Abstract
Introduction:
In 2010, the American Heart Association initiated Life’s Simple 7 with the goal of significantly improving cardiovascular health by the year 2020. The association of Life’s Simple 7 with risk of peripheral artery disease has not been thoroughly explored.
Methods:
Racially diverse individuals from the Multi-Ethnic Study of Atherosclerosis (2000–2012) were followed for incident peripheral artery disease (ankle brachial index ≤0.90) and decline in ankle brachial index (≥0.15) over approximately 10 years of follow-up. Cox and logistic regression were used to assess associations of individual Life’s Simple 7 components (score 0–2) and overall Life’s Simple 7 score (score 0–14) with incident peripheral artery disease and ankle brachial index decline, respectively, adjusted for age, sex, race/ethnicity, education, and income. Analyses were performed in 2016–2018.
Results:
Of 5,529 participants, 251 (4.5%) developed incident peripheral artery disease; 419 (9.8%) of 4,267 participants experienced a decline in ankle brachial index. Each point higher for the overall Life’s Simple 7 score was associated with a 17% lower rate of incident peripheral artery disease (hazard ratio=0.83, 95% CI=0.78, 0.88, p<0.001). Additionally, each point higher in overall Life’s Simple 7 was associated with a 0.94-fold lower odds of decline in ankle brachial index (OR=0.94, 95% CI=0.87, 0.97, p=0.003). Four components (smoking, physical activity, glucose, and blood pressure) were associated with incident peripheral artery disease and two (smoking and glucose) with decline in ankle brachial index.
Conclusions:
Better cardiovascular health as measured by Life’s Simple 7 is associated with lower incidence of peripheral artery disease and less decline in ankle brachial index. Use of the Life’s Simple 7 to target modifiable health behaviors may aid in decreasing the population burden of peripheral artery disease–related morbidity and mortality.
INTRODUCTION
Peripheral artery disease (PAD) is a significant public health problem, with approximately 8.5 million Americans currently affected.1 Over 10 years, the risk of cardiovascular (CV) mortality in those with PAD is 4.2 times higher in men and 3.5 times higher in women compared with those without PAD.2 In large meta-analyses, a low ankle brachial index (ABI; 0.90 or less), the standard clinical criterion for PAD diagnosis, was also associated with CV events and mortality, with approximately 1.5 to 2 times increased risk for myocardial infarction, stroke, CV mortality, and all-cause mortality when comparing those with low ABI with a normal ABI group (1.1–1.4).2,3 Functional decline, intermittent claudication, critical leg ischemia, and amputation resulting from PAD affect quality of life and lead to increased morbidity.4-6 As a result, useful preventive measures could decrease significant PAD-related morbidity and mortality.
In 2010, the American Heart Association’s (AHA’s) Strategic Planning Task Force and Statistics Committee designed the Life’s Simple 7 (LS7) metric for monitoring the AHA’s 2020 impact goal to improve CV health (CVH), which aims to improve the CVH of all Americans by 20% by the year 2020.7 LS7 can be used to calculate an overall CVH score comprised of an individual’s values for modifiable CV risk factors (blood pressure [BP], glucose, and cholesterol), and CV-related health behaviors (physical activity, BMI, diet, and smoking). Adherence to ideal CVH defined by LS7 has been associated with lower risk of stroke,8 diabetes,9 heart failure,10 and venous thromboembolism.11,12 In the Jackson Heart Study, having scored poorly on three or more LS7 components was associated with increased odds of prevalent PAD in African Americans.13 However, the extent to which this metric may be associated with preventing incident PAD or decline in ABI in a multi-ethnic population has not been fully established.
Thus, this investigation seeks to assess the association of the LS7 components and overall LS7, using a simple scoring method, with incident PAD and decline in ABI in non-Hispanic white, African American, Hispanic, and Asian men and women from the Multi-Ethnic Study of Atherosclerosis (MESA). Furthermore, this investigation seeks to determine whether these associations differed by race/ethnicity.
METHODS
Study Sample
MESA participants were recruited from six field sites in the U.S.: Forsyth County, North Carolina (Wake Forest); Northern Manhattan/Bronx, New York (Columbia); Baltimore/Baltimore County, Maryland (Johns Hopkins); St. Paul, Minnesota (University of Minnesota); Chicago, Illinois (Northwestern); and Los Angeles County, California (University of California, Los Angeles). Details of recruitment have been previously published.14 Briefly, MESA recruited 6,814 men and women ages 45 to 84 years free of clinical CV disease, with the cohort comprising 53% women and a racial/ethnic composition of approximately 38% non-Hispanic white, 28% African American, 23% Hispanic, and 11% Asian, primarily of Chinese descent. Exclusion criteria included a self-reported medical history of heart attack, angina, CV procedures, heart failure, cerebrovascular disease, active treatment for cancer, or pregnancy. The baseline exam (Exam 1) occurred from 2000 to 2002, with Exam 2 from 2002 to 2004, Exam 3 from 2004 to 2005, Exam 4 from 2005 to 2007, and Exam 5 from 2010 to 2012. MESA complies with the Declaration of Helsinki, and IRBs at each field site, as well as the Coordinating Center (University of Washington, Seattle), approved the study.
Measures
Systolic BP was measured in both the left and right brachial, dorsalis pedis, and posterior tibial arteries using a hand-held Doppler instrument with a 5-mHz probe. ABI was calculated for both the left and right sides as maximum systolic BP in the posterior tibial artery and dorsalis pedis, divided by the average of the left and right brachial pressures. As previous studies have shown a strong association between PAD and subclavian stenosis,15 in the event that left and right brachial pressures differed by ≥10 mmHg, the higher of the brachial pressures was used. If a pulse was detected when the cuff was inflated to 300 mmHg, ABI was classified as incompressible. For these analyses, the lower of an individual’s left and right leg ABI was used.
Incident PAD was defined as an ABI ≤0.90 at Exam 3 or Exam 5, and excluded participants with prevalent PAD (ABI <0.90) at the baseline exam, ABI>1.4 at Exams 1, 3, or 5, or without at least one follow-up ABI at Exam 3 or 5. An ABI >1.4 or incompressible is typically indicative of arterial stiffening caused by arterial medial calcification.16 This may distort the accuracy of the ABI measure when evaluating lower extremity occlusive disease. Prior work has demonstrated that a significant change in ABI ≥0.1517–19 may be clinically significant progression of disease and not indicative of measurement error.20 Thus, decline in ABI was defined as a decrease of ≥0.15 between Exam 1 and Exam 5, and excluded participants with missing ABI values at Exams 1, 5, or both exams. Participants with prevalent PAD (ABI ≤0.90) or ABI>1.4 were not excluded from the decline in ABI component of the analysis.
At Exam 1, baseline levels of LS7 metrics (smoking, BMI, physical activity, diet, BP, total cholesterol, and blood glucose) were measured and categorized into ideal, intermediate, and poor according to the AHA criteria7 with modifications in MESA as previously reported.21 Appendix Table 1 summarizes the definitions. Smoking status was assessed using questionnaires and participants were classified as current smokers, former smokers who quit within the past 12 months, quitters >12 months ago, or never smokers. BMI was calculated from height and weight measurements performed by study personnel. Physical activity was measured using a detailed questionnaire adapted from the Cross-Cultural Activity Participation Study.22 The questionnaire identifies the time and frequency spent in activities during a typical week in the previous month using 28 questions including household chores, lawn/yard/garden/farm, care of children/adults, transportation, walking (not at work), dancing and sport activities, conditioning activities, leisure activities, and occupational and volunteer activities. Minutes of walking, conditioning, and leisure activities were also included as exercise, and the minutes of moderate and vigorous exercise were calculated from the questionnaire. For BP, three measurements were taken after participants were seated and had rested for 5 minutes. The average of the last two measurements was used for analysis. Total cholesterol and blood glucose levels were obtained from fasting blood samples.14 Additionally, medication use in relation to BP, cholesterol, and glucose were verified by a medication inventory.
Diet assessment was performed using a validated 120-item food-frequency questionnaire modified from the Insulin Resistance Atherosclerosis Study instrument.23,24 Diet was defined according to AHA criteria using five components of healthy diet (high intake of fruits and vegetables, fish, whole grains, low intake of sodium and sugar-sweetened beverages).7 Of note, a coding error led to data being unavailable on the intake of 14 foods (fruit juice, dark/whole grains, fruit, salty snacks, other vegetables, leafy green vegetables, yogurt, potato, red meat, high fat and processed meat, high-fat dairy, desserts, cottage cheese, and legumes) in ~25% of MESA participants, the majority of whom were from two (Johns Hopkins and University of Minnesota) of six MESA field centers. Consequently, the data are not missing completely at random and may not be missing at random. To minimize biased analyses, imputation was conducted using sequential chained regression, implemented specifying multinomial or ordinal regression, with one model for all 14 items. Imputation models accounted for basic demographics and other auxiliary variables were selected based on high correlation with the food-frequency questionnaire or serving size items. There were a few values imputed for the other four study sites, but these were in very small numbers (<10% of the imputed data). The imputed dataset was used for this analysis.
This investigation used a previously defined scoring system12,21 where points were assigned to each LS7 component and summed: ideal=2 points, intermediate=1 point, and poor=0 points, for a total score ranging from 0 to 14 points.25 Study participants who scored 0 to 7 points were classified as having inadequate, those who scored 8 to 11 points were classified as having average, and participants who scored 12 to 14 points were classified as having optimum overall CVH.
Additional participant covariates were assessed by questionnaire at baseline and include participant age, sex, race/ethnicity, education (less than high school, high school graduate, some college, college graduate, or more than college graduate), and income (<$50,000 and ≥$50,000 per year).
Statistical Analysis
Unadjusted participant characteristics including age, sex, race/ethnicity, highest level of education completed, income, and LS7 scores were compared for incident PAD and decline in ABI groups using ANOVA for continuous variables and chi-square tests for categorical variables. Poisson distribution, a log-link function and an offset of ln(person-years) was used to calculate PAD incidence rates for LS7 individual component and overall CVH categories, using PROC GENMOD in SAS, version 9.3.
Each LS7 component was examined as a categorical variable: inadequate, average, and optimum. Overall LS7 score was assessed using the continuous 0–14 scale, as well as the overall categorical indications of inadequate, average, and optimum. Cox proportional hazard models were used to assess the association of individual LS7 components X overall LS7 with incident PAD. When defining incident PAD, time to event was the midpoint between exams at which the ABI was measured. To assess decline in ABI, logistic regression was performed after participants were dichotomized into those with ≥0.15 decline in ABI from Exam 1 to 5 versus those without. Models were adjusted for age, sex, race/ethnicity, education, and income. For models assessing decline in ABI, baseline ABI was included in the model.
Interactions of race/ethnicity X continuous LS7 score were assessed on a multiplicative scale for both incident PAD and decline in ABI outcomes, adjusted for age, sex, education, and income. A p-value <0.05 was used for statistical significance. All analyses were performed in 2016–2018 using SAS, version 9.3.
RESULTS
A total of 5,529 participants had complete LS7 information and met inclusion criteria to assess incident PAD. During a median follow-up time of 9.2 years, a total of 251 (4.5%) participants developed incident PAD. For decline in ABI, 4,267 participants had an ABI measure at both Exams 1 and 5, as well as complete LS7 information. During a median follow-up time of 9.4 years, 419 (9.8%) participants had a decline of≥0.15 in ABI. Baseline characteristics between those with and without incident PAD and those with and without a significant decline in ABI are presented in Table 1. Compared with those without incident PAD, participants who developed incident PAD were older, more likely to be female, African American, have less than a high school education, income <$50,000, have lower baseline ABI, and a lower total LS7 score. Participants who experienced a decline in their ABI were older, more likely to be African American, have less than a high school education, income <$50,000, have lower baseline ABI and lower total LS7 score compared with those without a significant change in ABI.
Table 1.
Exam 1 (2000–2002) Characteristics and Life’s Simple 7 Score by Incident PAD and Decline in the ABI in MESA
| Characteristic | No Incident PADa (n=5,278) |
Incident PADa (n=251) |
p-value | No decline in ABIb (n=3,848) |
Decline in ABIb (n=419) |
p-value |
|---|---|---|---|---|---|---|
| Age, years ± SD | 61 ± 10 | 68 ± 9 | <0.001 | 60 ± 9 | 63 ± 10 | <0.001 |
| Female, n (%) | 2,756 (52) | 155 (62) | 0.003 | 2,026 (53) | 213 (51) | 0.48 |
| Race/Ethnicity, n (%) | <0.001 | <0.001 | ||||
| Non-Hispanic white | 2,145 (41) | 82 (33) | 1,592 (41) | 169 (40) | ||
| Chinese | 676 (13) | 18 (7) | 495 (13) | 34 (8) | ||
| African American | 1,309 (25) | 105 (42) | 945 (25) | 136 (33) | ||
| Hispanic | 1,148 (22) | 46 (18) | 816 (21) | 81 (19) | ||
| Education, n (%) | 0.002 | 0.003 | ||||
| ≤High school | 1,764 (33) | 109 (43) | 1,167 (30) | 161 (38) | ||
| Some college | 1,503 (28) | 70 (28) | 1,123 (29) | 103 (25) | ||
| ≥Bachelor’s degree | 2,010 (38) | 72 (29) | 1,558 (41) | 155 (37) | ||
| Income >$50,000, n (%) | 2,196 (43) | 75 (32) | 0.001 | 1,737 (46) | 163 (41) | 0.03 |
| Life’s Simple 7 Scorec | 8.5 ± 2.0 | 7.5 ± 2.2 | <0.001 | 8.6 ± 2.1 | 8.2 ± 2.2 | <0.001 |
| Simple 7 categories, n (%) | <0.001 | <0.001 | ||||
| Inadequate (0–7 points) | 1,616 (31) | 128 (51) | 1,135 (30) | 157 (38) | ||
| Average (8–11 points) | 3,279 (62) | 118 (47) | 2,408 (63) | 242 (58) | ||
| Optimum (12–14 points) | 383 (7) | 5 (2) | 305 (8) | 20 (5) | ||
| Ankle Brachial Index ± SD | 1.13 ± 0.09 | 1.05 ± 0.09 | <0.001 | 1.12 ± 0.10 | 1.18 ± 0.13 | <0.001 |
Notes: Participant characteristics at Exam 1 (2000–2002) for those who developed incident PAD (ABI ≤0.9 any time after Exam 1) and/or experienced a decline in ABI (≥0.15 between Exam 1 [2002–2002] and Exam 5 [2010–2012]). Analyses include participants with complete information on Life’s Simple 7 components. Boldface indicates statistical significance (p≤0.05).
Incident PAD removes participants with prevalent PAD (ABI ≤0.9) at Exam 1 and those with ABI >1.4 at Exams 1, 3, or 5.
Decline in the ABI is defined as a decrease ≥0.15 in the ABI measure between Exam 1 and Exam 5.
On a 0–14 point scale.
PAD, peripheral artery disease; ABI, ankle brachial index; MESA, Multi-Ethnic Study of Atherosclerosis.
Adjusted rates of PAD per 1,000 person years were 17.8 for inadequate, 8.6 for average, and 4.1 for optimum CVH (Table 2). Each point higher on the continuous LS7 scale was associated with a 17% lower rate of incident PAD (hazard ratio [HR]=0.83, 95% CI=0.78, 0.88, p<0.001). Compared with inadequate CVH, participants with average and optimum health had a 49% lower rate (HR=0.51, 95% CI=0.38, 0.66, p<0.001) and a 76% lower rate (HR=0.24, 95% CI=0.10, 0.59, p<0.001) of incident PAD, respectively. Furthermore, each point higher on the continuous LS7 scale was associated with 0.94-fold lower odds of decline in ABI (OR=0.94, 95% CI=0.87, 0.97, p=0.003). Compared with those with inadequate CVH, participants with average and optimum health had a 0.84-fold lower odds (95% CI=0.58, 0.93, p=0.01) and a 0.54-fold lower odds (95% CI=0.32, 0.90, p=0.02) of decline in ABI over the follow-up period, respectively.
Table 2.
Associations of Life’s Simple 7 With Incident PAD and Decline in the ABI in MESA
| Variable | Incident PADb (n=5,529) |
Decline in ABIc (n=4,461) |
|||
|---|---|---|---|---|---|
| PAD incidence ratea (per 1,000-person years) |
HR (95% CI) | p-value | OR (95% CI) | p-value | |
| Per 1 point higher Categories | – | 0.83 (0.78, 0.88) | <0.001 | 0.94 (0.87, 0.97) | 0.003 |
| Inadequate (0–7 points) | 17.8 | 1.00 (ref) | 1.00 (ref) | ||
| Average (8–11 points) | 8.6 | 0.51 (0.38, 0.66) | <0.001 | 0.84 (0.58, 0.93) | 0.01 |
| Optimum (12–14 points) | 4.1 | 0.24 (0.10, 0.59) | <0.001 | 0.54 (0.32, 0.90) | 0.02 |
Notes: Presented are the incident rates (per 1,000-person years), hazard ratios for incident PAD (ABI ≤0.9 any time after Exam 1) and OR for experiencing a decline in ABI (≥0.15 between Exam 1 (2002–2002) and Exam 5 (2010–2012). All models adjusted for age, sex, and race/ethnicity. Boldface indicates statistical significance (p≤0.05).
Per 1-point increase on a 0–14 point scale.
Removes those with ABI >1.4 at Exams 1, 3, and 5. Models additionally adjusted for education and income.
Decline in the ABI is defined as a decrease ≥0.15 in the ABI measure between Exam 1 and Exam 5. Models additionally adjust for education, income, and baseline ABI.
PAD, peripheral artery disease; ABI, ankle brachial index; MESA, Multi-Ethnic Study of Atherosclerosis; HR, hazard ratio.
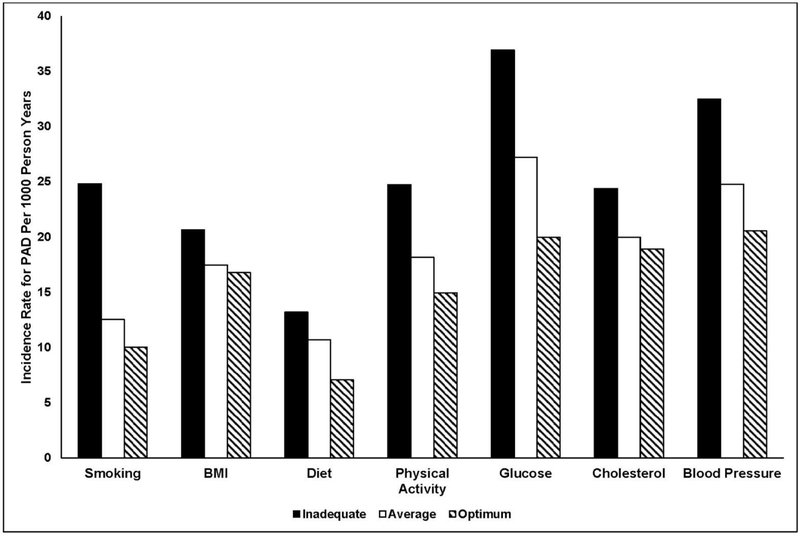
Adjusted PAD incidence rates varied across inadequate, average, and optimum categories for each individual LS7 component (Figure 1), with the highest rates in the inadequate category for all components. These associations appeared strongest for smoking, physical activity, glucose, and BP. Furthermore, after adjusting for age, sex, race/ethnicity, income, education and using the inadequate category as the reference group for each individual LS7 component, more optimal levels of smoking, physical activity, glucose, and BP were significantly associated with lower rates of incident PAD (Table 3). For decline in ABI, after adjusting for age, sex, race/ethnicity, income, education, and baseline ABI, more optimal levels of smoking, and glucose were significantly associated with lower odds of decline in ABI (Table 3). Categories of BMI, diet, and cholesterol were not associated with incident PAD or decline in ABI.
Figure 1. Incidence rates of PAD by individual Life’s Simple 7 components in MESA.
Notes: This figure displays the age, sex, and race-ethnicity adjusted incidence rates for PAD by each component of Life’s Simple 7. The y-axis displays the incidence rate for PAD per 1,000-person years, while the x-axis displays the inadequate, average, and optimum categories for each of the Life’s Simple 7 components.
PAD, peripheral artery disease; MESA, Multi-Ethnic Study of Atherosclerosis.
Table 3.
Associations of Individual Life’s Simple7 Components With Incident PAD and Decline in ABI in MESA
| Life’s Simple 7 component | At risk for incident PAD n/N |
Incident PADa HR (95% CI) |
Decline in ABIb OR (95% CI) |
|---|---|---|---|
| Smoking | |||
| Inadequate (Current smoker) | 49/696 | 1.00 (ref) | 1.00 (ref) |
| Average (Quit ≤12 months) | 91/1,927 | 0.53 (0.37, 0.77) | 0.52 (0.37, 0.71) |
| Optimum (Never/quit >12 months) | 111/2,906 | 0.43 (0.30, 0.61) | 0.45 (0.33, 0.61) |
| BMI | |||
| Inadequate (≥30 kg/m2) | 89/1,723 | 1.00 (ref) | 1.00 (ref) |
| Average (25–29.9 kg/m2) | 97/2,183 | 0.85 (0.64, 1.15) | 0.80 (0.62, 1.02) |
| Optimum (<25 kg/m2) | 65/1,623 | 0.81 (0.57, 1.15) | 0.93 (0.70, 1.23) |
| Healthy diet score | |||
| Inadequate (0–1 components) | 123/2,697 | 1.00 (ref) | 1.00 (ref) |
| Average (2–3 components) | 126/2,766 | 0.84 (0.64, 1.10) | 0.95 (0.86, 1.19) |
| Optimum (4–5 components) | 2/66 | 0.51 (0.13, 2.08) | 1.17 (0.44, 3.07) |
| Physical activity | |||
| Inadequate (None) | 75/1,195 | 1.00 (ref) | 1.00 (ref) |
| Average (<150 minutes/week) | 45/960 | 0.75 (0.51, 1.10) | 1.07 (0.76, 1.51) |
| Optimum (≥150 minutes/week) | 131/3,374 | 0.63 (0.47, 0.84) | 1.05 (0.80, 1.38) |
| Glucose | |||
| Inadequate (≥126 mg/dL) | 30/387 | 1.00 (ref) | 1.00 (ref) |
| Average (100–125mg/dL or treated to goal) | 57/954 | 0.78 (0.49, 1.23) | 0.51 (0.34, 0.78) |
| Optimum (<100 mg/dL) | 164/4,188 | 0.58 (0.38, 0.87) | 0.47 (0.33, 0.68) |
| Total cholesterol | |||
| Inadequate (≥240 mg/dL) | 30/520 | 1.00 (ref) | 1.00 (ref) |
| Average (200–239 mg/dL or treated to goal) | 112/2,383 | 0.81 (0.54, 1.22) | 0.94 (0.65, 1.36) |
| Optimum (<200 mg/dL) | 109/2,626 | 0.75 (0.49, 1.14) | 0.95 (0.66, 1.37) |
| Blood pressure | |||
| Inadequate (SBP ≥140 or DBP ≥90 mmHg) | 99/1,304 | 1.00 (ref) | 1.00 (ref) |
| Average (SBP 120–139 mmHg, DBP 80–89 mmHg, or treated to goal) | 107/2,313 | 0.79 (0.60, 1.05) | 0.93 (0.71, 1.22) |
| Optimum (SBP <120 mmHg and DBP <80mmHg) | 45/1,912 | 0.64 (0.45, 0.93) | 0.85 (0.63, 1.15) |
Notes: Presented are the incident PAD cases, hazard ratios for incident PAD (ABI ≤0.9 any time after Exam 1) and OR for experiencing a decline in ABI (≥0.15 between Exam 1 (2002–2002) and Exam 5 (2010–2012). All models adjusted for age, sex, and race/ethnicity. Boldface indicates statistical significance (p≤0.05).
Models also adjusted for income and education.
Models additionally adjusted for income, education, and baseline ABI
PAD, peripheral artery disease; ABI: ankle brachial index; MESA, Multi-Ethnic Study of Atherosclerosis; HR, hazard ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The strength of the continuous LS7 score X PAD association (Appendix Table 2) differed marginally by race/ethnicity (pinteraction=0.04) with the strongest associations found in non-Hispanics whites (HR=0.76, 95% CI=0.69, 0.85, p<0.001) and Hispanics (HR=0.78, 95% CI=0.67, 0.91, p=0.001). There were no significant interactions for decline in ABI (pinteraction=0.76).
DISCUSSION
In this prospective analysis of a multi-ethnic adult population-based cohort free of clinical CV disease at baseline, participants with higher LS7 scores of CVH were less likely to develop incident PAD or experience a decline in their ABI. Each one point higher on the LS7 score scale was associated with a lower rate of incident PAD and lower odds of decline in ABI (0.15 or more). Furthermore, participants with optimum and average CVH were significantly less likely to develop incident PAD or experience a decline in ABI over time compared with those with inadequate LS7 scores. These results were independent of age, sex, race/ethnicity, income, and education.
The AHA’s goal with the LS7 metric was to identify seven modifiable risk factors to optimize to promote and track individual and population progress in CVH improvement. The AHA goal of, “By 2020, to improve the cardiovascular health of all Americans by 20% while reducing deaths from cardiovascular diseases and stroke by 20%,”7 includes decreasing PAD-related death. PAD is associated with higher CV disease morbidity and mortality. For example, in a clinical vascular lab population, a significant decrease in ABI (0.15 or more) was found to be associated with greater CV and all-cause mortality.26 Although an ABI change of 0.15 or more is routinely used by vascular specialists to track progression of PAD, screening with the ABI is not routinely performed in asymptomatic patients in primary care clinics.27 Given that most individuals with PAD are asymptomatic,28 a measurable decline in ABI without reaching the PAD threshold may still indicate a significant risk. As a result, many high-risk individuals may not receive appropriate counseling and targeted therapies to reduce their CV risk. However, the results suggest that targeting the LS7 in adults should aid in preventing or slowing the progression of PAD.
Although higher overall LS7 scores were associated with lower rates of PAD and less decline in ABI, not all the individual LS7 components were significantly associated with these outcomes. In the analysis, LS7 categories for smoking, glucose level, BP, and physical activity were significantly associated with incident PAD. Not surprisingly, smoking and diabetes are consistently two of the strongest risk factors for the development of PAD.28 Kennedy et al.17 found that age, current cigarette use, diabetes, hypertension, and elevated cholesterol were associated with the development of PAD and decline in ABI. Additionally, lower levels of physical activity have been shown to be associated with prevalent PAD and worse outcomes in those with PAD.29 Even though targeting all LS7 metrics would be ideal, choosing one or two of the highest-yield modifiable risks may be the only reasonable option in some individuals.
In the Jackson Heart Study, African Americans with three or more poor categories of the LS7 had 34% higher odds of having prevalent PAD.13 In addition to an association in African Americans, the results in this investigation showed a significant prospective association between LS7 and incident PAD in Hispanics and non-Hispanic whites. Although the association was not statistically significant in Chinese Americans in MESA, this was likely due to the low number of incident PAD cases (n=18). Additionally, the results for decline in ABI did not appear to differ across race/ethnicity. These results support the use of LS7 to prevent PAD and decline in ABI in multiple racial/ethnic groups, which is one of the stated goals from the AHA.7 Previous studies of heart failure,10 stroke,8 diabetes,9 and venous thromboembolism11,12,30 showed robust associations of the LS7 across multiple race/ethnic groups. The results are consistent with other publications demonstrating the powerful associations of the LS7 with prevention of multiple health outcomes. Important future directions include comparing the predictive capability of the LS7 score with more traditional risk factor assessment, such as the Framingham score or the more modern pooled cohort risk equations.31
Limitations
There are limitations to this study. First, LS7 scores were assessed at baseline and this investigation did not examine whether participants changed categories of LS7 after the baseline exam. Additionally, measures of physical activity and diet were self-reported and subject to recall or reporting bias. The results demonstrating an association between physical activity and PAD may represent reverse causality. The ABI may not be sensitive to mild PAD, and those with mild PAD may be less physically active, while also being at greater risk of progressing to PAD severe enough to be detected by ABI. Furthermore, a scoring system was applied to the LS7 system not originally intended when introduced by the AHA. However, this scoring method was chosen for simplicity of implementation, interpretation, and consistency with other publications. Interpretation of the effect sizes should take this into consideration. Lastly, although MESA is a large, ethnically diverse population-based cohort study including participants from across the U.S., it was not designed to be nationally representative. A future study could examine if longitudinal improvement in LS7 scores confers a protective benefit against incident PAD. Strengths of the study include use of a large diverse population, lengthy follow-up time and protocol-based measures of ABI.
CONCLUSIONS
Higher scores on the AHA LS7 were associated with lower incident PAD and less decline in ABI. Preventive measures targeting LS7 components could assist with reducing PAD-related morbidity and mortality.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the Multi-Ethnic Study of Atherosclerosis (MESA) study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) at NIH via MESA contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169, and R01 HL098077 to SJB, and by grants UL1-TR-000040 and UL1-RR-025005 from National Center for Research Resources. Additionally, we like to thank NHLBI for University of California, San Diego training grant support (2T32HL079891).
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135(12):e726–e779. 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankle Brachial Index Collaboration, Fowkes FG, Murray GD, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heald CL, Fowkes FG, Murray GD, Price JF, Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: systematic review. Atherosclerosis. 2006;189(1):61–69. 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Guralnik JM, Ferrucci L, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117(19):2484–2491. 10.1161/CIRCULATIONAHA.107.736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDermott MM, Guralnik JM, Tian L, et al. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study). J Am Coll Cardiol. 2009;53(12):1056–1062. 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott MM, Liu K, Guralnik JM, et al. Functional decline in patients with and without peripheral arterial disease: predictive value of annual changes in levels of C-reactive protein and D-dimer. J Gerontol A Biol Sci Med Sci. 2006;61(4):374–379. 10.1093/gerona/61.4.374. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121 (4):586–613. 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 8.Kulshreshtha A, Vaccarino V, Judd SE, et al. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44(7):1909–1914. 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fretts AM, Howard BV, McKnight B, et al. Life’s Simple 7 and incidence of diabetes among American Indians: the Strong Heart Family Study. Diabetes Care. 2014;37(8):2240–2245. 10.2337/dc13-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folsom AR, Shah AM, Lutsey PL, et al. American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128(9):970–976.e2. 10.1016/j.amjmed.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folsom AR, Olson NC, Lutsey PL, Roetker NS, Cushman M. American Heart Association’s Life’s Simple 7 and incidence of venous thromboembolism. Am J Hematol. 2015;90(5):E92 10.1002/ajh.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson NC, Cushman M, Judd SE, et al. American Heart Association’s Life’s Simple 7 and risk of venous thromboembolism: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2015;4(3):e001494 10.1161/JAHA.114.001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins TC, Slovut DP, Newton R Jr., et al. Ideal cardiovascular health and peripheral artery disease in African Americans: results from the Jackson Heart Study. Prev Med Rep. 2017;7:20–25. 10.1016/j.pmedr.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44(3):618–623. 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48(5):1197–1203. 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy M, Solomon C, Manolio TA, et al. Risk factors for declining ankle-brachial index in men and women 65 years or older: the Cardiovascular Health Study. Arch Intern Med. 2005;165(16):1896–1902. 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 18.Bird CE, Criqui MH, Fronek A, Denenberg JO, Klauber MR, Langer RD. Quantitative and qualitative progression of peripheral arterial disease by non-invasive testing. Vasc Med. 1999;4(1):15–21. 10.1177/1358836X9900400103. [DOI] [PubMed] [Google Scholar]
- 19.McLafferty RB, Moneta GL, Taylor LM Jr., Porter JM Ability of ankle-brachial index to detect lower-extremity atherosclerotic disease progression. Arch Surg. 1997; 132(8):836–840. [DOI] [PubMed] [Google Scholar]
- 20.Cronenwett JL, Warner KG, Zelenock GB, et al. Intermittent claudication. Current results of nonoperative management. Arch Surg. 1984;119(4):430–436. 10.1001/archsurg.1984.01390160060012. [DOI] [PubMed] [Google Scholar]
- 21.Unger E, Diez-Roux AV, Lloyd-Jones DM, et al. Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7(4):524–531. 10.1161/CIRCOUTCOMES.113.000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999; 8(6):805–813. 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 23.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. 10.1016/0895-4356(90)90099-B. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9(5):314–324. 10.1016/S1047-2797198100070-2. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones DM. Improving the cardiovascular health of the U.S. population. JAMA. 2012;307(12):1314–1316. 10.1001/jama.2012.361. [DOI] [PubMed] [Google Scholar]
- 26.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52(21):1736–1742. 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 28.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–1526. 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 29.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292(4):453–461. 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 30.Ogunmoroti O, Allen NB, Cushman M, et al. Association between Life’s Simple 7 and noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2016;5(10):e003954 10.1161/JAHA.116.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.