ABSTRACT
Immunosurveillance is generally conceived as a mechanism through which the immune system detects and eliminates (pre-)malignant cells, thus reducing the risk of developing cancer. A recent paper by Ovadya et al. demonstrates that knockout of the gene coding for perforin-1 causes accelerated accumulation of senescent cells in multiple mouse organs, thereby speeding up the aging process. These results suggest that immunosurveillance plays a much broader role in maintaining organismal health than it had been suspected.
KEYWORDS: Age-related disease, cytotoxic T cells, NK cells, immunosenescence, senescence
Traditionally, the purpose of the immune system has been thought to fight off infectious agents to keep our internal organs close-to-sterile. For this reason, immunological research has laid much emphasis on the interaction between microbes and immune cells, as well as on the pathogen-host co-evolution that most likely has shaped the phylogeny of the immune system. Nonetheless, the notion that the immune cells would only recognize external antigens/pathogens was eroded by the discovery that pattern recognition receptors do not only recognize microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs) but also react with host cell-intrinsic danger-associated molecular patterns (DAMPs).1 Beyond, autoimmunity, a pathogenic deviation of the rule that lymphocytes expressing cognate immune receptors (i.e. T cell receptor, TCR, and B cell receptor, BCR) should only destroy cells expressing non-self- (but not self-) antigens,2 it later became clear that the immune system may also eliminate pre-malignant or overtly malignant cells through a process called immunosurveillance.3 Although this process may involve the recognition and destruction of cells through innate immune effectors (not involving cognate recognition by TCR or BCR) that recognize abnormal stress linked to cellular transformation, it may also occur through the cognate (mostly TCR-mediated) recognition of tumor-associated antigens including so-called neo-antigens that arise from mutations in (pre-)malignant cells.4 One possible explanation for this capacity of immunity was to postulate that the immune system evolved to recognize virus-encoded antigens in the context of host cell DAMPs and that it ‘incidentally’ can be hijacked to recognize cancer neo-antigen plus DAMPs, although it probably had not been evolutionarily selected to do so (given that cancer is a modern disease mainly manifesting at an old, post-reproductive age).5 The idea that the original purpose of the immune system is to control infection is seemingly corroborated by the discovery that the composition of the intestinal microbiota plays a major role in determining the efficacy of therapeutically induced immunosurveillance, for instance in the context of immune checkpoint blockade targeting the PD-1/PD-L1 interaction.6
Beyond this pathogen-centric view of the immune system, a few papers published in the past suggested that immune effectors would also be involved in the clearance of senescent cells (for review see Ref. 77). Cellular senescence is defined by a (usually) irreversible arrest of the cell cycle and is coupled with alterations in cellular morphology and function including the ‘senescence-associated secretory pattern’ (SASP). Ever accumulating evidence indicates that the loss of cellular functionality coupled with pro-inflammatory reactions resulting from SASP contributes to the acquisition of the aging phenotype. Thus, genetic manipulations or pharmacological agents that eliminate senescent cells (‘senolysis’) can prolong the median life span of mice and retard (and perhaps even reverse) age-related pathologies.8
A recent paper by Ovadya et al.9 now provides strong evidence for a direct link between failing immunosurveillance and accelerated aging. The authors show that perforin-1 deficient mice (genotype: Prf1−/-), which lack functional cytotoxic (T, NK or NKT) lymphocytes accumulate more senescent cells (phenotype: positive for senescence-associated-β-galactosidase, p16/Cdkn2a, p15/Cdkn2b, p53, p53BP1 foci, γH2AX foci, nuclear p65/RelA subunit of NF-κb, DcR2; negative for HMGB1) in multiple organs (such as bronchial epithelia, liver, pancreas and skin epidermis) along with signs of inflammation with overexpression of SASP components as well as increased infiltration of organs by T, NK and NKT cells, leukocytosis and splenomegaly. In addition, Prf1−/- mice exhibited multiple signs of premature and aggravated aging with increased fibrosis of kidney, liver, pancreas, and skin, glomerular sclerosis, loss of subcutaneous adiposity, reduced hair density, increased prevalence of gray hair, reduced muscle strength, exaggerated kyphosis, and a reduced median lifespan. Importantly, this phenotype was attenuated by treating mice with the senolytic agent ABT-737, which reduced the accumulation of senescent cells and decreased histological, functional and transcriptional signs of aging.9 Moreover, in a mouse model of Hutchinson–Gilford progeria syndrome (HGPS) with the LMNA+/G609G genotype, knockout of Prf1 accelerated the premature aging phenotype and this effect could again be alleviated by administration of ABT-737.9
Altogether, these agents argue in favor of the idea that (perforin-1-dependent) immunosurveillance plays a major role in eliminating senescent cells and in postponing the manifestations of aging. Numerous questions should be addressed in follow-up studies:
• Which are the immune effectors that express perforin-1 and that contribute to anti-senescence immunosurveillance? Are they T, NK or NKT cells? Do they belong to the innate lymphoid subsets? What do these cell types recognize? Is it cellular DAMPs (that might include the SASP) or is it the age-associated clonal expansion of cells carrying somatic mutations that occur as organisms age10?
• What is the link between immunosurveillance against senescent and (pre-)malignant cells? Senescence is one of the barriers aborting cellular transformation upon oncogene activation, and it has been speculated that subversion or reversal of senescence might be required for the development of cancer.11 As a result, it is possible that immunosurveillance against aging and transforming cells results from similar or overlapping processes that would be influenced by the gut microbiota composition.
• What is the molecular and cellular mode of action of senolysis by ABT-737? This agent, a BH3 mimetic, can induce both autophagy and apoptosis; and autophagy has prominent anti-aging, immunostimulatory and oncosuppressive effects.12 Thus, the mechanisms through which ABT-737 and other senolytic agents act require urgent clarification.
• Would it be possible to use immuno-oncological drugs such as immune checkpoint blockers or other non-specific immunostimulants for boosting anti-senescence immunosurveillance? In other words, is it possible to repurpose such agents for the treatment or prevention of age-related diseases?
Resolving these enigmas may have unprecedented implications for the triangulation between immunity, cancer, and aging (Figure 1), as well as for the development of therapeutic or prophylactic strategies for the cure or avoidance of major human diseases.
Figure 1.
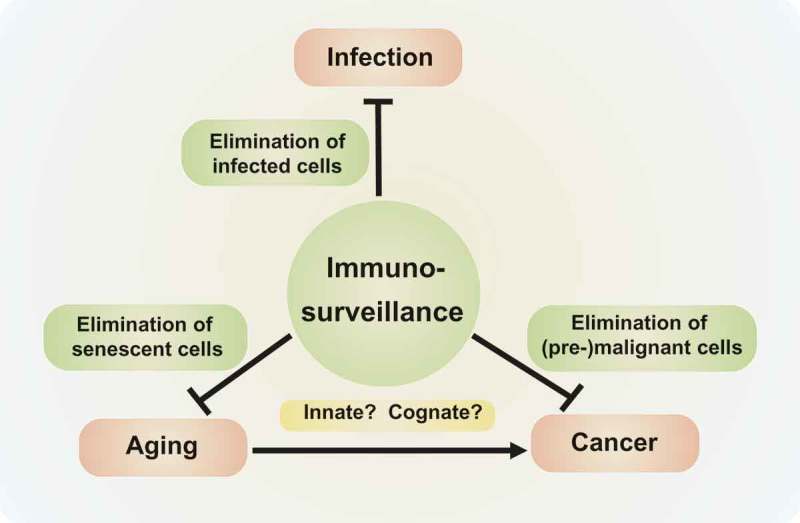
Triangulation of immunity, cancer, and aging.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM).
Disclosure of Potential Conflicts of Interest
GK is a scientific co-founder of Samsara Therapeutics. LZ and GK are scientific co-founders of everImmune.
References
- 1.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Martínez C.. The fail-safe paradigm of immunological self-tolerance. Lancet. 1991;338(8777):1246–1249. doi: 10.1016/0140-6736(91)92110-N. [DOI] [PubMed] [Google Scholar]
- 3.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29(1):235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen Y-S, Shea LK, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 6.Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15(6):382–396. doi: 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- 7.Senovilla L, Galluzzi L, Zitvogel L, Kroemer G. Immunosurveillance as a regulator of tissue homeostasis. Trends Immunol. 2013;34(10):471–481. doi: 10.1016/j.it.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoudi S, Xu L, Brunet A. Turning back time with emerging rejuvenation strategies. Nat Cell Biol. 2019;21(1):32–43. doi: 10.1038/s41556-018-0206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9(1):5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama A, Kakiuchi N, Yoshizato T, Nannya Y, Suzuki H, Takeuchi Y, Shiozawa Y, Sato Y, Aoki K, Kim SK, et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565(7739):312–317. doi: 10.1038/s41586-018-0811-x. [DOI] [PubMed] [Google Scholar]
- 11.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AHFM, Schlegelberger B, Stein H, Dörken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436(7051):660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 12.Rybstein MD, Bravo-San Pedro JM, Kroemer G, Galluzzi L. The autophagic network and cancer. Nat Cell Biol. 2018;20(3):243–251. doi: 10.1038/s41556-018-0042-2. [DOI] [PubMed] [Google Scholar]


