ABSTRACT
Cell elongation, which plays an important role in root penetration into the soil, responds to a variety of environmental factors. A previous study demonstrated that abscisic acid, a phytohormone involved in stress responses, inhibits root growth by delaying the onset of cell elongation. In contrast, we recently reported that cytokinins promote elongation of root cells by enhancing actin bundling. However, the control of root cell elongation through the interaction between abscisic acid and cytokinin signaling has not yet been uncovered. Here, we show that abscisic acid-induced delay in cell elongation requires inhibition of cytokinin signaling; further, stress is signaled to cell elongation by the pathway mediated by B-type ARABIDOPSIS RESPONSE REGULATOR 2 (ARR2), which retards root growth.
KEYWORDS: Cytokinin, abscisic acid, cell elongation, root
Results
The Arabidopsis root tip is divided into four distinct zones: the stem cell niche (SCN), the proximal meristem (PM), the transition zone (TZ), and the elongation/differentiation zone (EDZ).1,2 Cells produced in the SCN actively divide in the PM and accumulate in a longitudinal direction to form the root meristem. At the boundary between the PM and the TZ, the cell cycle is modified from the mitotic cycle to the endocycle. In the endocycle, DNA replication is repeated without mitosis or cytokinesis, causing DNA polyploidization.3 As a result, cell division ceases and rapid cell elongation occurs.4 Then, cells gradually expand in the TZ and eventually more rapid cell elongation is initiated in the EDZ. Recently, we reported that dynamic actin reorganization triggers the transition from the TZ to the EDZ.5 Therefore, Arabidopsis root cells undergo two modes of rapid cell elongation; the first mode is induced by the onset of endocycle at the border of the PM and the TZ, and the second mode results from actin reorganization at the border of the TZ and the EDZ.5
In addition, we observed that cytokinin (CK) signaling controls the number of TZ cells. The transmembrane receptors ARABIDOPSIS HISTIDINE KINASE2/3/4 (AHK2/3/4) perceive CKs. Further, a phosphorelay to the B-type ARABIDOPSIS RESPONSE REGULATORs (ARRs), transcription factors controlling CK-responsive genes, transmits the signal.6 At the boundary between the TZ and the EDZ, CK signaling that involves AHK3/AHK4 and ARR2 promotes actin bundling, which enables the second rapid cell elongation.5 Indeed, ahk3/4 and arr2 mutants have more TZ cells than wild type owing to the delayed onset of the second rapid elongation.5
Abscisic acid (ABA) is a phytohormone known as the “stress hormone”.7 ABA signaling mediates many types of abiotic stresses, such as drought, salinity, heat and cold stress.7 In roots, ABA plays an essential role in inhibiting cell division and elongation in response to soil conditions.8,9 Previously, Zhang et al. (2010) reported that ABA has a positive effect on the TZ length in Arabidopsis roots, a phenocopy observed in ahk3/4 and arr2 mutants.8 However, the crosstalk between ABA and CK signaling has not yet been analyzed, and it remains unknown whether abiotic stresses control root growth through modulation of CK signaling.
To examine the involvement of CK signaling in ABA-mediated control of the second rapid cell elongation, we first asked whether ABA controls the cell number in the TZ through the AHK3/AHK4-ARR2 pathway. Consistent with previous findings, 0.5 µM or 10 µM ABA treatment of Arabidopsis roots increased the TZ cell number, indicating an inhibition of the second rapid cell elongation (Figure 1).8 As we recently described, ahk3/4 and arr2 mutants had more TZ cells than wild type in the absence of ABA. Interestingly, these mutants were tolerant of ABA treatment in terms of an increase in TZ cell number (Figure 1).5 This result suggests that ABA-induced delay in the second rapid cell elongation requires inhibition of CK signaling through the AHK3/4-ARR2 pathway.
Figure 1.
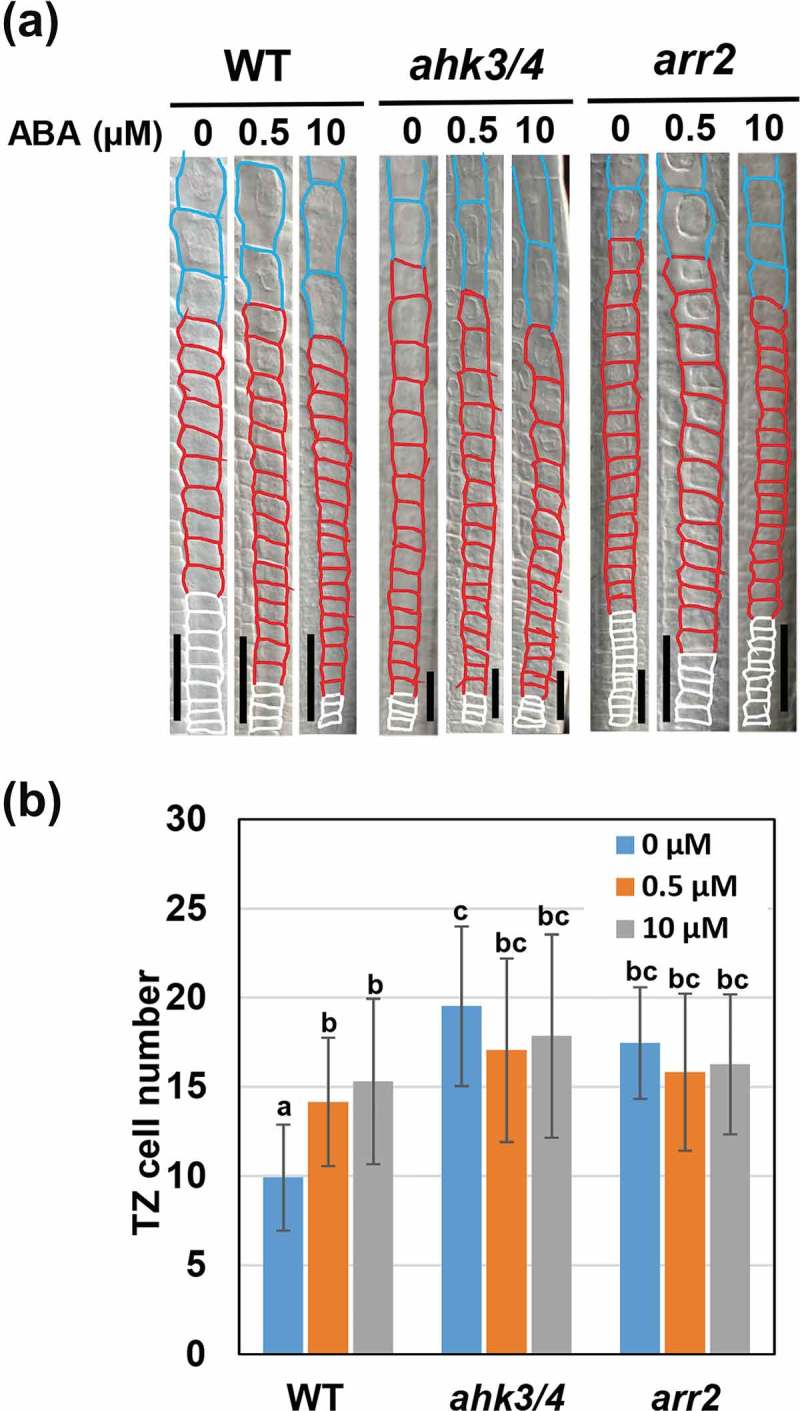
ABA increases the cell number in the TZ through AHK3/4 and ARR2. (a) Cortical cells around the TZ of wild-type, ahk3/4 and arr2. Three-day-old roots were treated with different concentrations of ABA for 4 days. The PM, TZ and EDZ cells are shown by white, red and blue outlines, respectively. The scale bars represent 50 µm. (b) The TZ cell number in wild-type, ahk3/4 and arr2 treated with or without 0.5 µM or 10 µM ABA. Data are presented as mean ± SD (n > 20). Bars with different letters differ significantly from each other. Significant differences were determined by Tukey’s test (P < 0.05).
Nguyen et al. (2016) reported that ABA treatment reduced the transcript levels of B-type ARRs, ARR1, ARR10, and ARR12, in Arabidopsis roots.10 However, whether ABA represses the expression of ARR2, which plays a major role in promoting the second rapid cell elongation, remains unknown. Therefore, we investigated the expression pattern of ARR2 fused to b-glucuronidase (GUS), which was expressed under the 2-kb ARR2 promoter, in the presence of ABA. A high GUS signal was observed in the area encompassing the TZ and the EDZ in the absence of ABA. However, the GUS signal was dramatically reduced by 0.5 µM ABA treatment; further, only a weak signal was detected in the vasculature of 10 µM ABA-treated roots (Figure 2a).5 As reported previously, ABA treatment reduced expression of pARR1:ARR1–GUS and pARR12:ARR12–GUS in a dose-dependent manner (Figure 2b), implying that ABA downregulates not only ARR2 but also other B-type ARRs.10 In contrast, neither 0.5 µM or 10 µM ABA treatment decreased the expression of pAHK3:AHK3-GUS or pAHK4:GUS (Figure 2c), suggesting that ABA signaling does not affect the expression of CK receptors.
Figure 2.
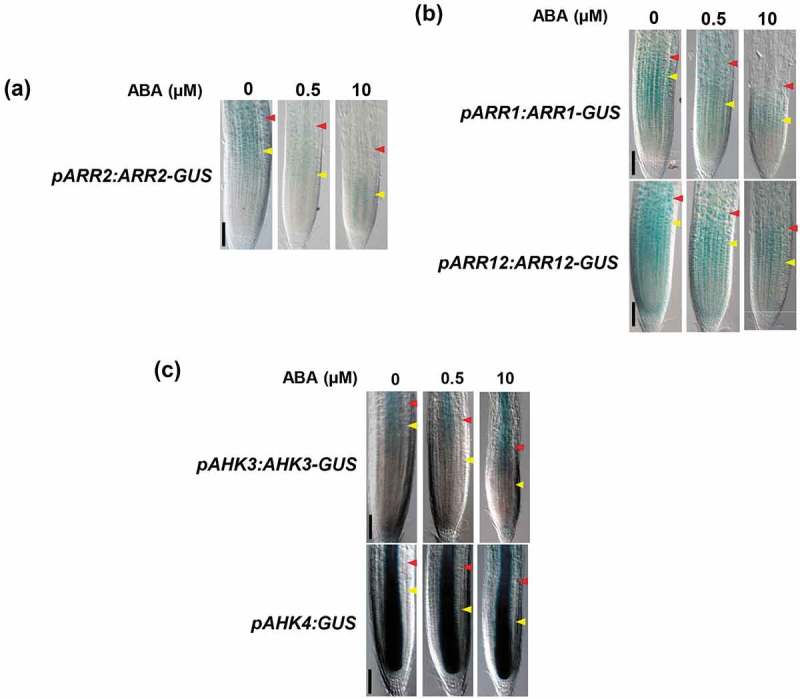
Expression patterns of ARR1, ARR2, ARR12, AHK3 and AHK4 in the presence of ABA. Three-day-old roots were treated with or without different concentrations of ABA for 4 days, and subjected to GUS staining. (a) pARR2:ARR2–GUS, (b) pARR1:ARR1–GUS and pARR12:ARR12–GUS, and (c) pAHK3:AHK3–GUS and pAHK4:GUS. Yellow and red arrowheads indicate the boundaries between the PM and the TZ, and between the TZ and the EDZ, respectively. The scale bars represent 100 µm.
ARR1, ARR2, and ARR12 are known to promote the first rapid cell elongation by enhancing the transition from the mitotic cell cycle to the endocycle.3 Therefore, downregulation of their expression by ABA may prevent mitotic cells from entering the endocycle, although mitosis is directly inhibited by ABA.8 However, our data suggest that ABA-triggered repression of ARR2 inhibits actin reorganization and delays the onset of the second rapid cell elongation, leading to inhibition of root growth. This result indicates that ABA controls cell elongation as well as cell division to effectively inhibit root growth under stressful conditions and that ARR2-mediated but cytokinin-independent pathway plays a crucial role in transmitting stress signals to actin-dependent cell elongation.
Funding Statement
This work was supported by MEXT KAKENHI under Grant number 17H03965; under Grant number 17H06470; and under Grant number 17H06477 to M.U., and MEXT KAKENHI under Grant number 17K15415 and the 2017 Inamori Research Grant Program to H.T.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Takatsuka H, Umeda M, Hormonal control of cell division and elongation along differentiation trajectories in roots. J Exp Bot. 2014;65:2633–2643. PMID: 24474807 https://academic.oup.com/jxb/article/65/10/2633/574854 [DOI] [PubMed] [Google Scholar]
- 2.Takatsuka H, Umeda M.. Epigenetic control of cell division and cell differentiation in the root apex. Front Plant Sci. 2015;24:1178 PMID: 25942995 https://www.frontiersin.org/articles/10.3389/fpls.2015.01178/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi N, Kajihara T, Okamura C, Kim Y, Katagiri Y, Okushima Y, Matsunaga S, Hwang I, Umeda M, Cytokinins control endocycle onset by promoting the expression of an APC/C activator in arabidopsis roots. Curr Biol. 2013;23:1812–1817. PMID: 24035544 https://www.cell.com/current-biology/fulltext/S0960-9822-13-00912-3 [DOI] [PubMed] [Google Scholar]
- 4.Beemster GTS, Baskin TI.. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 1998;116:1515–1526. PMID: 9536070 http://www.plantphysiol.org/content/116/4/1515.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takatsuka H, Higaki T, Umeda M, Actin reorganization triggers rapid cell elongation in roots. Plant Physiol. 2018;178:1130–1141. PMID: 30185441 http://www.plantphysiol.org/content/178/3/1130.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To JPC, Kieber JJ, Cytokinin signaling: two-components and more. Trends Plant Sci. 2008;13:85–92. PMID: 18262459 https://www.sciencedirect.com/science/article/pii/S1360138508000216?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 7.Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front Plant Sci. 2017;8:161 PMID: 28265276 https://www.frontiersin.org/articles/10.3389/fpls.2017.00161/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Han W, De Smet I, Talboys P, Loya R, Hassan A, Rong H, Jürgens G, Paul Knox J, Wang MH. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J. 2010;64:764–774. PMID: 21105924 https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-313X.2010.04367.x [DOI] [PubMed] [Google Scholar]
- 9.Sakaoka S, Mabuchi K, Morikami A, Tsukagoshi H. MYB30 regulates root cell elongation under abscisic acid signaling. Commun Integr Biol. 2018;11:e1526604 PMID: 30534346 https://www.tandfonline.com/doi/full/10.1080/19420889.2018.1526604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen KH, Van HC, Nishiyama R, Watanabe Y, Leyva-González MA, Fujita Y, Tran UT, Li W, Tanaka M, Seki M, et al. Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought. Proc Natl Acad Sci. 2016;113:3090–3095. PMID: 26884175 https://www.pnas.org/content/113/11/3090.long. [DOI] [PMC free article] [PubMed] [Google Scholar]


