Abstract
Regulations requiring a reduction of the nicotine content in cigarettes to minimally addictive levels could significantly reduce the public health impact of cigarette smoking. Clinical trials evaluating this strategy are ongoing and methods have been developed to use nicotine biomarkers to estimate compliance with use of very low nicotine content cigarettes (VLNCs). To date, these methods have not considered the potential contribution of nicotine absorption from environmental tobacco smoke (ETS) among research participants. This study used data from 100 randomly selected study completers in ongoing clinical trials of VLNCs (50 randomized to Usual Nicotine Content Cigarettes (UNCs) and 50 to VLNCs) to assess the use of plasma cotinine to estimate compliance. Plasma cotinine and smoking behavior were recorded at baseline after 2 weeks smoking UNC cigarettes, and then after 18 weeks of either continuing smoking UNCs or reducing the nicotine content such that the last 6 weeks comprised smoking VLNCs. Plasma cotinine remained stable (267 ng/ml) in the UNC group but reduced to 93 ng/ml in the VLNC group (P<0.01). Compliance with smoking VLNCs was first estimated by comparing the cotinine per cigarette on VLNCs with UNCs after allowing for potential compensatory smoking. We found that 29 (58%) of the VLNC group were compliant. Adjusting for potential ETS exposure estimated 32 (64%) to be compliant. This latter group (n=32) had a mean plasma cotinine on VLNCs of 7 ng/ml (range= 3–16.4 ng/ml). Adjusting for potential ETS exposure may improve identification of participants who plausibly complied with exclusive VLNC use.
Keywords: cigarette, smoking, reduced, nicotine, content, plasma, cotinine, compliance
INTRODUCTION
Cigarette smoking remains the major cause of premature death in the United States and many countries of the world (1,2). A comprehensive tobacco control strategy has reduced smoking steadily in many countries but tens of millions of smokers remain and young people continue to initiate smoking (1,2). One strategy that has been suggested as a way to more rapidly reduce smoking rates is to mandate a reduction in the permissible nicotine content in cigarettes down to a level that is minimally addictive (3,4,5). The U.S. Food and Drug Administration recently announced that reducing the addictiveness of cigarettes is central to their plans (6), and FDA/NIH has funded a series of studies to evaluate the feasibility and effects of such a strategy (e.g. 7,8,9).
Clinical trials of very low nicotine content cigarettes (VLNCs) typically recruit smokers with no immediate plans to quit and randomize some to switch to research cigarettes with a nicotine content similar to commercially manufactured cigarettes, and others to switch to very low nicotine content research cigarettes containing such a small amount of nicotine that they are very unlikely to be addictive (e.g. containing 0.5mg nicotine or less [3]). The smokers in each group are then followed and various measures of smoking behavior and toxicant exposure are assessed (e.g. 7,8,9,10). Participants in these research studies are provided with the research cigarettes at no cost and encouraged to smoke them exclusively during the study. However, although participants are asked if they used any other nicotine products during the course of the study, the investigators have limited ability to identify which participants smoked only the research cigarettes and which ones did not comply with the study protocol (e.g. by smoking their own brand cigarettes or using another nicotine product).
It is important for investigators to know which participants complied with smoking the VLNCs, partly as an indicator of feasibility for the whole nicotine reduction strategy, but also so that they can properly assess for adverse outcomes (e.g. increased or compensatory smoking, or nicotine withdrawal symptoms) in those who are potentially at greater risk of harm by virtue of switching to cigarettes delivering very little nicotine. For this reason, investigators have developed methods using nicotine metabolite biomarkers to estimate compliance with use of VLNCs. Benowitz et al, (11) proposed a method that involves comparing the within-subject ratios of plasma cotinine to cigarette consumption at baseline on either own brand or usual nicotine content cigarettes (UNCs) to the same ratio on VLNCs after taking into account the nicotine content of each cigarette type (11). The general aim of biochemical methods of estimating non-compliance is to estimate a level of a biochemical measure (e.g. plasma cotinine) for each individual above which they could not plausibly be smoking VLNCs exclusively and must have actively consumed nicotine from another source. The method recommended by Benowitz et al (11) took into account cigarette consumption and gave some latitude for the possibility that individuals could inhale more smoke per cigarette (up to 4 times as much per cigarette). In the Benowitz et al study (10), the baseline was based on “own brand” smoking and it was assumed that these cigarettes contained 10mg of nicotine, while the VLNCs in that study contained 0.5mg nicotine. Thus, using the equation below this meant that a smoker was judged noncompliant if the left side of the equation was greater than 0.2.
Many of the current studies examining the effects of switching to VLNCs use SPECTRUM research cigarettes with a UNC nicotine content of approximately 11.6mg as the UNC and a VLNC nicotine content of approximately 0.2mg based on the average of menthol and non-menthol cigarettes and an estimated 0.7g of tobacco per cigarette (12). Thus, if an individual smokes the same number of VLNCs in the same way as their UNCs their cotinine on VLNCs should be less than 2% of that on UNCs (0.2/11.6=0.0172). Even if they inhaled four times as much smoke per cigarette their cotinine level on VLNCs should be less than 7% of that on UNCs (0.0172×4=0.069).
The fact that the SPECTRUM VLNCs being used in current studies (7,8,9) contain a lower nicotine content than those in the original Benowitz et al (10) study raises the possibility that a research participant who is truly compliant, but is exposed to a plausible amount of environmental tobacco smoke (ETS), could be categorized as noncompliant when using the Benowitz et al method (11).
Given that the smokers being recruited for these studies are often low income smokers with little or no interest in quitting (8,9,13), they are frequently exposed to moderate or high levels of environmental tobacco smoke (ETS) from other friends, family and work colleagues who smoke. Noting this, it is reasonable to consider that they could obtain a plasma cotinine of 7ng/ml or higher (intake of around 0.6mg nicotine) from ETS exposure alone (14). The Society for Research on Nicotine and Tobacco recommended a cut-point of 15 ng/ml plasma cotinine to differentiate possible ETS exposure from active smoking (15), and Jarvis et al (16) recommended an optimal cut-point of 18 ng/ml for people of low income in England (saliva cotinine). We therefore propose an additional component to the Benowitz et al (2015) method whereby it is assumed that 15 ng/ml of the each individual’s plasma cotinine is due to ETS rather than active smoking and so this is subtracted (where possible) from their cotinine at both baseline on UNCs and later on VLNCs before dividing by the number of cigarettes smoked.
In order to demonstrate how this affects estimates of compliance with real data, we present here results from 100 participants from two randomized double-blind controlled trials of VLNCs based at Penn State University in Hershey, PA (8,9) using the Benowitz et al (2015) (11) method and separately adjusting for potential ETS exposure.
METHODS
The data analyzed here were sampled from two randomized controlled trials of VLNCs in people with less than a college degree (9) or people with mood and/or anxiety disorders (8). Briefly, adult smokers (age 18–65) of at least 5 cigarettes per day (CPD) who were not interested in making a quit attempt in the next 6 months were recruited for an 8-month trial and attended an assessment visit. After one week of data collection while smoking their own brands of cigarettes, there was a 2-week baseline period where all participants were provided with UNC SPECTRUMs (NRC600, non-menthol flavor or NRC601, menthol flavor) matched to their flavor preference. The research cigarettes were supplied by the NIDA Drug Supply Program (17). Each non-menthol SPECTRUM NRC600 cigarette is approximately 84 mm long, just under 0.7g of tobacco, has a filter ventilation of 27.3%, a tar yield of 10.5 mg, a nicotine yield of 0.8 mg, a nicotine content of 11.6 mg nicotine/cigarette, and a nicotine concentration of 16.5 mg/g of tobacco. Menthol flavored SPECTRUM NRC601 cigarettes have similar characteristics to non-menthols (12). The “baseline” (pre-randomization) measures reported here were collected at a visit following all participants smoking UNC SPECTRUMS for two weeks. Immediately after this data collection, participants entered the randomized phases of the trials, in which they either continued smoking UNC SPECTRUM cigarettes for 18 more weeks or switched to progressively lower nicotine content SPECTRUM cigarettes every three weeks, ending with 6 weeks on the VLNC cigarettes (Non-menthol code NRC 102 or menthol code NRC103) containing approximately 0.2mg nicotine. The VLNC data reported in this study were collected at the visit after participants had been smoking VLNCs for 6 weeks. Throughout the study participants were provided with the research cigarettes at no cost with the supply being approximately 150% greater than their original baseline cigarette consumption to ensure that participants did not run out of research cigarettes if compensatory smoking occurred or if their appointment was delayed. Participants were required to return all used (empty) and unused packs of research cigarettes and were incentivized ($100 bonus at the end of the study) for doing this and other data collection procedures consistently. Both of the parent trials were carried out in accordance with The Declaration of Helsinki and were approved by the Institutional Review Boards at the participating institutions.
Selection for this study
A study statistician (EW) randomly selected 50 participants from each trial from the Penn State Hershey site from all those who had (a) completed both the SPECTRUM baseline visit and the final visit and (b) provided both blood and urine samples at both of those visits. Once this eligibility was determined, 25 randomized to UNCs and 25 randomized to VLNCs were randomly subsampled from each trial (50/50 total).
This sub-sample is not intended to be representative of participants in the parent trials. Instead, it was chosen specifically to evaluate methodological questions about participant compliance prior to study completion and full data analyses. Therefore, group differences that may be found in the current random sub-sample may not reflect findings in each of these trials and should not be interpreted as final trial results.
Measures
A comprehensive battery of measures was collected at each visit (8,9) including:
A daily cigarette consumption log (including both research and non-research cigarettes). Participants were given a paper log designed to fit on the front of their cigarette pack with a pencil the size of a cigarette and were instructed to tally each cigarette (research and non-research) as they smoked it each day (9,18). At each visit, participants reported the past 6 days of tobacco use with a timeline follow back procedure aided by their logs. For each day, if the participant reported using nonresearch cigarettes (self-reported non-compliance), we recorded the number and included it in the total CPD count.
Measures of cigarette dependence including the Fagerstrom Test for Cigarette Dependence (19) and the Penn State Cigarette Dependence Index (20).
A measure designed to estimate recent exposure to ETS (21) in which participants reported the number of people in their home who smoke, the number of hours per day of smoke exposure at work, and the frequency of exposure to smoke in social settings outside of the home on 4-point Likert scale ranging from “Seldom” to “Daily”. The questions did not specify a time frame but rather invited participants to estimate current typical exposure. Self-reported smoke exposure was categorized as moderate to high if participants lived with a smoker who smokes inside the home, had greater than 1 hour a day of workplace exposure, or reported exposure during social activities several times a week or more. Otherwise smoke exposure was categorized as none to low.
Exhaled carbon monoxide (CO, in parts per million [ppm]) was measured with the Bedfont Pico+ Smokerlyzer which uses an electrochemical sensor, has a concentration range of 0–100 ppm, and has an accuracy of ±2% (22).
Blood was collected from participants during the pre and post-randomization study visits. Plasma cotinine levels were measured using a commercially available solid-phase, competitive enzyme-linked immunosorbent assay (ELISA) kit from Calbiotech (El Cajon, CA). The assay was carried out as directed by the manufacturers and has a detection limit of 4.3ng/mL (23). Assays below this level were assigned a value of 3 ng/ml, which was the level of detection (4.3 ng/ml) divided by the square root of two. This method has been used previously for plasma cotinine analysis in large clinical trials, including the National Health and Nutrition Examination Survey (NHANES) (24).
Data Analysis
All data analysis was conducted using SPSS Version 24. T-tests and Chi-square analyses were conducted to examine for group differences across all study variables. For variables collected at both the baseline UNC and VLNC visits, repeated measures ANOVA was used to assess for group by time interactions.
Compliance equations
Benowitz and colleagues (11) have proposed a method for determining compliance using a ratio of [plasma cotinine/CPD] after a period of smoking VLNC cigarettes to the baseline [plasma cotinine/CPD] while smoking usual nicotine content cigarettes. This compliance ratio provides an estimate of the reduction in cotinine per cigarette from usual to very low nicotine content cigarettes. Ratios of one or greater mean that the individual did not reduce their cotinine from baseline, while ratios of less than one mean that they did reduce their cotinine. Participants are categorized as non-compliant, if their compliance ratio was higher than the ratio of [VLNC nicotine content/UNC nicotine content] with a multiplicative factor of four to allow for potential compensation through greater nicotine inhalation or absorption per cigarette. The equation is as follows:
To adjust the equation for potential increases in cotinine due to environmental tobacco smoke exposure, both cotinine values in the compliance ratio were reduced by 15 ng/ml (where possible). The resulting equation was
- ETS-adjusted Method:
All participants with VLNC cotinine values below the limit of detection were classified as compliant. For the ETS-adjusted method, all participants with plasma cotinine values less than 15 ng/ml were classified as compliant.
RESULTS
The baseline participant characteristics in the two groups are shown in Table 1, demonstrating that the two groups were similar on demographic and smoking characteristics at baseline while smoking UNC cigarettes. There were no significant differences between groups in these baseline measures.
Table 1.
Participant demographic and cigarette characteristics by treatment group at SPECTRUM baseline smoking Usual Nicotine Cigarettes and at the final post-randomization visit (Hershey 2015–17)
| VLNC (n=50) | UNC (n=50) | |
|---|---|---|
| Demographics | ||
| Age, M(SD) | 43.6 (10.2) | 44.5 (11.5) |
| Female, N(%) | 34 (68) | 30 (60) |
| Race, N(%) | ||
| White | 39 (78) | 43 (86) |
| Black | 5 (10) | 4 (8) |
| Other | 6 (12) | 3 (6) |
| ≤ High school education, N(%) | 28 (56) | 29 (58) |
| Smoking Characteristics | ||
| Flavor, N(%) | ||
| Regular | 22 (44) | 21 (42) |
| Menthol | 28 (56) | 29 (58) |
| UNC Spectrum baseline study visit | ||
| Plasma cotinine, M(SD) | 243.9 (118.6) | 267.2 (137.1) |
| Exhaled CO, M(SD) | 36.6 (17.6) | 34.3 (15.9) |
| Cigarettes per day, M(SD) | 22.2 (9.3) | 25.4 (18.3) |
| FTND dependence score, M(SD) | 6.30 (2.0) | 6.26 (2.2) |
| PSCDI dependence score, M(SD) | 12.8 (3.4) | 13.4 (3.3) |
| ETS exposure, N(%) | ||
| None/Low | 16 (32) | 13 (26) |
| Moderate/High | 34 (68) | 37 (74) |
| Self-reported noncompliancec, N(%) | 0 (0) | 4 (8) |
| Post-randomization study visit | ||
| Plasma cotininec,d, M(SD) | 93.1 (160.8) | 267.7 (143.9) |
| Exhaled CO d, M(SD) | 29.2 (19.4) | 33.9 (14.7) |
| Cigarettes per day a, d, M(SD) | 19.8 (13.3) | 25.2 (14.0) |
| FTND dependence scoreb,c,d, M(SD) | 5.5 (2.9) | 6.6 (2.1) |
| PSCDI dependence scorec,d, M(SD) | 11.4 (4.4) | 13.6 (3.1) |
| ETS exposure, N(%) | ||
| None/Low | 17 (34) | 17 (34) |
| Moderate/High | 33 (66) | 33 (66) |
| Self-reported noncompliance, N(%) | 4 (8) | 6 (12) |
NOTE: VLNC = very low nicotine content; UNC = usual nicotine content; ETS = environmental tobacco smoke; CO = carbon monoxide; FTND = Fagerstrom Test for Nicotine Dependence; PSCDI = Penn State Cigarette Dependence Index.
UNC group n= 48
UNC group n= 49
Significant (p<.05) t-test or chi-square analysis indicating UNC > VLNC group
Significant (p<.05) group by time interaction in repeated measures ANOVA indicating ΔVLNC > ΔUNC
The baseline measures suggest this sample included predominantly moderate to heavy smokers, with a majority smoking mentholated cigarettes. Over two thirds were regularly exposed to environmental tobacco smoke.
Table 1 also shows the post-randomization results. These show very stable smoking patterns and measures of exposure in the group randomized to continue smoking UNC cigarettes over 18 weeks, but significant reductions in plasma cotinine, exhaled CO, and other measures of cigarette consumption and dependence in the group who were randomized to smoke VLNC cigarettes for the last 6 weeks.
Self-reported noncompliance
During the week prior to the SPECTRUM baseline study visit (the randomization visit), no participants in the VLNC group reported smoking non-study cigarettes, and four participants in the UNC group reported smoking a small number of non-study cigarettes in the prior week. At the post-randomization visit, 18 weeks later, four VLNC group participants (8%) reported smoking non-study cigarettes in the prior six days; while six UNC group participants (12%) reported smoking non-study cigarettes in the prior six days.
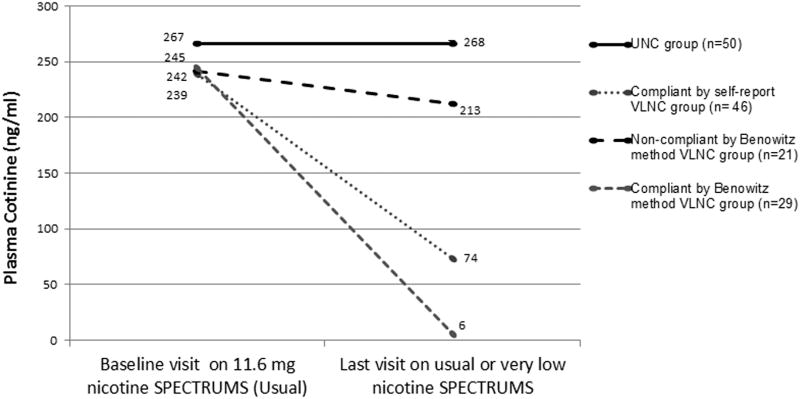
Compliance rates across methods
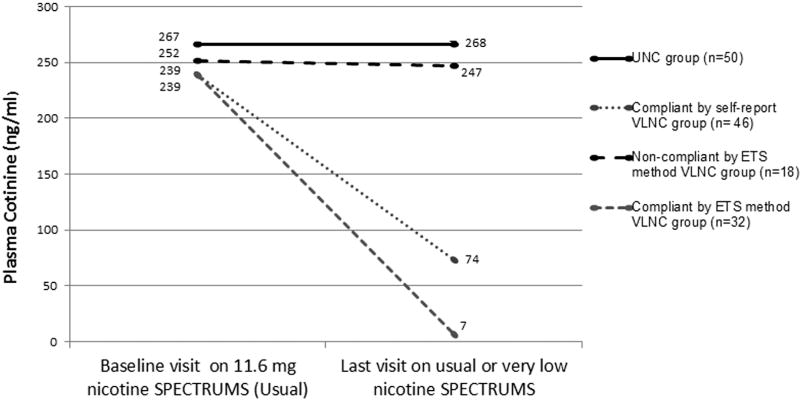
Using the Benowitz et al method (11), 29 (58%) of the VLNC participants were estimated to be compliant and 21 (42%) non-compliant. Using the same method adjusted for 15 ng/ml of ETS exposure, 32 (64%) were compliant and 18 (36%) were non-compliant. One participant who self-reported smoking non-study cigarettes three days prior to the VLNC study visit was categorized as compliant using both methods (cotinine=9.1 ng/ml). The three participants who all self-reported as compliant but were estimated as non-compliant using the Benowitz et al method and compliant using the ETS-adjusted method had cotinine concentrations of 8,13 and 15 ng/ml while smoking an average of 15.5, 40, and 16 VLNC cigarettes per day, respectively. Their baseline cotinine concentrations and cigarette consumption were all above 150 ng/ml and 18 cigarettes per day. Two out of these three participants reported moderate/high levels of ETS exposure prior to the VLNC visit.
Changes in cotinine across compliance methods
As shown in Figures 1 and 2 below, the average plasma cotinine for self-reported VLNC compliers (i.e. those who stated that they smoked zero non-study cigarettes in the prior six days), was higher than for those who qualified as compliant using either of the methods based on cotinine/cigarette ratios. Figure 2, which basically transfers 3 of the non-compliant group in Figure 1, to the compliant group due to their meeting the ETS-adjusted criteria for compliance, shows that when compliers are identified this way, it leaves a group of non-compliant smokers who had a negligible reduction in cotinine overall and in fact 8 of these 18 actually increased their plasma cotinine from their baseline on UNCs.
Figure 1.
Changes in mean cotinine by Usual Nicotine Content (UNC) group and Very Low Nicotine Content (VLNC) compliance groups categorized with the Benowitz method and self-reported compliance (10).
Figure 2.
Changes in mean cotinine by Usual Nicotine Content (UNC) group and Very Low Nicotine Content (VLNC) compliance groups determined with the environmental smoke exposure (ETS) adjusted method and self-reported compliance.
DISCUSSION
Decisions on whether to move forward with a nicotine regulation strategy for combustible tobacco products will be influenced by the scientific evidence from clinical trials of VLNCs. Interpretation of these trials depends to some extent on an accurate understanding of which research participants are largely complying with the trial protocol. This study described an attempt to compare an existing method of classifying compliance in participants who were smoking VLNCs (11) with a similar method that takes into account potential ETS exposure contributing to their cotinine concentrations. While the results of both methods showed a high degree of agreement, the ETS-adjusted method suggested that three additional participants were compliant.
Benowitz et al (11) also discussed a method that could be used in studies where no baseline cotinine to cigarettes ratio was available for usual nicotine cigarettes. Here it is assumed that in the average smoker each cigarette contributes 12 ng/ml to steady state plasma cotinine. We previously reported finding almost exactly that cotinine per cigarette when 341 smokers smoked UNC SPECTRUM cigarettes for two weeks (13). Using the same rationale presented in that paper (11), it can be estimated that each VLNC containing 0.2mg nicotine will contribute 0.24 ng/ml to steady state plasma cotinine. Assuming a smoker could potentially try to compensate by inhaling four times as much smoke per cigarette, the maximum plasma cotinine per cigarette would be just under 1 ng/ml. This method obtains very good agreement with the other two methods discussed here, but again we suggest that an allowance should be made for potential contribution to plasma cotinine from ETS. For example, this method (simply based on a maximum permissible one ng/ml of cotinine/cigarette) would estimate that someone smoking five VLNC cigarettes per day with a plasma cotinine of 6 ng/ml is likely non-compliant. These methods of estimation aim to count as non-compliant only those whose self-reported compliance is implausible. However, it is quite plausible that this individual could have absorbed some of the nicotine contributing to the 6 ng/ml plasma cotinine from ETS, rather than from smoking high nicotine cigarettes (14,15, 16).
Study limitations
Given that the actual tobacco and nicotine content per VLNC cigarette varies slightly by menthol content and by batch, one may question whether small but realistic changes in these numbers would change the results presented above. We repeated the analysis using more precise estimates of nicotine content based on the concentrations and precise tobacco weights for menthol and non-menthol research cigarettes published by Richter et al (12), and we found identical results. Similarly, one may question whether 15 ng/ml is a rather generous estimate for the contribution of ETS to plasma cotinine in research volunteers in these studies. We agree that this is at the high end of plausible estimates, and in fact only one participant in this study who we estimated to be compliant on VLNCs had a cotinine concentration greater than 15 ng/ml. We repeated the analyses assuming the contribution from ETS was only 5 ng/ml, and the results were identical.
The questions on ETS exposure used in this study were not designed to give a very precise estimate of total ETS exposure over the previous three or four days that contribute to a spot cotinine measure. So the fairly rough categorical index of average exposure presented here is not adequate to guide how much allowance for recent ETS should be made on an individual case basis. Rather the ETS exposure estimates are presented here simply to demonstrate that the smokers who volunteer for studies of VLNCs tend to have far higher ETS exposure than the non-smokers in whom this is typically studied. For example, in the original Nondahl et al study (21) using the same ETS exposure questionnaire, 8.6% of non-smoking participants had ETS exposure at home, whereas in the present sample 35% reported ETS exposure at home.
It is important to note that these preliminary analyses of clinical data are restricted only to research participants who completed participation in long trials, involving numerous visits spanning many months. Results may vary for participants who dropped out of these trials, for participants at other sites, and could also vary for participants who switched immediately to VLNCs rather than gradually as in these trials.
We agree with Benowitz et al (11) that assessing compliance in studies of the effects of VLNCs should not be based purely on self-report and we estimated that a high proportion (15/46, 32.6 %) of participants who claimed to be compliant with exclusive use of VLNCs most likely were not. We also agree that assessing smokers’ responses to switching to VLNCs should include separate analyses for compliant and non-compliant smokers, and we propose that the ETS-adjustment recommended here contributes a small improvement in precision in more accurately estimating compliance and should be used in future studies.
CONCLUSIONS
Measurement of nicotine metabolites and cigarette consumption can contribute to estimation of compliance in trials of VLNCs. Adjusting for potential ETS exposure may improve identification of participants who plausibly complied with exclusive VLNC use and should be used in future studies.
Highlights.
-
-
Plasma cotinine can estimate compliance in studies of very low nicotine cigarettes
-
-
A method adjusting for cigarette consumption estimated 58% of switchers complied
-
-
A new method additionally allowing for 15 ng/ml from ETS estimated 64% complied
-
-
Among those estimated compliant, cotinine fell from 239 to 7 ng/ml
-
-
This new method can be used to identify compliant users of low nicotine cigarettes
Acknowledgments
Funding: This study was funded by the National Institutes of Health (NIH) and the U.S. Food and Drug Adminstration (P50DA036107). The REDCap tools used in this project were supported by the National Center for Advancing Translational Sciences, National Institutes of Health through grant UL1TR000127 and TR00214. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Adminstration.
Conflicts of Interest: JF has done consulting work for pharmaceutical companies that manufacture smoking cessation products (e.g. Pfizer Inc) and has received a research contract from Pfizer Inc on a topic unrelated to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. A report of the Surgeon General. Atlanta (GA): (US); 2014. [Accessed 31 Oct 2016]. National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: The health consequences of smoking – 50 years of progress. https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf. [Google Scholar]
- 2.Eriksen M, Mackay J, Schluger N, Gomeshtapeh FI, Drope J. The Tobacco Atlas. American Cancer Society; Atlanta: 2015. [Google Scholar]
- 3.Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(Suppl 1):i14–17. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donny EC, Hatsukami DK, Benowitz NL, Sved AF, Tidey JW, Cassidy RN. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation. Prev Med. 2014;68:17–22. doi: 10.1016/j.ypmed.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO Study Group on Tobacco Product Regulation (TobReg) Geneva, Switzerland: World Health Organization; 2015. Advisory Note: Global Nicotine Reduction Strategy. [Google Scholar]
- 6.Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. doi: 10.1056/NEJMp1707409. [DOI] [PubMed] [Google Scholar]
- 7.Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen SI, Foulds J, Veldheer S, Pachas GN, Yingst J, Hrabovsky S, Cather C, Azzouz N, Hameed A, Richie J, Muscat J, Evins AE. A two-site, two-arm, 34-week, double-blind, parallel-group, randomized controlled trial of reduced nicotine cigarettes in smokers with mood and/or anxiety disorders: trial design and protocol. BMC Public Health. 2017 Jan 19;17(1):100. doi: 10.1186/s12889-016-3946-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krebs NM, Allen SI, Veldheer S, Martinez DJ, Horn K, Livelsberger C, Modesto J, Kuprewicz R, Wilhelm A, Hrabovsky S, Kazi A, Liao J, Zhu J, Wasserman E, Reilly S, Reinhart L, Trushin N, Moyer RE, Bascom R, Foulds J, Richie JP, Muscat J. Reduced nicotine content cigarettes in smokers of low socioeconomic status: study protocol for a randomized controlled trial. Trials. 2017;18:300. doi: 10.1186/s13063-017-2038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Smoking behavior and exposure to toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012 May;21(5):761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benowitz NL, Nardone N, Hatsukami DK, Donny EC. Biochemical estimation of noncompliance with smoking of very low nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2015 Feb;24(2):331–5. doi: 10.1158/1055-9965.EPI-14-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter P, Steven PR, Bravo R, Lisko JG, Damian M, Gonzalez-Jimenez N, Gray N, Keong LM, Kimbrell JB, Kuklenyik P, et al. Characterization of SPECTRUM Variable Nicotine Research Cigarettes. Tobacco Regulatory Science. 2016;2(2):94–105. doi: 10.18001/TRS.2.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldheer S, Midya V, Lester C, Liao J, Yingst J, Hrabovsky S, Allen S, Krebbs N, Evins AE, Horn K, Richie J, Muscat J, Foulds J. Acceptability of Spectrum research cigarettes among participants in trials of reduced nicotine cigarettes. Tobacco Regulatory Science. 4(1):573–585. doi: 10.18001/TRS.4.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis M, Foulds J, Feyerabend C. Exposure to passive smoking among bar staff. Brit J Addiction. 1992;87:111–113. doi: 10.1111/j.1360-0443.1992.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 15.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction. 2008 Sep;103(9):1553–61. doi: 10.1111/j.1360-0443.2008.02297.x. [DOI] [PubMed] [Google Scholar]
- 17.Nicotine Research Cigarettes Drug Supply Program. [Accessed February 1, 2018];NIDA Drug Supply Program. 2016 https://www.drugabuse.gov/nicotine-research-cigarette-drug-supply-program.
- 18.Foulds J, Stapleton J, Feyerabend C, et al. Effect of transdermal nicotine patches on cigarette smoking: a double blind crossover study. Psychopharmacology (Berl) 1992;106(3):421–427. doi: 10.1007/BF02245429. [DOI] [PubMed] [Google Scholar]
- 19.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 20.Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob Res. 2015;17(2):186–92. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nondahl DM, Cruickshanks KJ, Schubert CR. A questionnaire for assessing environmental tobacco smoke exposure. Environ Res. 2005;97(1):76–82. doi: 10.1016/j.envres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Bedfont Scientific Ltd. [Accessed May 1, 2017];PiCO+ Smokerlyzer Operating Manual. 2017 Available at: https://www.bedfont.com/file.php?f=ZmlsZSMjNzE0.
- 23.Calbiotech. [Accessed May 1, 2017];Cotinine ELISA. Available at: https://www.calbiotech.com/most-cited/104-cotinine.
- 24.Centers for Disease Control. [Accessed March 26, 2018];National Health and Nutrition Examination Survey 2009–2010 Data Documentation, Codebook, and Frequencies. 2011 Sep; https://wwwn.cdc.gov/nchs/nhanes/2009–2010/COTNAL_F.htm.