ABSTRACT
Calcineurin B-like protein 9 (CBL9) plays important roles in response to ABA, K+ deprivation in plants. However, whether CBL9 modulates plant adaptation to low-temperature stress is elusive. In this study, we demonstrated that the cbl9 mutants increased freezing tolerance under both cold-acclimating and nonacclimating conditions in Arabidopsis. Cold-induced changes of cytosolic free calcium concentration ([Ca2+]cyt) were then monitored by aequorin-expressed Arabidopsis plants. The results showed that the cold-triggered increases in [Ca2+]cyt levels in cbl9 mutants were clearly higher than those in wild type (WT) plants, while cold-affected changes in free calcium concentration within cytosolic microdomains adjacent to the vacuolar membrane ([Ca2+]md) in cbl9 mutants were similar to those in WT plants. In addition, treatments of seedlings with Ca2+ chelator ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) and Ca2+ channel blocker lanthanum chloride markedly inhibit changes of [Ca2+]cyt in cbl9 mutants, while the inhibition of calcium release by lithium chloride from intracellular pools demonstrated consistent suppression of [Ca2+]cyt in cbl9 mutants and WT plants. Together, these results indicate that CBL9 negatively modulates cold tolerance through decreasing [Ca2+]cyt in Arabidopsis.
KEYWORDS: Arabidopsis thaliana, calcium, CBL9, cold stress, aequorin
Introduction
Cold stress as an environmental factor restricts the growth and development of plant and remarkably reduces crop quality and productivity. Many temperate plant species can enhance their freezing tolerance after exposure to chilling temperature, the adaptive process known as cold acclimation.1 Complex physiological changes are involved in cold acclimation including enhancement stability of membranes and production of cryoprotective proteins and low-molecular-weight cryoprotectants.2 A set of marker genes are induced in cold acclimation. These genes include KIN (cold inducible), COR (cold regulated), RD (responsive to dehydration), and LTI (low temperature induced).3 Some proteins like enzymes, antifreeze polypeptide, and molecular chaperones can also increase tolerance to the dehydration caused by low temperature in plants.4,5
In plant cells, calcium functions as a second messenger in a wide range of signal transduction networks.6 It has been demonstrated that the earliest response to low temperature is a transient increase in [Ca2+]cyt. [Ca2+]cyt rise has been shown to be initiated by calcium influx through the plasma membrane from the extracellular calcium stores and by calcium release from intracellular calcium stores.7 In addition, electrophysiology study has shown that mechanosensitive Ca2+ channels are regulated by temperature in Arabidopsis mesophyll cells.8 The more important evidence of calcium behaves as a second messenger in low-temperature signaling is the prevention of cold acclimation by Ca2+ channel blockers and Ca2+ chelators.9 Thus, Ca2+ acts as a second messenger in response to low-temperature stress and cold acclimation.8
Calcium sensors unscramble the temporal and spatial changes of Ca2+ concentrations in calcium signaling molecular pathways.10 As calcium sensor protein, calcineurin B-like proteins (CBL) in plants are similar to calcineurin B (CNB) and neuronal calcium sensors from animals.11 CBL proteins containing EF-hand domains for calcium binding specifically interact with a set of serine–threonine protein kinases named as CBL-interacting protein kinases (CIPKs).12 Many types of research have confirmed a wide range of key functions of the CBL–CIPK network to cope with the environmental changes in plants. Different CBL–CIPK combination pairs appear to participate in specific signal transduction pathways and may have functional overlap. To date, at least ten Arabidopsis CBLs have been identified. The Arabidopsis CBLs share 20–90% amino acid sequence identity. It is supposed that CBL proteins would have high functional redundancy among closely related members while supporting functional specificity among highly divergent members.13 For example, SOS3 (CBL4) encodes a calcium sensor and is specifically involved in plant salt tolerance stress, whereas CBL1 has a positive role in regulating salt and drought stress and a negative role in regulating cold stress in Arabidopsis.14
Previous studies have shown that CBL9 negatively regulates ABA and osmotic stress responses and is involved in the absorption of potassium under low potassium conditions in plants.15,16 Arabidopsis CBL proteins are also responsible for numerous regulation of other stress in different plant signal transduction processes. Despite being involved in multiple stress responses, little is known about the function of CBL9 in responding to low temperatures in plants. Here, we reported that CBL9 decreased freezing tolerance through the depression of transient increase of [Ca2+]cyt induced by cold stress in Arabidopsis.
Results
CBL9 mutant was insensitive to freezing
To define the function of CBL9 in response to cold stress, we identified Salk_142774 that contained T-DNA insertion in the CBL9 gene.16 The site of T-DNA insertion is located in the promoter of CBL9 (Figure 1a) and was approved by PCR using the CBL9-specific primer and the T-DNA left border primer. RT-PCR results revealed that CBL9 was disrupted in the cbl9 mutant (Figure 1b). Under normal conditions, the growth and development of cbl9 mutants were not significantly different from WT plants (Figure 1c, d). However, the cbl9 mutants displayed freezing-insensitive phenotype compared with WT plants under both cold-acclimating and nonacclimating conditions (Figure 1c, d). Under nonacclimating conditions, 29.5 ± 8.26% of the WT plants survived the freezing test compared to 55.8 ± 10.38% of the cbl9 mutants (Figure 1e). Approximately 44.5 ± 8.13% of WT plants and 89.5 ± 7.52% of cbl9 mutants recovered from the freezing treatment after cold-acclimating (Figure 1f). These results indicate that cbl9 mutants are less sensitive to freezing stress than WT plants.
Figure 1.
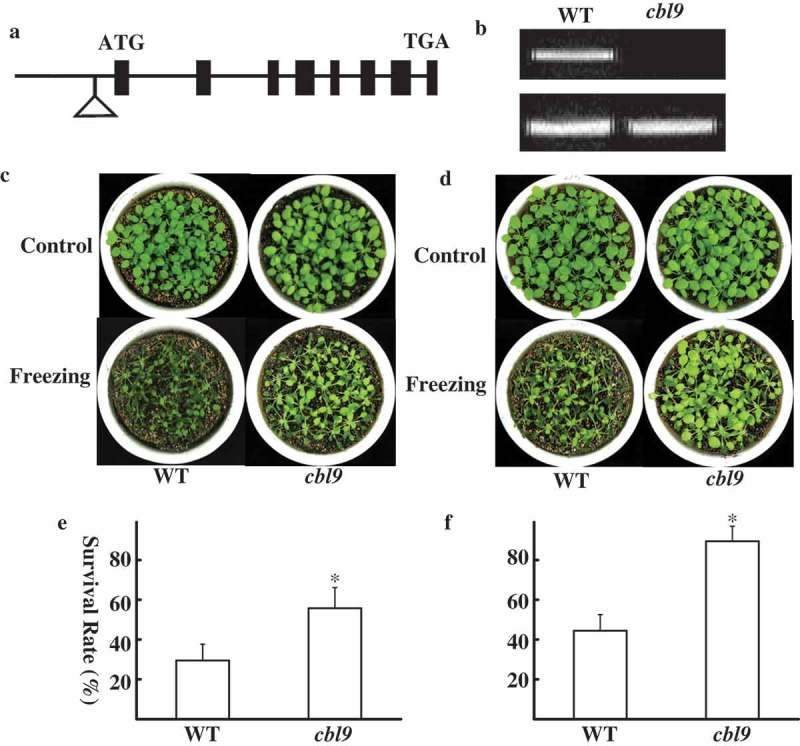
Mutation of CBL9 leads to stronger freezing tolerance in Arabidopsis thaliana.
(a) Insertion position of T-DNA in the CBL9 gene. Filled black boxes represent exons, lines represent introns, and the triangle represents T-DNA insertion. (b) RT-PCR analysis of CBL9 transcript levels. Actin2 was as a loading control. (c-d) Freezing phenotypes. Three-week-old seedlings grown in soil were subjected to the freezing assay. For nonacclimated treatment, the seedlings were directly subjected at −6°C for 8 h (c). For acclimated treatment (pretreated at 4°C for 4 d), the seedlings were subjected at −6°C for 8 h (d). The pictures were taken 7 d after treatments. The phenotype of seedlings before (upper) and after (bottom) freezing treatment was shown. (e-f) Survival rates of WT (left) and cbl9 (right) in (c-d). The data are the mean values of three replicates ± SD (n = 120). Asterisk indicates a significant difference (P < 0.05, t test) between WT and cbl9 mutants. Similar results were observed in three independent experiments.
Cold-induced increases in intracellular Ca2+ levels were promoted in cbl9
Previous data indicated that cold shock induced a transient calcium increase, which then triggered downstream responses.7 Thus, the freezing-insensitive phenotypes of cbl9 might be due to changes in calcium signature. To monitor cold stress-induced [Ca2+]cyt responses, we generated transgenic Arabidopsis plants stably transformed with the plasmid of MAQ2.4.17 As shown in Figure 2a, cbl9 mutants exhibited a higher peak of [Ca2+]cyt (1.96 ± 0.078 µM) than the WT plants (1.66 ± 0.057 µM). Statistical analysis showed that cbl9’s [Ca2+]cyt peak was significantly different from WT plants in response to cold shock (Figure 2b). Cold shock [Ca2+]cyt responses involve both a transmembrane influx of external calcium and calcium signaling events on the tonoplast.7 To investigate the source of intracellular calcium transient in response to cold, Arabidopsis was transformed with the plasmid of HVA1. HVA1 encodes a proton pyrophosphatase–apoaequorin fusion protein that expresses aequorin in the cytosolic microdomain adjacent to the vacuolar membrane.7 As shown in Figure 2c, after the cold shock, the [Ca2+]md peak of WT and cbl9 mutants achieved similar levels, with average peak height of 1.27 ± 0.03 µM and 1.34 ± 0.06 µM, respectively. Statistical analysis showed that [Ca2+]md peak in cbl9 mutants in response to cold shock was not significantly different from WT plants (Figure 2d). These data suggest at least in part contribution of calcium influx from extracellular but not from intracellular space induced by cold shock in cbl9 mutants that displayed enhanced freezing tolerance.
Figure 2.
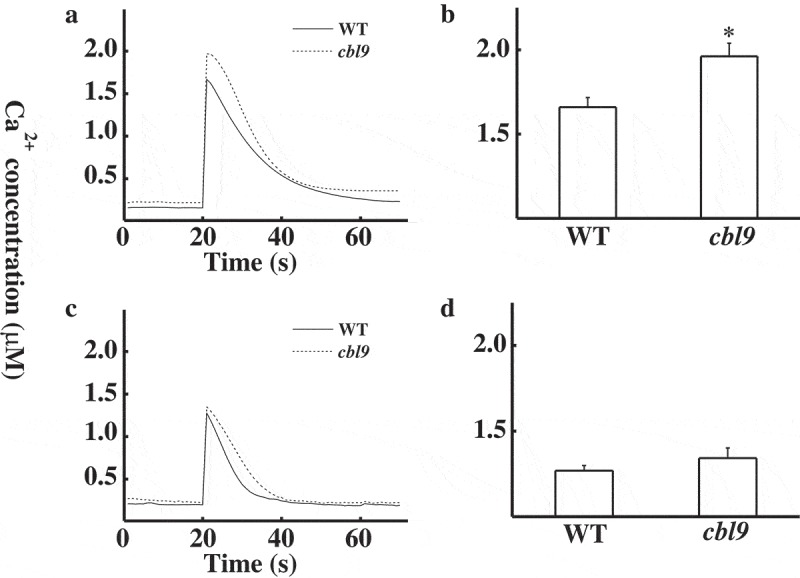
Kinetics of the cold-induced elevation of the [Ca2+] in WT and cbl9 mutants.
(a) Elevation of [Ca2+]cyt in response to cold shock in WT and cbl9 mutants. 7 d old seedlings expressing cytosolic aequorin were subjected to cold shock. A representative trace is shown. (b) The averages of [Ca2+]cyt at the peaks (WT, n = 7; cbl9, n = 8) were shown. Asterisk indicates a significant difference (P < 0.05, t test) between WT and cbl9 mutants. (c) Elevation of [Ca2+]md in response to cold shock in WT and cbl9 mutants. Seven-d-old seedlings expressing microdomain aequorin were subjected to cold shock. A representative trace is shown. (d) The averages of [Ca2+]md at the peaks (WT, n = 10; cbl9, n = 11) were shown.
Effects of various inhibitors on cold shock-induced [Ca2+]cyt transients
To further ascertain the involvement of extracellular or intracellular calcium in cold [Ca2+]cyt response in cbl9 mutants, the transformed MAQ2.4 Arabidopsis seedlings were pretreated with several calcium-signaling inhibitors. After reconstitution of aequorin, inhibitors were added for incubation as indicated in the figures. When plants were treated with Ca2+ antagonist LaCl3, both WT and cbl9 mutants exhibited reduced cold-induced increase in [Ca2+]cyt (Figure 3a). As shown in Figure 3b, the peak height of WT and cbl9 mutants was decreased from 1.69 ± 0.08 µM to 0.92 ± 0.05 µM and from 1.90 ± 0.07 µM to 0.79 ± 0.09 µM, respectively, and cold shock-induced [Ca2+]cyt increase tends to be consistent in WT and cbl9 mutants. Chelation of extracellular calcium by EGTA showed similar results (Figure 3c). The peak height of WT and cbl9 mutants was decreased from 1.67 ± 0.08 µM to 1.23 ± 0.11 µM and from 1.98 ± 0.01 µM to 1.18 ± 0.05 µM, respectively, and cold shock-induced [Ca2+]cyt increase also tends to be consistent (Figure 3d). The effect of EGTA and LaCl3 suggests that extracellular Ca2+ is at least, in part, an important source for the [Ca2+]cyt increase in the WT and cbl9 mutants under cold stress, most likely due to lower greater scope of cbl9 [Ca2+]cyt. IP3 was involved in a cold shock-induced calcium release from intracellular calcium pools. As a potent inhibitor of the phosphatidylinositol cycle, LiCl inhibits IP3 signaling and prevents calcium release from intracellular pools. 7 Plants were pretreated with LiCl to determine whether the different changes of cold-induced [Ca2+]cyt occurred from intracellular calcium pools. As shown in Figure 4a, LiCl led to a reduction in the peak of the Ca2+ concentration in response to cold shock. Moreover, the decreased extent of WT plants was notably different from that of cbl9 mutants. [Ca2+]cyt of WT peaked at 1.37 ± 0.04 µM compared with 1.71 ± 0.08 µM for the control, while [Ca2+]cyt of cbl9 peaked at 1.81 ± 0.05 µM compared with 1.98 ± 0.03 µM for the control. The cbl9 mutants also exhibited a higher peak than the WT did (Figure 4b). The fact that the peak of cbl9 mutants was affected to a greater extent by LaCl3 and EGTA inhibition than by IP3 signaling inhibition suggested that the higher [Ca2+]cyt elevation in cbl9 mutants mediated by cold shock suggested a significant contribution of extracellular calcium pools.
Figure 3.
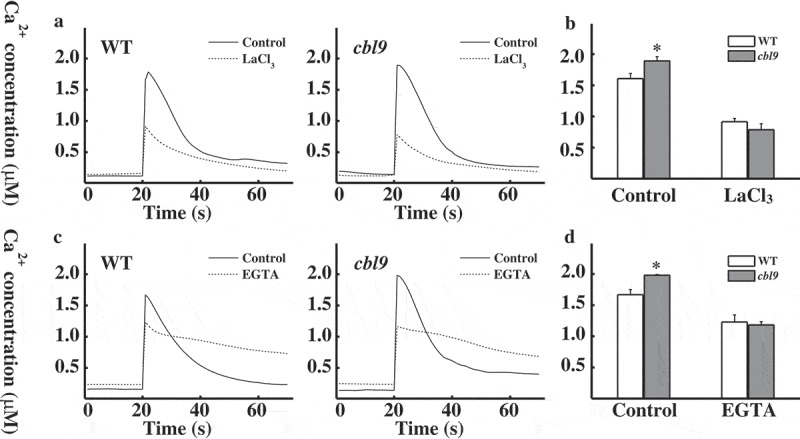
Effect of Ca2+ channel blocker LaCl3 and Ca2+ chelator EGTA on cold shock-induced [Ca2+]cyt transients in WT and cbl9 mutants.
(a) Effect of LaCl3 pretreatment for 1 h on cold shock-induced [Ca2+]cyt changes in WT and cbl9 mutants. A representative trace is shown. (b) The averages of [Ca2+]cyt at the peaks (WT, n = 6; cbl9, n = 6) in (a) were shown. (c) Effect of EGTA pretreatment for 1 h on cold shock-induced [Ca2+]cyt changes in WT and cbl9 mutants. A representative trace is shown. (d) The averages of [Ca2+]cyt at the peaks (WT, n = 9; cbl9, n = 10) in (c) were shown. In (b) and (d), asterisks indicate a significant difference (P < 0.05, t test) between WT and cbl9 mutants.
Figure 4.
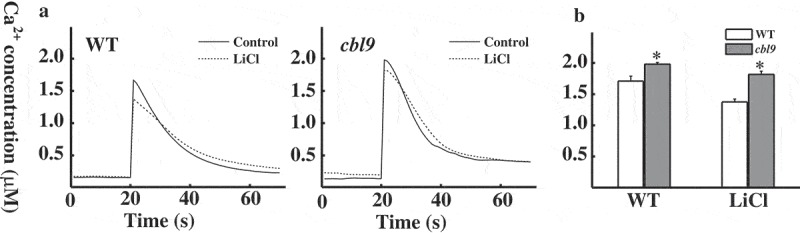
Effect of inhibiting IP3 metabolism on cold shock-induced [Ca2+]cyt transients in WT and cbl9 mutants.
(a) Effect of LiCl pretreatment for 30 min on cold shock-induced [Ca2+]cyt changes in WT and cbl9 mutants. A representative trace is shown. (b) The averages of [Ca2+]cyt at the peaks (WT, n = 8; cbl9, n = 8) in (a) were shown. Asterisks indicate a significant difference (P < 0.05, t test) between WT and cbl9 mutants.
Discussion
The geographical location of temperate and cultivated plants is limited by low temperatures, and freezing temperatures may significantly reduce crop production.1 To improve the freezing resistance of cultivated crops, traditional plant breeding approaches encounter many problems. Uncovering the molecular mechanisms that confer plant tolerance to freezing would not only improve the fundamental knowledge of how plants adapt to environmental changes but could have implications for the development of new strategies to increase the freezing tolerance of crop species.1,14 Previous evidence indicates that CBL9 is a negative regulator of ABA responses and osmotic stress, and its normal function desensitizes ABA effects on seed germination and seedling growth by reducing ABA-induced gene expression.15 Meanwhile, CBL9 and CBL1 bind to and activate protein kinase CIPK23 that directly phosphorylates the K+ transporter AKT1 and enhance K+ uptake in Arabidopsis roots.16 Here, our study provides evidence that CBL9 plays a negative role in freezing tolerance by decreasing [Ca2+]cyt. It was shown that the cbl9 mutants render plants insensitive to cold stress, thus supporting the importance of calcium sensor for adapting to cold stress.
CBL-type calcium sensors belong to small EF-hand proteins that constitute a distinctive family of calcium sensors. CBLs sense the calcium signals generated by a wide large of stresses and then transmit information to different kinds of protein kinases named CIPKs. It is suggested that Ca2+ sensors and their target protein are involved in decoding calcium signaling ‘specificity’. Ca2+ signature was decoded by various signal transduction pathways.18 For example, CBL1 positively regulates salt and drought responses and negatively regulates cold response, and CBL4/SOS3 modulates salt tolerance in plants.19 By contrast, CBL10 is involved in salt tolerance pathway by regulating AKT1 activity to affect ion homeostasis.20 Our studies have demonstrated that cbl9 mutants have increased tolerance to freezing stress under both cold-acclimating and nonacclimating conditions (Figure 1).
Ca2+ is an effective protecting agent, and so a [Ca2+]cyt rise is generally benefit for cold stress conditions. Ca2+ generally ameliorates stress injury on the cellular level.21 The plasma membrane is considered to be the primary site of injury when plants are subjected to cold stress.1 In addition, it is observed that Ca2+ tightens membranes, decreases passive ion fluxes, and renders membranes more hydrophobic. This suggests that Ca2+ has a universal role in membrane stability and cell integrity for increasing stress resistance.21 In contrast, the disaccumulation of membrane-associated Ca2+ can cause damage to membrane stability, and Ca2+ may protect the membrane from freezing stress.22 Maybe the different changes of Ca2+ concentration are the reason why the cbl9 mutants are more insensitive to freezing stress than WT.
It has been shown that the oscillation frequency of [Ca2+]cyt transmits the efficiency and specificity of cellular responses.23 It seems that subtle differences in the Ca2+ signatures are a requirement for eliciting physiological responses. Oxidative stress and extracellular Ca2+ elicited prolonged Ca2+ increases without oscillation in Arabidopsis V-ATPase mutant de-etiolated 3 (det3), whereas WT cells show [Ca2+]cyt oscillations. These data indicate that stimulation-induced specific Ca2+ oscillations are essential for long-term stomatal closure.24 However, there are little data on how these calcium sensors might decode calcium transients. As calcium-binding proteins, the CBL proteins are the most important relay for calcium signaling in plant. Thus, the CBL mutants provided a useful tool to investigate the regulation of calcium signaling. Cold-triggered calcium responses in cbl9 mutants were higher than those in WT in [Ca2+]cyt (Figure 2b) but were similar in [Ca2+]md (Figure 2d). Pharmacological studies have shown that such difference was mainly related to extracellular calcium transmembrane transport (Figures 3 and 4). CBL9 coupled with certain CIPK may mediate downstream signaling responses or feedback regulation of Ca2+ gradient during cold stress. As Ca2+ sensor relaying system, the CBL–CIPK network maintained Ca2+ homeostasis by a feedback mechanism. Arabidopsis CIPK19 was involved in regulating Ca2+ influx by mediating plasma membrane Ca2+ channels at the tip of the pollen tube.25 We speculated that CBL9 with certain CIPK act as a feedback mechanism to reduce the Ca2+ signaling strength when cytoplasmic Ca2+ concentration reaches a higher state level. Ca2+ networks with amplifying pathways and feedback loops induced Ca2+ oscillation.26 The downregulated Ca2+ transients by CBL9 may trigger Ca2+ oscillations. This hypothesis can also explain that the animal ER-type calcium pumps were regulated by a feedback system that maintains proper Ca2+ load and prevents cytotoxic Ca2+ overload.27 In the future, identifying CIPKs that interact with CBL9 will provide further insight into CBL9-mediated pathway regulating Ca2+ homeostasis in cold stress.
As a calcium sensor, CBL9 negatively modulates freezing tolerance in Arabidopsis. Although further evidence should be sought to uncover the participation of other calcium sensors in low-temperature signaling, this study reveals new insights into the molecular mechanism of response to low-temperature stress and could enable development of novel ways to enhance freezing tolerance of crop plants.
Materials and methods
Plant material and growth conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used as the WT plants in this study. Plants were grown at 22 ± 2°C under a 16-h-light/8-h-dark photoperiod at 100 µmol m−2 s−1 with 70% relative humidity in soil or on 1/2 Murashige and Skoog (MS) medium (Sigma) containing 0.8% agar and 1.5% sucrose.
Identification and isolation of cbl9 mutants
T-DNA insertion line of CBL9 (At5g47100), Salk_142774 was received from the Arabidopsis Biological Resource Center. The homozygous nature of the mutant was confirmed by PCR amplification according to http://signal.salk.edu/tdnaprimers.2.html. A recessive mutation of a single T-DNA insertion locus was confirmed as our laboratory previously described and approved from other studies .16,28
Freezing assay
Freezing tolerance assays were performed as described previously,29 with minor modifications. Three-week-old seedlings grown in soil were subjected to the freezing assay. For nonacclimated treatment, the seedlings were directly subjected to the freezing assay. For acclimated treatment, the seedlings were first grown at 4°C for 4 d under a 16-h-light/8-h-dark photoperiod and then subjected to the freezing assay. The freezing assays for both nonacclimated and acclimated seedlings were subjected at −6°C for 8 h. After treatment, the seedlings were transferred at 4°C for 12 h in the dark and then transferred to a growth chamber for another 7 d. After recovery, seedlings that could still grow new leaves were counted as survivors, and the survival rates were measured.
[Ca2+]cyt measurements in cbl9 and the WT plants
The plasmids of MAQ2.4 and HVA1 were transferred into Agrobacterium tumefaciens strain GV3101 and transformed, respectively, into WT and cbl9 mutants by floral infiltration. Transgenic Arabidopsis plants expressing MAQ2.4 were used for [Ca2+]cyt measurements. Plants expressing HVA1 were used for [Ca2+]md measurement. Aequorin was reconstituted in vivo by incubating 7–8 d Arabidopsis seedlings on water containing 2.5 µM native coelenterazine (Promega, WI, USA) for 12 h in the dark at 25°C ± 2°C. Cold shock-induced cytosolic Ca2+ concentration changes were measured according to Ref. 7. Luminescence counts were recorded by a digital luminometer (TD20/20n, Turner Biosystems, CA, USA), and luminescence values were converted into actual Ca2+ concentrations as described previously.7 Inhibitor experiments were performed by placing reconstituted seedlings in 10 mM LaCl3 for 1 h, in 20 mM EGTA for 1 h, and in 20 mM LiCl for 30 min (all Sigma, USA). After which, seedlings were removed from the inhibitor solution and put in luminometer for calcium concentration measurement as given above.
Funding Statement
This work was supported by the National Natural Science Foundation of China [U1204302].
Acknowledgments
We are grateful to Heather Knight and Marc R. Knight (Durham University) for the plasmids of MAQ2.4 and HVA1 and to Heather Knight for excellent technical assistance. We thank Dr. Jason Deng for critical reading of this manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C, Zhang Z, Xie S, Si T, Li Y, Zhu JK. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016;171:2744–2759. doi: 10.1104/pp.16.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Ding Y, Shi Y, Zhang X, Zhang S, Gong Z, Yang S. MPK3-and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev Cell. 2017;43:630–642. doi: 10.1016/j.devcel.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Janmohammadi M, Zolla L, Rinalducci S. Low temperature tolerance in plants: changes at the protein level. Phytochemistry. 2015;117:76–89. doi: 10.1016/j.phytochem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Jha UC, Bohra A, Jha R. Breeding approaches and genomics technologies to increase crop yield under low-temperature stress. Plant Cell Rep. 2017;36:1–35. doi: 10.1007/s00299-016-2073-0. [DOI] [PubMed] [Google Scholar]
- 6.Raffaello A, Mammucari C, Gherardi G, Rizzuto R. Calcium at the center of cell signaling: interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem Sci. 2016;41:1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori K, Renhu N, Naito M, Nakamura A, Shiba H, Yamamoto T, Suzaki T, Iida H, Miura K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci Rep. 2018;8:550. doi: 10.1038/s41598-017-17483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monroy AF, Sarhan F, Dhindsa RS. Cold-induced changes in freezing tolerance, protein phosphorylation, and gene expression (evidence for a role of calcium). Plant Physiol. 1993;102:1227–1235. doi: 10.1104/pp.102.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranty B, Aldon D, Cotelle V, Galaud JP, Thuleau P, Mazars C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front Plant Sci. 2016;7:327. doi: 10.3389/fpls.2016.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudla J, Xu Q, Harter K, Gruissem W, Luan S. Genes encoding calcineurin B-like protein in Arabidopsis are differentially regulated by stress signals. Proc Natl Acad Sci USA. 1999;96:4718–4723. doi: 10.1073/pnas.96.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Q, An L, Li W. The CBL-CIPK network mediates different signaling pathways in plants. Plant Cell Rep. 2014;33:203–214. doi: 10.1007/s00299-013-1507-1. [DOI] [PubMed] [Google Scholar]
- 13.Kolukisaoglu U, Weinl S, Blazevic D, Batistic O, Kudla J. Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004;134:43–58. doi: 10.1104/pp.103.033068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey GK, Cheong YH, Kim KN, Grant JJ, Li L, Hung W, D’Angelo C, Weinl S, Kudla J, Luan S. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell. 2004;16:1912–1924. doi: 10.1105/tpc.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporters AKT1 in Arabidopsis. Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- 18.Luan S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14:37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S. CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell. 2003;15:1833–1845. doi: 10.1105/tpc.012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monihan SM, Magness CA, Yadegari R, Smith SE, Schumaker KS. Arabidopsis CALCINEURIN B-LIKE10 functions independently of the SOS pathway during reproductive development in saline conditions. Plant Physiol. 2016;171:369–379. doi: 10.1104/pp.16.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plieth C. Plant calcium signaling and monitoring: pros and cons and recent experimental approaches. Protoplasma. 2001;218:1–23. doi: 10.1007/BF01288356. [DOI] [PubMed] [Google Scholar]
- 22.Hirschi KD. Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell. 1999;11:2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sneyd J, Han JM, Wang L, Chen J, Yang X, Tanimura A, Sanderson MJ, Kirk V, Yule DI. On the dynamical structure of calcium oscillations. Proc Natl Acad Sci USA. 2017;114:1456–1461. doi: 10.1073/pnas.1614613114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Lan W, Chen B, Fang W, Luan S. A calcium sensor-regulated protein kinase, CALCINEURIN B-LIKE PROTEIN-INTERACTING PROTEIN KINASE19, is required for pollen tube growth and polarity. Plant Physiol. 2015;167(4):1351–1360. doi: 10.1104/pp.114.256065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plieth C. Calcium: just another regulator in the machinery of life? Ann Bot. 2005;96:1–8. doi: 10.1093/aob/mci144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhogal MS, Colyer J. Depletion of Ca2+ from the sarcoplasmic reticulum of cardiac muscle prompts phosphorylation of phospholamban to stimulate store refilling. Proc Natl Acad Sci USA. 1998;95:1484–1489. doi: 10.1073/pnas.95.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai L, Zhang G, Zhou Y, Zhang Z, Wang W, Du Y, Wu Z, Song CP. Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca2+ signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant J. 2009;60:314–327. doi: 10.1111/j.1365-313X.2009.03956.x. [DOI] [PubMed] [Google Scholar]
- 29.Ji H, Wang Y, Cloix C, Li K, Jenkins GI, Wang S, Shang Z, Shi Y, Yang S, Li X. The Arabidopsis RCC1 family protein TCF1 regulates freezing tolerance and cold acclimation through modulating lignin biosynthesis. PLoS Genet. 2015;11:e1005471. doi: 10.1371/journal.pgen.1005471. [DOI] [PMC free article] [PubMed] [Google Scholar]


