Abstract
Hypertension (HTN) is an established risk factor for subsequent cardiovascular diseases, with Angiotensin II (Ang-II) playing a major role in mediating thrombotic and inflammatory abnormalities. Although T cells and interleukin-6 (IL-6) play an important role in adaptive immune responses, little is known about their role(s) in the thrombo-inflammatory responses associated with Ang-II. Here we show using intravital microscopy coupled with the light/dye injury model that Rag-1 deficient (Rag-1−/−) and IL-6 deficient (IL-6−/−) mice are afforded protection against Ang-II induced thrombosis. Blocking IL-6 receptors (using CD126 and gp130 antibodies) significantly diminished Ang-II-mediated thrombosis and inflammatory cell recruitment in mice. Furthermore, the adoptive transfer of IL-6−/−-derived T cells into Rag-1−/− mice failed to accelerate Ang-II-induced thrombosis compared to Rag-1−/− mice reconstituted with WT-derived T cells, suggesting T cell IL-6 mediates the thrombotic abnormalities associated Ang-II HTN. Interestingly, adoptive transfer of WT T cells into Rag-1−/−/Ang-II mice resulted in increased numbers of immature platelets, which constitutes a more active platelet population i.e. pro-thrombotic and pro-inflammatory. To translate our in vivo findings, we used clinical samples to demonstrate that IL-6 also predisposes platelets to an interaction with collagen receptors, thereby increasing the propensity for platelets to aggregate and cause thrombosis. In summary, we provide compelling evidence for the involvement of IL-6, IL-6R and T cell-dependent IL-6 signaling in Ang-II induced thrombo-inflammation, which may provide new therapeutic possibilities for drug discovery programs for the management of HTN.
Keywords: Hypertension, Interleukin-6, T-cells, Inflammation, Thrombosis
Introduction
Hypertension (HTN) is both a cardio- and cerebrovascular risk factor, pre-disposing hypertensive patients to both pro-inflammatory and pro-thrombotic vascular dysfunction,1–3 Furthermore, it is known that the pleiotropic molecule angiotensin II (Ang-II), the main effector of the renin-angiotensin system (RAS), plays a role in mediating elevated blood pressure and promoting vascular and endothelial cell dysfunction, hypertrophy and oxidative stress.1,4–10 Abnormalities in the functions of circulating immune cells which accompany the chronically elevated blood pressure associated with Ang-II5,7–9 and other models of HTN (e.g. high salt diet model9, 10 and the spontaneous HTN model11, 12) has been well-documented. However, it is not the elevated blood pressure per se that is the driving factor for thrombosis,9,13 but rather Ang-II itself.
T cells play an important role in adaptive immune responses and we and others have shown a major role for these cells in Ang-II induced HTN.5,9,14,15 Additionally, immunodeficient mice lacking both T- and B-lymphocytes (Rag-1 knockout [Rag-1−/−]) exhibit improvement in vasomotor dysfunction and leukocyte accumulation,9,15–17 and adoptive transfer of T cells, but not B cells, into Rag-1−/− mice restored the pro-thrombotic phenotype induced by Ang-II. These data confirm the major role that T cells play in mediating Ang-II induced accelerated microvascular thrombosis,9 however the mechanism by which T-cells induces this pro-thrombotic environment remains unknown.
Both Ang-II-induced HTN and T cells have a very strong relationship with the cytokine IL-6, which exerts its biological activities through two molecules: IL-6R (also known as IL-6Rα, gp80 or CD126) and gp130 (also referred to as IL-6Rβ or CD130).18–21 In the context of Ang-II HTN, reports have shown IL-6 to contribute to increased blood pressure, inflammatory cell recruitment, endothelial dysfunction,18,22–25 with a deficiency in either IL-6 or T cells providing protection.26,27 However, not only is the role that IL-6 plays in Ang-II-mediated thrombosis unknown, the actual role that T-cell-derived IL-6 signaling plays in the thrombo-inflammatory responses associated with Ang-II remains poorly defined. Thus, we herein tested the hypothesis that the chronic pro-thrombotic phenotype associated with Ang-II induced HTN is mediated by a mechanism that is dependent on both T cells and IL-6.
Our novel data reveal that IL-6 plays a major role in the T cell-dependent thrombo-inflammatory responses elicited by chronic Ang-II administration, with contributions by both IL-6Rα and gp130 receptors. We also found that IL-6 is able to potentiate platelets, pre-disposing them to stimulation/activation of the collagen receptor GPVI and contributing to platelet aggregation (by increasing GPIIbIIIa expression). In summary, T cell dependent IL-6 signaling mediates microvascular thrombotic and inflammatory responses associated with chronically elevated levels of Ang-II. Our compelling data suggests that new therapeutic strategies for drug discovery programs based on T cell dependent IL-6 signaling pathways may provide a previously unknown therapeutic strategy for the management of the thrombo-inflammatory complications that accompany HTN.
Materials and methods.
All data supporting the findings of this study are available within the article and its Supplementary files, or are available from the corresponding author upon reasonable request. All studies were done blinded and performed Male wild-type (WT, C57BL/6), IL-6 deficient (IL-6−/− B6.129S6-IL6tml Kopf,), and Rag-1 deficient (Rag-1−/−. B6;129S7-Rag1tmMom/J) mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 6–8 weeks of age. Housing and all of the animal experiments were performed in accordance with experimental procedures approved by the Louisiana State University Health Science Center Institutional Animal Care and Use Committee and in compliance with the guidelines of the American Physiological Society. (See Supplemental Table S1 for groups.)
Human samples
The study was approved by the institutional review board of the LSUHSC-S (STUDY00000261) and conducted in accordance with the Declaration of Helsinki. The consent form was discussed and after permission, blood was obtained from control volunteers. See Supplemental Materials for details.
Drug treatments.
See Supplemental Materials for details.
Osmotic pump implantation
Ang-II (1 μg/kg/min) loaded micro-osmotic pumps (Alzet, Cupertino, CA, model 1002) were implanted for 14 days subcutaneously (intrascapular region) under isofluorane anesthesia.7–9,17 See Supplemental Materials for more details.
Blood Pressure Measurement
Systemic arterial blood pressure was measured before and during the experiment as previously described.28 See Supplemental Materials for more details.
T cell isolation and reconstitution
T cell isolation and adoptive transfer was performed as described previously.9,16,17 See Supplemental Materials for more details.
Intravital Microscopy: Light/dye-induced thrombosis
Surgical procedures and light/dye-endothelial cell injury model were performed accordingly as described previously.8,9 See Supplemental Materials for more details.
Intravital Microscopy: Cremaster muscle
Separate groups of mice were subjected to intravital microscopy to evaluate leukocyte adhesion and emigration and platelet adhesion in cremaster muscle.7,17 See Supplemental Materials for more details.
Immunoblockade of IL-6 Receptors
Rat anti-mouse IL-6Ra (CD126)-blocking antibody and mouse gp130 were injected i.p. in 100 μl saline at doses of 100μg and 20μg/mouse 24 h prior to photo-illumination of the cremaster muscle microvessels.24 Light/dye–induced thrombosis and leukocyte recruitment were recorded in separate groups of experimental mice.
Blood cell counts
Platelet and leukocyte counts were performed manually using hemocytometer (Reichert Hemacytometer, New York Microscope Company, NY). See Supplemental Materials for more details.
In-vivo biotinylation method for platelet life-span measurement
The in-vivo biotinylation method29,30 as used to establish a platelet life-span and identify/quantify newly released platelets (platelet production) in WT-Saline and WT mice chronically infused with Ang-II. Biotin and TO (tiozole orange) administration and labeling procedures were performed as previously described.31,32 Newly released platelets were distinguished from other platelets based on SA (streptavidin conjugated with phycoerithrin (SA-PE; eBiosciences, San Diego, CA) binding to biotin. Newly released platelets were biotin negative by definition, representing a population CD41+SA−. The percentage of biotinylated platelets for every mouse during five days was determined by flow cytometry and lifespan converted into hours, as described previously.29,31,33 See Supplemental Materials for more details.
Assessment of activated platelets by flow cytometry
Human venous blood was collected from healthy volunteers for platelet isolation. 1×106 platelets were treated with 20 ng of IL-6 for 15 min labeled with P-selectin (CD62P) and/or CD41/CD61 (PAC-1 clone. Activated αIIbβ3) to assess platelet activation. Platelets were stimulated with either 0.01U of thrombin or 1 ng of convulxin (CVX). See Supplemental Materials for more details.
Enzyme linked immunosorbent assay (ELISA)
A commercially available cytometric bead array (CBA) kit was used to measure the concentration of IL-6 in serum as per the manufacturer’s instructions. See Supplemental Materials for more details.
Statistical analyses
All values are reported as mean ± SEM. Data within groups were compared using a Student’s t-test (two groups) or an analysis of variance (one-way ANOVA) with a Newman-Keuls post hoc correction for multiple comparisons. Analysis was performed using Graph Pad Prism5 software (San Diego, USA). Data are shown as mean values ± standard error of the mean (SEM). Differences were considered statistically significant at a value of p < 0.05.
Results
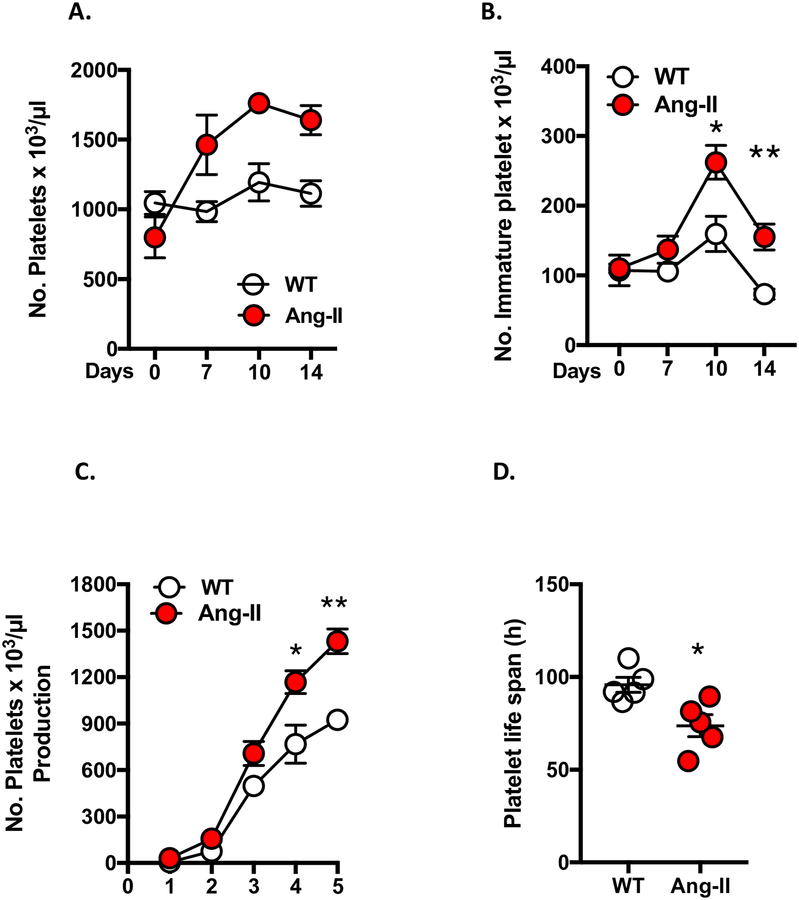
Ang-II infusion is associated with elevated platelet counts, increased levels of immature platelets and a shortened platelet life-span.
Although it is known that immature platelets cause accelerated thrombosis and heightened inflammation, this paradigm is undetermined in the context of Ang-II. We first sought to examine platelet counts, blood pressure and thrombus formation following infusion with varying doses (0–1000 μg/kg/min) of Ang-II. We found that the 1000 ng/kg/min and 1 μg/kg/min dose was associated with increased circulating platelet counts and elevated blood pressure, which were coupled with heightened thrombosis two weeks after pump implantation (Supplemental Figures S1A+B. Supplemental Tables S2+3). These results suggested a potential role for platelets in the accelerated thrombosis associated with Ang-II (Supplemental Figures S1C+D). It is important to note that our findings here are not related to elevated blood pressure per se, but rather Ang-II-mediated effects on thrombus formation. Previously we published data in an alternative model of HTN (deoxycorticosterone acetate [DOCA] salt-induced HTN), which had no effect on thrombus formation and CD40−/− mice implanted with AngII-loaded pumps exhibit protection against Ang-II-mediated thrombosis but remain hypertensive,34 further supporting our findings here that elevated blood pressure per se is mediating the effects.
Next, we evaluated the presence of reticulated (immature) platelets (which constitutes a more active platelet population i.e. pro-thrombotic and pro-inflammatory) as a marker of accelerated thrombus formation. WT mice were infused with 1 μg/kg/min for 14 days and platelet counts recorded concomitantly with immature platelet levels recorded after one week of either saline or Ang-II infusion (Figures 1 A+B). Ang-II infusion resulted in increased total platelet counts and elevated levels of immature platelets coupled with increased platelet production (Figure 1C). Additionally, we found for the first time that Ang-II chronic infusion caused a decrease in platelet life-span (Figure 1D). Collectively, these results demonstrated that chronic Ang-II–infusion is associated with increased platelet numbers, elevated immature platelet counts (thrombocytosis), enhanced platelet production and shorter platelet life-span. These effects both reflect increased elimination of platelets from the circulation as a result of increased pro-thrombotic activity (immature platelets represent hyperactive platelet population and are indicators of accelerated pro-thrombotic responses).
Figure 1. Ang-II infusion is associated with elevated platelet counts, increased levels of immature platelet and a shortened platelet life-span.
Wild type (WT) mice were implanted with either Ang-II (1 μg/kg/min) or control (saline) loaded micro-osmotic mini pumps for up to 14 days. Flow cytometry was performed to quantify platelet counts, immature platelet levels, newly released platelet levels and platelet life-span. A) Total platelet levels and B) immature platelets levels after 1 week of Ang-II infusion (expressed as a platelet number per μl of whole blood on day 0, 7, 10 and 14). C) Platelets produced following Ang-II infusion were monitored after day 7 and numbers of newly released platelets (biotin negative: CD41+SA−) were quantified daily. D) Platelet lifespan was determined in saline (WT) and Ang-II-infused groups (expressed in hours). Data are mean ± SEM of 3–6 mice per group. *p < 0.05, **p < 0.01 vs. WT mice.
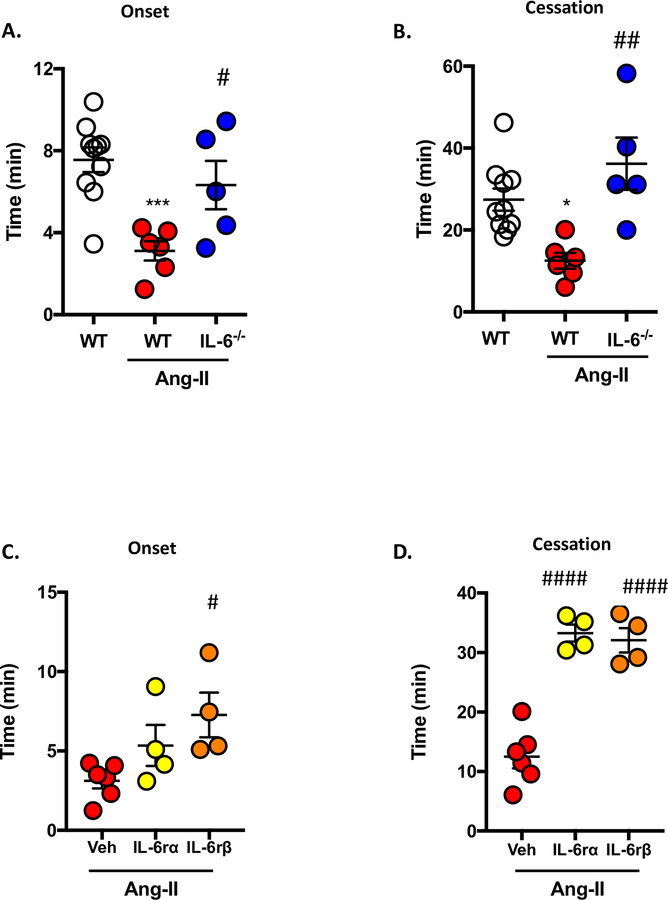
Immunodeficiency and Immunoblocking of IL-6 receptors mitigate Ang-II enhanced thrombosis.
Previously, we have demonstrated that IL-6 plays a dominant role in dextran sodium sulfate (DSS)-induced colonic thrombo-inflammation.31 As such, we wanted to assess whether IL-6 had a similar impact on thrombus formation in mice lacking IL-6 and chronically infused with Ang-II. In our experiments, compared to WT/Ang-II mice, IL-6−/− mice implanted with Ang-II-loaded pumps demonstrated a more dramatic restoration in both onset (two times higher than WT mice) and cessation (2.89 times higher than WT mice) (Figures 2A+B), suggesting that IL-6 genetic deficiency results in an amelioration of Ang-II-induced microvascular thrombosis in arterioles. These effects were also coupled with increased serum levels of IL-6 (4.0 ± 1.5 pg/ml vs. 25.4 ± 13.0 pg/ml WT and WT/Ang-II respectively) (Supplemental Figure S2).
Figure 2. IL-6 deficiency protects against Ang-II-induced microvascular thrombosis.
Wild-type (WT) and IL-6 deficient (IL-6−/−) mice were implanted with Ang-II (1 μg/kg/min) or control (saline) loaded micro-osmotic pumps for up to 14 days and light/dye induced thrombosis model was performed. The following were quantified: A) time in minutes (min) for onset (initial platelet deposition) and B) cessation (occlusion) of blood flow. C) and D) show the time for onset of a thrombus and cessation of blood in WT/Ang-II and WT/Ang-II groups treated 24 h prior to light/dye–induced thrombosis with IL-6Rα (20 μg/mouse) or IL-6Rβ (gp130. 100 μg/mouse). Data are mean ± SEM of 5–10 mice per group. *p < 0.05, ***p < 0.001 vs. WT mice. #p < 0.05, ##p < 0.01, ####p < 0.0001 vs. WT/Ang-II mice (Veh).
Since Ang-II-induced endothelial dysfunction has been linked to elevated IL-6 and having shown here that IL-6−/−/Ang-II mice afford protection against Ang-II induced thrombosis (Figures 2A+B), we next sought to determine the involvement of IL-6 receptors (IL-6Rα and gp130) in these interactions. Figures 2C+D show the effect of exogenous administration of IL6Rα and gp130 mAbs, which block either the −α or −β subunit of IL-6 receptor. Interestingly, whilst blocking IL-6Rβ (gp130) prolonged both onset and cessation times in WT-Ang-II mice (no affect in WT mice, data not shown), blocking of IL-6Rα affected onset only. These data suggest that IL-6Rα may play more of a role in thrombogenesis (i.e. initial platelet recruitment to the vessel), whereas IL-6Rβ is involved in both thrombogenesis and thrombosis.
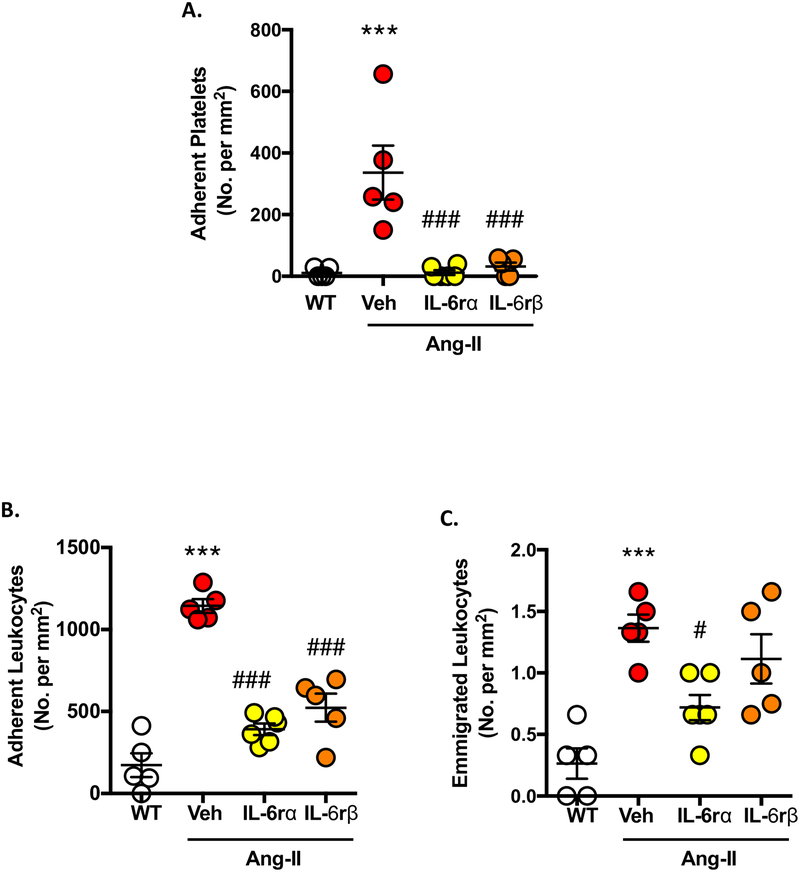
IL-6 receptors are involved in Ang-II-enhanced inflammatory responses in cremasteric venules.
Having shown the involvement of IL-6 receptors in thrombosis and knowing a crosstalk exists between thrombosis and inflammation, we next wanted to investigate whether blocking IL-6 receptors would reduce Ang-II induced inflammatory cell influx. Both IL-6Rα and gp130 immunoblocking blunted Ang-II enhanced platelet adhesion (assessed using intravital video microscopy), restoring levels back to levels seen in WT mice (Figure 3A). Figures 3B+C show that WT-Ang-II mice display heightened leukocyte adhesion and emigration when compared to controls, with the administration of IL-6Rα and gp130 mAbs significantly blunting enhanced leukocyte adhesion in cremaster muscle venules. In the case of emigrated leukocytes only gp130 mAb treatment, but not IL-6Rα mAb, was effective (Figure 3B+C). (No effects were observed in arterioles, data not shown). These data suggest that immunoblockade of IL-6 receptors effectively protects against Ang-II-induced platelet and immune cell accumulation within cremaster venules. Further supporting the concept that IL-6 is intimately involved in thrombo-inflammation associated with Ang-II induced HTN.
Figure 3. Blocking IL-6 receptors mitigates immune cell recruitment in Ang-II-induced inflammation.
Wild-type (WT) mice were implanted with Ang-II (1 μg/kg/min) or control (saline) loaded micro-osmotic pumps for up to 14 days before undergoing intravital microscopy (IVM) of the cremaster muscle microcirculation to quantify leukocyte and platelet interactions. WT/Ang-II mice were treated 24 h prior to IVM with IL-6Rα (20 μg/mouse) or IL-6Rβ (gp130. 100 μg/mouse). The following parameters were quantified: A) adherent platelets (≥ 2 sec), B) adherent leukocytes (stationary for ≥ 30 sec) and C) emigrated leukocytes. Data are mean ± SEM of 5–6 mice per group. ***p < 0.001 vs. WT mice. #p < 0.05, ###p < 0.001 vs. WT/Ang-II mice (Veh).
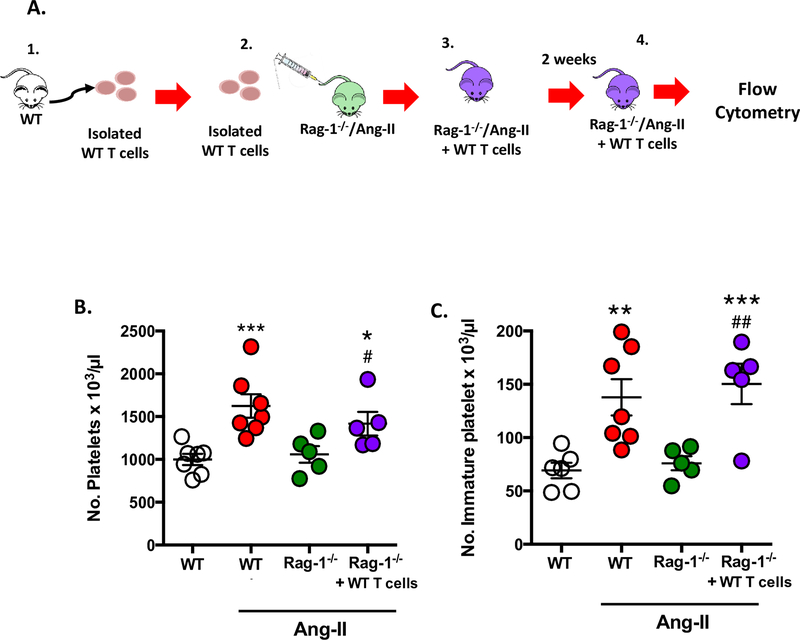
T cell adoptive transfer into immunodeficient Rag-1/Ang-II mice increases immature platelet population.
As we had provided clear evidence of the involvement of IL-6 in the thrombo-inflammatory state associated with Ang-II and knowing that IL-6−/− mice have lower levels of lymphocytes, which could in part explain their afforded protection (Figure 2), we next tested the role of T cells in our study (Figure 4A). Figure 4B shows for the first time that although Ang-II infused mice exhibit significantly elevated platelet numbers compared to WT mice, chronically elevated levels of Ang-II failed to increase the number of circulating platelets in Rag-1−/−/Ang-II mice when compared with WT/Ang-II mice, suggesting that lymphocyte deficiency is protective against Ang-II-mediated elevation in platelet counts (WT: 1026.0 ± 64.3; Ang-II: 1746.0 ± 101.9; Rag-1−/−: 1261.0 ± 139.4, and Rag-1−/−/Ang-II: 1137.0 ± 111.1 platelets per 103/μl). These previously unknown findings regarding platelet counts concur with our earlier published findings that Rag-1−/−/Ang-II mice are protected from thrombosis.9
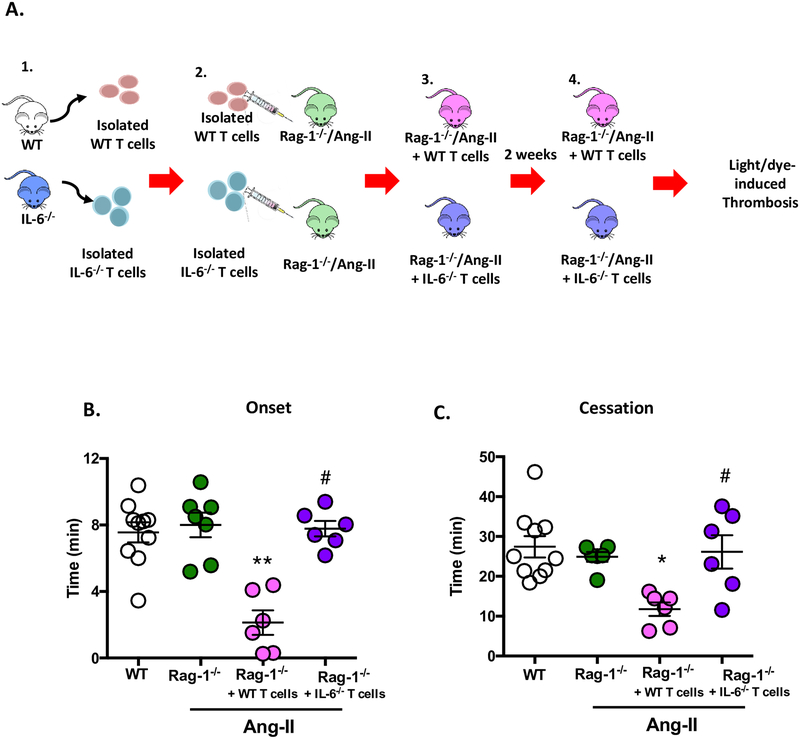
Figure 4. T cell adoptive transfer into immunodeficient Rag-1−/−/Ang-II mice increases immature platelet population.
Wild-type (WT) mice and immunodeficient Rag-1 (Rag-1−/−) mice were implanted with Ang-II (1 μg/kg/min) loaded micro-osmotic pumps for up to 14 days. A separate group of Rag-1−/−/Ang-II mice were also reconstituted with wild type (WT)-derived T cells (Rag-1−/−/Ang-II+WT T cells). A) Schematic representation of: 1. WT T cells being isolated; 2. Injected into Rag-1−/−/Ang-II mouse to make; 3. Rag-1−/−/Ang-II+WT T cell mouse; 4. After two weeks blood was collected from Rag-1−/−/Ang-II+WT T cell mouse and was subjected to platelet assessment by flow cytometry. B) Circulating platelet numbers and C) the number of immature platelets were counted. Data are mean ± SEM of 3–6 mice per group. *p < 0.05, ***p < 0.001 vs. WT. ##p < 0.01 vs. Rag-1−/−/Ang-II mice.
Since immunodeficient Rag-1−/−/Ang-II mice have lower circulating levels of platelets, we next sought to determine whether T cells were mediating the elevation in circulating platelet counts. We employed the use of adoptive transfer of WT T cells for a period of two weeks in Rag-1−/−/Ang-II mice. Figure 4B+C shows that the adoptive transfer of WT T cells into Rag-1−/−/Ang-II mice resulted in increased total platelet count and an increase in immature platelet count, representing a more active platelet population (i.e. pro-thrombotic),31,35–38 which may result in a majority of hyperactive platelets in the total platelet count compared to control. Additionally, we also confirmed that the Rag-1−/−/Ang-II mice reconstituted with WT-derived T cells, displayed elevated T cell counts (Supplementary Figure S3) (0.84 ± 0.2 % in Rag-1−/−/Ang-II vs. 10.6 ± 2.0 in Rag-1−/−/Ang-II reconstituted with WT derived T cells). Thus, these results suggest that T cells contribute to the alteration in platelets responses.
Platelets are the primary participants of arterial thrombosis and increased immature platelets and accumulation within the vasculature leads to an increased risk of thrombotic events. To determine whether increased immature platelets and elevated T cell count predisposes Rag-1−/−/Ang-II+WT T cells to Ang-II-mediated thrombotic responses, we next employed the light/dye induced thrombosis model (Figure 5A). Figures 5B+C confirms our hypothesis, clearly demonstrating that these mice do indeed display a heightened thrombotic response (i.e. shorter blood flow onset and cessation times) in cremaster arterioles (onset: Rag-1−/−/Ang-II 8.0 ± 0.7 min vs. Rag-1−/−/Ang-II-WT T cells 2.1 ± 0.7 min and for cessation: Rag-1−/−/Ang-II 24.9 ± 1.2 min vs. Rag-1−/−/Ang-II-WT T cells 11.8 ± 1.7 min). (No effect in venules. Data not shown.)
Figure 5. IL-6 deficient T cells adoptive transfer do not accelerate thrombotic responses elicited by Ang-II infusion.
Wild type (WT) mice or immunodeficient Rag-1 (Rag-1−/−) mice were implanted with Ang-II (1 μg/kg/min) loaded micro-osmotic pumps for up to 14 days. A) schematic representation of: 1. WT T or IL-6−/− T cells being isolated; 2. Injected into Rag-1−/−/Ang-II donor mouse to make; 3. Rag-1−/−/Ang-II+WT T cell mouse or Rag-1−/−/Ang-II+IL-6−/− T cell mouse; 4. After two weeks (14 days) mice were subjected to the light/dye induced thrombosis model. Time of B) onset and C) cessation was quantified in cremasteric arterioles. Data are mean ± SEM of 6–10 mice per group. *p < 0.05, **p < 0.01 vs. Rag-1−/−/Ang-II. #p < 0.05 vs. Rag-1−/−/Ang-II+WT T cells.
IL-6 deficient T cells do not accelerate thrombotic responses elicited by Ang-II infusion.
Having assessed the novel anti-thrombo-inflammatory effects of IL-6 immunoblocking (as well as and IL-6 deficiency) and the role that T cells play in Ang-II HTN, we wanted to address whether T cell dependent IL-6 was actually driving the protection. Figures 5B+C shows that when recipient Rag-1−/−/Ang-II mice were reconstituted with T cells obtained from IL-6−/− donor mice they displayed protection against thrombosis (quantified as increased times of onset and cessation). These data suggest that T cell IL-6 mediates the thrombotic abnormalities associated Ang-II HTN.
IL-6 primes platelet activation in response to collagen receptor GPVI.
Finally, to explore the translational relevance of the in-vivo findings, we ascertained the direct effect of IL-6 on human isolated platelets following the GPVI collagen receptor agonist convulxin (CVX) or the serine protease thrombin. Figure 6 shows that CVX enhanced the surface levels of active αIIbβ3 (as assessed by PAC-1 binding), P-selectin (CD62P) and double positive active αIIbβ3-P-selectin (a population of platelets that are more likely to being involved in adhesion and aggregation) on platelets. Stimulation with IL-6 alone did not affect active αIIbβ3 or P-selectin but potentiated these markers of platelet activation when in the presence of CVX. Interestingly, neither platelets incubated with IL-6 and stimulated with thrombin display any differences in percentage of αIIbβ3, P-selectin or vs. thrombin stimulation alone (Supplemental Figure S4). These data provide further evidence that not only is IL-6 able to enhance the pro-thrombotic ability of platelets in-vivo, but it also predisposes platelets to an interaction with collagen receptors and in so doing, increases the propensity for platelets to aggregate and cause thrombosis.
Figure 6. IL-6 increases platelet activation via GPVI pathway.
Human platelets were isolated, washed and 1×106 pre-incubated with vehicle (saline) or IL-6 (20 ng) for 15 min at 370C, and stimulated with and without convulxin (CVX. 1 ng per 1×106 platelets). A) Representative flow cytometric analysis for % expression of P-selectin (CD62P-FITC) and αIIbβ3 (CD41/CD61 Alexa eFluor 647) for baseline, IL-6 incubated platelets, CVX stimulated and IL-6 incubated and stimulated with CVX. The following were quantified: B) % of activated integrin αIIbβ3 (PAC-1), C) % P-selectin expression (CD62P) and D) % of double positive platelet population for activated integrin αIIbβ3 (PAC-1) and P-selectin (CD62P) expression. Data are mean ± SEM of 4–7 individual donors per group. ***p < 0.001, ****p < 0.0001 vs. unstimulated platelets (baseline), ###p < 0.001 and ####p < 0.0001 vs. CVX-stimulated platelets.
Discussion
We present herein several novel key conceptual findings which we believe advances knowledge and understanding in the field of Ang-II-mediated thrombo-inflammation. Specifically, we found that IL-6−/− mice afforded are protection against Ang-II-induced thrombosis. Both IL-6Rα and IL-6Rβ are involved in Ang-II induced thrombosis, with IL-6 being able to potentiate platelets, pre-disposing the platelets to stimulation/activation of the collagen receptor GPVI, which is a primary receptor for adhesion (e.g. increased P-selectin) and contributing to platelet aggregation increasing GPIIbIIIa expression). T cells obtained from IL-6−/− mice did not accelerate thrombosis, while WT-derived T cells induced thrombotic responses. Additionally, the adoptive transfer of WT T cells resulted in heightened platelet levels and increased numbers of immature platelets, which constitutes a more active platelet population and thereby pre-disposing a pro-thrombotic and pro-inflammatory environment elicited by Ang-II infusion. Finally, chronic Ang-II infusion also resulted in a shorter platelet life-span.
Over the past decade, animal and clinical studies have revealed the involvement of both the innate and adaptive immune system in the development and pathophysiological consequences of CVD and its risk factors such as HTN.5,14,15,36 It is well documented that Ang-II, the main effector hormone of renin-angiotensin system, induces HTN and is a direct mediator of both thrombotic abnormalities and vascular inflammatory responses.
Several cytokines (e.g. TNFα, IFNγ, IL-1β, IL-6)31, 34, 37 are known to contribute to the thrombo-inflammatory responses associated with acute and chronic inflammation, with IL-6 being considered a clinical biomarker of CVD.38,39 The normal range of IL-6 in plasma is about 5–7 pg/ml in human population37 and its secretion is upregulated in response to Ang-II, oxidative stress and vascular injury,19,38–40 which concurs with increased IL-6 levels in these studies. However, the role that IL-6 plays in Ang-II-mediated thrombosis, along with the role that T-cell-derived IL-6 signaling plays in thrombo-inflammatory responses associated with Ang-II, are currently unkown. Here we questioned whether we could exploit these two immune players for drug discovery programs targeting both thrombotic and inflammatory responses in CVD especially HTN.
Cells which have IL-6R (CD126, gp80) are able to bind IL-6 directly and are able to shed their IL-6R after cleavage by protein kinase C, forming soluble IL-6R, which in turn may bind IL-6.41,42 This complex (sIL-6R/IL-6) can cause homodimerization of gp130 and subsequent signal transduction in cells that lack the IL-6R.41,43 Here we showed that that genetic IL-6 deletion affords protection against Ang-II mediated thrombus formation in cremaster arterioles. As IL-6 exerts its effects by interacting with its receptor complex composed of a specific α subunit, IL-6Rα (gp80, CD126 or IL-6R) and a signal transduction subunit gp130 (also referred to as IL-6Rβ or CD130),21,44 we wanted to ascertain the contribution of IL-6Rα (classic cis signaling pathway) and/or gp130 from interacting with IL-6R/IL-6 (inhibiting trans-signaling pathway) to the Ang-II thrombo-inflammatory responses. We found that acute immunoblocking of IL-6 receptors21,45 in WT mice infused with Ang-II elicited a protective effect, as demonstrated by decreased arterial thrombosis and reduced platelet recruitment and leukocyte adhesion in venules. The different responses observed in arterioles and venules may lie in both the composition of each vessel type e.g. shear rates, coupled with the differences associated with thrombotic vs. inflammatory responses (e.g. arterial thrombi are rich in aggregated platelets46 and leukocyte and platelet accumulation typically occur in the venules, not arterioles). These data may suggest that either genetic deficiency of IL-6 or immunoblocking of IL6Rα and IL-6Rβ are protective against thrombo-inflammatory responses. Additionally, our findings concur with a number of other in vitro and in vivo models of inflammation showing IL-6 trans-signaling to regulates the expression of trafficking molecules that mediate leukocyte primary adhesion (E-selectin and VCAM-1), chemokine activation (CCL2, CXCL10, CCL4, CCL5, CCL11, and CCL17), as well as secondary firm adhesion and trans-endothelial migration (ICAM-1 and VCAM-1).41
From a clinical point of view several studies have already shown that blocking IL-6R using immunoblocking antibodies such as tocilizumab, siltuximab, sarilumab47 prevents IL-6 from exerting its pro-inflammatory effects and as such have shown to be promising therapies for different pathological conditions such as rheumatoid arthritis and cancer, conditions which are accompanied by enhanced thrombotic complications, increased immature platelet population (i.e. hyperactive platelets) and heighted inflammatory responses e.g. increased levels of IL-6.47,48 Additionally, it has recently been published that blocking IL-6 signaling also improves pulmonary arterial HTN, with IL-6 signaling considered as a new therapeutic target.49,50 In the context of HTN, several reports indicate that increased levels of IL-6 due to Ang-II activate gp130-linked signaling and contribute to Ang-II-induced hypertrophy.51 Our study demonstrates that IL-6 levels are linked to increased immature platelet production and thrombosis, and a reduction in levels of IL-6 results in a reduction of thrombotic complications. Future/ongoing studies are needed to fully address the role of IL-6 signaling in HTN and the therapeutic potential of targeting the IL-6-STAT3 axis for the management of thrombo-inflammatory diseases such as HTN, beyond more traditional methods such as blood pressure and Ang-II lowering.
Although we have previously demonstrated that immunodeficient mice (Rag-1−/−), which lack both T and B cells, exhibit a complete protection against Ang-II accelerated thrombosis and that CD4+ T cells are involved in the thrombotic responses observed within the microvasculature,9 the actual mechanism remained undefined. Interestingly, we found here that chronically elevated levels of Ang-II resulted in not only increased platelet counts, but resulted in heightened numbers of immature platelets (or reticulated platelets).34,35 Rag-1−/− mice however were protected against this increase and adoptive transfer of T cells isolated from WT mice and transferred into Rag-1−/− recipients restored increased levels of immature platelets, suggesting that a reciprocal crosstalk exists between these two cell types: T cells and platelets.
Having shown previously that Ang-II-induced microvascular thrombosis is mediated via T cells,9 and having demonstrated here that a crosstalk exists between T cells and platelets in chronic Ang-II infusion, we next sought to investigate the involvement of IL-6 in this immune cell relationship. It has been shown that IL-6 is required for the chemotactic activity and migration of T cells,52,53 although its role in thrombosis was less well defined. Interestingly, we found for the first time that T cells isolated from IL-6−/− mice and injected into Rag-1−/− mice were protected against Ang-II induced thrombosis suggesting, that T cell IL-6 plays a key role in the thrombotic process.
Finally, having studied the effects of T cell IL-6 and the role they play in Ang-II induced thrombosis, we next turned our focus to the platelet in order to translate the findings to a clinical setting. As such, we assessed the effect of IL-6 on human platelet function (P‐selectin and αIIbβ3 receptor expression) following stimulation with thrombin and the GPVI collagen receptor agonist CVX. We found that IL-6 increased both P-selectin and αIIbβ3 receptor expression following CVX treatment, but this effect was not produced with thrombin. It is well known that GPVI plays an important role in collagen-mediated platelet aggregation and adhesion especially in terms of conditions of arterial thrombosis where platelet activity induced by vascular inflammation is a known risk factor for arterial thrombosis.54,55 Ang-II causes heightened arterial thrombosis and platelet activation signaling plays a critical role in the function of platelets in hemostasis and thrombosis. Our novel findings showed that IL-6 is able to prime platelets predisposing the platelets to collagen receptor GPVI activation, which may lead to platelet adhesion and thrombotic responses via changes in P-selectin (critical for platelet activation and hetero- and homo-typic platelet interactions) and αIIbβ3 receptor (involved in aggregation and platelet plug stabilization) expression. These effects promote platelets to adhere and stabilize the platelet plug, thereby pre-disposing platelets to accumulate in arterioles. Indeed, IL-6 may be changing the intraplatelet GPVI receptor pools which are involved in platelet-collagen interactions.56–58 GPVI is localized on the platelet surface plasma membrane and also on the membranes of the surfaces connected to the open canalicular system (OCS), an elaborate system of tunneling invaginations of the cell membrane unique to the platelet59 and the α-granules in resting platelets. During platelet activation, the release of GPVI pools are redistributed (i.e. ultrastructural changes occur which lead to an increase in GPVI on the activated platelet surface, accompanied by a decrease in interior expression),58 a process in which IL-6 could be involved via its ability to initiate STAT3 dependent signaling which interacts with Syk and PLCγ2, thereby enhancing collagen-induced platelet activation and aggregation.60,61 One could speculate that a critical implication of this molecular interaction could be to facilitate a crosstalk between collagen-induced and inflammatory cytokine-induced signal pathways on platelets pre-disposing them to adhesion and aggregation.
In summary, using genetic and pharmacological approaches, we have found that innate and adaptive immune responses to Ang-II involve the interactions between platelets and T-cells. We report a previously unknown effect of T cell dependent IL-6 to alter platelet and leukocyte adhesion and thrombus formation via interacting with its IL-6 receptors, demonstrating a key role of this cytokine as a mediator of Ang-II induced thrombo-inflammation. These novel and compelling data provide new therapeutic possibilities based on T cell dependent IL-6 signaling pathways for drug discovery programs for the management of HTN.
Supplementary Material
Perspectives.
The main effector of the renin-angiotensin system (RAS) is angiotensin II (Ang-II), which is a critical determinant of the pro-thrombotic and pro-inflammatory environment associated with HTN. The chronic pro-thrombotic phenotype associated with Ang-II induced HTN is mediated by a mechanism that is dependent on both T cells and IL-6. Drug discovery programs based on T cell dependent IL-6 signaling pathways may provide a previously unknown therapeutic strategy for the management of the thrombo-inflammatory complications that accompany HTN.
Novelty and Significance.
What is New?
The main effector of the renin-angiotensin system (RAS) is angiotensin II (Ang-II), which is a critical determinant of the pro-thrombotic and pro-inflammatory environment associated with HTN. T cells and IL-6 play key roles in these processes driving forward the Ang-II associated thrombo-inflammation.
What is Relevant?
The chronic pro-thrombotic phenotype associated with Ang-II induced HTN is mediated by a mechanism that is dependent on both T cells and IL-6.
Summary
This study, using pharmacological and genetic approaches, coupled with murine and clinical samples is the first to show that T-cell-derived IL-6 signaling plays a key role in the thrombo-inflammatory responses associated with Ang-II.
Acknowledgments
Dr. Gavins acknowledges the financial support of the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI (HL134959–01A1)). Dr. Senchenkova acknowledges the financial support of fellowship from the Malcom Feist Cardiovascular Endowment fund at Louisiana State University Health Science Center.
Footnotes
Conflicts of interest
None.
References
- 1.Brown NJ, Vaughan DE. Prothrombotic effects of angiotensin. Adv Intern Med. 2000;45:419–429. [PubMed] [Google Scholar]
- 2.Kjeldsen SE, Julius S. Hypertension mega-trials with cardiovascular end points: effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Am Heart J. 2004;148(5):747–754. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest.1995;95(3):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadar S, Lip GY. The prothrombotic state in hypertension and the effects of antihypertensive treatment. Curr Pharm Des. 2003;9(21):1715–1732. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DG, Guzik TJ, Lob H, Madhur M, Marvar PJ, Thabet S, Vinh A, Weyand C. Inflammation, immunity, and hypertension. Hypertension. 2011; 57(2):132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celi A, Cianchetti S, Dell’Omo G, Pedrinelli R. Angiotensin II, tissue factor and the thrombotic paradox of hypertension. Expert Rev Cardiovasc Ther. 2010; 8(12):1723–1729. [DOI] [PubMed] [Google Scholar]
- 7.Yildirim A, Russell J, Yan LS, Senchenkova EY, Granger DN. Leukocyte-dependent responses of the microvasculature to chronic angiotensin II exposure. Hypertension. 2012;60(6):1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senchenkova E, Russell J, Almeida-Paula LD, Harding JW, Granger DN. Angiotensin II-mediated microvascular thrombosis. Hypertension. 2010;56:1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senchenkova EY, Russell J, Kurmaeva E, Ostanin D, Granger DN. Role of T lymphocytes in angiotensin II-mediated microvascular thrombosis. Hypertension. 2011; 58(5):959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dmitrieva NI, Burg MB. Elevated Sodium and Dehydration Stimulate Inflammatory Signaling in Endothelial Cells and Promote Atherosclerosis. PLoS One. 2015; 10(6): e0128870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabielska E, Pawlak R, Golatowski J, Rółkowski R, Pawlak D, Buczko W. Losartan inhibits experimental venous thrombosis in spontaneously hypertensive rats. Thromb Res. 1998;90(6):271–278. [DOI] [PubMed] [Google Scholar]
- 12.Lominadze D, Joshua IG, Schuschke DA. In vivo platelet thrombus formation in microvessels of spontaneously hypertensive rats. Am J Hypertens. 1997;10(10 Pt 1):1140–1146. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues SF, Almeida-Paula LD, Granger DN. Synergistic effects of high blood cholesterol and hypertension on leukocyte and platelet recruitment in the cerebral microcirculation. Hypertension. 2014; 63(4):747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007; 204(10):2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol. 2012;3:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113(17):2105–2112. [DOI] [PubMed] [Google Scholar]
- 17.Stokes KY, Gurwara S, Granger DN. T-cell derived interferon-gamma contributes to arteriolar dysfunction during acute hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2007;27:1998–2004. [DOI] [PubMed] [Google Scholar]
- 18.Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, Jeunemaitre X, Thomas A. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens. 2011; 24(10):1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomolak JR, Didion Sean P.. Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front Physiol. Front Physiol 2014; 5: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M.IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122(4):143–159. [DOI] [PubMed] [Google Scholar]
- 22.Grignani G, Maiolo A. Cytokines and hemostasis. Haematologica. 2000;85(9):967–972. [PubMed] [Google Scholar]
- 23.Ishibashi T, Kimura H, Shikama Y, Uchida T, Kariyone S, Hirano T, Kishimoto T, Takatsuki F, Akiyama Y. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989;74(4):1241–1244. [PubMed] [Google Scholar]
- 24.Hozumi H, Russell J, Vital S, Granger DN. IL-6 Mediates the Intestinal Microvascular Thrombosis Associated with Experimental Colitis. Inflamm Bowel Dis. 2016;22(3):560–568. [DOI] [PubMed] [Google Scholar]
- 25.Tang YH, Vital S, Russell J, Seifert H, Granger DN. Interleukin-6 mediates enhanced thrombus development in cerebral arterioles following a brief period of focal brain ischemia. Exp Neurol. 2015; 271:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010; 56(5):879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, Takahashi T, Makino S, Kato T, Ogawa S. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem. 2000;275(38):29717–29723. [DOI] [PubMed] [Google Scholar]
- 28.Cerwinka WH, Granger DN. Influence of hypercholesterolemia and hypertension on ischemia-reperfusion induced P-selectin expression. Atherosclerosis. 2001; 154(2):337–344. [DOI] [PubMed] [Google Scholar]
- 29.Ault KA, Knowles C. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in the circulation. Experimental Hematology 1995; 23:996–1001. [PubMed] [Google Scholar]
- 30.Robinson M, Machin S, Mackie I, Harrison P. In vivo biotinylation studies: specificity of labeling of reticulated platelets by thiazole orange and mepacrine. Brit J Haematology. 2000;108:859–864. [DOI] [PubMed] [Google Scholar]
- 31.Senchenkova EY, Komoto S, Russell J, Almeida-Paula LD, Yan LS, Zhang S, Granger DN. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am J Pathol. 2013;183(1):173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matic GB, Chapman ES, Zaiss M, Rothe G, Schmitz G. Whole blood analysis of reticulated platelets: improvements of detection and assay stability. Cytometry. 1998;34(5):229–234. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Liu J, He C, Yan R, Zhou K, Cui Q, Meng X, Li X, Zhang Y, Nie Y, Zhang Y, Hu R, Liu Y, Zhao L, Chen M, Xiao W, Tian J, Zhao Y, Cao L, Zhou L, Lin A, Ruan C, Dai K. Protein kinase A determines platelet life-span and survival by regulating apoptosis. J Clin Invest. 2017;127(12):4338–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senchenkova EY, Russell J, Vital SA, Yildirim A, Orr AW, Granger DN, Gavins FNE. A critical role for both CD40 and VLA5 in angiotensin II-mediated thrombosis and inflammation. FASEB J. 2018;32(6):3448–3456. [DOI] [PubMed] [Google Scholar]
- 35.Yan SLS, Russell J, Granger DN. The platelet activation and platelet-leukocyte aggregation elicited in experimental colitis are mediated by interleukin-6. Inflamm Bowel Dis. 2014; 20(2): 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong PC, Hoefer T, Knowles RB, Tucker AT, Hayman MA, Ferreira PM, Chan MV, Warner TD. Newly formed reticulated platelets undermine pharmacokinetically short-lived antiplatelet therapies. Arterioscler Thromb Vasc Biol. 2017; 37(5):949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernández-Ruiz I Immune system and cardiovascular disease Nature Reviews Cardiology. 2016;13:503. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H, Russell J, Senchenkova EY, Almeida Paula LD, Granger DN. Interleukin-1beta mediates the extra-intestinal thrombosis associated with experimental colitis. Am J Pathol. 2010. December;177(6):2774–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J, Axelsson J, Machowska A, Heimbürger O, Bárány P, Lindholm B, Lindström K, Stenvinkel P,Qureshi AR. Biomarkers of cardiovascular disease and mortality tisk in patients with advanced CKD. Clin J Am Soc Nephrol. 2016; 11(7): 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou T, Tieu BC, Ray S, Recinos IA, Cui R, Tilton RG, Brasier AR. Roles of IL-6-gp130 Signaling in Vascular Inflammation. Curr Cardiol Rev. 2008; 4(3):179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001. 115(1):3–12. [DOI] [PubMed] [Google Scholar]
- 42.Garbers C, Rose-John S. Dissecting Interleukin-6 Classic- and Trans-Signaling in Inflammation and Cancer. Methods Mol Biol. 2018;1725:127–140. [DOI] [PubMed] [Google Scholar]
- 43.Peters M, Müller AM, Rose-John S. Interleukin-6 and soluble interleukin-6 receptor: direct stimulation of gp130 and hematopoiesis. Blood. 1998;92(10):3495–3504. [PubMed] [Google Scholar]
- 44.Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, Jeunemaitre X, Thomas A. Inflammation and Hypertension: The Interplay of Interleukin-6, Dietary Sodium and the Renin-Angiotensin System in Humans. Am J Hypertens. Am J Hypertens. 2011; 24(10):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang B, Song Z, Wu B, Gardner D, Shealy D, Song XY, Wooley PH. Evaluation of anti-IL-6 monoclonal antibody therapy using murine type II collagen-induced arthritis. J Inflamm (Lond). 2009;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rumbaut RE, Slaff DW, Burns AR. Microvascular thrombosis models in venules and arterioles in vivo. Microcirculation. 2005;12:259–274. [DOI] [PubMed] [Google Scholar]
- 47.Plushner SL. Tocilizumab: an interleukin-6 receptor inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother. 2008;42(11):1660–1668. [DOI] [PubMed] [Google Scholar]
- 48.https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/tocilizumab).
- 49.Pullamsetti SS, Seeger W, Savai R.Classical IL-6 signaling: a promising therapeutic target for pulmonary arterial hypertension. J Clin Invest. 2018;128(5):1720–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavine K Blocking IL-6 signaling deflates pulmonary arterial hypertension. Science Translational Medicine 2018;10(442),eaat8534. [Google Scholar]
- 51.Gomolak JR and Didion SP. Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front Physiol. 2014;5:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vardam TD, Zhou L, Appenheimer MM, Chen Q, Wang WC, Baumann H, Evans SS. Regulation of a lymphocyte-endothelial-IL-6 trans-signaling axis by fever-range thermal stress: hot spot of immune surveillance. Cytokine. 2007;39(1):84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibrahim H, Schutt RC, Hannawi B, DeLao T, Barker CM, Kleiman NS. Association of immature platelets with adverse cardiovascular outcomes. J Am Coll Cardiol. 2014; 64(20):2122–2129. [DOI] [PubMed] [Google Scholar]
- 54.Fager AM, Wood JP, Bouchard BA, Feng P, Tracy PB. Properties of procoagulant platelets: defining and characterizing the subpopulation binding a functional prothrombinase. Arterioscler Thromb Vasc Biol. 2010; 30(12):2400–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weissenbach M, Clahsen T, Weber C, Spitzer D, Wirth D, Vestweber D, Heinrich PC, Schaper F. Interleukin-6 is a direct mediator of T cell migration. Eur J Immunol. 2004; 34(10):2895–2906. [DOI] [PubMed] [Google Scholar]
- 56.Bacon K,Gearing A, Camp R. Induction of in vitro human lymphocyte migration by interleukin 3, interleukin 4, and interleukin 6. Cytokine. 1990; 2(2):100–5. [DOI] [PubMed] [Google Scholar]
- 57.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449–461. [DOI] [PubMed] [Google Scholar]
- 58.Xu XR, Carrim N, Neves MA, McKeown T, Stratton TW, Coelho RM, Lei X, Chen P, Xu J, Dai X, Li BX, Ni H. Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb J. 2016;14(Suppl 1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki H, Murasaki K, Kodama K, takayama h. Intracellular localization of glycoprotein VI in human platelets and its surface expression upon activation. Br J Haematol. 2003;121(6):904–12. [DOI] [PubMed] [Google Scholar]
- 60.Blair P, Flaumenhalf R. Platlet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009; 23(4):177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Z, Gushiken FC, Bolgiano D, Salsbery BJ, Aghakasiri N, Jing N, Wu X, Vijayan KV, Rumbaut RE, Adachi R, Lopez JA, Dong JF Signal transducer and activator of transcription 3 (STAT3) regulates collagen-induced platelet aggregation independently of its transcription factor activity. Circulation. 2013;127(4):476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.