Abstract
Background
Acute myocardial infarction (AMI) with no evidence of relevant stenosis of the coronary artery, known as myocardial infarction (MI) with nonobstructive coronary arteries (MINOCA), has a prevalence of up to 14%. The various causes of MINOCA lead to damage of the myocardium, and there are marked differences in diagnoses, prognoses, and treatments. Although the number of patients affected is considerable owing to the high prevalence of acute coronary syndrome (ACS), the causes of MINOCA have received little attention with the result that some patients may not receive appropriate treatment. Awareness of this disease among clinicians has started only to improve since the beginning of the current century. The aim of this study was to develop a score that enables patients with MINOCA to be distinguished from patients with MI with coronary artery disease (MI-CAD) and thus to facilitate appropriate diagnosis and therapy.
Patients and methods
A multicenter observational cohort study was designed. All patients aged ≥18 years from the ARIAM-SEMICYUC (Analysis of Delay in AMI-Spanish Society of Intensive Care Medicine and Coronary Unit) registry, diagnosed with AMI, and admitted to critical care units or coronary care units (CCUs) were included. Patients were classified into two groups: MINOCA, comprising patients with no significant lesions on angiography, and MI-CAD, comprising patients with lesions of the coronary artery tree.
Results
A score based on standard variables to assess the probability of MINOCA on admission was designed, showing a maximum value corresponding to a 40% probability of MINOCA. The discriminative power of the model was 0.756 (P-value for the Hosmer–Lemeshow test was >0.05). At 30-day follow-up, the mortality rate was higher for MI-CAD patients.
Conclusion
Patients with MINOCA constitute a population that differs from other patients with AMI. Their differential characteristics require a certain diagnostic effort to align therapy with the disease causing the ischemic event. This score could prove useful in establishing additional diagnostic procedures.
Keywords: MINOCA, acute myocardial infarction, nonobstructive coronary disease, coronary syndrome, score
Introduction
Coronary heart disease is responsible for one-third of deaths in patients aged more than 35 years in developed countries.1 In Europe, cardiovascular diseases cause approximately 4 million deaths annually, mainly owing to coronary heart disease. The disease generates costs of around €196,000 million per year, ie, 54% of all health care spending.2
The European Society of Cardiology defines myocardial infarction (MI) as an alteration of markers of myocardial damage, mainly cardiac troponin (cTn), together with other clinical, electrocardiographic, and imaging data compatible with myocardial ischemia, although this definition is constantly changing.3
Acute myocardial infarction (AMI) with no evidence of relevant stenosis of the coronary artery is known as MI with nonobstructive coronary arteries (MINOCA). Its prevalence ranges from 1% to 14% with a mean of 6%.4,5 The various causes of MINOCA lead to damage of the myocardium, and there are marked differences in diagnoses, prognoses, and treatments. Although the number of patients affected is considerable owing to the high prevalence of acute coronary syndrome (ACS), the causes of MINOCA have received little attention with the result that some patients may not receive appropriate treatment. Awareness of this disease among clinicians has started only to improve since the beginning of the current century, when the results of the first studies were published.
The causes of MINOCA are extremely varied. It can be classed as disease affecting the epicardial arteries and disease affecting the microcirculation. Other causes that do not directly involve coronary circulation include myocarditis, various prothrombotic diseases, and diseases preventing successful functioning of the heart.6 Since affected patients are not candidates for coronary revascularization, other therapeutic strategies are needed to improve health and quality of life.
Given the heterogeneous nature of the causes of MINOCA, prognostic studies are of limited value and may be influenced by the etiology and by the degree of damage associated with AMI. In any case, the disease is associated with greater mortality and risk of cardiovascular disease than in the general population, in both the short term and in the medium term.4,5
Patients with MINOCA differ clinically from all other patients with AMI. They are more frequently younger, and the disease affects both sexes equally, unlike MI with coronary artery disease (MI-CAD). Patients with MINOCA tend to have fewer cardiovascular risk factors, and their serum markers of myocardial damage peak at lower levels.4,5,7–11
In Spain, the Spanish Society of Intensive Care Medicine and Coronary Unit (SEMICYUC) keeps a registry of acute coronary heart disease, the ARIAM (Analysis of Delay in AMI) registry, which has been kept running without interruption since 1994.12 The registry comprises clinical, diagnostic, and analytical data as well as prognostic tools, which form the basis of the present study.
Our aim was to develop a score that allows us to distinguish between patients with MINOCA and other patients with coronary disease on admission to hospital to reach a diagnosis and apply appropriate therapy. The secondary objective was to describe the characteristics of patients with MINOCA and their clinical course during admission and in the short term (30 days).
Patients and methods
We performed a multicenter observational cohort study involving 69 hospitals in Spain and Andorra. Patients from the ARIAM-SEMICYUC registry were included prospectively and analyzed retrospectively. The registry includes all patients who were admitted to the intensive care unit (ICU) and coronary care unit (CCU) with a diagnosis of ACS.
The study included all patients aged ≥18 years and diagnosed with AMI within the previous 48 hours. The criteria for AMI and for the diagnosis of MINOCA were established according to those of the European Society of Cardiology in force.13 Patients that were not diagnosed with having AMI, minors with a personal history of coronary obstructive pathology, and those who did not undergo diagnostic coronary angiography during admission were excluded from the study.
We classified patients diagnosed with AMI into two groups: MINOCA, comprising patients with no previous diagnosis of coronary heart disease and no significant lesions (ie, no coronary artery stenosis ≥50%) on the angiogram at admission, and MI-CAD, comprising patients with previously known lesions of the coronary artery tree at admission or coronary obstructive disease on the coronary angiogram taken during admission and/or the need for coronary angioplasty.
The study period was from January 2010 to March 2015. Patients were followed for 30 days after discharge from the ICU or CCU.
The ARIAM-SEMICYUC registry comprises hundreds of variables adjusted to the recommendations of national guidelines and European guidelines (the Cardiology Audit and Registration Data Standards [CARDS] and European Data Standards for Clinical Cardiology Practice), the manuals of which can be accessed from the registry web page.14,15 The variables selected for the study were those that could be used to identify the onset of MINOCA, according to previously published studies.
Statistical analyses
Categorical variables were expressed as absolute numbers and percentages. The chi-squared test was used to identify differences between the MINOCA and MI-CAD groups. Continuous variables are expressed as median (IQR). The Student’s t-test was used to identify significant differences. Non-normal continuous variables were log transformed before the test was applied.
Actual survival was evaluated on censored data by the Kaplan–Meier (KM) function estimator. Cox proportional hazards regression models were used for multivariate analyses and to build a nomogram. The predictors analyzed initially included all variables that were statistically significant in bivariate analysis; nevertheless, to develop the final model, a backward step-down selection method was performed. The nomogram was created; meanwhile, the reduced model with only significant variables was included. Internal validation of nomogram was performed by discrimination and calibration analysis. For that aim, Harrell’s concordance index (c-index) was used for quantifying the nomogram accuracy and, similarly, calibration plots were presented to further validate the nomogram. Calibration was assessed by grouping patients with respect to their nomogram-predicted probabilities and comparing the mean of the observed KM estimate of mortality at 30 days. The regression model was validated by analyzing the discriminative power of the model using the C-statistic (area under the receiver operating characteristic [ROC] curve) and the Hosmer–Lemeshow test.
The software used for the statistical analyses was STATA v.12© (StataCorp LP, College Station, TX, USA).
Ethics
The study was approved by SEMICYUC for the use of the ARIAM-SEMICYUC registry. The rules for appropriate use of the registry were followed. The registry data that were released for the research were anonymized from aggregated public data. The ARIAM-SEMICYUC adheres to Spanish legislation on observational post-authorization studies for medicines for human use and the Spanish data protection law. Local clinical research ethics committee did not require patients to give their informed consent.
Results
Characteristics of patients with MINOCA in the ARIAM-SEMICYUC registry
During the study period, a total of 12,899 patients with AMI were included in the ARIAM-SEMICYUC project. Of these, 9,993 underwent coronary angiography; 9,241 were classed as MI-CAD and 752 as MINOCA. In this group, we excluded 130 patients who had a history of occlusive ischemic heart disease. The MINOCA group thus comprised 622 patients (Figure 1).
Figure 1.
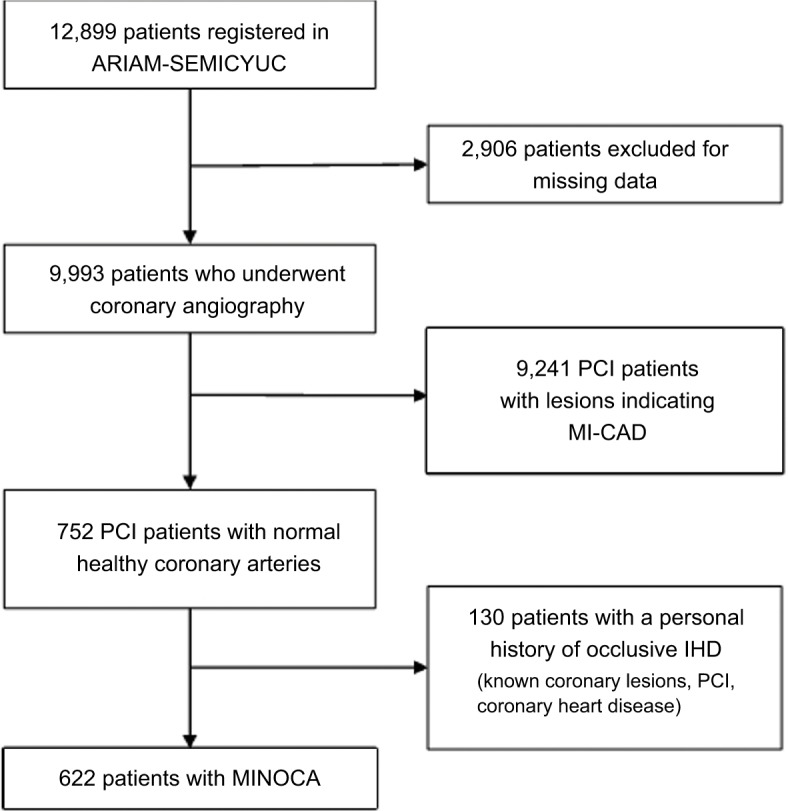
Flowchart of the selection process.
Abbreviations: AMI, acute myocardial infarction; ARIAM, Analysis of Delay in AMI; IHD, ischemic heart disease; MI-CAD, myocardial infarction with coronary artery disease; MINOCA, myocardial infarction with nonobstructive coronary arteries; PCI, percutaneous coronary intervention; SEMICYUC, Spanish Society of Intensive Care Medicine and Coronary Unit.
The characteristics of the study patients and their baseline characteristics are summarized in Table 1. Patients from the MINOCA group were younger, had a higher rate of female gender, and had less cardiovascular risk factors than patients from the MI-CAD group. In addition, cardiovascular drugs were administered more frequently to patients from the MI-CAD group.
Table 1.
Demographic and baseline variables
| Variables | MI-CAD (N=9,241) N (%) | MINOCA (N=622) N (%) | P-value |
|---|---|---|---|
| Gender, female | 2,019 (21.9) | 305 (49.0) | <0.001 |
| Age (years), median (IQR) | 65 (55–75) | 63 (50–74) | <0.001 |
| BMI, median (IQR) | 27.3 (25.0–29.9) | 27.0 (24.5–29.4) | 0.32 |
| Ex-smoker | 2,207 (23.9) | 87 (14) | <0.001 |
| Current smoker | 3,360 (36.4) | 169 (27.1) | <0.001 |
| Nonsmoker | 2,839 (30.7) | 294 (47.3) | <0.001 |
| Hypertension | 5,443 (58.9) | 326 (52.4) | 0.001 |
| Known CAD | 1,396 (15.1) | 0 (0.0) | <0.001 |
| Family history of ischemic heart disease | 1,088 (11.7) | 71 (11.4) | 0.594 |
| Dyslipidemia | 4,722 (51.1) | 275 (44.2) | <0.001 |
| Diabetes mellitus | 2,590 (28.0) | 116 (18.7) | <0.001 |
| Consumption of cocaine | 96 (1.0) | 11 (1.8) | 0.069 |
| Previous angina | 1,695 (18.3) | 89 (14.3) | 0.012 |
| Previous AMI | 1,554 (16.8) | 29 (4.7) | <0.001 |
| Stroke | 514 (5.5) | 25 (4.0) | 0.1 |
| Peripheral artery disease | 593 (6.4) | 14 (2.2) | <0.001 |
| Heart failure | 280 (3.0) | 19 (3.1) | 0.965 |
| COPD | 701 (7.6) | 39 (6.3) | 0.227 |
| Chronic kidney disease | 445 (4.8) | 19 (3.1) | 0.046 |
| Treatment before admission | |||
| Acetylsalicylic acid | 2,396 (25.9) | 88 (14.2) | <0.001 |
| Other antiplatelet agents | |||
| None | 8,318 (90.0) | 600 (96.5) | <0.001 |
| Clopidogrel | 784 (8.5) | 21 (3.4) | <0.001 |
| Prasugrel | 35 (0.4) | 0 (0.0) | 0.168 |
| Ticagrelor | 21 (0.2) | 0 (0.0) | 0.64 |
| Others | 71 (0.8) | 1 (0.1) | 0.089 |
| Nitrates | 656 (7.1) | 25 (4.0) | 0.003 |
| Beta-blockers | 1,743 (18.9) | 91 (14.6) | 0.008 |
| Calcium channel antagonists | 1,168 (12.7) | 59 (9.5) | 0.02 |
| ACEI/ARA-II | 3,318 (35.9) | 200 (32.1) | 0.054 |
| Statins/other lipid-lowering drugs | 3,294 (35.6) | 176 (28.3) | <0.001 |
| Oral anticoagulants | 379 (4.1) | 38 (6.1) | 0.017 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; AMI, acute myocardial infarction; ARA-II, angiotensin receptor antagonists-II; BMI, body mass index; CAD, coronary artery disease; MI-CAD, myocardial infarction with CAD; MINOCA, myocardial infarction with nonobstructive coronary arteries.
At baseline, all patients underwent a hemodynamic workup and electrocardiogram (ECG) and were classified according to the Killip class.16 Risk was assessed using the Global Registry of Acute Coronary Events (GRACE) and thrombolysis In myocardial infarction (TIMI) scores, as summarized in Table 2.17,18 At the time of admission, the MINOCA group had less severe changes in the ECG and lower values in the Killip class, as well as in the TIMI and GRACE scores, than patients with MI-CAD.
Table 2.
Assessment on admission
| Variables | MI-CAD (N=9,241) N (%) | MINOCA (N=622) N (%) | P-value |
|---|---|---|---|
| ECG at admission | |||
| ECG normal | 462 (5.0) | 67 (10.8) | <0.001 |
| New LBBB | 73 (0.8) | 10 (1.6) | 0.018 |
| ST segment depression <0.5 mm | 408 (4.4) | 37 (6.0) | 0.03 |
| ST depression ≥0.5 mm | 1,057 (11.4) | 72 (11.6) | 0.513 |
| ST elevation <2 mm | 924 (10.0) | 76 (12.2) | 0.019 |
| ST elevation ≥2 mm | 4,194 (45.4) | 119 (19.1) | <0.001 |
| Transient ST elevation (<20 minutes) | 339 (3.7) | 37 (6.0) | 0.001 |
| T wave inversion | 807 (8.7) | 112 (18.0) | <0.001 |
| LMCA/multivessel pattern | 150 (1.6) | 2 (0.3) | 0.009 |
| Others/unknown | 827 (9.0) | 90 (14.4) | <0.001 |
| Hemodynamic values | |||
| Killip class I | 7,280 (78.8) | 516 (83.0) | 0.014 |
| Killip class II | 1,218 (13.2) | 70 (11.2) | 0.166 |
| Killip class III | 479 (5.2) | 29 (4.7) | 0.567 |
| Killip class IV | 259 (2.8) | 7 (1.1) | 0.012 |
| Systolic pressure (mmHg), median (IQR) | 135 (118–153) | 134 (120–150) | 0.934 |
| Diastolic pressure (mmHg), median (IQR) | 75 (65.5–88) | 75 (65–87) | 0.141 |
| Heart rate (bpm), median (IQR) | 75 (65–89) | 78 (67–90) | <0.001 |
| Laboratory values | |||
| Creatinine (mg/dL), median (IQR) | 0.93 (0.8–1.1) | 0.90 (0.7–1.1) | <0.001 |
| Platelet count (×103/mL), median (IQR) | 189 (155–228) | 199 (169–237) | 0.005 |
| Total cholesterol (mg/dL), median (IQR) | 168 (139–199) | 170.5 (140.5–199.5) | 0.611 |
| HDL cholesterol (mg/dL), median (IQR) | 39 (33–47) | 45 (36–54) | <0.001 |
| Triglycerides (mg/dL), median (IQR) | 119 (89–163) | 111 (82.5–154) | 0.012 |
| Hematocrit (%), median (IQR) | 42 (38.1–45) | 41.2 (37.8–43.4) | 0.024 |
| Hemoglobin (g/L), median (IQR) | 14 (12.9–15.2) | 13.9 (12.7–14.8) | 0.086 |
| Evaluation of risk | |||
| TIMI score, median (IQR) | 3 (2–4) | 2 (1–3) | <0.001 |
| GRACE score, median (IQR) | 142 (120–168) | 126.5 (100.5–153) | <0.001 |
Abbreviations: ECG, electrocardiogram; GRACE, Global Registry of Acute Coronary Events; HDL, high-density lipoprotein; LBBB, left bundle branch block; LMCA, left mean coronary artery; MI-CAD, myocardial infarction with coronary artery disease; MINOCA, myocardial infarction with nonobstructive coronary arteries; TIMI, thrombolysis in myocardial infarction.
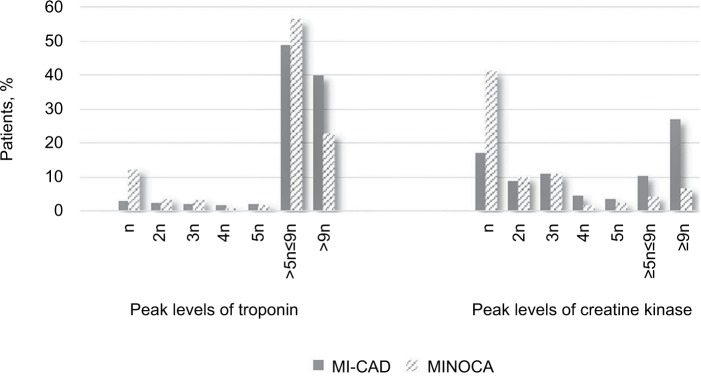
Table 3 summarizes the occurrence of complications during ICU/CCU admission and at 30 days. Cardiovascular complications were more frequently present in the MI-CAD group during ICU/CCU stay, with special relevance of acute kidney failure (4% vs 1.3%, P<0.001), cardiogenic shock (5.5% vs 2.2%, P<0.001), and death (2.8% vs 1.1%, P=0.045). Compared with the MI-CAD group, fewer patients in the MINOCA group had higher levels (more than nine times the reference limit used by each center) of serum markers of myocardial damage (Figure 2), indicating a more extensive myocardial lesion.
Table 3.
Outcomes
| Complications | MI-CAD (N=9,241) N (%) | MINOCA (N=622) N (%) | P-value |
|---|---|---|---|
| During ICU/CCU admission | |||
| Hemorrhage requiring transfusion or with hemodynamic instability | 55 (0.6) | 1 (0.2) | 0.261 |
| Stroke | |||
| Hemorrhagic | 9 (0.1) | 0 (0.0) | 1 |
| Ischemic | 38 (0.4) | 0 (0.0) | 0.169 |
| Acute kidney failure | 367 (4.0) | 8 (1.3) | 0.001 |
| Reinfarction | 138 (1.5) | 1 (0.2) | 0.003 |
| Post-AMI angina | 212 (2.3) | 4 (0.6) | 0.005 |
| Cardiac tamponade | 47 (0.5) | 1 (0.2) | 0.368 |
| Pulmonary embolism | 4 (0.1) | 3 (0.5) | 0.007 |
| Right heart failure | 72 (0.8) | 1 (0.2) | 0.131 |
| Cardiogenic shock | 509 (5.5) | 14 (2.2) | <0.001 |
| Death | 263 (2.8) | 7 (1.1) | 0.045 |
| At 30 days | |||
| NYHA functional class | |||
| NYHA I | 3,502 (37.9) | 276 (44.4) | 0.044 |
| NYHA II | 1,435 (15.5) | 81 (13.0) | 0.008 |
| NYHA III | 367 (4.0) | 32 (5.1) | 0.323 |
| NYHA IV | 95 (1.0) | 6 (1.0) | 0.724 |
| Need for coronary angiography | 247 (2.7) | 5 (0.8) | 0.001 |
| Need for PCI | 136 (1.5) | 0 (0.0) | <0.001 |
| Readmission | 468 (5.1) | 27 (4.4) | 0.256 |
| New AMI | 64 (0.7) | 0 (0.0) | 0.021 |
| CPR | 11 (0.1) | 0 (0.0) | 1 |
| Death | 69 (0.8) | 3 (0.5) | 0.632 |
Abbreviations: AMI, acute myocardial infarction; CCU, coronary care unit; CPR, cardiopulmonary resuscitation; ICU, intensive care unit; MI-CAD, myocardial infarction with coronary artery disease; MINOCA, myocardial infarction with nonobstructive coronary arteries; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Figure 2.
Plasma levels of markers of myocardial damage.
Notes: The proportion of patients with different peaks of the level of markers of myocardial injury is shown. n denotes the limit of the laboratory normal range.
Abbreviations: MI-CAD, myocardial infarction with coronary artery disease; MINOCA, myocardial infarction with nonobstructive coronary arteries.
At 30 days of follow-up, more complications related to cardiovascular problems were recorded in the MI-CAD group. The NYHA functional class was better in the MIN-OCA group.
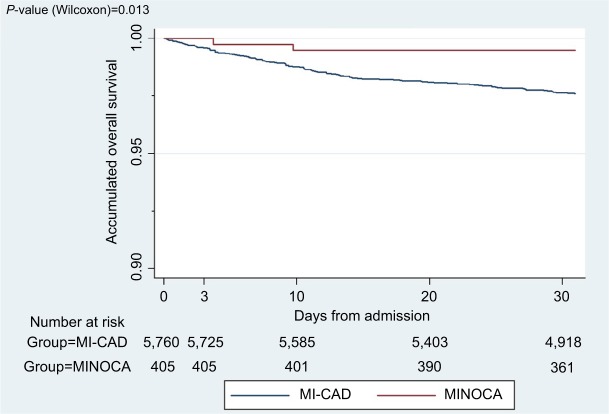
Survival rate at 30 days was higher for the MI-CAD group, without statistical significance (P=0.632). The P-value of Wilcoxon test for the survival curves was 0.013 (Figure 3).
Figure 3.
Mortality at 30 days of follow-up.
Note: Patients with follow-up completed at 30 days are included in the figure.
Abbreviations: MI-CAD, myocardial infarction with coronary artery disease; MINOCA, myocardial infarction with nonobstructive coronary arteries.
Creation of the MINOCA predictive score
A binomial logistic regression model was created using the stepwise method so that only significant variables were included in the final models. The dependent variable was the MINOCA/MI-CAD group; the independent variables were all those that were statistically significant in the bivariate analysis.
MINOCA was predicted by calculating the OR with its 95% CI and P-value. The results are presented in Table 4, showing that the variables were used to construct the nomogram.
Table 4.
Statistically significant variables
| Variables | OR | P-value | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender, female | 3.52 | <0.001 | 2.58 | 4.81 |
| Age >50 years | 0.37 | <0.001 | 0.25 | 0.53 |
| Troponin >9-fold | 0.53 | <0.001 | 0.38 | 0.74 |
| Smoker | 0.62 | <0.001 | 0.51 | 0.75 |
| Hypertension | 0.63 | 0.004 | 0.46 | 0.86 |
| Diabetes mellitus | 0.59 | 0.012 | 0.4 | 0.89 |
| Previous infarction | 0.4 | 0.009 | 0.2 | 0.79 |
| P-value (Hosmer–Lemeshow) | 0.157 | |||
| C-statistic | 0.756 | |||
We found that the typical MINOCA patient was a nonsmoking woman aged <50 years with cTn <9 times the limit of the normal range and no personal history of arterial hypertension, diabetes mellitus, or AMI. The discriminative power was 0.756, which is close to unity and therefore confirms that the model can detect patients with MINOCA. Furthermore, the P-value for the Hosmer–Lemeshow test was >0.05, thus indicating a good predictive adjustment of the regression model.
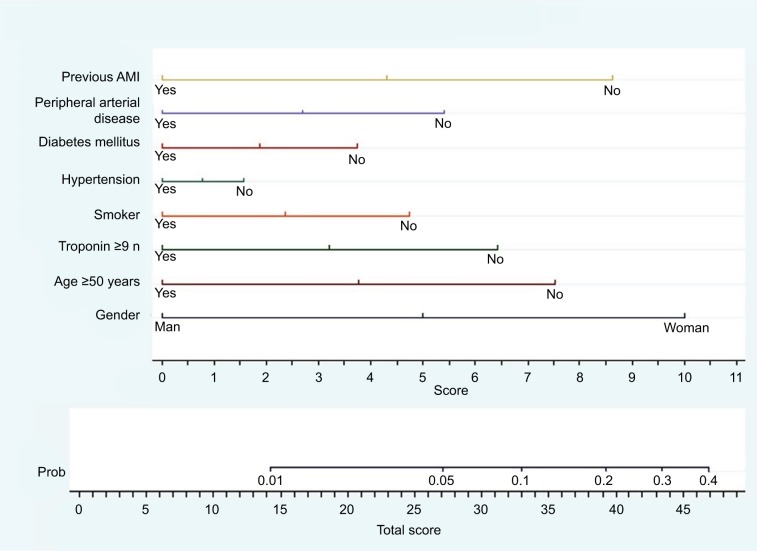
The MINOCA predictive score was created using the previous regression model, where the coefficients calculated in the final model were used to construct the predictive model in the form of a nomogram, that is, a graphical representation of the relative impact of each prognostic factor within the global model (Figure 3).
The predictive capacity of the final model was evaluated using calibration and the discriminative power that enabled the internal validation of the final model. The model was validated by means of the following tasks:
A prognostic index (PI) was created using the following model: IP = α x + α x 1 × 1+... + αkxk, that is, we kept the variable resulting from the model in our database. The sample was divided into risk groups by applying the cutoff points to the PI based on specific percentiles of patients with an event.
The KM curves were constructed for the groups (observed survival). These were compared with those predicted for the model for the mean values of the PI in the groups.
Figure 4 shows the probability that a patient with AMI has MINOCA at admission. The score value was obtained by summing the scores for the variables that comprised the nomogram. The values ranged from 0 to 48. The maximum value corresponded to a 40% probability of MINOCA.
Figure 4.
Nomogram for predicting MINOCA.
Note: Impact of each prognostic factor within the global model. n denotes the limit of the laboratory normal range.
Abbreviations: AMI, acute myocardial infarction; MINOCA, myocardial infarction with nonobstructive coronary arteries; Prob, probability.
Discussion
The ARIAM project has become one of the most important registries of ACS in the world with data from more than 160,000 patients throughout Spain and Andorra.19–22
The data obtained show that patients with MINOCA account for 6% of all cases of AMI diagnosed in Spain. The main characteristics of these patients are as follows: younger age than patients with obstructive coronary disease with no differences in distribution by gender; fewer risk factors for cardiovascular disease or known cardiovascular conditions before admission; less severe disease during admission and, therefore, a lower rate of complications, including death.
Although these characteristics had already been reported elsewhere, they had never been reviewed in Spain. As our findings are consistent with previously published data, they can be generalized to the whole of Spain.4,5,9–11,23–25
As shown in Table 5, the rate of MINOCA was about 6% in most reported series of patients in agreement with our results. The high incidence of coronary artery disease throughout the world means that the number of patients with MINOCA is also high. If we take the available data from the hospital morbidity survey of the Spanish National Statistics Institute (ie, 54,090 discharges with a diagnosis of AMI), and given a prevalence of around 6% of patients with AMI, then approximately 3,400 patients had MINOCA in Spain in 2014.26
Table 5.
Prevalence of MINOCA in most relevant studies
| Authors of the studies / year of publication | Proportion (95% CI) |
|---|---|
| Larsen et al32 / 2013 | 0.04 (0.03 – 0.04) |
| Collste et al31 / 2013 | 0.06 (0.06 – 0.07) |
| Sun et al33 / 2012 | 0.02 (0.00 – 0.03) |
| Rhew et al34 / 2012 | 0.08 (0.07 – 0.10) |
| Hamdan et al35 / 2012 | 0.09 (0.04 – 0.14) |
| Aldrovandi et al36 / 2012 | 0.04 (0.03 – 0.04) |
| Agewall et al9 / 2012 | 0.07 (0.03 – 0.11) |
| Tritto et al37 / 2011 | 0.05 (0.04 – 0.06) |
| Leurent et al38 / 2011 | 0.13 (0.11 – 0.16) |
| Kang et al39 / 2011 | 0.04 (0.04 – 0.12) |
| Uchida et al40 / 2010 | 0.08 (0.04 – 0.12) |
| Frycz-Kurek et al41 / 2010 | 0.03 (0.03 – 0.03) |
| Gehrie et al42 / 2009 | 0.10 (0.07 – 0.13) |
| Baccouche et al43 / 2009 | 0.14 (0.12 – 0.16) |
| Ong et al44 / 2008 | 0.10 (0.07 – 0.13) |
| Ahmar et al45 / 2008 | 0.06 (0.05 – 0.07) |
| Larson et al46 / 2007 | 0.04 (0.03 – 0.05) |
| Widimsky et al47 / 2006 | 0.03 (0.02 – 0.04) |
| Strunk et al48 / 2006 | 0.08 (0.05 – 0.10) |
| Patel et al11 / 2006 | 0.09 (0.08 – 0.09) |
| Larsen et al49 / 2005 | 0.07 (0.07 – 0.08) |
| Germing et al50 / 2005 | 0.06 (0.04 – 0.08) |
| Hung et al51 / 2003 | 0.10 (0.06 – 0.14) |
| Gehani et al52 / 2001 | 0.05 (0.04 – 0.06) |
| Hochman et al53 / 1999 | 0.07 (0.06 – 0.07) |
| Zimmerman et al54 / 1995 | 0.04 (0.04 – 0.05) |
| Sharifi et al55 / 1995 | 0.01 (0.00 – 0.02 |
| Overall | 0.06 (0.05 – 0.07) |
| Ballesteros et al | 0.06 (0.06 – 0.07) |
Notes: Prevalence of MINOCA in various studies (overall 6%, in red text; systematic review from Pasupathy et al4) compared with the present study (blue text), showing 6.3% of patients with MINOCA diagnosis.
Abbreviations: MINOCA, myocardial infarction with nonobstructive coronary arteries.
Although no registry data are available, medication is usually withdrawn from MINOCA patients before the end of the workup, thus underestimating the importance of the process without reaching a definitive diagnosis, as these patients are not thought to be at risk of death. This entails consequences for the patients since their prognosis is not that of the general population.27 Unlike this assumption for patients with MINOCA, morbidity and mortality are increased in the short, medium, and long terms, with the onset of new episodes of acute ischemic heart disease and death. In the ACUITY study, the adjusted risk of death at 1 year was 5.2%, which the researchers attributed to the poorer prognosis of these patients and the possible lack of assessment and treatment of the origin of the increase in AMI after coronary angiography.28 Therefore, some of the patients did not receive appropriate treatment with the possibility that the disease could progress or the event could recur.
Patients with MINOCA presented an event that led to myocardial ischemia with necrosis of myocardium. The present tool enables clinicians to suspect the presence of nonobstructive disease and act earlier to schedule new diagnostic tests and to prescribe appropriate therapy.
In 2000, Roe et al24 published the results of a study where they attempted to differentiate between patients with ACS without ST segment elevation who had obstructive disease and those who did not. The authors aimed to be able to distinguish between patients on administering treatment with anti-IIb/IIIa antiplatelet therapy. Their results show that patients with non-obstructive disease share characteristics with the MINOCA patients in our study. The authors designed a predictive score based on the patients’ baseline characteristics with a C-statistic value of 0.827, indicating that the model can reliably predict the presence of coronary artery disease without significant obstruction. Furthermore, when the predictive model was applied to the patients in the GUSTO-IIb trial, the C-statistic was 0.796, indicating excellent discrimination of the model when applied to a different population.29 In contrast with this algorithm, our score is aimed at patients with a diagnosis of AMI, and it is also easier to perform, since it is done with values that are easily obtained at the patient’s admision.
MINOCA is currently diagnosed based on coronary angiography to rule out coronary obstruction. In the case of patients who fulfill the diagnostic criteria for MINOCA, every attempt should be made to determine the etiology to manage the condition appropriately, since the origin may not even be cardiac, thus necessitating specific assessment and therapy.29 In this way, the application of a score elaborated with immediate clinical data could allow clinicians to choose patients for the accomplishment of minimally invasive diagnostic tests, which allow them to exclude coronary disease.30 Recent reports have demonstrated that computed tomography (CT) coronary angiography can target planned interventions in patients with angina, including coronary angiography, improving outcome.56,57 It is needed to carry out a study aimed to show a reduction in coronary angiography of patients with suspected MINOCA through less invasive procedure such as CT coronary angiography or other imaging tests.
Limitations of the study
The main limitation of our study is the lack of alternative diagnosis for MINOCA patients. Due to the short-term follow-up of the ARIAM registry, additional studies and definitive diagnosis are not known for these patients.
Another limitation of our score is the relatively low predictive value (40% probability of MINOCA with the highest score), although other widely used scores show similar values. The TIMI risk score for unstable angina and non-ST segment elevation showed a probability of 40.9% for all-cause mortality, MI, and severe recurrent ischemia prompting urgent revascularization through 14 days for the maximum score value.58 The risk prediction tool from the GRACE study showed C-statistics of 0.81 for predicting death and 0.73 for death or MI from admission to 6 months after discharge, which is similar to our results.59
Conclusion
In our registry, patients with MINOCA present characteristics which differentiate them early from those with MI-CAD. The diagnosis of MINOCA does not suppose the exclusion of an unfavorable outcome or future complications.
The score we present can accurately predict the diagnosis of MINOCA, encouraging clinicians to perform alternative tests for the correct diagnosis of these patients and allowing a correct treatment. This score is determined easily and quickly, not requiring tests in addition to those performed in the current ACS protocols. Further trials to establish definitive diagnosis for these patients are needed.
Supplementary Materials
Acknowledgments
The present study was funded in part by a grant from Fundación Universidad Alfonso X El Sabio–Santander (FUAX). The ARIAM-SEMICYUC registry is fully funded by SEMI-CYUC. The authors would also like to acknowledge the ARIAM-SEMICYUC Group.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe: epidemiological update. Eur Heart J. 2013;34(39):3028–3034. doi: 10.1093/eurheartj/eht356. [DOI] [PubMed] [Google Scholar]
- 3.Ibanez B, James S, Agewall S. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2017;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 4.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131(10):861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 5.Pizzi C, Xhyheri B, Costa GM, et al. Nonobstructive Versus Obstructive Coronary Artery Disease in Acute Coronary Syndrome: A Meta- Analysis. J Am Heart Assoc. 2016;5(12):e004185. doi: 10.1161/JAHA.116.004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36(8):475–481. doi: 10.1093/eurheartj/ehu469. [DOI] [PubMed] [Google Scholar]
- 7.Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302(8):874–882. doi: 10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw LJ, Shaw RE, Merz CN. American College of Cardiology-National Cardiovascular Data Registry Investigators. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117(14):1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 9.Agewall S, Daniel M, Eurenius L, et al. Risk factors for myocardial infarction with normal coronary arteries and myocarditis compared with myocardial infarction with coronary artery stenosis. Angiology. 2012;63(7):500–503. doi: 10.1177/0003319711429560. [DOI] [PubMed] [Google Scholar]
- 10.Ohlow MA, Wong V, Brunelli M, et al. Acute coronary syndrome without critical epicardial coronary disease: prevalence, characteristics, and outcome. Am J Emerg Med. 2015;33(2):150–154. doi: 10.1016/j.ajem.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Chen AY, Peterson ED, et al. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152(4):641–647. doi: 10.1016/j.ahj.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 12.Spanish Society of Intensive Medicine and Coronary Units Sociedad Española de Medicina Intensiva y Unidades Coronarias (SEMICYUC) [Accessed on June 14, 2018]. [homepage on the Internet]. Available from: http://www.semicyuc.org/temas/investigacion/registros/ariam.
- 13.Agewall S, Beltrame JF, Reynolds H, et al. On behalf of the WG on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38(3):143–153. doi: 10.1093/eurheartj/ehw149. [DOI] [PubMed] [Google Scholar]
- 14.Flynn MR, Barrett C, Cosío FG, et al. The Cardiology Audit and Registration Data Standards (CARDS), European data standards for clinical cardiology practice. Eur Heart J. 2005;26(3):308–313. doi: 10.1093/eurheartj/ehi079. [DOI] [PubMed] [Google Scholar]
- 15.Analysis of the Delay in Acute Myocardial Infarction Análisis del Retraso en el infarto Agudo de Miocardio (ARIAM) [Accessed on May 02, 2016]. [homepage on the Internet]. Available from: https://ariam.investigacion-intensivos.org/login.php.
- 16.Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20(4):457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 17.Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: a multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J. 2001;141(2):190–9. doi: 10.1067/mhj.2001.112404. [DOI] [PubMed] [Google Scholar]
- 18.Morrow DA, Antman EM, Snapinn SM, Mccabe CH, Theroux P, Braunwald E. An integrated clinical approach to predicting the benefit of tirofiban in non-ST elevation acute coronary syndromes. Application of the TIMI Risk Score for UA/NSTEMI in PRISM-PLUS. Eur Heart J. 2002;23(3):223–229. doi: 10.1053/euhj.2001.2738. [DOI] [PubMed] [Google Scholar]
- 19.Huynh T, Cox JL, Massel D, et al. Predictors of intracranial hemorrhage with fibrinolytic therapy in unselected community patients: a report from the FASTRAK II project. Am Heart J. 2004;148(1):86–91. doi: 10.1016/j.ahj.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb S. Mortality trends in men and women with acute myocardial infarction in coronary care units in Israel. A comparison between 1981–1983 and 1992–1994. Eur Heart J. 2000;21(4):284–295. doi: 10.1053/euhj.1999.1868. [DOI] [PubMed] [Google Scholar]
- 21.Rustige J, Schiele R, Burczyk U, et al. The 60 minutes myocardial infarction project. Treatment and clinical outcome of patients with acute myocardial infarction in Germany. Eur Heart J. 1997;18(9):1438–1446. doi: 10.1093/oxfordjournals.eurheartj.a015470. [DOI] [PubMed] [Google Scholar]
- 22.Tunstall-Pedoe H, Kuulasmaa K, Mähönen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA Project populations. Lancet. 1999;353(9164):1547–1557. doi: 10.1016/s0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- 23.Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998;339:436–443. [Google Scholar]
- 24.Roe MT, Harrington RA, Prosper DM, et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation. 2000;102(10):1101–1106. doi: 10.1161/01.cir.102.10.1101. [DOI] [PubMed] [Google Scholar]
- 25.Ferrer Gracia MC, Hernández-Antolín RA, Pérez-Vizcayno MJ, Conde Vela C, Alfonso Manterola F, Macaya Miguel C. Myocardial infarction with ST segment elevation and angiographically normal coronary arteries: epidemiology and mid-term follow-up. Med Clin. 2007;129(18):694–696. doi: 10.1157/13112511. [DOI] [PubMed] [Google Scholar]
- 26.National Institute of Statistics (Spain) Survey of hospital morbidity 2015. National results. Hospital admissions and according to main diagnosis. [Instituto Nacional de Estadística (INE). Encuesta de morbilidad hospitalaria 2015. Resultados nacionales. Altas hospitalarias y según diagnóstico principal] [Accessed on May 02, 2016]. [homepage on the Internet]. Available from: http://www.ine.es/jaxi/Datos.htm?path=/
- 27.Nordenskjöld AM, Baron T, Eggers KM, Jernberg T, Lindahl B. Predictors of adverse outcome in patients with myocardial infarction with non-obstructive coronary artery (MINOCA) disease. Int J Cardiol. 2018;261:18–23. doi: 10.1016/j.ijcard.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 28.Stone GW, Bertrand M, Colombo A, et al. Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale. Am Heart J. 2004;148(5):764–775. doi: 10.1016/j.ahj.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 29.Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb Investigators A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. N Engl J Med. 1996;335(11):775–82. doi: 10.1056/NEJM199609123351103. [DOI] [PubMed] [Google Scholar]
- 30.Pasupathy S, Tavella R, Beltrame JF. The What, When, Who, Why, How and Where of Myocardial Infarction With Non-Obstructive Coronary Arteries (MINOCA) Circ J. 2016;80(1):11–16. doi: 10.1253/circj.CJ-15-1096. [DOI] [PubMed] [Google Scholar]
- 31.Collste O, Sörensson P, Frick M, et al. Myocardial infarction with normal coronary arteries is common and associated with normal findings on cardiovascular magnetic resonance imaging: results from the Stockholm Myocardial Infarction with Normal Coronaries study. J Intern Med. 2013;273(2):189–196. doi: 10.1111/j.1365-2796.2012.02567.x. [DOI] [PubMed] [Google Scholar]
- 32.Larsen AI, Nilsen DW, Yu J, et al. Long-term prognosis of patients presenting with ST-segment elevation myocardial infarction with no significant coronary artery disease from the HORIZONS-AMI trial. Am J Cardiol. 2013 Mar 1;111(5):643–648. doi: 10.1016/j.amjcard.2012.11.011. Epub 2012 Dec 19. [DOI] [PubMed] [Google Scholar]
- 33.Sun WX, Yan JH, Zhao XY, Ruan YP, Fan ZJ. Risk factors and coronary angiography characteristics of female patients with acute coronary syndrome. Zhonghua Xin Xue Guan Bing Za Zhi. 2012 Nov;40(11):897–901. [PubMed] [Google Scholar]
- 34.Rhew SH, Ahn Y, Kim MC, et al. Is Myocardial Infarction in Patients without Significant Stenosis on a Coronary Angiogram as Benign as Believed? Chonnam Med J. 2012 Apr;48(1):39–46. doi: 10.4068/cmj.2012.48.1.39. Epub 2012 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamdan R, Frangieh A, Kadri Z, et al. What do we know about myocardial infarction with normal coronary arteries? Gazzetta Medica Italiana Archivio per le Scienze Mediche. 2012;171:7–12. [Google Scholar]
- 36.Aldrovandi A, Cademartiri F, Arduini D, et al. Computed tomography coronary angiography in patients with acute myocardial infarction without significant coronary stenosis. Circulation. 2012 Dec 18;126(25):3000–7. doi: 10.1161/CIRCULATIONAHA.112.117598. Epub 2012 Nov 20. [DOI] [PubMed] [Google Scholar]
- 37.Tritto I, Conti MG, Ambrosio G. Ischemia with normal coronary arteries: a puzzle and an opportunity. J Cardiovasc Med (Hagerstown) 2011 May;12(5):309–310. doi: 10.2459/JCM.0b013e32834588fe. [DOI] [PubMed] [Google Scholar]
- 38.Leurent G, Langella B, Fougerou C, et al. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch Cardiovasc Dis. 2011 Mar;104(3):161–70. doi: 10.1016/j.acvd.2011.01.005. Epub 2011 Apr 2. [DOI] [PubMed] [Google Scholar]
- 39.Kang WY, Jeong MH, Ahn YK, et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol. 2011 Jan 21;146(2):207–12. doi: 10.1016/j.ijcard.2009.07.001. Epub 2009 Aug 7. [DOI] [PubMed] [Google Scholar]
- 40.Uchida Y, Uchida Y, Sakurai T, et al. Fluffy luminal surface of the non-stenotic culprit coronary artery in patients with acute coronary syndrome: An angioscopic study. Circ J. 2010;74:2379–2385. doi: 10.1253/circj.cj-10-0422. [DOI] [PubMed] [Google Scholar]
- 41.Frycz-Kurek AM, Gierlotka M, Gąsior M, et al. Patients with no significant lesions in coronary arteries and ST-segment elevation myocardial infarction have worse outcome than patients with non-ST-segment elevation myocardial infarction: analysis from PL-ACS Registry. Kardiol Pol. 2010 Nov;68(11):1211–1217. [PubMed] [Google Scholar]
- 42.Gehrie ER, Reynolds HR, Chen AY, et al. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J. 2009 Oct;158(4):688–94. doi: 10.1016/j.ahj.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Baccouche H, Mahrholdt H, Meinhardt G, et al. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J. 2009 Dec;30(23):2869–2879. doi: 10.1093/eurheartj/ehp328. Epub 2009 Aug 20. [DOI] [PubMed] [Google Scholar]
- 44.Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008 Aug 12;52(7):523–7. doi: 10.1016/j.jacc.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 45.Ahmar W, Lefkovits J. Acute ST elevation myocardial infarction with angiographically normal coronary arteries: causes and outcomes. Int J Cardiol. 2008 Aug 1;128(1):131–3. doi: 10.1016/j.ijcard.2007.05.053. Epub 2007 Aug 8. [DOI] [PubMed] [Google Scholar]
- 46.Larson DM, Menssen KM, Sharkey SW, et al. “False-positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA. 2007 Dec 19;298(23):2754–60. doi: 10.1001/jama.298.23.2754. [DOI] [PubMed] [Google Scholar]
- 47.Widimsky P, Stellova B, Groch L, et al. PRAGUE Study Group Investigators Prevalence of normal coronary angiography in the acute phase of suspected ST-elevation myocardial infarction: experience from the PRAGUE studies. Can J Cardiol. 2006 Nov;22(13):1147–1152. doi: 10.1016/s0828-282x(06)70952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strunk B, Shaw RE, Bull S, et al. High incidence of focal left ventricular wall motion abnormalities and normal coronary arteries in patients with myocardial infarctions presenting to a community hospital. J Invasive Cardiol. 2006 Aug;18(8):376–381. [PubMed] [Google Scholar]
- 49.Larsen AI, Galbraith PD, Ghali WA, et al. APPROACH Investigators Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol. 2005 Jan 15;95(2):261–3. doi: 10.1016/j.amjcard.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Germing A, Lindstaedt M, Ulrich S, et al. Normal angiogram in acute coronary syndrome-preangiographic risk stratification, angiographic findings and follow-up. Int J Cardiol. 2005 Mar 10;99(1):19–23. doi: 10.1016/j.ijcard.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Hung MJ, Cherng WJ. Comparison of white blood cell counts in acute myocardial infarction patients with significant versus insignificant coronary artery disease. Am J Cardiol. 2003 Jun 1;91(11):1339–42. doi: 10.1016/s0002-9149(03)00325-4. [DOI] [PubMed] [Google Scholar]
- 52.Gehani AA, al-Mulla AW, Chaikhouni A, et al. Myocardial infarction with normal coronary angiography compared with severe coronary artery disease without myocardial infarction: the crucial role of smoking. J Cardiovasc Risk. 2001 Feb;8(1):1–8. doi: 10.1177/174182670100800101. [DOI] [PubMed] [Google Scholar]
- 53.Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med. 1999 Jul 22;341(4):226–32. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 54.Zimmerman FH, Cameron A, Fisher LD, Ng G. Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (Coronary Artery Surgery Study Registry) J Am Coll Cardiol. 1995 Sep;26(3):654–61. doi: 10.1016/0735-1097(95)00254-2. [DOI] [PubMed] [Google Scholar]
- 55.Sharifi M, Frohlich TG, Silverman IM. Myocardial infarction with angiographically normal coronary arteries. Chest. 1995 Jan;107(1):36–40. doi: 10.1378/chest.107.1.36. [DOI] [PubMed] [Google Scholar]
- 56.The SCOT-HEART investigators CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385(9985):2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 57.The SCOT-HEART investigators Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 58.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 59.Kaa F, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333:1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.