SUMMARY
Congenital HCMV infection is a leading infectious cause of long-term neurodevelopmental sequelae. Infection of newborn mice with MCMV intraperitoneally is a well-established model of congenital HCMV infection, which best recapitulates the hematogenous route of virus spread to brain and subsequent pathology. Here we used this model to investigate the role, dynamics and phenotype of CD8+ T cells in brain following infection of newborn mice. We show that CD8+ T cells infiltrate the brain and form a pool of tissue-resident memory T cells (TRM cells) that persist for lifetime. Adoptively transferred virus-specific CD8+ T cells provide protection against primary MCMV infection in newborn mice, reduce brain pathology and remain in the brain as TRM cells. Brain CD8+ TRM cells were long-lived, slowly proliferating cells able to respond to local challenge infection. Importantly, brain CD8+ TRM cells controlled latent MCMV and their depletion resulted in virus reactivation and enhanced inflammation in brain.
Keywords: mouse cytomegalovirus, tissue-resident memory T cells, brain pathology, microglia, congenital CMV infection
INTRODUCTION
Human cytomegalovirus (HCMV) is a highly prevalent herpesvirus infecting a large proportion of individuals worldwide. Primary infection of healthy individuals is usually asymptomatic, followed by the establishment of latency. However, HCMV infection or reactivation in immunocompromised or immunologically immature individuals can lead to severe or life-threatening disease (Ljungman et al., 2010, Kotton, 2010, Nigro and Adler, 2011). Congenital HCMV infection is a leading infectious cause of long-term neurodevelopmental sequelae, including mental retardation and sensorineural hearing loss (Dreher et al., 2014, McCormick and Mocarski, 2015, Boppana et al., 2013, Pati et al., 2013). HCMV is strictly species-specific virus; therefore, animal cytomegaloviruses are often used to investigate the immunobiology and pathogenesis of infection. Infection of mice with mouse cytomegalovirus (MCMV) is the most common model of HCMV infection (Reddehase et al., 2008). Since MCMV does not cross the placenta, we employ a model of congenital infection in which newborn mice are infected with MCMV intraperitoneally at 1st postnatal day (PND 1) (Koontz et al., 2008). It is worth mentioning that the central nervous system (CNS) in newborn mice is developmentally equivalent to the human fetus at 15 weeks of gestation, a time period when HCMV infection in humans is most frequently acquired during pregnancy (Enders et al., 2011, Clancy et al., 2001). Upon MCMV infection of newborn mice, the virus disseminates to various tissues, including the brain where infection results in widespread, focal, non-necrotizing encephalitis (Koontz et al., 2008). CNS pathology in infected newborn mice closely recapitulates the pathology occurring during the congenital HCMV infection, most evident in smaller cerebellum size and thicker external granular layer (Koontz et al., 2008, Cekinović et al., 2008). Moreover, the infection causes hearing loss associated with inner ear inflammation and loss of spiral ganglia neurons (Bradford et al., 2015). We and others have shown that MCMV infection in newborn mice induces a strong inflammatory response in the brain characterized by the activation of microglia, recruitment of activated peripheral immune cells and the expression of pro-inflammatory cytokines (Slavuljica et al., 2015, Koontz et al., 2008, Kosmac et al., 2013, Seleme et al., 2017). In fact, virus-induced inflammation, rather than the cytopathic effect of virus on infected cells, is responsible for neurodevelopmental abnormalities (Kosmac et al., 2013, Seleme et al., 2017). The dominant population of lymphocytes infiltrating brain upon infection are CD8+ T cells (Bantug et al., 2008). We have shown previously that CD8+ T cells are essential for control of MCMV infection in the brain and for the survival of newborn mice upon infection (Bantug et al., 2008). Virus-specific CD8+ T cells remain in the brain even after resolution of productive infection (Bantug et al., 2008, Venturi et al., 2016, Mutnal et al., 2011). However, the dynamics of these cells and their biology remain poorly characterized.
Memory CD8+ T cells are classically divided into two groups: central memory (TCM) and effector memory (TEM) (Sallusto et al., 1999). TCM circulate in between the lymph nodes and the blood and give rise to the new effector cells upon antigen re-encounter, while TEM circulate between lymphoid and nonlymphoid tissues and can perform immediate effector functions upon antigen encounter (Masopust and Schenkel, 2013). A new group of memory CD8+ T cells, tissue-resident memory T cells (TRM cells), has been identified that provides superior protection against local secondary infections (Gebhardt et al., 2009, Hawke et al., 1998, Masopust et al., 2001, Khanna et al., 2003). In fact, the majority of memory T cells is located in nonlymphoid tissues (Marshall et al., 2001). TRM cells are characterized by the expression of surface markers CD69 and CD103, and distinct molecular, functional and metabolic features (Mueller and Mackay, 2016, Pan et al., 2017, Mackay et al., 2015, Wakim et al., 2010). Even though the CNS is considered as immune privileged site, infections of the CNS with several viruses have been associated with the infiltration of lymphocytes and the development of TRM cells (Rosato et al., 2017). This is particularly important for chronic persistent infections that provide a constant antigen supply that can boost already primed memory T cells. In such conditions, a delicate balance between virus control and avoidance of brain pathology needs to be established.
Here we characterized CD8+ T cells that persist in the brain of mice infected perinatally with MCMV. We show that upon MCMV infection of newborn mice, virus-specific CD8+ T cells form a pool of TRM cells that are retained in the brain for lifetime. Adoptively transferred virus-specific CD8+ T cells provide protection against primary MCMV infection, reduce brain pathology and remain in the brain as TRM cells. Importantly, CD8+ TRM cells respond to challenge and prevent reactivation of latent virus as well as reactivation-induced pathology.
RESULTS
MCMV-specific CD8+ T cells persist in the brain of perinatally infected mice
Upon MCMV infection, various leukocyte populations infiltrate the brain of newborn mice. CD8+ T cells are the predominant lymphocytes recruited to the brain and are essential for virus clearance (Bantug et al., 2008). However, the long term fate of these cells has not been assessed in detail.
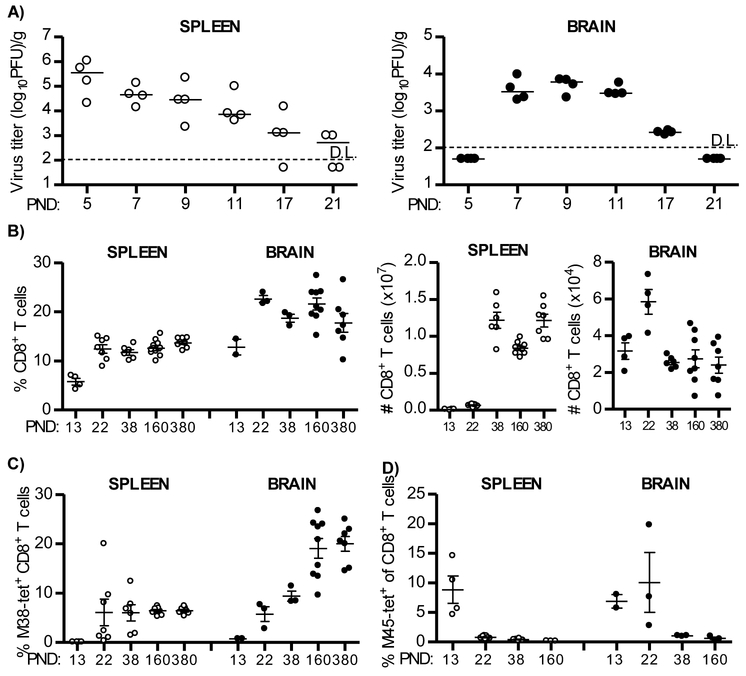
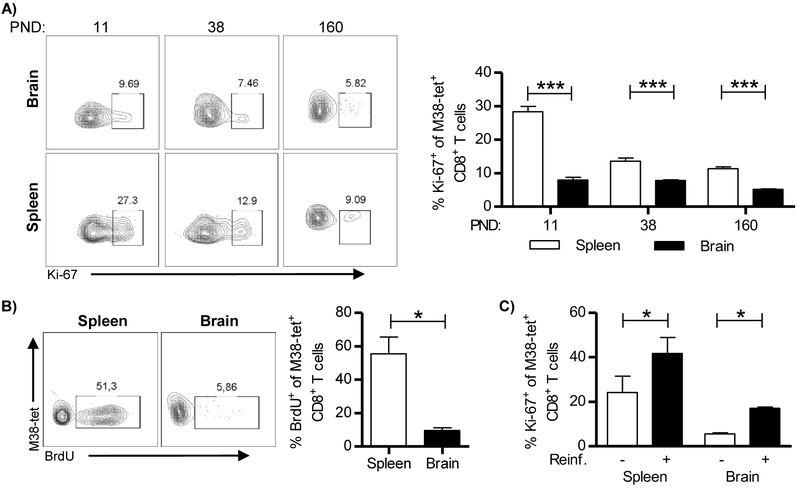
In order to follow CD8+ T cells in the brain we infected newborn C57BL/6 mice intraperitoneally (i.p.) with 200 PFU of MCMV on PND 1. As previously reported, infectious virus could be detected in the brain between days 7 and 17 p.i. (Figure 1A right panel, (Cekinović et al., 2008, Koontz et al., 2008)). Brain-infiltrating CD8+ T lymphocytes were strongly activated, as shown by their expression of CD43 (Figure S1). Notably, the expression of this activation marker gradually decreased and the kinetics of this decrease corresponded to the kinetics of virus clearance in the brain. However, despite successful clearance of productive virus infection, CD8+ T lymphocytes persisted essentially for lifetime (Figure 1B). Of note, the percentage of CD8+ T cells among lymphocytes was higher in the brain compared to the spleen of MCMV-infected newborn mice (Figure 1B).
Figure 1. Virus specific CD8+ T cells are recruited and persist in the brain of MCMV-infected newborn mice.
Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1. (A) Brains and spleens were collected at the indicated days and viral titers were determined by plaque assay. Titers in organ of individual mice are shown (circles); horizontal bars indicate the median values; D.L., detection limit. (B) At indicated time points frequencies (left) and total numbers (right) of CD8+ T cells were analyzed in spleen and brain. (C, D) The frequencies of M38-specific (C) and M45-specific (D) CD8+ T cells were analyzed in spleen and brain. Values for individual animals are shown (circles); Mean values +/− SEM are shown. Results from one of the three independent experiments are shown. n=4-10 animals.
CD8+ T cell responses to MCMV are characterized by the development of conventional and inflationary virus-specific CD8+ T cells (Munks et al., 2006, Klenerman and Oxenius, 2016). Inflationary M38-specific CD8+ T cells reached a frequency on average of 20% of the total number of CD8+ T cells in the brain of MCMV-infected newborn mice (Figure 1C). Similarly, the frequency of splenic M38-specific CD8+ T cells were maintained; albeit, at a much lower frequency (Figure 1C). The frequencies of CD8+ T cells specific for the non-inflationary epitope M45 reached the peak on PND 22 in brain, whereas in the spleen the peak was observed earlier, on PND 13 (Figure 1D). As expected, the frequency of M45-specific CD8+ T cells decreased during course of MCMV infection in brain and spleen which correlated with viral clearance (Figures 1A left panel and ID, (Munks et al., 2006)). Altogether, these data demonstrate that during perinatal MCMV infection virus-specific CD8+ T cells infiltrate the brain and persist in the organ for lifetime despite the resolution of productive infection.
MCMV-specific CD8+ T cells that persist in the brain of MCMV-infected newborn mice are tissue-resident memory T cells
TRM cells have been identified in many non-lymphoid organs, including brain (Rosato et al., 2017). Since CD8+ T cells persisted in the brain of MCMV-infected newborn mice for more than one year, we assumed that they acquired the characteristics of TRM cells.
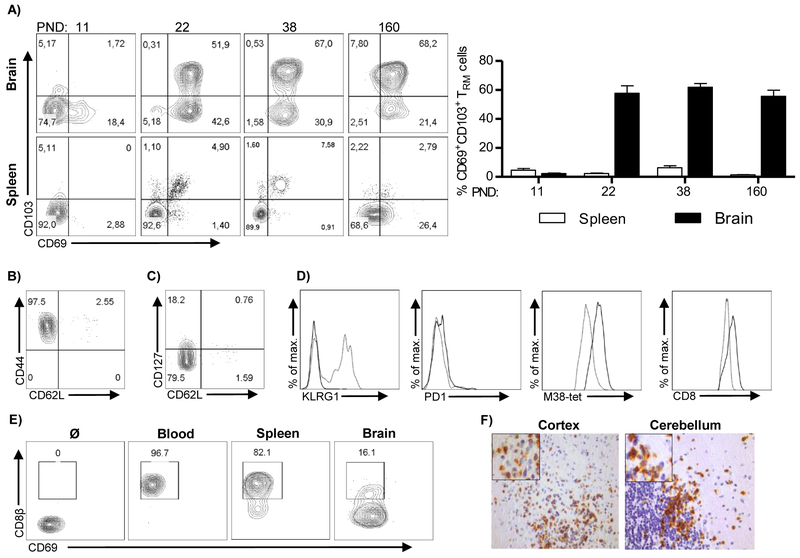
To answer this question, we analyzed expression of markers of tissue residency, CD69 and CD103, on M38-specific CD8+ T cells that persisted in the brain of perinatally infected mice (Figure 2A, (Mueller and Mackay, 2016)). In contrast to the spleen where the vast majority of virus-specific CD8+ T cells did not express CD69 or CD103, more than 90% of the brain M38-specific CD8+ T cells upregulated CD69 (Figure 2A), and on average 60% of brain memory CD8+ T cells co-expressed CD103, i.e. were double positive (CD69+CD103+) as early as three weeks p.i. (Figure 2A). A similar pattern was observed when another immunodominant epitope of MCMV (IE3) was analyzed, or when the total population of CD8+ T cells in the brain was analyzed for the expression of CD69 and CD103 (Figure S2A, S2B and S2C). Additionally, CD8+ T cells in the brain did not have the phenotype of central memory or effector memory T cells, as they lacked CD62L and KLRG1, had low expression of CD127, but expressed high levels of CD44 (Figures 2B-D). Furthermore, CD8+ T cells in the brain expressed higher levels of PD1, had higher TCR density and expressed more of co-receptor molecule CD8 compared to splenic CD8+ T cells (Figure 2D). Altogether, these data show that brain CD8+ T cells are phenotypically TRM cells.
Figure 2. Virus specific CD8+ T cells that persist in the brain of MCMV-infected newborn mice are tissue resident.
Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1. (A) The expression of CD103 and CD69 on M38-specific CD8+ T cells in the brain and the spleen was analyzed by flow cytometry at indicated time points p.i. Representative FACS plots showing expression of CD103 and CD69 (left) and quantification of frequency of CD69+CD103+ cells (right) are shown. Mean values + SEM are shown (n=3-4 animals). (B, C) Expression of CD44, CD62L and CD127 on brain M38-specific CD8+ T cells is shown. (D) Representative histograms showing expression of KLRG1, PD1, TCR and CD8 by M38-specific CD8+ T cells in brain (thick lines) and spleen (dashed lines) are shown. (E) Mice were injected with anti-CD8β-FITC antibody i.v. and the frequencies of labelled cells were assessed by flow cytometry in blood, spleen and brain. (F) Immunohistological staining of CD8+ T cells (brown) in brain cortex and cerebellum on PND 21. Results from one of the three independent experiments are shown.
To confirm that the persisting CD8+ T cells isolated from the brain are indeed TRM cells, we injected intravenously a monoclonal antibody specific for CD8β labeled with fluorochrome FITC (Figure 2E). The vast majority of CD8+ T cells isolated from blood and spleen were labeled with the antibody, while a majority of CD8+ T cells isolated from the brain remained unlabeled, indicating that brain CD8+ T cells are not in a direct contact with blood, but are rather located in the tissue. We have verified this assumption by immunohistochemistry of CD8+ T cells in brain and show that they are located in the parenchyma in different regions of brain (Figure 2F).
In addition to CD8+ T cells, a population of CD4+ T cells persisted in the brain of mice infected perinatally with MCMV (Figure S2D). Even though the population of CD4+ T cells was much smaller in frequency compared to CD8+ T cells, CD4+ T cells in the brain also acquired TRM phenotype as shown by the expression of CD69 and CD11a (Figure S2E). Yet, the depletion of CD4+ T cells during primary MCMV infection in newborn mice resulted in reduced frequency of CD103+ CD8+ TRM cells in brain (Figure S2F).
Altogether, these data show that persistent virus-specific CD8+ T cells induced by perinatal MCMV infection become TRM cells localized in the parenchyma.
Productive virus replication is required for generation of brain CD8+ TRM cells
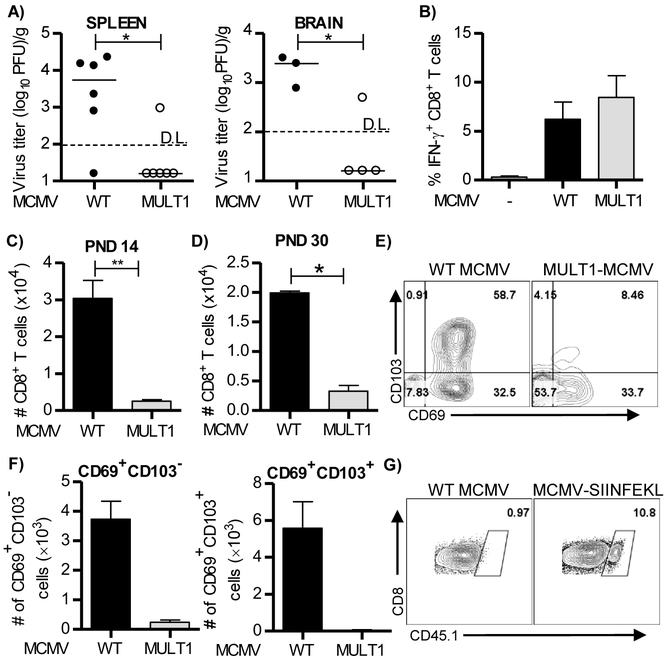
It has been shown that CD8+ TRM cells can develop also without antigen being present in the tissue (Jiang et al., 2012, Mackay et al., 2012). Upon intravenous infection of adult mice, MCMV does not reach the brain and this is associated with absence of CD8+ T cells in the brain tissue (data not shown). To assess whether CD8+ TRM cells can be generated in the brain of MCMV-infected newborn mice without productive virus replication in the tissue we used recombinant MCMV expressing NKG2D ligand MULT1 (MULT1-MCMV) which is heavily attenuated even in newborn mice (Figure 3A). Thus, the virus is hardly able to infect the brain. In spite of the strong attenuation both in the spleen and the brain, this virus induced virus-specific CD8+ T cell response equivalent to the WT MCMV in lymphoid organs (Figure 3B). In contrast, the infiltration of CD8+ T cells in brain of mice infected with MULT1-MCMV was dramatically reduced when compared to WT MCMV-infected mice (Figure 3C). Similar was found on PND 30 (Figure 3D), when majority of cells in brain become tissue resident in WT MCMV infected newborn mice. The number of CD8+ T cells in the brain of MULT1-MCMV infected mice was not only dramatically reduced, but these cells also failed to express CD103 and CD69 (Figure 3E and 3F). Altogether, these data indicate that virus replication is required in the brain for development of CD8+ TRM cells.
Figure 3. Active virus replication is required in the brain of newborn mice to induce optimal CD8+ T cell response.
(A-F) Newborn C57BL/6 mice were injected i.p. with 200 PFU of WT MCMV or MULT1-MCMV on PND 1. (A) Brains and spleens were harvested on PND 8 and virus titers were determined by plaque assay. Titers in organs of individual mice are shown (circles); horizontal bars indicate the median values; D.L., detection limit. *, P < 0.05; **, P < 0.01. (B) On PND 14 splenic lymphocytes were isolated and pulsed with M38 peptide. Frequency of IFN-γ producing CD8+ T cells is shown. (C, D) Numbers of CD8+ T cells in the brain on PND 14 (C) and PND 30 (D) are shown. Mean values + SEM are shown (n=4-5 animals). (E, F) Representative dot plots showing expression of CD69 and CD103 by brain CD8+ T cells (E) and quantification of CD69+CD103− and CD69+CD103+ CD8+ T cells are shown (F). (G) Newborn C57BL/6 mice (CD45.2+) were injected i.p. with 200 PFU of WT MCMV or MCMV-SIINFEKL on PND 1, followed by injection of 10,000 OT-1 CD8+ T cells (CD45.1+) on PND 7. The representative dot plots showing frequency of OT-1 cells in brain on PND 18 in brain is shown. Results from one of 2-3 independent experiments are shown.
To answer the question whether antigen is required to generate brain CD8+ TRM cells we have used newborn mice which were infected with MCMV expressing SIINFEKL (MCMV-SIINFEKL) or WT MCMV (Figure 3G). Both groups of infected newborn mice received OT-1 cells 6 days p.i., and generation of OT-1 TRM cells in brain has been followed. OT-1 cells could not be found in mice infected with WT MCMV 20 days p.i., while they were readily detected in mice infected with MCMV-SIINFEKL. These data suggest that antigen is required in the brain to generate CD8+ TRM cells.
Adoptively transferred MCMV-specific CD8+ T cells are protective and form a TRM pool of cells
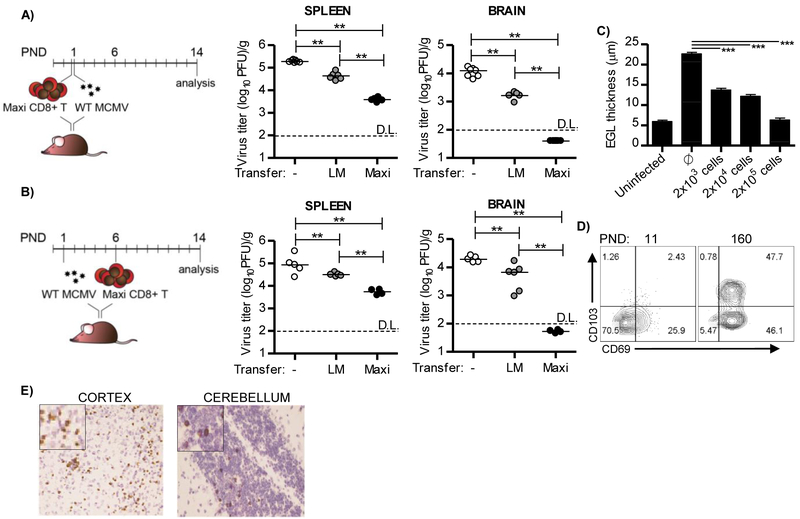
Adoptive transfer of CMV-specific CD8+ T cells has significant clinical potential (Riddell et al., 1992, Busch et al., 2016, Smith et al., 2016). We have previously shown that adoptive transfer of polyclonal virus-specific CD8+ T cells reduces the viral load in MCMV-infected newborn mice (Bantug et al., 2008). However, it remains unknown whether the adoptively transferred MCMV-specific CD8+ T cells form a TRM pool. To address this question we employed monoclonal Ly5.1+ (CD45.1+) TCR transgenic CD8+ T cells, which are specific for the immunodominant MCMV epitope M38316–323 (Maxi CD8+ T cells; (Torti et al., 2011)). In order to assess the protective capacity of M38-specific CD8+ T cells we performed adoptive transfer experiments. Newborn mice were infected with 200 PFU of MCMV and injected with 10,000 Maxi CD8+ T cells isolated from naive transgenic mice. Adoptive transfer experiments were performed either on day 0 (prophylactic protocol), or on the day 5 p.i. (therapeutic protocol) and virus titers were analyzed on PND 14 (Figures 4A and 4B). Transferred cells were readily found in both the brain and spleen of MCMV-infected newborn mice (Figure S3). Importantly, transferred Maxi CD8+ T cells provided protection against MCMV in both the prophylactic and therapeutic protocols (Figures 4A and 4B). Of note, transferred cells derived from littermate mice also reduced virus load, however to a much lower extent as compared to transferred Maxi CD8+ T cells.
Figure 4. Adoptively transferred M38-specific CD8+ T cells are protective in the brain of perinatally infected mice and acquire tissue-resident phenotype.
Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1. (A, B) Newborn mice were i.p. injected with 10,000 of Maxi CD8+ T cells, six hours before infection (A, prophylactic protocol) or on PND 6 (B, therapeutic protocol). The same number of CD8+ T cells isolated from non-transgenic littermate mice was used as a control. On PND 14 mice were sacrificed and viral titers in organs were determined by plaque assay. Titers in organ of individual mice are shown (circles); LM, littermates; horizontal bars indicate the median values; D.L., detection limit. (C) Infected newborn mice received indicated numbers of Maxi CD8+ T cells on PND 6. Mice were sacrificed and thickness of external granular layer was determined on PND 14. Mean values + SEM are shown. (D) Infected newborn mice received 10,000 Maxi CD8+ T cells on PND 6. The expression of CD103 and CD69 on adoptively transferred Maxi CD8+ T cells in brain was analyzed by flow cytometry at indicated postnatal days. (E) Localization of adoptively transferred CD45.1+ Maxi CD8+ T cells in cortex and cerebellum on day 21 p.i. is shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results from one of 2-3 independent experiments are shown.
Perinatal MCMV infection results in altered brain development in newborn mice, similar to what has been described in infants that underwent congenital HCMV infection (Slavuljica et al., 2015, Fowler et al., 1992, Koontz et al., 2008). Since the majority of the previously published data in this model used BALB/c mice and since Maxi mice are on a C57BL/6 background, we first characterized the impact of perinatal MCMV infection on brain development in C57BL/6 mice (Figure S4). Similar to what has been observed in BALB/c mice, perinatal MCMV infection induced detectable changes in external granular layer (EGL) thickness, cerebellum area and circumference in C57BL/6 mice (Figure S4). Adoptive transfer of increasing numbers of Maxi CD8+ T cells in infected newborn mice resulted in reduced brain pathology as observed by reduced EGL thickness that correlated with cell dose (Figure 4C).
Transferred Maxi CD8+ T cells persisted in the brain of perinatally infected mice, and expressed CD69 and CD103 to a similar extent as endogenous M38-specific CD8+ T cells in the brain, indicating their differentiation to tissue-resident cells (Figure 4D and 2A). In addition, the distribution of Maxi CD8+ T cells in the brain was similar to the endogenous CD8+ T cells (Figure 4E and 2C). Similarly, adoptive transfer of OT-1 cells into newborn mice infected with MCMV-SIINFEKL provided protection and OT-1 cells formed a pool of TRM cells (Figure S5).
Altogether, these findings show that adoptively transferred naive MCMV-specific CD8+ T cells are recruited to the brain of infected newborn mice, efficiently control infection, prevent brain pathology and establish a TRM pool of cells.
MCMV-specific brain CD8+ TRM cells are long-lived quiescent cells
It is currently considered that the TRM pool of CD8+ T cells is not replenished by circulating memory CD8+ T cells, but instead that these cells have the ability for self-renewal (Mueller and Mackay, 2016, Gebhardt et al., 2009, Jiang et al., 2012, Fan and Rudensky, 2016).
To assess the proliferative potential of virus-specific CD8+ TRM cells in the brain induced after perinatal MCMV infection we analyzed their Ki-67 expression and compared it with that of splenic CD8+ T cells. At all time-points analyzed splenic M38-specific CD8+ T cells expressed higher levels of Ki-67 indicating higher turnover, as compared to brain-resident (CD69+) M38-specific CD8+ T cells (Figure 5A). BrdU is commonly used to detect proliferating cells and it can pass blood-brain barrier (Taupin, 2007). To confirm that brain TRM cells cycle at lower rate at steady state as compared to splenic CD8+ T cells, 2-4 month old mice that were infected with MCMV as newborns were given BrdU in the drinking water for two weeks, before sacrificing the mice for analysis (Figure 5B). We found that the majority of splenic M38-specific CD8+ T cells incorporated BrdU, while only a small percentage of brain CD8+ TRM cells was BrdU-positive, indicating that these cells proliferate at a low rate. This suggested that brain TRM cells are long-lived. Furthermore, the low level of BrdU-positive cells in the brain indicated that repopulation of brain TRM pool by peripheral cells, if occurs, happens at a very low rate. To confirm that brain CD8+ TRM cells are not replenished by peripheral memory cells, we first generated memory Maxi CD8+ T cells by transferring naive Maxi CD8+ T cells into MCMV infected newborn C57BL/6 mice (Figure S6). Three months after infection, we have isolated memory Maxi CD8+ T cells (CD45.1+) and transferred them to CD45.2+ adult C57BL/6 mice that were infected as newborns. Two weeks after transfer, recipient mice were sacrificed and their organs were analyzed for the presence of Maxi cells (CD45.1+). While Maxi cells were readily detected in the periphery (Figure S6), they were absent in the brain, suggesting against repopulation of brain TRM cells by peripheral recirculating T cells.
Figure 5. Brain-resident memory T cells are long lived, slowly proliferating cells capable of responding to challenge.
C57BL/6 newborn mice were injected i.p. with 200 PFU of MCMV on PND 1. (A) The expression of Ki-67 by M38-specific CD8+ T cells in brain and spleen was analyzed by flow cytometry at indicated time points. Representative FACS plots (left) and quantification of Ki-67 expression (right) are shown. (B) Representative plots of BrdU incorporation by M38-specific CD8+ T cells in brain and spleen (left) and quantification of BrdU incorporation (right) are shown. (C) Two months old mice were re-infected with 50,000 PFU of salivary gland derived MCMV. Five days post re-infection Ki-67 expression by M38-specific CD8+ T cells was determined in brain and spleen. Mean values + SEM are shown (n=3-5 animals). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results from one of two independent experiments are shown.
To understand whether these cells can proliferate upon stimulation, 2-4 month old mice that were infected perinatally were injected with highly virulent salivary gland-derived MCMV (Figure 5C). Both splenic and brain M38-specific CD8+ T cells upregulated Ki-67 upon reinfection, even though the infectious virus was not detected in the brain or in any other organ analyzed (Figure 5C and Figure S7). Altogether, these data indicate that brain resident memory CD8+ T cells are long-lived, slowly proliferating cells capable of responding to stimulation in vivo.
MCMV-induced brain CD8+ TRM cells are functionally competent
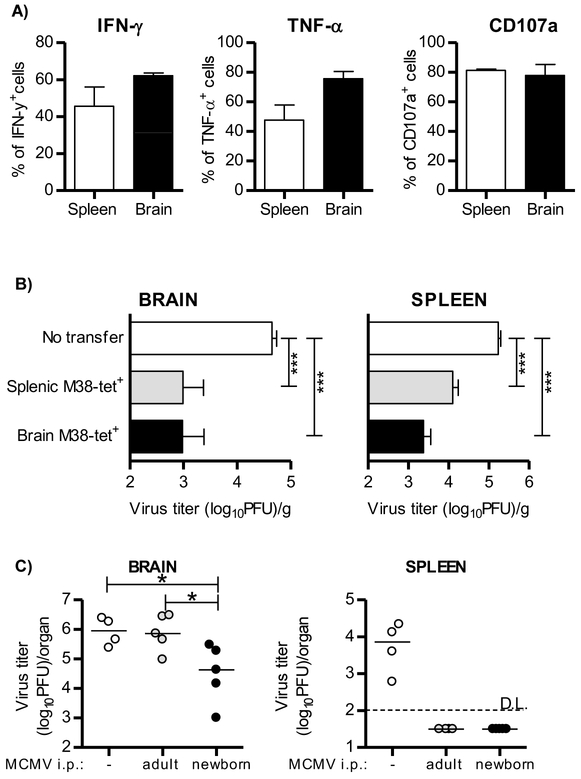
The major role of tissue-resident lymphocytes is the control of local reinfection or reactivation (Rosato et al., 2017). To understand the functional potential of MCMV-specific brain CD8+ TRM cells, we have stimulated lymphocytes isolated from the brain and the spleen of 2-4 month old mice with the M38 peptide in vitro (Figure 6A). Both splenic and brain M38-specific CD8+ T cells produced cytokines IFN-γ and TNF-α to similar extent. In addition, they were able to degranulate upon stimulation as indicated by expression of CD107a, demonstrating their cytotoxic ability (Figure 6A).
Figure 6. Virus-specific CD8+ TRM cells in brain are fully functional.
(A) Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1 and 10,000 Maxi CD8+ T cells on PND6. Two months p.i. brain and splenic lymphocytes were isolated and pulsed with M38 peptide. Frequency of IFN-γ, TNF-α and CD107a by Maxi CD8+ T cells is shown (n=3 animals). (B) C57BL/6 newborn mice were i.p. injected with 200 PFU of MCMV on PND 1. On PND 6 mice received 2×103 of sorted memory Maxi CD8+ T cells isolated from spleen or brain of 2 month old perinatally infected C57BL/6 mice which received naive Maxi CD8+ T cells 5 days p.i.. On PND 14 newborn mice were sacrificed and viral titers in organs were determined by standard plaque assay. Mean values + SEM are shown (n=8 animals). (C) C57BL/6JHT/JHT mice were infected as newborns (200 PFU of MCMV) or adults (2×105 PFU of MCMV) and left to establish latency. Mice were intracranially challenged with 105 PFU of MCMV. Non-infected C57BL/6JHT/JHT mice served as a control. All mice received FTY720 (1μg/g) i.p. daily starting 3 days prior i.c. challenge. Virus titer was determined 3 days post i.c. challenge. (n=4-5 animals). ***, P < 0.001.
To analyze the protective capacity of brain-resident memory CD8+ T cells we first infected newborn mice, and adoptively transferred Maxi CD8+ T cells on PND 6 (Figure 6B). Two months post infection, we sorted brain resident or splenic memory Maxi CD8+ T cells and adoptively transferred them into new infected pups 5 days p.i.. Transfer of either brain or splenic memory M38-specific CD8+ T cells reduced the viral load in both the spleen and the brain indicating that brain and splenic memory T cells are equally protective against MCMV infection. In addition, this demonstrates that the protective capacity of CD8+ TRM cells derived from brain is not restricted to the tissue of their origin. Altogether, these data show that brain CD8+ TRM cells are functionally competent and able to provide protection against the rechallenge.
To test the protective capacity of CD8+ TRM cells in brain tissue, mice MCMV-infected as newborns or mice MCMV-infected as adults, both in latent stage of infection, were challenged by intracranial (i.c.) injection of MCMV and viral titers were determined 3 days later (Figure 6C). Intracranially infected naive mice were used as controls. To prevent infiltration of circulating lymphocytes, mice were treated with FTY720 (Brinkmann et al., 2002). Intracranial injection of MCMV in naive mice resulted in a high virus titer in brain, but also in a simultaneous virus spread to periphery, including the spleen. Surprisingly, mice infected as adults and challenged intracranially had a similarly high titer of MCMV in the brain as naive mice receiving the virus, but were completely protected in periphery, suggesting once again that the immune response to primary infection of adult immunocompetent mice does not result in tissue resident immunity in brain. However, mice infected as newborns, i.e. having the virus specific TRM cells in brain, showed a significantly lower virus titer upon intracranial infection compared to mice infected as adults. Essentially the same results were obtained in agammaglobulinemic (C57BL/6JHT/JHT) (Figure 6C) and normal C57BL/6 mice (Figure S8) suggesting against any role of antiviral antibodies in protection of brain tissue.
Collectively, these findings demonstrated the capacity of brain resident T cells in protection against local virus challenge.
Functional capacity of CD103+ and CD103− subset of brain TRM cells
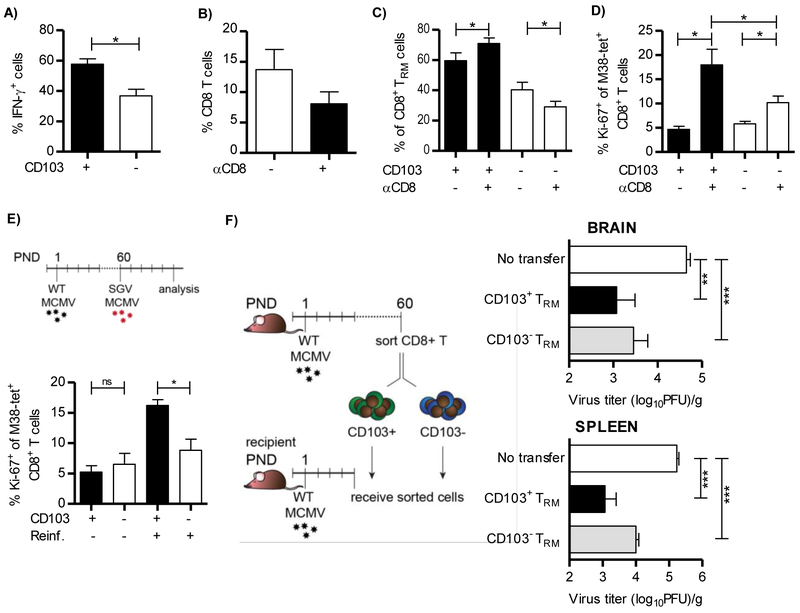
Two distinct populations of TRM cells, CD103+ and CD103−, persist in the brain of MCMV-infected newborn mice (Figure 2A). To compare these two populations in a direct manner, we first used M38 peptide to stimulate lymphocytes isolated from brain of 2-4 month old mice infected perinatally. Both populations produced IFN-γ upon stimulation, but a higher fraction of CD103+ M38-specific CD8+ TRM cells produced IFN-γ as compared to the CD103− subset (Figure 7A). Upon injection of CD8+ T cell depleting antibody into perinatally infected mice, a partial depletion of brain CD8+ TRM cells was observed three weeks after treatment (Figure 7B). The proportion of CD103+ CD8+ TRM cells increased after depletion (Figure 7C). In addition, upon depletion CD103+ cells induced Ki-67 expression to higher levels than did CD103− cells (Figure 7D). Furthermore, we re-infected perinatally-infected 2-4 month-old mice with highly virulent salivary gland-derived MCMV (Figure 7E). Even though at steady state these two populations express equal levels of Ki-67, five days after re-infection with salivary gland MCMV CD103+ M38-specific CD8+ T cells increased Ki-67 to higher levels than the CD103− population.
Figure 7. CD103+ brain-resident memory CD8+ T cells show enhanced functional potential as compared to CD103− subset.
(A-E) Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1 and 10,000 Maxi CD8+ T cells on PND6. A) Lymphocytes were isolated from brain of 2-4 month old mice and pulsed with M38 peptide. Frequency of IFN-γ producing CD103+ and CD103− Maxi CD8+ T cells is shown (n=5 animals). (B-D) Two month old C57BL/6 mice that were infected perinatally were injected with depleting anti-CD8 antibody. Frequencies of CD8+ T cells (B), CD103+ and CD103− m38+ CD8 T cells (C) and Ki-67 expressing CD103+ and CD103− M38-specific CD8+ T cells in brain (D) after depletion of CD8+ T cells are shown (n=4 animals). (E) Two months old perinatally-infected mice were re-infected with 5×104 PFU of salivary gland-derived MCMV. Five days post re-infection frequency of Ki-67 expressing CD103+ and CD103− M38-specific CD8+ T cells were determined in the brain (n=4 animals). (F) C57BL/6 newborn mice were i.p. injected with 200 PFU of MCMV on PND 1. On PND 6 newborn mice received 2,000 of sorted memory CD103+ and CD103− Maxi CD8+ T cells isolated from brain of 2-4 month old perinatally infected C57BL/6 mice which received naive Maxi cells 5 days p.i.. On PND 14 newborn mice were sacrificed and viral titers in organs were determined by standard plaque assay. Mean values + SEM are shown (n=8 animals). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results from one of two independent experiments are shown.
To analyze the protective capacity of CD103+ and CD103− subpopulations of the brain CD8+ TRM cells we first infected newborn mice, followed by adoptive transfer of Maxi CD8+ T cells on PND 6. Two months p.i., we sorted brain resident CD103+ and CD103− subsets of Maxi CD8+ T cells, and adoptively transferred them into infected newborn mice (Figure S9 and 7F). Both CD103+ and CD103− subsets of CD8+ TRM cells provided protection against MCMV, but the CD103+ subset of brain-resident cells was slightly better. Altogether, these data show that both CD103+ and CD103− CD8+ TRM populations are functionally competent.
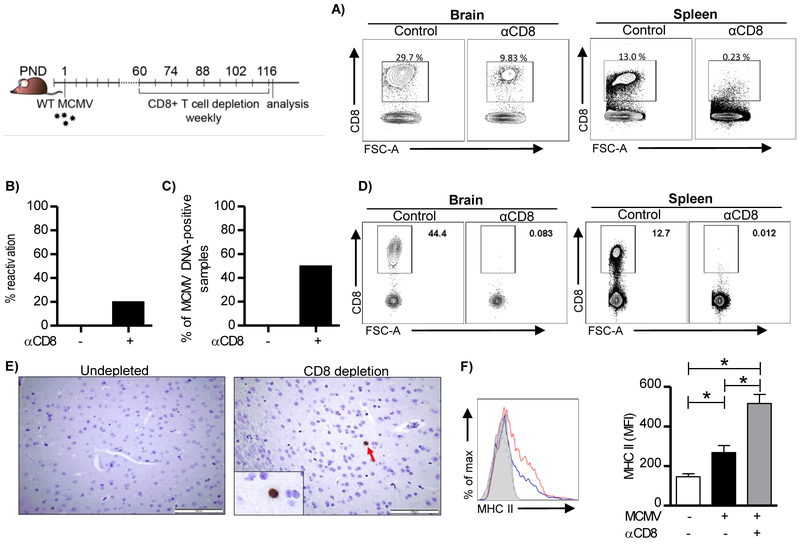
CD8+ TRM cells control MCMV reactivation in the brain and regulate the activation state of microglia
TRM cells not only control the specific pathogen but also secrete cytokines that influence local tissue inflammation and cellular polarization. CD8+ T cells are major regulators of MHC II upregulation on microglia during acute MCMV infection of the brain through the secretion of IFN-γ (Mutnal et al., 2011). However, the impact of brain CD8+ TRM cells on microglial polarization and virus reactivation during latency remains elusive. A single injection of depleting anti-CD8 antibodies only partially reduces the number of brain TRM cells (Figure 7). To achieve better depletion of brain resident memory CD8+ T cells we have injected anti-CD8 depleting antibodies once a week into 2-4 month old perinatally-infected mice for subsequent 8 weeks (Figure 8). This depletion approach resulted in significant reduction in the frequency of brain resident memory CD8+ T cells, even though the depletion was still not complete (Figure 8A). Productive virus infection was detected in the brain of some mice that were subject of CD8+ T cell depletion, whereas no virus could be detected in the brains of control mice (Figure 8B). In addition, a significant proportion of brains was positive for MCMV genome in mice that received anti-CD8 depleting antibodies, while MCMV genomes in non-depleted latently infected group were under the detection limit (Figure 8C). Encouraged by these results we extended depletion regime to four months (Figures 8D). CD8+ T cells were no longer detected in mice that received depleting anti-CD8 antibodies. Furthermore, we were able to detect individual cells in the brain parenchyma expressing immediate early 1 (IE1) protein of MCMV in mice depleted of CD8+ T cells, but not in undepleted control mice, additionally confirming conclusion that virus reactivates locally (Figures 8E). Surprisingly, the relative expression of MHC II on microglia increased significantly upon CD8+ T cell depletion (Figure 8F), indicating that brain resident memory CD8+ T cells, in addition to their role in virus control, are also involved in containment of the proinflammatory state of microglia. This is probably due to the viral reactivation as a consequence of reduced frequency of TRM cells.
Figure 8. Brain-resident memory CD8+ T cells control MCMV reactivation and activation state of microglia.
C57BL/6 newborn mice were injected i.p. with 200 PFU of MCMV on PND 1. Two months p.i. mice were injected with depleting CD8 antibodies. CD8+ T cells were depleted once a week for 8 weeks. (A) Representative plots of CD8+ T cells in the brain and the spleen after depletion of CD8+ T cells. (B) Brain homogenates were placed on MEF cells and four days later frequency of brains with re-activated virus was determined. C) Viral genome presence in DNA isolated from brain tissue was determined by quantitative PCR. The frequency of MCMV DNA-positive brains is shown (n=5 animals). D) CD8+ T cells were depleted once a week for 16 weeks. Representative plots of CD8+ T cells in the brain and the spleen after depletion of CD8+ T cells are shown. (E) Representative MCMV IE-1 (dark brown) staining is shown in paraffin-embedded brain sections. Altogether 120 brain sections were analyzed per group (24 sections per brain). (F) MHC II expression on microglia (CD45+CD11b+) in brain after depletion of CD8+ T cells. Representative histogram is shown (left). Dotted line represents uninfected animal, blue line infected animal, and red line infected animal depleted of CD8+ T cells. Quantification of MHC II expression on microglia is shown on the right. Mean values + SEM are shown (n=5 animals). *, P < 0.05. Results from one of two independent experiments are shown.
Discussion
The CNS was for a long time considered an immune privileged organ. However, in different inflammatory conditions, immune cells can infiltrate brain and provide protection against invading pathogens, but can also induce tissue damage (Ellwardt et al., 2016, Rosato et al., 2017). The importance of immune control in the CNS is well illustrated by congenital CMV infection, however, clearance of the virus is accompanied by inflammatory conditions and neurodevelopmental pathology (reviewed in (Slavuljica et al., 2015)).
The neurodevelopmental status of newborn mice is nearly equivalent to that of a late second trimester human fetus (Clancy et al., 2001). We have used this fact to develop a model of congenital HCMV infection by infecting newborn mice with MCMV intraperitoneally (Koontz et al., 2008). This model recapitulates the hematogenous route of virus spread to CNS following intrauterine HCMV infection of the human fetus and the subsequent brain pathology. We used this model to investigate the role of persistent CD8+ T cells in the brain of mice infected with MCMV as newborns. We showed that CD8+ T cells reach the brain following perinatal infection and form the pool of TRM cells that persist for lifetime. These cells express signature markers of TRM cells, they are not in a direct contact with circulation and could be found in different regions of the brain. Adoptively transferred naive virus-specific CD8+ T cells provided protection against MCMV in newborn mice, reduced brain pathology and formed a pool of TRM cells. MCMV-specific brain TRM cells are long-lived, slowly proliferating cells that are able to respond to rechallenge. Upon adoptive transfer, TRM cells protect the host against MCMV infection, not only in the brain but also in spleen suggesting their functional plasticity. Importantly, we also show that brain CD8+ TRM cells control latent virus and their depletion results in virus reactivation accompanied by an enhanced proinflammatory response of microglia.
All CD8+ T cells isolated from brain of mice infected perinatally with MCMV expressed CD69 and a majority of them expressed CD103 in addition (Figure 2A). Expression of CD69 by itself has a role in the maintenance of TRM cells in tissues by interfering with surface expression of S1P1, and therefore blocking their ability to egress from tissue (Bankovich et al., 2010, Matloubian et al., 2004). The integrin CD103 is a ligand of E-cadherin, but since E-cadherin is not expressed in the CNS the role of CD103 in retention of TRM cells in the brain remains unclear (Shimamura and Takeichi, 1992). In some tissues, binding to E-cadherin was not important for tissue residency (Park and Kupper, 2015), however, CD103 expression is beneficial for retention of TRM cells in some models (Wakim et al., 2010, Mackay et al., 2013, Casey et al., 2012). Many additional signals are central in differentiation and survival of TRM cells, for example TGF-β, IL-15, TNF-α and IL-33 (Mackay et al., 2013, Skon et al., 2013). Upon intracerebral infection of adult mice the PD-1/PDL-1 pathway seems to contribute to the generation of CD8+ TRM cells (Prasad et al., 2017). It will be of interest to investigate whether and how these signals contribute to the establishment of TRM cells upon MCMV infection of newborn mice, especially taking into account the immaturity of the immune system, the developmental changes that occur simultaneously and disparate cytokine milieu in newborn mice as compared to adult animals.
We have shown that active MCMV infection is required in the brain for establishment of CD8+ TRM cells (Figure 3). Namely, infection of newborn mice with a highly attenuated MULT1-MCMV, which fails to infect the brain, resulted in dramatically reduced number of CD8+ T cells in the brain, despite generation of a CD8+ T cell response in lymphoid organs, which was equivalent to the response obtained by virulent WT MCMV. Furthermore, the CD8+ T cells that were detected in the brain of MULT1-MCMV infected mice did not express signature markers of TRM cells indicating that these cells are most likely circulating cells. Whether or not virus persistence is required for long-term maintenance of CD8+ T cells in brain remains less clear. However, our results clearly demonstrated that antigen is required in the brain to generate TRM cells, as illustrated by failure of adoptively transferred OT-1 cells to colonize the brain of mice infected with WT MCMV (Figure 3). Others have shown that antigen is not required for the maintenance of brain TRM cells generated upon VSV infection, but is required for establishment of CD8+ TRM cells (Wakim et al., 2010). As VSV is not persistent virus it is not easy to draw parallels between the two models. In the case of other tissues, resident cells could be generated and maintained even without antigen (Wakim et al., 2010, Thom and Oxenius, 2016).
The protective capacity of TRM cells relies on several mechanisms including cytotoxicity and cytokine secretion (Rosato et al., 2017). We showed here that brain CD8+ TRM cells in mice infected perinatally can produce IFN-γ and TNF-α and can degranulate (Figure 6). Following adoptive transfer into infected newborn mice, brain CD8+ T cells control virus to similar extent as splenic memory CD8+ T cells. At a first glance, this finding seems in contrast with the finding that brain resident CD8+ T cells induced by VSV infection cannot survive when removed from their site of residence (Wakim et al., 2010, Wakim et al., 2012). However, one should not rule out the possibility that the microenvironment in MCMV-infected newborn mice could give advantage for the maintenance of TRM cells in order to provide protection against deleterious virus infection. As suggested also by other studies, here we show that both CD103− and CD103+ subsets of brain CD8+ T cells are functionally competent, although CD103+ cells showed slightly enhanced functional potential. The strongest evidence for direct antiviral capacity of TRM cells generated after perinatal MCMV infection was provided by intracranial challenge infection with MCMV (Figure 6C).
The requirement of CD4+ T cells for establishment and maintenance of CD8+ TRM cells is still an open question. Jiang et al have shown previously that CD4+ T cells are not required for generation of CD8+ TRM cells during Vaccinia virus infection in skin (Jiang et al., 2012). However, CD4+ T cells were required for the generation of CD103+ CD8+ TRM cells in Influenza infected lungs as well as in brain upon intracranial injection of MCMV in adult mice (Prasad et al., 2015, Laidlaw et al., 2014). In the present study, we showed that CD4+ T cells are not essential for generation of CD8+ TRM cells in brain of MCMV-infected newborn mice (Figure S2). Yet, in absence of CD4+ T cells the frequency of CD69+CD103+ cells is significantly reduced. Furthermore, a small population of CD4+ TRM cells remain long-term following infection of newborn mice (Figure S2). One possible role of brain-resident memory CD4+ T cells could be to prevent tissue damage during virus reactivation, as is the case with Treg cells during acute intracranial MCMV infection of adult mice (Prasad et al., 2015). Indeed, many mechanisms are involved in regulation of CNS inflammation including expression of inhibitory receptors TIGIT and PD-1 on immune cells, or release of cytokines IL-10 and TGF-β (Ellwardt et al., 2016, Schachtele et al., 2014). It will be of interest to determine which mechanisms attenuate the potent immune response in brain of mice infected perinatally with MCMV.
The role of CD8+ T cells in control of acute MCMV infection and latency is well-established (Reddehase et al., 1987, Klenerman and Oxenius, 2016, Simon et al., 2006, Podlech et al., 1998). However, the role of brain-resident CD8+ T cells that persist after resolution of perinatal infection remained undefined. Even though the infectious virus cannot be detected in the brain of MCMV-infected newborns following PND 17, clearance of the viral genome is not achieved, leaving the possibility for periodic reactivations (Slavuljica et al., 2015, Reddehase et al., 1994). Indeed, even upon partial depletion of brain resident CD8+ T cells, replicating virus could be detected in brain of infected mice (Figure 8). Even after primary infection of naïve adult animals MCMV fails to reach the brain, most likely due to efficient blood-brain barrier and early virus control in periphery (Figure S7). Thus, it is highly unlikely that the virus detected in brain of CD8+ TRM cell depleted mice was derived from peripherally reactivated cells. In addition, these mice contain antiviral antibodies that are potent in preventing virus spread (Polić et al., 1998). Furthermore, we were able to detect individual reactivating cells in the brain parenchyma in mice depleted of CD8+ T cells. These findings demonstrate a crucial role of brain resident CD8+ T cells in the control of latent virus in situ. In addition, we demonstrate that in absence of CD8+ TRM cells, resident microglia shifts towards proinflammatory phenotype most likely because of virus reactivation. Other viruses can also induce TRM cells in the brain (Rosato et al., 2017). For instance, intranasal VSV infection and intracranial infection with LCMV induced CD8+ TRM cells in the brain which were able to respond to a challenge infection (Wakim et al., 2010, Wakim et al., 2012, Steinbach et al., 2016). Furthermore, TRM cells provide superior protection against antigen re-encounter in other tissues as well (Park and Kupper, 2015, Mueller and Mackay, 2016, Rosato et al., 2017). In addition to brain, MCMV infection induces virus-specific TRM cells in the salivary gland, an organ important for virus persistence and horizontal spread (Thom et al., 2015, Jonjic et al., 1989, Smith et al., 2015). However, unlike in the brain, CD8+ T cells are not required for termination of MCMV persistence in salivary glands, but they can provide protection upon reinfection (Lucin et al., 1992, Walton et al., 2011, Thom et al., 2015). The importance of these findings is further highlighted by the fact that HCMV specific TRM cells can be found in various tissues in humans (Gordon et al., 2017).
Here we demonstrated that adoptively transferred virus-specific CD8+ T cells in infected newborn mice contribute to virus clearance and form TRM cells that persist for lifetime in the brain of MCMV-infected mice. The protective capacity of adoptively transferred cells is also demonstrated by the reduced developmental abnormalities. However, the putative side effects of the long-term persistence of CD8+ T cells and ongoing inflammation cannot be excluded. Therefore, the ideal situation would be to control the virus before reaching the brain and inducing inflammatory response. It is well established that even seropositivity in pregnant women does not guarantee protection against congenital HCMV infection, implying the need for vaccines which would induce more efficient humoral and cellular immunity, and prevent fetal infection or at least prevent virus to reach the brain undergoing development (Britt, 2017). There is significant development in this field, especially after the identification of a pentameric complex that seems to be essential target for vaccination (Plotkin, 2015, Macagno et al., 2010). However, additional approaches will be necessary to develop a HCMV vaccine which will be efficient against congenital infection. We have already shown in MCMV model that by exploiting viral immunoevasins one can generate highly attenuated vaccine vectors which are at the same time able to induce potent cellular immunity (Slavuljica et al., 2010, Trsan et al., 2013, Trsan et al., 2017). One important question that remains unanswered is whether CMV evades the development of more efficient antibody response. As CMVs evade most of the immune defense mechanisms (Powers et al., 2008, Brizic et al., 2014, Lemmermann et al., 2011, Lisnić et al., 2015), we presume that the antibody response is also targeted by viral immunomodulatory genes.
In conclusion, we have described the dynamics and biology of brain-resident CD8+ T cells in a mouse model that recapitulates congenital HCMV infection. In addition, we demonstrated that brain-resident CD8+ T cells are efficient in controlling the re-emergence of latent virus in the brain and in the prevention of viral pathology. The results of this study have important implications in designing new approaches for the prevention and therapy of congenital CMV infection and the generation of anti-CMV vaccines.
Materials and methods
Mice and viruses
C57BL/6, OT-1, C57BL/6JHT/JHT and Maxi mice (Torti et al., 2011) were housed and bred under specific pathogen–free conditions at the Central Animal Facility, Faculty of Medicine, University of Rijeka in accordance with the guidelines contained in the International Guiding Principles for Biomedical Research Involving Animals. The Ethics Committee at the University of Rijeka and National ethics committee approved all animal experiments. Newborn mice and 8–12 week-old mice were used.
Tissue culture derived WT MCMV reconstituted from BAC pSM3fr-MCK-2fl (Jordan et al., 2011) was used for majority of the experiments. In addition, MULT1-MCMV (MULT1 is inserted into a place of its viral inhibitor m145) and MCMV-SIINFEKL were used for some experiments (Trsan et al., 2013, Slavuljica et al., 2010). Virus stocks for newborn mice were aliquoted, frozen at −80 °C and titrated on murine embryonic fibroblasts (MEFs) using standard procedures (Cekinovic et al., 2014). For some experiments salivary gland derived virus was used (Jonjic et al., 2008). Viral titers in organs were determined by standard plaque assay (Jonjic et al., 2008). To detect virus reactivation, we performed extensive titration. Briefly, organs were mechanically homogenized and resuspended in 5 mL of DMEM with 3% FCS. Diluted homogenate (200 μL per well) was added on MEF cells in 24-well plate. Infection was enhanced with centrifugation for 30 min, 800 × g. Following centrifugation, 1 mL of DMEM containing 3% FCS was added per well and plates were incubated at 37°C. Viral titer in organs was determined 4 days after titration.
Intracranial injection of virus (2 μL) was performed using Angle two small animal stereotaxic instrument (Leica Biosystems).
Flow cytometry
Lymphocytes from brain were isolated using a previously described protocol (Lane et al., 2000). Briefly, mice were perfused with cold PBS and each brain was collected in RPMI 1640 with 3% FCS and mechanically dissociated. A 30% Percoll/brain homogenate suspension was underlaid with 70% Percoll in PBS and then centrifuged at 1050 g for 25 min. Cells in the interphase were collected for further analysis. Splenic leukocytes were prepared using standard protocols. Before staining of lymphocytes Fc receptors were blocked with 2.4G2 antibody (Yokoyama and Kim, 2008). The following antibodies, purchased from eBioscience were used: CD8α (53-6.7), CD8β (eBioH35-17.2), CD45 (30-F11), CD43 (eBio R2/60), CD45.1 (A20), CD4 (RM4-5), CD69 (H1.2F3), CD103 (2E7), CD11b (M1/70), IFN-γ (XMG1.2), TNF-α (MP6-XT22), CD107a (H4A3), GzmB (NGZB), CD11a (M17/4), Ki-67 (SolA15), MHC II (M5/114.15.2), KLRG1 (2F1), PD1 (J43) and fluorochrome-labeled streptavidin. M45, m139 and IE3 tetramers were synthesized by the National Institutes of Health tetramer core facility. Fixable Viability Dye (eBioscience) was used to exclude dead cells. For detection of IFN-γ, TNF-α and CD107a expression by CD8+ T cells, incubation was performed in RPMI medium supplemented with 10% of FCS (Gibco) and 1 μg/well of H-2Kb-restricted M38-derived peptide (316SSPPMFRV323) for 5 h at 37°C with 1 αg/ml of brefeldin A (eBioscience) added for the last 4 h of incubation. Intracellular staining of IFN-γ and TNF-α was performed using Intracellular fixation and permeabilization buffer set (eBioscience). Ki-67 staining was performed by using FoxP3 staining buffer set (eBioscience). Cell proliferation assay was performed by providing mice with 0.8 mg/ml BrdU in the drinking water for two weeks. To detect incorporated BrdU, cells were stained according to the manufacturer's protocol (BrdU flow kit; BD Pharmingen). Flow cytometry was performed on FACSAriaIIu and data were analyzed using FlowJo v10 (Tree Star) software.
Intravascular CD8 staining
Mice were injected i.v. with 6 μg of FITC-labeled anti-CD8b (H35-17.2) according to previously described protocol (Anderson et al., 2014). 3 min later, peripheral blood samples from tail were taken, and mice were anesthetized and perfused with PBS. Brains and spleens were dissected and processed immediately (<10 min after antibody injection) for leukocyte isolation
Depletion of immune cell subsets and adoptive transfers
Depletion of CD8+ T cells was performed by i.p. injection of 150 μg of anti-CD8 antibody (YTS 169.4). Long term depletion of CD8+ T cells was performed by i.p. injection of depletion antibodies once a week for eight weeks. In the first two weeks 150 μg of rat antimouse CD8 antibody (YTS 169.4) was injected, and in the remaining six weeks 200 μg of mouse anti-mouse Lyt2.2 depleting antibody was injected. Depletion of CD4+ T cells in newborn mice was performed by injecting 50 μg of CD4 depleting antibody (YTS 191.1) at 3 day interval, starting on PND3.
For adoptive transfer experiments of naive CD8+ T cells, we used splenocytes from MHC-I-restricted TCR-transgenic mice with specificity for the inflationary M38 epitope (Maxi mice (Torti et al., 2011)) or OT-1 mice (Hogquist et al., 1994). Control splenocytes were isolated from littermate mice. CD4 T cells and NK cells were antibody depleted from both Maxi and littermate mice the day before spleen harvesting by injecting i.p. 150 μg of anti-CD4 antibody (YTS 191.1) and anti-NK1.1 antibody (PK136). The number of CD8 T cells within the total splenocyte population was determined by FACS, and Maxi CD8+ T cells or littermate CD8+ T cells were i.p. injected either few hours before infection or 5 days p.i. Animals were sacrificed 14 days p.i.
For adoptive transfer experiments of memory CD8+ T cells, lymphocytes were isolated from brain and spleen as described. Afterwards, lymphocytes were stained with CD45.1 and CD8 antibodies and Maxi cells were sorted by using FACSAria II (BD) using high-speed sorting into RPMI supplemented with 20% FCS. Purity and viability of sorted cells was analyzed immediately post sorting by the addition of PI to a sample of sorted cells. In all experiments the purity exceeded 98%. Sorted cells were washed and injected i.p. in 50 μL of pure DMEM.
Immunohistochemical analysis
Serial sagittal sections (4 μm thick) were prepared from PFA-fixed, paraffin-embedded organs. EGL thickness and cerebellar areas were measured and calculated on serial brain sections stained with Cresyl Violet. For analysis of leukocytes, brains were frozen on dry ice and kept at −80 °C. 8-10 μm thick sections were prepared and stained against CD8 (YTS169.4.2). Slides were developed using biotinylated secondary antibodies and streptavidin-peroxidase, followed by AEC or DAB detection.
MCMV IE1 staining was performed on formalin–fixed, paraffin-embedded tissue sections (3 μm). After de-paraffinization and rehydration, antigen retrieval was performed in sodium citrate buffer (pH 6.0). Endogenous peroxidases were blocked with 3% H2O2 in 50% Methanol/PBS solution. Biotinylated anti-IE1 antibody (IE1.01) and streptavidin-POD (Jackson ImmunoResearch). DAB (Dako) was used as substrate. Samples were counterstained with hematoxylin.
The analysis was performed using the Olympus BX40 microscope and Olympus digital camera (C-3030).
Quantitative RT-PCR
For detection of viral genomes using quantitative PCR, genomic DNA was isolated from brain using Qiagen’s DNEasy Blood&Tissue kit according to manufacturer’s instructions. To amplify low amounts of viral DNA in latently infected organs (which were below level of detection), viral M86 and housekeeping GAPDH amplicons were pre-amplified for 20 cycles using the same primers as in qPCR reaction and New England Biolab’s standard Taq polymerase. The expression of GAPDH was previously demonstrated to be minimally changed during CMV infection (Watson et al., 2007). Because GAPDH is a highly abundant cellular gene, only 1 μL of total DNA was preamplified with GAPDH primers while 5 μL of 1:1000 dilution of GAPDH preamplification reaction was used in the qPCR reaction. To detect viral DNA, 15 μL of DNA was preamplified with M86 primers and 5 μL/well of M86 preamplified, undiluted reaction was used in qPCR reactions. No template controls were also preamplified with GAPDH and M86 primers. For positive control, cca 20 molecules of the M86 amplicon was also pre-amplified and quantified in the same manner as brain samples. Specificity of M86 primer and probe set was determined by measuring the amount of M86 in mock infected, pre-amplified brain samples. We have detected no M86 in any mock-infected controls, even with 30 cycles of preamplification. Quantitative PCR was performed on Applied Biosystem’s 7500 FAST instrument, in total reaction volume of 20 μL, using Eurogentec’s Takyon mastermix for probe-based assays, GAPDH Taqman assay (Applied Biosystem’s, cat.no. Mm99999915_g1), while M86 was detected with previously published primer and probe set (Seckert et al., 2009) (F: GGTCGTGGGCAGCTGGTT; R: CCTACAGCACGGCGGAGAA, probe: TCGGCCGTGTCCACCAGTTTGATCT). The efficiency of amplification for both GAPDH and M86 primers and probes was tested with Takyon master mix and was between 90-110%.
Statistical analysis
Raw data values were inspected for normal distribution using the Prism 5 software (GraphPad Software Inc.) using available tests for normality. The selection of the appropriate test was dependent on the number and distribution of animals per group (Mann-Whitney test or Student's t-test). A level of P <0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments:
We thank Edvard Ražić, Dijana Rumora, Miro Samsa, Edvard Marinović, Ante Miše and Tina Rudančić for the excellent technical support. The study was supported by European Research Council Advanced Grant (no. 322693) (to S.J.), Federal Ministry of Science and Education in 2016 (to JA), by NIH (1RO1AI089956-01A1) (to W.J.B. and S.J.) and by the European Regional Development Fund (KK.01.1.1.01.0006) (to S.J.).
References:
- ANDERSON KG, MAYER-BARBER K, SUNG H, BEURA L, JAMES BR, TAYLOR JJ, QUNAJ L, GRIFFITH TS, VEZYS V, BARBER DL & MASOPUST D 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc, 9, 209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANKOVICH AJ, SHIOW LR & CYSTER JG 2010. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem, 285, 22328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANTUG GR, CEKINOVIC D, BRADFORD R, KOONTZ T, JONJIC S & BRITT WJ 2008. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J Immunol, 181, 2111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOPPANA SB, ROSS SA & FOWLER KB 2013. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis, 57 Suppl 4, SI78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD RD, YOO YG, GOLEMAC M, PUGEL EP, JONJIC S & BRITT WJ 2015. Murine CMV-induced hearing loss is associated with inner ear inflammation and loss of spiral ganglia neurons. PLoS Pathog, 11, e1004774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINKMANN V, DAVIS MD, HEISE CE, ALBERT R, COTTENS S, HOF R, BRUNS C, PRIESCHL E, BAUMRUKER T, HIESTAND P, FOSTER CA, ZOLLINGER M & LYNCH KR 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem, 277, 21453–7. [DOI] [PubMed] [Google Scholar]
- BRITT WJ 2017. Congenital HCMV infection and the enigma of maternal immunity. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIZIC I, ROVIS TL, KRMPOTIC A & JONJIC S 2014. MCMV avoidance of recognition and control by NK cells. Seminars in Inmmnopathology, 36, 641–650. [DOI] [PubMed] [Google Scholar]
- BUSCH DH, FRÄßLE SP, SOMMERMEYER D, BUCHHOLZ VR & RIDDELL SR 2016. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol, 28, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASEY KA, FRASER KA, SCHENKEL JM, MORAN A, ABT MC, BEURA LK, LUCAS PJ, ARTIS D, WHERRY EJ, HOGQUIST K, VEZYS V & MASOPUST D 2012. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol, 188, 4866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEKINOVIC D, LISNIC VJ & JONJIC S 2014. Rodent models of congenital cytomegalovirus infection. Methods Mol Biol, 1119, 289–310. [DOI] [PubMed] [Google Scholar]
- CEKINOVIć D, GOLEMAC M, PUGEL EP, TOMAC J, CICIN-SAIN L, SLAVULJICA I, BRADFORD R, MISCH S, WINKLER TH, MACH M, BRITT WJ & JONJIć S 2008. Passive immunization reduces murine cytomegalovirus-induced brain pathology in newborn mice. J Virol, 82, 12172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLANCY B, DARLINGTON RB & FINLAY BL 2001. Translating developmental time across mammalian species. Neuroscience, 105, 7–17. [DOI] [PubMed] [Google Scholar]
- DREHER AM, ARORA N, FOWLER KB, NOVAK Z, BRITT WJ, BOPPANA SB & ROSS SA 2014. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr, 164, 855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLWARDT E, WALSH JT, KIPNIS J & ZIPP F 2016. Understanding the Role of T Cells in CNS Homeostasis. Trends Immunol, 37, 154–65. [DOI] [PubMed] [Google Scholar]
- ENDERS G, DAIMINGER A, BÄDER U, EXLER S & ENDERS M 2011. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol, 52, 244–6. [DOI] [PubMed] [Google Scholar]
- FAN X & RUDENSKY AY 2016. Hallmarks of Tissue-Resident Lymphocytes. Cell, 164, 1198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER KB, STAGNO S, PASS RF, BRITT WJ, BOLL TJ & ALFORD CA 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med, 326, 663–7. [DOI] [PubMed] [Google Scholar]
- GEBHARDT T, WAKIM LM, EIDSMO L, READING PC, HEATH WR & CARBONE FR 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol, 10, 524–30. [DOI] [PubMed] [Google Scholar]
- GORDON CL, MIRON M, THOME JJ, MATSUOKA N, WEINER J, RAK MA, IGARASHI S, GRANOT T, LERNER H, GOODRUM F & FARBER DL 2017. Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J Exp Med, 214, 651–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWKE S, STEVENSON PG, FREEMAN S & BANGHAM CR 1998. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med, 187, 1575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGQUIST KA, JAMESON SC, HEATH WR, HOWARD JL, BEVAN MJ & CARBONE FR 1994. T cell receptor antagonist peptides induce positive selection. Cell, 76, 17–27. [DOI] [PubMed] [Google Scholar]
- JIANG X, CLARK RA, LIU L, WAGERS AJ, FUHLBRIGGE RC & KUPPER TS 2012. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature, 483, 227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONJIC S, KRMPOTIC A, ARAPOVIC J & KOSZINOWSKI UH 2008. Dissection of the antiviral NK cell response by MCMV mutants. Methods Mol Biol, 415, 127–49. [DOI] [PubMed] [Google Scholar]
- JONJIC S, MUTTER W, WEILAND F, REDDEHASE MJ & KOSZINOWSKI UH 1989. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med, 169, 1199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN S, KRAUSE J, PRAGER A, MITROVIC M, JONJIC S, KOSZINOWSKI UH & ADLER B 2011. Virus progeny of murine cytomegalovirus bacterial artificial chromosome pSM3fr show reduced growth in salivary Glands due to a fixed mutation of MCK-2. J Virol, 85, 10346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHANNA KM, BONNEAU RH, KINCHINGTON PR & HENDRICKS RL 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity, 18, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLENERMAN P & OXENIUS A 2016. T cell responses to cytomegalovirus. Nat Rev Immunol, 16, 367–77. [DOI] [PubMed] [Google Scholar]
- KOONTZ T, BRALIC M, TOMAC I, PERNJAK-PUGEL E, BANTUG G, JONJIC S & BRITT WJ 2008. Altered development of the brain after focal herpesvirus infection of the central nervous system. J Exp Med, 205, 423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSMAC K, BANTUG GR, PUGEL EP, CEKINOVIC D, JONJIC S & BRITT WJ 2013. Glucocorticoid treatment of MCMV infected newborn mice attenuates CNS inflammation and limits deficits in cerebellar development. PLoS Pathog, 9, e1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTTON CN 2010. Management of cytomegalovirus infection in solid organ transplantation. Nat Rev Nephrol, 6,711–21. [DOI] [PubMed] [Google Scholar]
- LAIDLAW BJ, ZHANG N, MARSHALL HD, STARON MM, GUAN T, HU Y, CAULEY LS, CRAFT J & KAECH SM 2014. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity, 41, 633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANE TE, LIU MT, CHEN BP, ASENSIO VC, SAMAWI RM, PAOLETTI A, CAMPBELL IL, KUNKEL SL, FOX HS & BUCHMEIER MJ 2000. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol, 74, 1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMMERMANN NA, BÖHM V, HOLTAPPELS R & REDDEHASE MJ 2011. In vivo impact of cytomegalovirus evasion of CD8 T-cell immunity: facts and thoughts based on murine models. Virus Res, 157, 161–74. [DOI] [PubMed] [Google Scholar]
- LISNIć B, LISNIć VJ & JONJIć S 2015. NK cell interplay with cytomegaloviruses. Curr Opin Virol, 15, 9–18. [DOI] [PubMed] [Google Scholar]
- LJUNGMAN P, HAKKI M & BOECKH M 2010. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am, 24, 319–37. [DOI] [PubMed] [Google Scholar]
- LUCIN P, PAVIć I, POLIć B, JONJIć S & KOSZINOWSKI UH 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol, 66, 1977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACAGNO A, BERNASCONI NL, VANZETTA F, DANDER E, SARASINI A, REVELLO MG, GERNA G, SALLUSTO F & LANZAVECCHIA A 2010. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol, 84, 1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKAY LK, RAHIMPOUR A, MA JZ, COLLINS N, STOCK AT, HAFON ML, VEGA-RAMOS J, LAUZURICA P, MUELLER SN, STEFANOVIC T, TSCHARKE DC, HEATH WR, INOUYE M, CARBONE FR & GEBHARDT T 2013. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol, 14, 1294–301. [DOI] [PubMed] [Google Scholar]
- MACKAY LK, STOCK AT, MA JZ, JONES CM, KENT SJ, MUELLER SN, HEATH WR, CARBONE FR & GEBHARDT T 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA, 109, 7037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKAY LK, WYNNE-JONES E, FREESTONE D, PELLICCI DG, MIELKE LA, NEWMAN DM, BRAUN A, MASSON F, RALLIES A, BELZ GT & CARBONE FR 2015. T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity, 43, 1101–11. [DOI] [PubMed] [Google Scholar]
- MARSHALL DR, TURNER SJ, BELZ GT, WINGO S, ANDREANSKY S, SANGSTER MY, RIBERDY JM, LIU T, TAN M & DOHERTY PC 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA, 98, 6313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASOPUST D & SCHENKEL JM 2013. The integration of T cell migration, differentiation and function. Nat Rev Immunol, 13, 309–20. [DOI] [PubMed] [Google Scholar]
- MASOPUST D, VEZYS V, MARZO AL & LEFRANÇOIS L 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science, 291, 2413–7. [DOI] [PubMed] [Google Scholar]
- MATLOUBIAN M, LO CG, CINAMON G, LESNESKI MI, XU Y, BRINKMANN V, ALLENDE ML, PROIA RL & CYSTER IG 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature, 427, 355–60. [DOI] [PubMed] [Google Scholar]
- MCCORMICK AL & MOCARSKI ES 2015. The immunological underpinnings of vaccinations to prevent cytomegalovirus disease. Cell Mol Immunol, 12, 170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER SN & MACKAY LK 2016. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol, 16, 79–89. [DOI] [PubMed] [Google Scholar]
- MUNKS MW, CHO KS, PINTO AK, SIERRO S, KLENERMAN P & HILL AB 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol, 111, 450–8. [DOI] [PubMed] [Google Scholar]
- MUTNAL MB, HU S, LITTLE MR & LOKENSGARD IR 2011. Memory T cells persisting in the brain following MCMV infection induce long-term microglial activation via interferon-γ. J Neurovirol, 17, 424–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGRO G & ADLER SP 2011. Cytomegalovirus infections during pregnancy. Curr Opin Obstet Gynecol, 23, 123–8. [DOI] [PubMed] [Google Scholar]
- PAN Y, TIAN T, PARK CO, LOFFTUS SY, MEI S, LIU X, LUO C, O'MALLEY IT, GEHAD A, TEAGUE IE, DIVITO SI, FUHLBRIGGE R, PUIGSERVER P, KRUEGER IG, HOTAMISLIGIL GS, CLARK RA & KUPPER TS 2017. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature, 543, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK CO & KUPPER TS 2015. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med, 21, 688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATI SK, PINNINTI S, NOVAK Z, CHOWDHURY N, PATRO RK, FOWLER K, ROSS S, BOPPANA S & INVESTIGATORS NCS 2013. Genotypic diversity and mixed infection in newborn disease and hearing loss in congenital cytomegalovirus infection. Pediatr Infect Dis J, 32, 1050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOTKIN S 2015. The history of vaccination against cytomegalovirus. Med Microbiol Immunol, 204, 247–54. [DOI] [PubMed] [Google Scholar]
- PODLECH I, HOLTAPPELS R, WIRTZ N, STEFFENS HP & REDDEHASE MI 1998. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol, 79 (Pt 9), 2099–104. [DOI] [PubMed] [Google Scholar]
- POLIć B, HENGEL H, KRMPOTIć A, TRGOVCICH I, PAVIć I, LUCCARONIN P, JONJIć S & KOSZINOWSKI UH 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med, 188, 1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWERS C, DEFILIPPIS V, MALOULI D & FRÜH K 2008. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol, 325, 333–59. [DOI] [PubMed] [Google Scholar]
- PRASAD S, HU S, SHENG WS, CHAUHAN P, SINGH A & LOKENSGARD JR 2017. The PD-1: PD-L1 pathway promotes development of brain-resident memory T cells following acute viral encephalitis. J Neuroinflammation, 14, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRASAD S, HU S, SHENG WS, SINGH A & LOKENSGARD JR 2015. Tregs Modulate Lymphocyte Proliferation, Activation, and Resident-Memory T-Cell Accumulation within the Brain during MCMV Infection. PLoS One, 10, e0145457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDEHASE MJ, BALTHESEN M, RAPP M, JONJIć S, PAVIć I & KOSZINOWSKI UH 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med, 179, 185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDEHASE MJ, MUTTER W, MÜNCH K, BÜHRING HJ & KOSZINOWSKI UH 1987. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol, 61, 3102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDEHASE MJ, SIMON CO, SECKERT CK, LEMMERMANN N & GRZIMEK NK 2008. Murine model of cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol, 325, 315–31. [DOI] [PubMed] [Google Scholar]
- RIDDELL SR, WATANABE KS, GOODRICH JM, LI CR, AGHA ME & GREENBERG PD 1992. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science, 257, 238–41. [DOI] [PubMed] [Google Scholar]
- ROSATO PC, BEURA LK & MASOPUST D 2017. Tissue resident memory T cells and viral immunity. Curr Opin Virol, 22, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALLUSTO F, LENIG D, FÖRSTER R, LIPP M & LANZAVECCHIA A 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature, 401, 708–12. [DOI] [PubMed] [Google Scholar]
- SCHACHTELE SJ, HU S, SHENG WS, MUTNAL MB & LOKENSGARD JR 2014. Glial cells suppress postencephalitic CD8+ T lymphocytes through PD-L1. Glia, 62, 1582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SECKERT CK, RENZAHO A, TERVO HM, KRAUSE C, DEEGEN P, KÜHNAPFEL B, REDDEHASE MJ & GRZIMEK NK 2009. Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation. J Virol, 83, 8869–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELEME MC, KOSMAC K, JONJIC S & BRITT WJ 2017. Tumor Necrosis Factor Alpha-Induced Recruitment of Inflammatory Mononuclear Cells Leads to Inflammation and Altered Brain Development in Murine Cytomegalovirus-Infected Newborn Mice. J Virol, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEMAMURA K & TAKEICHI M 1992. Local and transient expression of E-cadherin involved in mouse embryonic brain morphogenesis. Development, 116, 1011–9. [DOI] [PubMed] [Google Scholar]
- SIMON CO, HOLTAPPELS R, TERVO HM, BÖHM V, DÄUBNER T, OEHRLEIN-KARPI SA, KÜHNAPFEL B, RENZAHO A, STRAND D, PODLECH J, REDDEHASE MJ & GRZIMEK NK 2006. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol, 80, 10436–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKON CN, LEE JY, ANDERSON KG, MASOPUST D, HOGQUIST KA & JAMESON SC 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol, 14, 1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAVULJICA I, BUSCHE A, BABIć M, MITROVIć M, GAŠPAROVIć I, CEKINOVICć D, MARKOVA CAR E, PERNJAK PUGEL E, CIKOVIć A, LISNIć VJ, BRITT WJ, KOSZINOWSKI U, MESSERLE M, KRMPOTIć A & JONJIć S 2010. Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest, 120, 4532–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAVULJICA I, KVEŠTAK D, HUSZTHY PC, KOSMAC K, BRITT WJ & JONJIć S 2015. Immunobiology of congenital cytomegalovirus infection of the central nervous system—the murine cytomegalovirus model. Cell Mol Immunol, 12, 180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH CJ, CALDEIRA-DANTAS S, TURULA H & SNYDER CM 2015. Murine CMV Infection Induces the Continuous Production of Mucosal Resident T Cells. Cell Rep, 13, 1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH CJ, QUINN M & SNYDER CM 2016. CMV-Specific CD8 T Cell Differentiation and Localization: Implications for Adoptive Therapies. Front Immunol, 7, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBACH K, VINCENTI I, KREUTZFELDT M, PAGE N, MUSCHAWECKH A, WAGNER I, DREXLER I, PINSCHEWER D, KORN T & MERKLER D 2016. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med, 213, 1571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUPIN P 2007. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev, 53, 198–214. [DOI] [PubMed] [Google Scholar]
- THOM JT & OXENIUS A 2016. Tissue-resident memory T cells in cytomegalovirus infection. Curr Opin Virol, 16, 63–9. [DOI] [PubMed] [Google Scholar]
- THOM JT, WEBER TC, WALTON SM, TORTI N & OXENIUS A 2015. The Salivary Gland Acts as a Sink for Tissue-Resident Memory CD8(+) T Cells, Facilitating Protection from Local Cytomegalovirus Infection. Cell Rep, 13, 1125–36. [DOI] [PubMed] [Google Scholar]
- TORTI N, WALTON SM, BROCKER T, RÜLICKE T & OXENIUS A 2011. Non-hematopoietic cells in lymph nodes drive memory CD8 T cell inflation during murine cytomegalovirus infection. PLoS Pathog, 7, e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRSAN T, BUSCHE A, ABRAM M, WENSVEEN FM, LEMMERMANN NA, ARAPOVIC M, BABIC M, TOMIC A, GOLEMAC M, BRINKMANN MM, JAGER W, OXENIUS A, POLIC B, KRMPOTIC A, MESSERLE M & JONJIC S 2013. Superior induction and maintenance of protective CD8 T cells in mice infected with mouse cytomegalovirus vector expressing RAE-1γ. Proc Natl Acad Sci U S A, 110, 16550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRŠAN T, VUKOVIć K, FILIPOVIć P, BRIZIć AL, LEMMERMANN NAW, SCHOBER K, BUSCH DH, BRITT WJ, MESSERLE M, KRMPOTIć A & JONJIć S 2017. Cytomegalovirus vector expressing RAE-1γ induces enhanced antitumor capacity of murine CD8(+) T cells. Eur J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENTURI V, NZINGHA K, AMOS TG, CHARLES WC, DEKHTIARENKO I, CICIN-SAIN L, DAVENPORT MP & RUDD BD 2016. The Neonatal CD8+ T Cell Repertoire Rapidly Diversifies during Persistent Viral Infection. J Immunol, 196, 1604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIM LM, WOODWARD-DAVIS A & BEVAN MJ 2010. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A, 107, 17872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIM LM, WOODWARD-DAVIS A, LIU R, HU Y, VILLADANGOS J, SMYTH G & BEVAN MJ 2012. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol, 189, 3462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTON SM, MANDARIC S, TORTI N, ZIMMERMANN A, HENGEL H & OXENIUS A 2011. Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS Pathog, 7, e1002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON S, MERCIER S, BYE C, WILKINSON J, CUNNINGHAM AL & HARMAN AN 2007. Determination of suitable housekeeping genes for normalisation of quantitative real time PCR analysis of cells infected with human immunodeficiency virus and herpes viruses. Virol J, 4, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOYAMA WM & KIM S 2008. Analysis of individual natural killer cell responses. Methods Mol Biol, 415, 179–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.