ABSTRACT
Malignant pleural effusions, arising from either primary mesotheliomas or secondary malignancies, heralds advanced disease and poor prognosis. Current treatments, including therapeutic thoracentesis and tube thoracostomy, are largely palliative. The immunosuppressive environment within the pleural cavity includes myeloid derived suppressor cells, T-regulatory cells, and dysfunctional T cells. The advent of effective immunotherapy with checkpoint inhibitors and adoptive cell therapies for lung cancer and other malignancies suggests a renewed examination of local and systemic therapies for this malady. Prior strategies reporting remarkable success, including instillation of the cytokine interleukin-2, perhaps coupled with checkpoint inhibitors, should be further evaluated in the modern era.
Keywords: Malignant pleural effusion (MPE), non-small cell lung cancer (NSCLC), mesothelioma (MM), damage associated molecular pattern molecules (DAMPs), cancer immunotherapies, interleukin-2 (IL-2), adoptive cell therapy (ACT)
Introduction
Advanced thoracic malignancies are commonly associated with malignant pleural effusions (MPE), which are defined as significant accumulations of fluid exudate, containing tumor tissue or cells, within the pleural cavity.1,2 The incidence of MPE is over 200,000 cases/year in the US, of which non-small cell lung cancer (NSCLC) (36.0%), breast carcinoma (26%), and lymphoma (13.0%) are the most common etiologies.3 MPEs are also found in over 90% of patients with malignant pleural mesothelioma (MM), an equally devastating disease on the rise in developing countries.4,5 MPEs may present as the first sign of malignancy in 15–40% of asymptomatic patients but are commonly indicative of advanced cancer and poor prognosis.3 The median survival of patients with MPE from NSCLC is less than five months, and not only is MPE an independent predictor of lower overall survival due to advanced disease, but the effects of diminished breathing capacity can limit a patient’s ability to tolerate systemic treatment.3,6 Uncontrolled MPE can result in hypoxia and ultimately be a primary cause of death. Despite improvements in cancer therapies, patients with MPE are largely only palliated to provide symptomatic relief1. There is currently no effective definitive treatment for metastatic pleural disease, but a multimodal therapeutic approach is commonly offered to patients with early MM.7
Proposed pathophysiological mechanisms include fluid development following primary parietal pleura invasion, limiting the normal function of the rich lymphatic drainage present within the parietal pleura. Other mechanisms include visceral pleural invasion enabling fluid egress via the pulmonary vasculature followed by secondary parietal pleural dissemination, as found in patients with advanced lung malignancy.8 In other diseases, such as lymphoma or breast cancer, lymphatic dissemination or direct tumor invasion of the chest wall, diaphragm, or lung are possible. Tumor viability and immune resistance is dependent on the cancer’s adherence to the mesothelium, allowing immune evasion, while enabling nutritional access and growth stimuli of the neo-tumor environment. In early MM, tumor nodules may be evident in the parietal and visceral pleura at the same time. Pleural fluid formation results from disrupted lymphatic drainage, increased capillary and pleura permeability, and increased fluid production.2,9
Research on the pathophysiology of MPE has also shed light on the immune landscape of the malignant pleural space and has helped to convey critical clinical and prognostic information. MPE harbors immunogenic exosomes, immune cells, and immune factors, but are functionally ‘cold’ with progression of tumor.10 This review defines the role of the innate and adaptive immune system in primary and secondary MPE. We highlight treatments that could transform MPE to be functionally ‘hot’ and drive effective immunotherapy.11-13
Current treatments for malignant pleural effusion
The current standards for MPE treatment are largely palliative: drainage via thoracentesis, tube thoracostomy with or without pleurodesis, pleuroperitoneal shunt, tunneled pleural catheter, or other less common procedures (Table 1).
Table 1.
Existing MPE therapeutic options.
| Therapy | Advantages | Disadvantages | Mortality (30 days) | Morbidity | Success Rate | Ref. |
|---|---|---|---|---|---|---|
| Therapeutic Thoracentesis | Outpatient procedure, limited anesthesia is required; technical simplicity; drain large volume of fluid (approximately 1.5 liters without risk of reexpansion pulmonary edema) | Recurrent pleural effusion; 96% failure rate in 30 days | 37% | <1% | 4% | 14,15 |
| General Chemical Pleurodesis | Minimal insertion of tubes and decreased risk of frequent thoracentesis | Associated pain and fever; prolonged hospitalization (median time: 4 days); pleurodesis failure | 32% | 6–33% | 68–85% | 1,16-19 |
| Talc Pleurodesis | Minimal drainage following instillation; superior agent in comparison to bleomycin, doxycycline, and tetracycline; comparable to chemical pleurodesis, regarding: quality of life and symptomatic relief | Risk of ARDS ranging between 1–9%; pleurodesis failure; pain is a common post-operative complaint | 2% | 9–38% | 98% | 20-22 |
| Indwelling Pleural Catheter | Indicated for lung entrapment syndrome and failed pleurodesis; technical simplicity; outpatient management; drainage guided by symptoms (patients have more autonomy) | Risk of infection is higher than chemical pleurodesis; increased risk for catheter-tract metastases in patients with mesothelioma | x | 10% | 48–58% | 23-26 |
| Indwelling Pleural Catheter and Talc Pleurodesis | Outpatient management | Pain, empyema, hydropneumothorax are known adverse effects; remains under study. | x | 9% | 43–92% | 25,27,28 |
| Pleuro-peritoneal Shunt | Useful in refractory MPE or trapped lung; post-operative morbidity is low | Infectious risk due to infection of the peritoneal cavity with infected pleural fluid; shunt occlusion (12–25%), tumor seeding into the peritoneal cavity. | 21% | 14% | 95% | 29-32 |
| Thoracoscopy and Pleurodesis | Video-assisted thoracoscopic surgery allows surgeon to assess pleura, diaphragm and pericardium for tumor implants; perform concurrent procedures (mediastinal lymphadenectomy, pleurectomy, etc.); visualize pleural effusion; shorter interval for chest drainage in comparison to chest tube thoracostomy | Patient has to tolerate single lung ventilation; post-operative complications (3%-25%); Prolonged hospitalization (7–10 days) | 2.8% | 2.8% | 90% | 26,30,33 |
| Pleurectomy with Decortication/Extrapleural Pneumonectomy |
Indicated in refractory MPE and mesothelioma | Invasive; 12% mortality risk; prolonged hospitalization; offered based on patient selection per hospital and surgical experience; not standard of care | 4–12% | 10–19% | x | 14,34,35 |
| Chemotherapy | Intrapleural chemotherapy (IC) can treat the underlying malignancy and pleural effusion and has been used in mesothelioma; chemosenstive malignancies with associated MPE, may respond to chemotherapy | IC maybe inferior to existing chemical pleurodesis; patient may not tolerate systemic chemotherapy given functional and physiologic status | 50% at 1 year | 7–40% | 30–70% | 36-38 |
| Radiotherapy, alone | Reduce risk of needle tract metastasis; radiation targeted at underlying malignancy may treat associated MPE; used in multi-modal treatment approach for mesothelioma | Radiation pneumonitis; limited studies on efficacy for MPE and secondary malignant pleural effusions | 17% at 1 year | x | x | 39,40 |
| Immunotherapy | Most current studies involve mesothelioma; immune checkpoint inhibition appears very promising strategy in MM; IL-2 installation could be reconsidered for local therapy | Toxicity; limited studies regarding efficacy | x | 7–90% | 10–20% | 41,42 |
Management choice is guided by the patient’s prognosis, preference, functional status, rate of pleural effusion accumulation and resolution, failed therapeutic options, and the surgical team’s experience. To date, there are no established criteria for selecting from the available therapeutic options. The decision to undergo pleurodesis is often based upon an anticipated survival of longer than three to four months.16 Talc pleurodesis was previously the mainstay of treatment. The mechanism of action involves promoting local inflammation following installation of a sclerosing pleurodesis agent to promote pleural symphysis and prevent recurrent fluid collection.16 Despite the potential therapeutic benefits, pleurodesis failure remains a major drawback. A meta-analysis of 62 randomized trials involving over 3,000 patients compared and ranked agents based on pleurodesis efficacy.16 Talc poudrage was identified as the superior method when compared to bleomycin, mepacrine, or iodine installation. There was no evidence of survival benefit associated with any of the individual types of pleurodesis. Failure of lung expansion remains a contraindication for chemical pleurodesis and the introduction of the intrapleural catheter has served as an initial suitable remedy for lung entrapment.43
Intrapleural catheters (IPC) have more recently become the primary means for managing MPEs because of their technical simplicity and cost effectiveness. A retrospective study assessing the financial benefits of outpatient management for patients with MPE versus inpatient management was conducted. Outpatient management was in fact, more cost effective without any change in survival or increased risk for complications.44 Additional studies that have assessed IPC include the Australasian Malignant Pleural Effusion (AMPLE) Trial. This multicenter randomized study compared IPC and talc pleurodesis for the management of MPE regardless of oncologic source.17 Total number of days spent in the hospital was the primary endpoint, and secondary endpoints included diminished hospital days specific to pleural effusion management, adverse events, self-reported symptoms, and quality-of-life scores. The patients who underwent IPC placement had a shorter median length of stay (10 days) versus talc pleurodesis (12 days), but the quality of life, symptoms, and survival were not different. A follow-up AMPLE-2 study assessing optimal IPC drainage schedule found that daily drainage is more effective in promoting spontaneous pleurodesis than symptom guided regimens.45 Alongside the comparative studies between IPC and chemical pleurodesis, different types of IPCs have been studied and compared. PleurXTM catheters are commonly used, but other types of IPCs (Aspira) have shown comparable efficacy, safety, and complication rates.46 Novel catheter designs, allowing for frequent installation of chemotherapeutic and immunologic agents, continuous infusion of therapeutic agents, and withdrawal of excessive fluid should be considered. These strategies would also allow regular sampling of pleural fluid including tumor and host cells, enabling timely assessment of immunotherapies and their application.
There is also a role for the surgical diagnosis and treatment of patients with primary MPE. Extrapleural pneumonectomy (EPP) and pleurectomy/decortication (P/D) can achieve macroscopic complete resection in early stage MM7. Improved quality of life and safety has been demonstrated in selected patients undergoing P/D for MM, but the procedure has a 6–12% risk of operative mortality.47 The survival benefit of EPP remains widely debated, with some trials suggesting added morbidity without survival benefit.48 Improved quality of life at three months has been reported.49,50
Despite advances in approaches to palliative care for MPE, local treatment continues to lag. Intrapleural chemotherapy (i.e. doxorubicin, methotrexate, 5-fluorouracil, etc.) has been slowly adopted as an adjunctive treatment for MPE.51 The impact of intrapleural chemotherapy with cisplatin and cytarabine has been studied in the presence of MPE secondary to NSCLC, and treatment responses have been associated with decidedly mixed outcomes. The Lung Cancer Study Group (LCSG 861) showed that IPC with cisplatin and cytarabine has a relatively low response rate (49%). Park’s group demonstrated a more favorable response rate (97.3%) and durability of benefit (12 month median duration of response).52 Intrapleural docetaxel was studied in a phase I clinical trial and was proven to be safe with low toxicity and with reasonable radiographic control.53 Currently, intrapleural chemotherapy is not used as a monotherapy but is integrated into other treatment strategies for secondary MPE. Chemotherapy response is estimated at 15% following single agent use in MM. Application of combined chemotherapy agents (cisplatin and pemetrexed) improves both quality of life and survival (9.3 −13.3 month median survival).7 While radiotherapy has minimal efficacy as a single agent in MM, there may be a survival benefit when used as an adjunct following surgery, with median survivals of 33.8 months for stage I and II but limited to 10 months for stage III and IV tumors.54
Given the existing limitations in treating MPE, new therapeutic options, especially application of modern immunotherapy, may enhance development of palliative and potentially curative therapies (Figure 1).
Figure 1.
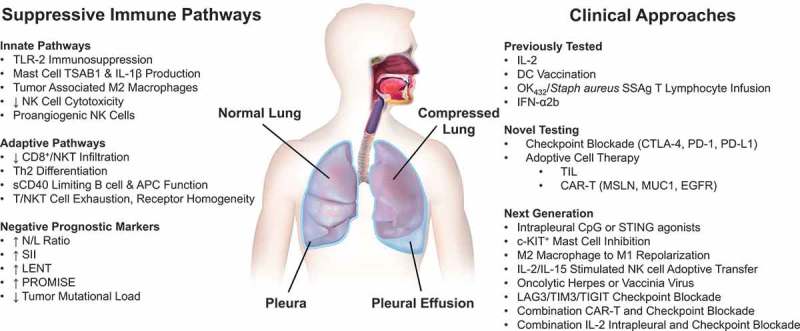
Immunotherapy for patients with malignant pleural effusions. Malignant pleural effusions are an inflammatory condition within the chest containing immunologically active but most often exhausted cells associated with both bulk tumor and tumor cells in suspension. Thus, they are a functionally ‘cold’ site. Several suppressive innate and adaptive pathways have been identified. MPE pathophysiology is closely correlated with the upregulation of these inflammatory pathways. Systemic measures associated with acute inflammation (neutrophils) and chronic inflammation – immunity (lymphocytes) can be utilized as prognostic indicators of disease outcome and for disease stratification. Previously tested clinical approaches, including IL-2 therapy and DC vaccination hold much promise, and should be further investigated in the context of trials evaluating checkpoint inhibition and adoptive cell therapy. Next generation immunotherapies may enable personalized treatments, leverage improved cell-based therapies, and modulate the local suppressive tumor microenvironment. Local therapies could be more effectively deployed.
Innate immunity within the MPE
Innate immune cells are recruited to the sites of tumor cells undergoing unscheduled cell death associated with release of damage associated molecular pattern molecules (DAMPs).55,56 Initial recruitment and activation of neutrophils, macrophages, mast cells, dendritic cells (DCs), and natural killer (NK) cells, follows elaboration of chemokines and cytokines in response to signals of tissue injury. Cancer cells and their products are distinguished from healthy tissues through pattern recognition receptors (PRRs) on the surface and within the cytoplasm of innate immune cells. PRRs recognize pathogen associated molecular pattern molecules (PAMPs) and DAMPs. These signals initiate the inflammatory and subsequent immune response to tumor.57-60 Frequently members of the Toll-like receptor (TLR) family, which recognizes conserved microbial and endogenous motifs,61 constitute the primary means for response to so-called ‘danger’ or ‘stranger’ signals.62-64 Dysregulated inflammatory innate immune networks support MPE development and tumor cell immune evasion (Figure 2).
Figure 2.
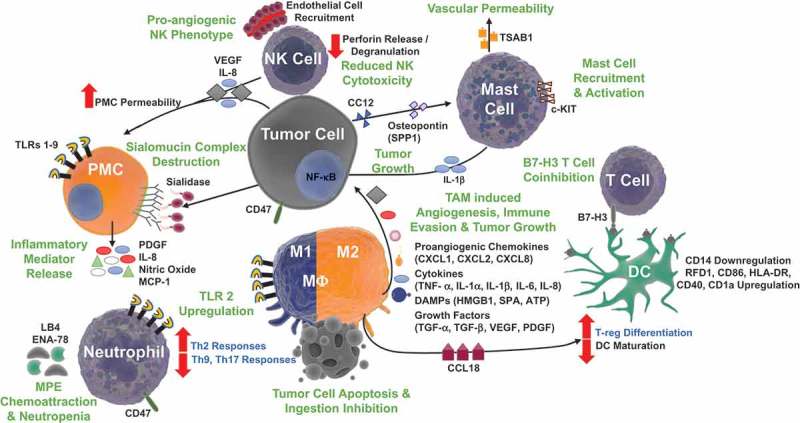
Innate immune signaling pathways in malignant pleural effusions. The altered tumor microenvironment enables MPE formation by enhancing angiogenesis, promoting vascular permeability, driving tumor growth, unscheduled cell death, and releasing damage associated molecular pattern molecules (DAMPs). Natural Killer (NK) cells exhibit a proangiogenic phenotype, with reduced cytotoxicity, increasing endothelial cell recruitment with production of IL-8 and vascular endothelial growth factor (VEGF). Pleural Mesothelial Cells (PMCs) respond to pleural cavity DAMPs and other ligands via toll-like receptor (TLR) 1–9 signaling, promoting recruitment of inflammatory cells with release of platelet derived growth factor (PDGF), IL-8, nitric oxide, and monocyte chemotactic protein 1 (MCP-1). Tumor cell production of VEGF and sialidase promote vascular permeability with destruction of the protective extracellular PMC sialomucin complex. Leukotriene B4 (LB4) and epithelial neutrophil-activating peptide-78 (ENA-78) increase neutrophil recruitment to the pleural space and neutropenia is sustained with CD47 mediated inhibition of neutrophil apoptosis. Accompanying neutrophil and macrophage (MΦ) TLR2 upregulation support MPE formation with a skewed Th2 response. Tumor associated macrophages (TAMs), favoring M2 polarization protect tumor cells from apoptosis and promote angiogenesis, immune evasion, and tumor growth with a milieu of proangiogenic chemokines, cytokines, DAMPs, and growth factors. TAMs also produce chemokine ligand 18 (CCL18), which promotes T regulatory cell (T-reg) differentiation and limits dendritic cell (DC) maturation. DCs facilitate immunosuppression via B7-H3 T cell coinhibition and upregulation of RFD1, CD86, HLA-DR, CD40, and CD1a expression. Tumor cells avoid ingestion by expressing CD47, a ‘don’t eat me’ signal and produce CC12 and osteopontin, inducing mast cell recruitment and c-KIT activation respectively. Increased mast cells are found to be associated with MPE formation with tumor growth promoting IL-1β and tryptase alpha/beta-1 (TSAB1) mediated enhanced vascular permeability.
Pleural mesothelial cells (PMCs) reside within the parietal and visceral pleura and are essential to pleural homeostasis. Dysregulation of PMC responses leads to activation and formation of MPE.65 Pleural cavity injury during infection, trauma, or malignancy is initially recognized by PMCs and results in a response-specific inflammatory cascade.65 In addition to their negatively charged surface glycoconjugates that limit errant cells,66 PMCs express TLRs 1–967 and mediate inflammation via release of platelet derived growth factor (PDGF), interleukin-8 (IL-8), monocyte chemotactic peptide (MCP-1), and nitric oxide.68 Sialidases on malignant cells remove the defensive sialomucin complex layer on PMCs.66 Release of vascular endothelial growth factor (VEGF) increases permeability of PMCs, allowing for leakage of high molecular weight proteins, promoting migration of cells into the pleural space.65,69 A retrospective analysis of 21 patients with NSCLC associated MPE treated with bevacizumab, an anti-VEGF antibody, and chemotherapy demonstrated a remarkable 71.4% response rate of MPE to antibody treatment.70
In an in vivo model of MPE, enhanced TLR-2 expression on recruited CD14+ inflammatory macrophages, Ly6G+ neutrophils, CD4+/CD8+ T cells, and CD19+ B cells accelerated the development of MPE and death in mice bearing MPE by suppressing Th9 and Th17 cell differentiation, while promoting Th2 differentiation.71 A clinical study evaluating TLR-2 expression following treatment of MPE with talc pleurodesis demonstrated decreased granulocyte TLR-2 expression directly and 24 hours following talc pleurodesis compared to pretreatment levels.72 Accordingly, soluble levels of TLR-2 were significantly increased following pleurodesis. Interestingly, patients retrospectively sorted into a lower prognostic scoring group (higher thorascore, larger pretreatment pleural fluid, and recurrence of MPE) had lowered levels of soluble TLR-2 following treatment compared to the prognostically favorable group. TLR-2 is a critical PRR at the interface of microbial and sterile inflammation in MPE. Questions remain concerning the mechanisms of its upregulation in MPE. Unlike TLR-2, TLR-4 expression appears to be immunoprotective in the formation of MPE. TLR-4-/- mice with MPE had augmented Th1 differentiation, via enhanced STAT1 signaling, and suppressed STAT3 dependent Th17 cells, accelerating the death of mice with MPE.73 The role of other PRRs, such as NOD-like receptors, RIG-I like receptors, AIM-2 like receptors, and C type lectin receptors (CLRs) remains to be investigated in MPE. CLRs have been identified as a molecular switch of the inflammatory response to tuberculosis associated pleural fluid, suggesting a potential role in the setting of MPE.74
Mast Cells are typically activated during allergic responses, and are among the first cells to infiltrate the tumor microenvironment and promote tumor progression via inflammatory and tumor angiogenesis signaling.75 Thought relatively sparse, mast cells are surprisingly elevated in MPE compared to benign effusions and are critical to MPE development.76 Pleural adenocarcinomas mobilize mast cells into the pleural space during MPE development through elaboration of CC family chemokine 12 (CCL12).76 In addition to its vasoactive components, tumor originating osteopontin, encoded by the secreted phosphoprotein 1 (SPP1) gene, promotes c-KIT+ mast cell activation and degranulation, leading to MPE formation with release of tryptase alpha/beta-1 (TSAB1) and IL-1β, causing vascular permeability and NF-κB mediated tumor growth respectively.76 Treatment with the clinically available imatinib mesylate, a mast cell c-KIT inhibitor, hampered mast cell pleural accumulation, vascular leakiness, and limited effusion development in murine models of MPE.76 Mast cells and their identified intermediary signaling molecules, CCL2, SPP1, TPSAB1, and IL-1β should be further investigated for more targeted approaches to MPE treatments.
Macrophages are phagocytic, antigen presenting cells (APCs) that serve as a bridge between innate and adaptive immunity.77 The polarized macrophage model describes macrophage activation in response to differing environmental and inflammatory triggers. M1 polarization promotes macrophages capable of producing proinflammatory cytokines (IFN-γ, TNF- α, IL-1α, IL-1β, IL-6) and cytotoxic reactive oxygen and nitrogen species (ROS, NRS) while M2 polarization directs an immunoregulatory and wound healing response that promotes Th2 responses critical for the development of cancer.78-80 Macrophages constitute over half of all the cells found in the pleural space. In the setting of MPE, they modulate T cell proliferation and differentiation with release of IL-1β, TNF-α, and IL-8.81,82 Tumor associated macrophages (TAMs) have decreased cytotoxicity and promote tumor cell growth and immune evasion.83 In MPE, TAMs protect cancer cells from apoptosis,84 ingest those that are apoptotic, and promote angiogenesis with release of proangiogenic chemokines (CXCL1, CXCL2, CXCL8), cytokines (TNF- α, IL-1α, IL-1 β, IL-6), DAMPs (high mobility group box 1 (HMGB1)), and growth factors (TGF-α, VEGF, PDGF, angiopoietins).85 Upregulation of MM CD47, a ‘don’t eat me signal’ that inhibits macrophage phagocytosis, promotes tumor cell immune evasion.86,87 In the respiratory tract, surfactant protein-A (SPA) is another important DAMP that is upregulated in human NSCLC MPE compared to non-malignant pleural effusion. Elevations in SPA positively correlate with increases in M2 polarized macrophages with TLR-2 and TLR-4 expression.88 Decreased CD163+ TAMs independently predict better NSCLC MPE progression free survival (PFS). Increased levels of M2 polarized TAMs correlate with poor prognosis.89 Interestingly, treatment with Pseudomonas aeruginosa-mannose sensitive hemagglutinin (PA-MSHA) for lung cancer MPE helped to re-educate M2 macrophages into an M1 phenotype in vitro.89 This pathway was TLR-4 mediated as treatment with a TLR-4 blocking antibody reversed M1 polarization. Inhibition of TAMs or repolarization of M2 macrophages are compelling therapeutic strategies for MPE.
Neutrophils are the body’s most abundant immune cell and are the major mediators of inflammation, the seventh hallmark of cancer.90,91 Leukotriene B4 and epithelial neutrophil-activating peptide-78, potent inflammatory chemoattractants, are present in exudative pleural effusions, and actively contribute to neutrophil recruitment to the pleural space.92,93 Flow cytometric analysis of patient NSCLC tumor specimens showed a robust immune response (50% of cellular content within tumor were CD45+), with neutrophils (20%) comprising the most abundant immune cell subset. The same study showed significant correlations between increased neutrophil counts and decreased lymphocytes, indicative of neutrophils potential to suppress lymphocytes within the tumor environment.94 NSCLC neutrophilia may be explained by increased neutrophil CD47 expression, which is associated with a delay in neutrophil apoptosis and phagocytic clearance.95 Neutrophil elastase is an inflammatory serine protease and mediates neutrophil induced proliferation of lung cancer cells in vitro in a COX-2 dependent fashion.96 Moreover, depletion of neutrophils with anti-Ly6g antibodies decreased tumor formation in a urethane model of murine lung cancer.97 Neutrophils are also a major source of IL-1β, which promotes lung cancer tumorigenesis and is indicative of poor survival in NSCLC patients.97,98 Neutrophils isolated from NSCLC patients with chronic obstructive pulmonary disease produce more APRIL (A proliferation-inducing ligand), an inflammatory regulator that promotes NSCLC growth and development.99,100 Neutrophil Extracellular Trap (NET) formation, a cell death mechanism that releases intracellular DNA, histone, elastase, and granule proteins, promotes lung cancer cell adhesion in vitro and increases in vivo micrometastases.101-103 Neutrophils and circulating neutrophil microRNAs serve as biomarkers for detection of NLSLC and are an independent negative predictor of survival in patients with advanced disease.104-106 Mechanistic studies on the role of neutrophils in MPE are warranted given their immunosuppressive role in NSCLC and adverse prognostic value in MPE.107
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that cross-present antigens, induce adaptive immune responses, and regulate the immune system by modulating T and B lymphocyte antigen specific responses via their major histocompatibility (MHC) class I and II receptors and ability to produce IL-12 family cytokines.108 A meta-analysis evaluating the significance of immune cells in NSCLC showed that increased tumor conventional dendritic cells (cDCs) were associated with improved overall survival (hazard ratio 0.55; 95% confidence interval 0.44–0.68).109 Increased stromal cDCs were also prognostic for increased disease-specific survival (hazard ratio 0.62; 0.47–0.83).109 However, DCs also have an immunosuppressive role. B7-H3, a potent T cell coinhibitory molecule,110 is expressed on NSCLC tumor-derived DCs and significantly reduced T cell proliferation during in vitro coculture experiments.111 Interestingly, soluble B7-H3 is upregulated in NSCLC associated MPE and correlates with advanced tumor staging, indicating the need for follow-up studies.112 CC chemokine ligand 18 (CCL18), implicated in immature DC and lymphocyte trafficking during homeostasis and inflammation, is present at a high level in NSCLC MPE and inhibits DC maturation, subsequently stimulating CD25+, FOXP3+ regulatory T cell (T-reg) differentiation.113,114 Accumulation of DCs in MPE (15.2%) is significantly greater than that found in benign pleural effusions (7.1%), demonstrating the chronic inflammatory nature of MPE.115 In both firmly adherent or loosely adherent mononuclear cells isolated from bronchogenic carcinoma MPEs, >80% of cells are HLA-DR+ immunosuppressive RFD1+ DCs.116 DCs isolated from lung cancer MPE patients expressed higher levels of CD86, HLA-DR, CD40, CD1a, and but lower CD14 compared to DCs from benign effusions.117 DCs from MPE increased stimulation of allogenic lymphocyte proliferation and subsequent IFN-γ production compared with control DCs.117 These findings contrast with tumor derived DCs isolated from NSCLC tissues, which exhibit a semi-mature phenotype with poor APC function.118 Given the preliminary success of DC vaccination trials in MM associated MPE,119-121 further study is warranted.
Natural Killer (NK) cells play four major roles in tumor biology and immunosurveillance: 1) promoting lysis of genomically unstable and stressed tumor cells; 2) producing cytokines (IL-8, IL-10, VEGF, HMGB1, and IFN-γ) to shape the inflammatory and proangiogenic response; 3) driving DC maturation in secondary lymphoid sites; and 4) promoting and initiating autophagy. NK cells from human MPE exhibit poor cytotoxicity, having impaired degranulation and reduced perforin release.122-124 In the malignant pleural environment, NK cells exhibit pro-angiogenic function, supporting capillary-like structures from recruited endothelial cells and produce angiogenic and vascular permeability inducing factors, including VEGF.122 Interestingly, CD56dim and CD56bright NK cells isolated from human MPE cultured with IL-2 for 72 hours had potent antitumor cytolytic activity.125 The same group found that IL-15 activated pleural effusion NK cells exhibited increased in vitro cytotoxicity and in vivo tumor clearance in mice.126 Interestingly, an earlier study showed that NK cells from MPE lung cancer patients stimulated with IL-2 and IL-12 produced more IFN-γ and IL-10 than blood mononuclear cells.127 These findings suggest that IL-2 and IL-15 activated NK cells from pleural fluid, normally discarded during thoracentesis, represent a potential source of effector cells that could be activated productively with cytokine instillation into the pleural space as part of an immunotherapeutic regimen, perhaps coupled with checkpoint inhibitors delivered systemically.128 This would also allow for sequential monitoring of changes in the non-sclerosed pleural space.
Systemic inflammatory indicators in MPE patients
Differentiating MPE from benign effusions can be difficult, although identification of malignant cells by pleural fluid cytology or pleural tissue histology defines the condition. Inflammatory measures, including neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) are easily obtained hematological parameters that can aid in MPE diagnosis and, more importantly, disease prognosis (Table 2).
Table 2.
Prognostic indicators in lung cancer amenable for use in MPE.
| Index and Definition | Patient Population | Prognostic Cutoff Value | Outcome Group | Ref |
|---|---|---|---|---|
| NLR | 382 (NSCLC) |
Low: NLR < 1.5 Intermediate: 1.5 ≤ NLR < 3.5 High: NLR ≥ 3.5 |
5 yr OS: Low 76 (77) Intermediate: 172 (70) High: 22 (58) p=0.033 |
257 |
| NLR | 88 (advanced NSCLC) |
Low: NLR ≤ 4x109 High: NLR > 4x109 | Median Survival: Low 21.4 months High 6.8 months p=0.019 DCR (Week 8): Low 28 (32) High 25 (28) p=0.025 |
258 |
| NLR | 109 (advanced NSCLC) |
Low: NLR <5: High: NLR ≥5 |
Median OS (months) Pre-treatment NLR:Low: 26.4High: 25.8p = 0.1Post-treatment NLR:Low: 29.1 High: 24.2p<0.001 | 259 |
| SII | 183 Low 149 High (NSCLC) |
660 × 109 | CR (PR): Low 76 (77) High 73 (62) SD (PD): Low 23 (23) High 45 (38) p<0.018 Median OS (months): Low 30 High 10 p<0.001 |
108 |
| SII | 140 Low: 71 male 69 female 270 High: 196 male 74 female (NSCLC) |
395.4 × 109 | Male 5 yr % OS: Low (53.4) High (35.4) p<0.0008 Female 5 yr % OS Low (63.3) High (30.9) p<0.0001 |
109 |
| SII | 214 Low 127 High (NSCLC) |
471.2 × 109 | 5 yr % OS: Low 83.61% High 60.39% p<0.0001 |
260 |
| SII ALI NLR PLR PNI |
381 (NSCLC) |
Low SII < 471.2 × 109 High SII ≥ 471.2 × 109 Low ALI ≥ 37.66 High ALI < 37.66 Low NLR < 5:1 High NLR ≥ 5:1 PLR 0 < 150:1 PLR 1 = 150 - 300 PLR 2 > 300:1 PNI 0 ≥ 45 PNI 1 < 45 |
5 yr OS (all p< 0.001): Low SII (83.61) High SII (60.39) Low ALI (57.61) High ALI (84.20) Low NLR (76.75) High NLR (40.18) PLR 0 (80.92) PLR 1-2 (62.89) PNI 0 (67.40)PNI 1 (79.01) |
261 |
| LENT | 43 Low-risk 129 Moderate-risk 31 High-risk (MPE) |
Low-risk LENT score: IQR 228–549 Moderate-risk LENT score: IQR 47–467 High-risk LENT score: IQR 22–77 |
Median survival (days) Low-risk: 319 HR (95% CI): not specified Moderate-risk: 130 HR (95% CI): 1.49 High-risk: 44 HR (95% CI): 5.97 |
110 |
| LENT | 36 High-risk 34 Moderate-risk (lung adenocarcinoma presenting with MPE) |
High-risk score: ≥ 5 Moderate-risk score: 2-4 | Median survival: High-risk: 190.5 days Moderate-risk: 346 days p<0.05 |
111 |
| PROMISE | 162 (stage IV cancer with MPE) |
Categories A: 0%-24% risk B: 25%-49% risk C: 50%-74% risk D:75%-100% risk |
3 month survival C statistic value Internal validation: 0.78 External validation: 0.89 p<0.05 |
133 |
Key: Systemic Immune-Inflammation Index (SII), Neutrophil to Lymphocyte Ratio (NLR), Non-Small Cell Lung Cancer (NSCLC), Malignant Pleural Effusion (MPE), Interquartile Range (IQR), Complete Response (CR), Partial Response (PR), Stabile Disease (SD), Progressive Disease (PD), Overall Survival (OS), Disease Control Rate (DCR), Advanced Lung Cancer Inflammation Index (ALI), Prognostic Nutritional Index (PNI), Platelet to Lymphocyte Ratio (PLR)
Increased NLR is a poor predictor of overall survival (OS) in patients with NSCLC139 and most other malignancies. This finding is presumably related to the exuberant release of DAMPs recruiting neutrophils but limiting lymphocyte expansion and survival. NLR assessment alone is not always an effective diagnostic metric. A study specifically assessing NLR in the MPE fluid (mNLR), demonstrated that mNLR values could not reliably distinguish malignant and benign pleural effusions.107 A subsequent study investigating NLR as a prognostic indicator in lung cancer patients with MPE, showed that blood NLR (NLR), in combination with the effusion NLR score was an independent predictor of OS.140 Moreover, a combination of platelet and lymphocyte to monocyte ratio (COP-LMR) is an independent predictor of shorter OS in stage IV NSCLC and MPE.141 Neither NLR nor the COP-LMR alone completely reflect the overall host inflammatory and hematopoietic response.
The systemic immune-inflammation index (SII), based on peripheral lymphocyte (L), neutrophil (N), and platelet (P) counts (SII = P x N/L), was first described in the context of hepatocellular carcinoma.142 SII’s predicative capability was shown to be greater than other conventional parameters such as tumor staging, tumor differentiation, and tumor number.142 SII is a powerful prognostic indicator, which when elevated, confers a poor outcome in patients with various cancers.143,144 SII is an independent prognostic indicator of poor outcomes for patients with stage III NSCLC and is a superior prognostic indicator to other inflammation-based indices, including NLR and PLR.130 Its low cost, easy determination, and high reproducibility from a simple complete blood count and differential make SII a promising tool for individualized lung cancer treatment strategies.131 The efficacy of SII as a prognostic indicator in patients with MPE is yet to be determined.
Developed in 2014, the LENT prognostic score, which incorporates pleural fluid lactate dehydrogenase, Eastern Cooperative Oncology Group performance score (ECOG PS), NLR, and tumor type, is another predictor of progression free survival (PFS) that is superior to ECOG PS in MPE.129 The LENT underestimates survival in patients having MPE secondary to lung adenocarcinoma.133,145 A more robust prognostic indicator was developed earlier in 2018. The eight variable PROMISE score, comprising hemoglobin level, C-reactive protein, white blood cell count, ECOG PS, cancer type, pleural fluid tissue inhibitor of metalloproteinases 1 (TIMP1) concentration, and previous chemotherapy or radiotherapy, is the first prospectively validated prognostic model for MPE that accurately estimates 3-month mortality that will aid in the development of more personalized treatment plans and enable stratification in randomized studies.138 Unfortunately, the PROMISE study was unable to identify markers of successful pleurodesis treatment, indicating the need to further specify prognostic criteria to improve clinical decision making in the setting of MPE.146
Adaptive immunity within MPE
The adaptive immune system comprises both antigen-specific antibody and cell mediated responses.147 B cells produce various immunoglobulins, act as APCs, and support T-reg cell differentiation and cytokine liberation.148 Upon recognition of cognate antigen and activation of costimulatory receptors, CD8+ T lymphocytes can directly recognize and destroy tumor cells or virally infected cells. Upon stimulation and exposure to varying microenvironmental signals, CD4+ helper T cells sustain and regulate adaptive responses.148 T-regs and naïve B cells in the MPE environment support tumor cell proliferation and immune evasion (Figure 3).
Figure 3.
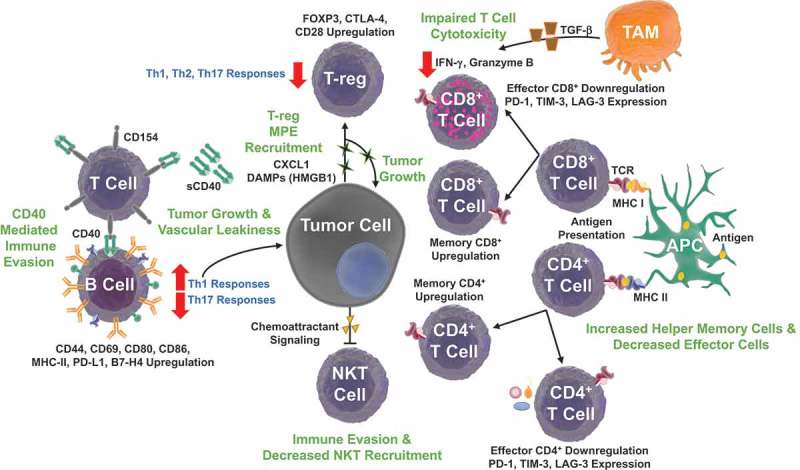
Adaptive immune signaling pathways in malignant pleural effusions. The immunosuppressive MPE environment modulates the ‘adaptome’, B, NKT, αβ T cell and γδ T lymphocyte biology to promote tumor growth and immune evasion. Tumor cells secrete chemokine ligand 1 (CXCL1) and DAMPs, promoting further cell proliferation and T-regulatory (T-reg) cell recruitment. T-regs promote an immunosuppressive environment, inhibiting Th1, Th2, Th9, and Th17 responses. Moreover, T lymphocytes display a high CD4+/CD8+ ratio with an increased percentage of central memory CD4+ T cells and decreased CD8+ effector-memory T cells. T cell programmed cell death protein 1 (PD-1), T cell immunoglobulin and mucin domain 3 (TIM-3), and Lymphocyte-activation gene 3 (LAG-3) immune checkpoint expression inhibit lymphocyte activity. Tumor Associated Macrophage (TAM) transforming growth factor beta (TGF-β) production decreases effector T cell cytotoxicity with reduced production of interferon gamma (IFN-γ) and granzyme B. These changes are accompanied with decreased NKT recruitment to the effusion site. Increased soluble CD40 (sCD40) levels inhibit B cell function by competing for CD154 (CD40 ligand) on T lymphocytes. Despite decreased B cell density, increased expression of CD80, CD86, MHC II, CD44, CD69, and programmed death ligand 1 (PD-L1) promote Th2 responses that can also support MPE formation.
T cell populations vary between healthy and malignant pleural fluid, both in phenotype and function.149 Healthy subjects display mainly effector-memory phenotypes within both CD4+ and CD8+ T cell subsets with a low CD4+/CD8+ ratio (0.59) in pleural fluid. Patients with MPE, however, show a high CD4+/CD8+ ratio (>2.2) in the pleural fluid with an increased percentage of central memory CD4+ T cells and decreased CD8+ effector-memory T cells, indicative of potential immune escape by tumor cells.149 Despite the stoichiometric changes in T cell population in MPE, no differences are observed in relative abundance of T lymphocytes based on receptor expression (TCRα/β or TCRγ/δ) compared to benign effusions.150 However, another group did find that TCRζ chain downregulation was associated with T cell apoptosis in MPE and related to the abundance of high monocyte populations in effusions.151
Despite increased levels of circulating natural killer T (NKT) cells, NKT recruitment to the pleural cavity is decreased in MPE patients.149 It is hypothesized that chemo-attractant tumor signaling may disrupt local effector T cell recruitment which contributes to immune response evasion.149,152 Another notion suggests that rapid apoptosis through activation-induced cell death of effector CD8+ T cells, leads to the accumulation of memory cells and a depletion of effector cells.149,153 Impaired T cell cytotoxicity, as measured by production of IFN-γ and granzyme B, has been attributed to TAM production of TGF-β, and presents a valuable target for MPE cell therapy.154
T regulatory cells are elevated in MPE with CD4+ CD25+ cells expressing high mRNA levels of FOXP3, CTLA-4, and CD28 compared to those found in benign effusions.155 Decreased expression of the micro-RNA 141, causing increased production of CXCL1, recruits T-regs into the MPE pleural environment through CXCR2 and CCL22 chemokine signaling.156,157 Moreover, increased MPE T-regs correlates with a decrease in the overall percentage of lymphocytes and an increase in expression of CD4+/CD4+CD25+ T cells, which were present at the highest frequency in patients with the most advanced clinical stage of lung cancer.156,158,159 CD39+ T-regs have also been implicated in inhibiting the generation and differentiation of Th17 cells, through a TGF-β1 latency associated peptide mechanism.160 While Th1 differentiation has traditionally thought to promote antitumor responses, IFN-γ deficient mice, devoid of Th1 and rich in Th17 cells, were protected from MPE development, while IL-17A deficient mice, rich in Th1 and devoid of Th17 cells, had enhanced pleural tumor cell proliferation and vascular leakiness.161 These studies showcase the special local MPE environment and the need for additional studies to understand the interplay between tumor induced inflammatory cascades and lymphocyte activity.
B Cells are decreased in number in MPEs compared to peripheral blood, however, MPE B cells express higher levels of CD80, CD86, MHC-II, CD44, CD69, and PD-L1 molecules.162 Tumor cells in this environment have abundant expression of MHC class I molecules but lack the B7-1 co-stimulatory molecule, important for B and T cell activation. In murine models, B-/- animals had decreased Th1 and increased Th17 responses, which increased survival time and decreased effusion volume.162 Adoptive transfer of activated naïve B cells reverses this trend, increasing Th1 and inhibiting Th17 expansion by targeting the PD-1/PD-L1 pathway.162 Coculture of naïve B cells with CD4+ Th1 or Th17 conditions resulted in increased Th1 cell expansion, but decreased Th17 expansion; however, treatment with anti-PD-L1 mAb reversed Th17 expansion.162 Thus, by regulating Th1/Th17 cell responses and skewing antitumor cellular immunity, naïve B cells support MPE formation and are an attractive therapeutic target.162 The costimulatory protein, CD40, is expressed on B lymphocytes and DCs. The CD40 ligand, CD154, is expressed by T lymphocytes following initial T cell receptor cross-linking as well as being expressed on platelets.163 Soluble CD40 (sCD40) levels are increased in MPE and are indicative of poor prognosis.164 High concentrations of sCD40 inhibit B cell function by competing for CD154 on T lymphocytes, limiting T cell help and promoting another means of immune evasion.164 B cell B7-H4 expression is also elevated and is associated with poor prognosis for patients with metastatic pleural adenocarcinoma.165 Interestingly, anti-B7-H4 mAb effectively suppressed pleural effusion formation in a mouse model of MPE.165
Completed MPE clinical trials promoting adaptive immune responses
Adaptive immunotherapies that involve the selection, activation, and expansion of T cells to elicit a tumor specific response with immunological memory hold much promise in treating MPE. Though many MPEs share similar characteristics, the primary site of disease appears to influence the composition of the effusion as demonstrated when comparing MM and breast/lung cancer patients.166 In addition to T cells, the pleural space harbors other immunomodulatory elements that vary within patients, suggesting a personalized approach to restoring a functional ‘hot’ immune tumor microenvironment, amenable to immunotherapy. In general, ‘hot’ immune infiltrated tumors are those within which intimate contact between T cells can be observed in the tumor. Dysfunctional T cells found within effusions are dispersed, like tumor cells, in the pleural fluid.167 Local administration of agents such as TLR-9 agonists (CpG’s), stimulator of interferon genes (STING) agonists, or installation of oncolytic viruses would seem to be logical approaches and need to be evaluated. Sequential studies of the pleural fluid should enable detailed mechanistic studies in this setting.
Interleukin 2 therapy
In 1993, a phase I trial of intrapleural recombinant IL-2 infusion examined 22 MPE patients with various cancers.168 This trial aimed to exploit IL-2 efficacy in treating MPE patients with disease stemming from MM (15), adenocarcinomas (6), or squamous cell carcinoma (1). The treatment resulted in 1 complete response (CR) and 9 partial responses (PR) with acceptable side effects. A subsequent study also assessed IL-2 intrapleural treatment, promoting stimulation of lymphokine activated killer (LAK) cell activity. In the treated patient population, a remarkable 30 of 33 patients responded to treatment; with 18 CR, 12 PR, and 3 NR.169 IL-2 administration has been shown to function by reversing the exhaustion phenotype of CD8+ T cells found within MPEs.170 This loss of effector function is proposed to be due to prolonged exposure and stimulation by nominal tumor antigens.171 A trial exploring this exhaustion phenotype showed initially low levels of granzyme B, IFN-γ, and CD8+ T cell proliferation paired with high PD-1 expression, that were then reversed with the IL-2 therapy.170 Toxicity was dose-dependent, ranging from fever to transient abnormal renal function. IL-2 has been approved for treatment of MPE in China since 1998; a recent meta-analysis of 18 IL-2 MPE trials found that despite increases in treatment related fever, thoracic injection of IL-2 plus cisplatin had higher objective response rates (4.1x greater), disease control rates (7.86x greater), quality of life (2.75x greater), and lower nonresponse rates than cisplatin alone.172 Moreover, IL-2 treatment reduced T cell expression of PD-1, while reversing exhaustion phenotypes: increasing proliferation and expression of granzyme B and IFN-γ.170 Following the introduction of IL-2 as a primary treatment regimen for patients with melanoma and renal cancer, as well as the exploration of its use in treating MPE patients, the field has since turned to other novel immunotherapeutic options for MPEs (Table 3). Although approved in China for therapy of MPE, investigators in Europe and the US were less experienced in the use of locally applied ‘microdose’ IL-2, associated with limited and acceptable outpatient toxicity. IL-2 should be reexamined in this setting, perhaps as part of a rationally designed combination therapy with systemic checkpoint inhibitors or novel cellular therapies.
Table 3.
Previous MPE immunotherapy clinical trials.
| Treatment | Design | Patients | Response # (%) | Adverse Events | Ref |
|---|---|---|---|---|---|
| IL-2 | Phase 1 trial of continuous intrapleural rIL-2 infusion | n = 22 adeno-6, meso-15, squam-1 |
CR 1 (5) PR 9 (41) |
Accepted tolerance with some side effects: fever/chills, 2 meso died | 168 |
| IL-2 and LAK | Lymphokine activated killer cells used with IL-2 to treat effusions from lung cancer | n = 33 | CR 18 (55) PR 12 (36) NR 3 (9) |
No serious side effects | 169 |
| IL-2 | Intrapleural and follow up subcutaneous administration of IL-2 providing palliation of pleural effusion and on primary tumor | n = 31 | ORR 7 (22) SD 10 (32) PD 14 (24) |
Manageable toxicity | 173 |
| IL-2 | IL-2 therapy reverses the exhaustion phenotype of MPE CD8 + T cells. Initially low Granzyme B, IFN- γ, and proliferation with high PD-1 expression is reversed to reduce IL-2 expression and increase the others in MPE. Carcino-embryonic antigen reduced by IL-2 | n = 35 (lung cancer) and 12 non-MPE |
IL‐2 treatment reduced the expression of PD‐1, increased the expression of Granzyme B and IFN-γ and enhanced the proliferation of CD8+ T cells in MPE | Dose-dependent severity ranging from fever to abnormal renal function | 170 |
| OK432 | Pilot study using autologous lymphocytes activated ex vivo and monocyte-derived dendritic cells in combination with low-dose OK432 | n = 5 | Decreased effusion production in all pts. | No severe AEs | 174 |
| IFN-α2b | Comparing bleomycin (chemotherapy) to IFN-α2b (immunotherapy). Effusion drained and then given either treatment. Second dose of bleomycin administered for nonresponsive patients. Treatment groups were randomly assigned | n = 160 (83 bleomycin, 77 IFN-α2b) |
30 Day Response Bleomycin: 70 (84.3) IFN-α2b: 48 (62.3) |
None listed | 175 |
| IFN-β | Phase 1 ranging single-dose intrapleural IFN-beta gene transfer by adenoviral vector through indwelling pleural catheter. Evaluates toxicity, gene transfer, and immune, and tumor responses. | n = 10, 7 MPM, 3 MPE |
Gene transfer 7 (70) Antitumor immune response 7 (70) SD 4 (40) PR 4 (40) |
Well tolerated, transient lymphopenia most common. Max tolerated 9E11 viral particles |
176 |
| DCs | Open label pilot study treating MPE patients with DCs derived from autologous CD34+ stem cells stimulated with IL-4, GM-CSF, TNF-α, | n = 26 | CR 1 (3.8) PR 13 (50) SD 10 (38.5) PD 5 (19.2) |
No severe AEs | 119 |
| DCs and cyclophosphamide | Pilot studying using cyclophosphamide and autologous DCs pulsed with MM tumor lysate prior to chemotherapy and P/D. | n = 10 | CR 1 (10) SD 4 (40) PD 2 (20) N/A 3 (30) 8/10 Radiographic improvement Median OS 26 Months |
Well tolerated without systemic toxicity, except for transient fatigue and low-grade fever on the day of the DC injection. No grade 3 or higher AEs. |
120,121 |
| αCD25 +IL-2 +OK432 |
Low-dose anti-CD25 antibody to target T-reg cells. Low concentration of basiliximab augments) production in combination with IL-2. Can also be followed by administration of OK432. Foxp3 expression of ELs (effusion lymphocytes) not definitively changed. Aims to evaluate efficacy of basiliximab followed by OK432 administration (day 0 or 1) | n = 12 | CR 2 (16.7) PR 5 (41.7) |
Safe and well tolerated | 177 |
| CIK Cell Therapy | DC and CIK (cytokine-induced killer) cells treat MPE | n = 16 and 15 control |
CR 8 (50) PR 5 (31.3) NR 3 (18.8) |
Grade III or below, most commonly fever in both groups | 178 |
| Viral Therapy | Phase 1 dose escalation of GMCI strategy with vector for thymidine-kinase gene followed by valacyclovir with chemotherapy, celecoxib added to reduce CRS. | n = 19, 17 evaluable | PR 4 (23.5) SD 9 (52.9) PD 4 (23.5) |
Well tolerated without DLTs | 179 |
Key: Interleukin-2 (IL-2), Recombinant IL-2 (rIL-2), Lymphocyte Activated Killer Cell (LAK), Complete Response (CR), Partial Response (PR), No Response (NR), Progressive Disease (PD), Adverse Events (AEs), Pleurectomy with Decortication(P/D) Interferon Gamma (IFN- γ), Programmed Death Receptor 1 (PD-1), Dendritic Cells (DCs), Els, CIK, GMCI (gene-mediated cytotoxic immune), Cytokine Release Syndrome (CRS), DLTs (Disease Limiting Toxicities).
Dendritic cell therapies
One of the extensively tested immunotherapy strategies that harnesses the specificity and memory of the immune system uses DCs to present tumor associated antigens to elicit responses. Treatment of 26 MPE patients with DCs derived from autologous peripherical CD34+ stem cells with IL-4, GM-CSF, and TNF-α stimulation resulted in no severe side effects, a 54% overall response rate, and improvement of the Karnofsky performance score (KPS) in 15 patients.119 In another 10 patient MM pilot study using autologous DCs pulsed with tumor cell lysate, intradermal or intravenous vaccination following cytoreductive chemotherapy was well tolerated and resulted in antigen specific DC proliferation and stimulation of granzyme B-associated antitumor T cell activity.120 While six patients progressed, three attained a PR, and one had stable disease, with a median OS of 19 months.120 In a follow-up pilot study by the same group, 10 patients with MM were treated with DCs pulsed with autologous tumor lysate combined with T-reg targeting cyclophosphamide following conventional chemotherapy and or P/D.121 This treatment strategy significantly decreased CD4+ T-reg populations, resulted in radiographic disease control in eight patients, and encouraging OS gains. Seven of the 10 patients survived more than 24 months.121 This highly selected patient population had documented stable disease or a partial response to prior chemotherapy. This promising finding warrants larger-scale clinical trials.121,180
Nonspecific immunostimulants
A number of early studies examined nonspecific immunostimulants. For example, infusion of ex vivo activated lymphocytes, monocyte derived DCs and OK432 (lyophilized Streptococcus pyogenes) decreased cancer cell counts in MPE’s in all five patients within a pilot study.174 No severe adverse events were observed and increased IFN-γ levels were found in three of five patients. Additionally, intrapleural injection of the immunostimulant, Staphylococcus aureus superantigen (SSAg) was hypothesized to stimulate T cells and resolve MPE.181 A trial of 14 NSCLC MPE patients with poor pre-treatment status (KPS = 40), received SSAg infusions up to two times a week until the effusion resolved while assessing toxicities. CR and PR were observed in 11 and 3 patients, respectively, without significant adverse effects. The median recurrence was five months and the median survival was 7.9 months compared to a 2.5-month median survival for 18 talc poudrage-treated patients. 9 of 14 SSAg treated patients survived more than six months, while no talc treated patients survived that long.181 Again, investigation in modern local therapies including installation of CpG or STING agonists seems warranted.
Interferon therapy
In addition to nonspecific immunostimulants, the cytokine IFN-α2b increases the cytotoxic activity of NK and T cells, when produced by infected cells, and directly inhibits tumor proliferation.182 However, comparing IFN-α2b immunotherapy to standard bleomycin chemotherapy demonstrated that bleomycin was more effective in MPE patients at 30 days.175 Out of 83 bleomycin and 77 IFN-α2b patients, 84.3% and 62.3%, respectively, responded. This trial demonstrated no survival advantage but did offer palliation and effusion control. Targeting another type I interferon known to inhibit tumor growth and boost the immune system, a phase I trial evaluating a single dose intrapleural IFN-β gene transfer using an adenoviral vector (Ad.IFN-β) showed no increases in pleural cell infiltrate, but was well tolerated and did elicit antitumor immune responses (7/10 patients).176 Another targeted treatment involved low-dose anti-CD25 (basiliximab) antibody to target T-regs and preserve CD4+CD25dim activated T cells in MPE patients. The treatment was given in combination with IL-2 or followed by OK432. 7 of 12 patients treated with OK432 and basiliximab responded, two of which were CR with acceptable adverse events.177
Other therapies
Trials of combined therapies showed that combination of autologous DC and cytokine-induced killer (CIK) cells to treat effusions had comparably mild side effects but only modest efficacy.178 An anecdotal report of intrapleural administration of tumor infiltrating lymphocyte (TIL) infusion compared to traditional cisplatin therapy demonstrated a claimed but very modest greater response rate (33.3% vs 28.57%) and disease control (71.43% vs 66.67%) than MPE patients given cisplatin.183 Certainly, local and systemic TIL therapy is an area worthy of further consideration. Another combination therapy, intrapleural gene-mediated cytotoxic therapy (GMCI) utilizing a thymidine-kinase gene expressed by an adenovirus-based vector was followed with anti-herpetic prodrug valacyclovir and chemotherapy in a phase I trial of patients with MPE.179 The addition of celecoxib decreased cytokine release syndrome as experienced by some patients. Of the 17 evaluable patients, treatment was safe and well tolerated with encouraging preliminary treatment responses: PR 4, SD 9, PD 4, with 3 patients alive 23–33 months after GMCI.179 The current approval of oncolytic herpes virus therapy for patients with melanoma,184 suggests that utilizing herpes or vaccinia virus,185-187 might be considered useful in the future treatment of patients with MPE. Local therapies such as these may enable dissemination of memory T cells, capable of controlling disease systemically.
Current antibody and cell therapies
Great strides continue to be taken in improving the efficacy of immunotherapy, including application of check-point inhibitors and Chimeric Antigen Receptor (CAR)-T cells. The use of adoptive therapy with TIL or alternative sources of T cells including the use of lymph nodes or peripheral blood-derived tumor reactive cells is currently under-developed.
Checkpoint inhibition
The immune checkpoint proteins are co-receptors expressed on the surface of T cells that interact with their corresponding ligands on APCs, which in turn effect T cell activation and subsequently may limit cancer cell elimination following T cell recognition.188 Checkpoint antibody inhibitors prevent receptor and ligand binding and interaction, disrupting immunosuppressive signaling; their administration has resulted in improved survival outcomes for patients with solid tumors,189 especially in patients with lung cancer (Table 4). Interestingly, almost no information about MPE and checkpoint treatment success (or failure) is currently available.
Table 4.
Checkpoint inhibitor clinical trials of NSCLC and MM.
| Immunotherapy | Patient Population | Response # (%) | Ref |
|---|---|---|---|
| BMS-936559 (anti-PD-L1) |
49 NSCLC | ORR: 5 (10.2) SD: 6 (12) PFS: (31) at 24 weeks |
190 |
| Chemotherapy + Ipilimumab (anti-CTLA-4) | 338 NSCLC (Stage IV or recurrent) | Median OS: 13.4 months Median PFS: 5.6 months AE (chemotherapy + ipilimumab): 173 (51) |
191 |
| BMS-936558 (anti-PD-1) |
76 NSCLC | ORR: 14 (18) SD: 5 (7) PFS: 20 (26) AE: 11 (14) |
192 |
| Pembrolizumab (anti-PD-1) |
690 NSCLC: 344 given 2 mg/kg 346 given 10 mg/kg |
2 mg/kg group: Median OS: 14.9 months Median PFS: 3.9 months 10 mg/kg group: Median OS: 17.3 months Median PFS: 4.0 months |
193 |
| Pembrolizumab (anti-PD-1) |
154 NSCLC: 29 squamous 125 non-squamous (18 Brain metastasis) |
ORR: 69 (45) Median time to respond: 2.2 months PFS: median 10.3 months (62.1% at 6 months) OS: 124 (80.2) at 6 months AE: 113 (73.4) |
194 |
| Nivolumab (anti-PD-1) |
135 NSCLC (non-squamous) (9 CNS metastasis) | ORR: 27 (20) PFS: median 3.5 months OS: 57 (42) 1 yr AE: 78 (58) |
195 |
| Nivolumab (anti-PD-1) |
52 NSCLC: 13 squamous 39 non-squamous (7 metastatic disease) |
ORR: 2 (15) squamous 10 (26) non-squamous AE: 10 (19) PFS: 21 (41) at 24 weeks OS: 38 (73) 1 yr |
196 |
| Nivolumab + ipilumab | 77 NSCLC | ORR: 33 (42.9) | 197 |
| Durvalumab (anti-PD-L1) |
473 NSCLC | Median PFS: 16.8 months PFS at 12 months: 264 (55.9) PFS at 18 months: 209 (44.2) ORR: 134 (28.4) |
198 |
| Nivolumab (anti-PD-1) |
292 NSCLC (non-squamous) | ORR: 56 (19) Median time to respond: 2.1 months OS: 149 (51) 1 yr (median 12.2 moths) PFS: median 2.3 months (19% at 12 months) |
199 |
| Pembrolizumab (anti-PD-1) |
495 NSCLC | ORR: 97 (19.4) Median time of response: 12.5 moths AE: 351 (70.9) SD: 21.8 (4.4) PFS: 3.7 months OS: median 12 months |
200 |
| Nivolumab and Nivolumab + Ipilimumab | 125 MPM: 63 given Nivo 62 given Nivo + Ipi |
ORR: Nivolumab: 11 (17.5) Nivolumab + Ipilimumab: 15 (24.2) |
201 |
| Nivolumab (anti-PD-1) |
34 MPM | DCR at 12 weeks: 17 (50) PR at 12 weeks: 5 (14.7) SD at 12 weeks: 12 (35.3 PD at 12 weeks: 17 (50) |
202 |
Key: Programmed Cell Death Protein-1 (PD-1), Programmed Death Receptor Ligand (PD-L1), Objective Response Rate (ORR), Stable Disease (SD), Progression Free Survival (PFS), Partial Response (PR), Complete Response (CR), Disease Control Rate (DCR), Stable Disease (SD), Progressive Disease (PD).
CTLA-4 antibody
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), an inhibitory receptor that down-modulates the initial stages of T cell activation, was the first clinically validated checkpoint pathway target.190,203 When CTLA-4 is mobilized in T cells from cytosolic stores, it binds its counter-receptors CD80 and CD86 on APCs, mediating direct inhibitory effects on the MHC-TCR pathway and decreasing T cell effector function.204,205 Anti-CTLA-4 monoclonal antibodies prevent this binding, amplifying T cell responses against tumors.206,207 In a Phase III study of advanced NSCLC, administration of anti-CTLA-4 mAb ipilimumab (Yervoy) with first-line chemotherapy, such as paclitaxel or carboplatin, did not prolong OS (median OS 13.4 vs 12.4 months) compared with chemotherapy alone.191
PD-1 antibody
Programmed cell death protein 1 (PD-1) is a key immune-checkpoint receptor expressed by activated T, B, and NK cells that mediates immunosuppression.192 When PD-1 binds to its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC), expressed by tumor and stromal cells, it moves the T cell receptor out of the so-called central supramolecular activation cluster (CSMAC) with a target, reducing T cell survival, and inhibiting T cell proliferation and production of IFN-γ, TNF-α, and IL-2210. Monoclonal antibodies that target PD-1, such as pembrolizumab (Keytruda) and nivolumab (Opdivo), are human IgG4 PD-1 immune-checkpoint–inhibitor antibodies that disrupt PD-1–mediated signaling and have a demonstrated ability to reverse suppression of T cell function and restore antitumor immunity.211,212
The phase II/III KEYNOTE-010 study, which included patients with advanced NSCLC and at least 1% PD-L1 positive tumors, showed that treatment with pembrolizumab prolongs OS compared to conventional docetaxel chemotherapy (12.7 vs 8.5 months).193 In the follow-up KEYNOTE-024 trial, patients who had PD-L1 expression on at least 50% of tumor cells treated with pembrolizumab had a significantly higher PFS and response rate than those treated docetaxel, leading to US Food and Drug Administration approval of Pembrolizumab as first-line therapy for patients with NSCLC with high PD-L1 expression.194 In patients with advanced NSCLC, the response rate (20% vs 9%), PFS (3.5 vs 2.8 months), OS (9.2 vs 6.0 months) are longer with nivolumab treatment than with docetaxel.185,195,196 Although 37% of patients experienced Grade 3–4 treatment related AEs, combination therapy of nivolumab and ipilimumab demonstrated encouraging clinical responses in NSCLC (47% confirmed objective response) and survival (median PFS 8.1 months) that should be tested against anti-PD-1 monotherapy in future trials.197 Interestingly, in NSCLC and across all cancer types, patients with greater tumor mutational load, the total number of coding mutations per megabase of the tumor genome, correlated with greater response to PD-1 therapy, suggesting additional biomarkers for treatment stratification, moving forward.208,209
PD-L1 antibody
Atezolizumab (Tecentriq) is a humanised engineered IgG1 monocolonal antibody that targets PD-L1, inhibiting both PD-L1/PD-1 and PD-L1/B7-1 binding, which might further enhance immune responses to cancer cells.213 In a randomized, open-label, phase III trial (OAK Study) evaluating Atezolizumab in patients with previously treated NSCLC, PD-L1 inhibition increased median overall survival compared to docetaxel (13.8 vs 9.6 months) with fewer grade III or IV AEs (15 vs 43%).214 Similarly, the phase III placebo controlled PACIFIC trial demonstrated that durvalumab (anti-PD-L1) is an efficacious consolidation therapy (median PFS 16.8 vs 5.6 months) in patients with stage III NSCLC who did not have disease progression after two or more cycles of platinum-based chemoradiotherapy, which resulted in the approval of durvalumab by the US Food and Drug Administration for use as a maintenance therapy following the completion of platinum-based chemoradiation in unresectable lung cancer.198
A few studies are also testing the combination effects of checkpoint inhibitors with anti-human vascular endothelial growth factor (anti-VEGF) receptors. Preliminary results from the ongoing CheckMate 012 phase I trial evaluating the efficacy and safety of switching to nivolumab combined with bevacizumab (anti-VEGF) as maintenance treatment, in a cohort of advanced NSCLC patients with no disease progression to first-line platinum-based chemotherapy reported median PFS (37.1 weeks) comparable to approved agents.215 Another clinical trial currently evaluating the safety and preliminary efficacy of pembrolizumab plus ramucirumab (anti-VEGFR-2) in patients with locally advanced and unresectable or metastatic NSCLC found no unexpected safety signals.216
Additional checkpoint targets
A number of novel T cell and NK cells checkpoint targets are being tested in the clinic, including antibodies to LAG3, TIM3, TIGIT, KIR2DL1-3, NKG2A, and others. Lymphocyte-activating gene 3 (LAG-3), for example, is expressed in cytosolic stores and on NK cells,217 DCs,218 B cells,219 and TIL.220 LAG-3 encodes a protein that binds a nonholomorphic region of MHC class II with greater affinity than CD4 and novel target fibroblast activating protein 1, and inhibits T cell proliferation, activation, and homeostasis.221,222 TIL from NSCLCs patients express LAG-3, positively correlating with PD-1/PD-L1 expression, consistent with a poor prognosis.223 T cell immunoglobulin domain and mucin domain-3 (TIM-3) is another immune checkpoint receptor that is expressed on IFN-γ producing CD4+ Th1 and CD8+ T cells and has an inhibitory role in T cell responses.224 Interaction between TIM-3 and its ligands, including HMGB1/DNA, phospatidylserine, and galectin-9, inhibits Th1 and Th17 responses and induces peripheral tolerance.225 TIM-3 is expressed on TIL of NSCLC patients and correlates with poor clinicopathological parameters such as nodal metastasis and advanced cancer stages.226,227
The current efficacy of checkpoint inhibition in MPE remains to be seen. There have been several clinical trials of anti-PD-1 in lung cancer patients, but its effect on MPE are not specifically reported.194,195,199,200 PD-L1 is upregulated in tumor cells and normal MRC-5 lung fibroblasts, and PD-L1 blockade restores in vitro CD8+ T-cell granzyme B expression. Interestingly, rather than being exhausted, CD8+ TIL in MPE are not completely differentiated and are negatively regulated by PD-L1.228 Abundant expression of immune checkpoints, PD-1, PD-L1, LAG-3, and TIM-3 have been identified in MPE resident immune and tumor cells, suggesting additional targets in novel therapeutic interventions.229,230
Modern adoptive cell therapy
Adoptive cell transfer (ACT) is a promising approach to immunotherapy that most often utilizes a patient’s own immune cells to treat their cancer. Chimeric antigen receptor (CAR) T cell therapy is an ACT with the most advanced clinical development. TIL within a tumor microenvironment often become exhausted, anergic, and nonfunctional.231 CAR-T cell receptors are specifically engineered to activate in response to tumor specific antigens (TSA) expressed on the cell surface, but most often target common structures on cells, including CD19 expressed on most B-cells.232 CARs are synthetic receptors most frequently grafted with antibody specificities onto TCR signaling domains.233 First generation CAR-T cells bound to TSAs with a single chain variable fragment (scFv) fused to the CD3ζ domain demonstrated in vitro cytotoxicity but limited in vivo capabilities due to CD3ζ’s inability to prolong activation of resting T cells.234,235 More recently, second and third generation CAR-T cells that contain the scFv with multiple costimulatory signaling domains (CD28 and 4-1BB) in addition to the CD3ζ chain have demonstrated in vivo antitumor efficacy with increased cytokine production and T cell proliferation and persistence.236,237 CAR-T cell therapy has had substantial clinical success in treating hematological patients with malignancies including acute lymphoblastic leukemia,238,239 chronic lymphocytic leukemia239 and non-Hodgkin lymphomas.240,241 CAR-T cell therapy is under active investigation for the treatment of solid tumors including NSCLC and MM, as outlined in Table 5. None of these specifically target MPE’s although local application is an attractive approach.
Table 5.
Current CAR-T cell clinical trials for thoracic malignancies.
| Target Antigen | Indication | Phase | Location | Status | NCT# |
|---|---|---|---|---|---|
| CEA | Lung, Colorectal, Gastric, Breast, Pancreatic |
I | China | Recruiting | NCT02349724 |
| EGFR | Cholangiocarcinoma, Colorectal, NSCLC, Ovarian, Pancreatic, Renal | I/II | China | Recruiting | NCT01869166 |
| FAP | Mesothelioma | I | Zurich | Recruiting | NCT01722149 |
| GD2 | Solid tumors | I/II | China | Recruiting | NCT02992210 |
| GPC3 | Hepatocellular Carcinoma, Squamous Cell Lung | I | China | Recruiting | NCT03198546 |
| GPC3 | Lung Squamous Cell Carcinoma | I | China | Recruiting | NCT02876978 |
| HER2 | Breast, Ovarian, Lung, Gastric, Colorectal, Glioma, Pancreatic | I/II | China | Recruiting | NCT02713984 |
| MSLN | Lung Adenocarcinoma, Ovarian, Peritoneal Carcinoma, Mesotheliomas | I | UPENN | Active, not recruiting | NCT03054298 |
| MSLN | Cervical, Pancreatic, Ovarian, Mesothelioma, Lung | I/II | NCI | Recruiting | NCT01583686 |
| MSLN | Breast, Lung, Malignant Pleural Disease, Mesothelioma, Metastases | I | MSKCC | Recruiting | NCT02414269 |
| MSLN | Breast, Endometrial, Mesothelioma, Ovarian, Pancreatic | I | China | Recruiting | NCT02580747 |
| MSLN | Mesothelioma, Pancreatic, Ovaria, Metastatic | I | UPENN | Complete | NCT02159716 |
| MSLN | Mesothelioma | I | UPENN | Complete | NCT01355965 |
| MUC1 | Lung Neoplasm Malignant, NSCLC | I/II | China | Recruiting | NCT03525782 |
| MUC1 | Hepatocellular Carcinoma, NSCLC, Pancreatic, Triple-Negative Invasive Breast Carcinoma, Malignant Glioma, Colorectal, Gastric | I/II | China | Recruiting | NCT02587689 |
| MUC1 | Hepatocellular Carcinoma, NSCLC, Pancreatic Carcinoma Triple-Negative Invasive Breast Carcinoma, Malignant Glioma of Brain, Colorectal, Gastric | I/II | China | Recruiting | NCT02839954 |
| PD1 | Gastric, Lung, Liver | I/II | China | Recruiting | NCT02862028 |
| PDL1 | NSCLC | I | China | Not yet recruiting | NCT03330834 |
| PSCA/MUC1/PDL1/CD80/86 | Lung | I | China | Recruiting | NCT03198052 |
| ROR1 | Breast (including triple negative), Leukemias (ALL, CLL, mantle cell), NSCLC | I | NCI | Recruiting | NCT02706392 |
| VEGF Receptor 2 | Melanoma, Renal, Metastatic | I/II | NCI | Complete | NCT01218867 |
Key: Carcinoembryonic Antigen (CEA), Epidermal Growth Factor (EGFR), Fibroblast Activation Protein (FAP), Ganglioside (GD2), Glypican-3 (GPC3), Human Epidermal Growth Factor 2 (HER2), Mesothelin (MSLN), Transmembrane Glycoprotein Mucin 1 (MUC1), Program Cell Death Protein 1 (PD1), Programmed Cell Death Ligand 1 (PDL1), Prostate Stem Cell Antigen (PSCA), Vascular Endothelial Growth Factor (VEGF).
Finding suitable TSAs for CAR-T cell therapy is a substantial challenge. Candidate target antigens currently being investigated in clinical trials for lung cancer and MM include overexpressed tumor-associated antigens (TAAs) [carcinoembryonic antigen (CEA), ganglioside (GD2), glypican-3 (GPC3), human epidermal growth factor receptor 2 (HER2), mesothelin (MSLN), epidermal growth factor (EGFR), prostate stem cell antigen (PSCA), and receptor tyrosine-kinase-like orphan receptor (ROR1)]; abnormal glycosylation proteins [transmembrane glycoprotein mucin 1 (MUC1)]; immunomodulatory antigens [NKG2D ligands including MICA/MICB and ULBP1-3, PD-L1, CD80/CD86]; and stromal elements associated with the tumor microenvironment [fibroblast activation protein (FAP) and VEGF Receptor 2].233,242
EGFR and HER2 are receptor tyrosine kinases that are amplified or mutated in a variety of cancers, including over 15% of NSCLC patients in western nations and 45% of NSCLC patients in Asian countries.243,244 Second generation EGFR-CAR T cells (CD3+CD8+ T cells) demonstrated high proliferative capacity as well as specific and potent cytotoxicity against NSCLC cells in vitro and in vivo.245 A Phase I study (NCT01869166) at the Chinese PLA Hospital testing escalating doses of EGFR-CAR-T cell infusions in 11 patients with advanced relapsed NSCLC was well tolerated with pathological eradication of EGFR positive tumor cells and two patients obtaining PR and five patients demonstrating stable disease.246 Another Phase I/II study (NCT02713984) in the Southwest Hospital of China is testing HER2-CAR-T cells in various refractory malignancies including NSCLC. EGFR mutations can be detected in malignant pleural fluid but have lowered response rates to EGFR tyrosine kinase inhibitors than solid tumors.247
MSLN is a cell surface glycoprotein overexpressed in epithelial cancers, including MM and NSCLC that has been associated with poor OS and PFS.248-251 Several Phase 1 studies evaluating systemic and intrapleural administration of MSLN-CAR-T cells are currently underway (NCT01583686, NCT02414269, NCT02580747, NCT02159716, NCT01355965). An initial seven patient MM study at the University of Pennsylvania using transiently expressed second generation MSLN-CAR T cells containing the CD3ζ and 4-1BB signaling domains showed no “on target, off target” cytotoxicities.252 Early results from these studies indicate that MSLN-CAR-T cells migrated to primary and metastatic tumor sites and elicited anti-tumor responses (NCT01355965).252 A follow-up study using escalating doses of MSLN-CAR-T cells and cyclophosphamide, as a lymphodepletion agent, was well tolerated and is nearing completion (NCT02159716).
MUC1 is a transmembrane glycoprotein that is aberrantly glycosylated in many cancers, including NSCLC.253,254 MUC1 and PSCA CAR-T cells showed independent and synergistic antitumor efficacy in a patient-derived xenograft model of NSCLC.255 The First People’s Hospital in Hefei, China is completing a Phase I/II study of MUC1-CAR T cells for patients with MUC1+ advanced refractory NSCLC (NCT02587689). The same group is also completing a similar study using MUC1-CAR-pNK cells, placing the CAR construct in placental derived NK cells in advanced refractory NSCLC patients (NCT02839954). Given that CAR-T cells are prone to acquiring a differentiated and exhausted phenotype with increased expression of PD-1 in the tumor microenvironment,256 another group at the First Affiliated Hospital of Guangdong Pharmaceutical University is testing MUC-1 CAR-T cells with PD-1 knockout in a randomized Phase I/II study of NSCLC (NCT03525782). There are three trials currently targeting immunomodulatory antigens in NSCLC, including PD-1 (NCT02862028), PD-L1 (NCT03330834), and a combination of PSCA, MUC1, PD-L1 or CD80/CD86 (NCT03198052). No published preclinical data exists of CAR-T cells that target PD-L1 or CD80/CD86. Since PD-1, PD-L1, and CD80/86 are expressed on normal immune cells, the possible off-tumor toxicities need to be carefully considered and reviewed.
FAP is an integral membrane gelatinase that controls fibroblast growth and epithelial-mesenchymal interactions that is activated in fibroblasts in 90% of epithelial cancers, including MM.257 The University of Zurich is recruiting MM MPE patients for a phase I single dose FAP CAR-T cell (NCT01722149) therapy. Local administration of anti-FAP (scFv F19) CAR-T cells, with CD28, Δ-CD28, and 4-1BB costimulatory domains, in combination with PD-1 inhibition provided transient tumor control and improved survival in a humanized mouse model of MM and also provided 1 year stable disease in a first-in-man clinical trial in MM with MPE.258 Given limited therapeutic responses to checkpoint blockade in MM, further clinical evaluation of combinations of FAP-specific CAR-T cells and checkpoint blockade is warranted to improve the T-cell repertoire to generate a therapeutic immune response.
Given the risk of normal tissue toxicity associated with CAR-T therapy targeting less restricted TSAs, new strategies to minimize CAR-T cytotoxicity are needed. Early approaches included use of kill switches that induce CAR-T cell apoptosis in case of severe toxicity.259,260 Unfortunately, these cell-suicide systems are irreversible and do not control T cell activation or expansion. A switch-controlled approach was recently developed to enable better control of reactivity and safety of CAR-T therapy for NSCLC.261 CAR-T cells targeting fluorescein isothiocyanate (FITC), were generated in conjunction with an intermediate antigen switch composed of folate bound to FITC, thereby binding the folate receptor α (FRα) chain, a cell surface protein that is expressed in over 70% of lung adenocarcinomas.262,263 This established a pseudoimmunological synapse with FITC-CAR T cells and cells expressing either FRα or FRβ, which is also highly expressed on TAMs in the tumor microenvironment.261 Potent antigen-specific and dose-dependent in vitro efficacy was demonstrated against NSCLC and macrophage cell lines. Further pre-clinical testing is required to determine the efficacy targeting both the tumor and tumor microenvironment in NSCLC. These bifunctional switches enable greater control of CAR-T cell antigen specificity and activity, which will help to greatly improve patient safety profiles during ACT treatment.264
Conclusion
MPEs represent a unique and understudied tumor microenvironment. Current treatment frequently involves palliative drainage of effusions, providing the opportunity to longitudinally access the evolution of neoplastic cells and the subsequent dynamics of innate and adaptive immunity in response to the disease. Nevertheless, even though immune cells are identified, host immunity has failed to contain malignancy. Given the urgency in this population with a short-expected survival time, only a small temporal window is available for gaining and sustaining therapeutic benefit. Further examination of the microenvironment of MPEs may provide insight into the mechanisms of tumor-mediated immunosuppression, and specifically, how these events change over time. As highlighted above, advances in our understanding of tumor immunology in conjunction with technological innovations have yielded novel immune-based treatments resulting in unprecedented clinical success, particularly in hematopoietic malignancies, melanoma, and lung cancer. Serial evaluation of response to CAR-T therapy, novel application alone or in combination with immunomodulators such as IL-2 and IL-15, employing factors to repolarize immunosuppressive leukocytes, oncolytic virotherapy, and T cell checkpoint inhibitors, alone or in rationally designed combinations may identify correlates of effective antitumor immunity and subsequent tumor immune escape mechanisms. This is remarkably possible in the setting of MPEs where serial evaluation of both the tumor and immune cells are realized. Notably, repeat evaluation of MPEs during immunotherapy may identify clinically actionable targets present within the tumor, immune, and/or stromal compartments, allowing for the development of more effective patient-specific treatments. Evaluation of locoregional IL-2 alone or with cisplatin installation in combination with checkpoint inhibitors would seem to be an early interesting and useful strategy.
Funding Statement
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under awards [T32CA113263-11] to Chigozirim Ekeke; [R01CA181450] to Michael Lotze; and [R01CA206012] to Michael Lotze. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interests.
References
- 1.Muduly D, Deo S, Subi T, Kallianpur A, Shukla N.. An update in the management of malignant pleural effusion. Indian J Palliat Care. 2011;17(2):98–103. doi: 10.4103/0973-1075.84529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Psallidas I, Kalomenidis I, Porcel JM, Robinson BW, Stathopoulos GT.. Malignant pleural effusion: from bench to bedside. Eur Respir Rev. 2016;25(140):189–198. doi: 10.1183/16000617.0019-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamboni MM, Da Silva CT Jr., Baretta R, Cunha ET, Cardoso GP. Important prognostic factors for survival in patients with malignant pleural effusion. BMC Pulm Med. 2015;15:29. doi: 10.1186/s12890-015-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YC. Hunting for a pleural fluid test for mesothelioma: is soluble mesothelin the answer? Thorax. 2007;62(7):561–562. doi: 10.1136/thx.2006.076612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheah HM, Lansley SM, Varano Della Vergiliana JF, Tan AL, Thomas R, Leong SL, Creaney J, Lee YC. Malignant pleural fluid from mesothelioma has potent biological activities. Respirology. 2017;22(1):192–199. doi: 10.1111/resp.12874. [DOI] [PubMed] [Google Scholar]
- 6.Morgensztern D, Waqar S, Subramanian J, Trinkaus K, Govindan R. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7(10):1485–1489. doi: 10.1097/JTO.0b013e318267223a. [DOI] [PubMed] [Google Scholar]
- 7.Opitz I. Management of malignant pleural mesothelioma-the european experience. J Thorac Dis. 2014;6(Suppl 2):S238–252. doi: 10.3978/j.issn.2072-1439.2014.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21(5):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antony VB. Immunological mechanisms in pleural disease. Eur Respir J. 2003;21(3):539–544. [DOI] [PubMed] [Google Scholar]
- 10.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 11.Horton B, Spranger S. A tumor cell-intrinsic yin-yang determining immune evasion. Immunity. 2018;49(1):11–13. doi: 10.1016/j.immuni.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18(3):139–147. doi: 10.1038/nrc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer immunotherapy targets based on understanding the t cell-inflamed versus non-t cell-inflamed tumor microenvironment. Adv Exp Med Biol. 2017;1036:19–31. doi: 10.1007/978-3-319-67577-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ, Group BTSPDG. Management of a malignant pleural effusion: british thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii32–40. doi: 10.1136/thx.2010.136994. [DOI] [PubMed] [Google Scholar]
- 15.DeBiasi EM, Pisani MA, Murphy TE, Araujo K, Kookoolis A, Argento AC, Puchalski J. Mortality among patients with pleural effusion undergoing thoracentesis. Eur Respir J. 2015;46(2):495–502. doi: 10.1183/09031936.00217114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016;(5):CD010529 https://pdfs.semanticscholar.org/02ee/3861721ef176cc66c5700c0796f0064eab92.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fysh ET, Thomas R, Read CA, Kwan BC, Yap E, Horwood FC, Lee P, Piccolo F, Shrestha R, Garske LA, et al. Protocol of the australasian malignant pleural effusion (ample) trial: A multicentre randomised study comparing indwelling pleural catheter versus talc pleurodesis. BMJ Open. 2014;4(11):e006757. doi: 10.1136/bmjopen-2014-006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, Davies CW, Grayez J, Harrison R, Prasad A, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the time2 randomized controlled trial. JAMA. 2012;307(22):2383–2389. doi: 10.1001/jama.2012.5535. [DOI] [PubMed] [Google Scholar]
- 19.Shouman W, Elgazzar A, Hussien RM, ElShaaray M, Light RW. Chemical pleurodesis for malignant pleural effusion. Egypt J Chest Dis Tuberc. 2012;61(3):115–120. doi: 10.1016/j.ejcdt.2012.10.013. [DOI] [Google Scholar]
- 20.Kilic D, Akay H, Kavukcu S, Kutlay H, Cangir AK, Enon S, Kadilar C. Management of recurrent malignant pleural effusion with chemical pleurodesis. Surg Today. 2005;35(8):634–638. doi: 10.1007/s00595-005-2996-5. [DOI] [PubMed] [Google Scholar]
- 21.Colt HG, Russack V, Chiu Y, Konopka RG, Chiles PG, Pedersen CA, Kapelanski D. A comparison of thoracoscopic talc insufflation, slurry, and mechanical abrasion pleurodesis. Chest. 1997;111(2):442–448. [DOI] [PubMed] [Google Scholar]
- 22.Barbetakis N, Asteriou C, Papadopoulou F, Samanidis G, Paliouras D, Kleontas A, Lyriti K, Katsikas I, Tsilikas C. Early and late morbidity and mortality and life expectancy following thoracoscopic talc insufflation for control of malignant pleural effusions: A review of 400 cases. J Cardiothorac Surg. 2010;5:27. doi: 10.1186/1749-8090-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putnam JB Jr., Light RW, Rodriguez RM, Ponn R, Olak J, Pollak JS, Lee RB, Payne DK, Graeber G, Kovitz KL. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86(10):1992–1999. [PubMed] [Google Scholar]
- 24.Thomas R, Budgeon CA, Kuok YJ, Read C, Fysh ETH, Bydder S, Lee YCG. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146(3):557–562. doi: 10.1378/chest.13-3057. [DOI] [PubMed] [Google Scholar]
- 25.Bhatnagar R, Keenan EK, Morley AJ, Kahan BC, Stanton AE, Haris M, Harrison RN, Mustafa RA, Bishop LJ, Ahmed L, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378(14):1313–1322. doi: 10.1056/NEJMoa1716883. [DOI] [PubMed] [Google Scholar]
- 26.Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med. 2008;102(7):939–948. doi: 10.1016/j.rmed.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Bhatnagar R, Kahan BC, Morley AJ, Keenan EK, Miller RF, Rahman NM, Maskell NA. The efficacy of indwelling pleural catheter placement versus placement plus talc sclerosant in patients with malignant pleural effusions managed exclusively as outpatients (ipc-plus): study protocol for a randomised controlled trial. Trials. 2015;16:48. doi: 10.1186/s13063-015-0731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed L, Ip H, Rao D, Patel N, Noorzad F. Talc pleurodesis through indwelling pleural catheters for malignant pleural effusions: retrospective case series of a novel clinical pathway. Chest. 2014;146(6):e190–e194. doi: 10.1378/chest.14-0394. [DOI] [PubMed] [Google Scholar]
- 29.Petrou M, Kaplan D, Goldstraw P. Management of recurrent malignant pleural effusions. The complementary role talc pleurodesis and pleuroperitoneal shunting. Cancer. 1995;75(3):801–805. [DOI] [PubMed] [Google Scholar]
- 30.Schulze M, Boehle AS, Kurdow R, Dohrmann P, Henne-Bruns D. Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg. 2001;71(6):1809–1812. [DOI] [PubMed] [Google Scholar]
- 31.Aydin Y, Turkyilmaz A, Intepe YS, Eroglu A. Malignant pleural effusions: appropriate treatment approaches. Eurasian J Med. 2009;41(3):186–193. [PMC free article] [PubMed] [Google Scholar]
- 32.Genc O, Petrou M, Ladas G, Goldstraw P. The long-term morbidity of pleuroperitoneal shunts in the management of recurrent malignant effusions. Eur J Cardiothorac Surg. 2000;18(2):143–146. [DOI] [PubMed] [Google Scholar]
- 33.Penz E, Watt KN, Hergott CA, Rahman NM, Psallidas I. Management of malignant pleural effusion: challenges and solutions. Cancer Manag Res. 2017;9:229–241. doi: 10.2147/CMAR.S95663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams T, Duraid H, Watson S, Durkin A, Todd K, Kindler HL, Vigneswaran WT. Extended pleurectomy and decortication for malignant pleural mesothelioma is an effective and safe cytoreductive surgery in the elderly. Ann Thorac Surg. 2015;100(5):1868–1874. doi: 10.1016/j.athoracsur.2015.04.151. [DOI] [PubMed] [Google Scholar]
- 35.Fry WA, Khandekar JD. Parietal pleurectomy for malignant pleural effusion. Ann Surg Oncol. 1995;2(2):160–164. [DOI] [PubMed] [Google Scholar]
- 36.Figlin R, Mendoza E, Piantadosi S, Rusch V. Intrapleural chemotherapy without pleurodesis for malignant pleural effusions. Lcsg trial 861. Chest. 1994;106(6 Suppl):363S–366S. [PubMed] [Google Scholar]
- 37.Fujita A, Takabatake H, Tagaki S, Sekine K. Combination chemotherapy in patients with malignant pleural effusions from non-small cell lung cancer: cisplatin, ifosfamide, and irinotecan with recombinant human granulocyte colony-stimulating factor support. Chest. 2001;119(2):340–343. [DOI] [PubMed] [Google Scholar]
- 38.Perng RP, Chen YM, Wu MF, Chou KC, Lin WC, Liu JM, Whang-Peng J. Phase ii trial of intrapleural paclitaxel injection for non-small-cell lung cancer patients with malignant pleural effusions. Respir Med. 1998;92(3):473–479. [DOI] [PubMed] [Google Scholar]
- 39.Chenyang Liu QQ, Geng S, Shi Y. Palliative treatment of malignant pleural effusion. Cancer Trans Med. 2015;1(4):131–136. doi: 10.4103/2395-3977.163804. [DOI] [Google Scholar]
- 40.Davis SR, Tan L, Ball DL. Radiotherapy in the treatment of malignant mesothelioma of the pleura, with special reference to its use in palliation. Australas Radiol. 1994;38(3):212–214. [DOI] [PubMed] [Google Scholar]
- 41.Calabro L, Morra A, Fonsatti E, Cutaia O, Fazio C, Annesi D, Lenoci M, Amato G, Danielli R, Altomonte M, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3(4):301–309. doi: 10.1016/S2213-2600(15)00092-2. [DOI] [PubMed] [Google Scholar]
- 42.Alley EW, Katz SI, Cengel KA, Simone CB 2nd.. Immunotherapy and radiation therapy for malignant pleural mesothelioma. Transl Lung Cancer Res. 2017;6(2):212–219. doi: 10.21037/tlcr.2017.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pien GW, Gant MJ, Washam CL, Sterman DH. Use of an implantable pleural catheter for trapped lung syndrome in patients with malignant pleural effusion. Chest. 2001;119(6):1641–1646. [DOI] [PubMed] [Google Scholar]
- 44.Putnam JB Jr., Walsh GL, Swisher SG, Roth JA, Suell DM, Vaporciyan AA, Smythe WR, Merriman KW, DeFord LL. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg. 2000;69(2):369–375. [DOI] [PubMed] [Google Scholar]
- 45.Muruganandan S, Azzopardi M, Fitzgerald DB, Shrestha R, Kwan BCH, Lam DCL, De Chaneet CC, Rashid Ali MRS, Yap E, Tobin CL, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (ample-2): an open-label randomised trial. Lancet Respir Med. 2018. doi: 10.1016/S2213-2600(18)30288-1. [DOI] [PubMed] [Google Scholar]
- 46.Gupta SS, Floudas CS, Chandra AB. A comparison between two types of indwelling pleural catheters for management of malignant pleural effusions. J Thorac Dis. 2018;10(5):2976–2980. doi: 10.21037/jtd.2018.05.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mollberg NM, Vigneswaran Y, Kindler HL, Warnes C, Salgia R, Husain AN, Vigneswaran WT. Quality of life after radical pleurectomy decortication for malignant pleural mesothelioma. Ann Thorac Surg. 2012;94(4):1086–1092. doi: 10.1016/j.athoracsur.2012.05.102. [DOI] [PubMed] [Google Scholar]
- 48.Treasure T, Lang-Lazdunski L, Waller D, Bliss JM, Tan C, Entwisle J, Snee M, O’Brien M, Thomas G, Senan S, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the mesothelioma and radical surgery (mars) randomised feasibility study. Lancet Oncol. 2011;12(8):763–772. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao C, Krog Andvik SK, Yan TD, Kennedy C, Bannon PG, McCaughan BC. Staging of patients after extrapleural pneumonectomy for malignant pleural mesothelioma–institutional review and current update. Interact Cardiovasc Thorac Surg. 2011;12(5):754–757. doi: 10.1510/icvts.2010.262972. [DOI] [PubMed] [Google Scholar]
- 50.Ambrogi V, Mineo D, Gatti A, Pompeo E, Mineo TC. Symptomatic and quality of life changes after extrapleural pneumonectomy for malignant pleural mesothelioma. J Surg Oncol. 2009;100(3):199–204. doi: 10.1002/jso.21261. [DOI] [PubMed] [Google Scholar]
- 51.Du N, Li X, Li F, Zhao H, Fan Z, Ma J, Fu Y, Kang H. Intrapleural combination therapy with bevacizumab and cisplatin for non-small cell lung cancermediated malignant pleural effusion. Oncol Rep. 2013;29(6):2332–2340. doi: 10.3892/or.2013.2349. [DOI] [PubMed] [Google Scholar]
- 52.Kim KW, Park SY, Kim MS, Kim SC, Lee EH, Shin SY, Lee JH, Kweon JB, Park K. Intrapleural chemotherapy with cisplatin and cytarabine in the management of malignant pleural effusion. Cancer Res Treat. 2004;36(1):68–71. doi: 10.4143/crt.2004.36.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones DR, Taylor MD, Petroni GR, Shu J, Burks SG, Daniel TM, Gillenwater HH. Phase i trial of intrapleural docetaxel administered through an implantable catheter in subjects with a malignant pleural effusion. J Thorac Oncol. 2010;5(1):75–81. doi: 10.1097/JTO.0b013e3181c07ddc. [DOI] [PubMed] [Google Scholar]
- 54.Rusch VW, Rosenzweig K, Venkatraman E, Leon L, Raben A, Harrison L, Bains MS, Downey RJ, Ginsberg RJ. A phase ii trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;122(4):788–795. doi: 10.1067/mtc.2001.116560. [DOI] [PubMed] [Google Scholar]
- 55.Li G, Liang X, Lotze MT. Hmgb1: the central cytokine for all lymphoid cells. Front Immunol. 2013;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, Pass HI, Gaudino G, Carbone M, Yang H. Cancer cell secretion of the damp protein hmgb1 supports progression in malignant mesothelioma. Cancer Res. 2012;72(13):3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anunobi R, Boone BA, Cheh N, Tang D, Kang R, Loux T, Lotze MT, Zeh HJ. Extracellular DNA promotes colorectal tumor cell survival after cytotoxic chemotherapy. J Surg Res. 2018;226:181–191. doi: 10.1016/j.jss.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 58.Lotfi R, Kaltenmeier C, Lotze MT, Bergmann C. Until death do us part: necrosis and oxidation promote the tumor microenvironment. Transfus Med Hemother. 2016;43(2):120–132. doi: 10.1159/000444941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang J, Xie Y, Sun X, Zeh HJ 3rd, Kang R, Lotze MT, Tang D. Damps, ageing, and cancer: the ‘damp hypothesis’. Ageing Res. Rev. 2015;24(Pt A):3–16. doi: 10.1016/j.arr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou W, Zhang Q, Yan Z, Chen R, Zeh Iii HJ, Kang R, Lotze MT, Tang D. Strange attractors: damps and autophagy link tumor cell death and immunity. Cell Death Dis. 2013;4:e966. doi: 10.1038/cddis.2013.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erridge C. Endogenous ligands of tlr2 and tlr4: agonists or assistants? J Leukoc Biol. 2010;87(6):989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 62.Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21(13):R488–493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 63.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. Pamps and damps: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249(1):158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289(51):35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Batra H, Antony VB. Pleural mesothelial cells in pleural and lung diseases. J Thorac Dis. 2015;7(6):964–980. doi: 10.3978/j.issn.2072-1439.2015.02.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma RK, Mohammed KA, Nasreen N, Hardwick J, Van Horn RD, Ramirez-Icaza C, Antony VB. Defensive role of pleural mesothelial cell sialomucins in tumor metastasis. Chest. 2003;124(2):682–687. [PubMed] [Google Scholar]
- 67.Hussain T, Nasreen N, Lai Y, Bellew BF, Antony VB, Mohammed KA. Innate immune responses in murine pleural mesothelial cells: toll-like receptor-2 dependent induction of beta-defensin-2 by staphylococcal peptidoglycan. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L461–470. doi: 10.1152/ajplung.00276.2007. [DOI] [PubMed] [Google Scholar]
- 68.Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J. 1997;10(10):2411–2418. [DOI] [PubMed] [Google Scholar]
- 69.Sriram PS, Mohammed KA, Nasreen N, Hardwick J, Van Horn R, Sanders K, Antony VB. Adherence of ovarian cancer cells induces pleural mesothelial cell (pmc) permeability. Oncol Res. 2002;13(2):79–85. [PubMed] [Google Scholar]
- 70.Masago K, Fujimoto D, Fujita S, Hata A, Kaji R, Ohtsuka K, Okuda C, Takeshita J, Katakami N. Response to bevacizumab combination chemotherapy of malignant pleural effusions associated with non-squamous non-small-cell lung cancer. Mol Clin Oncol. 2015;3(2):415–419. doi: 10.3892/mco.2014.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu XZ, Zhou Q, Lin H, Zhai K, Wang XJ, Yang WB, Shi HZ. Immune regulation of toll-like receptor 2 engagement on cd4(+) t cells in murine models of malignant pleural effusion. Am J Respir Cell Mol Biol. 2017;56(3):342–352. doi: 10.1165/rcmb.2015-0396OC. [DOI] [PubMed] [Google Scholar]
- 72.Jankovicova K, Kondelkova K, Habal P, Andrys C, Krejsek J, Mandak J. Tlr2 in pleural fluid is modulated by talc particles during pleurodesis. Clin Dev Immunol. 2012;2012:158287. doi: 10.1155/2012/158287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu QQ, Zhou Q, Xu LL, Lin H, Wang XJ, Ma WL, Zhai K, Tong ZH, Su Y, Shi HZ. Toll-like receptor 4 signaling inhibits malignant pleural effusion by altering th1/th17 responses. Cell Biol Int. 2015;39(10):1120–1130. doi: 10.1002/cbin.10485. [DOI] [PubMed] [Google Scholar]
- 74.Lugo-Villarino G, Troegeler A, Balboa L, Lastrucci C, Duval C, Mercier I, Benard A, Capilla F, Al Saati T, Poincloux R, et al. The c-type lectin receptor dc-sign has an anti-inflammatory role in human m(il-4) macrophages in response to mycobacterium tuberculosis. Front Immunol. 2018;9:1123. doi: 10.3389/fimmu.2018.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dalton DK, Noelle RJ. The roles of mast cells in anticancer immunity. Cancer Immunol Immunother. 2012;61(9):1511–1520. doi: 10.1007/s00262-012-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giannou AD, Marazioti A, Spella M, Kanellakis NI, Apostolopoulou H, Psallidas I, Prijovich ZM, Vreka M, Zazara DE, Lilis I, et al. Mast cells mediate malignant pleural effusion formation. J Clin Invest. 2015;125(6):2317–2334. doi: 10.1172/JCI79840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gordon S. The role of the macrophage in immune regulation. Res Immunol. 1998;149(7–8):685–688. [DOI] [PubMed] [Google Scholar]
- 78.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–212. [DOI] [PubMed] [Google Scholar]
- 79.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 81.Park JS, Kim YS, Jee YK, Myong NH, Lee KY. Interleukin-8 production in tuberculous pleurisy: role of mesothelial cells stimulated by cytokine network involving tumour necrosis factor-alpha and interleukin-1 beta. Scand J Immunol. 2003;57(5):463–469. [DOI] [PubMed] [Google Scholar]
- 82.Kaczmarek M, Sikora J. Macrophages in malignant pleural effusions - alternatively activated tumor associated macrophages. Contemp Oncol (Pozn). 2012;16(4):279–284. doi: 10.5114/wo.2012.30054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim R, Emi M, Tanabe K. Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol Ther. 2005;4(9):924–933. [DOI] [PubMed] [Google Scholar]
- 84.Kaczmarek M, Frydrychowicz M, Nowicka A, Kozlowska M, Batura-Gabryel H, Sikora J, Zeromski J. Influence of pleural macrophages on proliferative activity and apoptosis regulating proteins of malignant cells. J Physiol Pharmacol. 2008;59(Suppl 6):321–330. [PubMed] [Google Scholar]
- 85.Ellis LM, Liu W, Ahmad SA, Fan F, Jung YD, Shaheen RM, Reinmuth N. Overview of angiogenesis: biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28(5 Suppl 16):94–104. [DOI] [PubMed] [Google Scholar]
- 86.Schürch CM, Frido Brühl SF, Yang SH, Felley-Bosco E, Hewer E. The “don’t eat me” signal cd47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology. 2018;7(1):e1373235. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tong B, Wang M. Cd47 is a novel potent immunotherapy target in human malignancies: current studies and future promises. Future Oncol. 2018;14(21):2179–2188. doi: 10.2217/fon-2018-0035. [DOI] [PubMed] [Google Scholar]
- 88.Kaczmarek M, Lagiedo M, Masztalerz A, Kozlowska M, Nowicka A, Brajer B, Batura-Gabryel H, Sikora J. Concentrations of sp-a and hsp70 are associated with polarization of macrophages in pleural effusions of non-small cell lung cancer. Immunobiology. 2018;223(2):200–209. doi: 10.1016/j.imbio.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 89.Wang F, Yang L, Gao Q, Huang L, Wang L, Wang J, Wang S, Zhang B, Zhang Y. Cd163+cd14+ macrophages, a potential immune biomarker for malignant pleural effusion. Cancer Immunol Immunother. 2015;64(8):965–976. doi: 10.1007/s00262-015-1701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balkwill F, Mantovani A. Inflammation and cancer: back to virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 91.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 92.Pace E, Profita M, Melis M, Bonanno A, Paterno A, Mody CH, Spatafora M, Ferraro M, Siena L, Vignola AM, et al. Ltb4 is present in exudative pleural effusions and contributes actively to neutrophil recruitment in the inflamed pleural space. Clin Exp Immunol. 2004;135(3):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu GN, Shi HZ, Xie ZH, Shen HH, Huang HQ, Deng JM, Liang QL, Wu YB. Epithelial neutrophil-activating peptide-78 recruits neutrophils into pleural effusion. Eur Respir J. 2009;34(1):184–190. doi: 10.1183/09031936.00111908. [DOI] [PubMed] [Google Scholar]
- 94.Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, Hubbard JJ, Lee SM, Madtes DK, McIntosh MW, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8:14381. doi: 10.1038/ncomms14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barrera L, Montes-Servin E, Hernandez-Martinez JM, Garcia-Vicente MLA, Montes-Servin E, Herrera-Martinez M, Crispin JC, Borbolla-Escoboza JR, Arrieta O. Cd47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer. 2017;117(3):385–397. doi: 10.1038/bjc.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hattar K, Franz K, Ludwig M, Sibelius U, Wilhelm J, Lohmeyer J, Savai R, Subtil FS, Dahlem G, Eul B, et al. Interactions between neutrophils and non-small cell lung cancer cells: enhancement of tumor proliferation and inflammatory mediator synthesis. Cancer Immunol Immunother. 2014;63(12):1297–1306. doi: 10.1007/s00262-014-1606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McLoed AG, Sherrill TP, Cheng DS, Han W, Saxon JA, Gleaves LA, Wu P, Polosukhin VV, Karin M, Yull FE, et al. Neutrophil-derived il-1beta impairs the efficacy of nf-kappab inhibitors against lung cancer. Cell Rep. 2016;16(1):120–132. doi: 10.1016/j.celrep.2016.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo B, Fu S, Zhang J, Liu B, Li Z. Targeting inflammasome/il-1 pathways for cancer immunotherapy. Sci Rep. 2016;6:36107. doi: 10.1038/srep36107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Polverino F, Laucho-Contreras M, Rojas Quintero J, Divo M, Pinto-Plata V, Sholl L, de-Torres JP, Celli BR, Owen CA. Increased expression of a proliferation-inducing ligand (april) in lung leukocytes and alveolar epithelial cells in copd patients with non small cell lung cancer: A possible link between copd and lung cancer? Multidiscip Respir Med. 2016;11:17. doi: 10.1186/s40248-016-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dou H, Yan Z, Zhang M, Xu X. April promotes non-small cell lung cancer growth and metastasis by targeting erk1/2 signaling. Oncotarget. 2017;8(65):109289–109300. doi: 10.18632/oncotarget.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, Wagner DD. Priming of neutrophils toward netosis promotes tumor growth. Oncoimmunology. 2016;5(5):e1134073. doi: 10.1080/2162402X.2015.1134073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138. doi: 10.1126/scitranslmed.aaf0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma J, Li N, Lin Y, Gupta C, Jiang F. Circulating neutrophil micrornas as biomarkers for the detection of lung cancer. Biomark Cancer. 2016;8:1–7. doi: 10.4137/BIC.S37333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Catacchio I, Scattone A, Silvestris N, Mangia A. Immune prophets of lung cancer: the prognostic and predictive landscape of cellular and molecular immune markers. Transl Oncol. 2018;11(3):825–835. doi: 10.1016/j.tranon.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barrera L, Montes-Servin E, Hernandez-Martinez JM, Orozco-Morales M, Montes-Servin E, Michel-Tello D, Morales-Flores RA, Flores-Estrada D, Arrieta O. Levels of peripheral blood polymorphonuclear myeloid-derived suppressor cells and selected cytokines are potentially prognostic of disease progression for patients with non-small cell lung cancer. Cancer Immunol Immunother. 2018;67:1393–1406. doi: 10.1007/s00262-018-2196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akturk UA, Ernam D, Akbay MO, Kocak ND, Ogur E, Irmak I. Role of the neutrophil-lymphocyte ratio in the differential diagnosis of exudative pleural effusion. Clinics (Sao Paulo). 2016;71(10):611–616. doi: 10.6061/clinics/2016(10)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. 2013;1(3):145–149. doi: 10.1158/2326-6066.CIR-13-0102. [DOI] [PubMed] [Google Scholar]
- 109.Soo RA, Chen Z, Yan Teng RS, Tan HL, Iacopetta B, Tai BC, Soong R. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget. 2018;9(37):24801–24820. doi: 10.18632/oncotarget.24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greenwald RJ, Freeman GJ, Sharpe AH. The b7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 111.Schneider T, Hoffmann H, Dienemann H, Schnabel PA, Enk AH, Ring S, Mahnke K. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating b7-h3. J Thorac Oncol. 2011;6(7):1162–1168. doi: 10.1097/JTO.0b013e31821c421d. [DOI] [PubMed] [Google Scholar]
- 112.Chen L, Zhang G, Sheng S, Zhou Q, Pan Y, Guan S. Upregulation of soluble b7-h3 in nsclc-derived malignant pleural effusion: A potential diagnostic biomarker correlated with nsclc staging. Clin Chim Acta. 2016;457:81–85. doi: 10.1016/j.cca.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 113.Schutyser E, Richmond A, Van Damme J. Involvement of cc chemokine ligand 18 (ccl18) in normal and pathological processes. J Leukoc Biol. 2005;78(1):14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen L, Zhou Q, Zhong F, Wang Q, Fang Y, Yang K, Guan S. [expression of cc-chemokine ligand 18 (ccl18) in the serum and pleural effusion of non-small-cell lung cancer patients and its regulatory effect on the differentiation of monocyte-derived dendritic cells]. Zhonghua Zhong Liu Za Zhi. 2014;36(11):823–827. [PubMed] [Google Scholar]
- 115.Lieser EA, Croghan GA, Nevala WK, Bradshaw MJ, Markovic SN, Mansfield AS. Up-regulation of pro-angiogenic factors and establishment of tolerance in malignant pleural effusions. Lung Cancer. 2013;82(1):63–68. doi: 10.1016/j.lungcan.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 116.Gjomarkaj M, Pace E, Melis M, Spatafora M, D’Amico D, Toews GB. Dendritic cells with a potent accessory activity are present in human exudative malignant pleural effusions. Eur Respir J. 1997;10(3):592–597. [PubMed] [Google Scholar]
- 117.Zhong H, Han BH, Dong QG, Feng JX, Bao GL, Sha HF, Su JZ. [efficient generation and functional characteristics of dendritic cells from malignant pleural effusion in patients with lung cancer]. Zhonghua Jie He He Hu Xi Za Zhi. 2004;27(8):542–545. [PubMed] [Google Scholar]
- 118.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, Lebecque S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178(5):2763–2769. [DOI] [PubMed] [Google Scholar]
- 119.Di LJ, Ren J, Song GH, Yu J, Fang J, Che L, Zhu YL. [treatment of malignant effusions with the injection of dendritic cells derived from autologous peripheral cd34+ stem cells]. Beijing Da Xue Xue Bao Yi Xue Ban. 2008;40(5):486–488. [PubMed] [Google Scholar]
- 120.Hegmans JP, Veltman JD, Lambers ME, de Vries IJ, Figdor CG, Hendriks RW, Hoogsteden HC, Lambrecht BN, Aerts JG. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med. 2010;181(12):1383–1390. doi: 10.1164/rccm.200909-1465OC. [DOI] [PubMed] [Google Scholar]
- 121.Cornelissen R, Hegmans JP, Maat AP, Kaijen-Lambers ME, Bezemer K, Hendriks RW, Hoogsteden HC, Aerts JG. Extended tumor control after dendritic cell vaccination with low-dose cyclophosphamide as adjuvant treatment in patients with malignant pleural mesothelioma. Am J Respir Crit Care Med. 2016;193(9):1023–1031. doi: 10.1164/rccm.201508-1573OC. [DOI] [PubMed] [Google Scholar]
- 122.Bosi A, Zanellato S, Bassani B, Albini A, Musco A, Cattoni M, Desio M, Nardecchia E, D’Urso DG, Imperatori A, et al. Natural killer cells from malignant pleural effusion are endowed with a decidual-like proangiogenic polarization. J Immunol Res. 2018;2018:2438598. doi: 10.1155/2018/2438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pace E, Di Sano C, Ferraro M, Tipa A, Olivieri D, Spatafora M, Santagata R, Bellia V, Gjomarkaj M. Altered cd94/nkg2a and perforin expression reduce the cytotoxic activity in malignant pleural effusions. Eur J Cancer. 2011;47(2):296–304. doi: 10.1016/j.ejca.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 124.Yin T, Wang G, He S, Shen G, Su C, Zhang Y, Wei X, Ye T, Li L, Yang S, et al. Malignant pleural effusion and ascites induce epithelial-mesenchymal transition and cancer stem-like cell properties via the vascular endothelial growth factor (vegf)/phosphatidylinositol 3-kinase (pi3k)/akt/mechanistic target of rapamycin (mtor) pathway. J Biol Chem. 2016;291(52):26750–26761. doi: 10.1074/jbc.M116.753236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vacca P, Martini S, Garelli V, Passalacqua G, Moretta L, Mingari MC. Nk cells from malignant pleural effusions are not anergic but produce cytokines and display strong antitumor activity on short-term il-2 activation. Eur J Immunol. 2013;43(2):550–561. doi: 10.1002/eji.201242783. [DOI] [PubMed] [Google Scholar]
- 126.Croxatto D, Martini S, Chiossone L, Scordamaglia F, Simonassi CF, Moretta L, Mingari MC, Vacca P. Il15 induces a potent antitumor activity in nk cells isolated from malignant pleural effusions and overcomes the inhibitory effect of pleural fluid. Oncoimmunology. 2017;6(4):e1293210. doi: 10.1080/2162402X.2017.1293210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takeuchi E, Yanagawa H, Suzuki Y, Shinkawa K, Ohmoto Y, Bando H, Sone S. Il-12-induced production of il-10 and interferon-gamma by mononuclear cells in lung cancer-associated malignant pleural effusions. Lung Cancer. 2002;35(2):171–177. [DOI] [PubMed] [Google Scholar]
- 128.Vacca P, Martini S, Mingari MC, Moretta L. Nk cells from malignant pleural effusions are potent antitumor effectors: A clue for adoptive immunotherapy? Oncoimmunology. 2013;2(4):e23638. doi: 10.4161/onci.23638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, Bintcliffe OJ, Boshuizen RC, Fysh ET, Tobin CL, et al. Predicting survival in malignant pleural effusion: development and validation of the lent prognostic score. Thorax. 2014;69(12):1098–1104. doi: 10.1136/thoraxjnl-2014-205285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage iii non-small cell lung cancer. J Transl Med. 2017;15(1):221. doi: 10.1186/s12967-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta. 2018;484:272–277. doi: 10.1016/j.cca.2018.05.059. [DOI] [PubMed] [Google Scholar]
- 132.Tomita M, Ayabe T, Maeda R, Nakamura K. Systemic immune-inflammation index predicts survival of patients after curative resection for non-small cell lung cancer. In Vivo. 2018;32(3):663–667. doi: 10.21873/invivo.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abisheganaden J, Verma A, Dagaonkar RS, Light RW. An observational study evaluating the performance of lent score in the selected population of malignant pleural effusion from lung adenocarcinoma in singapore. Respiration. 2018;96(4):308–313. doi: 10.1159/000489315. https://www.ncbi.nlm.nih.gov/pubmed/29945142. [DOI] [PubMed] [Google Scholar]
- 134.Mizuguchi S, Izumi N, Tsukioka T, Komatsu H, Nishiyama N. Neutrophil-lymphocyte ratio predicts recurrence in patients with resected stage 1 non-small cell lung cancer. J Cardiothorac Surg. 2018;13(1):78. doi: 10.1186/s13019-018-0763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zer A, Sung MR, Walia P, Khoja L, Maganti M, Labbe C, Shepherd FA, Bradbury PA, Feld R, Liu G, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with pd-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018. doi: 10.1016/j.cllc.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 136.Tomita M, Ayabe T, Maeda R, Nakamura K. Comparison of inflammation-based prognostic scores in patients undergoing curative resection for non-small cell lung cancer. World J Oncol. 2018;9(3):85–90. doi: 10.14740/wjon1097w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khunger M, Patil PD, Khunger A, Li M, Hu B, Rakshit S, Basu A, Pennell N, Stevenson JP, Elson P, Panchabhai TS, et al. Post-treatment changes in hematological parameters predict response to nivolumab monotherappy in non-smal cell lung cancer patients. PLose One. 2018;13(10)e0197743. doi: 10.1371/journal.pone.0197743 https://www.ncbi.nlm.nih.gov/pubmed/30359383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Psallidas I, Kanellakis NI, Gerry S, Thezenas ML, Charles PD, Samsonova A, Schiller HB, Fischer R, Asciak R, Hallifax RJ, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (promise): A multicohort analysis. Lancet Oncol. 2018;19(7):930–939. doi: 10.1016/S1470-2045(18)30294-8. [DOI] [PubMed] [Google Scholar]
- 139.Yin Y, Wang J, Wang X, Gu L, Pei H, Kuai S, Zhang Y, Shang Z. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo). 2015;70(7):524–530. doi: 10.6061/clinics/2015(07)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee YS, Nam HS, Lim JH, Kim JS, Moon Y, Cho JH, Ryu JS, Kwak SM, Lee HL. Prognostic impact of a new score using neutrophil-to-lymphocyte ratios in the serum and malignant pleural effusion in lung cancer patients. BMC Cancer. 2017;17(1):557. doi: 10.1186/s12885-017-3550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lim JU, Yeo CD, Kang HS, Park CK, Kim JS, Kim JW, Kim SJ, Lee SH. Prognostic value of platelet count and lymphocyte to monocyte ratio combination in stage iv non-small cell lung cancer with malignant pleural effusion. PLoS ONE. 2018;13(7):e0200341. doi: 10.1371/journal.pone.0200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 143.Farolfi A, Petrone M, Scarpi E, Galla V, Greco F, Casanova C, Longo L, Cormio G, Orditura M, Bologna A, et al. Inflammatory indexes as prognostic and predictive factors in ovarian cancer treated with chemotherapy alone or together with bevacizumab. A multicenter, retrospective analysis by the mito group (mito 24). Target Oncol. 2018;13:469–479. doi: 10.1007/s11523-018-0574-1. [DOI] [PubMed] [Google Scholar]
- 144.Ishibashi Y, Tsujimoto H, Hiraki S, Kumano I, Yaguchi Y, Horiguchi H, Nomura S, Ito N, Shinto E, Aosasa S, et al. Prognostic value of preoperative systemic immunoinflammatory measures in patients with esophageal cancer. Ann Surg Oncol. 2018;25:3288–3299. doi: 10.1245/s10434-018-6651-y. [DOI] [PubMed] [Google Scholar]
- 145.Lui MM, Fitzgerald DB, Lee YC. Phenotyping malignant pleural effusions. Curr Opin Pulm Med. 2016;22(4):350–355. doi: 10.1097/MCP.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 146.Baas P, Burgers S. Malignant pleural effusions: will promise make its name true? Lancet Oncol. 2018;19(7):853–855. doi: 10.1016/S1470-2045(18)30361-9. [DOI] [PubMed] [Google Scholar]
- 147.Bruce Alberts AJ, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. New York: Garland Science; 2002. [Google Scholar]
- 148.Luz Elena R, Cano HDEL. Autoimmunity: from bench to bedside. Bogota, Colombia: El Rosario University Press; 2013. [PubMed] [Google Scholar]
- 149.Scherpereel A, Grigoriu BD, Noppen M, Gey T, Chahine B, Baldacci S, Trauet J, Copin MC, Dessaint JP, Porte H, et al. Defect in recruiting effector memory cd8+ t-cells in malignant pleural effusions compared to normal pleural fluid. BMC Cancer. 2013;13:324. doi: 10.1186/1471-2407-13-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cornfield DB, Gheith SM. Flow cytometric quantitation of natural killer cells and t lymphocytes expressing t-cell receptors alpha/beta and gamma/delta is not helpful in distinguishing benign from malignant body cavity effusions. Cytometry B Clin Cytom. 2009;76(3):213–217. doi: 10.1002/cyto.b.20455. [DOI] [PubMed] [Google Scholar]
- 151.Sikora J, Dworacki G, Giersz R, Zeromski J. The role of monocytes/macrophages in tcr-zeta chain downregulation and apoptosis of t lymphocytes in malignant pleural effusions. J Biol Regul Homeost Agents. 2004;18(1):26–32. [PubMed] [Google Scholar]
- 152.Atanackovic D, Block A, de Weerth A, Faltz C, Hossfeld DK, Hegewisch-Becker S. Characterization of effusion-infiltrating t cells: benign versus malignant effusions. Clin Cancer Res. 2004;10(8):2600–2608. [DOI] [PubMed] [Google Scholar]
- 153.Prado-Garcia H, Romero-Garcia S, Morales-Fuentes J, Aguilar-Cazares D, Lopez-Gonzalez JS. Activation-induced cell death of memory cd8+ t cells from pleural effusion of lung cancer patients is mediated by the type ii fas-induced apoptotic pathway. Cancer Immunol Immunother. 2012;61(7):1065–1080. doi: 10.1007/s00262-011-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li L, Yang L, Wang L, Wang F, Zhang Z, Li J, Yue D, Chen X, Ping Y, Huang L, et al. Impaired t cell function in malignant pleural effusion is caused by tgf-beta derived predominantly from macrophages. Int J Cancer. 2016;139(10):2261–2269. doi: 10.1002/ijc.30289. [DOI] [PubMed] [Google Scholar]
- 155.Budna J, Kaczmarek M, Kolecka-Bednarczyk A, Spychalski L, Zawierucha P, Gozdzik-Spychalska J, Nowicki M, Batura-Gabryel H, Sikora J. Enhanced suppressive activity of regulatory t cells in the microenvironment of malignant pleural effusions. J Immunol Res. 2018;2018:9876014. doi: 10.1155/2018/9876014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y, Liu B, Hou Y, Wang T. Mir141-cxcl1-cxcr2 signaling-induced treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol Cancer Ther. 2014;13(12):3152–3162. doi: 10.1158/1535-7163.MCT-14-0448. [DOI] [PubMed] [Google Scholar]
- 157.Qin XJ, Shi HZ, Deng JM, Liang QL, Jiang J, Ye ZJ. Ccl22 recruits cd4-positive cd25-positive regulatory t cells into malignant pleural effusion. Clin Cancer Res. 2009;15(7):2231–2237. doi: 10.1158/1078-0432.CCR-08-2641. [DOI] [PubMed] [Google Scholar]
- 158.Budna J, Spychalski L, Kaczmarek M, Frydrychowicz M, Gozdzik-Spychalska J, Batura-Gabryel H, Sikora J. Regulatory t cells in malignant pleural effusions subsequent to lung carcinoma and their impact on the course of the disease. Immunobiology. 2017;222(3):499–505. doi: 10.1016/j.imbio.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 159.Lievense LA, Cornelissen R, Bezemer K, Kaijen-Lambers ME, Hegmans JP, Aerts JG. Pleural effusion of patients with malignant mesothelioma induces macrophage-mediated t cell suppression. J Thorac Oncol. 2016;11(10):1755–1764. doi: 10.1016/j.jtho.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 160.Ye ZJ, Zhou Q, Zhang JC, Li X, Wu C, Qin SM, Xin JB, Shi HZ. Cd39+ regulatory t cells suppress generation and differentiation of th17 cells in human malignant pleural effusion via a lap-dependent mechanism. Respir Res. 2011;12:77. doi: 10.1186/1465-9921-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lin H, Tong ZH, Xu QQ, Wu XZ, Wang XJ, Jin XG, Ma WL, Cheng X, Zhou Q, Shi HZ. Interplay of th1 and th17 cells in murine models of malignant pleural effusion. Am J Respir Crit Care Med. 2014;189(6):697–706. doi: 10.1164/rccm.201310-1776OC. [DOI] [PubMed] [Google Scholar]
- 162.Wu XZ, Shi XY, Zhai K, Yi FS, Wang Z, Wang W, Pei XB, Xu LL, Wang Z, Shi HZ. Activated naive b cells promote development of malignant pleural effusion by different regulation of th1 and th17 response. Am J Physiol Lung Cell Mol Physiol. 2018;315:L443–L455. doi: 10.1152/ajplung.00120.2018. [DOI] [PubMed] [Google Scholar]
- 163.Yaftian M, Yari F, Ghasemzadeh M, Fallah Azad V, Haghighi M. Induction of apoptosis in cancer cells of pre-b all patients after exposure to platelets, platelet-derived microparticles and soluble cd40 ligand. Cell J. 2018;20(1):120–126. doi: 10.22074/cellj.2018.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mu CY, Qin PX, Qu QX, Chen C, Huang JA. Soluble cd40 in plasma and malignant pleural effusion with non-small cell lung cancer: A potential marker of prognosis. Chronic Dis Transl Med. 2015;1(1):36–41. doi: 10.1016/j.cdtm.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen C, Qu QX, Xie F, Zhu WD, Zhu YH, Huang JA. Analysis of b7-h4 expression in metastatic pleural adenocarcinoma and therapeutic potential of its antagonists. BMC Cancer. 2017;17(1):652. doi: 10.1186/s12885-017-3615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.DeLong P, Carroll RG, Henry AC, Tanaka T, Ahmad S, Leibowitz MS, Sterman DH, June CH, Albelda SM, Vonderheide RH. Regulatory t cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther. 2005;4(3):342–346. [DOI] [PubMed] [Google Scholar]
- 167.Mellman I, Hubbard-Lucey VM, Tontonoz MJ, Kalos MD, Chen DS, Allison JP, Drake CG, Levitsky H, Lonberg N, van der Burg SH, et al. De-risking immunotherapy: report of a consensus workshop of the cancer immunotherapy consortium of the cancer research institute. Cancer Immunol Res. 2016;4(4):279–288. doi: 10.1158/2326-6066.CIR-16-0045. [DOI] [PubMed] [Google Scholar]
- 168.Astoul P, Viallat JR, Laurent JC, Brandely M, Boutin C. Intrapleural recombinant il-2 in passive immunotherapy for malignant pleural effusion. Chest. 1993;103(1):209–213. [DOI] [PubMed] [Google Scholar]
- 169.Mao G, Gao Z, Wang Q. [intrapleural administration of lak cells combined with ril2 in the treatment of advanced lung cancer with malignant pleural effusion]. Zhonghua Jie He He Hu Xi Za Zhi. 1995;18(2):83–84. 127. [PubMed] [Google Scholar]
- 170.Hu CY, Zhang YH, Wang T, Chen L, Gong ZH, Wan YS, Li QJ, Li YS, Zhu B. Interleukin-2 reverses cd8(+) t cell exhaustion in clinical malignant pleural effusion of lung cancer. Clin Exp Immunol. 2016;186(1):106–114. doi: 10.1111/cei.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129(4):474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Han L, Jiang Q, Yao W, Fu T, Zeng Q. Thoracic injection of low-dose interleukin-2 as an adjuvant therapy improves the control of the malignant pleural effusions: A systematic review and meta-analysis base on chinese patients. BMC Cancer. 2018;18(1):725. doi: 10.1186/s12885-018-4242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Castagneto B, Zai S, Mutti L, Lazzaro A, Ridolfi R, Piccolini E, Ardizzoni A, Fumagalli L, Valsuani G, Botta M. Palliative and therapeutic activity of il-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: results of a phase ii study on 31 consecutive patients. Lung Cancer. 2001;31(2–3):303–310. [DOI] [PubMed] [Google Scholar]
- 174.Morisaki T, Matsumoto K, Kuroki H, Kubo M, Baba E, Onishi H, Tasaki A, Nakamura M, Inaba S, Katano M. Combined immunotherapy with intracavital injection of activated lymphocytes, monocyte-derived dendritic cells and low-dose ok-432 in patients with malignant effusion. Anticancer Res. 2003;23(6a):4459–4465. [PubMed] [Google Scholar]
- 175.Sartori S, Tassinari D, Ceccotti P, Tombesi P, Nielsen I, Trevisani L, Abbasciano V. Prospective randomized trial of intrapleural bleomycin versus interferon alfa-2b via ultrasound-guided small-bore chest tube in the palliative treatment of malignant pleural effusions. J Clin Oncol. 2004;22(7):1228–1233. doi: 10.1200/JCO.2004.09.164. [DOI] [PubMed] [Google Scholar]
- 176.Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL, et al. A phase i clinical trial of single-dose intrapleural ifn-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13(15 Pt 1):4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 177.Okawaki M, Yamaguchi Y, Okita R, Ohara M, Okada M. Dose-finding study of anti-cd25 antibody for targeting regulatory t cells in locoregional immunotherapy of malignant effusion. Hiroshima J Med Sci. 2008;57(1):37–46. [PubMed] [Google Scholar]
- 178.Wang H, Cui Y, Wang S, Zhao R, Sun M. Curative effects of dendritic cells combined with cytokine-induced killer cells in patients with malignant pericardial effusion. Med Sci Monit. 2016;22:4159–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Aggarwal C, Haas AR, Metzger S, Aguilar LK, Aguilar-Cordova E, Manzanera AG, Gomez-Hernandez G, Katz SI, Alley EW, Evans TL, et al. Phase i study of intrapleural gene-mediated cytotoxic immunotherapy in patients with malignant pleural effusion. Mol Ther. 2018;26(5):1198–1205. doi: 10.1016/j.ymthe.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Peikert T, Sterman DH. Harnessing the power of the host: improving dendritic cell vaccines for malignant pleural mesothelioma. Am J Respir Crit Care Med. 2016;193(9):943–945. doi: 10.1164/rccm.201512-2444ED. [DOI] [PubMed] [Google Scholar]
- 181.Ren S, Terman DS, Bohach G, Silvers A, Hansen C, Colt H, Sahn SA. Intrapleural staphylococcal superantigen induces resolution of malignant pleural effusions and a survival benefit in non-small cell lung cancer. Chest. 2004;126(5):1529–1539. doi: 10.1378/chest.126.5.1529. [DOI] [PubMed] [Google Scholar]
- 182.Alvarez-Mon M, Molto LM, Manzano L, Olivier C, Carballido JA. Immunomodulatory effect of interferon-alpha 2b on natural killer cells and t lymphocytes from patients with transitional cell carcinoma of the bladder. Anticancer Drugs. 1992;3(Suppl 1):5–8. [DOI] [PubMed] [Google Scholar]
- 183.Chu H, Du F, Gong Z, Lian P, Wang Z, Li P, Hu B, Chi C, Chen J. Better clinical efficiency of tils for malignant pleural effusion and ascites than cisplatin through intrapleural and intraperitoneal infusion. Anticancer Res. 2017;37(8):4587–4591. doi: 10.21873/anticanres.11857. [DOI] [PubMed] [Google Scholar]
- 184.Watanabe D, Goshima F. Oncolytic virotherapy by hsv. Adv Exp Med Biol. 2018;1045:63–84. doi: 10.1007/978-981-10-7230-7_4. [DOI] [PubMed] [Google Scholar]
- 185.Moon EK, Wang LS, Bekdache K, Lynn RC, Lo A, Thorne SH, Albelda SM. Intra-tumoral delivery of cxcl11 via a vaccinia virus, but not by modified t cells, enhances the efficacy of adoptive t cell therapy and vaccines. Oncoimmunology. 2018;7(3):e1395997. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fend L, Yamazaki T, Remy C, Fahrner C, Gantzer M, Nourtier V, Preville X, Quemeneur E, Kepp O, Adam J, et al. Immune checkpoint blockade, immunogenic chemotherapy or ifn-alpha blockade boost the local and abscopal effects of oncolytic virotherapy. Cancer Res. 2017;77(15):4146–4157. doi: 10.1158/0008-5472.CAN-16-2165. [DOI] [PubMed] [Google Scholar]
- 187.Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and pd-l1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8:14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs. 2017;4(2):127–135. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-pd-l1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J, Vladimirov V, et al. Phase iii trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 192.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-pd-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, pd-l1-positive, advanced non-small-cell lung cancer (keynote-010): A randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 194.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for pd-l1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 195.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2980–2987. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (checkmate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after chemoradiotherapy in stage iii non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 199.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 201.Scherpereel A, Mazieres J, Greillier L, Dô P, Bylicki O, Monnet I, Corre R, Audigier-Valette C, Locatelli-Sanchez M, Molinier O, et al. Second- or third-line nivolumab (nivo) versus nivo plus ipilimumab (ipi) in malignant pleural mesothelioma (mpm) patients: results of the ifct-1501 maps2 randomized phase ii trial. J Clin Oncol. 2017;35(18_suppl):LBA8507–LBA8507. doi: 10.1200/JCO.2017.35.15_suppl.LBA8507. [DOI] [Google Scholar]
- 202.Josine Quispel-Janssen GZ, Schouten R, Buikhuisen W, Monkhorst K, Thunissen E, Baas P. Oa13.01 a phase ii study of nivolumab in malignant pleural mesothelioma (nivomes): with translational research (tr) biopies. J Thorac Oncol. 2017;12(1). doi: 10.1016/j.jtho.2016.09.002. [DOI] [Google Scholar]
- 203.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. Cancer regression and autoimmunity induced by cytotoxic t lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U.S.A. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat Rev Immunol. 2002;2(6):439–446. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 205.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific t cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165(2):302–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Intlekofer AM, Thompson CB. At the bench: preclinical rationale for ctla-4 and pd-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94(1):25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Callahan MK, Wolchok JD. At the bedside: Ctla-4- and pd-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94(1):41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Offin M, Rizvi H, Tenet M, Ni A, Sanchez-Vega F, Li BT, Drilon A, Kris MG, Rudin CM, Schultz N, et al. Tumor mutation burden and efficacy of egfr-tyrosine kinase inhibitors in patients with egfr-mutant lung cancers. Clin Cancer Res. 2018. doi: 10.1158/1078-0432.CCR-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. Pd-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 212.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted cd8 t cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 213.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–6587. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 214.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rizvi NA, Antonia SJ, Shepherd FA, Chow LQ, Goldman J, Shen Y, Chen AC, Gettinger S. Nivolumab (anti-pd-1; bms-936558, ono-4538) maintenance as monotherapy or in combination with bevacizumab (bev) for non-small cell lung cancer (nsclc) previously treated with chemotherapy. Int J Radiat Oncol. 2014;90(5):S32. doi: 10.1016/j.ijrobp.2014.08.206. [DOI] [Google Scholar]
- 216.Herbst RS, Bendell JC, Isambert N, Calvo E, Santana-Davila R, Cassier P, Perez-Gracia JL, Yang J, Rege J, Ferry D, et al. A phase 1 study of ramucirumab (r) plus pembrolizumab (p) in patients (pts) with advanced gastric or gastroesophageal junction (g/gej) adenocarcinoma, non-small cell lung cancer (nsclc), or urothelial carcinoma (uc): phase 1a results. J Clin Oncol. 2016;34(15_suppl):3056. doi: 10.1200/JCO.2016.34.15_suppl.3056. [DOI] [Google Scholar]
- 217.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. Lag-3, a novel lymphocyte activation gene closely related to cd4. J Exp Med. 1990;171(5):1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. Lag-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182(4):1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (lag-3) on b cells is induced by t cells. Eur J Immunol. 2005;35(7):2081–2088. doi: 10.1002/eji.200526090. [DOI] [PubMed] [Google Scholar]
- 220.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, et al. Lag-3 regulates cd8+ t cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117(11):3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Workman CJ, Vignali DA. The cd4-related molecule, lag-3 (cd223), regulates the expansion of activated t cells. Eur J Immunol. 2003;33(4):970–979. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- 222.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (cd223) regulates the size of the expanding t cell population following antigen activation in vivo. J Immunol. 2004;172(9):5450–5455. [DOI] [PubMed] [Google Scholar]
- 223.He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, Suda K, Ren S, Wu C, Hou L, et al. Lag-3 protein expression in non-small cell lung cancer and its relationship with pd-1/pd-l1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017;12(5):814–823. doi: 10.1016/j.jtho.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 224.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 225.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of tim-3 and tim-3 ligand regulates t helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4(11):1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 226.He Y, Rozeboom L, Rivard C, Dziadziuszko R, Ellison K, Yu H, Zhou C, Hirsch FR. Pub044 tim-3 protein expression in non-small cell lung cancer and its relationship with pd-1/pd-l1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2016;12(1):S1473. doi: 10.1016/j.jtho.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 227.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. Tim-3 expression characterizes regulatory t cells in tumor tissues and is associated with lung cancer progression. PLoS ONE. 2012;7(2):e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Prado-Garcia H, Romero-Garcia S, Puerto-Aquino A, Rumbo-Nava U. The pd-l1/pd-1 pathway promotes dysfunction, but not “exhaustion”, in tumor-responding t cells from pleural effusions in lung cancer patients. Cancer Immunol Immunother. 2017;66(6):765–776. doi: 10.1007/s00262-017-1979-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Marcq E, Waele J, Audenaerde JV, Lion E, Santermans E, Hens N, Pauwels P, van Meerbeeck JP, Smits ELJ. Abundant expression of tim-3, lag-3, pd-1 and pd-l1 as immunotherapy checkpoint targets in effusions of mesothelioma patients. Oncotarget. 2017;8(52):89722–89735. doi: 10.18632/oncotarget.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Khanna S, Thomas A, Abate-Daga D, Zhang J, Morrow B, Steinberg SM, Orlandi A, Ferroni P, Schlom J, Guadagni F, et al. Malignant mesothelioma effusions are infiltrated by cd3(+) t cells highly expressing pd-l1 and the pd-l1(+) tumor cells within these effusions are susceptible to adcc by the anti-pd-l1 antibody avelumab. J Thorac Oncol. 2016;11(11):1993–2005. doi: 10.1016/j.jtho.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cohen IJ, Blasberg R. Impact of the tumor microenvironment on tumor-infiltrating lymphocytes: focus on breast cancer. Breast Cancer (Auckl). 2017;11:1178223417731565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary t cells with chimeric receptors: costimulation from cd28, inducible costimulator, cd134, and cd137 in series with signals from the tcr zeta chain. J Immunol. 2004;172(1):104–113. [DOI] [PubMed] [Google Scholar]
- 233.Zeltsman M, Dozier J, McGee E, Ngai D, Adusumilli PS. Car t-cell therapy for lung cancer and malignant pleural mesothelioma. Transl Res. 2017;187:1–10. doi: 10.1016/j.trsl.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.van der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14(7):499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced t lymphocytes. Nat Rev Cancer. 2003;3(1):35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 236.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1bb and cd28 signaling domains augment pi3kinase/akt/bcl-xl activation and cd8+ t cell-mediated tumor eradication. Mol Ther. 2010;18(2):413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, et al. Control of large, established tumor xenografts with genetically retargeted human t cells containing cd28 and cd137 domains. Proc Natl Acad Sci U.S.A. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. Cd19-targeted t cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified t cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-cd19 chimeric-antigen-receptor-transduced t cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, et al. Chemotherapy-refractory diffuse large b-cell lymphoma and indolent b-cell malignancies can be effectively treated with autologous t cells expressing an anti-cd19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kiesgen S, Chicaybam L, Chintala NK, Adusumilli PS. Chimeric antigen receptor (car) t-cell therapy for thoracic malignancies. J Thorac Oncol. 2018;13(1):16–26. doi: 10.1016/j.jtho.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ke EE, Wu YL. Egfr as a pharmacological target in egfr-mutant non-small-cell lung cancer: where do we stand now? Trends Pharmacol Sci. 2016;37(11):887–903. doi: 10.1016/j.tips.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 244.Midha A, Dearden S, McCormack R. Egfr mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutmapii). Am J Cancer Res. 2015;5(9):2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 245.Li H, Huang Y, Jiang DQ, Cui LZ, He Z, Wang C, Zhang ZW, Zhu HL, Ding YM, Li LF, et al. Antitumor activity of egfr-specific car t cells against non-small-cell lung cancer cells in vitro and in mice. Cell Death Dis. 2018;9(2):177. doi: 10.1038/s41419-018-1111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H, Han W. Chimeric antigen receptor-modified t cells for the immunotherapy of patients with egfr-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59(5):468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 247.Yang J, Lee OJ, Son SM, Woo CG, Jeong Y, Yang Y, Kwon J, Lee KH, Han HS. Egfr mutation status in lung adenocarcinoma-associated malignant pleural effusion and efficacy of egfr tyrosine kinase inhibitors. Cancer Res Treat. 2018;50(3):908–916. doi: 10.4143/crt.2017.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Thomas A. Should anti-mesothelin therapies be explored in lung cancer? Expert Rev Anticancer Ther. 2016;16(7):677–679. doi: 10.1080/14737140.2016.1192472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, Chou J, Sima CS, Vertes E, Rusch VW, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res. 2014;20(4):1020–1028. doi: 10.1158/1078-0432.CCR-13-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Thomas A, Chen Y, Steinberg SM, Luo J, Pack S, Raffeld M, Abdullaev Z, Alewine C, Rajan A, Giaccone G, et al. High mesothelin expression in advanced lung adenocarcinoma is associated with kras mutations and a poor prognosis. Oncotarget. 2015;6(13):11694–11703. doi: 10.18632/oncotarget.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Klampatsa A, Haas AR, Moon EK, Albelda SM. Chimeric antigen receptor (car) t cell therapy for malignant pleural mesothelioma (mpm). Cancers (Basel). 2017;9(9)115. doi: 10.3390/cancers9090115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al. Mesothelin-specific chimeric antigen receptor mrna-engineered t cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Xu T, Li D, Wang H, Zheng T, Wang G, Xin Y. Muc1 downregulation inhibits non-small cell lung cancer progression in human cell lines. Exp Ther Med. 2017;14(5):4443–4447. doi: 10.3892/etm.2017.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Xu M, Wang X. Critical roles of mucin-1 in sensitivity of lung cancer cells to tumor necrosis factor-alpha and dexamethasone. Cell Biol Toxico. 2017;33(4):361–371. doi: 10.1007/s10565-017-9393-x. [DOI] [PubMed] [Google Scholar]
- 255.Wei X, Lai Y, Li J, Qin L, Xu Y, Zhao R, Li B, Lin S, Wang S, Wu Q, et al. Psca and muc1 in non-small-cell lung cancer as targets of chimeric antigen receptor t cells. Oncoimmunology. 2017;6(3):e1284722. doi: 10.1080/2162402X.2017.1284722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yoon DH, Osborn MJ, Tolar J, Kim CJ. Incorporation of immune checkpoint blockade into chimeric antigen receptor t cells (car-ts): combination or built-in car-t. Int J Mol Sci. 2018;19(2). doi: 10.3390/ijms19020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Petrausch U, Schuberth PC, Hagedorn C, Soltermann A, Tomaszek S, Stahel R, Weder W, Renner C. Re-directed t cells for the treatment of fibroblast activation protein (fap)-positive malignant pleural mesothelioma (fapme-1). BMC Cancer. 2012;12:615. doi: 10.1186/1471-2407-12-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gulati P, Ruhl J, Kannan A, Pircher M, Schuberth P, Nytko KJ, Pruschy M, Sulser S, Haefner M, Jensen S, et al. Aberrant lck signal via cd28 costimulation augments antigen-specific functionality and tumor control by redirected t cells with pd-1 blockade in humanized mice. Clin Cancer Res. 2018;24(16):3981–3993. doi: 10.1158/1078-0432.CCR-17-1788. [DOI] [PubMed] [Google Scholar]
- 259.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for t-cell therapy. Blood. 2005;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chu W, Zhou Y, Tang Q, Wang M, Ji Y, Yan J, Yin D, Zhang S, Lu H, Shen J. Bi-specific ligand-controlled chimeric antigen receptor t-cell therapy for non-small cell lung cancer. Biosci Trends. 2018. doi: 10.5582/bst.2018.01048. [DOI] [PubMed] [Google Scholar]
- 262.Shen J, Hu Y, Putt KS, Singhal S, Han H, Visscher DW, Murphy LM, Low PS. Assessment of folate receptor alpha and beta expression in selection of lung and pancreatic cancer patients for receptor targeted therapies. Oncotarget. 2018;9(4):4485–4495. doi: 10.18632/oncotarget.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Boogerd LS, Boonstra MC, Beck AJ, Charehbili A, Hoogstins CE, Prevoo HA, Singhal S, Low PS, van de Velde CJ, Vahrmeijer AL. Concordance of folate receptor-alpha expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget. 2016;7(14):17442–17454. doi: 10.18632/oncotarget.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, et al. Switch-mediated activation and retargeting of car-t cells for b-cell malignancies. Proc Natl Acad Sci U.S.A. 2016;113(4):E459–468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]


