ABSTRACT
Exogenous application of γ-aminobutyric acid (GABA) could improve plant tolerance to environmental stresses such as hypoxia, heat, cold, drought, and salt stress. However, the mechanism by which GABA relieves stress is poorly understood. Here, we studied the uptake and metabolism of exogenous GABA in citrus leaves. Leaves were incubated in GABA solutions and the levels of endogenous GABA, succinic acid, and fumaric acid were investigated. Interestingly, the levels of endogenous GABA, succinic acid, and fumaric acid were quickly increased in GABA-treated leaves. This result indicated that GABA was taken up by the leaf, metabolized to succinic acid, and fed into the tricarboxylic acid cycle (TCA). This finding suggested that exogenous GABA could enhance plant resistance to biotic and abiotic stresses by generating more energy through the activation of the GABA shunt pathway and TCA cycle.
KEYWORDS: Gamma-aminobutyric acid, GABA, succinic acid, TCA, glutamic acid
Although more than 250 non-proteinogenic amino acids (NPAA) have been identified in plants, γ-aminobutyric acid (GABA) has received the highest attention in plants science. GABA is implicated in cytosolic regulation of pH, controlling of carbon and nitrogen metabolism, protection against biotic and abiotic stresses, and it could act as a signaling molecule. The level of GABA increases in plants under abiotic stresses (e.g. acidosis, mechanical damage, anoxia, heat and cold shock, drought, and salt stress) and biotic stresses (e.g. insect, bacterial, and viral attack).1 GABA is mainly synthesized from glutamate by glutamate decarboxylase (GAD), which is induced under stresses. Previous studies also showed that exogenous GABA could relieve stress symptoms caused by abiotic stresses including salt stress, hypoxia, heat, and drought.1
Recently, we found that the levels of several phytohormones were increased in GABA-treated citrus plants seven days post-treatment (dpt).2 In the same manner, the level of endogenous GABA was also increased in GABA-treated plants seven dpt, but it declined to its normal level 14 dpt. This result indicates that GABA was translocated to the citrus leaves and was metabolized.
Although exogenous GABA has been applied to many plants, the uptake and fate of exogenous GABA has not been investigated. Here, we investigated the fate of exogenous GABA in citrus using detached Mexican lime (Citrus aurantifolia) leaves. The leaf petiole was immersed in 10 mM GABA solution for 0, 1, 2, 4, and 6 h (Figure 1(a)). Three leaves were used in each treatment (15 leaves total). At the end of the incubation time, the petioles were excluded, and the leaf blades were washed with water and dried. Then, the leaves were ground in liquid nitrogen. GABA and other metabolites were extracted and analyzed using gas chromatography-mass spectrometry as described by Hijaz et al.2
Figure 1.
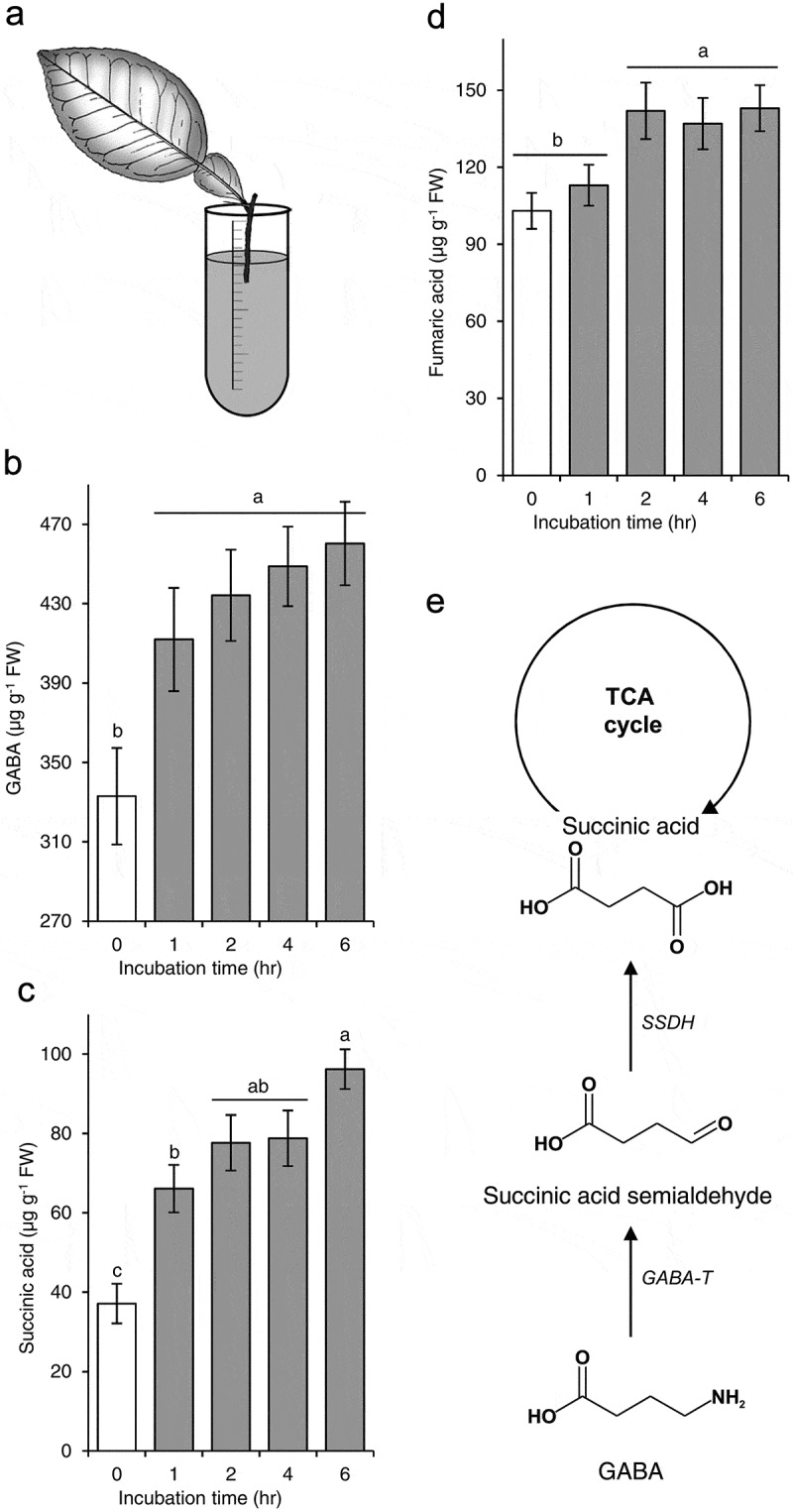
Citrus leaf incubation in GABA (a), effect of exogenous GABA on the level of endogenous GABA (b), succinic acid (c), and fumaric acid (d), and a schematic diagram for the conversion of GABA to succinic acid.
Averages (n = 3) with different letters are significantly different (P-value < 0.05) using Tukey’s Honest Significant Difference test. GABA-T: GABA-transaminase, SSDH: semialdehyde dehydrogenase.
The level of endogenous GABA increased with the increase of incubation time (Figure 1(b)), indicating that GABA was taken up by citrus leaves. Interestingly, the level of succinic acid also increased in GABA-treated leaves (Figure 1(c). In addition, the level of fumaric acid was slightly increased in GABA-treated leaves (Figure 1(d)). Taken together, these results indicated that GABA was metabolized to succinic acid and fed into the TCA cycle (Figure 1(e)).
In agreement with our results, the gene expression level of GABA-transaminase (GABA-T), succinic semialdehyde dehydrogenase (SSADH), malate dehydrogenase, and succinic dehydrogenase were upregulated in GABA-treated citrus plants.2 These results indicated that GABA was converted to succinate and fed into the TCA cycle. Using [14C]-glutamate, Tuin and Shelp showed that glutamic acid was metabolized to carbon dioxide, 2-oxoglutarate, GABA, succinate, fumarate, malate, citrate, and some amino acids.3 The formation of TCA cycle intermediates suggested that glutamic acid was converted to GABA and then to succinic acid.3
The citrus genome possesses a putative GABA permease (CsGABAP), which connects the GABA-shunt with TCA cycle.4 CsGABP could play a key role in connecting the GABA-shunt to the TCA cycle, by transporting cytosolic GABA to the mitochondria. Interestingly, the gene expression of CsGABP was highly induced in Candidatus Liberibacter asiaticus (CLas)-infected and Diaphorina citri-infested citrus plants, indicating an increase in GABA transport from cytosol to mitochondria.4 The level of succinic acid was also enhanced in CLas-infected and D. citri-infested plants, indicating a conversion of GABA to succinic acid directly.4 The activation of GABA shunt pathway and TCA cycle by exogenous GABA could enhance plant resistance to biotic and abiotic stresses by generating more energy.5 More research is still needed to understand the possible roles of GABA in enhancing plant resistance against biotic stresses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Shelp BJ, Bown AW, Zarei A.. 4-Aminobutyrate (GABA): ametabolite and signal with practical significance. Botany. 2017;95:1015–1032. doi: 10.1139/cjb-2017-0135. [DOI] [Google Scholar]
- 2.Hijaz F, Nehela Y, Killiny N.. Application of gamma-aminobutyric acid increased the level of phytohormones in Citrus sinensis. Planta. 2018;248:909–918. doi: 10.1007/s00425-018-2947-1. [DOI] [PubMed] [Google Scholar]
- 3.Tuin LG, Shelp BJ. In situ [14C]Glutamate metabolism by developing soybean Cotyledons I. metabolic routes. J Plant Physiol. 1994;143:1–7. doi: 10.1016/S0176-1617(11)82089-4. [DOI] [Google Scholar]
- 4.Nehela Y, Killiny N. Candidatus Liberibacter asiaticus and its vector, Diaphorina citri, augments the TCA cycle of their host via the GABA shunt and polyamines pathway. Mol Plant-Microbe Interact. 2018. MPMI-09-18-0238-R doi: 10.1094/MPMI-09-18-0238-R [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Sun C, Zhang Y, Fu D, Zheng X, Yu T. Induced resistance in tomato fruit by γ-aminobutyric acid for the control of alternaria rot caused by Alternaria alternata. Food Chem. 2017;221:1014–1020. doi: 10.1016/j.foodchem.2016.11.061. [DOI] [PubMed] [Google Scholar]


