Abstract
Introduction
Diabetic nephropathy (DN) represents one of the main causes of end-stage renal disease in type 2 diabetes mellitus (DM) patients. Galectin-3 has been implicated in pathogenesis of many pathological conditions. To date, there are limited data regarding the relationship between galectin-3 and DN.
Aim of the study
Evaluation of serum galectin-3 as a novel prognostic biomarker in patients with DN.
Patients and methods
This prospective study was carried out in the Internal Medicine and Clinical Pathology Departments, Tanta University Hospital, Egypt, from March 2015 to March 2018 on 300 patients with type 2 DM. Patients were divided into three groups: group I included 100 patients with albumin/creatinine ratio (ACR) <30 mg/g (normoalbuminuria), group II included 100 patients with ACR within 30–300 mg/g (microalbuminuria), and group III included 100 patients with ACR >300 mg/g (macroalbuminuria). All patients were subjected to the following: full history taking, clinical examination, and laboratory evaluation (HbA1c, creatinine, estimated glomerular filtration rate, ACR, and serum galectin-3).
Results
The mean levels of galectin-3 were significantly higher in patients with macroalbuminuria than in those with microalbuminuria and normoalbuminuria. Galectin-3 was a significant predictor for progression to microalbuminuria, macroalbuminuria, dialysis, and death among patients with type 2 DM.
Conclusion
Based on this single center prospective study, serum galectin-3 is considered a significant predictor for DN progression among patients with type 2 DM.
Keywords: diabetic nephropathy, galectin-3, albumin/creatinine ratio, microalbuminuria, macroalbuminuria, dialysis, diabetes mellitus
Introduction
Type 2 diabetes mellitus (DM) is a common health problem; its incidence has been increased worldwide.1 Diabetic nephropathy (DN) is one of the microvascular complications, which represents the main cause of end-stage renal disease (ESRD) in patients with DM.2 Early identification and treatment of this chronic complication may reduce the rates of morbidity and mortality.3 The American Diabetic Association, 2019 guidelines for screening of chronic kidney disease (CKD) in diabetic patients recommends the following: at least once a year, assess urinary albumin (eg, spot urinary albumin/creatinine ratio) and estimated glomerular filtration rate (eGFR) in patients with type 1 DM with duration ≥5 years, in all patients with type 2 DM, and comorbid hypertension.4
Although microalbuminuria serves as an early indicator of glomerular disease and can predict progression to overt nephropathy and ESRD in diabetic patients, it has some limitations. Urinary albumin excretion may remain within the normal range and clinically DN remains undetectable at this stage. Also, not all patients with microalbuminuria will progress to nephropathy.5–8 KDIGO Conference 2012 addressed a number of areas in the management of patients with DM and chronic kidney disease (CKD), including aspects of screening for CKD with measurements of urinary albumin/creatinine ratio (ACR) and eGFR. ACR is considered more sensitive and reliable biomarker to identify the patients who are at risk for progression to DN.9
Galectin-3 is a 32- to 35-kD glycoprotein that is a member of the multifunctional lectin family. It is found in both intracellular and extracellular space. Extracellular galectin-3 interacts with the β-galactoside residues of extracellular matrix and cell surface glycoproteins via its C-terminal carbohydrate-recognition domain. In addition, the intracellular galectin-3 interacts via peptide–peptide associations mediated by its N-terminus domain. These structural and localization properties enable galectin-3 to achieve multiple functions; it is involved in cell adhesion, growth, differentiation, apoptosis, and angiogenesis.10–14 Additionally, galectin-3 has been recently considered an important player in the development and progression of many pathological conditions, including inflammatory response, immunity, tissue fibrosis, and tumorigenesis.15–17
The role of galectin-3 in renal disease is complex and still unclear. Increased galectin-3 expression has been associated with renal fibrosis.18 However, galectin-3 may have a beneficial effect in some experimental studies as being a clearance receptor for the advanced glycation end products (AGEs).19,20
To date, there are limited published data regarding the relationship between serum galectin-3 levels and initiation and progression of DN. The purpose of this study is to evaluate serum galectin-3 as a novel prognostic biomarker in patients with DN.
Patients and methods
This prospective study was carried out from the period of March 2015 to March 2018. Three hundred diabetic patients were enrolled in this study; they were invited to participate in our study when they came to the endocrinology clinic, Internal Medicine Department, Tanta University Hospital, Egypt, for regular follow-up. The study was approved by the Hospital Ethical Committee according to the Principles of the Declaration of Helsinki; a written informed consent of the patient was obtained.
The studied patients were divided into three groups according to KDIGO 2012: group I included 100 patients with type 2 DM with albumin/creatinine ratio (ACR) <30 mg/g (normoalbuminuria), group II included 100 patients with type 2 DM with ACR within 30–300 mg/g (microalbuminuria), and group III included 100 patients with type 2 DM with ACR >300 mg/g (macroalbuminuria).
Exclusion criteria were as follows: patients presented with type 1 DM, eGFR <30 mL/min, on renal replacement therapy, pregnancy, obesity, smoking, any acute/chronic inflammatory conditions, malignancies, and autoimmune conditions.
All patients were subjected to the following: full history taking, full clinical examination, including (cardiovascular, neurological, and fundus examination), routine laboratory evaluation, including HbA1c, ACR, creatinine, and eGFR and serum galectin-3 measurement. All patients were followed up for a period of 3 years with regular visit at 3-month interval, till the progression to microalbuminuria, macroalbuminuria, ESRD, or death of the patients, whichever occurred first.
DN was diagnosed in patients with DM by ACR, serum creatinine, and eGFR after exclusion of other causes of proteinuria.
Blood and urine collection and laboratory assay
Specimens
Laboratory assays were performed at Clinical Chemistry Unit, Clinical Pathology Department, Tanta University Hospital, Egypt. Whole blood was collected by standard venipuncture in VACUETTE® blood collection tubes (Greiner Bio-One, Kremsmünster, Austria) containing K2EDTA and containing clot activator/sep. Serum samples were left to clot for 5–10 minutes at room temperature and were centrifuged at 1,000× g for 10 minutes. Serum samples were used for routine laboratory investigations. Whole EDTA blood samples were used for HbA1c. EDTA plasma samples were separated by centrifugation at 1,000× g for 10 minutes and were stored at –20°C till the time of galectin-3 assay. Serum creatinine was performed on fully automated chemistry analyzer (Konelab Prime 60i, Konelab, Helsinki, Finland) and eGFR was calculated using Cockcroft–Gault formula.21 HbA1c was performed on (Tosoh Bioscience, Tokyo, Japan). Urinary albumin excretion was evaluated by albumin/creatinine ratio (ACR) (mg/g). Galectin-3 levels were measured by Human Galectin-3 Platinum ELISA kit (ready-to-use Sandwich ELISA) (Catalog No: BMS279/2/BMS279/2TEN, Ebioscience, Vienna, Austria).
ACR measurement
Urinary albumin was measured by immunoturbidimetric method using Microalbuminria kit (REF 31924), Biosystems, Barcelona, Spain. Creatinine in urine was measured by JAFFE method using Creatinine kit (REF 11502), Biosystems.
Galectin-3 assay
Principle of the assay: Human galectin-3 present in the sample or standard bound to antibodies adsorbed to the microwells, a biotin-conjugated anti-human galectin-3 antibody was added and bound to human galectin-3 captured by the first antibody (first incubation). Following this step, unbound biotin-conjugated anti-human galectin-3 antibody was removed by washing. Streptavidin-horseradish peroxidase (HRP) was added and bound to the biotin-conjugated anti-human galectin-3 antibody (second incubation). Following this step, unbound Streptavidin-HRP was removed by washing and substrate solution reactive with HRP was added to the wells (third incubation). A colored product was formed in proportion to the amount of human galectin-3 present in the sample or standard. The reaction was terminated by the addition of acid and absorbance was measured at 450 nm. A standard curve was prepared and human galectin-3 concentrations were determined.
Statistical analysis
The collected data were organized, tabulated, and statistically analyzed using SPSS software (version 19, SPSS Inc. Chicago, IL, USA). For quantitative data, the range, mean, and SD were calculated. For qualitative data, which describe a categorical set of data by frequency, percentage of each category, comparison between two groups and more was done using chi-squared test (χ2). For comparison between more than two means of parametric data, F value of ANOVA test was calculated. For comparison between more than two means of non-parametric data, Kruskal–Wallis (χ2 value) was calculated. Correlation between variables was evaluated using Pearson’s correlation coefficient (r).
Cox regression analysis was done, where logistic regression coefficients (B) are calculated and used to estimate ORs (EX [B]) for galectin-3 as an independent predictor for prognosis to microalbuminuria, macroalbuminuria, dialysis, and death among patients with type 2 DM.
The receiver operating characteristic (ROC) curve is used to detect the sensitivity and specificity of ACR and galectin-3 as prognostic biomarkers in the studied patients. The cumulative probability of death over 36 months of follow-up among the studied patients was estimated by Kaplan–Meier curve.
Significance was adopted at P<0.05 for interpretation of results of tests of significance.22
Results
Regarding baseline characteristics of the studied patients with type 2 DM, there was no significant difference between the three groups with respect to age, gender, body mass index (BMI), and family history of DM (P>0.05). Patients with macroalbuminuria had significantly higher mean DM duration (years) and mean SBP and DBP (mmHg) than the other groups of patients (P<0.05) as shown in Table 1. Regarding the complications of type 2 DM, there was no significant difference between the three groups with respect to neuropathy (P>0.05), while there was a significant difference between the three groups regarding retinopathy, coronary artery disease and peripheral artery disease (P<0.05) as shown in Table 1.
Table 1.
Baseline characteristics of the studied patients with type 2 DM (n=300)
| Variables | Group I Normoalbuminuria (n=100) |
Group II Microalbuminuria (n=100) |
Group III Macroalbuminuria (n=100) |
P-value |
|---|---|---|---|---|
|
| ||||
| Age (years) | ||||
| Range | 42–69 | 43–68 | 46–66 | 0.135 |
| Mean ± SD | 54.93±7.13 | 53.93±5.63 | 55.57±4.36 | |
| Gender (%) | ||||
| Female | 56 (56%) | 58 (58%) | 52 (52%) | 0.685 |
| Male | 44 (44%) | 42 (42%) | 48 (48%) | |
| BMI (kg/m2) | ||||
| Range | 25.00–29.80 | 25.70–29.80 | 25.30–29.90 | 0.098 |
| Mean ± SD | 28.08±1.42 | 28.07±1.33 | 28.43±1.30 | |
| Family history of DM (%) | ||||
| Negative | 58 (58%) | 66 (66%) | 62 (62%) | 0.507 |
| Positive | 42 (42%) | 34 (34%) | 38 (38%) | |
| DM duration (years) | ||||
| • Range | 6.0–19.0 | 6.0–19.0 | 11.0–19.0 | <0.001* |
| • Mean ± SD | 10.90±4.07 | 9.70±3.18 | 12.84±2.56 | |
| SBP (mmHg) | ||||
| Range | 125–135 | 135–146 | 144–160 | <0.001* |
| Mean ± SD | 129.75±2.75 | 139.48±3.28 | 152.28±5.24 | |
| DBP (mmHg) | ||||
| Range | 65–80 | 70–87 | 80–95 | <0.001* |
| Mean ± SD | 71.9 5±3.64 | 78.09±4.72 | 89.31±4.37 | |
| Neuropathy (%) | ||||
| Negative | 81 (81%) | 78 (78%) | 77 (77%) | 0.772 |
| Positive | 19 (19%) | 22 (22%) | 23 (23%) | |
| Retinopathy (%) | ||||
| Negative | 72 (72%) | 60 (60%) | 49 (49%) | 0.004* |
| Positive | 28 (28%) | 40 (40%) | 51 (51%) | |
| CAD (%) | ||||
| Negative | 88 (88%) | 79 (79%) | 73 (73%) | 0.028* |
| Positive | 12 (12%) | 21 (21%) | 27 (27%) | |
| PAD (%) | ||||
| Negative | 89 (89%) | 80 (80%) | 72 (72%) | 0.010* |
| Positive | 11 (11%) | 20 (20%) | 28 (28%) | |
Note:
Significant (P<0.05).
Abbreviations: BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; PAD, peripheral artery disease.
As expected, patients with macroalbuminuria had significantly higher mean ACR and creatinine levels than the other groups of patients (P<0.05) and significantly lower mean eGFR levels than the other groups of patients (P<0.05). Interestingly, the mean levels of galectin-3 were significantly higher in patients with macroalbuminuria than patients with microalbuminuria, and in patients with microalbuminuria than patients with normoalbuminuria (P<0.05) as shown in Table 2.
Table 2.
Laboratory data of the studied patients with type 2 diabetes mellitus (n=300)
| Variables | Group I Normoalbuminuria (n=100) |
Group II Microalbuminuria (n=100) |
Group III Macroalbuminuria (n=100) |
P-value |
|---|---|---|---|---|
|
| ||||
| HbA1c (%) | ||||
| Range | 6.70–7.90 | 6.70–7.80 | 6.70–7.90 | 0.056 |
| Mean ± SD | 7.22±0.32 | 7.28±0.32 | 7.34±0.36 | |
| Creatinine (mg/dL) | ||||
| Range | 0.62–0.92 | 0.66–1.21 | 1.09–1.89 | <0.001* |
| Mean ± SD | 0.75±0.08 | 0.80±0.13 | 1.57±0.19 | |
| eGFR (mL/min) | ||||
| Range | 67–95 | 61–82 | 40–66 | <0.001* |
| Mean ± SD | 79.49±7.93 | 68.34±5.58 | 51.08±5.93 | |
| ACR (mg/g) | ||||
| Range | 7.00–16.40 | 55.30–212.30 | 498.00–1623 | <0.001* |
| Mean ± SD | 10.75±2.30 | 114.74±41.23 | 908.24±283.37 | |
| Galectin-3 (ng/mL) | ||||
| Range | 5.00–15.40 | 9.90–21.30 | 14.80–25.50 | <0.001* |
| Mean ± SD | 9.02±3.55 | 14.48±3.78 | 19.15±2.85 | |
Note:
Significant (P<0.05).
Abbreviations: ACR, albumin/creatinine ratio; eGFR, estimated glomerular filtration rate.
Galectin-3 levels were significantly correlated with the age of the patients, BMI, the duration of DM, SBP, DBP, HbA1c, creatinine, eGFR, and ACR levels (P<0.05) as shown in Table 3.
Table 3.
Correlation between galectin-3 levels and levels of other parameters among the studied patients with type 2 diabetes mellitus (n=300)
| Variables | Galectin-3 (ng/mL) among the studied type 2 diabetes mellitus patients (n=300) | |
|---|---|---|
|
| ||
| R | P | |
|
| ||
| Age (years) | 0.398 | 0.0001* |
| Duration of diabetes mellitus (years) | 0.607 | 0.0001* |
| BMI (kg/m2) | 0.332 | 0.0001* |
| SBP (mmHg) | 0.765 | 0.0001* |
| DBP (mmHg) | 0.688 | 0.0001* |
| HbA1c (%) | 0.200 | 0.0001* |
| Creatinine (mg/dL) | 0.651 | 0.0001* |
| eGFR (mL/min) | –0.816 | 0.0001* |
| ACR (mg/g) | 0.664 | 0.0001* |
Note:
Significant (P<0.05).
Abbreviations: ACR, albumin/creatinine ratio; BMI, body mass index; eGFR, estimated glomerular filtration rate; P, significance level; R, correlation coefficient.
By Cox regression analysis, galectin-3 was a significant predictor for progression to microalbuminuria, macroalbuminuria, dialysis, and death among patients with type 2 DM (P<0.05) as shown in Table 4.
Table 4.
Cox regression analysis of the galectin-3 as a predictor for progression to microalbuminuria, macroalbuminuria, dialysis, and death among the studied patients with type 2 diabetes mellitus (n=300)
| Galectin-3 | B | SE | (P-value) | EX (B) | 95% CI
|
|
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
|
| ||||||
| Microalbuminuria | 0.682 | 0.133 | 0.0001* | 1.978 | 1.525 | 2.567 |
| Macroalbuminuria | 0.628 | 0.110 | 0.0001* | 1.874 | 1.512 | 2.324 |
| Dialysis | 0.360 | 0.113 | 0.001* | 1.433 | 1.148 | 1.788 |
| Death | 0.625 | 0.120 | 0.0001* | 1.868 | 1.475 | 2.364 |
Note:
Significant (P<0.05).
Abbreviations: B, logistic regression coefficient; EX (B), estimated odds ratio; P, significance level; SE, standard error of B.
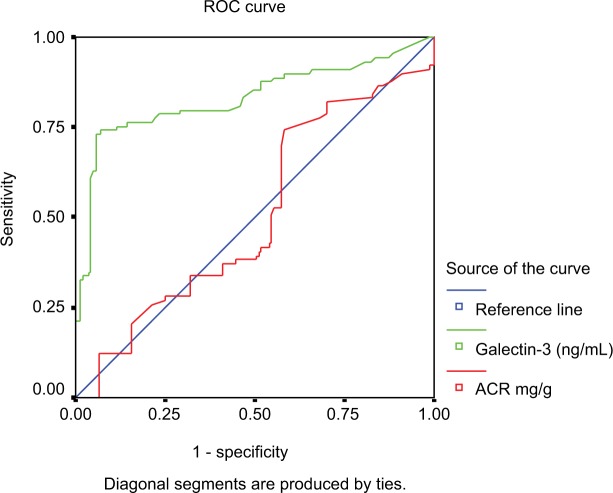
Interestingly, ROC curve illustrated the sensitivity and specificity of ACR and galectin-3 as prognostic biomarkers in the studied patients. The best cut-off level of ACR was 300 (mg/g) with an area under the curve (AUC) of 0.549 yielding sensitivity of 60%, specificity of 83%, positive predictive value (PPV) of 90, negative predictive value (NPV) of 47, and accuracy of 67%. The best cut-off level of galectin-3 was 15 (ng/mL) with an AUC of 0.742 yielding sensitivity of 70%, specificity of 63%, PPV of 45, NPV of 83, and accuracy of 65%. In addition, combined analysis of both biomarkers yielded sensitivity of 74%, specificity of 89%, PPV of 94, NPV of 60, and accuracy of 70% as shown in Figure 1.
Figure 1.
ROC curve of ACR and galectin-3 of the studied patients with type 2 diabetes mellitus.
Abbreviations: ACR, albumin/creatinine ratio; ROC, receiver operating characteristic.
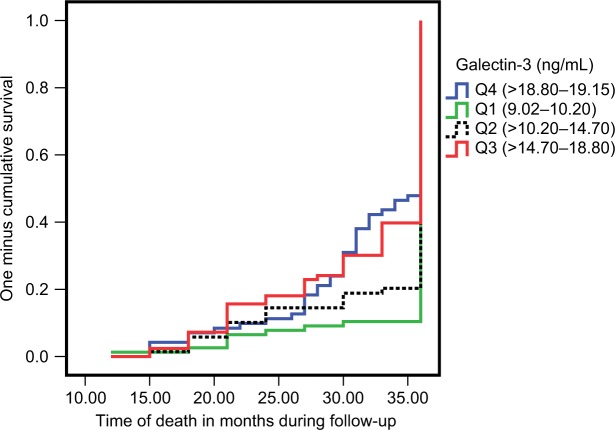
By Kaplan–Meier curve analysis, the cumulative probability of death was higher among patients with higher levels of galectin-3; it was highest among levels more than three quartiles and became lower in the lower quartiles as shown in Figure 2.
Figure 2.
Kaplan–Meier method of the studied patients with type 2 diabetes mellitus according to quartiles (Q) of galectin-3 levels over 36 months of follow-up.
At the end of follow-up period, 9 patients suffered from ESRD, and 15 died.
Discussion
Considerable attention has been directed to the role of galectin-3 in the development and progression of CKD in DM. To date, the role of galectin-3 in the pathogenesis of DN remains controversial and the exact mechanism of elevated serum galectin-3 levels in DN remains unclear.23 The key finding in our study is that the mean levels of galectin-3 were significantly higher in patients with macroalbuminuria than those with microalbuminuria, and in patients with microalbuminuria than those with normoalbuminuria, suggesting that the increase in circulating galectin-3 plays an essential role in development and progression of DN.
Our results were in harmony with that of Tan et al who concluded that galectin-3 is a mediator of initiation and progression of DN in type 2 DM patients. Other evidences, in agreement with our findings, were obtained by Kikuchi et al who revealed that galectin-3 had a role in pathogenesis of DN.24,25 They demonstrated that the number of galectin-3-positive cells, in renal biopsy specimens from individuals with DN, were significantly increased in the glomeruli compared with other glomerular diseases. There was also a strongly significant correlation between the number of galectin-3-positive cells and urinary protein excretion in those patients with DM. On contrary to our results, Vlassara et al found that galectin-3 has the ability to bind AGEs and advanced lipoxidation end products that accumulate in the kidney. With this respect, galectin-3 was involved in the elimination of these toxic compounds and seems to have a protective effect toward the development of DN.26
Results obtained from the present study revealed that galectin-3 was significantly correlated with the age of the patients, BMI, the duration of DM, SBP, DBP, HbA1c, creatinine levels, eGFR, and ACR (P<0.05). Quiet near to our results, Song et al showed that increased serum galectin-3 levels were significantly correlated with fasting plasma glucose (FPG), HbA1c, SBP levels, ACR, increased serum creatinine, and decreased eGFR levels in DN patients.27 Tan et al as well revealed that serum galectin-3 levels were significantly correlated with age, eGFR, urine ACR, and HbA1c, but no correlation was seen with BMI, duration of diabetes, or SBP.24 Far from our results, Yücel et al concluded that no relation was between galectin-3 and the parameters of DM, such as duration of DM, HbA1c, or FPG.28
It is noteworthy that after a period of 3 years follow-up, galectin-3 was a significant predictor for progression to microalbuminuria, macroalbuminuria, dialysis, and death among patients with type 2 DM. In accordance with our results, a large cohort study performed by Tan et al showed that galectin-3 was independently associated with progression of DN (eg, macroalbuminuria), deterioration of renal function (eg, doubling of serum creatinine), as well as initiation of renal replacement therapy after a mean follow-up of 9 years.24 On the other hand, Yücel et al concluded that serum galectin-3 values had no relation to the levels of urinary albumin excretion in patients with DM.28
At cut-off value of 15 ng/mL, the sensitivity and specificity of galectin-3 as a prognostic biomarker in the studied patients were 70% and 63%, respectively. According to Yilmaz et al, galectin-3 is a promising biomarker for detecting diabetes.29 In ROC curve analysis, a galectin-3 cut-off value of 803.55 pg/mL diagnoses DM with a sensitivity and specificity of 80.7% and 85.5%, respectively. Moreover, Chen et al demonstrated that galectin-3 has a predictive value in patients with chronic heart failure.30 At cut-off level of 7.52 ng/mL, the sensitivity and specificity of galectin-3 to predict chronic heart failure were 62.9% and 90%, respectively.
Conclusion
Based on this single center prospective study, serum galectin-3 is considered a significant predictor for DN progression (eg, progression to microalbuminuria, macroalbuminuria, dialysis, and death) among patients with type 2 DM.
We recommend large-scale multicenter studies to assess the clinical application of galectin-3 as an independently novel prognostic biomarker in patients with DN.
Limitations of the study
This study involved a relatively small number of patients, our study period was only 3 years follow-up, thus, we may have missed patients who subsequently have macroalbuminuria, dialysis, or died afterwards.
Acknowledgments
The authors would like to thank the nurses at the Internal Medicine and Clinical Pathology Departments, Tanta University Hospital, for their assistance in conducting the study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Satirapoj B, Adler SG. Prevalence and management of diabetic nephropathy in Western countries. Kidney Dis (Basel) 2015;1(1):61–70. doi: 10.1159/000382028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S124–S138. doi: 10.2337/dc19-S011. [DOI] [PubMed] [Google Scholar]
- 5.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 6.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61–e99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murussi M, Baglio P, Gross JL, Silveiro SP. Risk factors for microalbuminuria and macroalbuminuria in type 2 diabetic patients: a 9-year follow-up study. Diabetes Care. 2002;25(6):1101–1103. doi: 10.2337/diacare.25.6.1101. [DOI] [PubMed] [Google Scholar]
- 8.Jh A, Cho YM, Hg Y, et al. The clinical characteristics of normoalbuminuric renal insufficiency in Korean type 2 diabetic patients: a possible early stage renal complication. J Korean Med Sci. 2009;24:S75–81. doi: 10.3346/jkms.2009.24.S1.S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements. 2013;3(1):1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 10.Haudek KC, Spronk KJ, Voss PG, Patterson RJ, Wang JL, Arnoys EJ. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim Biophys Acta. 2010;1800(2):181–189. doi: 10.1016/j.bbagen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newlaczyl AU, Yu LG. Galectin-3 – a jack-of-all-trades in cancer. Canc Lett. 2011;313(2):123–128. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Nio-Kobayashi J. Tissue- and cell-specific localization of galectins, β-galactose-binding animal lectins, and their potential functions in health and disease. Anat Sci Int. 2017;92(1):25–36. doi: 10.1007/s12565-016-0366-6. [DOI] [PubMed] [Google Scholar]
- 13.Pugliese G, Iacobini C, Pesce CM, Menini S. Galectin-3: an emerging all-out player in metabolic disorders and their complications. Glycobiology. 2015;25(2):136–150. doi: 10.1093/glycob/cwu111. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese G, Iacobini C, Ricci C, Blasetti Fantauzzi C, Menini S. Galectin-3 in diabetic patients. Clin Chem Lab Med. 2014;52(10):1413–1423. doi: 10.1515/cclm-2014-0187. [DOI] [PubMed] [Google Scholar]
- 15.Li LC, Li J, Gao J. Functions of galectin-3 and its role in fibrotic diseases. J Pharmacol Exp Ther. 2014;351(2):336–343. doi: 10.1124/jpet.114.218370. [DOI] [PubMed] [Google Scholar]
- 16.Díaz-Alvarez L, Ortega E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediators Inflamm. 2017;2017(8):1–10. doi: 10.1155/2017/9247574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira FL, Gatto M, Bassi N, et al. Galectin-3 in autoimmunity and autoimmune diseases. Exp Biol Med (Maywood) 2015;240(8):1019–1028. doi: 10.1177/1535370215593826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SC, Kuo PL. The role of galectin-3 in the kidneys. Int J Mol Sci. 2016;17(4):565. doi: 10.3390/ijms17040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmedt V, Desmedt S, Delanghe JR, Speeckaert R, Speeckaert MM. Galectin-3 in renal pathology: more than just an innocent bystander. Am J Nephrol. 2016;43(5):305–317. doi: 10.1159/000446376. [DOI] [PubMed] [Google Scholar]
- 20.Drechsler C, Delgado G, Wanner C, et al. Galectin-3, renal function, and clinical outcomes: results from the luric and 4D studies. J Am Soc Nephrol. 2015;26(9):2213–2221. doi: 10.1681/ASN.2014010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Dawson BD, Trapp RG, editors. Basic & Clinical Biostatistics. 4th edition. New York: Lange Medical Books-McGraw-Hill Medical Publication Division; 2001. Reading the medical literature; pp. 161–218. [Google Scholar]
- 23.O’Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol. 2013;24(9):1470–1477. doi: 10.1681/ASN.2012090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan KCB, Cheung CL, Lee ACH, Lam JKY, Wong Y, Shiu SWM. Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia. 2018;61(5):1212–1219. doi: 10.1007/s00125-018-4552-z. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi Y, Kobayashi S, Hemmi N, et al. Galectin-3-positive cell infiltration in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19(3):602–607. doi: 10.1093/ndt/gfg603. [DOI] [PubMed] [Google Scholar]
- 26.Vlassara H, Li YM, Imani F, et al. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995;1(6):634–646. [PMC free article] [PubMed] [Google Scholar]
- 27.Song G, Sun H, Han P, et al. Increased serum galectin-3 levels are associated with the development of type 2 diabetic nephropathy: a novel marker for progression? Int J Clin Exp Med. 2018;11(7):7156–7164. [Google Scholar]
- 28.Yücel N, Çakır Madenci Ö, Bölük A, et al. Is galectin-3 associated with urinary albumin excretion in type 2 diabetes? Endokrynol Pol. 2016;67(6):580–584. doi: 10.5603/EP.a2016.0036. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz H, Cakmak M, Inan O, Darcin T, Akcay A. Increased levels of galectin-3 were associated with prediabetes and diabetes: new risk factor? J Endocrinol Invest. 2015;38(5):527–533. doi: 10.1007/s40618-014-0222-2. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Jiang RJ, Wang CQ, et al. Predictive value of plasma galectin-3 in patients with chronic heart failure. Eur Rev Med Pharmacol Sci. 2013;17(8):1005–1011. [PubMed] [Google Scholar]