Abstract
Colorectal cancer commonly metastasizes. The liver is the most frequent site of metastases and dominates the length of survival for this disease. As surgical and systemic therapies have become accepted and now are proven to be potentially curative, other sites of metastases have become more clinically relevant in terms of clinical symptoms and influence on survival. Treatment of extrahepatic metastases by surgical and ablative procedures is increasingly accepted and is proving to be effective at palliating symptoms, as well as life prolonging. In this review, we will first summarize key issues with metastatic colorectal cancer to the liver and available treatments. We will then discuss surgical and ablative treatments of other sites of disease including lung, lymph nodes, peritoneum, bone, and brain. Best available evidence for treatment strategies will be presented as well as potential new directions.
Keywords: adjuvant, chemotherapy, HIPEC, microwave ablation, neoadjuvant, PIPAC, radiofrequency ablation, radiation therapy, recurrence pattern, surgical outcome
In Brief
Colorectal cancer commonly metastasizes. Most commonly this occurs by five means: direct extension, lymphatic spread, portal venous spread to liver, peritoneal dissemination, and vascular spread to distant organs including lung, bone, and brain. The liver is the most frequent site of metastases and dominates the length of survival for this disease. Nearly one-half of patients diagnosed with colorectal cancer will be found to have liver metastases at some point during their disease. When untreated, patients with liver metastases have a median survival of 6–9 months. Even with the best chemotherapy, median survival of unresectable disease is 13–18 months. In the last three decades, treatment of extrahepatic metastases by surgical and ablative procedures has proven to be effective. It is increasingly accepted and is effective at palliating symptoms, prolongs life, and can be potentially curative. The fact that liver resection is affecting outcome is also highlighted by the fact that over 70% of patients with unresectable liver metastases die of their liver metastases. In patients treated by hepatectomy, approximately 30% ultimately die of liver metastases.
The median survival of patients after hepatectomy for stage IV metastatic colorectal cancer in the liver is over forty months. Consequently, other sites of metastases are not only more likely to become apparent, but also more likely to cause symptoms and influence survival. Thus, management of liver metastases has made enough progress so that other sites of metastases have become more clinically relevant. In this review, we will first summarize the natural history of colorectal cancer metastases. We then will address key issues with metastatic colorectal cancer to the liver and available treatments. This is followed by a discussion of surgical and ablative treatments of other sites of disease including lung, peritoneum, bone, lymph nodes and brain. Best available evidence for treatment strategies will be presented as well as potential new directions.
At presentation, 20–25% of patients will have distant metastases, most to the liver. Another 20–25% will later develop liver metastases. Of patients who succumb to the disease, 49% will have liver dominant disease, and 83% will have some liver involvement. Disease specific survival is also significantly shorter for those who die of liver metastasis, compared to patients who die from other metastatic sites. Thus, addressing liver metastases initially is the most clinically relevant, since this is the most life limiting. Currently, patients who do not undergo surgical treatment of liver metastases typically live less than 18 months, with no 5-year survivors. By comparison, those who are resected but recur have a median survival of 40 months, and have a 17% 5-year survival. As such, liver directed therapies shift the cause of death to other sites at a later time point. For this reason, having metastases at other sites does not change survival for patients with liver metastases, as long as they are candidates for surgery. Understanding patient prognosis after treatment of liver metastases goes beyond American Joint Committee on Cancer (AJCC) staging due to patient heterogeneity. Clinical risk scores have been developed to facilitate this, and show that survival is based on primary cancer metastases to lymph nodes, length of disease free interval (if liver disease was not identified at diagnosis), number of metastases within the liver, and serum carcinoembryonic antigen level.
Most major centers report operative mortality of <5% for those undergoing hepatectomy for colorectal cancer liver metastases. Indications for surgery are expanding, which is no longer limited to younger patients without comorbidities. Number of liver metastases is less important for determining resectability than is the existence of adequate vascular inflow and outflow of the remaining liver remnant. Smaller lesions within the planned liver remnant can be treated with microwave ablation or irreversible electroporation. The post-operative functional liver remnant must, however, be 20–40% of the pre-operative liver volume depending on hepatocyte functionality. This is dependent on exposure to previous chemotherapy and pre-existing cirrhosis. If the functional liver remnant is insufficient at presentation, it can be augmented by pre-operative portal venous embolization. Expanding on this concept, some surgeons perform a two-staged procedure that begins with liver partition and portal vein ligation to promote growth of the functional liver remnant, which can initially be as small as a single liver segment. If metastatic disease is limited to the liver, but is too extensive to resect, a hepatic artery infusion pump that delivers floxuridine directly and only to the liver may also be considered. Patients who are not considered operative candidates up front can be converted to resectable with neoadjuvant chemotherapy. It should be noted that 70% of patients who receive neoadjuvant chemotherapy and have so called “disappearing liver metastases” will have microscopic residual foci of disease in the liver, which is the site of local recurrence in 59% of these patients.
Surgical treatment of liver metastases can be performed synchronous with resection of the primary disease, or at different times. If resectability of the colorectal disease is in question, this should be performed first to ensure an R0 resection prior to addressing the metastatic disease. If the colorectal disease is clearly resectable, then the liver should be approached first to ensure low central venous pressure during this portion of the procedure without compromising blood flow to an intestinal anastomosis. A minimally invasive approach may be appropriate depending on the location of planned resections. Given the number of variables that go into determining optimal treatment of colorectal cancer liver metastases, decisions must be made in a multidisciplinary environment that includes team members with expertise in radiology, interventional radiology, chemotherapy, and surgery. Currently, there are wide discrepancies in referral of patients for surgical intervention versus patients who are considered resectable by liver surgeons, reinforcing that multidisciplinary care is of utmost importance.
Lung metastases are the second most common site of colorectal cancer metastases, but are rarely (<10%) found in isolation. Five-year survival is best for patients who have lung metastases resected, compared to patients where the lung disease is left in situ and only liver disease is removed (13% vs. 57%). Existing data, however, are largely retrospective. A randomized phase III trial to examine the effect of concurrent lung metastasectomy (PulMiCC trial) is currently underway. Similar to liver disease, criteria for resectability have expanded in the last few decades. It is currently considered acceptable to treat colorectal cancer lung metastases if there is complete treatment of the primary tumor as well as complete resection of all pulmonary metastases while maintaining adequate pulmonary function. Pre-operative lung function tests are used to assess anticipated post-operative pulmonary function and need for supplemental oxygen. Treatment consists of removal of the minimum amount of lung necessary to completely remove the metastatic deposit. Some consider thoracotomy superior to video assisted thoracoscopic surgery due to the ability to palpate the lung for additional deposits. No studies, however, show a survival advantage with an open compared to a thoracoscopic approach. Propensity score matched retrospective studies in fact suggest the opposite. Methylene blue staining of nodules via CT guidance or navigational bronchoscopy can also be used to assist with intraoperative tumor identification. Approach to thoracic lymph nodes is variable and of uncertain survival benefit. Positive lymph nodes do indicate significantly poorer prognosis. For this reason, lymph node examination is generally recommended for completing staging and to help determine prognosis, which may guide further therapies. Patients who have unresectable disease can alternatively be treated with radiofrequency, microwave, or cryoablation, or stereotactic radiation.
Of patients who die of metastatic colorectal disease, it is believed that 25% have peritoneal carcinomatosis. Treatment of peritoneal carcinomatosis by cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) is of particular interest because progression leads to patient suffering from malignant bowel obstruction, weight loss, and symptomatic ascites. This can result in chemotherapy interruptions and repeated hospitalizations. Peritoneal carcinomatosis occurs more often in patients with right sided colon cancers, T3 tumors, involved mesenteric lymph nodes, and those who developed obstruction or perforation. Identification of peritoneal disease is variable because it is difficult to assess by existing imaging modalities, but is suggested by omental caking, scalloping of the diaphragm, peritoneal nodules, and ascites. [18F]-Fluoro-deoxyglucose positron emission tomography (FDG-PET) can be used to confirm inconclusive imaging findings, but the gold standard of assessment is operative exploration. A study of high risk patients with negative imaging identified peritoneal carcinomatosis in 68%. Diagnostic laparoscopy may be used to avoid exploration in patients without carcinomatosis, or with too extensive carcinomatosis to treat surgically (often because of disease within the mesentery and porta hepatis). Two prospective systematic second look operations studies (CEA Second Look and PROPHYLOCHIP) failed, however, to show a survival advantage. A randomized trial of cytoreduction and HIPEC versus systemic chemotherapy alone showed near doubling of survival in the cytoreduction and HIPEC group. This study was published prior to the widespread use of oxaliplatin and irinotecan though, limiting its applicability to our current patient population. A more recent randomized trial of systemic chemotherapy with cytoreduction with or without HIPEC failed to show a survival advantage in the HIPEC group, but showed a remarkable median survival of 41 months without HIPEC. Of note, no randomized trial to date shows the additive benefit of cytoreductive surgery to modern chemotherapy. Cytoreduction and HIPEC for peritoneal disease remains a popular strategy in select centers because of acceptable morbidity and mortality, along with reported 5-year survival of 27%.
Bone metastases are important to address due to the pain they cause impacting patient quality of life. Tumor related factors not only affect the bone where they are deposited, but also can make adjacent nerves more sensitive to painful stimuli. Further, local therapy can be used in situations where most of the patient’s disease is controlled with systemic therapy, but bone lesions progress. External beam radiation therapy (EBRT) is first line because it is non-invasive and operator independent, but can only be used in patients who can tolerate long periods of immobility while the therapy is being delivered. Pain relief is experienced by 60–80% of patients after EBRT, but can take up to 6 weeks for full effect, and recurs in 50% of patients by 18 weeks. Radiofrequency and microwave ablation can be used for local bone metastases when EBRT is not possible or fails, and improve symptoms in 90% of patients. Radiofrequency ablation has several limitations including dependence on tissue conductivity and predisposition to heat loss from adjacent blood vessels. Microwave ablation can achieve higher temperatures and generate larger ablation zones, however prospective data comparing radiofrequency to microwave ablation for this purpose are lacking. Cryoablation alternatively can be used to freeze tumor tissue. Advantages with cryoablation include a readily visible ablation zone, with less procedurally related pain compared to hyperthermic techniques. Disadvantages include absence of vessel coagulation that can lead to bleeding, and longer procedural time needed for repeat freeze-thaw cycles that are required. High intensity focused ultrasound (HIFU) is a newer technology that uses targeted high energy ultrasound waves on a focused point to induce thermal injury. It is non-invasive and is performed under magnetic resonance imaging (MRI) guidance allowing for real time assessment of thermal ablation. Its use is limited in locations that abut critical organs that could be affected by patient motion during treatment, and can only treat small tissue volumes at a time.
Data is also accumulating that selective resection of distant lymph node metastases may have therapeutic benefit. Patients found to have positive peri-hepatic lymph nodes have lower 3-year overall survival compared to those with negative peri-hepatic lymph nodes (25 vs. 75%). Para-aortic lymph nodes exist between the left renal vein and aortic bifurcation; clearance is associated with a survival advantage in retrospective series. They are positive in 38% of patients with suspicious pre-operative imaging, and when positive are associated with survival similar to that of distant metastatic disease. Similar findings have been reported for lateral pelvic lymph node resections in rectal cancer. While there is no survival difference between patients with and without a lateral pelvic lymph node dissection, there is decreased survival for those who have positive lateral pelvic lymph nodes. Existing data on extended lymphadenectomy is mostly from Eastern countries, and thus may not be well applied to Western populations. As such, current recommendations are to perform extended lymph node clearance only in select patients for prognostic purposes.
Colorectal cancer brain metastases are rarely (<1%) the first metastatic site. Brain metastases are most often identified in patients with metastases to 3 or more other sites, with an average interval from diagnosis of colorectal cancer to detection of 20–40 months. One-year survival after diagnosis is 30%. Patients often present with headache and gait changes; fewer (24%) present with seizure. Contrast enhanced MRI is the diagnostic modality of choice. Treatment begins with management of seizures and cerebral edema if present, but should not be prophylactic. Reviews of patients treated for colorectal cancer brain metastases consistently show that treatment lengthens life. This is most true for patients with good performance status; those with no impairment of performance status have a median survival of 13.5 months. Treatment with surgical resection via craniotomy is used for more superficial tumors that are larger, and when tissue is needed to confirm the diagnosis. Stereotactic radiosurgery alternatively can be used for smaller tumors that are located more deeply in the brain, and can be used in combination with surgical resection when needed. Whole brain radiation has been used after open surgery and stereotactic radiosurgery for metastases in the past, but a recent randomized trial showed no difference in survival with this additional treatment. In this study, 22% of each group were alive and functionally independent at 2 years.
To conclude, there are now many accepted effective ways to treat colorectal cancer metastases to the liver, lung, peritoneum, bone, distant lymph nodes, and brain. These treatments can be used to palliate symptoms and also to prolong life. Due to the complexity and multifactorial nature of the decision making that goes into optimal patient care, treatment of colorectal cancer metastases should routinely be performed in a multidisciplinary environment to maximize patient benefit.
Introduction
Colorectal cancer metastasizes by five means: direct extension, lymphatic spread, portal venous spread to liver, peritoneal dissemination, and vascular spread to distant organs including lung, bone, and brain. The liver is one of the most common sites of metastases. Nearly one-half of patients diagnosed with colorectal cancer will be found to have liver metastases at some point during their disease. This site, when involved by tumor, dominates the disease. When untreated, patients with liver metastases have a median survival of 6–9 months (1). Even with the best chemotherapy, median survival of unresectable disease is 13–18 months (2, 3). Thus, the hepatic site of disease usually dominates the clinical picture.
In the last three decades, liver resection has been proven to be effective and potentially curative therapy for liver metastases (4). Thus, many more individuals are now living longer than 1–2 years from diagnosis. Consequently, other sites of metastases are not only more likely to become apparent, but also more likely to cause symptoms and influence survival.
In this review, we will present data on the natural history of various sites of metastases. We will then follow with a discussion of potential surgical and ablative therapies for these sites. We will consider the indications, risks, and outcomes for treatments of metastases to liver, lung, distant nodes, peritoneum, bone, and brain.
Natural History of Colorectal Cancer Metastases
At presentation, one third of the patients will have nodal metastases, and 20–25 % will have distant metastases, with most of those cases involving the liver (5). Another 20–25% of patients will be found to have metachronous liver metastases. Thus, the liver is the most common site of systemic metastases. The reason for this is anatomic, since all venous blood from the colon and rectum drains through the portal circulation to the liver. Until the 1970s, stage IV liver metastases were thought to be inoperable. Patients did poorly and generally died 6–12 months after diagnosis.
Over the last three decades, however, much data has become available to justify surgical treatment of liver metastases. It has become clear that most tumor cells arriving at the liver do not implant and develop the vasculature necessary to survive (6). Thus, only a few liver tumors may become clinically apparent even if millions of tumor cells enter the portal circulation. Clinical data tracking outcomes of liver resections and other liver directed tumor therapies (Table 1) have proven these interventions extend survival for patients with hepatic colorectal metastases. Liver directed therapies have clearly changed the course of disease in colorectal cancer.
Table 1.
Forms of Liver Directed Therapies.
| Potentially Curative Therapies | Palliative Therapies |
|---|---|
|
|
Causes of death in patients with colorectal cancer liver metastasis: Impact of surgical resection on the natural history and rationale for treating other sites
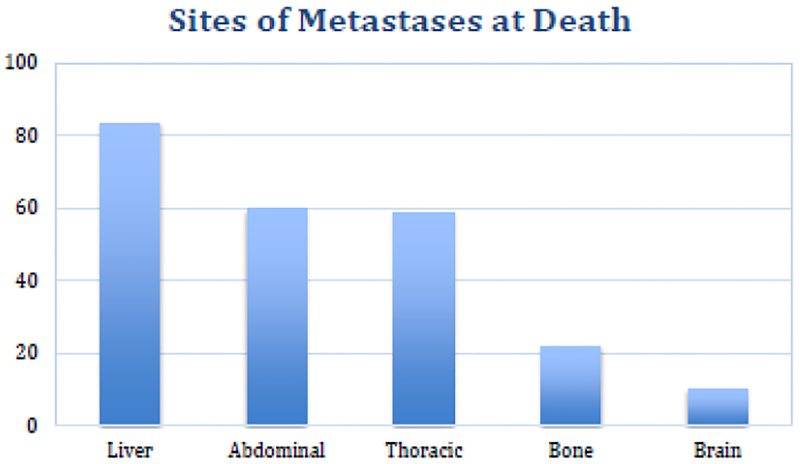
In order to determine the course of disease for patients with systemic dissemination of cancer, we recently looked at causes of death in 476 patients with stage IV colorectal cancer followed until death. Of the 476 patients in this study, 275 (58%) patients had hepatectomies and 201 (42%) patients had unresectable liver metastases. At death, we found that this disease is generally widely disseminated. Liver involvement is found at death in 83% of cases, while intra-abdominal recurrences and lung disease are each found in 59% of cases. Bone metastases are found in 22%, while brain metastases are found in 10% of cases (Figure 1). This further supports the role of the liver as a good filter, preventing portal venous tumor cells from bypassing the liver onto other sites in many cases.
Figure 1.
Sites of metastases at death. Data from 476 patients with colorectal cancer followed until death.
In a ten-year follow-up study, the most common dominant metastatic site prior to death was the liver (49%) followed by other intraabdominal (16%) sites, intrathoracic (11%) sites, the brain (7%), and bone (6%) (1). Patients who underwent hepatectomy died from liver metastasis less frequently compared with unresectable patients (32% vs. 71%; p<0.0001). Disease-specific survival of patients who died of liver metastasis (17.3±1.5 months) was shorter than for patients who died of other intraabdominal disease (29.7±4 months; p<0.0001), intrathoracic disease (39.3±5 months; p<0.0001), or brain metastasis (35.6±5.3 months; p<0.0001). Hepatic resection altered not only length of survival but also eventual cause of death. Hepatic cytoreduction allowed other, more indolent sites of metastatic disease to become clinically evident and important.
Dominant metastatic site prior to death (cause of death)
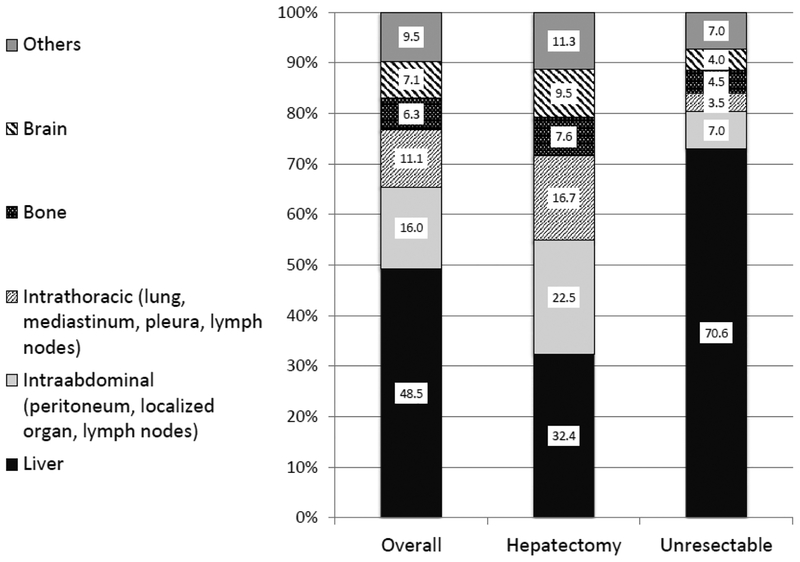
Figure 2 shows the dominant metastatic site at the last evaluation just prior to death in all patients as well as in the hepatectomy and unresectable groups. Overall, the most common dominant metastatic site prior to death was the liver (231 patients, 49%) followed by intraabdominal sites (76 patients, 16%), intrathoracic sites (53 patients, 11%), the brain (34 patients, 7%), bone (30 patients, 6%), and others (52 patients, 11%). Patients who underwent hepatectomy died from liver metastasis less frequently compared with unresectable patients (32.4% vs. 70.6%; p <0.0001).
Figure 2.
Dominant sites of disease/ causes of death for 476 patients with stage IV colorectal cancer followed until death.
Symptoms, signs, and laboratory data in patients with each dominant metastatic site are summarized in Table 2. Patients with dominant liver metastases had worse liver function tests (LFTs) prior to death compared with patients with other dominant metastatic sites. As would be expected, albumin was lower (2.7 vs. 3.0 g/dL; p=0.001), while aspartate transaminase (79.5 vs. 41.0 U/L; p=0.0001), alanine transaminase (43.0 vs. 26.0 U/L; p=0.0001), alkaline phosphatase (383 vs. 228 U/L; p=0.001), and bilirubin (6.0 vs. 1.4 mg/dL) were higher in liver-dominant disease cases. In 24 patients (10.4% of the liver-dominant group), liver failure (hepatic encephalopathy and/or coagulopathy along with an LFT abnormality) was evident. Symptoms and signs from hepatic dysfunction were usually due to the mass effect of tumor (replacement of liver parenchyma) or portal hypertension.
Table 2.
Chief complaints, signs, and liver function tests of patients with colorectal cancer liver metastasis who died of disease, by dominant metastatic site.
| Liver Dominant (n=231) | N | % |
| LFT abnormality | 183 | 79.2 |
| Liver failure | 24 | 10.4 |
| Symptoms/Signs | ||
| Jaundice | 66 | 28.6 |
| Cachectic | 40 | 17.3 |
| Hepatic encephalopathy | 21 | 9.1 |
| Abdominal distension | 20 | 8.7 |
| Hepatomegaly | 26 | 11.3 |
| Pain | 24 | 10.4 |
| Infectious disease | 13 | 5.6 |
| Respiratory distress | 18 | 7.8 |
| GI bleeding | 3 | 1.3 |
| Intraabdominal Sites Dominant (n=76) | N | % |
| Liver metastases exist | 50 | 65.8 |
| LFT abnormality | 35 | 46.1 |
| Liver failure | 0 | 0.0 |
| Symptoms/Signs | ||
| Peritonitis carcinomatosis | 20 | 26.3 |
| Pain | 13 | 17.1 |
| Abdominal distension | 12 | 15.8 |
| Cachectic | 16 | 21.1 |
| Infectious disease | 4 | 5.3 |
| Respiratory distress | 2 | 2.6 |
| GI bleeding | 2 | 2.6 |
| Vaginal bleeding | 2 | 2.6 |
| Others | 5 | 6.6 |
| Intrathoracic Sites Dominant (n=53) | N | % |
| Liver metastases exist | 27 | 50.9 |
| LFT abnormality | 11 | 20.8 |
| Liver failure | 0 | 0.0 |
| Symptoms/signs | ||
| Respiratory distress | 30 | 56.6 |
| Cachectic | 8 | 15.1 |
| Pain | 5 | 9.4 |
| Infectious disease | 4 | 7.5 |
| Hemoptysis | 1 | 1.9 |
| Others | 4 | 7.5 |
| Bone Dominant (n=30) | N | % |
| Liver metastases exist | 18 | 60.0 |
| LFT abnormality | 12 | 40.0 |
| Liver failure | 0 | 0.0 |
| Symptoms/Signs | ||
| Pain | 20 | 66.7 |
| Spinal cord compression | 8 | 26.7 |
| Cachectic | 1 | 3.3 |
| Respiratory distress | 1 | 3.3 |
| Brain Dominant (n=34) | N | % |
| Liver metastases exist | 24 | 70.6 |
| LFT abnormality | 15 | 44.1 |
| Liver failure | 0 | 0.0 |
| Symptoms/Signs | ||
| Focal neurologic symptoms | 20 | 58.8 |
| Altered mental status | 3 | 8.8 |
| Pain | 3 | 8.8 |
| Respiratory distress | 2 | 5.9 |
| Headache | 2 | 5.9 |
| Cachectic | 2 | 5.9 |
| Others | 2 | 5.9 |
Notes: GI, gastrointestinal; LFT, liver function test
Intraabdominal metastatic disease was the second most common dominant metastatic site. Among this group, liver metastasis co-existed in 50 patients (66%). Thirty-five patients (46%) had abnormal LFTs; however, no patients had liver failure. The most common presentation in this patient group was peritoneal carcinomatosis. Bowel obstruction or massive malignant ascites caused abdominal distension, and in some cases respiratory distress. These patients typically had poor oral intake and were cachectic.
Intrathoracic metastatic disease was the third most common dominant metastatic site prior to death. About half of this group also had liver metastases (27 patients, 51%) with some LFT abnormalities and liver failure. Massive involvement of lung or malignant pleural effusion caused respiratory distress. Mediastinal lymph node disease at times invaded into the bronchus and caused hemoptysis. Pleural metastases that extended into the chest wall caused severe pain.
Although not many patients died with dominant bone metastasis, the clinical course of these patients was miserable. Pathologic fractures or epidural invasion caused severe pain and neurologic symptoms. Spinal cord compression led to myelopathy. Typically, these patients required huge amounts of narcotics, and the disease compromised patients’ respiratory function and/or mental status as well. Sixty percent of these patients (n=18) had concomitant liver metastasis.
Patients with dominant brain metastasis usually presented with focal neurologic findings. Once mental status was compromised, respiration was suppressed. The general condition rapidly deteriorated. Most of these patients had some liver disease.
Site of metastases and cause of death
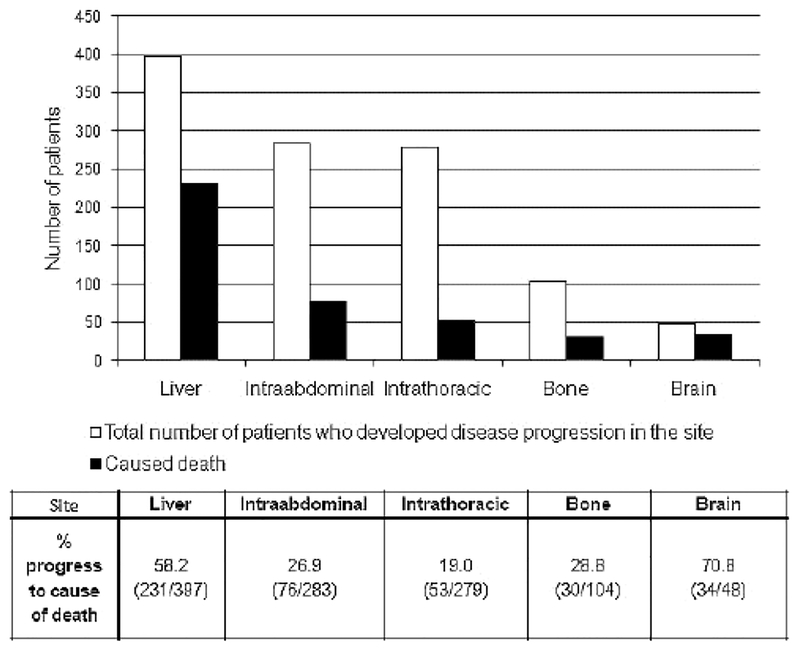
The chance of the metastatic site progressing to cause death was analyzed and is shown in Figure 3. Disease progression in the liver was observed in 397 patients (83% overall). Among them, 231 patients (58%) had dominant disease in the liver prior to death. There were 283 patients (59% overall) who experienced disease progression in the intraabdominal site, and, of this group, 76 (27%) died due to intraabdominal site disease progression. In the intrathoracic site, 279 patients developed disease progression (59% overall); thoracic disease in 53 of these patients (19%) progressed to cause death. In bone metastases, 104 patients (22% overall) developed disease progression, and in 30 (29%), bone disease progressed to cause death. In brain metastases, 48 patients (10% overall) developed disease progression and 34 (71%) progressed to death from this site of disease.
Figure 3.
Sites of cancer progression and likelihood of site of disease causing death.
Timing of presentation at various metastatic sites and related survival
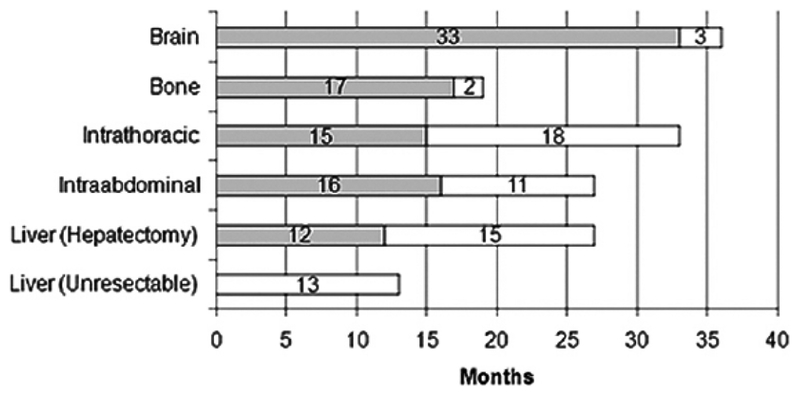
Figure 4 shows the median time of presentation of each metastatic site and the median survival after presentation for each metastatic site of disease. These data verified that if liver disease is unresectable, it generally dominates the clinical picture and most patients die within 1.5 years. Patients with intrathoracic recurrence usually live a fair bit of time, and most die because of a concurrent recurrence at another site.
Figure 4.
Median time of presentation of each metastatic site (gray bars) and median survival after presentation (white bars) for each metastatic site of disease.
The late presentation of brain metastases suggests that these are secondary metastases in the setting of widely disseminated cancer. Most patients die quickly. These data also emphasize that even though patients are likely to die of liver disease, they suffer from the bone and intraabdominal recurrences. These data document the causes of death in patients resected of their colorectal metastases, as well as patients with radiographically resectable disease found unresectable. There is no doubt that hepatectomy resulted in a change in the natural history of this disease, and was associated with a much-prolonged survival even in patients with recurrence after resection (median survival: unresectable = 13 months; resectable but with recurrence = 40 months), (5-year survival: unresectable = 1%; resectable but with recurrence = 17%). There was also a difference in causes of death.
The overwhelming number of unresectable patients died of liver disease (71%), with only a small percentage dying of extrahepatic disease (abdominal = 7%, bone = 5%, lung = 4%). However, for patients initially resected of their hepatic disease, even if they recurred, the causes of death shifted. Only 32% died of hepatic disease. The extrahepatic sites then took on much more important roles as the causes of death (abdominal = 23%; lung = 17%; bone = 8%; brain = 10%). These other disease sites seemed to be more indolent or were secondary metastases; the median times to clinical presentation of bone or brain metastases were 17 and 33 months, respectively. Symptomatic presentation of brain or bone metastases was associated with very poor prognosis, with median survival of only 2–3 months. Survival times after recurrence at other sites were similar, with a median survival of 12–18 months. In fact, survival measured from time of recurrence at these other sites was similar to survival of unresectable patients.
There is increasing data advocating liver tumor cytoreduction even in the presence of gross extrahepatic disease (7–10). There has long been evidence that extrahepatic disease portends poor prognosis (11–25). Nevertheless, there have been various series showing that selected patients with lung metastases or hepatic nodal disease could have prolonged survival. Furthermore, recent advances in chemotherapy may further improve outcomes of hepatectomy in the presence of extrahepatic disease. Adam et al.(7) reported the outcome of 138 patients who underwent hepatectomy for colorectal cancer liver metastases (CRCLM) after downstaging by chemotherapy. There were 52 patients (38%) with extrahepatic disease. Among them, 41 (30%) underwent extrahepatic resection (lung, peritoneal nodules, portal lymph nodes, ovary, kidney, and local recurrence of colorectal cancer). The analysis showed the presence of extrahepatic disease was not associated with worse prognosis (5-year survival rate 33% vs. 34%; p=0.67). Similarly, a study from Minagawa et al. (2000) analyzing 235 patients who underwent hepatectomy for CRCLM showed no significant difference in survival between patients who had extrahepatic disease and those who did not (8). These previous papers and the data in the current study demonstrating that liver resection even with subsequent extrahepatic recurrence can be associated with prolonged survival are highly encouraging of future studies of the efficacy of hepatic cytoreduction in the setting of minimal extrahepatic disease. These data also encourage selective cytoreduction in extrahepatic sites.
Colorectal Liver Metastases
As discussed above, there was a time when stage IV colorectal cancer in the liver was considered a death sentence. Surgeons and other innovators pushed the envelope and we have now arrived in an era where even disease that is not liver-limited can be considered for aggressive metastasectomy. With boundaries being stretched well past what was previously acceptable, it is good to contextualize what is considered innovation in liver metastasectomy and what was thought of as “too far” just a short time ago.
Partial hepatectomy is a safe and effective therapy for colorectal cancer liver metastases
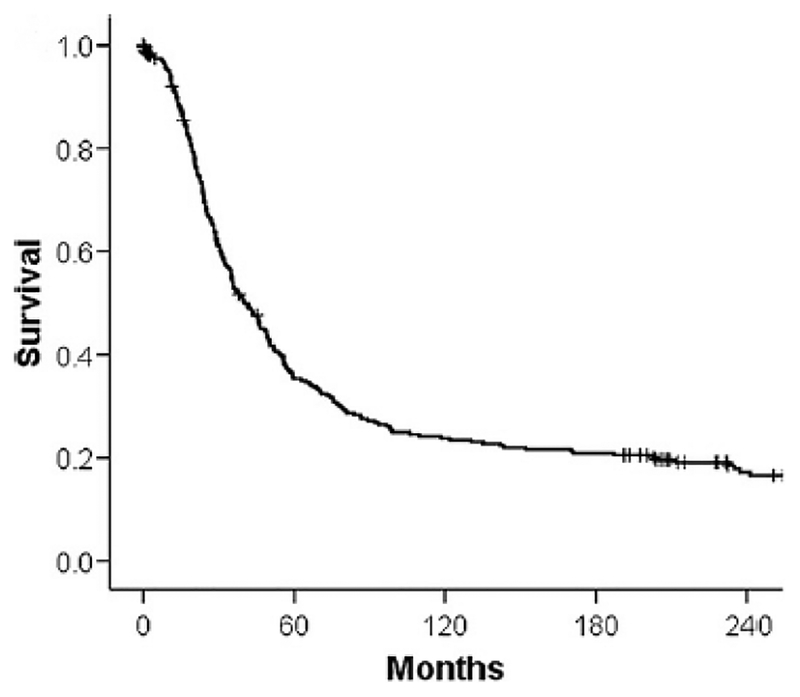
The birth of surgical therapy for liver metastases can be traced back to autopsy studies demonstrating that deceased patients may have the metastatic disease confined to the liver and to CT scanners documenting disease confined to the liver in many cases. With these data, surgeons began to resect hepatic colorectal metastases (16, 26, 27). A large body of data has since accumulated proving surgical resection to be safe and effective therapy (Table 3) (8, 11, 15, 26–38) (39–41). At present, most major centers report operative mortality less than 5% for hepatectomy, and 5-yr survival for over one-third of patients. An interesting study with complete 25-year follow-up of patients treated by liver resection by a pioneer in the field has demonstrated that surgery alone can provide cure in approximately 20% of patients (Figure 5) (42). These results are accomplished with short hospital stays (30–32, 43), and with patients recovering quickly and returning to normal life (44). Since then, liver resection has become the standard of care for patients with hepatic colorectal metastases.
Table 3.
Overall survival among studies reporting outcomes of hepatic metastasectomy in colorectal cancer patients.
| Study | n | Operative Mortality % | 1-y Survival % | 3-y Survival % | 5-y Survival % | 10-y Survival % | Median Months |
|---|---|---|---|---|---|---|---|
| Gayowski et al. 1994 (11) | 204 | 0 | 91 | -- | 32 | -- | 33 |
| Scheele et al. 1995 (26) | 434 | 4 | 85 | 45 | 33 | 20 | 40 |
| Nordlinger et al. 1996 (16) | 1568 | 2 | 80 | -- | 28 | -- | 40 |
| Fong et al. 1999 (27) | 1001 | 2.8 | 89 | 57 | 36 | 22 | 42 |
| Minagawa et al. 2000 (43) | 235 | 0.85 | - | 51 | 38 | 26 | |
| Adam et al. 2001 (32) | 335 | 1 | 91 | 66 | 48 | 30 | 52 |
| Choti et al. 2002 (30) | 226 | 1 | 93 | 57 | 40 | 26 | 46 |
| Kato et al. 2003 (31) | 585 | 0 | - | - | 33 | - | - |
| Figueras et al. 2007 (35) | 501 | 4.0 | 88 | 67 | 42 | 36 | 44 |
| Tomlinson et al. 2007 (36) | 612 | -- | -- | -- | -- | 17% | 44 |
| Rees et al. 2008 (37) | 929 | 1.5 | -- | -- | 36 | 23 | 43 |
| De Jong et al. 2009 (39) | 1669 | -- | -- | -- | 47 | -- | 36 |
| Robertson et al. 2009 (40) | 3957 | 8.2 | -- | -- | 25.5 | -- | -- |
| House et al. 2010 (33) | 563 | 1 | - | 69 | 51 | 37* | 64 |
| Nathan et al. 2010 (38) | 949 | 0.9 | -- | 65 | 45 | 22 | 52 |
| Hwang et al. 2014 (41) | 3481 | 4.2 | -- | 42.4 | 28 | -- | 30.5 |
Note:
8-y survival
Figure 5.
Survival of patients subjected to liver resection for hepatic colorectal metastases in the pre-adjuvant chemotherapy era. Data represents actual 25-year survival. Adapted from Fortner and Fong, 2009 (4).
Need for useful clinical staging criteria
Hepatic colorectal metastases are considered stage IV by American Joint Committee on Cancer (AJCC) criteria. The population of patients offered hepatectomy for colorectal metastases is heterogeneous. Thus, there has been a need for better clinical staging criteria for this patient population to assist in patient selection for surgery, for adjuvant therapies and trials, and for comparison of data from various institutions.
Building on many prior studies, two very large patient studies in the 1990s conceived similar scoring systems for staging patients with hepatic metastases. Both systems utilize variables related to the primary cancer and the liver metastases (16, 27). The five common elements to both systems have been popularized as the Clinical Risk Score (CRS) (Table 4)(27): 1) nodal metastases from primary cancer (45); 2) short disease-free interval (17, 46–48); 3) size of the largest liver tumor (17, 49); 4) more than one liver metastasis (17, 48); and 5) high carcinoembryonic antigen (CEA) (16, 17). This scoring system has been independently verified by investigators from many nations (50–52). The CRS has been found to also predict prognosis after resection or ablation. It has also been used to select patients for the extent of preoperative diagnostic work-up to optimize yield while minimizing cost (53, 54). Most of all, the simplicity of the CRS has led to its widespread use.
Table 4.
Prognostic variables for hepatic colorectal metastases.
| Prognostic Variables | Author |
|---|---|
| Clinical indicators | |
| *Node-positive primary tumor | Fong et al. 1999 (13) |
| *Size of largest lesion >5 cm | |
| *Carcinoembryonic antigen >200 ng/dl | |
| Extrahepatic disease | Poultsides et al. 2012(55) |
| Fibrotic response to chemotherapy | |
| Pathologic indicators | |
| Margin positive resection | Turcotte et al. 2014 (56) |
| Molecular indicators | |
| CXCR4 | Yopp et al. 2014 (57) |
| HumanHT-12 gene Chip/MRS panel | Ito et al. 2013 (59) |
| kRAS | Kemeny et al. 2014 (293) |
Notes:
components of the CRS scoring system (One point assigned for each positive criterion. Sum of points is CRS).
Investigators have attempted to improve upon the CRS by adding parameters such as response to chemotherapy (55), immune cell infiltration index such as for TILs (56), molecular measures of tumor “stemness” such as CXCR4 (57), and angiogenic indices as measured by VEGF, EGFR (58), or biomarker panels (59). While these add additional discriminating effect, most of these molecular analyses are not universally employed. These will remain of use mainly in tertiary centers.
Patient selection for hepatectomy
In the 1980s, only healthy patients with limited liver disease (generally solitary lesions or less than 4 lesions in the same lobe of liver) were considered candidates for resection. With such limited indications, less than 10% of patients were candidates for surgery. With increasing safety and documented favorable long-term cancer outcomes, medical and oncologic indications have broadened. Advanced chronologic age is no longer a complete contraindication (60, 61). Compensated medical co-morbidities are no longer a contraindication. Patients with extensive disease, including synchronous disease, bilobar disease, and extensive numbers of nodules, are now considered for aggressive surgery (62). It is estimated that over 50% of patients are now candidates for hepatectomy. It should be noted that the overall survival of patients at major institutions has not worsened despite the expanding indications for surgery.
While there is not a defined criterion for making the statement of “innumerable”, it appears acceptable for radiologists to refer to metastases numbering more than 10 as “innumerable”(63). This lags behind current surgical ethos of CRCLM management, which essentially states that number and lobar location of metastases are far less relevant to determination of resectability than adequate inflow, outflow, and functional liver remnant (64–66), and emphasizes the need for image review by a radiologist well-versed in liver imaging. Indeed, even extrahepatic disease no longer precludes appropriate clearance of CRCLM. It is also important that decisions for resection are made in a multidisciplinary setting, bearing in mind high-risk features and other considerations like current response to chemotherapy and candidacy for immunotherapy (65). Any discussion of when and upon whom to operate should include a multidisciplinary team (including medical oncology, surgical oncology, radiology, pathology, interventional radiology, radiation oncology, and genetics), and goes beyond simply thinking about what can be done (Table 5).
Table 5:
Required elements of assessments for resection.
| Component required | Information acquisition |
|---|---|
| Assessment of disease extent |
|
| Assessment of need for chemotherapy or response to previous chemotherapy |
|
| Optimizing surgical decisions - assessment of resectability |
|
Notes: CEA, carcinoembryonic antigen; CT, computed tomography; FLR, functional liver remnant; MRI, magnetic resonance imaging; PVE, portal vein embolization
Functional liver remnants (FLR) should make up at least 20% of estimated liver volume in chemotherapy naïve livers, 30% in chemotherapy-treated livers, and 40% in livers with any evidence of cirrhosis or fibrosis (65). In order to achieve adequate FLR, techniques like portal vein embolization (PVE) with and without concurrent transarterial chemoembolization (TACE) can be pursued. Portal vein embolization is a technique for producing growth of remnant liver prior to resection. By transcutaneous puncture of the portal vein and filling the vein on the side of planned future resection with embolic material, ipsilateral atrophy and contralateral hypertrophy occurs. Future remnant liver is grown and peri-operative outcome improved (67). When anticipating a formal hepatectomy, PVE is successful the vast majority of the time, and mean increase in FLR remnant size is roughly 35% (68). When considering whether adequate FLR hypertrophy has occurred following PVE, an absolute increase of 5% and a growth rate of at least 2% per week should be considered (69).
Appropriate preoperative imaging assessment involves liver-protocoled CT or magnetic resonance imaging (MRI) depending upon state of the liver.(69, 70) Livers that are cirrhotic or have elements of steatohepatitis (chemotherapy-induced or otherwise) are typically more optimally imaged with MRI (70, 71). Despite improvements in currently available axial image clarity and consistency, employment of intraoperative ultrasound remains a component of disease assessment. Historically, IOUS frequently altered the pre-operative surgical plan (72). Although the advent of Eovist use and more readily available MRI protocoling designed to best evaluate the liver have somewhat reduced the element of operative surprise, IOUS still has a critical role in real-time delineation of anatomic structures and confirmation of equivocal findings on axial imaging (73–75).
Timing of primary resection for synchronous metastases
One quarter of cases of colorectal cancer will present with synchronous liver metastases. As liver surgical morbidity and mortality have decreased, many groups are pursuing single stage intervention for patients presenting with synchronous metastases, resecting the primary and hepatic lesions in the same operation. In general, concurrent resection is well tolerated. In well-selected patients, simultaneous resections are safe, and allow for reduced time to recovery and to start of appropriate adjuvant chemotherapy (76–78). Most recently, simultaneous major hepatectomy and rectal resection has also been shown to be safe (79).
There are a few rules of thumb that should be observed. Firstly, when the primary lesion is asymptomatic, liver surgery should be performed first as this requires a lower central venous pressure and typically presents higher morbidity. Moreover, if the liver lesions cannot be cleared, there is no known survival benefit to resecting the primary. Second, in the setting of rectal cancer wherein an R0 resection is not assured, the order of resection should prioritize a rectum-first approach in case of unresectability. Data supporting these principles are ever-evolving and each patient’s case should be considered on a case-by-case basis.(80)
Contraindications to simultaneous resection remain major medical co-morbidities, bowel obstruction, bowel perforation, and lack of technical expertise to perform both the liver and colorectal resection.
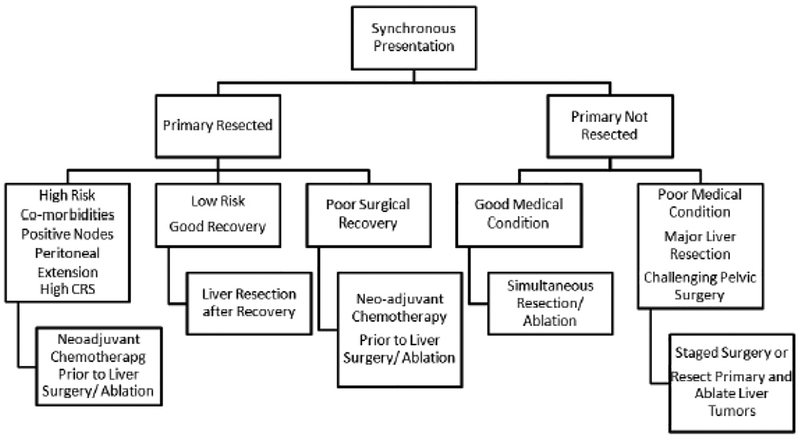
For colorectal cancer presenting with synchronous liver metastases a suggested algorithm of care is presented in Figure 6. If the patient has synchronous primary and metastatic disease that can be safely removed in the same operation, a combined resection is justified (76, 81). For those cases where the primary colorectal cancer has been resected, delay in resection of the liver metastases is justified if the patient’s comorbidities dictate optimization of medical condition.
Figure 6.
Algorithm of treatment for patients with synchronous hepatic colorectal metastases.
Number of metastases and presence of extrahepatic disease
Although number of metastases does not necessarily permit or preclude safe resection, extent of disease burden can and should play a role in surgical decision making. A recent review of the 15-year experience at two high volume European centers divided patients into those with less than 8 metastases or more than 8 (82). Among the group with more than 8 lesions, there were survival differentials seen from 8–10, 11–15, and greater than 15 lesions (82). Higher risk features included extrahepatic disease, failed response to chemotherapy, and primary rectal cancer (82). Patients with two or more risk factors had very poor outcomes indicating possible futility of surgical resection. However, patients in the greater than 8 group with no risk factors had similar survival to those in the less than 8 group (5-year overall survival (OS) rate of 44.0% vs. 44.2%) (82). Thus, the number of lesions alone should not limit potential for resection.
Recent data from Memorial Sloan Kettering ascribed importance to number of metastases in the context of resection with curative intent of hepatic metastases along with extrahepatic disease (83). They developed a novel score ascribing one point to each of three variables (largest CRCLM >3cm, >5 CRCLM, and unfavorable extrahepatic disease site) with a resulting score that was prognostic of overall and recurrence-free survival (83). In this study, portal and retroperitoneal lymph node metastases as well as multiple sites of extrahepatic disease were considered “unfavorable” and were associated with decreased overall and progression-free survival (83). Nevertheless, there were some true 10-year survivors in this cohort (83). Thus, neither number of metastases nor presence of extrahepatic metastases should preclude consideration for resection. It is clear that at least with currently available data, treatment decisions must be made in a multidisciplinary fashion with consideration of patient goals and individual characteristics.
In addition to misconceptions regarding prognostic weight of number of metastases, it can be challenging to get resectable patients referred for surgical intervention. Recent publications have shown substantial discrepancies between referring medical oncologists and expert hepatic surgeons in terms of what is thought of as resectable (84, 85). Moreover, hospital and surgeon practice patterns of what is considered resectable also vary widely (86). Educational initiatives are needed both to help patients advocate for themselves and to educate referring providers about what are broadly accepted criteria for resectability to induce more consistent referral patterns (87).
Associated liver partition and portal vein ligation for staged hepatectomy (ALPPS) and Two-staged hepatectomy
With continued progress, expanded indications are giving way to new operative strategies such as two-stage hepatectomy and associated liver partition and portal vein ligation for staged hepatectomy (ALPPS). These techniques are employed with a FLR that would be prohibitively small using standard PVE and resection techniques. Some are even able to offer single segment FLR for patients with these techniques. That said, the risks are real and should not be discounted. ALPPS is essentially a short-term version of a two-stage procedure wherein re-intervention is planned within days (typically 7–10) of initial procedure. In the case of two-staged hepatectomy, the less-extensively invaded hepatic lobe is cleared of disease with resection or ablation, and the patient then undergoes PVE to induce FLR hypertrophy. At second stage, the other lobe is resected or cleared depending upon the situation. The primary tumor can be resected during either of these stages in an attempt to render the patient disease-free (88–90). Whether or not chemotherapy is employed between stages varies institutionally. A 2015 study of this approach reported a failure rate of 35% with commensurately low overall survival in the failed group (91). Risk factors for failure are well-aligned with those of the CRS and include CEA >30ng/mL, tumor size >40mm, 3 or more metastases in the FLR, more than 12 preoperative chemotherapy cycles, and disease progression during first line chemotherapy (91). Initial reports of ALPPS and two-stage hepatectomy results yielded what many viewed to be unacceptably high morbidity and mortality rates. However, proponents of ALPPS have now created an international registry. Initial reports of early survival and safety compiled from this multi-national, multi-institutional registry have shown that 141 (70%) of the 202 included patients had CRCLM with a median FLR of 21% that increased by 80% within a median of 7 days (92). Nevertheless, the major morbidity (27%) and 90-day mortality of 9% remain quite high (92). Authors report that independent factors associated with higher morbidity rates included age over 60, operative time greater than 5 hours and non-CRCLM (92). Factors associated with less FLR hypertrophy included use of the Pringle maneuver and age (92).
Role of minimally invasive surgery
Laparoscopic liver resection has been the accepted standard of care for peripheral lesions in the so-called “laparoscopic segments” II, III, V, and VI for more than a decade (93). However, use of minimally invasive surgery (MIS) for hepatic lobectomy is more limited and has been much slower to achieve adoption. The advent of robotic liver surgery has seen an increase in the employment of MIS for all liver resections, but has specifically demonstrated usefulness in facilitating completion of procedures like major lobectomies that have a higher conversion rate to open surgery when attempted laparoscopically (94). Finally, robotic liver surgery is exceedingly helpful in wedge resections in what would otherwise be considered incision-dominant cases (95), meaning those where a minor wedge is required in a hard-to-reach segment or posterior section as shown in Figure 7. A robotic approach in these cases allows safe resection of tumor while minimizing morbidity.
Figure 7.
Incision dominant case of liver metastases. Located in segment 7 of the liver, this small lesion (circled) requires a large incision for open surgery. It is also difficult to reach this with routine laparoscopy. This is a lesion ideal for out-patient robotic hepatectomy.
Importance of ablation
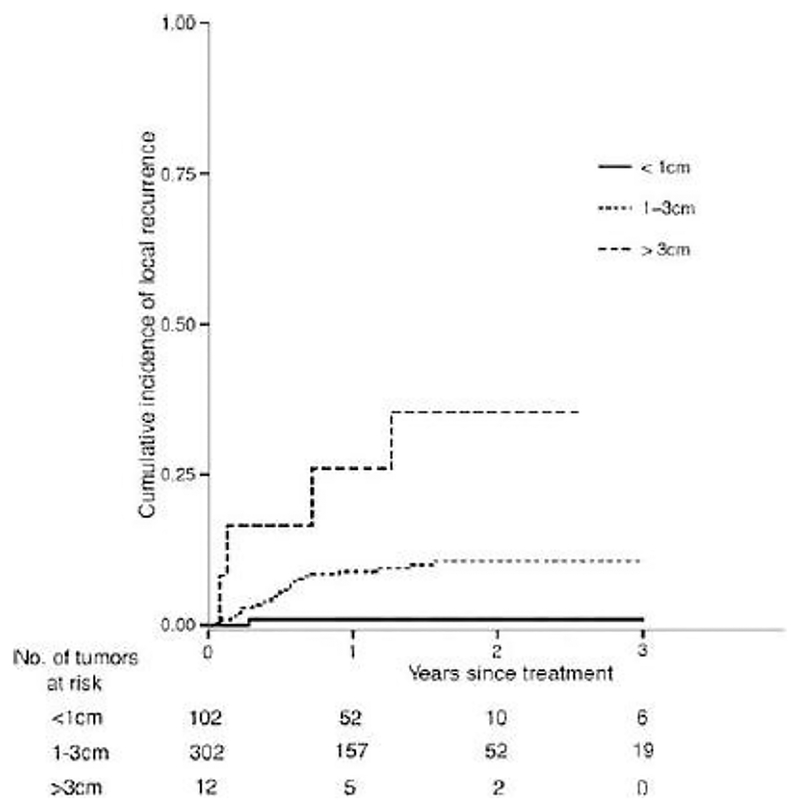
While initial reports of radiofrequency ablation for CRCLM demonstrated unacceptable rates of local recurrence and in some cases increased rates of lung metastases, improved provider experience with ablative techniques along with improved ablative technologies like microwave ablation and enhanced radiofrequency ablation machines have made these local control modalities an important part of the “toolbox” that can be used to attempt to render patients disease free or to prolong survival (96). Moreover, improved interventional radiology and surgical skill with these techniques have yielded more modern series demonstrating acceptable efficacy of ablation of smaller lesions. The current generation of 2.45 GHz MW ablation units now delivers durable ablations for small and medium sized lesions. In a recent publication reporting 465 ablations, microwave destruction of cancer was shown to be highly effective, and durable (97). For tumors 1 cm or less, ablation completely killed cancer in 99% of the time (Figure 8) (97).
Figure 8.
Long-term results of microwave ablation for cancer. For tumors less than 1 cm in size, recurrence was 1%. For those > 3cm in size, recurrence rate was 9%. Adapted from Leung et al., 2015 (97).
Irreversible Electroporation (IRE, Nanoknife)
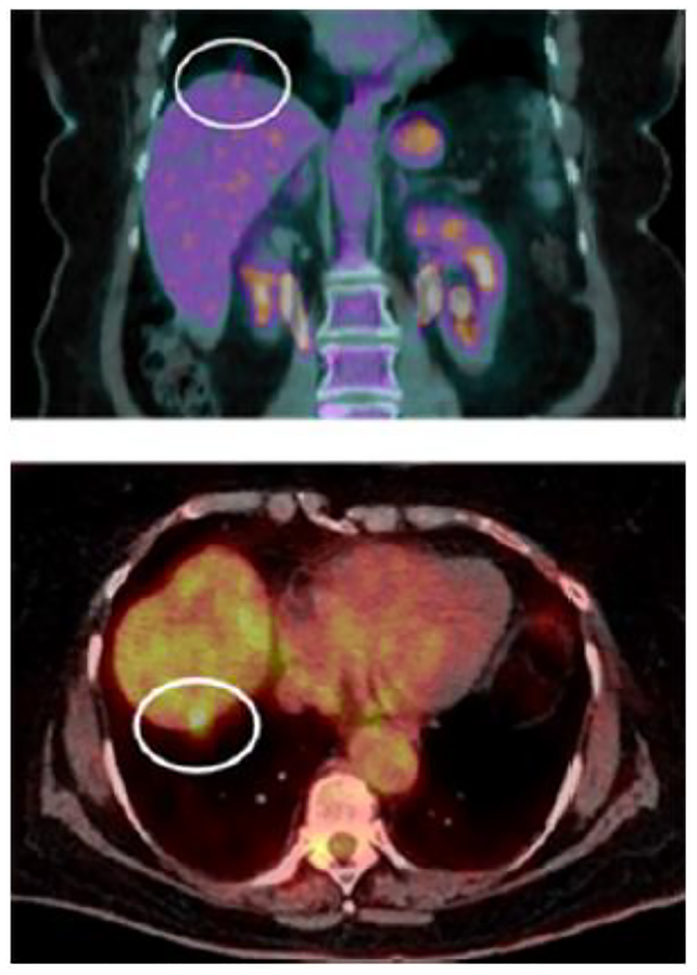
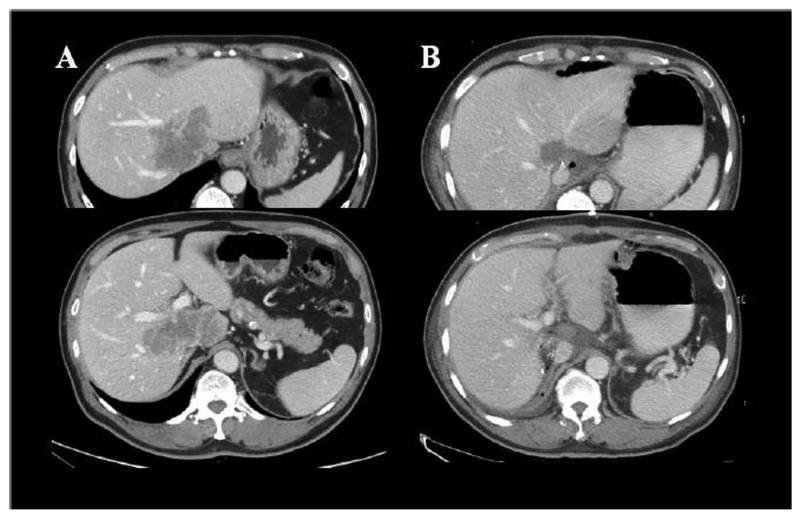
Irreversible Electroporation is a relatively new technology that uses a series of rapid micro- to millisecond pulses (70 – 90 pulses) of high energy (1000–2500 V/cm2) direct current to kill cancer. Such deposited energy causes formation of permanent nanopores within the cellular membrane, triggering cell death through cellular apoptotic pathways (98–100). Because IRE is essentially non-thermal (101, 102), it is not as susceptible to heat sink effects of nearby vessels as radiofrequency and microwave ablation, and can be used with relative safety adjacent to heat-sensitive structures, such as vessels, bile ducts and nerves (Figure 2) (103, 104). The use of IRE in humans was first reported to be safe by Thomson et al. in 2011 (104). Highlighting the safety of IRE, Kingham and colleagues studied the use of IRE in patients (n=28) with small tumors (median diameter = 1cm, range = 0.5 – 5 cm), all of which were close to a major vascular structure (< 1cm). No life-threatening events were described (1 patient developed supraventricular tachycardia and another developed portal vein thrombosis). Of the 65 tumors treated, only 1 tumor demonstrated persistent disease and 3 tumors locally recurred (105). Eller et al. similarly studied local control of liver tumors in perivascular locations (106). It is promising in allowing ablation of anatomic sites previously prohibitively dangerous for thermal ablation (Figure 9).
Figure 9.
Patients with ill-placed lesion involving both portal veins, and all three hepatic veins (A). Combined treatment with microwave and IRE resulted in FDG-PET-negative scan 2 years later (B).
Combined liver resection and tumor ablation
Focal ablation techniques are also allowing for treatment of extensive bilateral liver cancers (107, 108). While most of the early adaptors of such combined usage employed it as a last resort, the philosophy has shifted to using such combined resection and ablation as a liver parenchymal preservation method of choice. Karanicolas and colleagues demonstrated that combining resection and ablation achieves favorable cancer related outcomes while decreasing operative time and blood loss (109). An international consortium of four centers recently confirmed these findings and formulated the acronym CARe (Combined Ablation and Resection) as a name for this next phase of natural evolution in the local eradication of cancer while preserving functional liver (110).
In the era of parenchymal preservation, many surgeons will employ ablation in deeper parenchymal lesions where attempted resection would render an unacceptably small FLR or when trying to achieve limited resections. Current ethos is that this approach in conjunction with appropriately timed systemic therapy can render the possibility of cure or at least a significant disease-free interval. For example, the recent CLOCC trial was a randomized phase II trial that was terminated early after demonstration that combined surgery with RFA of otherwise unresectable tumors in conjunction with systemic therapy was associated with significant overall survival improvement (111).
Role of hepatic arterial infusion (HAI)
Hepatic arterial infusion (HAI) pumps are an important part of the surgical armamentarium against liver limited disease that is unable to be cleared with resection. The rationale for HAI use is predicated upon the anatomic blood supply of liver metastases, which is known to come from the hepatic arterial system rather than the portal venous one. Furthermore, drugs such as floxuridine (FUDR) that has high first pass liver clearance can be delivered intrahepatically in high doses with minimal systemic toxicity (112).
HAI of chemotherapy is typically administered via a surgical implanted subcutaneous pump with a catheter placed in the gastroduodenal artery, and is given concurrently with systemic chemotherapy. A substantial body of level one evidence supports the regular employment of HAI pumps against CRCLM. Several Phase III randomized control trials have demonstrated success of this modality in prolonging both OS in the unresectable setting and recurrence free survival in setting of surgery and pump placement versus surgery alone (113–115). The predominant critique of these trials is that they pre-date modern chemotherapeutics. Thus, Phase I and II trials of HAI in combination with modern systemic therapies have been performed, and showed favorable results (116–120).
A recent propensity-score matched comparison of patients receiving HAI and modern chemotherapy in comparison with controls at MSKCC showed a substantial survival benefit (67 months vs. 47 months), in addition to a pronounced survival advantage for patients with node-negative disease and a low CRS of 0–2 (121). Moreover, amalgamated data from four prospective trials of HAI combined with systemic chemotherapy after liver resection have demonstrated excellent long-term survival with modern era patients demonstrating 5-year survival rates up to 78% and 10 year survival rates of 61% (122). Nevertheless, HAI perhaps owing to its technical difficulty, is still not in wide use outside of selected high volume specialty centers.
Disappearing liver metastases
In the era of modern chemotherapeutics, treatment effects can result in CRCLM disappearance on standard pre-operative imaging and even on Eovist-based MRI. Chemotherapy-associated hepatic changes can make these lesions hard to see even on intra-operative ultrasound. Standard historical teaching has been to resect all areas of known disease – quiescent or otherwise, meaning that if it was seen originally on scan it should be included in the field of resection. Placement of fiducial markers prior to chemotherapy initiation, however, while safe and effective, is not widely employed (123).
Moreover, in the era of parenchymal preservation and increasing use of effective percutaneous ablation, blind resection of all areas of prior disease without fiducials is not always completed. Nevertheless, even with use of Eovist, one can expect up to 40% of liver metastases to disappear and of those, up to 70% will contain at least microscopic residual foci of disease (124, 125). Others have noted that in patients with unidentified and untreated disappearing liver metastases, up to 59% develop local recurrence at the site of the original tumor (126). Moreover, a recent series indicates that use of Eovist-based MRI or contrast-enhanced ultrasound techniques can identify up to 55% of disappearing lesions, of which 69% will have residual disease (125). Thus, if disappearing liver metastases, remain unresected, close follow-up is warranted (124).
Downstaging chemotherapy for converting patients to resectable
Bismuth et al. first reported the possibility that chemotherapy may convert non-resectable disease to resectable (127). Since then, there have been many reports of using FOLFOX, FOLFIRI, or regional FUDR chemotherapy to convert disease to resectable (28, 32, 127–130). Approximately 15% of patients treated with systemic chemotherapy, and 30–50% of patients treated with regional chemotherapy are so converted (Table 6).
Table 6.
Results of downstaging chemotherapy.
| Author | n | Chemotherapeutic agent | N (%) converted to resectable | 5-y survival |
|---|---|---|---|---|
| SYSTEMIC CHEMOTHERAPY | ||||
| Bismuth et al. 1996 (127) | 330 | 5-FU, Leucovorin | 53 (16%) | OS: 40% |
| Adam et al. 2001 (32) | 701 | 5-FU, Leucovorin | 95 (13.5%) | OS: 35–60% large tumors, |
| Adam et al. 2004 (294) | 1104 | 5-FU + oxaliplatin (70%) | 138 (12.5%) | OS: 33% |
| 5-FU + both (4%) | DFS: 22% | |||
| Alberts et al. 2005 (130) | 42 | 5-FU, Leucovorin | 17 (40%) | (Median f/u 22 m) |
| Barone et al. 2007 (295) | 40 | 5-FU, Leucovorin | 19 (47.5%) | OS: 62% |
| REGIONAL CHEMOTHERAPY | ||||
| Clavien et al. 2002 (128) | 23 | HAI Floxuridine | 6 (26%) | -- |
| Kemeny et al. 2009 (129) | 49 | HAI Floxuridine | 23 (47%) | (median f/u 26 m) |
Notes: DFS, disease-free survival; 5-FU, fluorouracil; f/u, follow up; HAI, hepatic arterial infusion. OS, overall survival.
Of debate is how long a patient should stay on downstaging chemotherapy before resection. Some advocate for surgery as soon as the patient is resectable (32), while others push for maximum tumor response (median=4 months) (129). The timing can also depend on the need for PVE. It has been shown that chemotherapy does not retard such hypertrophy in a clinically appreciable way and prevents growth of tumors that may be present on the non-embolized side (131). We tend to perform the PVE early in the course of downstaging chemotherapy and wait for complete growth of future remnant (132). The subsequent removal of a very small, atrophied lobe of liver will have negligible impact on the patient’s physiology.
Multidisciplinary management
No discussion of surgical intervention for CRCLM is complete without an emphasis on the importance of multidisciplinary discussions and engagement and collaboration of medical oncology and surgical oncology in the management of these complex patients. Recent studies have shown widely disparate referral patterns among medical oncologists even in the same regions and towns, with some referring patients frequently for liver resection and others referring for resection only rarely (87). While some of this could be due in part to a failure of liver surgeons to come to consensus and more standard practice patterns, part of it also rests in the hands of medical oncologists. Early and frequent involvement and collaboration of medical and surgical oncologists with regular evaluation of staging scans in a tumor board setting is key to successful patient management.
Lung Metastases
More than half of patients who undergo surgical resection for colorectal cancer are expected to have a recurrence of the disease (133). After liver, lung is the second most common site of colorectal metastasis, accounting for approximately 10–15% of metastatic disease (134). Isolated pulmonary metastases are rare, however, ranging from 1.7–7.2% and are more common in rectal cancer patients than in colon cancer patients (135). In most cases, pulmonary metastases occur synchronously with liver metastases.
Five-year survival for stage IV colon cancer is 13.8%. In select patients, however, resection of pulmonary metastases that are either isolated or occur synchronously with liver metastases has been shown to result in durable long-term survival. In a review of institutional outcomes of surgical resection of pulmonary colorectal metastases, overall survival rate ranged from 32–61% at 5 years (Table 7) (133). In patients with synchronous liver and lung metastases, those who had chemotherapy only or had resection of liver metastases only had worse 5-year overall survival compared to patients who had resection of both liver and lung metastases (1.6% vs 13.1% vs. 56.9%, p <0.01) (136). As stated above, overall survival of patients undergoing resection of liver and lung metastases appears comparable to that of patients undergoing resection for isolated liver metastases.
Table 7.
Overall survival among studies reporting outcomes of pulmonary metastasectomy in colorectal cancer patients.
| Study (year) | Number of patients | 5-year survival (%) |
|---|---|---|
| Higashiyama et al. (2003) | 94 | 52 |
| Rena et al. (2002) | 80 | 41 |
| Melloni et al. (2006) | 74 | 44 |
| Saito et al. (2002) | 165 | 40 |
| Pfannschmidt et al. (2003) | 167 | 32 |
| Shiono et al. (2005) | 87 | 61 |
| Vogelsang et al. (2004) | 75 | 27 |
| Okumura et al. (2017) | 785 | 68 |
| Yokoyama et al. (2017) | 59 | 55 |
| Nanji et al. (2018) | 420 | 40 |
Prognostic factors that influence survival in patients undergoing resection for pulmonary metastases include patient demographics such as age and gender, primary tumor characteristics such as initial stage, histology, and colon or rectal origin, as well as characteristics of lung metastases including number and size of lesions, presence of simultaneous liver disease, extent of resection required, and thoracic lymph node involvement. Other reported prognostic factors found on multivariate analysis to affect overall survival include pre-resection CEA value, disease-free interval prior to metastases, and different histologic characteristics of the primary and lung metastases. The data is predominantly retrospective though, and due to the inherent selection bias for metastasectomy, the true survival benefit for resection of pulmonary metastases is difficult to accurately measure. A Randomized Trial of Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC trial) is a phase III trial currently underway that randomizes patients to pulmonary metastasectomy or best medical therapy that will hopefully provide more information for which patients will benefit from surgery (137).
Principles of resection
The first successful pulmonary and chest wall resection for metastatic rib sarcoma disease was performed by Weinlechner in 1882. Before 1970, lung metastasectomy was performed only in highly selected patients. Aberg et al. (1980) published a series on 70 patients who underwent resection for solitary lung metastases from 1961–1978 and used the following criteria: 1) removal of primary tumor; 2) no extrapulmonary disease; 3) only one lung involved; and 4) lung tumor was operable (138). As experience increased, the selection criteria for lung resection became less stringent and in 1991, the International Registry of Lung metastases was established and accrued 5,206 patients from Europe and North America who underwent resection of pulmonary metastases (133). In 1997, it published evidence that complete pulmonary metastasectomy was associated with improved survival. Selection criteria for the registry included “eradication of the primary tumor and absence or effective treatment of metastases in other organs before or concurrent with pulmonary metastasectomy” (139).
According to the National Comprehensive Cancer Network (NCCN) guidelines, criteria of resectability include complete treatment of the primary tumor as well as complete resection of all pulmonary metastases based on number and location of lesions while maintaining adequate pulmonary function. Additionally, the presence of resectable extrapulmonary disease, such as hepatic metastases does not preclude lung resection and select patients may be eligible for reresection (140).
Preoperative evaluation
Similar to hepatic metastasectomy, pulmonary metastasectomy should be considered only in a multidisciplinary discussion among radiologists, surgeons, oncologists, radiation oncologists, and pathologists. Preoperative imaging should be carefully reviewed to determine resectability. When necessary, a preoperative biopsy can be obtained prior to resection, but this is often not necessary when the appearance and growth of lung lesions are highly suggestive of metastatic disease.
The most sensitive and specific imaging modality for detection of pulmonary metastases is high resolution computed tomography (CT) of the chest with thin slices. The sensitivity for detection of pulmonary nodules on CT alone ranges from 34–97%, on positron emission tomography (PET)/CT 66–67.5% and up to 75% for high resolution CT. The specificity of helical CT for identifying pulmonary nodules is 54–93%. The use of thoracotomy for resection of pulmonary metastases has the advantage of meticulously palpating the ipsilateral lung and finding more nodules than on CT scan. However up to 49% of these palpated nodules, when resected, were found to be false positives (benign nodules) (141). In fact, with increasing number of nodules, the concordance of CT-detected malignant lung nodules with histologically confirmed malignant lesions significantly decreased (142). The impact of unresected occult pulmonary metastases on survival is unknown as is the morbidity of resecting benign lesions, especially in the setting of thoracotomy (143).
Preoperative evaluation of patients undergoing resection for colorectal lung metastases must take into consideration functional status of the patient and residual lung volume. Pulmonary function tests should be obtained on all patients, especially those undergoing reresection or those who may have more than one lesion. Those who may become oxygen dependent after complete pulmonary metastasectomy may benefit from nonsurgical treatment modalities or combined surgical resection with alternative ablative therapies for oligometastatic disease, such as stereotactic body radiotherapy or percutaneous ablation (microwave, radiofrequency, or cryoablation).
Pulmonary metastases, when discovered synchronously with the primary colorectal tumor or with extrapulmonary metastases such as in the liver, may be resected simultaneously or using a staged approach. The sequence and coordination of multiple surgeries for the colorectal cancer patient with pulmonary metastases is another reason why a multidisciplinary approach is crucial to favorable patient outcomes.
Treatment
The most common thoracic surgeries performed for colorectal metastases to the lung are wedge resection and segmentectomy (133). In general, surgeons should attempt to resect the minimum amount of lung necessary to completely remove the tumor. Modern video-assisted thoracoscopic surgery (VATS) is being used with increasing frequency, up to 40% according to a survey of thoracic surgeons taken in 2008 by the European Society of Thoracic Surgery, which is likely higher today (144). This has decreased the number of thoracotomies, which some still consider the gold standard for treatment of colorectal metastatic disease to the lung due to the ability to perform bimanual palpation of the ipsilateral lung, with identification and resection of occult metastatic disease not seen on imaging (145). However, to date, no studies have shown that thoracotomy offers a survival benefit over VATS. In fact, a recent Japanese multi-institutional retrospective study using propensity score adjustment compared open surgery to VATS for resection of colorectal metastases and found that patients undergoing VATS had better survival than those undergoing open approach. Additionally, the difference between radiographic nodule number and resected nodule number was insignificant between the two approaches after propensity score matching (146).
With the improvement of CT and PET/CT imaging as well as minimally invasive surgical technique, the benefit of thoracotomy for the resection of occult metastatic disease for colorectal lung metastases is uncertain, especially when weighed against the morbidity of thoracotomy and the potential need for future re-resection. VATS technique varies according to surgeon preference, but is usually performed in lateral decubitus position with 2–3 incisions, allowing for introduction of a thoracoscopic camera, instruments, and occasionally a finger to identify and resect the lesion. The specimen is typically removed using an endoscopic bag and may be sent for frozen section margins.
Robotic-assisted thoracoscopic surgery is also being used with increasing frequency for resection of primary lung cancer. However, its role in resection of pulmonary metastases is not well-defined. The advantages of robotic-assisted surgery over traditional VATS include 3-dimensional visualization and wristed instruments, which greatly facilitates lymph node dissection (discussed below). The main disadvantage is the loss of haptic feedback, which can make identification of a pulmonary nodule difficult. However, the localization of pulmonary nodules using methylene blue, delivered either by CT-guidance or navigational bronchoscopy followed by robotic resection, has been reported as safe and effective in the treatment of primary lung cancer (147). We have found this approach can help us identify small metastases more efficiently.
The posterolateral thoracotomy is the most commonly performed open approach to pulmonary metastasectomy and allows ample exposure to the ipsilateral lung for bimanual palpation and resection of metastatic disease. We typically use a muscle-sparing thoracotomy. For bilateral metastatic disease, surgical approaches include median sternotomy and sequenced thoracotomy (148).
In unresectable disease, or multifocal disease where resection would compromise pulmonary function, the NCCN guidelines state that various ablative techniques such as radiofrequency ablation, cryoablation, or microwave ablation may be used alone or in conjunction with surgery. Furthermore, stereotactic body radiation therapy may also be used as an alternative to surgery (8). The discussion and coordination of these procedures with and around surgery is again another reason why the treatment of these patients is best decided in the setting of a multidisciplinary tumor board.
Management of nodal disease
Thoracic lymph node metastases occur in 10 to 32% of colorectal cancer patients who undergo pulmonary metastasectomy and is considered a poor prognostic factor with a 5-year survival of 0 – 34% compared to 39% - 71% for patients without thoracic lymph node involvement. Lymph node examination during pulmonary metastasectomy includes sampling or complete dissection of at least three N2 stations and is selectively performed and variably reported in the literature (133). The survival benefit of thoracic nodal examination during pulmonary resection for colorectal metastases is unknown, but it is generally recommended for the sake of completing staging and to determine prognosis to guide additional therapies (149). Techniques include open, traditional VATS, as well as robot-assisted. As mentioned previously, the robot is an excellent platform for mediastinal lymph node dissection.
Conclusion
Many retrospective studies have demonstrated a survival benefit of resecting pulmonary metastases in colorectal cancer patients; however, no prospective studies have been done comparing pulmonary metastasectomy to best medical therapy. Preoperative workup for resection of colorectal metastasis to the lung includes discussion in a multidisciplinary context, preoperative CT or PET/CT, pulmonary function tests, and coordinating for synchronous or sequential surgery if resectable extrapulmonary metastases are present. More thoracic surgeons are turning to less invasive VATS and robot-assisted approaches to perform wedge resections and segmentectomies. Nonsurgical therapies are being used in lieu of or in combination with surgery. Nodal disease in the setting of colorectal pulmonary metastases is a poor prognostic factor and thoracic lymph node evaluation is indicated for completing staging and determining prognosis.
Peritoneal Cytoreduction For Colorectal Cancer Carcinomatosis
Spread of cancer to the peritoneal surfaces – carcinomatosis – is common and is negatively associated with survival of patients with colorectal cancer (150, 151). This process generates a lot of suffering for the patients and caregivers by causing malignant bowel obstruction, weight loss, obstructive nephropathy and symptomatic ascites. Unlike other sites of metastases, carcinomatosis is frequently symptomatic causing chemotherapy interruptions and repeated hospitalizations.
The true incidence of carcinomatosis is unknown because current imaging technologies are unable to detect small peritoneal deposits. The best evidence comes from an autopsy study of 5,817 patients who were diagnosed with colorectal cancer and underwent an autopsy between 1991 – 2010 in the Netherlands (150). Based on this study the overall incidence of peritoneal carcinomatosis was 25%. Of these, 30% had metastases limited to peritoneal cavity and the rest had additional organ involvement. It is known that patients with peritoneal metastases have a higher adjusted risk of death compared to patients with non-peritoneal metastases when given modern chemotherapy regimens (Hazard ratio 1.32, 95% Confidence Interval 1.15 – 1.50) (152). These observations underscore the need for improvement in detection and treatment of peritoneal carcinomatosis in colorectal cancer patients.
Clinical presentation of peritoneal carcinomatosis
The majority of peritoneal carcinomatosis is incidentally discovered on staging CT or MRI scans or at the time of surgical exploration for primary tumor resection. At times, symptoms from carcinomatosis prompt the diagnosis of malignancy. Signs and symptoms from carcinomatosis are not only dictated by the burden of disease but also the location of disease (153). Typical symptoms include: abdominal distension leading to discomfort from ascites; weight loss; fatigue; gastroesophageal reflux; early satiety; nausea and vomiting from bowel obstruction; peripheral edema or anasarca from protein calorie malnutrition; and shortness of breath from ascites. Occasionally patients present with obstructive nephropathy. Diagnosis of patients with symptomatic carcinomatosis can be delayed if the patients do not develop a bowel obstruction. Many times, these patients are thought to have central obesity and are initiated on dietary modifications. Patients with poor appetite and enlarging abdominal girth should raise the concern for peritoneal carcinomatosis.
Risk factors for peritoneal carcinomatosis
Risk of peritoneal carcinomatosis is dependent on several clinical and pathologic factors. A large population-based study of patients in the Stockholm County Council Registry demonstrated that of the 11,124 patients with colorectal cancer from 1995–2007, 4.3% developed synchronous and 4.2% developed metachronous peritoneal carcinomatosis (154). Independent risk factors for metachronous peritoneal carcinomatosis included: right-sided colon cancer (Odds Ratio (OR) 1.77), T3 stage (OR 3.8), T4 stage (OR 10), N0 with <12 lymph nodes removed (OR 1.74), N1 with 12+ lymph nodes removed (OR 2.1), N1 with <12 nodes removed (OR 3.8), N2 with >12 lymph nodes removed (OR 4.7), N2 nodal status with <12 lymph nodes removed (OR 7.4), R1 resection (OR 2.0) and R2 resection (OR 2.7). Interestingly, older patients had a lower risk of metachronous peritoneal carcinomatosis (OR 0.69). In an analysis of institutional database from Singapore General Hospital, a total of 3,019 patients with colorectal cancer were evaluated (155). In this study, additional independent risk factors for metachronous peritoneal carcinomatosis included perineural invasion (OR 1.6) and venous invasion (OR 1.6). A case-control study from Massachusetts General Hospital identified obstruction (OR 7.3) and perforation (OR 5.5) to be associated with development of peritoneal carcinomatosis. Finally, adenocarcinomas predominantly metastasize to the liver, while mucinous and signet ring histologies more frequently had peritoneal metastases (150). Collectively, these patients are regarded as high-risk for development of peritoneal carcinomatosis and some of these variable have been used as inclusion criteria for prophylactic treatment strategies as discussed later (156).
Detection of peritoneal carcinomatosis
Peritoneal carcinomatosis is most commonly diagnosed using cross-sectional imaging during staging and surveillance. These modalities include contrast-enhanced multi-detector computed tomography (MDCT), dynamic contrast-enhanced and diffusion weighted MRI (DWMRI) and [18F]-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scans. Current imaging technologies are sub-optimal for detecting sub-centimeter peritoneal tumor nodules and therefore often underestimate the burden of disease. However, some features of advanced peritoneal dissemination can be readily detected such as: omental caking; scalloping of the diaphragm; mucinous ascites; and peritoneal nodules >1 cm.
In a study of patients with colorectal cancer carcinomatosis, Koh et. al. identified that the accuracy of MDCT depends on lesion size and location (140). For instance, MDCT detection of parietal peritoneal disease (i.e. non-small bowel/mesenteric disease) approaches 54–67%, whereas the detection of visceral peritoneal disease (i.e. small bowel/ mesenteric disease) is low (8–17%). Similarly, the MDCT detection of peritoneal nodules was strongly associated with lesion size (11% for <0.5 cm, 37% for 0.5–5cm, and 94% for >5 cm) (140). In addition, MDCT underestimates the size of the lesion in 33% of the nodules and overestimates in 7% of nodules compared to operative assessment.
The utility of DW-MRI has been evaluated in multiple small institutional studies in comparison to MDCT (157–160). However, none of the studies are restricted to colorectal cancer carcinomatosis patients. In a recent study, it was noted that the benefit of adding DW-MRI to MDCT was marginal (161). For instance, the detection of tumor implants on parietal peritoneum was marginally better for DW-MRI + MDCT vs. MDCT alone (74% vs. 63%, respectively). However, there was no difference in the detection of visceral peritoneal implants (DW-MRI + MDCT: 44% vs. MDCT: 41%). The relationship of lesion detection rate with DW-MRI and size of peritoneal implant is less clear but likely matches that of MDCT. Motion artifacts, peristalsis and wide variation in imaging protocols among institutions make it difficult to draw definite conclusions.
FDG-PET scans lack the ability to detect peritoneal implants less than 1 cm. However, when a PET-avid lesion is identified it is highly likely that it represents carcinomatosis. Therefore, PET scans have been used in clinical practice when cross-sectional imaging with MDCT or DW-MRI has been inconclusive. A recent meta-analysis of 7 studies included 513 patients with peritoneal carcinomatosis who underwent a FDG-PET scan with or without MDCT (162). While a remarkably high pooled estimate of sensitivity (72%), specificity (97%), and accuracy (88%) was noted, there was marked heterogeneity between the studies. The association of size of peritoneal implant and the ability to detect the lesion on FDG-PET was not evaluated. Further, FDG-PET is frequently non-avid in mucinous peritoneal carcinomatosis. An updated meta-analysis of 14 studies with 671 patients presented similar results (163). Further studies comparing these modalities, stratified by location and size of the peritoneal lesions are needed.
The limitations of imaging technologies has prompted the use of laparoscopy and second-look laparotomy to allow: 1) early detection of carcinomatosis in high-risk imaging negative patients and 2) accurate assessment of carcinomatosis burden in imaging positive patients to determine resectability. Exploratory laparotomy is the gold-standard for the assessment of carcinomatosis burden but requires a generous abdominal incision and a prolonged recovery time. In comparison, diagnostic laparoscopy can be performed in an outpatient setting but the visualization of peritoneal cavity can be limited especially in patients with high BMI, mucinous ascites and lack of intra-abdominal domain.
For detection of carcinomatosis in high-risk imaging-negative patients, diagnostic laparoscopy is a valuable strategy. A multi-center study of laparoscopic assessment of peritoneal carcinomatosis index (BIG-RENAPE) was recently published (164). In this study 50 patients who were at high risk of peritoneal carcinomatosis and had negative preoperative imaging underwent diagnostic laparoscopy followed by exploratory laparotomy. In total, 34 patients (68%) had peritoneal carcinomatosis on laparotomy. Laparoscopy detected carcinomatosis in 28 patients and missed it in 6 (12%). Laparoscopy was not feasible in 6 (12%) and was satisfactory in only 52% of the patients. Of the patients that underwent successful laparoscopy (n=44), peritoneal carcinomatosis index (PCI) was underestimated in 25% of patients (164). Because these patients have relatively limited burden of carcinomatosis, however, under-estimation of PCI is unlikely to impact decisions on surgical resectability.
For assessment of carcinomatosis burden to determine resectability, several institutional studies have demonstrated excellent correlation between laparoscopic and open assessment of PCI. More importantly, diagnostic laparoscopy avoided exploration in 7–41% of patients not amenable to complete cytoreduction (well summarized in Seshadri et al. (165)). In one study the rate of incomplete cytoreduction decreased from 44% before introduction of routine laparoscopic assessment to 30% after (166). The added value of laparoscopy to cross-sectional imaging lies in the ability to accurately assess mesenteric carcinomatosis and involvement of the porta hepatis which often precludes complete cytoreduction.
Treatment and outcomes
Treatment for isolated peritoneal carcinomatosis can be divided into three distinct categories based on assessment of peritoneal carcinomatosis burden: 1) No evidence of carcinomatosis on imaging but high risk of developing metachronous carcinomatosis; 2) Carcinomatosis that is amenable to complete cytoreduction; 3) Unresectable carcinomatosis.
1). Imaging negative high-risk patients:
Aggressive management with complete surgical extirpation in metastatic colorectal cancer has been debated for a long time. One of the earliest studies that questioned the aggressive pursuit of clinically non-evident metastatic disease was the CEA Second Look Trial. This was a randomized controlled trial of CEA-prompted reoperations for recurrent colorectal cancer. Patients were randomized to standard of care or second look laparotomy and cytoreduction (including liver or peritoneal implants). While this trial was started in the 1980s, it was not published until 2014 (167). In this trial, the overall survival of the two groups was not different. Over the course of the last three decades, there have been tremendous improvements in imaging modalities and standard of care chemotherapy. Based on the landmark MOSAIC trial, oxaliplatin-containing chemotherapy regimen has now become standard of care for stage III colon cancer patients (168, 169). Of note, these would be the patients at high risk for not only distant but also peritoneal recurrence. Unfortunately, during this time there have been no new trials evaluating the role of second look laparotomy and cytoreduction for imaging negative disease until recently (Table 8). The PROPHYLOCHIP trial results were recently reported at the American Society of Clinical Oncology 2018 meeting (170). In this trial, patients at high risk of developing carcinomatosis (defined as minimal carcinomatosis resected with the primary, or history of ovarian metastases, or perforated primary tumor) completed 6 months of standard adjuvant therapy. They were then randomized to (1) surveillance, (2) systematic second-look surgery plus heated intraperitoneal chemotherapy (HIPEC) using oxaliplatin. For patients that underwent laparotomy, about half had peritoneal disease. This trial demonstrated that a pro-active strategy including a systematic second-look surgery plus HIPEC failed to improve survival, in comparison to an adequate surveillance. Results from other trials with varying inclusion criteria are awaited.
Table 8.
Ongoing and completed clinical trials for peritoneal carcinomatosis - prophylactic intent.
| Prophylactic Intent Trial Title | Status | Interventions | Locations |
|---|---|---|---|
| APEC: Adjuvant Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Versus no HIPEC in Locally Advanced Colorectal Cancer | Recruiting | Arm 1: Standard adjuvant systemic chemotherapy (mFOLFOX6/CapeOx/sLV5FU2/Cape) | China |
| Arm 3: Hyperthermic intraperitoneal chemotherapy (HIPEC) with oxaliplatin | |||
| Pilot Study: Prophylactic Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Colorectal Cancers at High Risk of Developing Peritoneal Metastases | Recruiting | Arm 1: HIPEC with Mitomycin C at the time of primary resection | Singapore |
| Randomized Phase 2 Study Comparing Second Look Laparoscopy to Standard Follow up in Patients With no Radiologic Evidence of Disease at 6 Months After Complete Resection of Colorectal Mucinous Carcinoma | Recruiting | Arm 1: Second look laparoscopy; PCI >20 systemic therapy; PCI<20 cytoreduction and HIPEC (IV: 5-FU/LV, IP: Oxaliplatin) | Italy |
| HIPECT4: Clinical Trial to Evaluate Safety and Efficacy of Hyperthermic Intra-peritoneal Chemotherapy (HIPEC) With Mitomycin C Used During Surgery for Treatment of Locally Advanced Colorectal Carcinoma | Recruiting | Arm 1: HIPEC with Mitomycin C followed by standard adjuvant chemotherapy | Spain |
| COLOPEC: Adjuvant HIPEC in High Risk Colon Cancer | Active, not recruiting | Arm 1: HIPEC (IP - Oxaliplatin; IV - 5FU/LV) followed by standard adjuvant chemotherapy | Netherlands |
| Adjuvant HIPEC to Prevent Colorectal Peritoneal Metastases in High-risk Patients | Completed | Arm 1: HIPEC with Cisplatin and Mitomycin | Italy |
| PROPHYLOCHIP: Trial Comparing Simple Follow-up to Exploratory Laparotomy Plus “in Principle” (Hyperthermic Intraperitoneal Chemotherapy) HIPEC in Colorectal Patients | Completed | 6 months of Adjuvant therapy followed by: | France |
| Arm 2: Systematic second look and HIPEC (oxaliplatin) | |||
| Randomized Multicentric Phase III Trial Comparing Simple Surgery to Surgery Plus HIPEC (Hyperthermic Intraperitoneal Chemotherapy) With MMC in Colorectal Patients Who Have a High Risk of Developing Colorectal Peritoneal Carcinomatosis | Recruiting | Arm 1: Surveillance | China |
| Both arms will then receive 6 months of adjuvant therapy | |||
| Feasibility of Intraperitoneal Chemotherapy in the Surgical Treatment of Colon Cancer pT4 | Recruiting | Arm 1: Exploratory Laparotomy and Mitomycin C | Russia |
| Adjuvant Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in Resected High Risk Colon Cancer Patients - The PIPAC-OPC3 CC Trial | Recruiting | Single arm: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) treatments with oxaliplatin after primary resection and standard adjuvant chemotherapy (if indicated) for colon cancer | Denmark |
Notes: CapeOx, capecitabine + oxaliplatin; 5-FU, fluorouracil; LV, leucovorin.
2). Carcinomatosis amenable to cytoreduction:
It has long been recognized that untreated carcinomatosis is uniformly fatal with a historical median survival of 6 months (155). Surgical treatment of carcinomatosis from gastrointestinal cancers including colorectal cancer was championed by Dr. Paul H. Sugarbaker (171). Based on Dr. Sugarbaker’s original report of selected 47 patients with limited carcinomatosis burden treated with cytoreduction followed by intraperitoneal chemotherapy that could achieve long-term survival, there have been multiple institutional programs around the world that have evaluated this strategy and have achieved similar results. Despite growing experience, it remained unclear if the benefit in these retrospective analyses is a result of patient selection, aggressive cytoreduction, hyperthermia or chemotherapy. In the first of its kind, Veerwal et al. conducted a randomized trial of cytoreduction/HIPEC + systemic chemotherapy (5- fluorouracil/leucovorin) vs. systemic chemotherapy (5- fluorouracil/leucovorin) and palliative surgery (172, 173). This trial demonstrated that the cytoreduction and HIPEC nearly doubled the median survival of colorectal carcinomatosis patients compared to systemic therapy alone (22.3 months vs. 12 months, p=0.032). However, around this time, oxaliplatin and irinotecan were introduced in chemotherapy regimens of metastatic colorectal and were found to be significantly superior to 5-fluorouridine with leucovorin alone questioning the utility of cytoreduction/HIPEC (174).
Despite lack of evidence from randomized clinical trials, cytoreductive surgery and HIPEC has remained a popular strategy in select centers with expertise. A multi-institutional French study by Elias et al. included 523 selected patients with peritoneal carcinomatosis from 23 centers (175). In this retrospective analysis, 5-year survival of 27% was noted. More importantly, this study along with the multi-institutional study by Glehen et al. demonstrated that the mortality rate (3–4%) and morbidity rate (23–31%) of cytoreductive surgery/ HIPEC was acceptable (176). The continued skepticism and opposition from medical oncologists prompted a randomized clinical trial that included patients with peritoneal carcinomatosis with a PCI <25. The patients were randomized to systemic chemotherapy and cytoreduction with vs. without HIPEC (177). The results of this trial were recently reported and demonstrated that there was no overall survival benefit of adding HIPEC to cytoreduction in the context of modern systemic chemotherapy. In addition, this trial demonstrated a remarkable median survival of patients undergoing cytoreductive surgery of 41 months. This compares favorably to the median overall survival of 30 months reported in the modern systemic therapy trials in combination with targeted therapy (178). It is important to note however, that there is no evidence from a randomized trial that confirms the additive benefit of cytoreductive surgery to modern systemic therapy. Ongoing and recently completed therapeutic intent clinical trials are summarized in Table 9.
Table 9.
Ongoing and completed clinical trials for peritoneal carcinomatosis - therapeutic intent.
| Therapeutic Intent Trial Title | Status | Interventions | Locations |
|---|---|---|---|
| HIPEC and Systemic Chemotherapy in Unresectable Peritoneal Metastases From Colorectal Cancer | Recruiting | Single Arm: HIPEC with Raltitrexed followed by systemic (Oxaliplatin and Capecitabine) then second look surgery with possible cytoreduction | China |
| HIPEC Using High Intra-abdominal Pressure | Completed | HIPEC with cisplatin | Italy |
| Immunotoxin in Peritoneal Carcinomatosis-ImmunoPeCa Trial | Completed | Arm 1: Low intra-abdominal pressure 8–12mmHg | Norway |
| ICARuS Post-operative Intraperitoneal Chemotherapy (EPIC) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) After Optimal Cytoreductive Surgery (CRS) for Neoplasms of the Appendix, Colon or Rectum With Isolated Peritoneal Metastasis | Recruiting | Arm 2: High intra-abdominal pressure 18–22mmHg” | United States |
| Comparing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) Using Mitomycin-C Versus Melphalan for Colorectal Peritoneal Carcinomatosis | Recruiting | Single arm: Cytoreduction and HIPEC followed by MOC31PE Immunotoxin (targeting EpCAM positive cells) | United States |
| The Influence of Perioperative Administration of Dexmedetomidine on Inflammation Response and Postoperative Outcomes in Patients Undergoing Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Cytoreductive Surgery; Double Blind Randomized Controlled Trial | Recruiting | Arm 1: Cytoreduction then HIPEC with Mitomycin C | South Korea |
| Thalidomide in Treating Patients Who Have Undergone Surgery and Chemotherapy for Cancer That Has Spread Throughout the Abdomen Due to Colorectal Cancer or Appendix Cancer | Completed | Arm 2: Cytoreduction then EPIC with FUDR and Leucovorin | United States |
| CAIRO6 trial: Perioperative Systemic Therapy and Surgery Versus Surgery Alone for Resectable Colorectal Peritoneal Metastases. | Recruiting | Arm 1: Cytoreduction then HIPEC with Mitomycin C | Netherlands |
| PRODIGE 7: A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC) | Completed | Arm 2: Cytoreduction then HIPEC with Melphalan | France |
| Histopathological Response to FOLFOXIRI + Bevacizumab in Peritoneal Metastasis From Colorectal Cancer | Recruiting | Both arms get Cytoreduction then HIPEC. | Austria |
Note: FUDR, floxuridine
3). Unresectable Carcinomatosis:
For patients with unresectable disease confined to the peritoneal cavity, combination chemotherapy with or without targeted therapy has become the standard of care (152). However, patients with peritoneal carcinomatosis are under-represented in metastatic colorectal cancer trials leading to limitations in extrapolating data from these trials to patients with peritoneal carcinomatosis (151). Alternatively, multiple observational studies have demonstrated that incomplete cytoreduction with gross residual disease does not offer survival benefit in this setting and is not recommended (175, 176). Increased purposeful representation of peritoneal carcinomatosis patients is needed in phase 3 and 4 systemic trials because these patients fare worse than those patients without peritoneal carcinomatosis (151). One explanation is the higher proportion of patients with an aggressive BRAF mutant genotype. The other possibility is that these patients are more likely to be symptomatic leading to hospitalizations, procedures, therapy interruptions and dose reductions. More recently, pressurized intraperitoneal aerosol chemotherapy (PIPAC) has been developed and evaluated for unresectable carcinomatosis patients (179). The premise of this therapy is based in the notion that aerosolized chemotherapy has more even distribution and in pre-clinical studies is shown to have enhanced penetration depth. Typically, oxaliplatin has been used for colorectal carcinomatosis and repeat procedures are feasible. The efficacy of PIPAC needs to be further evaluated in prospective randomized trials before wide adoption.
Conclusions
Peritoneal cytoreduction has become safe in expert hands. In patients with completely cyto-reducible disease, there is also increasing evidence that it may be efficacious. Whether HIPEC will prove to be useful as an adjunct to surgical cytoreduction awaits further randomized trials that stratify additional levels of tumor burden. In the meantime, PIPEC, peritoneal treatment with novel agents including oncolytic viruses, nanoparticles, and radioimmune therapies are actively being pursued.
Treatment of Bone Metastases
The skeletal system is the most common site of metastatic disease in cancer patients (180, 181). In the past, bone metastases from colorectal cancer were infrequently seen (182). Due to improved systemic treatments for metastatic colorectal cancer, and surgical treatments for liver metastases, patient survival has increased. Longer patient survival has led to an increase in the previously more rarely seen complications of the disease and specifically an increasing prevalence of metastatic bone tumors (183). Most patients with bone metastases will develop symptoms over the course of their disease, including pain, instability, nerve compression, hypercalcemia and pathologic fracture all of which significantly contribute to morbidity and decreased quality of life (184, 185). Therefore, it is imperative that the oncology community develops new methods for treating these patients.
When patients present with widespread bone metastases, standard treatment includes chemotherapy and bisphosphonates. However, there are several instances where local therapy for bone metastases may provide additional benefit to patients. Focal pain is the most common presenting symptom in patients with metastatic bone lesions (184). Tumor cells produce a variety of cytokines and tumor derived factors stimulate bone resorption, weakening the effected bone, and also leading to structural damage, periosteal irritation and nerve compression. Additionally, these tumor factors can sensitize adjacent nerves and potentiate painful stimuli (186–189). Local tumor control of osseous metastases can arrest this process by destroying the inciting cancer cells and allowing bone remodeling with functional restoration. Additionally, in the absence of bone related symptoms, certain situations may still warrant local tumor control. Up to 35% of patients with metastatic colorectal cancer may demonstrate a mixed response to systemic chemotherapy, likely due to interlesional genetic heterogeneity (190). In situations where the bulk of the patient’s disease burden is controlled on systemic therapy, if metastatic progression is restricted to a limited number of bone lesions, local therapy can provide a means of disease control and obviate the need to alter systemic treatment.
External beam radiation therapy (EBRT) has traditionally been the first line treatment for most patients with symptomatic osseous metastases, with multiple randomized trials demonstrating safety and efficacy (191, 192). EBRT also has the benefit of being noninvasive, operator independent, and applicable to most skeletal locations. However, there are several limitations to radiation therapy. Firstly, for patients to undergo radiation therapy, they must be able to tolerate relatively long periods of immobility while therapy is delivered. This may be particularly challenging in patients with painful osseous metastases. Additionally, between 20–40% of patients fail to achieve optimal pain relief from palliative external beam radiotherapy (192, 193). Moreover, even for those that ultimately achieve adequate pain relief, the onset and durability of pain relief is variable, and the full benefit of radiation therapy may not be achieved for up to 6 weeks following treatment. Finally, the response to treatment typically wanes over time, with nearly half of the patients who initially respond to EBRT ultimately going on to develop a painful relapse by 18 weeks (194).
A variety of alterative modalities now exist for the local treatment of bone metastases. Broadly, these treatments may be referred to as “image guided ablative therapies.” These treatments encompass a variety of technologies that utilize medical imaging to focally target and destroy areas of tumor involvement. Tumor ablation has become a standard treatment for a variety of primary and metastatic tumors. It provides high rates of local tumor control and may be curative in several early stage tumors such as the lung, liver, and kidney (195–201).
Ablation technologies
A. Radiofrequency ablation
Radiofrequency ablation (RFA) is a method of heat-based thermal ablation. In RFA, a probe is placed within a volume of tissue and used to generate a rapid alternating electrical current. As the current travels through the tissue, local frictional agitation generates heat around the RF probe resulting in a rise in tissue temperature. When temperatures reach a critical threshold, cells undergo coagulative necrosis and cell death (202). This technology has been utilized successfully in a variety of tissue types and produces reliable and predictable ablation zones (203). This method has been proven highly clinically effective and comes in a variety of commercially available platforms (203).
Several technical limitations exist when employing RFA in the treatment of osseous lesions. RFA relies on both electrical and thermal conduction to cause tissue necrosis. Therefore, variations in tissue characteristics, such as tissue water content and blood flow, can greatly impact radiofrequency ablation zones (204, 205). This is particularly important in bone, due to its inherently low electrical conductivity. Additionally, vascular soft tissue lesions within bone may cause local cooling effects that can limit thermal conduction. Both of these factors may result in less predictable and thus less effective ablation zones (206). Despite these limitations, RFA has demonstrated efficacy in the treatment of a variety of painful bone disorders and is a viable first line treatment option for certain primary bone lesions (207–210). Several clinical studies have also demonstrated it to effectively reduce the pain from osseous metastatic disease, reduce opioid use, and provide durable symptom reduction. Additionally, RFA has been shown to provide pain relief in patients that have failed other focal therapies, such as external beam radiotherapy, and may be effectively repeated in cases of recurrent pain after initial successful ablation (211–213). In cases where the structural integrity of the bone is compromised, RFA may be effectively combined with percutaneous cement injection to stabilize the bone and help prevent future fracture (214–216).
B. Microwave ablation
Microwave ablation (MWA), like RFA, causes tissue destruction by targeted heating of tissue, resulting in coagulative necrosis and cell death. However, the mechanism of tissue heating with microwave ablation is unique and offers several potential clinical benefits over other forms of heat-based ablation (217). Microwave spectrum energy causes local tissue heating by means of frictional energy generated from water molecule oscillation. Due to this, MWA does not rely on electrical conductivity, and is therefore much less susceptible to local tissue characteristics. Furthermore, MWA relies primarily on active tissue heating, rather than tissue conduction, to produce cellular death. This can allow for more uniform heating, higher temperatures, and larger ablation zones (218, 219). Additionally, unlike RFA, where multiple applicators may cause electrical interference, multiple MW probes may be combined to produce larger, confluent ablation zones (220–222).
The advantages of MWA may be particularly useful in certain types of metastatic bone lesions. Given its ability to generate large ablation zones, MWA may be well suited to treat larger lesions, where multiple overlapping RFA zones would be needed (219, 223). Additionally, all hyperthermic ablations may be attenuated by local tissue cooling caused by medium and large sized blood vessels within the ablation zone (205). MWA, however, due both to its ability to achieve higher temperatures and its lesser reliance on thermal conduction, can often overcome these cooling zones and achieve adequate ablative temperatures surrounding larger blood vessels (219, 224). Blastic osseous lesions also present a challenge for RFA. Sclerotic bone is a poor electrical conductor and high areas of electrical impedance within blastic bone lesions may limit the propagation of RF energy and hinder effective ablation. MW energy, on the other hand, propagates through all biologic tissues and can effectively treat both lytic and blastic lesions. Finally, MWA, due in large part to its reliance on active heating, as opposed to tissue conduction, can achieve similar sized ablations in substantially less time than required of other ablative modalities (218, 219, 223, 225).
Despite these potential advantages, careful consideration must be taken prior to MWA. To date, only limited data exists regarding MWA of bone lesions. Several retrospective series have demonstrated comparable efficacy to RFA, with symptom improvement in greater than 90% of patients (223, 225). However, no prospective trials have evaluated MWA in comparison to RFA for this purpose. Furthermore, due to its ability to achieve higher temperatures in shorter amounts of time, there is a greater risk of potential non-target ablation resulting in thermal injuries to surrounding tissues.
C. Cryoablation
In contradistinction to the hyperthermic modalities, cryoablation causes cellular death by means of tissue freezing and the creation of intracellular and extracellular ice. In cryoablation, temperatures beyond −40°C are created surrounding the ablation probe by pumping a gas, typically argon, through the probe to its distal end, where it is allowed to expand rapidly. This expansion causes a rapid decrease in temperature within the probe by means of the Joule-Thomson effect. The low temperature then propagates to the surrounding tissue through thermal conduction (202). As the cells freeze, cell membranes are damaged. Additionally, the ice causes osmotic changes in the extracellular space that results in cell dehydration. Both of these ultimately contribute to a well-defined area of cell death (226, 227).
Cryoablation has demonstrated efficacy and cost-effectiveness in the treatment of a variety of primary and metastatic tumors (198, 199, 228). In several studies of musculoskeletal metastases, cryoablation has repeatedly demonstrated the ability to provide local tumor control, durable pain relief and results in significant reduction of adjunctive narcotic use (229–232). There are several potential advantages of cryoablation for the treatment of musculoskeletal metastases. First, unlike RF and MW ablation, the lethal zone of tissue destruction, or “lethal ice”, is readily visible with conventional imaging modalities, such as CT and ultrasound. On CT, this is seen as an ovoid hypodense zone. This visualization allows more confident prediction of ablation zones and more reliable protection of adjacent critical structures, such as bowel and nerves. Additionally, cryoablation allows the operator to employ multiple probes simultaneously. This allows for both larger ablation zones and the ability to design customized ablation zones by placing the probes in varied and unique configurations that optimize tumor coverage (233). Furthermore, the hyperthermic methods of ablation have been shown to induce short-term irritation of local sensory nerves, which may potentially result in initial, acute worsening of pain (213, 234). However, this phenomenon appears to be much less common with cryoablation, where both acute and chronic nerve damage appears to be more rare (235, 236). Consequently, patients undergoing cryoablation may potentially receive more immediate pain relief and require a shorter post-procedure hospitalization. This benefit may also allow ablations to be performed under a lower level of sedation, which can be beneficial in patients with medical comorbidities (233, 237–239).
Nonetheless, there are several limitations to cryoablation as well. Unlike RFA or MWA, vessels within the ablation zone are not coagulated by the ablation itself, potentially leading to higher rates of post-procedure bleeding (232). Additionally, bleeding risk is further complicated by the typically larger needle diameter of the cryoprobes compared to that of either RFA or MWA (202). Also, unlike RFA or MWA, cryoablation requires a two-staged ablation protocol, with two freeze cycles separated by a period of tissue thawing. Due to this, ablation times for cryoablation typically exceed two times that of the hyperthermic modalities (233).
D. High intensity focused ultrasound
High intensity focused ultrasound (HIFU) is a relatively new technology being utilized for bone and soft tissue ablation. This modality has demonstrated great promise, achieving high rates of symptom palliation and local tumor control in treating both primary and metastatic bone and soft tissue tumors (240–243). Unlike the previously described modes of ablation, HIFU is performed completely non-invasively. In this form of ablation, high energy ultrasound waves are focused on a target volume of tissue producing local molecular friction and acoustic cavitation. This in turn causes local heating with temperatures reaching 65–100° C within 1 second (244–246).
There are several key advantages of HIFU compared with the other forms of ablation. Firstly, unlike the other forms of ablation, HIFU is performed completely non-invasively, thereby eliminating the procedural-related risk of bleeding or unintentional organ injury that exists from percutaneous needle placement. Additionally, unlike the previously described forms of ablation, HIFU is primarily performed using MRI guidance. MRI provides superior soft tissue contrast compared to CT or ultrasound and can therefore often better delineate and quantify soft tissue tumor extension. Therefore, by utilizing MRI guidance, HIFU allows real time assessment of the lesion during ablation. Additionally, certain MRI systems allow for accurate MR thermometry, which can be used to provide real-time monitoring of the ablation zone (247).
Despite these advantages, several issues limit the widespread use of HIFU for the treatment of painful osseous lesions at this time. If tumors closely abut critical organs or structures, motion during treatment may alter the position of the targeted lesion and potentially interfere with safe delivery of the ultrasound waves to the targeted lesion (241, 247). In addition to this, each application of HIFU can only encompass a small volume of tissue. Therefore, treatment of larger lesions can be quite cumbersome, requiring sequential treatments of multiple small volumes of tissue, resulting in long ablation and anesthesia times (241, 243, 248).
Conclusion
Osseous metastases from colorectal cancer remain an infrequent occurrence, often present late in the course of the disease, and typically portend a poor prognosis. However, as treatment strategies evolve and survival improves, increasingly patients may present with symptomatic or limited bone lesions that warrant local therapy. A wide variety of treatment strategies now exist to address these lesions and should be incorporated into the multidisciplinary care of this population.
Distant Lymph Node Metastases
Lymphadenectomy is critical to the appropriate treatment of patients with primary colorectal cancer. Evaluation of the Intergroup INT-0089 trial demonstrated that patient survival increased as more lymph nodes were analyzed, even when no lymph nodes were involved (249). Lymph node involvement beyond the mesenteric basin is considered stage IV metastatic disease. Salvage surgery had previously been avoided; however, as chemotherapy and radiation therapy improve, the question of how best to address metastases to distant lymph nodes has again been raised.
Peri-hepatic lymph nodes
Resection of colorectal liver metastases is now considered within the standard of care. The best method for management of peri-hepatic lymph nodes, however, is less clear. An earlier study from Memorial Sloan Kettering Cancer Center examined 59 patients who had peri-hepatic lymph node clearance during liver resection for metastatic colorectal cancer from 2002–2004, 50/59 (85%) of whom had prior chemotherapy (Table 10). The mean number of peri-hepatic nodes harvested was 3, and 22/59 (37%) had metastases identified within these nodes. More than half of the cases of metastatic disease were missed on hematoxylin and eosin (H&E) staining, and were identified by immunohistochemical (IHC) analysis alone. Further, only 12/22 positive lymph nodes were considered “suspicious” for disease involvement by the attending surgeon. Patients with positive peri-hepatic lymph nodes by H&E had lower 3-year overall survival (25%), whereas those detected by IHC had similar outcomes to those with negative nodes (3-year overall survival 76% and 75%, respectively) (250).
Table 10.
Studies examining peri-hepatic lymphadenectomy in patients undergoing liver resection for metastatic colon cancer
| Study (n=lymphadenectomy patients) | Neoadjuvant chemo | # LN harvested (range) | % with (+)PHLN | OS (−) PHLN | OS (+) PHLN |
|---|---|---|---|---|---|
| Bennett et al. 2008 (n=59) | 50/59 (85%) | 3.2 (1–11) | 22/59 (37%) | 3-year 75% | 3-year 25% (H&E+), 76% (IHC+) |
| Okuno et al. 2017 (n=174) | 154/174 (89%) | NR | 54/174 (31%) | 71 months | 13.8 months |
| Nanji et al. 2016 (n=103) | 22/103 (21%) | 2.2 (1–15) | 30/103 (29%) | 46 months | 25 months |
| Bradatsch et al. 2016 (n=20) | 15/20 (75%) | 5 (NR) | 0/20 (0%) | NR | NA |
| Pindak et al. 2017 (n=59) | 0/59 (0%) | 4.6 (0–13) | 7/59 (12%) | 67 months | 30 months |
Notes: LN, lymph node; NA, not applicable; NR, not reported; OS, median overall survival; PHLN, peri-hepatic lymph node
A similar more recent study from MD Anderson Cancer Center examined 174 patients treated with peri-hepatic lymphadenectomy during liver resection from 2003–2014 (251). Of these patients, 154/174 (89%) were treated with modern neoadjuvant chemotherapy, and 54/174 (31%) patients displayed distal lymph node involvement. Of note, only 2/35 (6%) who had lymphadenectomy for staging without evidence of involvement had metastases on final pathology. Patients that had a peri-hepatic lymphadenectomy but no metastases identified had longer overall survival compared to those who did not undergo lymphadenectomy (71 vs 56 months, p=0.03). Patients with positive hepato-duodenal lymph nodes also had shorter overall survival compared to those with para-aortic lymph node involvement (16 vs 58 months, p=0.01), as did those with 3 or more positive peri-hepatic lymph (p=0.04). Another recent study corroborated inferior survival in patients with positive peri-hepatic lymph nodes, and reported that they were more strongly associated with survival than size or number of hepatic metastases in adjusted analysis (252).
In contrast to these retrospective studies, Bradatsch et al. (2016) prospectively performed peri-hepatic lymph node clearance (including hepatoduodenal ligament, posterior superior pancreaticoduodenal region, and hepatic artery/celiac regions) in patients from 2012–2015 (253). Of these patients, 15/20 (75%) received modern chemotherapy. The mean number of lymph nodes harvested was 5, and remarkably, zero patients had metastatic spread to these lymph nodes by H&E or IHC. Despite this, the authors concluded that while the lymphadenectomy did not change survival, it may provide a prognostic tool. Another study compared surgical teams that routinely performed peri-hepatic lymphadenectomy to those that only performed it selectively. While a difference in survival was identified for those with positive lymph nodes, the frequency of peri-hepatic lymph node metastasis was low (7/81, 9%). Overall, the performance of routine peri-hepatic lymphadenectomy did not correlate to a difference in overall survival between the groups (254). A recent case report was published on the feasibility of sentinel lymph node mapping for peri-hepatic lymph nodes, however, there is no further data on this methodology for metastatic colorectal cancer to the liver (255). Thus, the current data support selective use of peri-hepatic lymphadenectomy for patients with pre-operative suspicion of lymph node involvement for prognostic purposes only. It should also be recognized that the number of lymph nodes harvested is generally low.
Para-aortic lymph nodes
Resection of para-aortic lymph nodes is more common in the far East than in the United States, and is considered stage III regional disease by some (256). These lymph nodes lie between the left renal vein and the division of the aorta into the common iliac arteries. Surgical treatment consists of removing all lymphatic tissue along the aorta between these landmark vessels (257). Lymph nodes within this region that are within 1 cm of the main vascular pedicle are also referred to as apical lymph nodes. Apical lymph node involvement has been reported to be an independent risk factor for distant metastases, and shorter overall survival (258).
A recent review of 4 studies examining synchronous para-aortic lymph node dissections reported a 5-year overall survival range of 23–66% (257). Bae et al. (2016) examined 129 patients who underwent primary para-aortic lymphadenectomy in the largest study on this topic. Para-aortic lymph node dissection was performed for suspected metastases based on pre-operative imaging by CT and/or PET. These patients were compared to 953 others who underwent standard regional lymphadenectomy for colon cancer, and 91 patients who underwent synchronous liver resection for metastatic disease. Para-aortic lymph nodes were positive in 4.5% of all patients, and in 38% of patients who had a para-aortic lymph node dissection. Morbidity was similar between the standard and para-aortic lymphadenectomy groups (5.6% vs 7.8%, p=0.47), but 5-year overall survival was significantly better in the standard lymphadenectomy group (75% vs 34%, p<0.001). Interestingly, survival was similar between the para-aortic lymphadenectomy group and the group of patients who underwent synchronous liver resection (34% vs 39%). The authors concluded that para-aortic lymph node positivity represents distant metastatic disease, and that upfront lymph node clearance should only be considered for carefully selected low risk patients, whereas the remainder should be considered for neoadjuvant chemotherapy (259).
This same group later did further analysis on the patients who had positive para-aortic lymph nodes and found that the number of positive lymph nodes was an independent prognostic factor for overall survival. There were 7/49 patients who had >7 para-aortic lymph nodes positive for metastatic disease, and found that these patients had significantly shorter overall survival (14 months), compared to patients with <7 involved para-aortic lymph nodes involved (37 months, p=0.03) (260). It should be noted, however, that in the original manuscript, the mean number of para-aortic lymph nodes harvested was only 5 (259).
Choi et al. (2010) examined a cohort of 116 surgical patients with evidence of para-aortic lymph node metastases on imaging, and compared those who received a para-aortic lymphadenectomy (n=24, 35%) to those who did not (n=53, 78%). The decision to omit para-aortic lymphadenectomy was based on surgeon discretion in 73% of cases, and on patient preference or co-morbidities in the remainder. While the rate of surgical compilations and length of stay were similar between groups, the 5-year overall survival rates were significantly different; 57% for the para-aortic lymphadenectomy group and only 13% for the non-para-aortic lymphadenectomy group. Confounders for this study include retrospective selection bias, and that 96% of the para-aortic lymph node dissection group received neoadjuvant chemotherapy, compared to only 75% of the other group (Figure 10) (261). The authors concluded that paraaortic lymph node dissection may increase survival with acceptable morbidity in select patients.
Figure 10.
Survival of patients after peri-aortic lymph node dissection. Kaplan Meyer curves are shown for node dissection (D+) and control (D-) patients. Reproduced with permission from Choi et al., 2010 (261).
While the above studies were exclusively with open operations, Song et al. (2016) published data on 40 patients who underwent para-aortic lymph node dissections performed laparoscopically (262). The average operative time was 192+/−69 minutes, with an estimated blood loss of 65 +/− 52 mL. There was a 15% complication rate, including 5% anastomotic leak rate, and no complications related to the lymphadenectomy itself. Final pathology demonstrated a 40% rate of metastatic disease in the para-aortic lymph nodes, with an average of 7 para-aortic lymph nodes harvested per patient. Thus, it can again be concluded dissection may be considered in select patients with pre-operative evidence of disease for prognostic purposes, but there is not a definitive survival benefit to doing so. There is a paucity of data from Western nations, and as such the existing data may not be applicable to these populations.
Lateral lymph node resection for rectal cancer
A lateral pelvic lymph node dissection includes clearance of internal iliac, obturator, external iliac, and common iliac lymph nodes. This practice is more common for patients with rectal cancer in the far East, and is not typically performed in the United States (263). Retrospective evaluation of local failure rates in patients with T3/T4 rectal tumors were higher in patients who had larger pre-treatment lateral pelvic lymph nodes (4-year recurrence rate 33% vs 10%, p=0.03), despite getting chemoradiation to this location (264). This data suggests that non-surgical treatment of these lymph nodes may be insufficient. Multiple recent studies have examined the impact of lateral pelvic lymph node dissection on patients’ status post neoadjuvant chemoradiotherapy with enlarged lymph nodes on pre-treatment imaging. Imaging features consistent with metastatic disease on multivariate analysis included size ≥8 mm, size reduction <33% after treatment, and heterogeneous signal intensity (265). The accuracy of an 8 mm cut off on pre-treatment imaging was 52%, and increased to 66% after neoadjuvant chemoradiation (263). Positive lateral pelvic lymph nodes were also more common with larger primary tumors, and for patients with low-lying tumors <4 cm from the anal verge (263). Overall, lymph node positivity rates were 40–52% (263, 265). While there was no statistical difference in 5-year overall survival between those who received a lateral pelvic lymph node dissection and those who did not (81% vs 85%, p=0.46), there was a difference for those who had positive lateral pelvic lymph nodes compared to those who did not (60% vs 100%, p<0.01) (263). Another study compared open to laparoscopic lateral lymph node clearance for matched patients with stage II and III rectal cancer undergoing mesorectal excision. This study showed that while operative times were longer, estimated blood loss was lower, with similar complication and 3-year relapse free survival rates. This suggests that performing the procedure laparoscopically is both feasible and safe (266). These studies are limited by their retrospective nature, and are generally only out of the far East. Like the data on par-aortic lymph node clearance, applicability to Western populations may be limited.
Colorectal Brain Metastases
It is well known that brain metastases are the most common type of intracranial tumors. In colorectal cancer, however, brain metastases are less common than the other locations discussed. The incidence of brain metastases ranges from 0.6–2.9% (267). Amongst those with metastases, they account for 5% of patients with a colon primary tumor, and 8% of patients with a rectal primary cancer (268).
Risk factors for brain metastases
Brain metastases are rarely the first metastatic site (<1%) (269), and the average interval from diagnosis of colon cancer to identification of brain metastases is 20–40 months (267). More than half of these patients also have lung metastases at the time of diagnosis (268, 270). Brain metastases are more often found in patients with metastases to 3 or more sites (268). They are also more likely to occur in patients who are younger; the odds of developing a brain metastasis for a patient >79 years old is only 30–40% compared to a patient who is <60 years old. They are more common in patients with adenocarcinoma compared to those with signet ring or mucinous histology (268). RAS mutational status is also a factor; patients who are RAS mutants have a significantly higher rate of brain metastases compared to those who are RAS wild type (hazard radio 3.7). It has been suggested that the threshold for obtaining neuroimaging on patients with RAS mutant tumors should be lower to enhance early detection (271).
Initial presentation and management
When patients develop colorectal brain metastases, most present with headache (46–51%) and gait changes (52–59%) (272). Only 24% present with seizure. Very few (2%) are asymptomatic. Per the most recent guidelines from the European Association of Neuro-oncology (EANO), a contrast enhanced MRI is the method of choice for diagnosis of brain metastases (273). Metastases are typically found in the frontal lobe and cerebellum. When brain metastases are detected, the immediate priority is medical stabilization and prevention of neurological deterioration (273). This includes treatment of seizures and cerebral edema if present. Those who develop seizures should be treated with an anticonvulsant. Levetiracetam (Keppra ®) is the anticonvulsant of choice given the lack of interaction with the cytochrome P450 system (274). Those without seizures generally do not require long-term medication for prophylaxis. Patients with peri-lesional edema are treated with oral glucocorticoids, usually dexamethasone (273). It should be noted that patients with co-existing conditions requiring therapeutic anticoagulation can continue with enoxaparin treatment when brain metastases are identified. A recent matched cohort study demonstrated no difference in the frequency of intracranial hemorrhage between those treated with enoxaparin for one year. Intracranial hemorrhage in the setting of brain metastases is a frequent complication however, affecting ~20% of patients. (275).
Prognosis
There are a number of treatment options for patients with brain metastases, including chemotherapy, external beam whole brain radiation, stereotactic radiosurgery, and open surgery. Despite these options, the overall prognosis is generally regarded as poor. Compared with the other metastatic locations discussed, patients with brain metastases have the lowest one-year cause-specific survival – only 30%, as compared to 90% for patients without brain metastases (270). Synchronous presentations carry a worse prognosis (276), as do increased number of brain metastases. In 1996, the median survival was 4.8 months; without radiotherapy or surgery, the median survival was 1.9 months. By comparison, patients who underwent surgery alone had median survival of 10.5 months. There were 16% of patients who lived >1 year – nearly all of these patients presented with oligometastases (272). A more recent review of the literature reported that mean interval time between diagnosis of colorectal cancer and brain metastases was 28.3 months, 77% of these patients had extra-cranial metastases, and that median survival time after diagnosis of cerebral metastases was 5.3 months (276). This was longer for patients who had surgical resection compared to patients who had stereotactic radiosurgery, whole brain radiation, or were treated with supportive care alone; 24% of patients survived >1 year. This review of 23 studies published from 1995–2016 consistently showed that survival was lengthened by treatment. Case reports exist of patients living as long as 10 years with brain metastases (277), but these instances are the exception, not the rule. Median survival estimates based on treatment modality are presented in Table 11.
Table 11.
Median survival time for colorectal brain metastases by treatment modality.
| Median Survival (months) | SC | WBRT | SRS | S | WBRT +SRS | S+ SRS | S+ WBRT | S+ WBRT +SRS |
|---|---|---|---|---|---|---|---|---|
| Sperduto et al. 2010 (280) (n=211) | 2.9 | 7.3 | 7.1 | 9.8 | 10.4 | 7.9 | ||
| Silva et al. 2017 (276) (n=1,475) | 1.8 | 4.4 | 6.4 | 10.3 |
Notes: CI, confidence interval; S, surgery; SC, supportive care; SRS, sterotactic radiosurgery; WBRT, whole brain radiation therapy
Since expected outcomes can help determine the best treatment options for patients, a system of categorization was devised by Gaspar et al. using data from three randomized trials studying whole brain radiation (278). Recursive partitioning analysis, a method of building decision trees to model predictors, was used to create groups. The best outcomes were achieved for patients with good performance status (Karnofsky performance status at least 70) (279), who were younger than 65 years old, with controlled primary disease, and metastases to the brain only. These patients comprised 20% of enrolled subjects, and were considered recursive partitioning analysis (RPA) class I, with a median survival of 7.1 months after treatment (278). Patients with a Karnofsky performance status <70 were found to have the poorest outcomes, with a median survival of only 2.3 months. They were 15% of the study cohort, and were considered RPA class III. All other patients (65%) fell into the RPA class II category.
Of note, the RPA class system does not distinguish between different primary disease sites. A follow up to this was the development of the diagnosis-specific graded prognostic assessment score (DS-GPA) based on the retrospective review of 4,259 patients in a multi-institutional database (280). Scores ranged from 0–4; 0 correlated with the shortest survival, and 4 correlated with the longest survival. This study interestingly found that Karnofsky performance status was the only independent variable significantly associated with prognosis for patients with gastro-intestinal brain metastases. Prognostic factors such as age, and number of brain metastases were found only to important for patients with metastatic lung cancer, renal cell cancer, and melanoma. For gastro-intestinal cancer brain metastases, a DS-GPA score of 0 indicated Karnofsky performance status of <70%, and correlated with an overall survival of only 3.1 months. With each 10 point increase in Karnofsky performance status, the score increased by 1, as did the median overall survival, up to Karnofsky performance status of 100%, which had a DG-GPA score of 4.0, and correlated with a 13.5 month median overall survival. These data are summarized in Table 12. As such, patients with the highest Karnofsky performance status scores are the best suited for the most aggressive invasive treatments, whereas patients who have lower performance status derive less benefit.
Table 12.
Predictive scoring methods for brain metastases.
| Criteria | Median Overall Survival (months) | |
|---|---|---|
| RPA – all brain metastases (Gaspar et al.)(278) | ||
| Class I | KPS >70, age <65, controlled primary, Brain only metastasis | 7.1 |
| Class II | KPS >70, not meeting class I criteria | 4.2 |
| Class III | KPS <70 | 2.3 |
| DS-GPA – GI cancer (Sperduto et al.)(280) | ||
| GPA 0–1 | KPS <79 | 3.1 |
| GPA 2 | KPS 80 | 4.4 |
| GPA 3 | KPS 90 | 6.9 |
| GPA 4 | KPS 100 | 13.5 |
Notes: DS-GPA, disease specific graded prognostic assessment; KPS, Karnofsky performance status; RPA, recusive partitioning analysis
Surgical resection
Treatment of brain metastases depends on a number of variables. As stated above, patients treated with surgical resection have the longest survival. Brain metastases typically appear at the grey-white junction, making them both accessible and distinct from non-tumoral tissue, enabling complete excision. Surgery is generally considered for limited brain metastases for primary treatment of newly diagnosed disease, management of otherwise stable disease, or management of symptoms. This is based on the size, location, and symptomatology (281). Open surgery via craniotomy is most often offered for patients with solitary lesions that are larger than 3 cm. This is especially true for lesions in the posterior fossa, and for lesions that may be causing edema or compression on the 4th ventricle or brain stem. It is further used when a pathologic diagnosis is required, such as when it is the first site of failure. A tissue diagnosis is also recommended in patients with well-controlled systemic cancer when the imaging is not typical or there has been a long disease-free interval (273). Colon cancer is considered radio-resistant, and as such, surgical resection is given greater consideration (273).
There have been multiple randomized clinical trials comparing surgical excision followed by radiation with whole brain radiation alone for single brain metastases. Patchell et al. (1990) showed that surgical treatment decreased local recurrence from 52% to 20%, and that survival was extended from 3.5 months to 9.3 months. Importantly, patients also remained functionally independent for longer, from 1.9 months to 8.9 months (282). In a similar study, Vecht et al. (1993) showed that patients treated with surgery and radiation had a median survival of 10 months, compared to 6 months in the whole brain radiation alone group. They also noted that surgery did not influence survival for patients with progressive extra-cranial disease. This study, however, had no patients with metastases from colorectal primary tumors (283). Both studies combined patients with metastases from multiple locations, and were primarily composed of patients with lung metastases.
While simple craniotomy can be used for well-defined superficial lesions, methods of intra-operative stereotactic guidance based on pre-operative imaging (neuro-navigation) are now considered standard, and are most important for tumors that are deeper in the brain or in proximity to eloquent cortex. This is performed by registering anatomic landmarks that link to a probe that is used on the surgical field. This enables smaller craniotomies, with lower blood loss, and decreased operative time. Given the brain shift that occurs with cerebrospinal fluid release and initial tumor resection, there are additional more sophisticated methods for intra-operative location of brain metastases, including intra-operative ultrasound, MRI, and angiography, enabling resection of tumors in the most delicate locations (284). It has been reported that en bloc resection decreases local recurrence rates compared to piecemeal resection (285). A review of 1,033 single brain metastasectomies reported a 15% complication rate, with a 3% mortality rate. Complications included 10% neurologic complaints (4% focal motor deficits), with lower rates of meningitis, wound infections, and seizures. This series also reported complications were higher with piecemeal resection in eloquent locations of the brain, but were similar to resections in non-eloquent locations when resected en bloc (286).
Stereotactic radiosurgery
Stereotactic radiosurgery is an alternative to surgical resection of brain metastases. High doses of radiation are delivered to a precisely defined lesion and works best for lesions that are smaller than 2 cm in diameter, since lower doses must be used for larger lesions (287). While less invasive than surgical resection, complications still occur, including radiosurgery induced radionecrosis in 24%. This is symptomatic in 10% of the cases, and can result in seizures, motor deficits and cognitive deficits. Frequency of complications increases with greater volume of tumor treated. In addition, radiosurgery can induce vasogenic edema, and cause nausea, headache, hemorrhage into brain metastases, and Cushing syndrome (288). Considerations for surgery versus stereotactic radiosurgery include surgical accessibility, mass effect/symptoms, need for tissue diagnosis, tumor size, and of equipment availability (281). For patients with multiple lesions, stereotactic radiosurgery can be used in addition to surgical resection as well.
Randomized controlled trials have been attempted to compare surgical resection to stereotactic radiosurgery. Muacevic et al. compared surgical resection followed by whole brain radiation to stereotactic radiosurgery. Survival and neurologic death rates were similar. Length of hospitalization and toxicities were lower, and quality of life was better at 6 weeks in the stereotactic radiosurgery group, but distant brain recurrence was significantly more common in this group (289). Similarly, Roos et al. compared surgical resection to stereotactic radiosurgery, both followed by whole brain radiation. This study showed similar median overall survival and quality of life (290). Both studies, however, significantly under-accrued resulting early study closure and underpowered analysis.
A recent randomized trial compared stereotactic radiosurgery alone to stereotactic radiosurgery plus whole brain radiation for patients with 1–3 brain metastases smaller than 3 cm (291). Cognitive deterioration at 3 months was less frequent in the radiosurgery alone group (63% vs 92%); this included differences in performance in immediate memory, delayed memory, and verbal fluency. The stereotactic radiosurgery alone group also had better quality of life at 3 months. Of note, the stereotactic radiosurgery group had a greater incidence of progression of intracranial tumors compared to the stereotactic radiosurgery plus whole brain radiation group (25% vs 7%); however, this did not correlate with a change in survival. Median survival for the stereotactic radiosurgery group was 10.2 months, and was 7.4 months for the radiosurgery plus whole brain radiation group.
Whole brain radiation therapy has also been used as an adjunct to surgically treated patients in the past. A recent randomized trial examined patients with cerebral metastases treated with either surgery or stereotactic radiosurgery, and compared post-procedural whole brain radiation to observation. This study found no difference in survival between the whole brain radiation and observational arms, although patients in the whole brain radiation arm had lower rates of intracranial progression (48% vs 78%) at previously treated and new sites. Functional independence was also similar; at two years, 22% of each group were alive and functionally independent (292).
Conclusion
Safer and less invasive surgical and ablative procedures now allow for effective cytoreductive procedures for metastatic colorectal cancer. These procedures are now being deployed to palliate symptoms, increase survival, and provide potential cure for patients presenting with advanced disease. Liver and lung resections and ablations are now standard. Select patients with regional nodal recurrence and peritoneal recurrence may also benefit from surgical therapy. Treatments for brain and bone are only palliative. Appropriate use of these along with increasingly effective systemic therapies have translated multidisciplinary treatment plans into improved patient outcomes.
Highlights.
Survival with metastatic colorectal cancer is often based on liver resectability.
Cytoreduction for metastatic colorectal cancer can cure and prolong survival.
Treatment of metastatic colorectal cancer should be multidisciplinary.
Author biographies
Camille L. Stewart earned her medical degree from Tulane School of Medicine. During medical school she participated in a research fellowship at Boston Children’s Hospital, through the Sarnoff Cardiovascular Research Foundation. She then completed general surgical residency at University of Colorado, which also included two years of dedicated clinical research. She is currently a surgical oncology fellow at City of Hope National Cancer Center, in Duarte, California. Her clinical and research interests are in hepatopancreatobiliary and foregut malignancies.
Susanne Warner earned her medical degree from Texas A&M Health Science Center College of Medicine. She then completed general surgical residency at the Mayo Clinic in Phoenix, Arizona. During her surgical residency, she participated in a research fellowship under the mentorship of Dr. Yuman Fong at Memorial Sloan Kettering Cancer Center in New York, New York. She then completed a clinical fellowship in hepatopancreatobiliary and advanced gastrointestinal surgery at the University of Michigan, in Ann Arbor Michigan. She is currently an assistant professor of surgery in the division of surgical oncology at City of Hope National Cancer Center, in Duarte, California. Her research and clinical interests are in oncolytic viral therapies and in strategies to improve peri-operative patient-centric outcomes.
Kaori Ito earned her degree from the University of Tokyo. After a highly successful research fellowship at the Memorial Sloan-Kettering Cancer Center, she completed her internship at the Brigham and Women’s hospital, followed by a residency at the Michigan State University. She is now in practice.
Mustafa Raoof earned his medical degree from Aga Khan University in Pakistan. He studied immunology at Beth Israel Deaconess in Boston, Massachusetts, completed his surgical internship at Yale-New Haven Hospital, and completed his general surgical residency at the University of Arizona, in Tucson, Arizona. During his residency, he participated in a research fellowship at MD Anderson Cancer Center, in Houston, Texas. He then went on to complete a clinical fellowship in complex general surgical oncology at City of Hope National Cancer Center, in Duarte, California. He stayed on as faculty at City of Hope, where he is now an assistant professor in the Department of Surgery. His research and clinical interests are in immune therapies and the treatment of peritoneal surface malignancies.
Geena X. Wu earned her medical degree from University of California San Diego School of Medicine. She then completed general surgical residency at Maricopa Medical Center in Phoenix, Arizona. She participated in a thoracic oncology fellowship at City of Hope National Medical Center, in Duarte, California.
Jonathan Kessler earned his medical degree from University of Pennsylvania, in Philadelphia, Pennsylvania. He completed internship at Albert Einstein Medical Center, in Philadelphia, and then completed residency in radiology at University of California Los Angeles. He then continued his training with a vascular and radiology fellowship also at University of California Los Angeles. He then joined faculty at City of Hope National Medical Center, in Duarte, California, where he is now an assistant professor in the Department of Diagnostic Radiology.
Jae Y. Kim earned his medical degree at University of California San Francisco, and then stayed there to complete his general surgical residency. He then completed a fellowship in Thoracic Surgery at MD Anderson Cancer Center, in Houston, Texas. He then joined faculty at City of Hope National Medical Center in Duarte, California, where he is the Division Chief of Thoracic Surgery. His clinical and research interests are in DNA repair mechanisms in lung cancer.
Yuman Fong is a graduate of Brown University (BA, Medieval Literature, 1981) and Cornell University Medical College (MD, 1984). After surgical training at the New York Hospital/Cornell Medical Center and surgical oncology fellowship at the Memorial Sloan-Kettering Cancer Center, he was appointed to the faculty of the Memorial Sloan-Kettering Cancer Center. There he served on staff for over 20 years, and held the Murray F. Brennan Chair in Surgery. He is currently the Sangiacomo Chair and Chairman of the Department of Surgery at the City of Hope Medical Center. Dr. Fong is best known clinically for his extensive work in the field of liver and pancreatic surgery––especially for pioneering many laparoscopic, robotic and ablative therapies for these cancers. He has assisted in the design and deployment of many novel surgical tools. His work helped establish resection of colorectal metastases as a safe, effective, and potentially curative option even at stage IV. Dr. Fong has also been active in biologic bench investigation. His laboratory focus over the last 15 years has been in the field of gene therapy, designing and studying the use of genetically modified viruses for the killing of cancer. His leadership in this field on the national level has included serving as the Chair of the Recombinant DNA Advisory Committee (RAC) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors declare no conflict of interest.
References
- 1.Ito K, Govindarajan A, Ito H, Fong Y. Surgical treatment of hepatic colorectal metastasis: evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010;16(2):103–10. [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014. July; 25(7):1346–55. [DOI] [PubMed] [Google Scholar]
- 3.Van CE, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. NEnglJ Med. 2009;360(14):1408–17. [DOI] [PubMed] [Google Scholar]
- 4.Fortner JG, Fong Y. Twenty-five-year follow-up for liver resection: the personal series of Dr. Joseph G. Fortner. Ann Surg. 2009. December;250(6):908–13. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017. January;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 6.Picardo A, Karpoff HM, Ng B, Lee J, Brennan MF, Fong Y. Partial hepatectomy accelerates local tumor growth:potential roles of local cytokine activation. Surgery. 1998;124(1):57–64. [PubMed] [Google Scholar]
- 7.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004. October;240(4):644–57; discussion 57–8. Epub 2004/09/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000. April;231(4):487–99. Epub 2000/04/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias D, Liberale G, Vernerey D, Pocard M, Ducreux M, Boige V, et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005. November;12(11):900–9. Epub 2005/09/27. eng. [DOI] [PubMed] [Google Scholar]
- 10.Elias D, Ouellet JF, Bellon N, Pignon JP, Pocard M, Lasser P. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg. 2003. May;90(5):567–74. Epub 2003/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 11.Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994. October;116(4):703–10; discussion 10–1. Epub 1994/10/01. eng. [PMC free article] [PubMed] [Google Scholar]
- 12.Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Pattern of recurrence in liver resection for colorectal secondaries. World J Surg. 1987. August;11(4):541–7. Epub 1987/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 13.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994. June 4;343(8910):1405–10. Epub 1994/06/04. eng. [DOI] [PubMed] [Google Scholar]
- 15.Nordlinger B, Quilichini MA, Parc R, Hannoun L, Delva E, Huguet C. Hepatic resection for colorectal liver metastases. Influence on survival of preoperative factors and surgery for recurrences in 80 patients. Ann Surg. 1987. March;205(3):256–63. Epub 1987/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77(7):1254–62. [PubMed] [Google Scholar]
- 17.Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986. August;100(2):278–84. Epub 1986/08/01. eng. [PubMed] [Google Scholar]
- 18.Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986. September;73(9):727–31. Epub 1986/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 19.Cady B, McDermott WV. Major hepatic resection for metachronous metastases from colon cancer. Ann Surg. 1985. February;201(2):204–9. Epub 1985/02/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster JH. Survival after liver resection for secondary tumors. Am J Surg. 1978. March;135(3):389–94. Epub 1978/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 21.Adson MA. The resection of hepatic metastases. Another view. Arch Surg. 1989. September;124(9):1023–4. Epub 1989/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen IK, Burcharth F, Roikjaer O, Baden H. Resection of liver metastases from colorectal cancer. Indications and results. Dis Colon Rectum. 1994. November;37(11):1078–82. Epub 1994/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 23.Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997. May;132(5):505–10; discussion 11. Epub 1997/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins LT, Millikan KW, Bines SD, Staren ED, Doolas A. Hepatic resection for metastatic colorectal cancer. Am Surg. 1997. July;63(7):605–10. Epub 1997/07/01. eng. [PubMed] [Google Scholar]
- 25.Ohlsson B, Stenram U, Tranberg KG. Resection of colorectal liver metastases: 25-year experience. World J Surg. 1998. March;22(3):268–76; discussion 76–7. Epub 1998/03/12. eng. [DOI] [PubMed] [Google Scholar]
- 26.Scheele J Liver resection for colorectal metastases. World Journal of Surgery. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 27.Fong Y, Fortner J, Sun R, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. American Surgical Association Meeting, 1999. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adam R, Wicherts DA, de Haas RJ, Ciacio O, Levi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J ClinOncol. 2009;27(11):1829–35. [DOI] [PubMed] [Google Scholar]
- 29.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Annals Of Surgery. 2002;235(6):759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato T, Yasui K, Hirai T, Kanemitsu Y, Mori T, Sugihara K, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Diseases of the Colon & Rectum. 2003;46(10 Suppl):S22–S31. [DOI] [PubMed] [Google Scholar]
- 32.Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal [liver] metastases. Annals of Surgical Oncology. 2001;8(4):347–53. [DOI] [PubMed] [Google Scholar]
- 33.House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. JAmCollSurg. 2010;210(5):744–5. [DOI] [PubMed] [Google Scholar]
- 34.Strasberg SM, Dehdashti F, Siegel BA, Drebin JA, Linehan D. Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. AnnSurg. 2001;233(3):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figueras J, Torras J, Valls C, Llado L, Ramos E, Marti-Rague J, et al. Surgical resection of colorectal liver metastases in patients with expanded indications: a single-center experience with 501 patients. DisColon Rectum. 2007;50(4):478–88. [DOI] [PubMed] [Google Scholar]
- 36.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007. October 10;25(29):4575–80. [DOI] [PubMed] [Google Scholar]
- 37.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008. January;247(1):125–35. [DOI] [PubMed] [Google Scholar]
- 38.Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. Journal of the American College of Surgeons. 2010. May;210(5):755–64, 64–6. [DOI] [PubMed] [Google Scholar]
- 39.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009. September;250(3):440–8. [DOI] [PubMed] [Google Scholar]
- 40.Robertson DJ, Stukel TA, Gottlieb DJ, Sutherland JM, Fisher ES. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer. 2009. February 15;115(4):752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang M, Jayakrishnan TT, Green DE, George B, Thomas JP, Groeschl RT, et al. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease. Eur J Cancer. 2014. July;50(10):1747–57. [DOI] [PubMed] [Google Scholar]
- 42.Fortner JG, Fong Y. Twenty-five Year Follow-up for Liver Resection: The Personal Series of Dr. Joseph G. Fortner. AnnSurg. 2009. [DOI] [PubMed] [Google Scholar]
- 43.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Annals Of Surgery. 2000;231(4):487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fong Y, Gonen M, Rubin D, Radzyner D, Brennan MF. Long-term Survival is Superior after Resection for Cancer in High Volume Centers. Annals Of Surgery. 2005;242(4):540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes K, Scheele J, Sugarbaker PH. Surgery for colorectal cancer metastatic to the liver. Surgical Clinics Of North America. 1989;69:339–59. [DOI] [PubMed] [Google Scholar]
- 46.Ballantyne GH, Quin J. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer. 1993;71(S12):4252–66. [DOI] [PubMed] [Google Scholar]
- 47.Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 48.Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, Van Heerden JA, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. AnnSurg. 1992;216:492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephenson KR, Steinberg SM, Hughes KS, Vetto J, Sugarbaker PH. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastasis. Ann Surg. 1988;208:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mala T, Bohler G, Mathisen O, Bergan A, Soreide O. Hepatic resection for colorectal metastases: can preoperative scoring predict patient outcome? World Journal of Surgery. 2002;26(11):1348–53. [DOI] [PubMed] [Google Scholar]
- 51.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. AnnSurg. 2006;243(3):321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart C, and Fong Y. Clinical Scoring Systems for Colorectal Cancer Liver Metastases In: Correia MM, editor. Colorectal Cancer Liver Metastases: A Comprehensive Guide to Management. New York, NY: Springer Nature; 2018. (in press). [Google Scholar]
- 53.Schussler-Fiorenza CM, Mahvi DM, Niederhuber J, Rikkers LF, Weber SM. Clinical risk score correlates with yield of PET scan in patients with colorectal hepatic metastases. Journal of Gastrointestinal Surgery. 2004;8(2):150–7. [DOI] [PubMed] [Google Scholar]
- 54.Jarnagin WR, Conlon K, Bodniewicz J, Dougherty E, DeMatteo RP, Blumgart LH, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001;91(6):1121–8. [DOI] [PubMed] [Google Scholar]
- 55.Poultsides GA, Bao F, Servais EL, Hernandez-Boussard T, DeMatteo RP, Allen PJ, et al. Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol. 2012. September;19(9):2797–804. [DOI] [PubMed] [Google Scholar]
- 56.Turcotte S, Katz SC, Shia J, Jarnagin WR, Kingham TP, Allen PJ, et al. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer immunology research. 2014. June;2(6):530–7. Epub 2014/06/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yopp AC, Shia J, Butte JM, Allen PJ, Fong Y, Jarnagin WR, et al. CXCR4 expression predicts patient outcome and recurrence patterns after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2012. July;19 Suppl 3:S339–46. Epub 2011/05/18. eng. [DOI] [PubMed] [Google Scholar]
- 58.Crowe PJ, Yang JL, Berney CR, Erskine C, Ham JM, Fisher R, et al. Genetic markers of survival and liver recurrence after resection of liver metastases from colorectal cancer. World Journal of Surgery. 2001;25(8):996–1001. [DOI] [PubMed] [Google Scholar]
- 59.Ito H, Mo Q, Qin LX, Viale A, Maithel SK, Maker AV, et al. Gene expression profiles accurately predict outcome following liver resection in patients with metastatic colorectal cancer. PloS one. 2013;8(12):e81680 Epub 2013/12/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fong Y, Blumgart LH, Fortner JG, Brennan MF. Pancreatic or liver resection for malignancy is safe and effective in the elderly. AnnSurg. 1995;222(4):426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adam R, Frilling A, Elias D, Laurent C, Ramos E, Capussotti L, et al. Liver resection of colorectal metastases in elderly patients. BrJSurg. 2010;97(3):366–76. [DOI] [PubMed] [Google Scholar]
- 62.Weber SM, Jarnagin WR, DeMatteo RP, Blumgart LH, Fong Y. Survival after resection of multiple hepatic colorectal metastases. Annals of Surgical Oncology. 2000;7(9):643–50. [DOI] [PubMed] [Google Scholar]
- 63.Lee HJ, Lee CH, Kim JW, Park YS, Lee J, Kim KA. Use of hepatobiliary phase images in Gd-EOB-DTPA-enhanced MRI of breast cancer hepatic metastasis to predict response to chemotherapy. Clinical imaging. 2017. May-Jun;43:127–31. [DOI] [PubMed] [Google Scholar]
- 64.Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: The evolution of determining prognosis. World journal of gastrointestinal oncology. 2013. December 15;5(12):207–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN, et al. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2013. February;15(2):91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzeng CW, Aloia TA. Colorectal liver metastases. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2013. January;17(1):195–201; quiz p −2. [DOI] [PubMed] [Google Scholar]
- 67.Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, et al. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247(3):451–5. [DOI] [PubMed] [Google Scholar]
- 68.van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, et al. Portal vein embolization before liver resection: a systematic review. Cardiovascular and interventional radiology. 2013. February;36(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dhir M, Sasson AR. Surgical Management of Liver Metastases From Colorectal Cancer. Journal of oncology practice. 2016. January;12(1):33–9. [DOI] [PubMed] [Google Scholar]
- 70.Schulz A, Viktil E, Godt JC, Johansen CK, Dormagen JB, Holtedahl JE, et al. Diagnostic performance of CT, MRI and PET/CT in patients with suspected colorectal liver metastases: the superiority of MRI. Acta radiologica. 2016. September;57(9):1040–8. [DOI] [PubMed] [Google Scholar]
- 71.Park MJ, Hong N, Han K, Kim MJ, Lee YJ, Park YS, et al. Use of Imaging to Predict Complete Response of Colorectal Liver Metastases after Chemotherapy: MR Imaging versus CT Imaging. Radiology. 2017. August;284(2):423–31. [DOI] [PubMed] [Google Scholar]
- 72.Kruskal JB, Kane RA. Intraoperative US of the liver: techniques and clinical applications. Radiographics : a review publication of the Radiological Society of North America, Inc. 2006. Jul-Aug;26(4):1067–84. [DOI] [PubMed] [Google Scholar]
- 73.Sahani DV, Kalva SP, Tanabe KK, Hayat SM, O’Neill MJ, Halpern EF, et al. Intraoperative US in patients undergoing surgery for liver neoplasms: comparison with MR imaging. Radiology. 2004. September;232(3):810–4. [DOI] [PubMed] [Google Scholar]
- 74.Ellsmere J, Kane R, Grinbaum R, Edwards M, Schneider B, Jones D. Intraoperative ultrasonography during planned liver resections: why are we still performing it? Surgical endoscopy. 2007. August;21(8):1280–3. [DOI] [PubMed] [Google Scholar]
- 75.Tamandl D, Herberger B, Gruenberger B, Schoppmann SF, Puhalla H, Schindl M, et al. Adequate preoperative staging rarely leads to a change of intraoperative strategy in patients undergoing surgery for colorectal cancer liver metastases. Surgery. 2008. May;143(5):648–57. [DOI] [PubMed] [Google Scholar]
- 76.Martin R, Paty P, Fong Y, Grace A, Cohen A, DeMatteo R, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. Journal of the American College of Surgeons. 2003;197(2):233–41. [DOI] [PubMed] [Google Scholar]
- 77.Reddy S, Zorzi D, Lum YW, Barbas A, Pawlik T, Ribero D, et al. Timing of Multimodality Therapy for Resectable Synchronous Colorectal Liver Metastases: a Retrospective Multi-institutional Analysis. Ann Surg Oncol. 2009. [DOI] [PubMed] [Google Scholar]
- 78.Martin RC, Augenstein V, Reuter NP, Scoggins CR, McMasters KM. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am CollSurg. 2009;208(5):842–50. [DOI] [PubMed] [Google Scholar]
- 79.Silberhumer GR, Paty PB, Temple LK, Araujo RL, Denton B, Gonen M, et al. Simultaneous resection for rectal cancer with synchronous liver metastasis is a safe procedure. Am J Surg. 2015. June;209(6):935–42. Epub 2015/01/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer treatment reviews. 2015. November;41(9):729–41. [DOI] [PubMed] [Google Scholar]
- 81.Silberhumer GRP, P. P; Temple LK; Araujo RLC; Denton B; Gonen M; Nash GM; Allen PJ; DeMatteo RP; Guillem J; Weiser MR; D’Angelica MI; Jarnagin WR; Wong WD; Fong Y Simultaneous Resection for Rectal Cancer with Synchronous Liver Metastasis Is a Safe Procedure. AmJSurg. 2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vigano L, Capussotti L, Majno P, Toso C, Ferrero A, De Rosa G, et al. Liver resection in patients with eight or more colorectal liver metastases. Br J Surg. 2015. January;102(1):92–101. [DOI] [PubMed] [Google Scholar]
- 83.Leung U, Gonen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, et al. Colorectal Cancer Liver Metastases and Concurrent Extrahepatic Disease Treated With Resection. Ann Surg. 2017. January;265(1):158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choti MA, Thomas M, Wong SL, Eaddy M, Pawlik TM, Hirose K, et al. Surgical Resection Preferences and Perceptions among Medical Oncologists Treating Liver Metastases from Colorectal Cancer. Ann Surg Oncol. 2016. February;23(2):375–81. [DOI] [PubMed] [Google Scholar]
- 85.Homayounfar K, Bleckmann A, Helms HJ, Lordick F, Ruschoff J, Conradi LC, et al. Discrepancies between medical oncologists and surgeons in assessment of resectability and indication for chemotherapy in patients with colorectal liver metastases. Br J Surg. 2014. April;101(5):550–7. [DOI] [PubMed] [Google Scholar]
- 86.Mohammad WM, Martel G, Mimeault R, Fairfull-Smith RJ, Auer RC, Balaa FK. Evaluating agreement regarding the resectability of colorectal liver metastases: a national case-based survey of hepatic surgeons. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2012. May;14(5):291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krell RW, Reames BN, Hendren S, Frankel TL, Pawlik TM, Chung M, et al. Surgical Referral for Colorectal Liver Metastases: A Population-Based Survey. Ann Surg Oncol. 2015. July;22(7):2179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chua TC, Liauw W, Chu F, Morris DL. Summary outcomes of two-stage resection for advanced colorectal liver metastases. Journal of surgical oncology. 2013. February;107(2):211–6. [DOI] [PubMed] [Google Scholar]
- 89.Karoui M, Vigano L, Goyer P, Ferrero A, Luciani A, Aglietta M, et al. Combined first-stage hepatectomy and colorectal resection in a two-stage hepatectomy strategy for bilobar synchronous liver metastases. Br J Surg. 2010. September;97(9):1354–62. [DOI] [PubMed] [Google Scholar]
- 90.Stella M, Dupre A, Chabaud S, Gandini A, Meeus P, Peyrat P, et al. A comparative study of patients with and without associated digestive surgery in a two-stage hepatectomy setting. Langenbeck’s archives of surgery. 2012. December;397(8):1289–96. [DOI] [PubMed] [Google Scholar]
- 91.Imai K, Benitez CC, Allard MA, Vibert E, Cunha AS, Cherqui D, et al. Failure to Achieve a 2-Stage Hepatectomy for Colorectal Liver Metastases: How to Prevent It? Ann Surg. 2015. November;262(5):772–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 92.Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014. November;260(5):829–36; discussion 36–8. [DOI] [PubMed] [Google Scholar]
- 93.Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250(5):825–30. [DOI] [PubMed] [Google Scholar]
- 94.Tsung A, Geller DA, Sukato DC, Sabbaghian S, Tohme S, Steel J, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg. 2014. March;259(3):549–55. [DOI] [PubMed] [Google Scholar]
- 95.Melstrom LG, Warner SG, Woo Y, Sun V, Lee B, Singh G, et al. Selecting incision-dominant cases for robotic liver resection: towards outpatient hepatectomy with rapid recovery. Hepatobiliary surgery and nutrition. 2018. April;7(2):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2016. August;27(8):1386–422. [DOI] [PubMed] [Google Scholar]
- 97.Leung U, Kuk D, D’Angelica MI, Kingham TP, Allen PJ, DeMatteo RP, et al. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015. January;102(1):85–91. Epub 2014/10/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255(2):426–33. [DOI] [PubMed] [Google Scholar]
- 99.Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver. 2010. September;4 Suppl 1:S99–S104. Epub 2010/11/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee EW, Wong D, Prikhodko SV, Perez A, Tran C, Loh CT, et al. Electron microscopic demonstration and evaluation of irreversible electroporation-induced nanopores on hepatocyte membranes. J Vasc Interv Radiol. 2012. January;23(1):107–13. Epub 2011/12/06. eng. [DOI] [PubMed] [Google Scholar]
- 101.Faroja M, Ahmed M, Appelbaum L, Ben-David E, Moussa M, Sosna J, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology 2013. February;266(2):462–70. Epub 2012/11/22. eng. [DOI] [PubMed] [Google Scholar]
- 102.Golberg A, Yarmush ML. Nonthermal irreversible electroporation: fundamentals, applications, and challenges. IEEE Trans Biomed Eng. 2013. March;60(3):707–14. Epub 2013/01/15. eng. [DOI] [PubMed] [Google Scholar]
- 103.Lee YJ, Lu DS, Osuagwu F, Lassman C. Irreversible electroporation in porcine liver: short- and long-term effect on the hepatic veins and adjacent tissue by CT with pathological correlation. Invest Radiol. 2012. November;47(11):671–5. Epub 2012/10/06. eng. [DOI] [PubMed] [Google Scholar]
- 104.Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011. May;22(5):611–21. Epub 2011/03/29. eng. [DOI] [PubMed] [Google Scholar]
- 105.Kingham TP, Karkar AM, D’Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. Journal of the American College of Surgeons. 2012. September;215(3):379–87. Epub 2012/06/19. eng. [DOI] [PubMed] [Google Scholar]
- 106.Eller A, Schmid A, Schmidt J, May M, Brand M, Saake M, et al. Local Control of Perivascular Malignant Liver Lesions Using Percutaneous Irreversible Electroporation: Initial Experiences. Cardiovascular and interventional radiology. 2014. May 6 Epub 2014/05/07. Eng. [DOI] [PubMed] [Google Scholar]
- 107.Rivoire M, De CF, Meeus P, Negrier S, Sebban H, Kaemmerlen P. Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer. 2002;95(11):2283–92. [DOI] [PubMed] [Google Scholar]
- 108.Abdalla EK, Vauthey JN. Technique and patient selection, not the needle, determine outcome of percutaneous intervention for hepatocellular carcinoma. Annals of Surgical Oncology. 2004;11(3):240–1. [DOI] [PubMed] [Google Scholar]
- 109.Karanicolas PJ, Jarnagin WR, Gonen M, Tuorto S, Allen PJ, DeMatteo RP, et al. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA surgery. 2013. July;148(7):597–601. Epub 2013/05/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Evrard S, Poston G, Kissmeyer-Nielsen P, Diallo A, Desolneux G, Brouste V, et al. Combined ablation and resection (CARe) as an effective parenchymal sparing treatment for extensive colorectal liver metastases. PloS one. 2014;9(12):e114404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruers T, Punt CJ, van Coevorden F, Pierie J-P, Borel Rinkes I, Ledermann JA, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): Long-term survival results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). American Society of Clinical Oncology; 2015. [Google Scholar]
- 112.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Seminars in oncology. 1983. June;10(2):176–82. [PubMed] [Google Scholar]
- 113.Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. The New England journal of medicine. 1999. December 30;341(27):2039–48. [DOI] [PubMed] [Google Scholar]
- 114.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. The New England journal of medicine. 2005. February 17;352(7):734–5. [DOI] [PubMed] [Google Scholar]
- 115.Kemeny MM, Adak S, Gray B, Macdonald JS, Smith T, Lipsitz S, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002. March 15;20(6):1499–505. [DOI] [PubMed] [Google Scholar]
- 116.Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006. March 20;24(9):1395–403. [DOI] [PubMed] [Google Scholar]
- 117.Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009. July 20;27(21):3465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.D’Angelica MI, Correa-Gallego C, Paty PB, Cercek A, Gewirtz AN, Chou JF, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015. February;261(2):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kemeny N, Capanu M, D’Angelica M, Jarnagin W, Haviland D, DeMatteo R, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20(7):1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kemeny N, Jarnagin W, Paty P, Gonen M, Schwartz L, Morse M, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(22):4888–96. [DOI] [PubMed] [Google Scholar]
- 121.Groot Koerkamp B, Sadot E, Kemeny NE, Gonen M, Leal JN, Allen PJ, et al. Perioperative Hepatic Arterial Infusion Pump Chemotherapy Is Associated With Longer Survival After Resection of Colorectal Liver Metastases: A Propensity Score Analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017. June 10;35(17):1938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kemeny NE, Chou JF, Boucher TM, Capanu M, DeMatteo RP, Jarnagin WR, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. Journal of surgical oncology. 2016. April;113(5):477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Passot G, Odisio BC, Zorzi D, Mahvash A, Gupta S, Wallace MJ, et al. Eradication of Missing Liver Metastases After Fiducial Placement. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2016. June;20(6):1173–8. [DOI] [PubMed] [Google Scholar]
- 124.Owen JW, Fowler KJ, Doyle MB, Saad NE, Linehan DC, Chapman WC. Colorectal liver metastases: disappearing lesions in the era of Eovist hepatobiliary magnetic resonance imaging. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2016. March;18(3):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tani K, Shindoh J, Akamatsu N, Arita J, Kaneko J, Sakamoto Y, et al. Management of disappearing lesions after chemotherapy for colorectal liver metastases: Relation between detectability and residual tumors. Journal of surgical oncology. 2018. February;117(2):191–7. [DOI] [PubMed] [Google Scholar]
- 126.van Vledder MG, de Jong MC, Pawlik TM, Schulick RD, Diaz LA, Choti MA. Disappearing colorectal liver metastases after chemotherapy: should we be concerned? Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010. November;14(11):1691–700. [DOI] [PubMed] [Google Scholar]
- 127.Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Annals Of Surgery. 1996;224(4):509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clavien PA, Selzner N, Morse M, Selzner M, Paulson E. Downstaging of hepatocellular carcinoma and liver metastases from colorectal cancer by selective intra-arterial chemotherapy. Surgery. 2002;131(4):433–42. [DOI] [PubMed] [Google Scholar]
- 129.Kemeny NE, Huitzil Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, et al. Conversion to Resectability Using Hepatic Artery Infusion Plus Systemic Chemotherapy for the Treatment of Unresectable Liver Metastases From Colorectal Carcinoma. J ClinOncol. 2009;27(21):3465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J ClinOncol. 2005;23(36):9243–9. [DOI] [PubMed] [Google Scholar]
- 131.Fischer C, Melstrom LG, Arnaoutakis D, Jarnagin W, Brown K, D’Angelica M, et al. Chemotherapy after portal vein embolization to protect against tumor growth during liver hypertrophy before hepatectomy. JAMA surgery. 2013. December;148(12):1103–8. Epub 2013/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 132.Correa D, Schwartz L, Jarnagin WR, Tuorto S, DeMatteo R, D’Angelica M, et al. Kinetics of liver volume changes in the first year after portal vein embolization. ArchSurg. 2010;145(4):351–4. [DOI] [PubMed] [Google Scholar]
- 133.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical Resection of Pulmonary Metastases From Colorectal Cancer: A Systemic Review of Published Series. Ann Thorac Surg. 2007;84:324–38. [DOI] [PubMed] [Google Scholar]
- 134.Parnaby CN, Bailey W, Balasingam A, Beckert L, Eglinton T, Fife J, et al. Pulmonary staging in colorectal cancer: a review. Colorectal Dis. 2012;14:660–70. [DOI] [PubMed] [Google Scholar]
- 135.Tan KK, Lopes Gde L Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2009;13(4):642–8. [DOI] [PubMed] [Google Scholar]
- 136.Mise Y, Kopetz S, Mehran RJ, Aloia TA, Conrad C, Brudvik KW, et al. Is complete liver resection without resection of synchronous lung metastases justified? Ann Surg Oncol. 2015;22(5):1585–92. [DOI] [PubMed] [Google Scholar]
- 137.Migliore M, Milosevic M, Lees B, Treasure T, Di Maria G. Finding the evidence for pulmonary metastasectomy in colorectal cancer: the PulMicc trial. Future Oncol. 2015;11(2 Suppl):15–8. [DOI] [PubMed] [Google Scholar]
- 138.Aberg T, Malmberg K, Nilsson B, Nou E. The Effect of Metastasectomy: Fact of Fiction? Ann Thorac Surg. 1980;30:378–84. [DOI] [PubMed] [Google Scholar]
- 139.Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997. January;113(1):37–49. Epub 1997/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 140.Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009. February;16(2):327–33. [DOI] [PubMed] [Google Scholar]
- 141.Macherey S, Doerr F, Heldwin M, Khekmat K. Is manual palpation of the lung necessary in patients undergoing pulmonary metastasectomy? Interact Cardiovasc Thorac Surg. 2016. March;22(3):351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marron C, Lora D, Rivas JJ, Embun R, Molins L, de la Cruz J, et al. Agreement Between Computed Tomography and Pathologic Nodule Counts in Colorectal Lung Metastases. Ann Thorac Surg. 2016. January;101(1):259–65. [DOI] [PubMed] [Google Scholar]
- 143.CR J, BA S, McCarty TP, Minnich DJ. A Prospective Study to Determine the Incidence of Non-Imaged Malignant Pulmonary Nodules in Patients Who Undergo Metastasectomy by Thoracotomy With Lung Palpation. Ann Thorac Surg. 2011. June;91(6):1696–700. [DOI] [PubMed] [Google Scholar]
- 144.Internullo E, Cassivi SD, Van Raemdonck D, Friedel G, Treasure T, Group EPMW. Pulmonary Metastasectomy: A Survey of Current Practice Amongst Members of the European Society of Thoracic Surgeons. J Thorac Onc. 2008. November;3(11):1257–66. [DOI] [PubMed] [Google Scholar]
- 145.Eckardt J, Licht PB. Thoracoscopic versus open pulmonary metastasectomy: a prospective, sequentially controlled study. Chest. 2012. December;142(6):1598–602. [DOI] [PubMed] [Google Scholar]
- 146.Murakawa T, Sato H, Okumura S, Nakajima J, Horio H, Ozeki Y, et al. Thoracoscopic surgery versus open surgery for lung metastases of colorectal cancer: a multi-institutional retrospective analysis using propensity score adjustment. Eur J Cardiothorac Surg. 2017. June;51(6):1157–63. [DOI] [PubMed] [Google Scholar]
- 147.Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg. 2014. August;98(2):471–5; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 148.Molnar TF, Gebitekin C, Turna A. What are the considerations in the surgical approach in pulmonary metastasectomy? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2010. June;5(6 Suppl 2):S140–4. [DOI] [PubMed] [Google Scholar]
- 149.Hamaji M, Cassivi SD, Shen KR, Allen MS, Nichols FC, Deschamps C, et al. Is lymph node dissection required in pulmonary metastasectomy for colorectal adenocarcinoma? Ann Thorac Surg. 2012. December;94(6):1796–800. [DOI] [PubMed] [Google Scholar]
- 150.Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014. March;25(3):651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tseng J, Bryan DS, Poli E, Sharma M, Polite BN, Turaga KK. Under-representation of peritoneal metastases in published clinical trials of metastatic colorectal cancer. Lancet Oncol. 2017. June;18(6):711–2. [DOI] [PubMed] [Google Scholar]
- 152.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012. January 20;30(3):263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Levinson KL, Belinson JL. Peritoneal carcinomatosis: signs and symptoms. Advances in the Management of Peritoneal Carcinomatosis. p. 6–15. [Google Scholar]
- 154.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012. May;99(5):699–705. [DOI] [PubMed] [Google Scholar]
- 155.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002. December;89(12):1545–50. [DOI] [PubMed] [Google Scholar]
- 156.Willett C, Tepper JE, Cohen A, Orlow E, Welch C. Obstructive and perforative colonic carcinoma: patterns of failure. J Clin Oncol. 1985. March;3(3):379–84. [DOI] [PubMed] [Google Scholar]
- 157.Fujii S, Matsusue E, Kanasaki Y, Kanamori Y, Nakanishi J, Sugihara S, et al. Detection of peritoneal dissemination in gynecological malignancy: evaluation by diffusion-weighted MR imaging. Eur Radiol. 2008. January;18(1):18–23. [DOI] [PubMed] [Google Scholar]
- 158.Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol. 2009. August;193(2):461–70. [DOI] [PubMed] [Google Scholar]
- 159.Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2012. May;19(5):1394–401. [DOI] [PubMed] [Google Scholar]
- 160.Fehniger J, Thomas S, Lengyel E, Liao C, Tenney M, Oto A, et al. A prospective study evaluating diffusion weighted magnetic resonance imaging (DW-MRI) in the detection of peritoneal carcinomatosis in suspected gynecologic malignancies. Gynecol Oncol. 2016. July;142(1):169–75. [DOI] [PubMed] [Google Scholar]
- 161.Dohan A, Hoeffel C, Soyer P, Jannot AS, Valette PJ, Thivolet A, et al. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br J Surg. 2017. August;104(9):1244–9. [DOI] [PubMed] [Google Scholar]
- 162.Chang MC, Chen JH, Liang JA, Huang WS, Cheng KY, Kao CH. PET or PET/CT for detection of peritoneal carcinomatosis: a meta-analysis. Clin Nucl Med. 2013. August;38(8):623–9. [DOI] [PubMed] [Google Scholar]
- 163.Kim SJ, Lee SW. Diagnostic accuracy of (18)F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis. Br J Radiol. 2018. January;91(1081):20170519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Passot G, Dumont F, Goere D, Arvieux C, Rousset P, Regimbeau JM, et al. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br J Surg. 2018. May;105(6):663–7. [DOI] [PubMed] [Google Scholar]
- 165.Seshadri RA, Hemanth Raj E. Diagnostic Laparoscopy in the Pre-operative Assessment of Patients Undergoing Cytoreductive Surgery and HIPEC for Peritoneal Surface Malignancies. Indian J Surg Oncol. 2016. June;7(2):230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Iversen LH, Rasmussen PC, Laurberg S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg. 2013. January;100(2):285–92. [DOI] [PubMed] [Google Scholar]
- 167.Treasure T, Monson K, Fiorentino F, Russell C. The CEA Second-Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ Open. 2014. May 13;4(5):e004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004. June 3;350(23):2343–51. [DOI] [PubMed] [Google Scholar]
- 169.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009. July 1;27(19):3109–16. [DOI] [PubMed] [Google Scholar]
- 170.Goere D, Glehen O, Quenet F, Ducreux M, Guilloit J-M, Texier M, et al. Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-NTC01226394). Journal of Clinical Oncology. 2018;36(15_suppl):3531-. [Google Scholar]
- 171.Sugarbaker PH. Surgical treatment of peritoneal carcinomatosis: 1988 Du Pont lecture. Can J Surg. 1989. May;32(3):164–70. [PubMed] [Google Scholar]
- 172.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008. September;15(9):2426–32. [DOI] [PubMed] [Google Scholar]
- 173.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003. October 15;21(20):3737–43. [DOI] [PubMed] [Google Scholar]
- 174.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004. January 1;22(1):23–30. [DOI] [PubMed] [Google Scholar]
- 175.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010. January 1;28(1):63–8. [DOI] [PubMed] [Google Scholar]
- 176.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004. August 15;22(16):3284–92. [DOI] [PubMed] [Google Scholar]
- 177.Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. Journal of Clinical Oncology. 2018;36(18_suppl):LBA3503–LBA. [Google Scholar]
- 178.Elez E, Argiles G, Tabernero J. First-Line Treatment of Metastatic Colorectal Cancer: Interpreting FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol. 2015. November;16(11):52. [DOI] [PubMed] [Google Scholar]
- 179.Nowacki M, Alyami M, Villeneuve L, Mercier F, Hubner M, Willaert W, et al. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: An international survey study. Eur J Surg Oncol. 2018. July;44(7):991–6. [DOI] [PubMed] [Google Scholar]
- 180.Togawa D, Lewandrowski K. The Pathophysiology of Spinal Metastases In: R M, R M, R B, M M, K L, Benzel EC, editors. Cancer in the Spine: Comprehensive Care (Current Clinical Oncology). Dordrecht Netherlands: Humana Press Incorporated; 2006. p. 17–23. [Google Scholar]
- 181.Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991. March;9(3):509–24. [DOI] [PubMed] [Google Scholar]
- 182.Kanthan R, Loewy J, Kanthan SC. Skeletal metastases in colorectal carcinomas: a Saskatchewan profile. Dis Colon Rectum. 1999. December;42(12):1592–7. [DOI] [PubMed] [Google Scholar]
- 183.Sundermeyer ML, Meropol NJ, Rogatko A, Wang H, Cohen SJ. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clinical colorectal cancer. 2005. July;5(2):108–13. [DOI] [PubMed] [Google Scholar]
- 184.Kawamura H, Yamaguchi T, Yano Y, Hozumi T, Takaki Y, Matsumoto H, et al. Characteristics and Prognostic Factors of Bone Metastasis in Patients With Colorectal Cancer. Dis Colon Rectum. 2018. June;61(6):673–8. [DOI] [PubMed] [Google Scholar]
- 185.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006. October 15;12(20 Pt 2):6243s–9s. [DOI] [PubMed] [Google Scholar]
- 186.Watkins LR, Goehler LE, Relton J, Brewer MT, Maier SF. Mechanisms of tumor necrosis factor-alpha (TNF-alpha) hyperalgesia. Brain research. 1995. September 18;692(1–2):244–50. [DOI] [PubMed] [Google Scholar]
- 187.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. British journal of pharmacology. 1997. June;121(3):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nature reviews Cancer. 2002. March;2(3):201–9. [DOI] [PubMed] [Google Scholar]
- 189.Orr FW, Lee J, Duivenvoorden WC, Singh G. Pathophysiologic interactions in skeletal metastasis. Cancer. 2000. June 15;88(12 Suppl):2912–8. [DOI] [PubMed] [Google Scholar]
- 190.van Kessel CS, Samim M, Koopman M, van den Bosch MA, Borel Rinkes IH, Punt CJ, et al. Radiological heterogeneity in response to chemotherapy is associated with poor survival in patients with colorectal liver metastases. Eur J Cancer. 2013. July;49(11):2486–93. [DOI] [PubMed] [Google Scholar]
- 191.Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar R, et al. NCCN Task Force Report: Bone Health In Cancer Care. Journal of the National Comprehensive Cancer Network : JNCCN. 2013. August;11 Suppl 3:S1–50; quiz S1. [DOI] [PubMed] [Google Scholar]
- 192.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clinical oncology. 2012. March;24(2):112–24. [DOI] [PubMed] [Google Scholar]
- 193.Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014. September 10;32(26):2913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.van der Linden YM, Steenland E, van Houwelingen HC, Post WJ, Oei B, Marijnen CA, et al. Patients with a favourable prognosis are equally palliated with single and multiple fraction radiotherapy: results on survival in the Dutch Bone Metastasis Study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2006. March;78(3):245–53. [DOI] [PubMed] [Google Scholar]
- 195.Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001. October;221(1):159–66. [DOI] [PubMed] [Google Scholar]
- 196.Healey TT, Dupuy DE. Radiofrequency ablation: a safe and effective treatment in nonoperative patients with early-stage lung cancer. Cancer journal. 2011. Jan-Feb;17(1):33–7. [DOI] [PubMed] [Google Scholar]
- 197.Psutka SP, McGovern FJ, Mueller P, McDougal WS, Gervais D, Feldman AS. Long-term durable oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012. February 10;30(5_suppl):384. [Google Scholar]
- 198.Bang HJ, Littrup PJ, Currier BP, Goodrich DJ, Aoun HD, Klein LC, et al. Percutaneous cryoablation of metastatic lesions from non-small-cell lung carcinoma: initial survival, local control, and cost observations. J Vasc Interv Radiol. 2012. June;23(6):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bang HJ, Littrup PJ, Goodrich DJ, Currier BP, Aoun HD, Heilbrun LK, et al. Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation. J Vasc Interv Radiol. 2012. June;23(6):770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barral M, Auperin A, Hakime A, Cartier V, Tacher V, Otmezguine Y, et al. Percutaneous Thermal Ablation of Breast Cancer Metastases in Oligometastatic Patients. Cardiovascular and interventional radiology. 2016. June;39(6):885–93. [DOI] [PubMed] [Google Scholar]
- 201.Erie AJ, Morris JM, Welch BT, Kurup AN, Weisbrod AJ, Atwell TD, et al. Retrospective Review of Percutaneous Image-Guided Ablation of Oligometastatic Prostate Cancer: A Single-Institution Experience. J Vasc Interv Radiol. 2017. July;28(7):987–92. [DOI] [PubMed] [Google Scholar]
- 202.Ahmed M, Brace CL, Lee FT Jr., Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011. February;258(2):351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McWilliams JP, Lee EW, Yamamoto S, Loh CT, Kee ST. Image-guided tumor ablation: emerging technologies and future directions. Seminars in interventional radiology. 2010. September;27(3):302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology. 2009. February;41(2):168–72. [DOI] [PubMed] [Google Scholar]
- 205.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002. January;178(1):47–51. [DOI] [PubMed] [Google Scholar]
- 206.Eckmann MS, Martinez MA, Lindauer S, Khan A, Ramamurthy S. Radiofrequency ablation near the bone-muscle interface alters soft tissue lesion dimensions. Regional anesthesia and pain medicine. 2015. May-Jun;40(3):270–5. [DOI] [PubMed] [Google Scholar]
- 207.Engel A, Rappard G, King W, Kennedy DJ, Standards Division of the International Spine Intervention S. The Effectiveness and Risks of Fluoroscopically-Guided Cervical Medial Branch Thermal Radiofrequency Neurotomy: A Systematic Review with Comprehensive Analysis of the Published Data. Pain medicine. 2016. April;17(4):658–69. [DOI] [PubMed] [Google Scholar]
- 208.Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain physician. 2013. April;16(2 Suppl):S49–283. [PubMed] [Google Scholar]
- 209.Lanza E, Thouvenin Y, Viala P, Sconfienza LM, Poretti D, Cornalba G, et al. Osteoid osteoma treated by percutaneous thermal ablation: when do we fail? A systematic review and guidelines for future reporting. Cardiovascular and interventional radiology. 2014. December;37(6):1530–9. [DOI] [PubMed] [Google Scholar]
- 210.Yamakado K, Matsumine A, Nakamura T, Nakatsuka A, Takaki H, Matsubara T, et al. Radiofrequency ablation for the treatment of recurrent bone and soft-tissue sarcomas in non-surgical candidates. International journal of clinical oncology. 2014. October;19(5):955–62. [DOI] [PubMed] [Google Scholar]
- 211.Callstrom MR, Charboneau JW, Goetz MP, Rubin J, Wong GY, Sloan JA, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002. July;224(1):87–97. [DOI] [PubMed] [Google Scholar]
- 212.Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004. January 15;22(2):300–6. [DOI] [PubMed] [Google Scholar]
- 213.Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010. February 15;116(4):989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Madaelil TP, Wallace AN, Jennings JW. Radiofrequency ablation alone or in combination with cementoplasty for local control and pain palliation of sacral metastases: preliminary results in 11 patients. Skeletal radiology. 2016. September;45(9):1213–9. [DOI] [PubMed] [Google Scholar]
- 215.Munk PL, Rashid F, Heran MK, Papirny M, Liu DM, Malfair D, et al. Combined cementoplasty and radiofrequency ablation in the treatment of painful neoplastic lesions of bone. J Vasc Interv Radiol. 2009. July;20(7):903–11. [DOI] [PubMed] [Google Scholar]
- 216.Hoffmann RT, Jakobs TF, Trumm C, Weber C, Helmberger TK, Reiser MF. Radiofrequency ablation in combination with osteoplasty in the treatment of painful metastatic bone disease. J Vasc Interv Radiol. 2008. March;19(3):419–25. [DOI] [PubMed] [Google Scholar]
- 217.Wells SA, Hinshaw JL, Lubner MG, Ziemlewicz TJ, Brace CL, Lee FT Jr. Liver Ablation: Best Practice. Radiologic clinics of North America. 2015. September;53(5):933–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Aubry S, Dubut J, Nueffer JP, Chaigneau L, Vidal C, Kastler B. Prospective 1-year follow-up pilot study of CT-guided microwave ablation in the treatment of bone and soft-tissue malignant tumours. Eur Radiol. 2017. April;27(4):1477–85. [DOI] [PubMed] [Google Scholar]
- 219.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Current problems in diagnostic radiology. 2009. May-Jun;38(3):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovascular and interventional radiology. 2013. April;36(2):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008. July;19(7):1087–92. [DOI] [PubMed] [Google Scholar]
- 222.Huang S, Yu J, Liang P, Yu X, Cheng Z, Han Z, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. European journal of radiology. 2014. March;83(3):552–8. [DOI] [PubMed] [Google Scholar]
- 223.Pusceddu C, Sotgia B, Fele RM, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol. 2013. February;24(2):229–33. [DOI] [PubMed] [Google Scholar]
- 224.Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr., Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics : a review publication of the Radiological Society of North America, Inc. 2014. Sep-Oct;34(5):1344–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kastler A, Alnassan H, Pereira PL, Alemann G, Barbe DA, Aubry S, et al. Analgesic effects of microwave ablation of bone and soft tissue tumors under local anesthesia. Pain medicine. 2013. December;14(12):1873–81. [DOI] [PubMed] [Google Scholar]
- 226.Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002. August;60(2 Suppl 1):40–9. [DOI] [PubMed] [Google Scholar]
- 227.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998. November;37(3):171–86. [DOI] [PubMed] [Google Scholar]
- 228.Welch BT, Callstrom MR, Morris JM, Kurup AN, Schmit GD, Weisbrod AJ, et al. Feasibility and oncologic control after percutaneous image guided ablation of metastatic renal cell carcinoma. The Journal of urology. 2014. August;192(2):357–63. [DOI] [PubMed] [Google Scholar]
- 229.Callstrom MR, Dupuy DE, Solomon SB, Beres RA, Littrup PJ, Davis KW, et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer. 2013. March 01;119(5):1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.McMenomy BP, Kurup AN, Johnson GB, Carter RE, McWilliams RR, Markovic SN, et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol. 2013. February;24(2):207–13. [DOI] [PubMed] [Google Scholar]
- 231.Prologo JD, Passalacqua M, Patel I, Bohnert N, Corn DJ. Image-guided cryoablation for the treatment of painful musculoskeletal metastatic disease: a single-center experience. Skeletal radiology. 2014. November;43(11):1551–9. [DOI] [PubMed] [Google Scholar]
- 232.Wallace AN, McWilliams SR, Connolly SE, Symanski JS, Vaswani D, Tomasian A, et al. Percutaneous Image-Guided Cryoablation of Musculoskeletal Metastases: Pain Palliation and Local Tumor Control. J Vasc Interv Radiol. 2016. December;27(12):1788–96. [DOI] [PubMed] [Google Scholar]
- 233.Callstrom MR, Kurup AN. Percutaneous ablation for bone and soft tissue metastases--why cryoablation? Skeletal radiology. 2009. September;38(9):835–9. [DOI] [PubMed] [Google Scholar]
- 234.Nazario J, Hernandez J, Tam AL. Thermal ablation of painful bone metastases. Techniques in vascular and interventional radiology. 2011. September;14(3):150–9. [DOI] [PubMed] [Google Scholar]
- 235.Bing F, Garnon J, Tsoumakidou G, Enescu I, Ramamurthy N, Gangi A. Imaging-guided percutaneous cryotherapy of bone and soft-tissue tumors: what is the impact on the muscles around the ablation site? AJR Am J Roentgenol. 2014. June;202(6):1361–5. [DOI] [PubMed] [Google Scholar]
- 236.Koethe Y, Mannes AJ, Wood BJ. Image-guided nerve cryoablation for post-thoracotomy pain syndrome. Cardiovascular and interventional radiology. 2014. June;37(3):843–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Thacker PG, Callstrom MR, Curry TB, Mandrekar JN, Atwell TD, Goetz MP, et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients’ immediate response to radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2011. August;197(2):510–5. [DOI] [PubMed] [Google Scholar]
- 238.Allaf ME, Varkarakis IM, Bhayani SB, Inagaki T, Kavoussi LR, Solomon SB. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation--initial observations. Radiology. 2005. October;237(1):366–70. [DOI] [PubMed] [Google Scholar]
- 239.Havez M, Lippa N, Al-Ammari S, Kind M, Stoeckle E, Italiano A, et al. Percutaneous image-guided cryoablation in inoperable extra-abdominal desmoid tumors: a study of tolerability and efficacy. Cardiovascular and interventional radiology. 2014. December;37(6):1500–6. [DOI] [PubMed] [Google Scholar]
- 240.Li C, Zhang W, Fan W, Huang J, Zhang F, Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer. 2010. August 15;116(16):3934–42. [DOI] [PubMed] [Google Scholar]
- 241.Chen W, Zhu H, Zhang L, Li K, Su H, Jin C, et al. Primary bone malignancy: effective treatment with high-intensity focused ultrasound ablation. Radiology. 2010. June;255(3):967–78. [DOI] [PubMed] [Google Scholar]
- 242.Thompson SM, Schmitz JJ, Schmit GD, Callstrom MR, Kurup AN. Image-Guided Thermal Ablative Therapies in the Treatment of Sarcoma. Curr Treat Options Oncol. 2017. April;18(4):25. [DOI] [PubMed] [Google Scholar]
- 243.Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. Journal of the National Cancer Institute. 2014. April 23;106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hynynen K MRI-guided focused ultrasound treatments. Ultrasonics. 2010. February;50(2):221–9. [DOI] [PubMed] [Google Scholar]
- 245.Kim YS, Rhim H, Choi MJ, Lim HK, Choi D. High-intensity focused ltrasound therapy: an overview for radiologists. Korean journal of radiology. 2008. Jul-Aug;9(4):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bucknor MD, Ozhinsky E, Shah R, Krug R, Rieke V. Effect of Sonication Duration and Power on Ablation Depth During MR-Guided Focused Ultrasound of Bone. Journal of magnetic resonance imaging : JMRI. 2017. February 22. [DOI] [PubMed] [Google Scholar]
- 247.Napoli A, Anzidei M, Marincola BC, Brachetti G, Noce V, Boni F, et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013. October;33(6):1555–68. [DOI] [PubMed] [Google Scholar]
- 248.Mavrogenis AF, Angelini A, Vottis C, Pala E, Calabro T, Papagelopoulos PJ, et al. Modern Palliative Treatments for Metastatic Bone Disease: Awareness of Advantages, Disadvantages, and Guidance. The Clinical journal of pain. 2016. April;32(4):337–50. [DOI] [PubMed] [Google Scholar]
- 249.Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003. August 1;21(15):2912–9. Epub 2003/07/30. eng. [DOI] [PubMed] [Google Scholar]
- 250.Bennett JJ, Schmidt CR, Klimstra DS, Grobmyer SR, Ishill NM, D’Angelica M, et al. Perihepatic lymph node micrometastases impact outcome after partial hepatectomy for colorectal metastases. Ann Surg Oncol. 2008. April;15(4):1130–6. Epub 2008/01/30. eng. [DOI] [PubMed] [Google Scholar]
- 251.Okuno M, Goumard C, Mizuno T, Kopetz S, Omichi K, Tzeng CD, et al. Prognostic impact of perihepatic lymph node metastases in patients with resectable colorectal liver metastases. Br J Surg. 2018. April 17 Epub 2018/04/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nanji S, Tsang ME, Wei X, Booth CM. Regional lymph node involvement in patients undergoing liver resection for colorectal cancer metastases. Eur J Surg Oncol. 2017. February;43(2):322–9. Epub 2017/01/07. eng. [DOI] [PubMed] [Google Scholar]
- 253.Bradatsch A, Kornprat P, Bacher H, Cerwenka H, Haybaeck J, Mischinger HJ. The Value of Lymph Node Dissection in the Surgery of Colorectal Cancer Liver Metastases. Anticancer research. 2016. June;36(6):2993–7. Epub 2016/06/09. eng. [PubMed] [Google Scholar]
- 254.Pindak D, Pavlendova J, Tomas M, Dolnik J, Duchon R, Pechan J. Selective versus routine lymphadenectomy in the treatment of liver metastasis from colorectal cancer: a retrospective cohort study. BMC surgery. 2017. April 4;17(1):34 Epub 2017/04/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Perini MV, Tai J, Muralidharan V, Christophi C. Sentinel lymph node mapping in liver resection for colorectal liver metastases. ANZ journal of surgery. 2018. February 8 Epub 2018/02/10. eng. [DOI] [PubMed] [Google Scholar]
- 256.Lu JY, Xu L, Xue HD, Zhou WX, Xu T, Qiu HZ, et al. The Radical Extent of lymphadenectomy - D2 dissection versus complete mesocolic excision of LAparoscopic Right Colectomy for right-sided colon cancer (RELARC) trial: study protocol for a randomized controlled trial. Trials. 2016. December 8;17(1):582 Epub 2016/12/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wong JS, Tan GH, Teo MC. Management of para-aortic lymph node metastasis in colorectal patients: A systemic review. Surgical oncology. 2016. December;25(4):411–8. Epub 2016/12/06. eng. [DOI] [PubMed] [Google Scholar]
- 258.Tsai HL, Chen YT, Yeh YS, Huang CW, Ma CJ, Wang JY. Apical Lymph Nodes in the Distant Metastases and Prognosis of Patients with Stage III Colorectal Cancer with Adequate Lymph Node Retrieval Following FOLFOX Adjuvant Chemotherapy. Pathology oncology research : POR. 2018. January 3 Epub 2018/01/05. eng. [DOI] [PubMed] [Google Scholar]
- 259.Bae SU, Han YD, Cho MS, Hur H, Min BS, Baik SH, et al. Oncologic Outcomes of Colon Cancer Patients with Extraregional Lymph Node Metastasis: Comparison of Isolated Paraaortic Lymph Node Metastasis with Resectable Liver Metastasis. Ann Surg Oncol. 2016. May;23(5):1562–8. Epub 2015/12/31. eng. [DOI] [PubMed] [Google Scholar]
- 260.Bae SU, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Which Patients with Isolated Para-Aortic Lymph Node Metastasis Will Truly Benefit from Extended Lymph Node Dissection for Colon Cancer? Cancer research and treatment : official journal of Korean Cancer Association. 2017. July 14 Epub 2017/07/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Choi PW, Kim HC, Kim AY, Jung SH, Yu CS, Kim JC. Extensive lymphadenectomy in colorectal cancer with isolated para-aortic lymph node metastasis below the level of renal vessels. J Surg Oncol. 2010. January 1;101(1):66–71. [DOI] [PubMed] [Google Scholar]
- 262.Song SH, Park SY, Park JS, Kim HJ, Yang CS, Choi GS. Laparoscopic para-aortic lymph node dissection for patients with primary colorectal cancer and clinically suspected para-aortic lymph nodes. Annals of surgical treatment and research. 2016. January;90(1):29–35. Epub 2016/01/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ishihara S, Kawai K, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, et al. Oncological Outcomes of Lateral Pelvic Lymph Node Metastasis in Rectal Cancer Treated With Preoperative Chemoradiotherapy. Dis Colon Rectum. 2017. May;60(5):469–76. Epub 2017/04/07. eng. [DOI] [PubMed] [Google Scholar]
- 264.Kusters M, Slater A, Muirhead R, Hompes R, Guy RJ, Jones OM, et al. What To Do With Lateral Nodal Disease in Low Locally Advanced Rectal Cancer? A Call for Further Reflection and Research. Dis Colon Rectum. 2017. June;60(6):577–85. Epub 2017/05/10. eng. [DOI] [PubMed] [Google Scholar]
- 265.Kim MJ, Hur BY, Lee ES, Park B, Joo J, Kim MJ, et al. Prediction of lateral pelvic lymph node metastasis in patients with locally advanced rectal cancer with preoperative chemoradiotherapy: Focus on MR imaging findings. PloS one. 2018;13(4):e0195815 Epub 2018/04/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yamaguchi T, Konishi T, Kinugasa Y, Yamamoto S, Akiyoshi T, Okamura R, et al. Laparoscopic Versus Open Lateral Lymph Node Dissection for Locally Advanced Low Rectal Cancer: A Subgroup Analysis of a Large Multicenter Cohort Study in Japan. Dis Colon Rectum. 2017. September;60(9):954–64. Epub 2017/08/11. eng. [DOI] [PubMed] [Google Scholar]
- 267.Christensen TD, Spindler KL, Palshof JA, Nielsen DL. Systematic review: brain metastases from colorectal cancer--Incidence and patient characteristics. BMC cancer. 2016. April 1;16:260 Epub 2016/04/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Scientific reports. 2016. July 15;6:29765 Epub 2016/07/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yaeger R, Cowell E, Chou JF, Gewirtz AN, Borsu L, Vakiani E, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015. April 15;121(8):1195–203. Epub 2014/12/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Qiu M, Hu J, Yang D, Cosgrove DP, Xu R. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015. November 17;6(36):38658–66. Epub 2015/10/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zoratto F, Loupakis F, Cremolini C, Salvatore L, Schirripa M, Antoniotti C, et al. Risk for developing brain metastases from colorectal cancer in long-term survivors with lung metastases and KRAS mutations. Journal of Clinical Oncology. 2013;31(15_suppl):e14574–e. [Google Scholar]
- 272.Farnell GF, Buckner JC, Cascino TL, O’Connell MJ, Schomberg PJ, Suman V. Brain metastases from colorectal carcinoma. The long term survivors. Cancer. 1996. August 15;78(4):711–6. Epub 1996/08/15. eng. [DOI] [PubMed] [Google Scholar]
- 273.Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro-oncology. 2017. February 1;19(2):162–74. Epub 2017/04/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sinha R, Sage W, Watts C. The evolving clinical management of cerebral metastases. Eur J Surg Oncol. 2017. July;43(7):1173–85. Epub 2016/12/18. eng. [DOI] [PubMed] [Google Scholar]
- 275.Donato J, Campigotto F, Uhlmann EJ, Coletti E, Neuberg D, Weber GM, et al. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood. 2015. July 23;126(4):494–9. Epub 2015/05/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Silva IL, Iskandarani M, Hotouras A, Murphy J, Bhan C, Adada B, et al. A systematic review to assess the management of patients with cerebral metastases secondary to colorectal cancer. Techniques in coloproctology. 2017. November;21(11):847–52. Epub 2017/11/11. eng. [DOI] [PubMed] [Google Scholar]
- 277.Morinaga N, Tanaka N, Shitara Y, Ishizaki M, Yoshida T, Kouga H, et al. Ten-Year Survival of a Patient Treated with Stereotactic Gamma Knife Radiosurgery for Brain Metastases from Colon Cancer with Ovarian and Lymph Node Metastases: A Case Report. Case reports in gastroenterology. 2016. Jan-Apr;10(1):199–206. Epub 2016/07/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. International journal of radiation oncology, biology, physics. 1997. March 1;37(4):745–51. Epub 1997/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 279.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1984. March;2(3):187–93. Epub 1984/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 280.Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. International journal of radiation oncology, biology, physics. 2010. July 1;77(3):655–61. Epub 2009/11/28. eng. [DOI] [PubMed] [Google Scholar]
- 281.Ewend MG, Morris DE, Carey LA, Ladha AM, Brem S. Guidelines for the initial management of metastatic brain tumors: role of surgery, radiosurgery, and radiation therapy. Journal of the National Comprehensive Cancer Network : JNCCN. 2008. May;6(5):505–13; quiz 14. [DOI] [PubMed] [Google Scholar]
- 282.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. The New England journal of medicine. 1990. February 22;322(8):494–500. Epub 1990/02/22. eng. [DOI] [PubMed] [Google Scholar]
- 283.Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Annals of neurology. 1993. June;33(6):583–90. Epub 1993/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 284.Kuzmik G, Long A, Bulent Omay S, Moliterno Günel J. The Usefulness of Stereotactic Neuro navigation Along with Intraoperative Imaging in Malignant Brain Tumor Surgery In: Moliterno Gunel J, Piepmeier JM, Baehring JM, editors. Malignant Brain Tumors : State-of the-Art Treatment. Cham: Springer International Publishing; 2017. p. 51–62. [Google Scholar]
- 285.Patel AJ, Suki D, Hatiboglu MA, Abouassi H, Shi W, Wildrick DM, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. Journal of neurosurgery. 2010. August;113(2):181–9. Epub 2009/12/29. eng. [DOI] [PubMed] [Google Scholar]
- 286.Patel AJ, Suki D, Hatiboglu MA, Rao VY, Fox BD, Sawaya R. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. Journal of neurosurgery. 2015. May;122(5):1132–43. Epub 2015/03/21. eng. [DOI] [PubMed] [Google Scholar]
- 287.Suh JH. Stereotactic radiosurgery for the management of brain metastases. The New England journal of medicine. 2010. March 25;362(12):1119–27. Epub 2010/03/26. eng. [DOI] [PubMed] [Google Scholar]
- 288.Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiation oncology (London, England). 2011. May 15;6:48 Epub 2011/05/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. Journal of neuro-oncology. 2008. May;87(3):299–307. Epub 2007/12/25. eng. [DOI] [PubMed] [Google Scholar]
- 290.Roos DE, Smith JG, Stephens SW. Radiosurgery versus surgery, both with adjuvant whole brain radiotherapy, for solitary brain metastases: a randomised controlled trial. Clinical oncology. 2011. November;23(9):646–51. Epub 2011/05/20. eng. [DOI] [PubMed] [Google Scholar]
- 291.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. Jama. 2016. July 26;316(4):401–9. Epub 2016/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011. January 10;29(2):134–41. Epub 2010/11/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kemeny NE, Chou JF, Capanu M, Gewirtz AN, Cercek A, Kingham TP, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer. 2014. August 25 Epub 2014/08/27. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Annals Of Surgery. 2004;240(4):644–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Barone C, Nuzzo G, Cassano A, Basso M, Schinzari G, Giuliante F, et al. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. BrJ Cancer. 2007;97(8):1035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]