Abstract
Objective:
Heterotaxy (HTX) congenital heart disease (CHD) patients with ciliary dysfunction (CD) have been shown to have increased postoperative respiratory morbidity. We hypothesized that non-HTX CHD infants with CD will also have increased postoperative morbidity, particularly respiratory complications.
Methods:
Sixty-three infants with non-HTX CHD undergoing cardiac surgery were enrolled. Tests commonly used to assess for CD, nasal nitric oxide (nNO) measurements and nasal epithelial ciliary motion (CM) assessment, were obtained. Baseline characteristics and postoperative outcomes were collected and analyzed.
Results:
Non-HTX CHD infants exhibited a high prevalence of abnormal CM (32%) and low nNO (39%). This was not correlated with demographics or surgical complexity. Infants with abnormal CM had increased odds of requiring non-invasive positive pressure ventilation (OR 6.5, CI 1.5–29.4, P = 0.016) and respiratory medication use (OR 4.4, CI 1.5–13.3, P = 0.01). In contrast, infants with low nNO showed evidence of abnormal pre- and postoperative systolic function (40% vs. 4%; P = 0.004, and 34% vs. 13%; P = 0.056, respectively) and had higher odds of acquiring infections (OR 4.9, CI 1.4–17, P = 0.014).
Conclusions:
Non-HTX CHD infants with abnormal CM showed increased postoperative morbidity associated with poor respiratory outcomes. In contrast, low nNO correlated with reduced hemodynamic function. These findings suggest screening for abnormal CM may allow perioperative interventions to reduce pulmonary morbidities. Whether low nNO may prognosticate poor hemodynamic function warrants further investigation.
Graphical abstract
INTRODUCTION
The survival of patients with critical congenital heart disease (CHD) has improved greatly with surgical advances and improvements in critical care. However, postoperative morbidity remains a significant problem, with respiratory complications being a substantial burden. A mechanistic link between CHD and the disturbance of airway clearance was suggested with the recent realization that motile cilia are required for both airway mucociliary clearance and embryonic development of visceral organ asymmetry, including the cardiovascular system1–4. Indeed, previous studies have shown CHD patients with heterotaxy (HTX), a birth defect involving randomized left-right patterning, have a high prevalence of motile ciliary dysfunction (CD), with those exhibiting CD suffering increased postoperative morbidity5–7.
Ciliary dysfunction in the HTX CHD patients was identified by measuring nasal nitric oxide (nNO) and obtaining nasal epithelial scrapes for ciliary videomicroscopy assessment, two tests commonly used to evaluate for primary ciliary dyskinesia (PCD), a sinopulmonary disease associated with very low nNO and immotile or dyskinetic airway ciliary motion (CM)8. Using these same tests, a study was conducted on outpatients with a broad spectrum of both HTX and non-HTX CHD revealing a similarly high prevalence of CD (combination of both abnormal CM and low nNO in 33% of HTX CHD and 17% of non-HTX CHD)9. Ciliary dysfunction in these patients was associated with increased sinopulmonary symptoms, with ciliary function status shown to be more important than HTX status for determining the risk of manifesting sinopulmonary symptoms9.
In this current study, we investigated the hypothesis that non-HTX CHD patients with CD have increased postoperative morbidity and more respiratory complications. With prior evidence that CHD patients with CD could have abnormal CM with normal nNO or low nNO with normal CM9, we further examined whether abnormal CM versus low nNO posed different risks for postoperative morbidity.
METHODS
Patient Recruitment
Patients <1 year of age with non-HTX CHD were prospectively enrolled from 2010 to 2014 at Children’s Hospital of Pittsburgh of UPMC (Pittsburgh, PA). This age group was chosen to minimize heterogeneity in the study population. This infant cohort is a subset of a larger database containing patients of all ages with all types of congenital heart disease. Informed consent was obtained from parents or guardians of patients. The study protocol was approved and performed in accordance with the Institutional Review Board of the University of Pittsburgh (PRO09090021).
Inclusion Criteria
CHD patients recruited in this study comprised those with malformations of the heart and/or great arteries without laterality defects. All non-HTX CHD patients who had at least one cardiac surgery prior to one year of age were eligible for inclusion in the study. Patients hospitalized greater than 3 months prior to surgical repair or those with iatrogenic airway injury were excluded from the study. Minor surgical encounters including thrombectomy, isolated vessel reconstruction, sternal closure, and extracorporeal membrane oxygenation (ECMO) cannulation and decannulation were excluded from analysis as independent surgical events.
Nasal Nitric Oxide Measurement
Nasal NO measurements were obtained preoperatively with a CLD 88 sp (Eco Physics AG; Ann Arbor, MI) NO analyzer according to established protocols with the tidal breath sampling technique10, 11. Infants were classified as having normal or low nNO based on the published prediction interval of normative infant nNO values12.
Ciliary Motion Analysis
Nasal epithelial tissue was obtained using rhino-probe curettage of the inferior nasal turbinate during the preoperative period. CM was examined using high-speed video microscopy using methods previously described6, 9. In order to rule out CM defects secondary to environmental insults like infection or allergy, nasal epithelia tissue was placed into culture after videomicroscopy for expansive growth and reciliation as described previously9. The reciliated tissue was examined by videomicroscopy to assess whether CM was normal or abnormal (Supplemental Methods). In cases where the initial scrape and reciliation results were discordant, the CM status was determined from the reciliation data.
Outcome Data Collection
We collected data from each major surgical encounter a patient experienced over the study period. All clinical staff were blinded to the patients’ CM and nNO assessments. Detailed demographic, surgical, and postoperative outcome data were abstracted from the electronic medical record utilizing patient discharge summaries, progress notes, operative reports, and daily flowsheets. Basic demographic data was collected including age at nNO and CM sampling, gender, race, and gestational age. Medical history and preoperative data were gathered including anatomic description of CHD, genetic evaluation (Supplemental Methods), comorbidities, age at surgery, preoperative weight, and preoperative systemic systolic ventricular function. Surgical mortality and risk adjustment scores were collected including Society of Thoracic Surgeons (STS) and European Association for Cardiothoracic Surgery (EACTS) Congenital Heart Surgery Database (STS-EACTS) categories, Aristotle Basic Complexity score (Aristotle), and Risk Adjustment in Congenital Heart Surgery (RACHS-1) score13–15.
Length of stay (LOS) parameters collected included postoperative LOS (PLOS), initial cardiac intensive care unit (CICU) LOS, and total CICU LOS, which included additional time spent in CICU due to readmission after initial transfer to the step-down unit. Ventilation parameters collected included mechanical ventilation (MV) days, non-invasive positive pressure ventilation (NIPPV) days, and supportive ventilation days, which included high-flow nasal cannula and nasal cannula. Duration of postoperative medication requirements were recorded after each major surgical encounter.
Data regarding postoperative complications were gathered including reintubations, development of pleural effusions and/or pneumothoraces, necrotizing enterocolitis, and infections. Failed extubation was defined as a patient being reintubated less than 24 hours after extubation. Pleural effusions were only counted if they required chest tube drainage. Intrinsic airway disease was noted for patients with endoscopic evidence of external or internal airway compression or vocal cord dysfunction. Pneumothorax was defined as clinical symptoms with radiographic evidence of air in the pleural space. Diaphragm paresis was noted for patients with sonographic or fluoroscopic evidence of paresis. Qualitative systemic ventricular function was abstracted from transthoracic echocardiography assessments by pediatric cardiologists who were blinded to all aspects of the study. Viral infection was defined as presence of clinical symptoms and positive nasal viral panel. Bacterial infection was defined as presence of clinical symptoms and positive blood culture and excluded pneumonia and tracheitis. Fungal infection was defined as presence of clinical symptoms and positive fungal culture. Pneumonia (bacterial) was defined as clinical symptoms, presence of infiltrate on chest x-ray, and infectious disease recommendation to treat with antimicrobials for bacterial pneumonia. Tracheitis was defined as clinical symptoms and positive tracheal culture.
Statistical Analysis
Summary statistics such as proportion and median with interquartile range (IQR) were used to describe patient characteristics and their postoperative outcomes. For patient characteristics, Chi-square test was used to compare categorical variables and Fisher’s exact test was applied when appropriate, and Wilcoxon rank-sum test was used to compare continuous variables between subgroups. For baseline surgical characteristics and postoperative outcomes, mixed-effects models were applied to adjust for the intra-subject correlation among multiple surgical events of individual patients. As the postoperative continuous outcomes were right- skewed, the linear mixed models (LMM) for repeated-measures were used to analyze the effect of CM and nNO on the log-transformed continuous outcomes that were approximately normal after transformation. The generalized linear mixed models (GLMM) for repeated-measures were used for assess the differences in the categorical outcomes between the abnormal and normal CM or nNO subgroups. Multivariate analysis of adverse postoperative outcomes based on GLMM models was performed to examine the effect of CM or nNO controlling for baseline surgical characteristics (age, weight, surgical severity, ventricular function, and single ventricle status). All tests were two-sided, and a P-value ≤0.01 was considered statistically significant. A more conservative significance level of 0.01 was used to adjust for multiple comparisons. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and STATA 12.1 (STATA Corp.; College Station, Texas).
RESULTS
Baseline Demographics
We recruited 63 infants with a broad spectrum of critical non-HTX CHD (Figure 1, Supplemental Table 1). Two patients had one of two surgical encounters excluded for hospitalization >3 months prior to surgical repair (1) and for iatrogenic tracheal injury (1). One patient was excluded due to hospitalization > 3 months prior to surgical repair. During the study period, the survival rate was 99% with only one patient death reported in the immediate postoperative period, precluding analysis of postoperative mortality.
Figure 1. Spectrum of CHD in study participants.
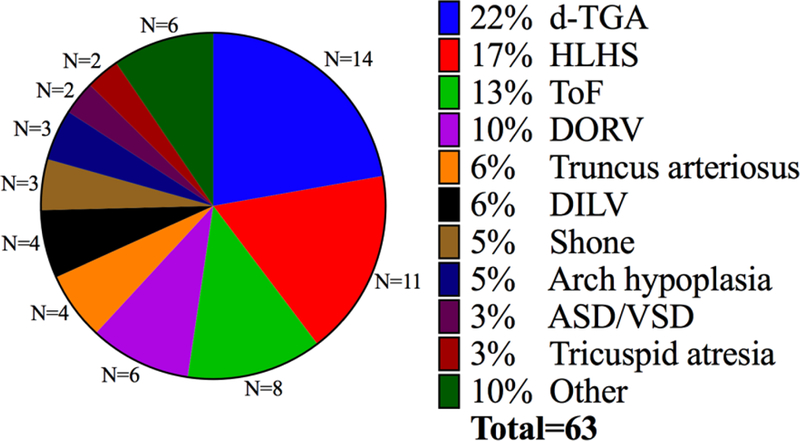
The distribution of non-HTX CHD observed in study participants is summarized in this pie chart. “Other” category includes one patient each with pulmonary atresia/intact ventricular septum, isolated infradiaphragmatic total anomalous pulmonary venous return, double outlet left ventricle, critical aortic stenosis, complete atrioventricular septal defect, and anomalous left coronary artery from the pulmonary artery.
The most common cardiac abnormalities in study participants were d-transposition of the great arteries (14 patients) and hypoplastic left heart syndrome (11 patients). Thirty-six (57%) patients exhibited conotruncal defects (Supplemental Table 2). The majority of the patients were male (67%), full term (97%) and Caucasian (87%), with the remainder being African American (6%), Hispanic (3%), Arab (2%) and Asian (2%) (Supplemental Table 2). Genetic evaluation, carried out by microarray analysis as the standard of care (Supplemental Methods), was abnormal in 24% of study participants (Supplemental Table 2). Detailed information regarding anatomic subtypes of CHD, comorbidities of each patient and genetic abnormalities is provided in (Supplemental Table 1).
Baseline Demographics Stratified by Ciliary Motion and nNO Status
Nasal scrapes were obtained in 59 (94%) patients for CM analysis and nNO measurements were performed in 56 (89%) (Figure 2A, (Supplemental Table 3. Abnormal CM was observed in 32% of the patients. Low nNO was observed in 39% of the patients. For patients (N = 52) with both CM and nNO assessments, 6% had both abnormal CM and low nNO (Figure 2B, (Supplemental Table 3). There was no significant difference in recruitment age or prematurity, gender, race, or presence of an abnormal genetic evaluation in patients with abnormal versus normal CM and low versus normal nNO (Supplemental Table 2). Patients with low nNO were recruited at an older age than patients with normal nNO (24 [6–53] days vs. 5 [3–21] days, respectively; P = 0.038). Conotruncal defects were significantly more common in patients with abnormal CM (84% vs. 48%, P = 0.01).
Figure 2. Summary of ciliary motion and nasal nitric oxide findings in non-HTX CHD patients.
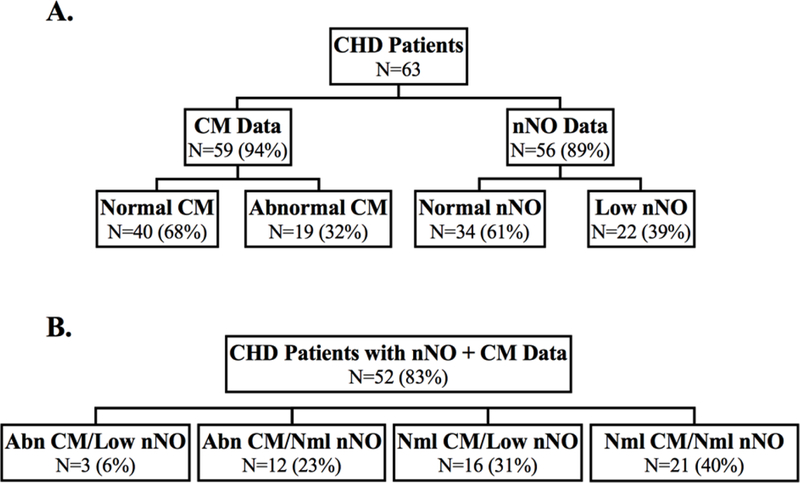
(A) Distribution of normal/abnormal CM and nNO in all non-HTX CHD patients.
(B) Distribution of normal/abnormal CM and nNO in non-HTX CHD patients with both CM and nNO data.
Preoperative Surgical Data
The 63 CHD infants recruited into this study collectively had 95 surgical encounters available for analysis. The median age of recruitment was 10 [3–29] days (Supplemental Table 2). The median age at surgery was 14 [7–91] days (Table 1). Single ventricle palliation was performed in 40 (42%) patient encounters with surgeon one (of two) performing 68 (72%) of the surgical cases.
Table 1.
Baseline surgical parameters in CHD patients by ciliary motion and nasal nitric oxide*
| All Surgical Events (n = 95) |
Events with CM Data (n=91) |
Events with nNO Data (n=83) |
|||
|---|---|---|---|---|---|
| Normal (n = 58) |
Abnormal (n = 33) |
Normal (n = 48) |
Low (n = 35) |
||
| Age at Surgery (days) | 14 (7–91) | 11 (6–84) | 30(9–127) | 9 (7–92) | 30 (8–91) |
| P= 0.060 | P=0.23 | ||||
| Preoperative Weight (kg) | 3.7 (3.1–5.0) | 3.4 (3.1–4.7) | 4.3 (3.2–6.1) | 3.7 (3.1–4.9) | 3.5 (3.1–5.1) |
| P= 0.054 | P= 0.99 | ||||
| STS-EACTS category† | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| P= 0.65 | P=0.47 | ||||
| Abn PreOp Sys Fxn‡, n (%) | 21 (22%) | 13 (22%) | 7 (21%) | 2 (4%) | 14 (40%) |
| P= 0.64 | P= 0.004 | ||||
| Single ventricle palliation, n (%) | 40 (42%) | 30 (52%) | 10 (30%) | 14 (29%) | 20 (57%) |
| P= 0.15 | P= 0.061 | ||||
| Surgeon 1, n (%) | 68 (72%) | 41 (71%) | 24 (73%) | 34 (71%) | 26 (74%) |
| P= 0.87 | P= 0.51 | ||||
| CPB Time (min) | 88 (62–115) | 89 (66–112) | 89 (55–134) | 86 (57–120) | 91(65–108) |
| P= 0.32 | P= 0.96 | ||||
| XC Time (min) | 27 (0–53) | 23 (0–44) | 44 (0–54) | 30 (0.8–55) | 26 (0–46) |
| P= 0.36 | P= 0.54 | ||||
| DHCA, n (%) | 28 (30%) | 21 (36%) | 7 (21%) | 14 (29%) | 11 (31%) |
| P= 0.15 | P= 0.83 | ||||
Data are median (IQR) or n (%). P values obtained from the linear mixed models and generalized linear mixed model for continuous and categorical variables, respectively.
Empirically based mortality stratification, categories of 1–5 with higher numbers implying higher mortality risk, based on of the Society of Thoracic Surgeons (STS) and European Association for Cardiothoracic Surgery (EACTS) Congenital Heart Surgery Database
Abn Preop Sys Fxn = abnormal preoperative systolic ventricular function of systemic ventricle. CPB = cardiopulmonary bypass, XC = aortic cross clamp, DHCA = deep hypothermic circulatory arrest
Of the 95 surgical events, 36% occurred in patients with abnormal CM, 42% in patients with low nNO, and 8% in patients with both abnormal CM and nNO. There was no difference in preoperative weight, STS-EACTS category, RACHS-1 or Aristotle scores, surgeon, median cardiopulmonary bypass time, cross-clamp time, or deep hypothermic circulatory arrest time among patients stratified either by CM or nNO status (Table 1). There was a trend towards younger age at surgery in patients with normal CM (11 [6–84] days vs. 30 [9–127] days; P= 0.06). No difference was found in preoperative systolic function by CM status. However, abnormal systolic function was significantly associated with low nNO (40% vs. 4%; P = 0.004) (Table 1).
Postoperative Outcome Assessments
Our patient cohort had a median PLOS of 15 [10–24] days (Table 2). The median total CICU LOS was 8 [5–11] days, and initial CICU LOS was 7 [5–11] days. In patients with low nNO, initial CICU LOS (10 [5–12] days vs. 7 [4–8] days; P = 0.042) and total CICU LOS (10 [512] days vs. 7 [4–9] days; P = 0.041) were longer (Table 3). These low nNO patients also had a higher prevalence of decreased postoperative systolic function (34% vs. 13%; P = 0.056). The decreased systolic function in the low nNO patients persisted in nine of 16 patients up to the time of hospital discharge. Low nNO was also correlated with an increase in number of days treated with milrinone (6 [4–8] days vs. 4 [3–6] days; P = 0.021), dexmedetomidine (6 [3–8] days vs. 3 [2–6] days; P = 0.002), and nicardipine requirement (34% vs. 6%; P = 0.034) (Table 3). All other postoperative medications used, including inhaled NO (iNO), were not different among patients with normal or low nNO.
Table 2.
Association between abnormal ciliary motion and postoperative outcomes*
| All Surgical Events (n=95) |
Surgical Events for patients with CM Data (n = 91) |
|||
|---|---|---|---|---|
| Normal CM (n=58) |
Abnormal CM (n=33) |
P | ||
| Postoperative LOS (days) | 15 (10–24) | 16 (11–26) | 13 (6–19) | 0.057 |
| Total CICU LOS (days) | 8 (5–11) | 8 (5–11) | 7(4–10) | 0.19 |
| Initial CICU LOS (days) | 7 (5–11) | 8 (5–11) | 7(4–10) | 0.19 |
| Abn PostOp Sys Fxn†, n (%) | 22 (23%) | 15 (26%) | 6 (18%) | 0.37 |
| ECMO, n (%) | 10 (11%) | 6 (10%) | 4 (12%) | 0.8 |
| Delayed sternal closure, n (%) | 58 (61%) | 39 (67%) | 17 (52%) | 0.15 |
| Mechanical ventilation, n (%) | 84 (88%) | 50 (86%) | 30 (91%) | 0.58 |
| Supportive ventilation, n (%) | 88 (93%) | 53 (91%) | 31 (94%) | 0.66 |
| NIPPV‡, n (%) | 24 (25%) | 9 (16%) | 14 (42%) | 0.012 |
| Reintubation, n (%) | 15 (16%) | 7 (12%) | 8 (24%) | 0.27 |
| Respiratory medication (any) use, n (%) | 31 (33%) | 11 (19%) | 17 (52%) | 0.004 |
| Albuterol use, n (%) | 22 (23%) | 8 (14%) | 14 (42%) | 0.007 |
| Dornase alfa use, n (%) | 3 (3%) | 0 (0%) | 2 (11%) | 0.10$ |
| Ipratropium use, n (%) | 3 (3%) | 0 (0%) | 3 (16%) | 0.03$ |
| Overall infection, n (%) | 28 (30%) | 15 (26%) | 12 (36%) | 0.3 |
| Bacterial Infection, n (%) | 25 (26%) | 14 (24%) | 11(33%) | 0.35 |
| Viral Infection, n (%) | 4 (4%) | 0 (0%) | 3 (16%) | 0.03$ |
Data are median (IQR) or n (%). P-values obtained by from the linear mixed models and generalized linear mixed model for continuous and categorical variables, respectively.
Abn Postop Sys Fxn = abnormal postoperative systolic ventricular function of systemic ventricle
NIPPV = Non-invasive positive pressure ventilation
P-value by Fisher’s exact test for testing whether the percentages are difference between the normal CM (n = 40) and abnormal CM (n = 19) patients.
Table 3.
Association between low nasal nitric oxide and postoperative outcomes*
| All Surgical Events (n=95) |
Surgical Events for patients with nNO Data (n = 83) |
|||
|---|---|---|---|---|
| Normal nNO (n=48) |
Low nNO (n=35) |
P | ||
| Postoperative LOS (days) | 15 (10–24) | 15 (10–20) | 18 (10–29) | 0.28 |
| Total CICU LOS (days) | 8 (5–11) | 7 (4–9) | 10 (5–12) | 0.041 |
| Initial CICU LOS (days) | 7 (5–11) | 7 (4–8) | 10 (5–12) | 0.042 |
| Abn PostOp Sys Fxn†, n (%) | 22 (23%) | 6 (13%) | 12 (34%) | 0.056 |
| ECMO, n (%) | 10 (11%) | 6 (13%) | 3 (9%) | 0.58 |
| Delayed sternal closure, n (%) | 58 (61%) | 28 (58%) | 23 (66%) | 0.50 |
| Mechanical ventilation, n (%) | 84 (88%) | 42 (88%) | 31 (89%) | 0.91 |
| Supportive ventilation, n (%) | 88 (93%) | 46 (96%) | 32 (91%) | 0.42 |
| NIPPV‡, n (%) | 24 (25%) | 11 (23%) | 8 (23%) | 0.92 |
| Reintubation, n (%) | 15 (16%) | 6 (13%) | 7 (20%) | 0.39 |
| Respiratory medication (any) use, n (%) | 31 (33%) | 13 (27%) | 12 (34%) | 0.50 |
| Dexmedetomidine (days) | 4 (2–7) | 3 (2–6) | 6(3–8) | 0.002 |
| Fentanyl (days) | 3 (2–5) | 3 (2–5) | 4(3–6) | 0.098 |
| Milrinone (days) | 5 (3–7) | 4 (3–6) | 6 (4–8) | 0.021 |
| Nicardipine use, n (%) | 17 (18%) | 3 (6%) | 12 (34%) | 0.034 |
| Overall infection, n (%) | 28 (30%) | 8 (17%) | 16 (46%) | 0.01 |
| Bacterial Infection, n (%) | 25 (26%) | 8 (17%) | 13 (37%) | 0.048 |
| Viral Infection, n (%) | 4 (4%) | 1 (2%) | 1 (3%) | 0.83 |
Data are median (IQR) or n (%). P-values were obtained from the linear mixed models and generalized linear mixed model for continuous and categorical variables, respectively.
Abn Postop Sys Fxn = abnormal postoperative systolic ventricular function of systemic ventricle
NIPPV = Non-invasive positive pressure ventilation
Patients with abnormal CM showed no difference in total or initial CICU LOS or postoperative systolic function. Neither abnormal CM nor low nNO was correlated with differences in PLOS, days of ECMO, or CICU readmission (Tables 2, 3). We also found no correlation between incidence of necrotizing enterocolitis or use of a nasogastric tube for nutrition at hospital discharge stratified by CM or nNO status (Supplemental Table 5). Patients exhibiting both abnormal CM and low nNO comprised only 3 patients and 6 surgical events, precluding any meaningful analysis of outcome parameters in this small subset of patients.
Postoperative Respiratory Outcome Assessments
There was no difference observed for the requirement of MV or other supportive ventilation including high flow nasal cannula and nasal cannula among patients with abnormal CM, but the requirement of NIPPV was higher (42% vs. 16%; P = 0.012) (Table 2). A significantly higher use of respiratory medications, inclusive of inhaled β-agonists, inhaled corticosteroids, mucolytics, and lung surfactant was observed in patients with abnormal CM (52% vs. 19%; P = 0.004) (Table 2). Multivariate analysis also indicated that the odds of requiring NIPPV, receiving β-agonist therapy, and receiving any type of respiratory medication were significantly higher in patients with abnormal CM compared to those for infants with normal CM (Table 4). In contrast, none of these parameters were significantly changed in patients with low nNO. Mechanical ventilation days, NIPPV, duration of supportive ventilation and respiratory medication use were not affected by low nNO (Table 3). Neither abnormal CM nor low nNO correlated with changes in the rate of reintubation, failed extubation, pneumothorax, incidence of diaphragm paralysis or tracheostomy, presence of intrinsic airway disease, presence of effusion, number of chest tube days or amount of chest tube drainage (Tables 2, 3; Supplemental Table 5).
Table 4.
Multivariate analysis of ciliary motion and nasal nitric oxide for adverse postoperative outcomes*.
| Outcomes | Adjusted Odds Ratio (95% CI) |
P |
|---|---|---|
| Abnormal vs. Normal CM | ||
| NIPPY † | 6.53 (1.47 – 29.4) | 0.016 |
| β-Agonist Use | 5.78 (1.60 – 20.8) | 0.009 |
| Respiratory Medication Use | 4.42 (1.47 – 13.3) | 0.01 |
| Low vs. Normal nNO | ||
| Overall Infection | 4.90 (1.42 – 17.0) | 0.014 |
| Bacterial Infection | 2.95 (0.82 – 10.6) | 0.094 |
For each postoperative outcome, a generalized linear mixed model with the logit-link was carried out to estimate the adjusted odds ratio for the CM or nNO group, controlling for the baseline characteristics (age at surgery, preoperative weight, STS-EACTS category, abnormal preoperative systolic ventricular function and surgical classification).
NIPPY = Non-invasive positive pressure ventilation.
Postoperative Infection Outcomes
A higher proportion of patients with abnormal CM had respiratory viral infections (3 of 19 patients [16%] vs. 0%; P = 0.03) (Table 2), but no difference was observed for bacterial infection or combined infection incidence (bacterial, viral, fungal infections, pneumonia, tracheitis) (Table 2;Supplemental Table 5). In contrast, patients with low nNO had an increased incidence of bacterial infections (37% vs. 17%; P = 0.048) (Table 3) with an increased combined infection incidence compared to those with normal nNO (46% vs. 17%; P = 0.01) (Table 3). As viral infections were seen only in patients with abnormal CM, this precluded the ability to calculate an odds ratio. Those with low nNO had nearly 5 times the odds of acquiring an infection (CI 1.42–17; P = 0.014) in the multivariate analysis controlling for baseline characteristics (Table 4). The overall rate of fungal infections, bacterial pneumonia and tracheitis was not different with abnormal CM or low nNO (Supplemental Table 5; Supplemental Table 6).
DISCUSSION
We found a high prevalence of abnormal airway CM and low nNO in non-HTX CHD infants undergoing cardiac surgery. Postoperative morbidity was significantly increased with abnormal CM or low nNO, with the postoperative parameters affected by abnormal CM differing from those affected by low nNO. Despite CM assessment and nNO measurement being standard, validated tests for PCD, the mechanism linking the two are unknown and there are reports of normal nNO levels in confirmed PCD patients16–18. Hence, the divergence in our CM and nNO findings in CHD patients is not entirely surprising.
Abnormal CM Correlated with Increased Respiratory Morbidity in Non-HTX CHD Infants
We showed non-HTX CHD infants with abnormal CM exhibited a significant increase in respiratory morbidity, including increased length of NIPPV, greater need for respiratory medications, and increased incidence of viral respiratory infections. These findings were observed despite abnormal CM infants being older at the time of surgery and more likely to have a biventricular repair, both of which have been associated with decreased postoperative morbidity/mortality19, 20.
Despite not sharing classic features of PCD, CHD infants with abnormal respiratory CM appear to have mucociliary clearance defects that may account for their increased postoperative respiratory morbidity. This is suggested by increased use of respiratory medications, including β-agonists and inhaled corticosteroids, and need for NIPPV. We plan to corroborate this finding in the future with direct assessments of mucociliary clearance in CHD infants with abnormal CM.
Our present study supports the previous work of Harden et al7, which showed increased postoperative use of inhaled β-agonists in HTX infants with CD. The replication of this finding in our cohort of exclusively non-HTX CHD infants at an independent institution suggests abnormal CM has broader relevance for postsurgical outcomes in infants with critical CHD, irrespective of the presence of concomitant HTX.
Association of Abnormal CM vs. Low nNO with Infections
The increased incidence of bacterial infections in infants with low nNO is consistent with the known inhibitory effect of nitric oxide on microbial growth, and its modulation of the immune/inflammatory response21, 22. NO is also known to stimulate ciliary beat frequency, predicting low nNO might compromise mucociliary clearance function in the airway. In contrast, we found infants with abnormal CM had increased respiratory viral infections, but not bacterial infections. This may reflect a propensity for viral entry via the ciliated epithelia, observations suggesting the cilium may be the point of viral entry23–25. Yet another study showed FUZ, a protein required for ciliogenesis, is also required for endocytosis-mediated viral entry25.
Low nNO Associated with Hemodynamic Alterations
Infants with low nNO exhibited a higher incidence of decreased systolic function immediately prior to surgery and at the time of hospital discharge. The low nNO preceded the decrease in systolic function, as the majority of infants exhibited normal systolic function at the time of nNO assessment, but their systolic function worsened by the time of surgery (median 8 days later). Abnormal systolic function in most of these infants persisted to the time of hospital discharge. Such infants also showed an increased need for intravenous supportive therapy and longer stay in the CICU. Interestingly, there was also a significant increase in use of systemic vasodilators in these infants. This finding raises the interesting possibility that defective vasoregulation may arise secondary to impaired endogenous NO production in such infants.
The alterations in hemodynamics seen in infants with low nNO are consistent with those reported by Yau et al, showing low nNO levels correlated with failing single ventricle physiology and a trend towards adverse outcomes in CHD infants with single ventricle lesions26. The benefits of adequate NO bioavailability have been further illustrated by James et al who showed a reduced incidence of post-cardiopulmonary bypass low cardiac output syndrome in children who received supplemental NO via the bypass oxygenator during surgery27. The mechanism driving the hemodynamic effects associated with low nNO is presently unknown. As cilia are thought to play a role in NO production and non-motile primary cilia have been shown to mediate calcium signaling and mechanosensory signal transduction, CHD infants with normal motile cilia function may nevertheless have primary cilia defects that may contribute to abnormal hemodynamic regulation28, 29. Further studies are needed to investigate whether low nNO may be correlated with low systemic plasma NO species. Such studies will be needed to elucidate the precise link between low nNO and reduction in systolic function.
Limitations
A limitation in our study is the relatively small infant sample size, which may have underestimated or failed to detect differences in certain outcome parameters. Our results are based on an observation study of 63 non-HTX CHD infants, thus the findings from comparisons of multiple outcomes are hypothesis generating and require validation in a larger cohort. It was particularly underpowered to detect a difference in outcome for infants with both abnormal CM and low nNO, or a mortality difference given the very low mortality rate in our study population. Reciliation data was not available for all of the patients included in our study and thus abnormal CM may have been somewhat overestimated. This may have biased our results towards the null hypothesis for outcome parameters that are more modestly affected by abnormal CM.
CONCLUSION
Non-HTX CHD infants with abnormal CM showed increased postoperative morbidity, with abnormalities in CM predictive of poor respiratory function. Those with low nNO were observed to exhibit hemodynamic perturbation. Longitudinal assessment is needed to examine more long-term outcomes of surgical palliation beyond the hospital admission period for the index surgical event. Further studies are also needed to investigate the mechanisms by which abnormal CM and low nNO drive the different outcome parameters. Together these findings suggest preoperative evaluation for CD in CHD infants may identify at-risk infants, thereby providing opportunities for perioperative interventions to reduce postoperative morbidity. This could improve outcomes for infants with critical CHD, many of whom must undergo multiple high-risk cardiac surgeries for palliation of their structural heart disease.
Supplementary Material
Video 1. Nasal epithelial cells from a patient with normal ciliary motion.
These nasal epithelial cells bear abundant cilia with long, synchronized, coordinated strokes. Note the ability of the beating cilia to move pericellular debris.
Video 2. Nasal epithelial cells from a patient with abnormal ciliary motion.
These nasal epithelial cells have stiff, dyskinetic, wavy cilia with uncoordinated strokes.
ACKNOWLEDGEMENTS
We thank Evonne Morell (Krushansky), DO, Brian Feingold, MD, Ricardo Munoz, MD, William Devine, BS, and Linda Leatherbury, MD for their insightful discussions in their fields of expertise. This study was supported by a Pennsylvania Department of Health; Sang C. Park Fellow Research Award (16060). PSA is supported in part by a training grant from the NIH (T32GM075770).
Funding: Pennsylvania Department of Health; Sang C. Park Fellow Research Award (16060). PSA is supported in part by a training grant from the NIH (T32GM075770).
Glossary:
- CD
ciliary dysfunction
- CHD
congenital heart disease
- CICU
cardiac intensive care unit
- CM
ciliary motion
- EACTS
European Association for Cardiothoracic Surgery
- HTX
heterotaxy
- LOS
length of stay
- NIPPV
non-invasive positive pressure ventilation
- nNO
nasal nitric oxide
- PCD
primary ciliary dyskinesia
- PLOS
postoperative length of stay
- STS
Society of Thoracic Surgeons
Footnotes
IRB approval: Institutional Review Board of the University of Pittsburgh, PR009090021. Approved 12/14/09, renewed 9/1/16.
Central Picture:
Ciliary axoneme of a human nasal epithelia cell visualized by immunostaining
Central Message:
Airway ciliary dysfunction is associated with increased pulmonary morbidity after congenital cardiac surgery in infants with non-heterotaxy congenital heart disease.
Perspective Statement:
Current evidence suggests airway ciliary dysfunction (CD) in congenital heart disease (CHD) patients with heterotaxy (HTX) is associated with increased postoperative pulmonary morbidity. Here, analysis of patients with a broad spectrum of non-HTX CHD yielded similar findings. This suggests preoperative screening for CD may allow therapeutic interventions to improve outcomes for at-risk CHD patients.
Conflict of interest statement: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bush A, Hogg C. Primary ciliary dyskinesia: recent advances in epidemiology, diagnosis, management and relationship with the expanding spectrum of ciliopathy. Expert Rev Respir Med. 2012;6(6):663–82. [DOI] [PubMed] [Google Scholar]
- 2.Shinohara K, Kawasumi A, Takamatsu A, Yoshiba S, Botilde Y, Motoyama N, et al. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat Commun. 2012;3:622. [DOI] [PubMed] [Google Scholar]
- 3.Tan SY, Rosenthal J, Zhao XQ, Francis RJ, Chatterjee B, Sabol SL, et al. Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. J Clin Invest. 2007;117(12):3742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Klena NT, Gabriel GC, Liu X, Kim AJ, Lemke K, et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature. 2015;521(7553):520–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swisher M, Jonas R, Tian X, Lee ES, Lo CW, Leatherbury L. Increased postoperative and respiratory complications in patients with congenital heart disease associated with heterotaxy. J Thorac Cardiovasc Surg. 2011;141(3):637–44, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakhleh N, Francis R, Giese RA, Tian X, Li Y, Zariwala MA, et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation. 2012;125(18):2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harden B, Tian X, Giese R, Nakhleh N, Kureshi S, Francis R, et al. Increased postoperative respiratory complications in heterotaxy congenital heart disease patients with respiratory ciliary dysfunction. J Thorac Cardiovasc Surg. 2014;147(4):1291–1298.e2. [DOI] [PubMed] [Google Scholar]
- 8.Leigh MW, Ferkol TW, Davis SD, Lee HS, Rosenfeld M, Dell SD, et al. Clinical Features and Associated Likelihood of Primary Ciliary Dyskinesia in Children and Adolescents. Ann Am Thorac Soc. 2016; 13(8): 1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrod AS, Zahid M, Tian X, Francis RJ, Khalifa O, Devine W, et al. Airway ciliary dysfunction and sinopulmonary symptoms in patients with congenital heart disease. Ann Am Thorac Soc. 2014;11(9):1426–32. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. [DOI] [PubMed] [Google Scholar]
- 11.Beydon N, Chambellan A, Alberti C, de Blic J, Clément A, Escudier E, et al. Technical and practical issues for tidal breathing measurements of nasal nitric oxide in children. Pediatr Pulmonol. 2015;50(12):1374–82. [DOI] [PubMed] [Google Scholar]
- 12.Adams PS, Tian X, Zahid M, Khalifa O, Leatherbury L, Lo CW. Establishing normative nasal nitric oxide values in infants. Respir Med. 2015;109(9):1126–30. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–53. [DOI] [PubMed] [Google Scholar]
- 14.Lacour-Gayet F, Clarke D, Jacobs J, Gaynor W, Hamilton L, Jacobs M, et al. The Aristotle score for congenital heart surgery. Seminars in thoracic and cardiovascular surgery Pediatric cardiac surgery annual. 2004;7:185–91. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–118. [DOI] [PubMed] [Google Scholar]
- 16.Marthin JK, Nielsen KG. Choice of nasal nitric oxide technique as first-line test for primary ciliary dyskinesia. Eur Respir J. 2011. March;37(3):559–65. [DOI] [PubMed] [Google Scholar]
- 17.Karadag B, James AJ, Gültekin E, Wilson NM, Bush A. Nasal and lower airway level of nitric oxide in children with primary ciliary dyskinesia. Eur Respir J. 1999. June;13(6):1402–5. [DOI] [PubMed] [Google Scholar]
- 18.Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013. December;10(6):574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natarajan G, Anne SR, Aggarwal S. Outcomes of congenital heart disease in late preterm infants: double jeopardy? Acta paediatrica. 2011;100:1104–7. [DOI] [PubMed] [Google Scholar]
- 20.Bogdan C Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–16. [DOI] [PubMed] [Google Scholar]
- 21.Uehara EU, Shida Bde S, de Brito CA. Role of nitric oxide in immune responses against viruses: beyond microbicidal activity. Inflamm Res. 2015;64(11):845–52. [DOI] [PubMed] [Google Scholar]
- 22.Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol. 2005;79(24): 15511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76(11):5654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, et al. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol. 2005;79(2):1113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ooi YS, Stiles KM, Liu CY, Taylor GM, Kielian M. Genome-wide RNAi screen identifies novel host proteins required for alphavirus entry. PLoS Pathog. 2013;9(12):e1003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau C, Zahid M, Khalifa O, Devine W, Leatherbury L, Wearden P, et al. Low nasal nitric oxide as a prognosticator of failing single ventricle physiology in congenital heart disease patients. Circulation. 2014;130:Suppl2 A16375. [Google Scholar]
- 27.James C, Millar J, Horton S, Brizard C, Molesworth C, Butt W. Nitric oxide administration during paediatric cardiopulmonary bypass: a randomised controlled trial. Intensive Care Med. 2016. November;42(11):1744–1752. [DOI] [PubMed] [Google Scholar]
- 28.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117(9):1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, et al. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104(7):860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Nasal epithelial cells from a patient with normal ciliary motion.
These nasal epithelial cells bear abundant cilia with long, synchronized, coordinated strokes. Note the ability of the beating cilia to move pericellular debris.
Video 2. Nasal epithelial cells from a patient with abnormal ciliary motion.
These nasal epithelial cells have stiff, dyskinetic, wavy cilia with uncoordinated strokes.


