, Powers BE, Mallinckrodt CH and Withrow SJ. Surgery and doxorubicin in dogs with hemangiosarcoma. J Vet Intern Med. 1996; 10(6): 379–84. [DOI] [PubMed] [Google Scholar]
- 20.Lana S, U’Ren L, Plaza S, Elmslie R, Gustafson D, Morley P and Dow S. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med. 2007; 21(4): 764–9. [DOI] [PubMed] [Google Scholar]
- 21.Sorenmo KU, Baez JL, Clifford CA, Mauldin E, Overley B, Skorupski K, Bachman R, Samluk M and Shofer F. Efficacy and toxicity of a dose-intensified doxorubicin protocol in canine hemangiosarcoma. J Vet Intern Med. 2004; 18(2): 209–13. [DOI] [PubMed] [Google Scholar]
- 22.U’Ren LW, Biller BJ, Elmslie RE, Thamm DH and Dow SW. Evaluation of a novel tumor vaccine in dogs with hemangiosarcoma. J Vet Intern Med. 2007; 21(1): 113–20. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura T, Qian BZ and Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015; 15(2): 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA and Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011; 475(7355): 222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J and Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015; 212(7): 1043–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DY, Carragher NO, Munro A, Chang A, Bresnick AR, Lang RA and Pollard JW. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015; 212(9): 1433–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng J, Zhang W, Wu J, Gao S, Ye H, Sun L, Chen Y, Yu K and Xing CY. Effect of initial absolute monocyte count on survival outcome of patients with de novo non-M3 acute myeloid leukemia. Leuk Lymphoma. 2016: 1–7. [DOI] [PubMed] [Google Scholar]
- 28.Izumi K, Mizokami A, Lin HP, Ho HM, Iwamoto H, Maolake A, Natsagdorj A, Kitagawa Y, Kadono Y, Miyamoto H, Huang CK, Namiki M and Lin WJ. Serum chemokine (CC motif) ligand 2 level as a diagnostic, predictive, and prognostic biomarker for prostate cancer. Oncotarget. 2016; 7(7): 8389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X, Qian CN, Mu YG, Li NW, Li S, Zhang HB, Li SW, Wang FL, Guo X and Xiang YQ. Serum CCL2 and serum TNF-alpha--two new biomarkers predict bone invasion, post-treatment distant metastasis and poor overall survival in nasopharyngeal carcinoma. Eur J Cancer. 2011; 47(3): 339–46. [DOI] [PubMed] [Google Scholar]
- 30.Nishijima TF, Muss HB, Shachar SS, Tamura K and Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015; 41(10): 971–8. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Tominaga M, Okunaga R, Shibata K, Ohta M and Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007; 11(5): 596–602. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J and von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005; 93(3): 273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassilakopoulos TP, Dimopoulou MN, Angelopoulou MK, Petevi K, Pangalis GA, Moschogiannis M, Dimou M, Boutsikas G, Kanellopoulos A, Gainaru G, Plata E, Flevari P, Koutsi K, Papageorgiou L, Telonis V, Tsaftaridis P, Sachanas S, Yiakoumis X, Tsirkinidis P, Viniou NA, Siakantaris MP, Variami E, Kyrtsonis MC, Meletis J, Panayiotidis P and Konstantopoulos K. Prognostic Implication of the Absolute Lymphocyte to Absolute Monocyte Count Ratio in Patients With Classical Hodgkin Lymphoma Treated With Doxorubicin, Bleomycin, Vinblastine, and Dacarbazine or Equivalent Regimens. Oncologist. 2016; 21(3): 343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry JA, Thamm DH, Eickhoff J, Avery AC and Dow SW. Increased monocyte chemotactic protein-1 concentration and monocyte count independently associate with a poor prognosis in dogs with lymphoma. Vet Comp Oncol. 2011; 9(1): 55–64. [DOI] [PubMed] [Google Scholar]
- 35.Sottnik JL, Rao S, Lafferty MH, Thamm DH, Morley PS, Withrow SJ and Dow SW. Association of blood monocyte and lymphocyte count and disease-free interval in dogs with osteosarcoma. J Vet Intern Med. 2010; 24(6): 1439–44. [DOI] [PubMed] [Google Scholar]
- 36.Boozer LB, Davis TW, Borst LB, Zseltvay KM, Olby NJ and Mariani CL. Characterization of immune cell infiltration into canine intracranial meningiomas. Vet Pathol. 2012; 49(5): 784–95. [DOI] [PubMed] [Google Scholar]
- 37.Gregorio H, Raposo TP, Queiroga FL, Prada J and Pires I. Investigating associations of cyclooxygenase-2 expression with angiogenesis, proliferation, macrophage and T-lymphocyte infiltration in canine melanocytic tumours. Melanoma Res. 2016. [DOI] [PubMed] [Google Scholar]
- 38.Grieco V, Rondena M, Romussi S, Stefanello D and Finazzi M. Immunohistochemical characterization of the leucocytic infiltrate associated with canine seminomas. J Comp Pathol. 2004; 130(4): 278–84. [DOI] [PubMed] [Google Scholar]
- 39.Raposo T, Gregorio H, Pires I, Prada J and Queiroga FL. Prognostic value of tumour-associated macrophages in canine mammary tumours. Vet Comp Oncol. 2014; 12(1): 10–9. [DOI] [PubMed] [Google Scholar]
- 40.Vanherberghen M, Day MJ, Delvaux F, Gabriel A, Clercx C and Peeters D. An immunohistochemical study of the inflammatory infiltrate associated with nasal carcinoma in dogs and cats. J Comp Pathol. 2009; 141(1): 17–26. [DOI] [PubMed] [Google Scholar]
- 41.Thamm DH, Dickerson EB, Akhtar N, Lewis R, Auerbach R, Helfand SC and MacEwen EG. Biological and molecular characterization of a canine hemangiosarcoma-derived cell line. Res Vet Sci. 2006; 81(1): 76–86. [DOI] [PubMed] [Google Scholar]
- 42.Duffy AL, Olea-Popelka FJ, Eucher J, Rice DM and Dow SW. Serum concentrations of monocyte chemoattractant protein-1 in healthy and critically ill dogs. Vet Clin Pathol. 2010; 39(3): 302–5. [DOI] [PubMed] [Google Scholar]
- 43.Giles AJ, Reid CM, Evans JD, Murgai M, Vicioso Y, Highfill SL, Kasai M, Vahdat L, Mackall CL, Lyden D, Wexler L and Kaplan RN. Activation of Hematopoietic Stem/Progenitor Cells Promotes Immunosuppression Within the Pre-metastatic Niche. Cancer Res. 2016; 76(6): 1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiratsuka S, Watanabe A, Aburatani H and Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006; 8(12): 1369–75. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S and Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005; 438(7069): 820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL and Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011; 1(1): 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan YX, Qiu J, Park J, Sinha P, Bissell MJ, Frengen E, Werb Z and Egeblad M. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012; 21(4): 488–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guth AM, Hafeman SD, Elmslie RE and Dow SW. Liposomal clodronate treatment for tumour macrophage depletion in dogs with soft-tissue sarcoma. Vet Comp Oncol. 2013; 11(4): 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overview of distribution of CD18+ cells within hemangiosarcoma metastases. Low-magnification image demonstrating CD18+ cells localized both circumferentially around the periphery of metastatic nodules (black arrows), as well as diffusely infiltrating through the tumor stroma and core of metastatic nodules. 10x magnification. Fast red chromogen. Hematoxylin counterstain.
Figure S2. Cross species validation of the anti-human CCL2 antibody and isotype control staining of pulmonary metastases for CCL2. (A) Cross-reactivity of the anti-human CCL2 antibody was confirmed via western blot against canine recombinant CCL2 (R&D systems Inc. Minneapolis, MN), which detected a band of ≈ 15 kDa (predicted M.W. of 8-12 kDa). (B) Concentration-matched, rabbit IgG negative control for CCL2 staining of FFPE metastatic tumor tissues demonstrating a complete lack of immunoreactivity within tumor tissues. 20x magnification. DAB chromogen, hematoxylin counterstain.
Figure S3. Validation of the ImageJ color deconvolution algorithm for quantitative assessment of CD18+ cell density. (A) Original 40x image of CD18+ immunolabeling, and (B) corresponding quality control, thresholded image mask of CD18 positive area as determined in ImageJ using the color deconvolution algorithm. Color deconvolution with the Fast red and Fast blue vectors followed by setting a lower threshold limit at the mean gray value of negative control images generated effective masks for accurate quantitative evaluation of CD18+ cellular infiltrates.
Figure S4. Significant amounts of CCL2 was also detected via ELISA in conditioned media from a second canine hemangiosarcoma cell line (SB-HSA). As described for the DEN-HSA cells, tumor cells were seeded in 24-well plates and allowed to grow for 24h prior to harvesting the supernatant for CCL2 measurement via ELISA assay. Data representative of Mean ± SD. **p=0.001 unpaired two-tailed t test.
Figure S5. CCL2 positive immunolabeling within other metastatic canine tumors. (A) Another case of hemangiosarcoma demonstrating strong CCL2 cytoplasmic immunoreactivity in the majority of tumor cells. (B) A transitional cell carcinoma metastasis demonstrating multifocal, strong CCL2 cytoplasmic immunoreactivity in tumor cells. (C) Weak to moderate positive immunolabeling for CCL2 within an osteosarcoma metastasis. (D) A melanoma metastasis that was negative for CCL2. 40x magnification. DAB chromogen. Hematoxylin counterstain.