Abstract
Freezing of gait (FOG) is a devastating axial motor symptom in Parkinson’s disease (PD) leading to falls, institutionalization, and even death. The response of FOG to dopaminergic medication and deep brain stimulation (DBS) is complex, variable, and yet to be optimized. Fundamental gaps in the knowledge of the underlying neurobiomechanical mechanisms of FOG render this symptom one of the unsolved challenges in the treatment of PD. Subcortical neural mechanisms of gait impairment and FOG in PD are largely unknown due to the challenge of accessing deep brain circuitry and measuring neural signals in real time in freely-moving subjects. Additionally, there is a lack of gait tasks that reliably elicit FOG. Since FOG is episodic, we hypothesized that dynamic features of subthalamic (STN) beta oscillations, or beta bursts, may contribute to the Freezer phenotype in PD during gait tasks that elicit FOG. We also investigated whether STN DBS at 60 Hz or 140 Hz affected beta burst dynamics and gait impairment differently in Freezers and Non-Freezers. Synchronized STN local field potentials, from an implanted, sensing neurostimulator (Activa® PC+S, Medtronic, Inc.), and gait kinematics were recorded in 12 PD subjects, off-medication during forward walking and stepping-in-place tasks under the following randomly presented conditions: NO, 60Hz, and 140Hz DBS. Prolonged movement band beta burst durations differentiated Freezers from Non-Freezers, were a pathological neural feature of FOG and were shortened during DBS which improved gait. Normal gait parameters, accompanied by shorter bursts in Non-Freezers, were unchanged during DBS. The difference between the mean burst duration between hemispheres (STNs) of all individuals strongly correlated with the difference in stride time between their legs but there was no correlation between mean burst duration of each STN and stride time of the contralateral leg, suggesting an interaction between hemispheres influences gait. These results suggest that prolonged STN beta burst durations measured during gait is an important biomarker for FOG and that STN DBS modulated long not short burst durations, thereby acting to restore physiological sensorimotor information processing, while improving gait.
Keywords: Parkinson’s disease, freezing of gait, deep brain stimulation, beta bursts, subthalamic nucleus
Introduction
Axial motor symptoms of Parkinson’s disease (PD) such as freezing of gait (FOG) and postural instability are major reasons for loss of autonomy, falls, institutionalization, and death in patients with PD (Giladi et al., 1992, 1997; Bloem et al., 2004; Vervoort et al., 2016). FOG frequently manifests as patients attempt to initiate walking, turning, and while navigating obstacles. It results in life-threatening falls or sudden immobility that renders patients helpless in dangerous situations such as crossing a street. The response of FOG to treatment is complex, variable, and has yet to be optimized. Both dopaminergic medication and subthalamic nucleus (STN) deep brain stimulation (DBS) at low and high frequencies may improve FOG, suggesting that neural mechanisms underlying FOG reside within the sensorimotor networks accessed by both medication and DBS (Moreau et al., 2008; Sidiropoulos et al., 2013; Annic et al., 2014; Khoo et al., 2014; Nantel and Bronte-Stewart, 2014; Vercruysse et al., 2014; Fasano et al., 2015; Xie et al., 2015). However, PD patients may still develop FOG while on medication and/or DBS even while the other cardinal motor signs of PD (tremor, rigidity, and bradykinesia) are well-treated (Lilleeng et al., 2015). Fundamental gaps in the knowledge of the underlying neurobiomechanical mechanisms of FOG make this symptom one of the unsolved challenges in the treatment of PD.
Subcortical neural mechanisms of gait impairment and FOG in PD are largely unknown due to the challenge of accessing deep brain circuitry and measuring neural signals in freely-moving subjects and in the lack of gait tasks that reliably elicit FOG. Until recently, subcortical local field potentials (LFPs) could only be recorded in the intra- or peri-operative period, when PD subjects were stationary and attached to cables (Thevathasan et al., 2012; Singh et al., 2013; Toledo et al., 2014). Additionally, there is a lack of knowledge concerning the contribution of each hemisphere to an axial task such as gait. Recent clinical reports demonstrated that unilateral STN DBS can be as efficacious as bilateral DBS for treating gait impairment but the neural mechanism underlying this finding remains unknown (Ricciardi et al., 2015; Lizarraga et al., 2017; Rizzone et al., 2017).
Advances in concurrent sensing and stimulating technology from an implanted sensing neurostimulator (Activa® PC+S, Medtronic Inc.) have made it possible to record synchronized subthalamic neural activity and quantitative kinematic data in freely-moving human PD subjects (Quinn et al., 2015; Blumenfeld et al., 2017; Hell et al., 2018). Using such technology we recently demonstrated that Freezers exhibited increased STN sample entropy during periods of FOG suggesting that temporal fluctuations of STN neural activity may play an important role in FOG (Syrkin-Nikolau et al., 2017). Short fluctuations in beta (13–30 Hz) oscillations (beta bursts) are a physiological feature of normal cortical and subcortical motor circuitry and it has been proposed that short duration beta bursts represent normal signal processing in the sensorimotor network (Murthy and Fetz, 1992, 1996; Courtemanche et al., 2003; Feingold et al., 2015). This hypothesis has been supported by evidence that longer duration beta bursts in the resting-state, correlated with PD motor disability and were reduced in number by therapeutic doses of dopaminergic medication (Tinkhauser, Pogosyan, Little, et al., 2017; Tinkhauser, Pogosyan, Tan, et al., 2017).
To date no study has investigated the nature of STN beta bursts during gait and freezing of gait in freely moving human subjects with PD, nor whether the nature of dynamic fluctuations in beta activity is different in PD subjects with FOG (Freezers) compared to Non-Freezers. Moreover, whether beta burst properties are modulated by STN low or high frequency DBS, during tasks that elicit FOG, has yet to be explored. To optimize therapies for the treatment of FOG, these gaps need to be addressed. Using synchronized neural and kinematic recordings and concurrent sensing and neurostimulation in freely-moving PD subjects, we demonstrate that STN beta burst duration, measured during novel gait tasks that elicited FOG in freely-moving PD subjects, differentiated Freezers from Non-Freezers during gait without freezing and were longer in Freezers during FOG. We also demonstrate that STN DBS, either at 60 Hz or 140 Hz shortened the pathological, longer beta burst durations and improved gait impairment in Freezers but left unchanged the shorter burst durations and normal gait parameters in Non-Freezers.
Materials and Methods
Human subjects
Twelve PD subjects (7 male) had bilateral implantation of DBS leads (model 3389, Medtronic, Inc.) in the sensorimotor region of the STN using a standard functional frameless stereotactic technique and multi-pass microelectrode recording (MER) (Bronte-Stewart et al., 2010; Quinn et al., 2015). Dorsal and ventral borders of each STN were determined during MER, and the base of electrode zero was placed at the ventral border of the STN. The two leads were connected to the implanted investigative neurostimulator (Activa® PC+S, Medtronic, Inc. FDA Investigator Device Exemption approved). The preoperative selection criteria, surgical technique, and assessment of subjects have been previously described (Bronte-Stewart et al., 2010; Quinn et al., 2015). All subjects gave their written informed consent to participate in the study, which was approved by the Food and Drug Administration (FDA) and the Stanford School of Medicine Institutional Review Board (IRB). Long-acting dopaminergic medication was withdrawn over 24 h (72 h for extended-release dopamine agonists), and short-acting medication was withdrawn over 12 h before all study visits. Subjects were classified as a freezer or non-freezer based on the clinical history of a subject’s symptoms and/or if the subject displayed freezing behavior during the tasks.
Experimental Protocol
Recordings were collected in the Stanford Human Motor Control and Balance Laboratory. Experiments were performed off-medication and following at least 21 months of continuous, high frequency DBS. Subjects performed two gait tasks: Stepping in Place (SIP) and Forward Walking (FW). The tasks were performed a mean of 23.42 days (range 1 – 78 days) apart. During the SIP task, subjects began with 30 seconds of standing, at rest, followed by 100 seconds of alternating, self-paced stepping on adjacent dual force plates. For the FW task, subjects walked forward for 10 m, turned around and returned, and repeated this for a total of 40 m of straight walking. Subjects performed each of the tasks during randomized presentations of no, 60 Hz and 140 Hz DBS and were blinded to each condition. Due to the limitations of sensing capabilities of the Activa® PC+S, stimulation was applied in a monopolar configuration through the electrode in between the recording electrode pair (Quinn et al., 2015; Trager et al., 2016; Blumenfeld et al., 2017). The stimulation voltage was determined by a clinician, who optimized the clinical DBS parameters by monitoring the subject for adverse effects but had no other involvement in the research. Voltage was held constant across frequencies for each subject’s STN since it has been previously demonstrated that the difference in STN LFPs between 60 and 140 Hz DBS was frequency specific and not power specific (Blumenfeld et al., 2015). At least a five minute break was allotted in between experiments to allow the subjects to rest. Prior to experiments, two minutes were spent with no DBS for the effects of previous stimulation to wash out. We have previously demonstrated that after six months of chronic DBS, the effects of DBS on neural activity would have been washed out after two minutes as there was no statistical difference in the off therapy LFP beta power from recordings taken fifteen seconds after DBS was turned off and one hour later (Trager et al., 2016).
Data Acquisition and Analysis
Ground reaction forces were captured at 1000 Hz with two force plates on the Bertec system (Bertec Corporation, Columbus, OH). The SIP cycle began with the initial contact of one foot with the ground and ended just prior to contact of the same foot with the ground, consistent with the usual definition of a gait cycle. As described in our previous studies, gait cycle parameters were calculated from the force plate data and freezing episodes (FEs) were identified by a validated computerized algorithm (Nantel et al., 2011). Leg or shank angular velocity was measured during the forward walking task using wearable inertial measurement units (IMUs, APDM, Inc., Portland, OR), which were positioned in a standardized manner for all subjects and tasks on the top of the feet, on both shanks, on the lumbar, and chest trunk regions. All signals from the IMU tri-axial gyroscope were sampled at 128 Hz. Care was taken to align the sensor on the shank, so that the positive Z-axis was lateral and picked up the gait angular velocity in the sagittal plane. The data were filtered using a zero phase 8th order low pass Butterworth filter with a 9 Hz cut-off frequency and principal component analysis was used to align the angular velocity with the sagittal plane. Using the aligned Z angular velocity, the beginning of the swing phase (positive slope zero crossing), end of swing phase (subsequent negative slope zero crossing), and peak angular velocities (first positive peak following the beginning of swing phase) were identified. From these times, swing and stride times (time between consecutive peak angular velocities) were calculated. Swing and stride times were then used to calculate arrhythmicity and asymmetry during periods when the subject was not freezing. Asymmetry and arrhythmicity were defined as: asymmetry = 100 × |ln(SSWT/LSWT)|, where SSWT and LSWT correspond to the leg with the shortest and longest mean swing time over the trials, respectively and arrhythmicity = the mean stride time coefficient of variation (CV) of both legs (Plotnik et al., 2005, 2007; Nantel et al., 2011). A large stride time CV is indicative of a less rhythmic gait. Analysis was performed in MATLAB (version 8.2, The MathWorks Inc. Natick, MA, USA).
STN LFPs were recorded from electrode pair 0–2 or 1–3 of the DBS lead; see Supplementary Table 1 for details for each subject. Pre-amplified LFP signals were high-pass filtered at 0.5 Hz and low pass filtered at 100 Hz. The sampling rate for the LFP data was 422 Hz (10-bit resolution). The gain and center frequency parameters chosen for each LFP recording which was determined using a standardized protocol, further detailed in Blumenfeld et al., (Supplementary Material). This protocol provides a robust method by which the channels from which we record can be configured to produce the most reliable neural data possible, by avoiding channel overload and center frequency mismatch. Only the quality and resolution of the recordings from the sensing components of the device during stimulation are impacted by these parameters and they have no impact on the therapy (Blumenfeld et al., 2017). Uncompressed LFP data were extracted via telemetry using the Activa® PC+S tablet programmer and transferred to a computer for offline analysis in MATLAB. Using the movement state, power spectral density curves were created. A peak detection algorithm was used to identify the peak frequency in the beta band region for each STN. LFP recordings from the Activa® PC+S all feature a distinct 1/f falloff which served as a baseline fit in the power spectral density curves (Connolly et al., 2015). Using this baseline, crossings were identified as the first interpolated 1/f crossings to the left and right of the identified peak in the beta band (13–30 Hz) region. To capture peak activity, we took half of the frequency bandwidth between these two 1/f crossings and centered it on the peak beta frequency. This half width centered about the peak frequency was chosen as the ‘movement band’. The raw LFP signals were then filtered using a zero-phase 8th order Butterworth bandpass filter around this subject-specific frequency band and was squared, Fig. 1A and B.
Figure 1:
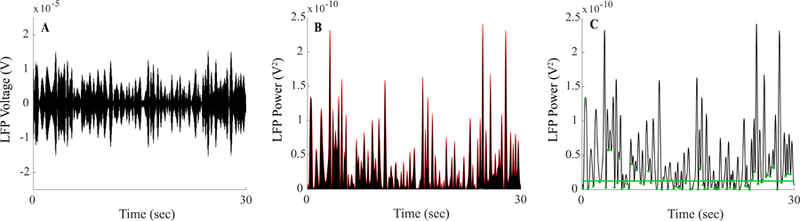
Beta burst determination process (A) Raw local field potential was band pass filtered around the STN specific frequency band of interest during 30 seconds of the movement state. (B) The filtered local field potential signal was squared and, an envelope, denoted in red, connected consecutive peaks from the squared signal. (C) The envelope of the filtered squared signal; green dots indicate the identified troughs of the envelope signal. The baseline of the envelope power, represented by the green line, was determined by taking two times the median of the trough power.
An amplitude envelope was calculated by linearly connecting consecutive peaks of the filtered and squared LFP cycle to form an outline of the maximum power, Fig. 1B. Bursts were identified as periods, during which the envelope amplitude was greater than a defined baseline. The baseline was determined by finding the troughs in the envelope and was set to two times the median trough power, Fig. 1C. The troughs were the local minima of the envelope and represented the local smallest peak amplitudes of the filtered and squared signal. The estimated noise of the device was calculated based on the bandwidth of the filter (BW) used. This was calculated using:
The ‘noise of the device’ refers to the estimated noise floor after the LFP has been filtered and squared. It has been reported in the literature as well as in the manufacturer datasheet that the Activa® PC+S neurostimulator has a nominal noise floor (150 nV/√Hz) which provides a lower limit, above which we can reliably detect LFP signals (Rouse et al., 2011; Stanslaski et al., 2012). The equation represents the calculation for total noise power based on our method of LFP signal processing for our burst analysis in which the raw LFP is bandpass filtered and squared. Troughs that fell below this estimated noise floor of the device were excluded from the baseline analysis. The duration of each burst was determined as the interval between successive crossings over the baseline of the amplitude envelope, Fig. 1C. The average power within each burst was calculated by taking the average power of the bandpass filtered and squared LFP over the duration of the burst. Since raw signal power can vary by multiple orders of magnitude, normalization of burst power was necessary to allow for comparison across subjects. Relative burst power for an individual burst was calculated by taking each burst’s average amplitude and dividing it by the mean of burst amplitudes across the resting-state in the subject’s 40–70 Hz band during no DBS. It has been previously reported and seen in this cohort that the LFP spectrum in the 40–70 Hz range in the resting-state (no DBS) was free of any artifact or peaks (Syrkin-Nikolau et al., 2017).
A three-dimensional spectrogram of the squared LFP, see Results, was constructed using a series of overlapping and consecutive envelope power calculations and was performed as follows: the envelope power was calculated in 1 Hz increments from 4 Hz to 34Hz using a 2Hz wide bandpass filter. The overlap has the benefit of capturing spectral events that occur at the edge of the filter but decreases the maximum spectral resolution to 3 Hz. Each envelope was then interpolated in time onto a rectilinear uniformly spaced grid and the power mapped to both the z-axis and the color heat map.
Synchronization of neural and kinematic recordings was achieved offline using internal and external instruments, using a data acquisition interface (Power1401) and Spike software (version 2.7, Cambridge Electronic Design, Ltd., Cambridge, England) (Quinn et al., 2015; Blumenfeld et al., 2017; Syrkin-Nikolau et al., 2017). Prior to the experiment, surface electrodes were attached to the skin. Subjects received a pulse of 20Hz/1.5V neurostimulation for a few seconds through either DBS lead. A signal artifact was detected by the implanted neurostimulator and Spike Software, the latter system using the surface electrode to record the stimulation artifact.
Statistics
Kruskal-Wallis One Way Analysis of Variance on Ranks was used for the comparison of beta burst dynamics between Freezers and Non-Freezers during no DBS. This decision was decided after a Normality Test (Shapiro-Wilk) was failed (P < 0.050) for these distributions. The effect of different stimulation frequencies on burst duration, the difference in burst duration during freezing episodes versus non-freezing episodes, and the difference in peak frequency in the spectral profile during movement was evaluated using one-way repeated measure ANOVAs. Following the normalization process, burst power was evaluated using the same statistical tests, as described for burst duration. The relationship between burst duration and power was investigated using a Pearson product moment correlation analysis to compare the distribution of each burst’s duration and power during no DBS on all subjects. The same analysis was used to investigate the relationship between stride time and burst duration.
To compare arrhythmicity, asymmetry, or stride time between groups, a one-way ANOVA was used. Paired t-tests were used to compare the effects of different stimulation frequencies on arrhythmicity, asymmetry, and stride time within each group for each task. Student’s t-tests were used for the comparison of demographics between the Freezer and Non-Freezer groups. Post hoc analyses were completed to compare between stimulation conditions. All statistical testing was performed in SigmaPlot (Systat Software, San Jose, CA) using two-tailed tests with significance levels of P < 0.05.
Results
Age, disease duration, preoperative off- and on- medication Unified Parkinson’s disease Rating Scale III (UPDRS III) scores were not significantly different between Freezers (N = 8) and Non-Freezer groups (N = 4), see Supplementary Table 2 for demographics. UPDRS III was also performed by the same experienced rater for each patient at each research visit, throughout the duration of the study. There was no significant difference in off medication / off stimulation UPDRS III at the time of the visit between the two groups (P = 0.103), see Supplementary Table 2. Out of the 24 hemispheres, 18 STNs were used for the neural analysis for the SIP task and 20 STNs were included in the neural analysis for the FW tasks when comparing the effects of DBS. One subject froze during the entire SIP task during all stimulation conditions. As a result, the subject was unable to complete the gait task and was excluded from the neural and kinematic portion of the analysis for that task. The remaining four hemispheres, from four subjects, were excluded due to either stimulation artifact that interfered with the beta band region of LFP recordings obtained from the respective lead or electrocardiogram (ECG) artifact.
Subject-specific movement band is conserved during different gait tasks
Fig. 2 demonstrates the neural and kinematic signals from a representative subject during the resting-state and during stepping in place and forward walking when no FOG occurred.
Figure 2:

State-dependent beta band profiles. Time frequency spectrograms and synchronized kinematic traces (A, D) and power spectral density (PSD) diagrams (E, F) from a representative subject during resting and movement states of the stepping in place (SIP) (A–C) and forward walking (FW) (D–F) tasks. Kinematics from the right and left foot of the subject are represented by the red and blue traces, respectively. Shaded blue region in PSD (B–C, E–F) diagram indicates movement band chosen for each STN.
For this subject, the LFP spectral profile was different during movement compared to the resting-state, in both STNs, but was similar between the SIP and FW tasks, Fig. 2B, C and E, F. This was representative of all subjects; in all cases, there was a change in the LFP profile from the resting to the movement-state, as we have demonstrated previously (Quinn et al., 2015; Syrkin-Nikolau et al., 2017). While the LFP profile during movement varied among subjects and between STNs, the peak frequency during movement was conserved between gait tasks in each STN (P > 0.05). A frequency band surrounding the peak during movement was chosen for each STN; see Methods, Supplementary Table 3 for the subject-specific frequency band for each task. We defined this frequency band as the movement band, which had an average bandwidth of 6.84 ± 2.08 Hz across subjects and was conserved between gait tasks for each STN (P > 0.05).
Freezers demonstrate longer duration beta bursts than non-freezers during ‘normal’ gait
Beta burst duration and power was calculated using movement bands, during both the resting and movement-states. Fig. 3 demonstrates that movement band burst durations and power was greater in a representative freezer compared to a representative non-freezer during ‘normal’ stepping without freezing episodes.
Figure 3:

Distribution of envelope power, burst duration and mean burst power with kinematics for Freezer and Non-Freezer. Beta burst distribution of a Non-Freezer and Freezer performing stepping in place (SIP) during no DBS. Envelope power of the squared LFP signal of a representative STN, with the green line indicating the baseline of the signal, is used to determine each individual beta bursts (represented by solid black circles) throughout the task (A, B). The distribution of burst duration (C, D) and relative burst power (E, F) is plotted along with synchronized kinematics (G, H).
The group analysis supported this observation; during SIP without FOG, burst durations were significantly longer in Freezers compared to Non-Freezers, (H(1) = 45.068, P <0.001). The same result was seen during the FW task without FOG (H(1) = 69.564, P <0.001). In contrast, there was no significant difference in burst duration between Freezers and Non-Freezers during rest. Freezers also had higher relative mean beta burst power than Non-Freezers while stepping without freezing (H(1) = 212.680, P < 0.001) but mean burst power was not different between the two groups during forward walking. In the entire cohort, a very weak relationship was seen between burst duration and burst power during rest, stepping in place and forward walking (R2 = 0.0625, P < 0.001; R2 = 0.108, P < 0.001; R2=0.006, respectively).
Freezers demonstrate more severe gait impairment during gait without freezing than Non-Freezers
Freezers exhibited longer average stride time (F(1) = 7.715, P = 0.012) and more arrhythmic stepping during SIP (F(1) = 6.194 P = 0.034) compared to Non-Freezers. Freezers demonstrated significantly more asymmetric gait during the FW task (F(1) = 6.640, P = 0.013) when compared to Non-Freezers. No significant difference between the two groups was seen in arrhythmicity or stride time during FW or in asymmetry during SIP.
Freezers exhibit longer burst durations during periods of FOG
The SIP task has provided a mechanism to investigate the difference in behavior between stepping without and with freezing episodes within the same trial (Nantel et al., 2011; Syrkin-Nikolau et al., 2017). Synchronized neural and kinematic recordings allow investigation of neural activity in real-time as the subject performs the task. Fig. 4 shows the envelope of power across the alpha/beta frequency spectrum presented as a three-dimensional spectrogram with the SIP traces, from a representative Freezer and Non-Freezer.
Figure 4:
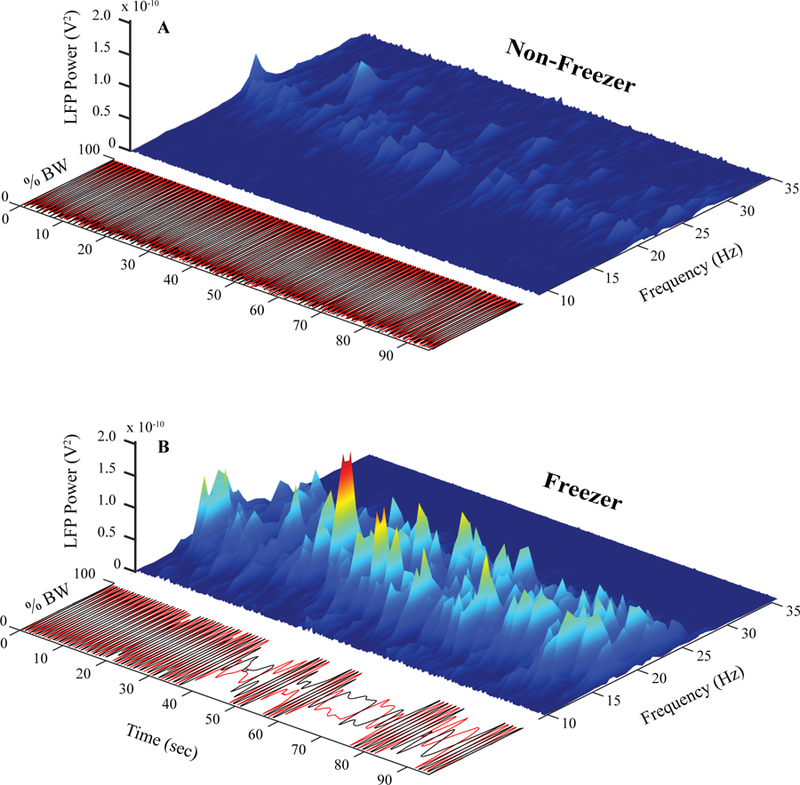
Three-dimensional spectrogram and kinematics during no DBS. Synchronized kinematics and three-dimensional spectrogram plotting time, frequency, and local field potential power for a representative Non-Freezer (A) and Freezer (B) throughout the duration of the stepping in place task during no DBS.
The Non-Freezer exhibited consistent alternating stepping during the task and there was evidence of low power bursts in the beta range that remained consistent throughout the task, Fig. 4A. In contrast the Freezer performed consistent stepping only for the first 20 seconds of the task, after which there was progressive degradation of the motor program leading to freezing episodes of varying durations, interspersed with short cycles of alternating stepping for the remainder of the task. There was evidence of bursting behavior that was variable in beta burst power and duration throughout the freezing behavior. Among the Freezers who froze during SIP (N= 5), burst duration was significantly longer during stepping with freezing compared to stepping when not freezing (F(1,815) = 13.114, P <0.001), but there was no significant difference in burst power during stepping with compared to without freezing episodes.
Bilateral hemispheric control of alternating stepping
Axial motor programs are influenced by neural activity from both cerebral hemispheres (Wagner et al., 2016; Pizzamiglio et al., 2018). However, Parkinson’s disease usually starts on one side of the body, which remains the more affected (MA) side and we have shown that resting-state beta band power is higher in the MA STN (Shreve et al., 2017). We investigated whether movement band burst durations varied between STNs, and whether this was related to gait impairment, during the SIP task.
Fig. 5A and B display the burst durations in the left (MA) and right (LA) STNs, respectively, of a representative Freezer during SIP. The mean (± 2 SD) of the burst durations of the Non-Freezers is shown on Fig. 5A and B (green lines). The mean burst duration in this subject was longer in the MA STN compared to the LA STN (0.85 sec, 0.55 sec respectively) during the periods of stepping without freezing, although both were longer than the mean Non-Freezer burst duration of 0.27 ± 0.04 sec. During the period of regular alternating stepping, 82.4% of the bursts in the MA STN were longer than those of the non-freezer group, Fig. 5A, whereas 66.7% of the burst durations in the LA STN were longer and outside the range of those of Non-Freezers, Fig. 5B. The burst durations in the LA STN increased 23 seconds after the start of the trial, after which the stepping performance became more impaired and freezing episodes occurred, Fig 5C. For the ten subjects, in whom bilateral STN data was available, the difference in the mean burst duration between hemispheres (STNs) of individuals strongly correlated with the difference in stride time between their legs (r = 0.853, P = 0.002), during SIP without freezing episodes, but there was no correlation between mean burst duration of each STN and mean stride time of the contralateral leg.
Figure 5:
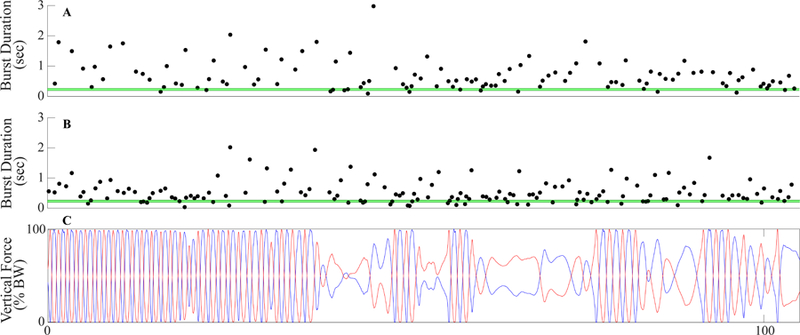
Bilateral beta burst duration distribution. Synchronized beta burst durations in the LSTN (MA) (A) and RSTN (LA) (B) with kinematics of the stepping in place task. The horizontal green lines represent the mean group burst duration for Non-Freezers ± two standard deviations.
Neurostimulation at 60 Hz and 140 Hz improves FOG and shortens beta burst durations
Subjects performed SIP and FW during randomized presentations of no DBS, 60Hz and 140 Hz STNDBS. Fig. 5 demonstrates the effect of 60 Hz and 140 Hz DBS on the gait of a representative Freezer during SIP.
During no DBS the subject exhibited asymmetric and arrhythmic alternating stepping and the algorithm identified three freezing episodes, Fig. 6A. Both 60 Hz and 140 Hz DBS improved the alternating stepping patterns; the subject spent 17.0% of the SIP task freezing during no DBS, 0% during 60 Hz DBS, and 4.5% during 140 Hz DBS. Table 1 summarizes the effects of 60 Hz and 140 Hz DBS on the kinematics of the entire FW and SIP tasks in the Freezer and Non-Freezer groups.
Figure 6:
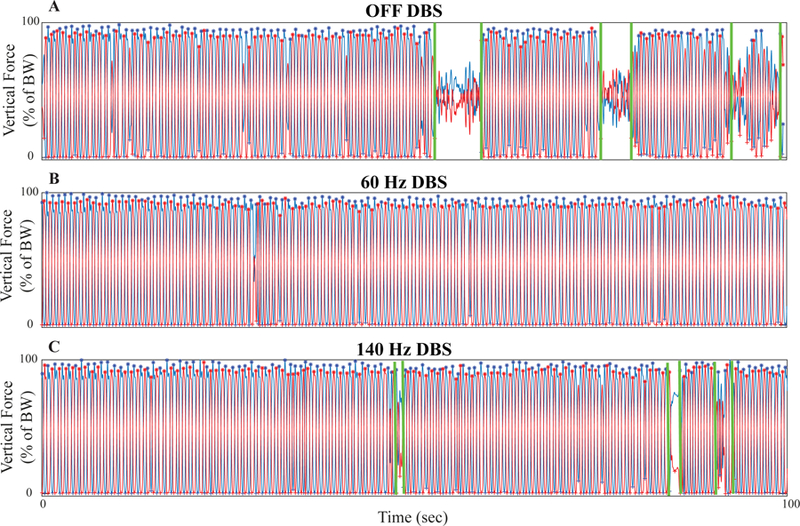
Stepping-in-place kinematics during no, 60 Hz, and 140 Hz STN DBS. Force plate traces from the stepping in place (SIP) task from a representative Freezer during the three stimulation conditions: (A) no DBS, (B) 60 Hz, and (C) 140 Hz DBS.
Table 1:
Kinematic Results for Freezers and Non-Freezers. Asymmetry, arrhythmicity, stride time, and percentage of task spent freezing (mean ± SD) for Freezers and Non-Freezers during the entire stepping in place (SIP) and forward walking (FW) tasks for the three stimulation conditions: no DBS, 60 Hz DBS, and 140 Hz DBS
| SIP | FW | ||||||
|---|---|---|---|---|---|---|---|
| OFF | 60 Hz | 140 Hz | OFF | 60 Hz | 140 Hz | ||
| Freezer | Asymmetry (%) | 26.74 ± 23.44 | 27.10 ± 21.42 | 15.99 ± 12.12 | 6.11 ± 4.11 | 5.01 ± 2.97 | 4.52 ± 3.39 |
| Arrhythmicity (CV %) | 54.04 ± 50.46 | 27.49 ± 33.23* | 29.34 ± 56.18 | 6.76 ± 3.29 | 5.18 ± 2.25*^ | 6.41 ± 2.68 | |
| Stride Time (sec) | 1.71 ± 0.68 | 1.44 ± 0.53*^ | 1.21 ± 0.44* | 1.14 ± 0.19 | 1.11 ± 0.15 | 1.10 ± 0.14 | |
| % of Task Freezing | 29.37 ± 38.27 | 11.01 ± 29.57 | 18.13 ± 34.58 | 0.17 ± 0.48 | 0% | 0% | |
| Non-Freezer | Asymmetry (%) | 9.54 ± 6.87 | 7.86 ± 7.10 | 7.25 ± 9.03 | 3.32 ± 1.88ǁ | 2.41 ± 2.50^ | 4.50 ± 2.23 |
| Arrhythmicity (CV) | 4.01 ± 0.81ǁ | 4.10 ± 1.40 | 4.00 ± 0.73 | 5.98 ± 4.37 | 4.96 ± 1.35 | 4.94 ± 1.38 | |
| Stride Time (sec) | 1.07 ± 0.15ǁ | 1.05 ± 0.14 | 1.06 ± 0.17 | 1.16 ± 0.06 | 1.15 ± 0.07 | 1.18 ± 0.10 | |
Significant difference between OFF during same task (P < 0.05)
Significant difference between 140 Hz during same task (P < 0.05)
Significant difference between Freezers in same task (P < 0.05)
During SIP without freezing, both 60- and 140 Hz DBS improved gait arrhythmicity in Freezers compared to no DBS (t(6) = −2.366, P = 0.016, t(6) = 2.994, P = 0.024, respectively). Stride time during SIP was significantly shorter during 140 Hz DBS compared to no DBS and during 60 Hz DBS (t(13) = 3.747, P = 0.002; t(13) = 3.727, P = 0.003). During SIP with freezing, 60 Hz DBS significant improved gait arrhythmicity compared to no DBS (t(6)=− 2.366, P = 0.016). In the FW task, 60 Hz DBS significantly improved gait arrhythmicity compared to both no DBS and to 140 Hz DBS (t(31) = 2.512, P = 0.017, t(31) = −2.787, P = 0.009). Among the eight Freezers, five exhibited FEs during no DBS, two during 60 Hz DBS, and two during 140 Hz DBS during SIP; subjects spent a total of 239.4 seconds freezing no DBS, 122 seconds during 60 Hz DBS, and 152.3 seconds during 140 Hz DBS. One subject experienced one FE during the no DBS condition of FW, this FE was excluded from the neural and kinematic analysis. None of the Freezers exhibited FEs during FW during either 60- or 140 Hz DBS. In Non-Freezers, gait parameters were similar to healthy controls (Nantel et al., 2011), DBS had no overall effect. Gait asymmetry during FW was lower during 60 Hz compared to 140 Hz DBS (t(15) = −2.276, p = 0.038).
STN DBS differentially affects burst duration and power in Freezers compared to Non-Freezers
There was a significant effect of stimulation conditions on burst duration among Freezers during stepping with freezing (F(2, 3007) = 24.27, P <0.001), stepping without freezing (F(2, 2803) = 22.247, P <0.001) and walking without freezing (F(2, 1202) = 3.376, P = 0.034). Both 60- and 140 Hz significantly shortened duration of bursts compared to no DBS but there was no significant difference in the effect of either frequency of DBS. Within the Non-Freezer group, there was no significant change in burst duration during no, 60 Hz, and 140 Hz DBS for either task.
Freezers experienced significant but different effects of stimulation conditions on burst power during the SIP (F(2, 2380) = 245.361, P <0.001) and FW tasks (F(2, 1195) = 136.211, P < 0.001). During SIP without freezing, both 60 Hz (P <0.001) and 140 Hz DBS (P <0.001) attenuated burst power compared to no DBS and 60 Hz attenuated burst power to a greater degree in comparison to 140 Hz DBS (P <0.001). Freezers during the FW task showed a similar attenuation of burst power during both 60 Hz (P <0.001) and 140 Hz (P <0.001) DBS compared to no DBS. Non-Freezers experienced a significant effect of stimulation on burst power in both SIP (F(2, 2282) = 89.353, P <0.001) and FW (F(2, 735) = 17.615, P <0.001) tasks. During the two gait tasks, 60 Hz (P <0.001) and 140 Hz DBS (P <0.001) attenuated burst power compared to no DBS and 140 Hz DBS significantly attenuated burst power compared to the 60 Hz DBS condition (P <0.001).
Discussion
For the first time, this study has demonstrated that beta burst duration and power, recorded during freely-moving gait, differentiated PD Freezers from Non-Freezers and was differentially modulated during low and high frequency STN DBS. STN beta burst durations were longer in Freezers compared to Non-Freezers during all gait tasks, in which gait was more impaired in Freezers. Within Freezers, burst durations were longer during periods of FOG compared to during gait without freezing. There was no difference in burst duration between the two groups in the resting-state and burst duration and was not correlated with mean power in either the resting or moving-state. DBS at 60- and 140 Hz shortened burst durations and improved FOG in Freezers and had no effect on the already shorter burst durations and normal SIP gait parameters in Non-Freezers.
Beta burst duration and power during gait is relevant to FOG
In this and in previous studies, it has been shown that STN LFP beta band power might attenuate but does not completely disappear during ongoing movement (Quinn et al., 2015; Blumenfeld et al., 2017; Syrkin-Nikolau et al., 2017; Hell et al., 2018). Instead, there is a distinct band of elevated power in the beta band region during both upper extremity movement and gait, which was the movement band. In this study, the movement band peak frequency was similar between the different gait tasks, suggesting conservation of the movement band in similar activity states; similarly, it has been demonstrated that the resting-state band is conserved in different resting-state postures (Quinn et al., 2015).
This is the first study to demonstrate the relevance of movement band beta burst duration and power during gait and FOG in freely-moving PD subjects. Previous studies investigating LFP beta burst durations and power have focused on the resting-state in both PD human subjects and in healthy, non-human primates (Murthy and Fetz, 1992, 1996; Feingold et al., 2015; Tinkhauser, Pogosyan, Little, et al., 2017; Tinkhauser, Pogosyan, Tan, et al., 2017). In those studies, longer resting-state beta burst durations correlated with increased motor disability in PD human subjects and dopaminergic medication shifted the distribution of burst durations toward shorter durations (Tinkhauser, Pogosyan, Little, et al., 2017; Tinkhauser, Pogosyan, Tan, et al., 2017). Longer durations of elevated STN beta band power reflect longer periods of neural oscillations and synchrony in beta frequency bands, which has been previously associated with the pathological Parkinsonian state (Kühn et al., 2006, 2008; Ray et al., 2008). Conversely, it has been proposed that short beta burst durations reflect normal sensorimotor processing (Murthy and Fetz, 1996; Courtemanche et al., 2003; Feingold et al., 2015; Tinkhauser, Pogosyan, Little, et al., 2017).
The state dependent nature of STN LFP beta activity and these findings highlight the value of identifying subject-specific movement bands to investigate the neural features of impaired movement such as FOG in PD, since there was no difference in burst duration between the two groups in the resting state. Previously, it has been shown that Freezers exhibited a lower average STN beta band power and higher beta entropy during gait without FOG than Non-Freezers (Syrkin-Nikolau et al., 2017). In this study, this is confirmed with distinct differences in subthalamic neural activity between Freezers and Non-Freezers using subject-specific dynamic features of the movement band during gait.
Bihemispheric control of gait - Can one hemisphere compensate to avoid FOG?
Parkinson’s disease is usually asymmetric as the disturbance of motor control (tremor, bradykinesia, rigidity) starts on one side of the body and that side usually remains the more affected side (Hoehn and Yahr, 1967; Louie et al., 2009). Similarly the pathological features of motor signs in PD, such as striatal dopamine denervation, also tend to be asymmetric and the resting-state alpha/beta band power is shown to be greater in the more affected STN in 112 STNs from 56 PD subjects (Marek et al., 1996; Shreve et al., 2017). Axial signs such as postural instability and FOG tend to occur after motor signs manifest on both sides of the body (Hoehn and Yahr, 1967). In this cohort, the mean movement band burst durations were different between STNs in individuals and the difference in mean burst duration between STNs during SIP without freezing was correlated with the mean difference in stride time between legs during the task. However, there was no correlation between individual STN burst durations and contralateral leg stride time. This suggests that there is bihemispheric influence on axial tasks such as SIP and that gait was more impaired when the difference in burst durations between hemispheres was greater. Our results support a network model of FOG suggesting that episodic-increased ‘crosstalk’ between frontostriatal circuits overloads the informational processing capacity in the dopamine depleted striatum, which leads to inhibition of brainstem locomotor regions and FOG and other studies proposing that gait asymmetry might be related to asymmetric neural dysfunction in the basal ganglia (Plotnik et al., 2005; Plotnik and Hausdorff, 2008; Peterson and Horak, 2016; Ehgoetz Martens et al., 2018). Burst durations varied during the SIP task, during which there were periods of relatively normal alternating stepping and periods of FOG. In certain Freezers, periods of SIP without FOG were associated with periods of relatively shorter burst durations in one STN, Fig. 5, and that the gait became more disordered leading to freezing episodes after burst durations became longer in both STNs. This suggests that the number of freezing episodes and severity of FOG could be mitigated by the compensatory influence of the STN with shorter burst durations, which are closer to those associated with normal sensorimotor processing (Feingold et al., 2015; Tinkhauser, Pogosyan, Little, et al., 2017; Tinkhauser, Pogosyan, Tan, et al., 2017).
The hypothesis that one hemisphere can compensate for the other in axial tasks such as gait has been supported by a recent study of unilateral versus bilateral STN DBS for gait, which demonstrated that there appeared to be a ‘dominant’ STN, unilateral stimulation of which was almost as efficacious as bilateral STN stimulation (Rizzone et al., 2017). Other studies have demonstrated that if the stimulation parameters of both STNs are adjusted to minimize the difference in stride times between legs, there was improvement in FOG (Fasano et al., 2011). It has also been demonstrated that the less affected hemisphere can compensate for the greater pathology of the more affected hemisphere in appendicular tasks such as finger tapping (Kishore et al., 2007; Trager et al., 2015). Furthermore, asymmetric therapies targeting the less affected side have been shown to be efficacious and comparable to more symmetrical therapies (Ricciardi et al., 2015; Lizarraga et al., 2017; Rizzone et al., 2017).
Pathological neural features and gait impairment in Freezers improve during STN DBS
Both neural and kinematic features of gait impairment and FOG improved with STN DBS compared to no DBS but there were differences between the effect of 60- and 140 Hz. The mean movement band burst durations of Freezers were shorter and mean burst power was attenuated during both 60- and 140 Hz DBS compared to no DBS. Furthermore, there was greater attenuation of burst power during 60 Hz compared to during 140 Hz. The gait arrhythmicity and stride time in SIP decreased during both 60- and 140 Hz DBS, and with greater improvement in stride time during 140 Hz compared to 60 Hz. The gait arrhythmicity of FW improved during 60 Hz but not during 140 Hz. There were fewer subjects, who exhibited FOG, and less overall time was spent freezing, during either frequency compared to no DBS.
This and our previous study investigating the effect of 60 Hz DBS on neural and kinematic features of progressive bradykinesia demonstrate that 60 Hz DBS promotes more regularity in ongoing movement (Blumenfeld et al., 2017). The irregularity of ongoing movement is the definition of progressive bradykinesia and of gait impairment, and may predict the transition from a Non-Freezer to a Freezer phenotype (Hausdorff et al., 2003; Plotnik and Hausdorff, 2008; Nantel et al., 2011). This provides a mechanism to explain the clinical studies showing that 60 Hz DBS improved FOG more than conventional high frequency DBS in PD subjects (Moreau et al., 2008; Xie et al., 2015).
Non-Freezers with less gait impairment and shorter burst durations exhibit modest changes during STN DBS
Non-Freezers’ gait parameters remained largely unchanged during either 60 Hz or 140 Hz DBS compared to no DBS. Similarly, the shorter movement band burst durations of both the SIP and FW tasks were not affected by either frequency of DBS. Mean burst power of both gait tasks was attenuated during 60- and 140 Hz DBS with the greater attenuation occurring during 140 Hz DBS. These results demonstrate that if the neural and kinematic features associated with FOG were not apparent then there was no change during either frequency DBS. Whereas in PD subjects exhibiting FOG, there was shift towards less pathological beta burst durations and gait impairment, during DBS.
A similar ‘if it isn’t broken, it doesn’t need fixing” effect of DBS was seen on aspects of postural instability (Bronte-Stewart et al., 2002; Shivitz et al., 2006). PD subjects with already normal postural sway in sensory deprived conditions showed no change after pallidotomy or during STN DBS. However, those demonstrating postural instability in these conditions improved after pallidotomy and during STN DBS. Gait parameters of the Non-Freezer were already within the range of normal values, as reported in previous studies, resulting in no significant change during DBS (Nantel et al., 2011; De la Casa-Fages et al., 2017). Together with the lack of neuromodulation of bursts, we propose there might be a more precise effect of DBS such that it preferentially modulates only pathological neural and kinematic aspects of behavior.
Significance of Neuromodulation of Beta Bursts
While continuous DBS has been an established therapy for PD, there have been promising studies that have successfully used neural driven adaptive DBS to improve behavioral outcomes (Little et al., 2013; Rosa et al., 2015; Arlotti et al., 2018). Recent studies have shown adaptive DBS shortened long beta bursts which were positively correlated with motor impairment (Tinkhauser, Pogosyan, Little, et al., 2017). By demonstrating for the first time that beta burst durations are relevant to gait impairment including FOG, this suggests that beta burst duration may be an important feature for adaptive DBS. Neural driven adaptive DBS systems that modulate longer beta bursts but not physiological short bursts may be a more efficient and effective therapy for FOG. These results contribute to the further development of control policy algorithms that can potentially use the dominant or more affected hemisphere to drive bihemispheric stimulation. Ultimately, these results bring us closer to optimizing a more patient-specific therapy for FOG.
Limitations
A limited number of Activa PC+S® investigative neurostimulators are allocated to centers which contributed to the small sample size of this cohort (Quinn et al., 2015; Blumenfeld et al., 2017; Syrkin-Nikolau et al., 2017). Additionally, there was an unequal distribution of subjects in the Freezer group compared to the Non-Freezer group. Despite our previous studies having a relatively even number of subjects in each group, over time some subjects have developed FOG and turned into Freezers (Syrkin-Nikolau et al., 2017). Progression into the freezer phenotype was not investigated in this study. In the future, we hope to further this question with a longitudinal study exploring the development of neural and kinematic features of FOG. Another limitation stems from the fact that these first-generation devices sometimes develop low-frequency ECG artifact in LFP recordings and/or experience sensing channel overload during stimulation (Blumenfeld et al., 2017); see Supplementary Material. Additionally, a longer habituation period following each stimulation paradigm could have produced alternate results. The interval used between no, 60 Hz and 140Hz DBS has been documented to be long enough for STN LFP power to return to baseline (Trager et al., 2016; Blumenfeld et al., 2017). We did not document that the performance of gait tasks returned to baseline but we randomized the order of the DBS frequencies and the no DBS condition and therefore, we do not believe that this affected the results. Our results reflect the acute effects of stimulation frequency and are congruent to what was reported in previous studies (Moreau et al., 2008; Xie et al., 2012; Vallabhajosula et al., 2015). Lastly, neither 60- nor 140 Hz DBS completely normalized neural or kinematic features of gait in Freezers. To enable concurrent sensing and stimulation through the DBS lead it was only possible to use monopolar stimulation and thus the DBS parameters may not have been optimal in comparison to the patient’s clinical stimulation settings.
Conclusion
The study demonstrates that longer duration of beta bursts in the movement band is a defining feature of freezing behavior in freely-moving PD subjects. Our results show that both 60- and 140Hz STN DBS can improve gait impairment in Freezers and shorten pathological beta burst durations. In contrast to Freezers, Non-Freezers exhibited shorter duration beta bursts and normal gait, which were unchanged on versus off DBS. These results support the hypothesis that in PD, pathological prolonged beta synchrony is superimposed upon physiological shorter periods of beta oscillations and synchrony which are critical to sensorimotor processing (Murthy and Fetz, 1992, 1996; Tinkhauser, Pogosyan, Little, et al., 2017). STN DBS acted to restore physiological sensorimotor processing by targeting pathological (longer) not physiological (short) beta burst durations, while improving gait in Freezers. The correlation between the difference in STN beta burst durations and the difference in leg stride times provided evidence that gait became more impaired when there was greater difference in burst duration between STNs. We also suggest that the STN with shorter burst durations may be able to compensate to maintain gait without freezing. With the unique opportunity to investigate the temporal dynamics of neural signals during gait, we have identified a potential biomarker for FOG that can instruct future closed-loop, adaptive DBS algorithms to prevent freezing behavior.
Supplementary Material
Highlights.
First study to investigate STN beta bursts during gait in freely moving PD subjects
Prolonged movement beta band burst durations were a pathological feature of FOG
STN DBS shortened pathological burst durations and improved gait in Freezers
Normal gait parameters, accompanied by shorter bursts, were not modulated by DBS
Greater difference in burst duration between STNs correlated with more impaired gait
Acknowledgements
We thank Varsha Prabhakar, Raumin Neuville, Tom Prieto, Amaris Martinez, Leanel Liwanag, Russell Mendonca, and Talora Martin for their support during the experiments and helpful comments. We would also like to thank our dedicated patient population who contributed their time to participating in our study (ClinicalTrials.gov Identifier: NCT02304848). This study was supported by the Michael J Fox Foundation, the NINDS Grant 5 R21 NS096398-02, the Robert and Ruth Halperin Foundation, the John A. Blume Foundation, the Helen M. Cahill Award for Research in Parkinson’s Disease, and Medtronic Inc., who provided the devices used in this study but no additional financial support.
Funding
This study was supported by the NINDS Grant 5 R21 NS096398-02, the Michael J Fox Foundation, the Robert and Ruth Halperin Foundation, the John A. Blume Foundation, the Helen M. Cahill Award for Research in Parkinson’s Disease, and Medtronic Inc., who provided the devices used in this study but no additional financial support.
Abbreviations
- FOG
freezing of gait
- DBS
deep brain stimulation
- LFP
local field potential
- STN
subthalamic nucleus
- SIP
stepping-in-place
- FW
forward walking
Footnotes
Declaration of interest
None of the authors have any conflicts of interest.
Supplementary Material
Supplementary data available in ‘Supplementary Material’ attached.
References
- Annic A, Moreau C, Salleron J, Devos D, Delval A, Dujardin K, et al. Predictive factors for improvement of gait by low-frequency stimulation in Parkinson’s disease. J. Park. Dis 2014; 4: 413–420. [DOI] [PubMed] [Google Scholar]
- Arlotti M, Marceglia S, Foffani G, Volkmann J, Lozano AM, Moro E, et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 2018; 90: e971–e976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov. Disord. Off. J. Mov. Disord. Soc 2004; 19: 871–884. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z, Koop MM, Prieto TE, Shreve LA, Velisar A, Quinn EJ, et al. Sixty-hertz stimulation improves bradykinesia and amplifies subthalamic low-frequency oscillations. Mov. Disord. Off. J. Mov. Disord. Soc 2017; 32: 80–88. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z, Velisar A, Koop MM, Hill BC, Shreve LA, Quinn EJ, et al. Sixty Hertz Neurostimulation Amplifies Subthalamic Neural Synchrony in Parkinson’s Disease. PLOS ONE 2015; 10: e0121067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte-Stewart H, Louie S, Batya S, Henderson JM. Clinical motor outcome of bilateral subthalamic nucleus deep-brain stimulation for Parkinson’s disease using image-guided frameless stereotaxy. Neurosurgery 2010; 67: 1088–1093; discussion 1093. [DOI] [PubMed] [Google Scholar]
- Bronte-Stewart HM, Minn AY, Rodrigues K, Buckley EL, Nashner LM. Postural instability in idiopathic Parkinson’s disease: the role of medication and unilateral pallidotomy. Brain J. Neurol 2002; 125: 2100–2114. [DOI] [PubMed] [Google Scholar]
- Connolly AT, Muralidharan A, Hendrix C, Johnson L, Gupta R, Stanslaski S, et al. Local field potential recordings in a non-human primate model of Parkinsons disease using the Activa PC + S neurostimulator. J. Neural Eng 2015; 12: 066012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J. Neurosci. Off. J. Soc. Neurosci 2003; 23: 11741–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Casa-Fages B, Alonso-Frech F, Grandas F. Effect of subthalamic nucleus deep brain stimulation on balance in Parkinson’s disease: A static posturographic analysis. Gait Posture 2017; 52: 374–380. [DOI] [PubMed] [Google Scholar]
- Ehgoetz Martens KA, Hall JM, Georgiades MJ, Gilat M, Walton CC, Matar E, et al. The functional network signature of heterogeneity in freezing of gait. Brain J. Neurol 2018; 141: 1145–1160. [DOI] [PubMed] [Google Scholar]
- Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat. Rev. Neurol 2015; 11: 98–110. [DOI] [PubMed] [Google Scholar]
- Fasano A, Herzog J, Seifert E, Stolze H, Falk D, Reese R, et al. Modulation of gait coordination by subthalamic stimulation improves freezing of gait. Mov. Disord. Off. J. Mov. Disord. Soc 2011; 26: 844–851. [DOI] [PubMed] [Google Scholar]
- Feingold J, Gibson DJ, DePasquale B, Graybiel AM. Bursts of beta oscillation differentiate postperformance activity in the striatum and motor cortex of monkeys performing movement tasks. Proc. Natl. Acad. Sci 2015; 112: 13687–13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N, Kao R, Fahn S. Freezing phenomenon in patients with parkinsonian syndromes. Mov. Disord. Off. J. Mov. Disord. Soc 1997; 12: 302–305. [DOI] [PubMed] [Google Scholar]
- Giladi N, McMahon D, Przedborski S, Flaster E, Guillory S, Kostic V, et al. Motor blocks in Parkinson’s disease. Neurology 1992; 42: 333–339. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp. Brain Res 2003; 149: 187–194. [DOI] [PubMed] [Google Scholar]
- Hell F, Taylor PCJ, Mehrkens JH, Bötzel K. Subthalamic stimulation, oscillatory activity and connectivity reveal functional role of STN and network mechanisms during decision making under conflict. NeuroImage 2018; 171: 222–233. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967; 17: 427–442. [DOI] [PubMed] [Google Scholar]
- Khoo HM, Kishima H, Hosomi K, Maruo T, Tani N, Oshino S, et al. Low-frequency subthalamic nucleus stimulation in Parkinson’s disease: a randomized clinical trial. Mov. Disord. Off. J. Mov. Disord. Soc 2014; 29: 270–274. [DOI] [PubMed] [Google Scholar]
- Kishore A, Espay AJ, Marras C, Al-Khairalla T, Arenovich T, Asante A, et al. Unilateral versus bilateral tasks in early asymmetric Parkinson’s disease: differential effects on bradykinesia. Mov. Disord. Off. J. Mov. Disord. Soc 2007; 22: 328–333. [DOI] [PubMed] [Google Scholar]
- Kühn A, Kupsch A, Schneider G-H, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur. J. Neurosci 2006; 23: 1956–1960. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kempf F, Brücke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J. Neurosci. Off. J. Soc. Neurosci 2008; 28: 6165–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilleeng B, Gjerstad M, Baardsen R, Dalen I, Larsen JP. Motor symptoms after deep brain stimulation of the subthalamic nucleus. Acta Neurol. Scand 2015; 131: 298–304. [DOI] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol 2013; 74: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga KJ, Luca CC, De Salles A, Gorgulho A, Lang AE, Fasano A. Asymmetric neuromodulation of motor circuits in Parkinson’s disease: The role of subthalamic deep brain stimulation. Surg. Neurol. Int 2017; 8: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie S, Koop MM, Frenklach A, Bronte-Stewart H. Quantitative lateralized measures of bradykinesia at different stages of Parkinson’s disease: the role of the less affected side. Mov. Disord. Off. J. Mov. Disord. Soc 2009; 24: 1991–1997. [DOI] [PubMed] [Google Scholar]
- Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, et al. [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology 1996; 46: 231–237. [DOI] [PubMed] [Google Scholar]
- Moreau C, Defebvre L, Destée A, Bleuse S, Clement F, Blatt JL, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 2008; 71: 80–84. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc. Natl. Acad. Sci. U. S. A 1992; 89: 5670–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Synchronization of neurons during local field potential oscillations in sensorimotor cortex of awake monkeys. J. Neurophysiol 1996; 76: 3968–3982. [DOI] [PubMed] [Google Scholar]
- Nantel J, Bronte-Stewart H. The effect of medication and the role of postural instability in different components of freezing of gait (FOG). Parkinsonism Relat. Disord 2014; 20: 447–451. [DOI] [PubMed] [Google Scholar]
- Nantel J, de Solages C, Bronte-Stewart H. Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson’s disease. Gait Posture 2011; 34: 329–333. [DOI] [PubMed] [Google Scholar]
- Peterson DS, Horak FB. Neural Control of Walking in People with Parkinsonism. Physiol. Bethesda Md 2016; 31: 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzamiglio S, Abdalla H, Naeem U, Turner DL. Neural predictors of gait stability when walking freely in the real-world. J. Neuroengineering Rehabil 2018; 15: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Ann. Neurol 2005; 57: 656–663. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Exp. Brain Res 2007; 181: 561–570. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Hausdorff JM. The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc 2008; 23 Suppl 2: S444–450. [DOI] [PubMed] [Google Scholar]
- Quinn EJ, Blumenfeld Z, Velisar A, Koop MM, Shreve LA, Trager MH, et al. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov. Disord. Off. J. Mov. Disord. Soc 2015; 30: 1750–1758. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, et al. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp. Neurol 2008; 213: 108–113. [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Ricciardi D, Lena F, Plotnik M, Petracca M, Barricella S, et al. Working on asymmetry in Parkinson’s disease: randomized, controlled pilot study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol 2015; 36: 1337–1343. [DOI] [PubMed] [Google Scholar]
- Rizzone MG, Ferrarin M, Lanotte MM, Lopiano L, Carpinella I. The Dominant-Subthalamic Nucleus Phenomenon in Bilateral Deep Brain Stimulation for Parkinson’s Disease: Evidence from a Gait Analysis Study. Front. Neurol 2017; 8: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M, Arlotti M, Ardolino G, Cogiamanian F, Marceglia S, Di Fonzo A, et al. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov. Disord. Off. J. Mov. Disord. Soc 2015; 30: 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse AG, Stanslaski SR, Cong P, Jensen RM, Afshar P, Ullestad D, et al. A chronic generalized bi-directional brain–machine interface. J. Neural Eng 2011; 8: 036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivitz N, Koop MM, Fahimi J, Heit G, Bronte-Stewart HM. Bilateral subthalamic nucleus deep brain stimulation improves certain aspects of postural control in Parkinson’s disease, whereas medication does not. Mov. Disord. Off. J. Mov. Disord. Soc 2006; 21: 1088–1097. [DOI] [PubMed] [Google Scholar]
- Shreve LA, Velisar A, Malekmohammadi M, Koop MM, Trager M, Quinn EJ, et al. Subthalamic oscillations and phase amplitude coupling are greater in the more affected hemisphere in Parkinson’s disease. Clin. Neurophysiol 2017; 128: 128–137. [DOI] [PubMed] [Google Scholar]
- Sidiropoulos C, Walsh R, Meaney C, Poon YY, Fallis M, Moro E. Low-frequency subthalamic nucleus deep brain stimulation for axial symptoms in advanced Parkinson’s disease. J. Neurol 2013; 260: 2306–2311. [DOI] [PubMed] [Google Scholar]
- Singh A, Plate A, Kammermeier S, Mehrkens JH, Ilmberger J, Bötzel K. Freezing of gait-related oscillatory activity in the human subthalamic nucleus. Basal Ganglia 2013; 3: 25–32. [Google Scholar]
- Stanslaski S, Afshar P, Cong P, Giftakis J, Stypulkowski P, Carlson D, et al. Design and Validation of a Fully Implantable, Chronic, Closed-Loop Neuromodulation Device With Concurrent Sensing and Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng 2012; 20: 410–421. [DOI] [PubMed] [Google Scholar]
- Syrkin-Nikolau J, Koop MM, Prieto T, Anidi C, Afzal MF, Velisar A, et al. Subthalamic neural entropy is a feature of freezing of gait in freely moving people with Parkinson’s disease. Neurobiol. Dis 2017; 108: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Foltynie T, Limousin P, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain 2012; 135: 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkhauser G, Pogosyan A, Little S, Beudel M, Herz DM, Tan H, et al. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain J. Neurol 2017a; 140: 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkhauser G, Pogosyan A, Tan H, Herz DM, Kühn AA, Brown P. Beta burst dynamics in Parkinson’s disease OFF and ON dopaminergic medication. Brain J. Neurol 2017b [DOI] [PMC free article] [PubMed]
- Toledo JB, López-Azcárate J, Garcia-Garcia D, Guridi J, Valencia M, Artieda J, et al. High beta activity in the subthalamic nucleus and freezing of gait in Parkinson’s disease. Neurobiol. Dis 2014; 64: 60–65. [DOI] [PubMed] [Google Scholar]
- Trager MH, Koop MM, Velisar A, Blumenfeld Z, Nikolau JS, Quinn EJ, et al. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson’s disease. Neurobiol. Dis 2016; 96: 22–30. [DOI] [PubMed] [Google Scholar]
- Trager MH, Velisar A, Koop MM, Shreve L, Quinn E, Bronte-Stewart H. Arrhythmokinesis is evident during unimanual not bimanual finger tapping in Parkinson’s disease [Internet]. J. Clin. Mov. Disord 2015; 2 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4711026/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhajosula S, Haq IU, Hwynn N, Oyama G, Okun M, Tillman MD, et al. Low-frequency Versus High-frequency Subthalamic Nucleus Deep Brain Stimulation on Postural Control and Gait in Parkinson’s Disease: A Quantitative Study. Brain Stimulat 2015; 8: 64–75. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Spildooren J, Heremans E, Wenderoth N, Swinnen SP, Vandenberghe W, et al. The neural correlates of upper limb motor blocks in Parkinson’s disease and their relation to freezing of gait. Cereb. Cortex N. Y. N 1991 2014; 24: 3154–3166. [DOI] [PubMed] [Google Scholar]
- Vervoort G, Bengevoord A, Strouwen C, Bekkers EMJ, Heremans E, Vandenberghe W, et al. Progression of postural control and gait deficits in Parkinson’s disease and freezing of gait: A longitudinal study. Parkinsonism Relat. Disord 2016; 28: 73–79. [DOI] [PubMed] [Google Scholar]
- Wagner J, Makeig S, Gola M, Neuper C, Müller-Putz G. Distinct β Band Oscillatory Networks Subserving Motor and Cognitive Control during Gait Adaptation. J. Neurosci. Off. J. Soc. Neurosci 2016; 36: 2212–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Kang UJ, Warnke P. Effect of stimulation frequency on immediate freezing of gait in newly activated STN DBS in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012; 83: 1015–1017. [DOI] [PubMed] [Google Scholar]
- Xie T, Vigil J, MacCracken E, Gasparaitis A, Young J, Kang W, et al. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology 2015; 84: 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


