Abstract
Purpose
The head-mounted automated perimeter imo® is a new portable perimeter that does not require a dark room and can be used to examine patients in any setting. In this study, imo 24plus (1-2) AIZE examinations were compared with previous Humphrey Field Analyzer (HFA) 30-2 (SITA standard) examinations within the same patient.
Patients and methods
imo examinations (either head-mounted [i-H] or fixed [i-F] type) were performed in patients with glaucoma or suspected glaucoma who had already experienced HFA five or more times. Measurement time and correlations of mean deviation (MD) and visual field index (VFI) values were compared between groups for HFA, i-H, i-F, and imo total (i-T). Fixation loss (FL), false-positive (FP), and false-negative (FN) detection rates were compared. The percentage of binocular random single-eye tests under possible non-occlusion conditions using imo was determined. Mann–Whitney U test was performed, and Spearman’s rank-order correlation coefficient was calculated.
Results
The inclusion period was July to December 2016. Among 273 subjects (543 eyes), 147 (292 eyes) were tested with i-H type and 126 (251 eyes) with i-F type. Mean MD values for HFA and i-T were -6.1±7.8 and -6.2±7.1 dB, respectively. Mean measurement times for HFA, i-H, i-F, and i-T were 15.23±2.07, 10.47±2.11, 11.04±2.31, and 10.54±2.19 minutes, respectively (P<0.01 for HFA vs i-H/i-F). Total mean measurement time was shorter by 30.8% for i-T vs HFA. Correlation coefficients of MD and VFI were R2>0.81 for HFA vs i-H and i-F. FP and FN detection rates were significantly higher with i-T than HFA; there was no significant difference in FL. Binocular random single-eye tests were possible in 85% of cases.
Conclusion
imo reduced measurement time by 30.8%. imo VFI and MD values were highly correlated with HFA. As i-F and i-H types produced similar results, imo can be used in accordance with the patient’s situation.
Keywords: visual field, glaucoma, automatic perimetry, mean deviation, visual field index, reliability index
Video abstract
Introduction
Glaucoma is one of the leading causes of blindness, both in developed and developing countries, and its prevalence is expected to increase in future.1,2 An estimated 4.5 million people are blind due to primary glaucoma, accounting for approximately 12% of global blindness.3 The WHO recognizes that early and appropriate treatment to halt progression is key to preventing avoidable blindness.3
The visual field examination is useful to diagnose glaucoma and assess progression. Static automatic perimeters (SAPs), such as the Humphrey Field Analyzer (HFA) (Carl Zeiss Meditec, Dublin, CA, USA) and Octopus perimeter (Haag-Streit, Koeniz, Switzerland), are widely used in the clinical setting and have become the international standard. Visual field examination is clinically important not only for diagnosis and staging4–8 but also for examination of various neurological disorders affecting vision.
The incidence of glaucoma increases with advancing age.9 Using conventional SAPs, subjects are required to maintain a fixed position for a period of time during the examination. However, it is often difficult for elderly people to maintain the same position or for wheelchair users to maintain their trunk in a given position. Hyperkinetic patients with neurological or psychiatric disorders, or patients with sarcopenia, can also experience difficulties.
For aging societies such as in Japan, the need exists for a portable perimeter that can be used for examination anytime and anywhere, without the need for patients to maintain a fixed position. Head-mounted perimeters have been developed for such a purpose. Although a study of a portable head-mounted perimetry system was reported previously,10 no device has yet demonstrated clinical utility. In recent years, more practical devices using virtual reality glasses have been developed. However, these devices show similarities to the SAP grayscale, and clinical data have yet to be collected.11–13 imo® (CREWT Medical Systems, Tokyo, Japan), the device used in this study, is the only head-mounted perimeter currently in use in clinical practice.14 Owing to the head-mounted design, examinations do not require a dark room or a large space to install equipment. Examinations can be performed at any location including patients’ homes, health care centers, or neurology offices. As such, the device may have extensive clinical application.
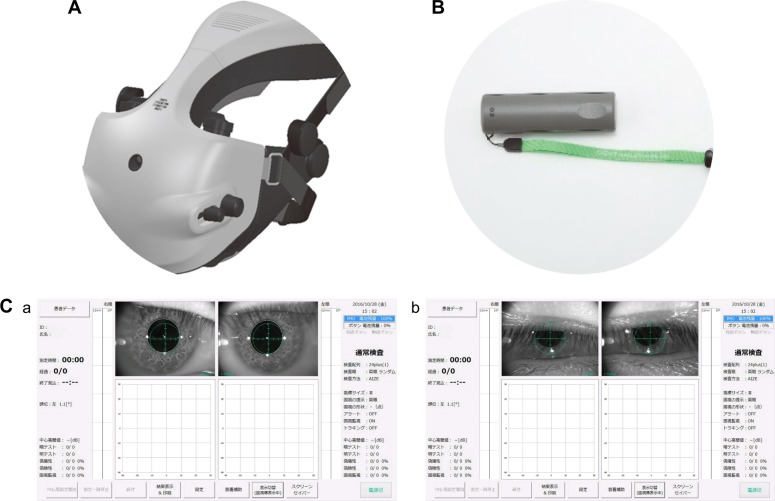
The basic structure and function of imo have been reported in detail by Matsumoto et al.14 Briefly, the device consists of a main body, a monitor, and a patient response button (Figure 1). The specifications of the imo perimeter unit are described in Table 1, and its main features are summarized below.
The head-mounted (i-H) perimeter is portable and can be used anywhere, including a patient’s bedside or wheelchair.
Generally, the i-H type is used (Figure 2A). However, if the patient can maintain a forward head posture, or if the patient prefers not to wear the head device for any reason, an adjustable fixed (i-F) type can be used (Figure 2B), also without the need for a dark room. The i-F-type perimeter is attached to a pillar, and patients place their face in contact with the device. By moving the device up and down to adjust for height, examinations can be performed in a comfortable forward posture.
imo’s removable magnetic cylindrical lens system allows for correction of astigmatism as required.
The distance between the center of the cornea surface and lens is 17.5 mm. Patients’ viewing distance is set at 1 m. Using the focus adjustment dial, distance can be adjusted within a control range of ±3 mm according to the shape of the examinee’s face.
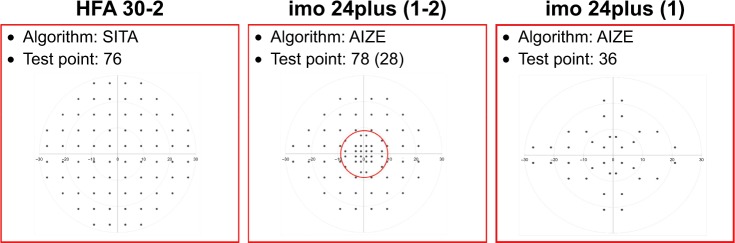
In addition to the 30-2, 10-2, and 24-2 test programs (as per HFA), imo includes 24plus (1-2) and 24plus (1) programs. In the peripheral area, imo 24plus (1-2) has similar measurement points (at 6° intervals) to those of 24-2, but has an additional 28 points at 2° intervals within the central 10° (Figure 3). 24plus (1-2) can thus detect a decrease in sensitivity in the vicinity of the fixation point that is overlooked with conventional 30-2 and 24-2 procedures and in less time than the conventional method since there are fewer measurement points. 24plus (1) extracts 36 points along the nerve fiber layer near the fixation point of 24-2 (Figure 3).
imo uses a modified Zippy Estimated by Sequential Testing (ZEST) algorithm called AIZE (Ambient Interactive ZEST). AIZE is a method of determining threshold value by adding ZEST to the effect of the periphery of the measurement point.15 AIZE has been reported to reduce test duration by about 70% compared with the conventional 4-2 dB bracketing strategy.14
imo can display a test target under the same conditions as HFA, using a full high-definition transmissive liquid crystal display (LCD) and separate high-intensity light-emitting diode backlights for the right and left eyes. imo is calibrated using a luminance meter. During calibration, imo displays the same gray level on the entire LCD.
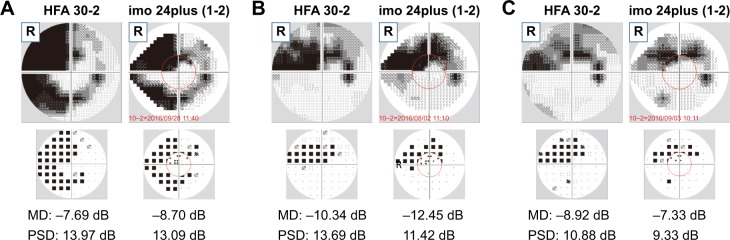
Mean deviation (MD), pattern SD (PSD),4 and visual field index (VFI)5 in imo are derived in the same manner as for HFA. Since examination results using imo are displayed in a similar manner to those of the single visual field analysis by HFA, no extra skill is required for interpretation. Examples of HFA 30-2 and imo 24plus (1-2) MD and PSD test results are shown in Figure 4.
imo enables examination under non-occlusion conditions, thus eliminating artifacts such as monocular fatigue and dark adaptation abnormality associated with monocular occlusion.
imo’s optical system mechanism permits separate testing of the right and left eyes. The test target is presented randomly to either eye with no awareness by the examinee of which eye is being tested. Target presentation and the examinee’s view during the binocular random single-eye test are shown in Figure 5. If the examinee is unable to achieve fusion on the right and left fixation targets for each eye at the start of the test, each eye can be examined separately without need for occlusion.
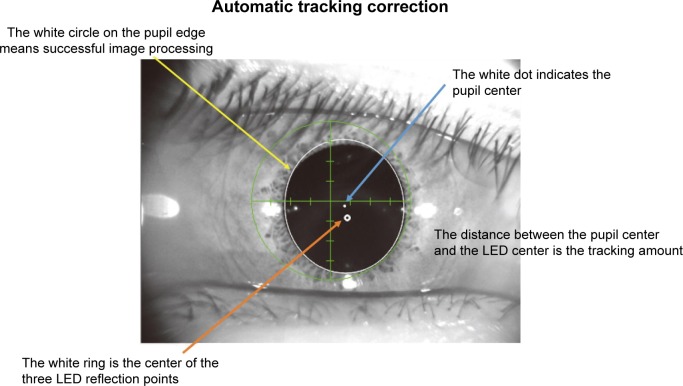
imo’s pupil tracking function monitors pupil deviation under certain conditions during the examination. For pupil deviation within 5°, the test targets are displayed correspondingly (Figure 6).
Although imo and HFA differ somewhat in defining reliability indices, the MD, PSD, and VFI values are displayed in the same manner. Measurement time is displayed in total for both eyes (binocular random single-eye test) or for one eye (monocular measurement).
Figure 1.
Components of imo®.
Notes: (A) Perimeter unit. (B) Patient response button. (C) Control tablet. If the pupil is detected at three points of the iris margin, pupil monitoring is automatically performed. If the pupil edge is not detected due to drowsiness, blepharoptosis, etc, pupil monitoring is not performed.
Table 1.
Characteristics of the head-mounted imo® perimeter unit
| Dimensions | Width 22 cm × depth 28 cm × height 24 cm |
|---|---|
| Weight | 1.8 kg |
| Spherical lens adjustment | −9 D to 3 D |
| Astigmatic lens adjustment | −3 D to 0 D |
| Pupil distance | 52–78 cm |
Figure 2.
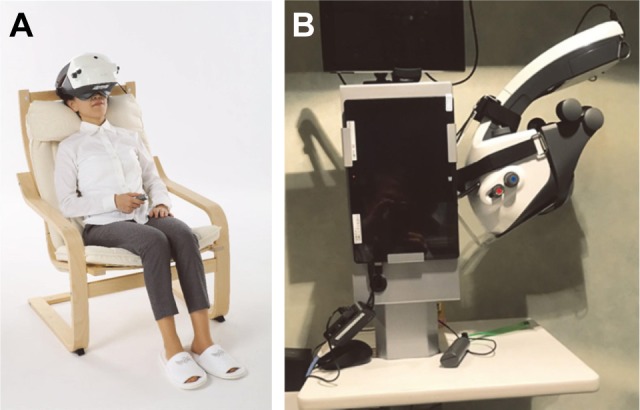
Types of imo®.
Notes: (A) The head-mounted type allows patients to sit back in a chair and be tested in a comfortable position. (B) The fixed position type can be moved up and down and allows the position to be determined according to a patient’s body type. No dark room is required for either type.
Figure 3.
Test points of the HFA 30-2 and the imo® 24plus (1-2) and 24plus (1) programs.
Notes: imo 24plus (1-2) has 28 points at 2° intervals within the central 10°. imo 24plus (1) has 36 points along the retinal nerve fiber layer.
Abbreviation: HFA, Humphrey Field Analyzer.
Figure 4.
Three representative cases.
Notes: (A) Case 1: 55-year-old female, normal tension glaucoma. (B) Case 2: 64-year-old male, primary open-angle glaucoma. (C) Case 3: 70-year-old male, normal tension glaucoma. Pattern deviation plots are shown below the HFA 30-2 and imo® 24plus (1-2) test results. For each case, MD and PSD values are found. The red circle indicates the central 10°.
Abbreviations: HFA, Humphrey Field Analyzer; MD, mean deviation; PSD, pattern SD.
Figure 5.
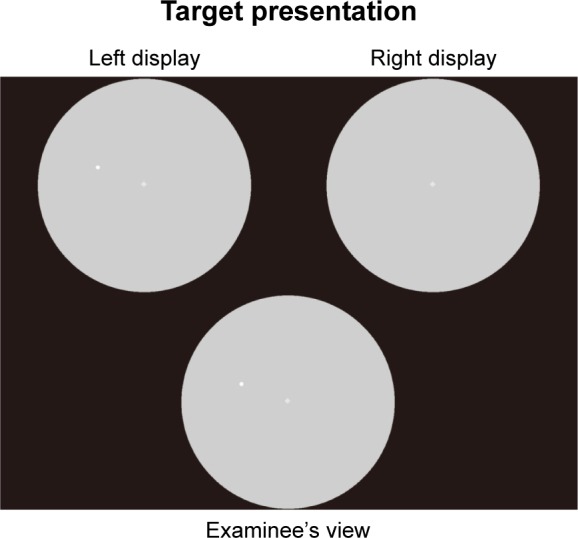
Target presentation and examinee’s view during the binocular random single-eye test.
Notes: The test target is presented randomly to either eye under non-occlusion conditions without the patient being aware of which eye is being tested. Copyright ©2016 Matsumoto et al. Reproduced from Matsumoto C, Yamao S, Nomoto H, et al. Visual Field Testing with Head-Mounted Perimeter ‘imo’. PLoS One. 2016;11(8):e0161974.14
Figure 6.
During the examination, each pupil may be individually and continuously monitored and the images are used for an eye tracking system.
Notes: The distance between the pupil center and the LED center is the tracking amount. If a fixation disparity occurs, the tracking function ensures test accuracy by following fixation and automatically correcting the target location (within a range of ±5°).
Abbreviation: LED, light-emitting diode.
Based on the above features, including new functions such as examination in a comfortable posture, binocular random single-eye test, and pupil tracking function, imo is expected to produce more accurate results than conventional SAP, although extreme fluctuations compared with previously accumulated HFA data are not expected. Previously, Matsumoto et al evaluated 40 eyes (20 subjects) with HFA 30-2 and imo 30-2 and reported similarities between the systems.14 Similarities between imo and SAP have also been reported in a case series of optic nerve disease.16
The purpose of this study was to evaluate possible benefits of the imo perimeter by analyzing the correlation between two visual field tests with overlapping measurement fields and almost the same number of measurement points, namely HFA SITA standard 30-2 (76 measurement points) and imo 24plus (1-2) AIZE (78 measurement points).
Patients and methods
This was a retrospective, cross-sectional study performed at the Ueno Eye Clinic. All participants were evaluated using imo 24plus (1-2) AIZE, and the results were compared with results previously recorded using HFA SITA standard 30-2. Examinations using HFA and imo were performed within a maximum interval of 6 months for each patient.
Patients were randomly assigned in turn to undergo evaluation by i-H and i-F during medical examination on clinic visits. Patients assigned to the i-H type could be tested anywhere in a comfortable position, although a few frail elderly patients who felt the device to be heavy/uncomfortable when trialing were switched to the i-F type.
Subjects
The study enrolled patients with glaucoma and those suspected of having glaucoma who had previously undergone testing with HFA 30-2 SITA at least five times with good reproducibility of results (MD value fluctuation<±3 dB). A diagnosis of glaucoma was made when the case satisfied Anderson’s criteria4 (ie, when one of the following three criteria was met: a cluster of three contiguous points with P<0.05, one of which had P<0.01 on a pattern deviation probability plot; corrected PSD/PSD with P<0.05; or a glaucoma hemifield test outside normal limits), and had findings consistent with enlarged optic disc cupping or nerve fiber layer defect (NFLD). If the case did not satisfy Anderson’s criteria, but NFLD was observed in optical coherence tomography, it was considered to be suspected glaucoma. Cases with varying reproducibility of HFA test results (≥±3.0 dB), cases that had undergone invasive treatment during the observation period, and cases with best-corrected visual acuity <0.7, spherical refraction <−8.0 D, spherical refraction >3.0 D, or astigmatic refraction >2.5 D were excluded. Other cases that the physician considered unsuitable for examination were also excluded. Glaucoma cases were included regardless of disease type. Cases seen within 1 week after introduction of imo were not included in the analysis to account for the examiner’s level of skill with each type of imo.
The study was planned in accordance with the tenets of the Declaration of Helsinki. Advance approval was granted by the Kinki University Ethics Committee (no 26-239). Written informed consent was obtained from all patients after thorough explanation of the study objectives. Data collection was performed in accordance with the ethical principles of the Declaration of Helsinki.
Assessments
Parameters examined and compared between HFA SITA standard 30-2 and imo 24plus (1-2) AIZE were:
Measurement time for both eyes, including binocular measurement time (in monocular examination, the sum of both eyes) and measurement time by age.
Mean values and correlations of MD and VFI values, including the coefficient of correlation between HFA 30-2 and imo total (i-T) and correlation between i-H type, i-F type, and HFA.
Reliability indices, including false-positive (FP), false-negative (FN), and fixation loss (FL) detection rates.
Percentage of binocular random single-eye tests possible using imo, as well as mean MD and examination time between cases in which the examination could or could not be performed.
Statistical analyses
Spearman’s rank-order correlation coefficient was calculated for correlations between HFA and i-H type, i-F type, and i-T. Bland–Altman plots were used to analyze the systematic errors of both; a straight line representing 2σ was described in the Bland–Altman plots. FP, FN, and FL detection rates were calculated using the Mann–Whitney U test. MD values and measurement times were compared between binocular random single-eye tests and monocular examinations using the Mann–Whitney U test. P<0.05 was regarded as a significant difference.
Results
The inclusion period was July to December 2016. Among 273 patients in total (543 eyes), 147 (292 eyes) were evaluated using i-H type and 126 (251 eyes) were evaluated using i-F type (Table 2). Diagnosis of the patient’s eye conditions were classified as primary open-angle glaucoma (n=118), normal tension glaucoma (n=250), primary angle closure glaucoma (n=25), secondary glaucoma (n=10), and preperimetric glaucoma (n=140). The average age of participants was 62.9 years, and there was a slight female preponderance.
Table 2.
Patient demographics
| Characteristics | imo® head-mounted | imo fixed | imo total |
|---|---|---|---|
| Age, years (mean ± SD) | 62.2±13.1 | 63.9±12.8 | 62.9±13.0 |
| N (cases:eyes) | 147:292 | 126:251 | 273:543 |
| Gender (male:female) | 59:88 | 58:68 | 117:156 |
Abbreviation: N, number.
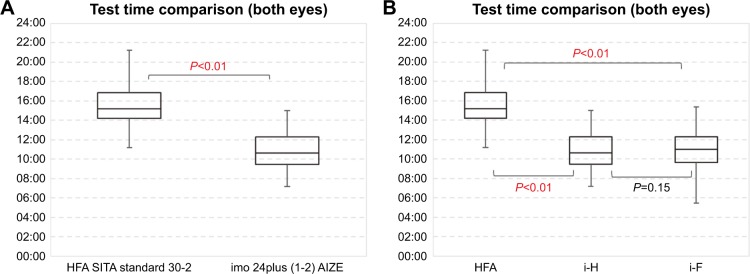
The main results of HFA and imo perimetry are summarized in Table 3. Mean MD was −6.1±7.8 dB for HFA and −6.2±7.1 dB for i-T. Mean measurement times were 15:23±2:07 and 10:54±2:19 minutes, respectively, representing a significant (P<0.01) 30.8% reduction using imo (i-T). The difference in measurement time between the i-H-type and i-F-type perimeters was not significant (Figure 7).
Table 3.
Results for HFA SITA standard 30-2 and imo® 24plus (1-2) AIZE examinations
| Parameters | imo head-mounted | imo fixed | imo total |
|---|---|---|---|
| Mean deviation (dB) | |||
| HFA | −6.0±7.3 | −6.2±8.3 | −6.1±7.8 |
| imo | −6.0±7.0 | −6.3±7.3 | −6.2±7.1 |
| Measurement time (minutes) | |||
| HFA | 15:32±2:00 | 15:13±2:14 | 15:23±2:07 |
| imo | 10:47±2:11** | 11:04±2:31** | 10:54±2:19** |
Notes: Data are presented as mean ± SD.
P<0.01 vs HFA.
Abbreviation: HFA, Humphrey Field Analyzer.
Figure 7.
Comparison of mean measurement time (SITA standard vs AIZE).
Notes: (A) HFA vs i-T. (B) HFA vs i-H and i-F. Total mean measurement time for imo® was 10:54±2:19 minutes, which was shorter by 30.8% compared to the mean of 15:23±2:07 minutes for HFA. There was no significant difference between i-H type and i-F type.
Abbreviations: HFA, Humphrey Field Analyzer; i-F, imo fixed type; i-H, imo head-mounted type; i-T, imo total.
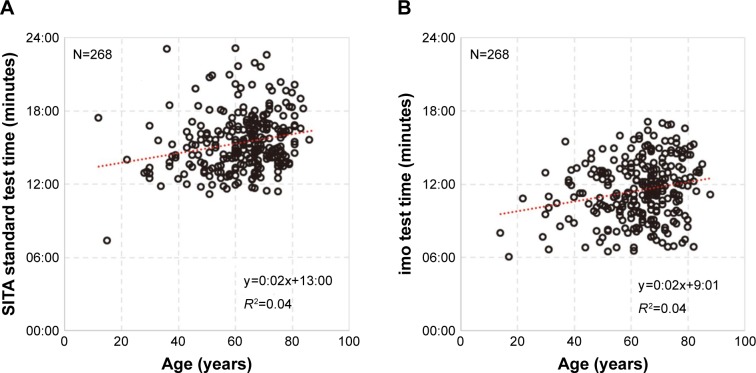
Evaluation of the correlation between measurement time and age showed correlation coefficients of R2=0.04 for HFA and imo (Figure 8). Although positive, the correlations were not significant. From the slope of the linear regression equation (0:02), it was calculated that visual field measurement time would be extended by 2 seconds with each year of aging.
Figure 8.
Age vs measurement time.
Notes: (A) HFA: red dotted regression line (r=0.04, P<0.001), slope 95% CI (−0:01 to +0:05). (B) imo®: red dotted regression line (r=0.04, P<0.001), slope 95% CI (−0:01 to +0:05).
Abbreviation: HFA, Humphrey Field Analyzer.
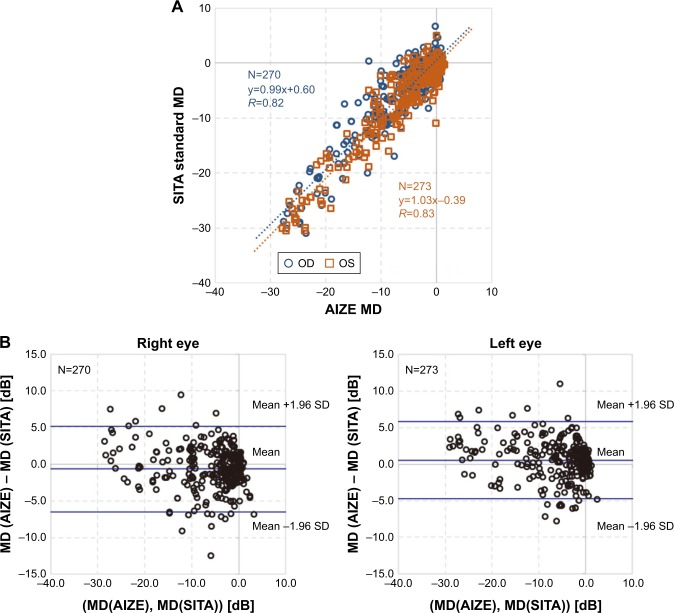
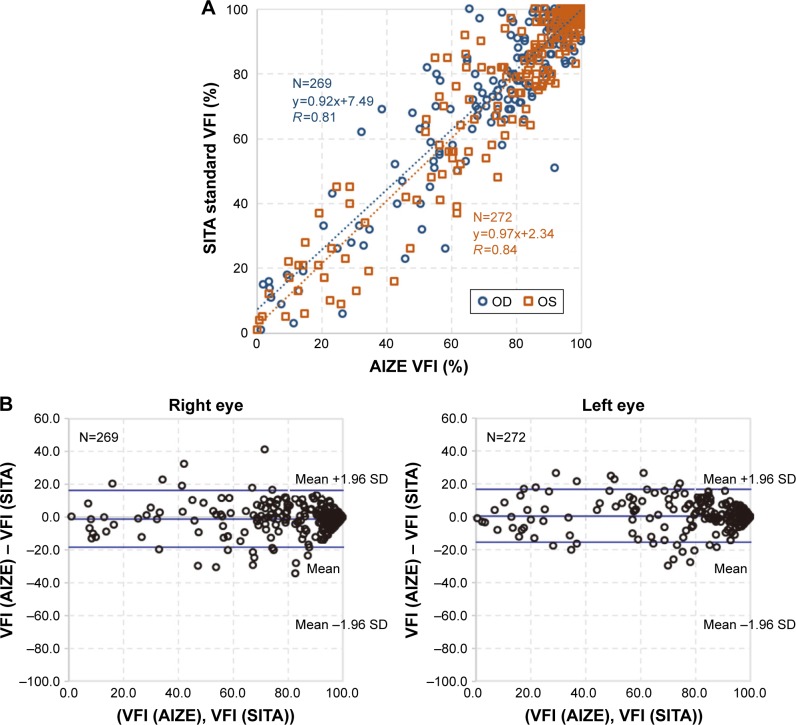
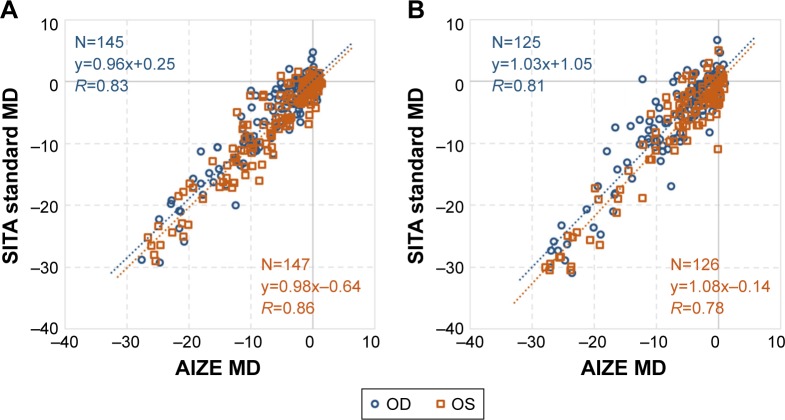
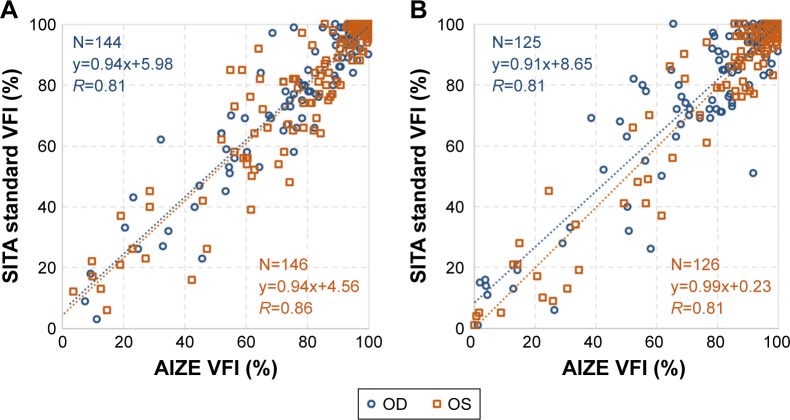
Comparison of MD values measured with HFA 30-2 and imo 24plus (1-2) in the same patient showed strong correlations in both the left and right eyes; Bland–Altman plots indicated no notable bias in the 2σ range in either eye (Figure 9). Likewise, comparison of VFI values showed a strong correlation between perimetry methods, with no notable deviation of 2σ in either eye based on Bland–Altman plots (Figure 10). Strong correlations between HFA and i-H type and between HFA and i-F type were observed for MD (Figure 11) and VFI (Figure 12) test results, with no significant differences between the devices (Table 4).
Figure 9.
MD correlation between HFA and imo®.
Notes: (A) Correlation between HFA 30-2 and imo 24plus (1-2). Blue dotted regression line (r=0.82, P<0.001): right eye slope, 95% CI (0.96–1.02); orange dotted regression line (r=0.83, P<0.001): left eye slope, 95% CI (1.00–1.06). (B) Bland–Altman plots: right eye mean value, 95% CI (−0.65, −6.49 to 5.19); left eye mean value, 95% CI (0.56, −4.70 to 5.81).
Abbreviations: HFA, Humphrey Field Analyzer; MD, mean deviation.
Figure 10.
VFI correlation between HFA and imo®.
Notes: (A) Correlation between HFA 30-2 vs imo 24plus (1-2). Blue dotted regression line (r=0.81, P<0.001): right eye slope, 95% CI (0.90–0.94); orange dotted regression line (r=0.84, P<0.001): left eye slope, 95% CI (0.95–0.99). (B) Bland–Altman plots: right eye mean value, 95% CI (−1.1, −18.4 to 16.2); left eye mean value, 95% CI (0.50, −15.6 to 16.7).
Abbreviations: HFA, Humphrey Field Analyzer; VFI, visual field index.
Figure 11.
MD correlation by imo® type.
Notes: (A) HFA vs i-H. Blue dotted regression line (r=0.83, P<0.001): right eye slope, 95% CI (0.92–1.00); orange dotted regression line (r=0.86, P<0.001): left eye slope, 95% CI (0.94–1.02). (B) HFA vs i-F. Blue dotted regression line (r=0.81, P<0.001): right eye slope, 95% CI (0.98–1.08); orange dotted regression line (r=0.78, P<0.001): left eye slope, 95% CI (1.04–1.12).
Abbreviations: HFA, Humphrey Field Analyzer; i-F, imo fixed type; i-H, imo head-mounted type; MD, mean deviation.
Figure 12.
VFI correlation between HFA and imo® type.
Notes: (A) HFA vs i-H. Blue dotted regression line (r=0.81, P<0.001): right eye slope, 95% CI (0.92–0.96); orange dotted regression line (r=0.86, P<0.001): left eye slope, 95% CI (0.92–0.96). (B) HFA vs i-F. Blue dotted regression line (r=0.81, P<0.001): right eye slope, 95% CI (0.89–0.93); orange dotted regression line (r=0.81, P<0.001): left eye slope, 95% CI (0.97–1.01).
Abbreviations: HFA, Humphrey Field Analyzer; i-F, imo fixed type; i-H, imo head-mounted type; VFI, visual field index.
Table 4.
Comparisons of mean deviation and visual field index test results for the imo® head-mounted-type and imo fixed-type perimeters
| Parameters | Comparison | P-value (right eye) | P-value (left eye) |
|---|---|---|---|
| Mean deviation | i-H vs i-F | 0.97 | 0.29 |
| Visual field index | i-H vs i-F | 0.97 | 0.29 |
Note: P-values are for Mann–Whitney U test.
Abbreviations: i-F, imo fixed type; i-H, imo head-mounted type.
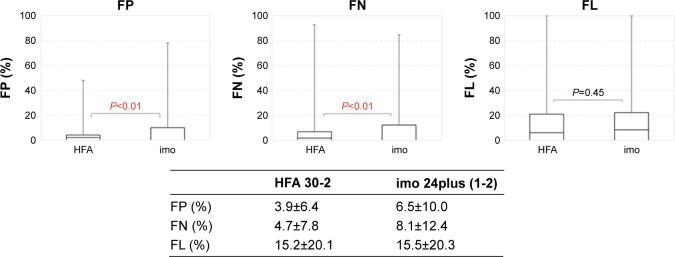
Comparison of the reliability indices FL, FP, and FN between imo 24plus (1-2) and HFA 30-2 indicated significant differences between methods for FP and FN (both P<0.01) but not FL (Figure 13).
Figure 13.
Reliability index: comparison of false positive, false negative, and fixation loss test results between HFA 30-2 and imo® 24plus (1-2) (total).
Abbreviations: FL, fixation loss; FN, false negative; FP, false positive; HFA, Humphrey Field Analyzer.
Among 273 cases, 85% could be examined using the imo binocular random single-eye test. Significant differences (both P<0.05) in favor of binocular random single-eye testing over monocular testing were observed in examination time and MD (Table 5).
Table 5.
Comparison of measurement time and mean deviation test results between the binocular random single-eye test and monocular test
| Condition | Number of cases (n=273) | Measurement time (mean ± SD) | Mean deviation (dB) |
|---|---|---|---|
| Binocular random single-eye test | 231 (85%) | 11:16±2:24* | −5.88* |
| Monocular test (sum of one eye) | 42 (15%) | 12:43±2:24 | −7.74 |
Notes:
P<0.05 vs monocular test; Mann–Whitney U test.
Discussion
To evaluate the possible benefits of imo perimetry for glaucoma, this study compared imo 24plus (1-2) AIZE with HFA SITA standard 30-2, two visual field tests with similar numbers of measurement points. Examinations were conducted in the usual outpatient environment and involved subjects with previous experience of visual field testing who had shown stable results. Despite first-time examination with imo 24plus (1-2), the perimeter was strongly and significantly correlated with HFA 30-2 in terms of MD and VFI and the modified AIZE algorithm was associated with significantly shorter measurement time compared with ZEST.
Matsumoto et al reported that imo’s 4-2 dB bracketing algorithm lengthened examination time for the binocular random single-eye test compared with HFA (17.3±1.25 vs 16.0±1.21 minutes).14 In the present study, binocular random single-eye testing with imo 24plus (1-2) AIZE was completed in just less than 11 minutes.
In this context, an explanation of the relationship between ZEST and AIZE might be useful. Among QUEST methods for determining final threshold,17 ZEST as Mean-Quest has been identified as efficient.15 ZEST updates the probability mass function of the visual field position using average value instead of mode value used in QUEST, and determines the threshold value from the Bayesian estimation method and maximum likelihood method. Its function is to shorten test time without lowering output quality. Nevertheless, additional scope exists for further reducing measurement time using AIZE. In simple terms, AIZE is a method of determining threshold value by adding ZEST to the effect of the periphery of the measurement point.15 As a result, measurement time using AIZE was shortened by 36% from the total point threshold measurement time reported by Matsumoto et al,14 and 30.8% from ZEST. Further studies on algorithms and testing strategies that place less burden on patients are warranted in future.
No statistically significant differences were observed between imo i-H-type and i-F-type perimeters in measurement time, MD, and VFI values in the current study, indicating that the devices can be selected and used according to the patient’s situation. For example, elderly patients or those with a small frame who find the 1.8 kg head-mounted device too heavy can use the i-F type without compromising benefit.
With respect to reliability indices, there were significant differences between imo 24plus (1-2) and HFA for FP or FN but not for FL. On account of imo’s superior eye tracking function, changes were made to the examination display point. FP and FN were thus expected to be lower for imo than for HFA, but this was not the case. This finding might be explained by differences between procedures in defining reliability indices (Table 6). HFA defines FP as a reaction to a test target within 0–0.2 seconds. In contrast, imo defines FP on the basis of responses to a 9 dB dark target relative to the determined threshold. Although the definitions of an FN are basically the same for HFA and imo (an FN is assumed on the basis of no response to a 9 dB bright target relative to the determined threshold),4 in HFA, the observation point is unknown whereas, in imo, all observation points are included except for those in the most peripheral area. This difference appears to be responsible for significant differences in FN detection rates. The possibility also exists that differences in the test programs, 30-2 for HFA and 24plus (1-2) for imo, affected the study results. In the former, measurement points are all at 6° intervals, while the latter has 28 observation points spaced 2° apart within the central 10° of the fixation point; FP and FN may differ within this region. Visual field examination under binocular open-eye conditions can be considered to affect various eye conditions. The influence on reactivity to FP and FN cannot be denied. Therefore, the analysis of visual field binocular open-eye examination in various conditions is awaited.
Table 6.
Reliability index definitions (false positive, false negative, and fixation loss)
| Reliability index | Humphrey Field Analyzer SITA Standard | imo® AIZE |
|---|---|---|
| False positive | Conversion from reaction time (response within 0.2 seconds) | Ratio of responding by presenting a 9 dB dark target to the determined threshold Binocular random conditions |
| False negative | Ratio of not responding by presenting a 9 dB bright target to the determined threshold Included observation point unknown Monocular | Ratio of not responding by presenting a 9 dB bright target to the determined threshold All observation points known except for outermost circumference Binocular random conditions |
| Fixation loss | Percentage in response to stimulus to blind spots | Percentage in response to stimulus to blind spots |
Although certain parameters showed approximately similar values for HFA and imo, some differences were observed between methods. Several factors may be implicated. For example, differences between AIZE13,14 and SITA18 algorithms, and differences in the test environment of the eye during examination, could affect the results. The bilateral eye is open in imo, whereas one eye is occluded in HFA; examination under binocular open-eye conditions without occlusion is a new environment for glaucoma patients.
It is recognized that binocular open-eye examinations cannot be performed in all situations, including cases of binocular vision dysfunction such as strabismus, anisometropia, nystagmus, and so on. Binocular open-eye and one-eye occlusion examinations are affected by various factors which, in turn, can affect measured parameters. In the current study, measurement time under binocular open-eye conditions was less than the sum of the monocular measurement time. It can thus be inferred that binocular open-eye examination is not the mere sum of monocular examination.
The issues associated with one-eye occlusion are well documented. There is known to be transient blank-out during bowl perimetry,19 and a decrease in sensitivity on frequency doubling technology in the non-occluded eye.20 Effects on the nondominant eye in dominant ocular occlusion, and functional changes in the non-occluded eye after one-eye occlusion, have also been reported.21–23 The phenomenon highlights the relationship with visual cortex function in the brain.24 The influence of visual function of one-eye occlusion and its mechanism are still under investigation. Analysis of the influence of examination under binocular open-eye conditions should be considered for future study.
In terms of differences between the HFA 30-2 and imo 24plus (1-2) test programs, there are no significant differences between 76-point and 78-point measurements; however, the difference in measurement points in the central 10° appears to be responsible for the differences observed in MD, VFI, and reliability indices. Nevertheless, the results for MD and VFI were fairly similar, and are similar to those of HFA accumulated empirically (a consequence of the consistency between imo 24plus (1-2) and SAP). Thus, the reliability of imo is considered to be high.
Finally, the issue of head tilt during examination with the i-H type must be considered, although the influence is reportedly small if tilting is within 20°.25 The i-F type may prove to be better suited for use in cases where head tilt is prominent or the head-mounted device is felt to be heavy. In this context, the ability to select the most appropriate type of device is another advantage.
Overall, imo 24plus (1-2) AIZE, which can perform examinations in a shorter time, and can detect a decrease in sensitivity and visual field of the peripheral area otherwise detected only by the 10-2 program, appears to be highly promising for clinical use. A logical next step would be to compare the sensitivity of the 10° internal field of imo 24plus (1-2) with the sensitivity of HFA 10-2 and imo 10-2.
A study limitation is selection bias given that patients included for examination with imo (and for comparison with accumulated HFA data) were all experienced with visual field testing. The results we obtained may therefore represent a best-case scenario.
Conclusion
The imo perimeter, a portable device with flexibility for use in any situation, is reliable with respect to clinical data. As the 24plus (1-2) program has the advantage of being able to measure peripheral vision and central visual field at the same time, its clinical usefulness is high. Detection of the same sensitivity in a shorter time than that of a conventional test program further supports its clinical utility. imo can perform separate visual field examination for each eye under non-occlusion conditions, without the subject aware of which eye is being examined. Measurement in a natural environment different from conventional visual field examination was possible in 85% of cases. Further evaluation of the clinical potential of imo by assessing various examination programs is anticipated in the future.
Acknowledgments
Editing assistance was provided by Content Ed Net.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cook C, Foster P. Epidemiology of glaucoma: what’s new? Can J Ophthalmol. 2012;47(3):223–226. doi: 10.1016/j.jcjo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization [homepage on the Internet] Blindness and vision impairment prevention. [Accessed December 27, 2018]. Available from: http://www.who.int/blindness/causes/priority/en/index6.html.
- 4.Anderson DR, Patella VM. Automated Static Perimetry. 2nd ed. St Louis, MO: Mosby; 1999. [Google Scholar]
- 5.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145(2):343–353. doi: 10.1016/j.ajo.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Japan Glaucoma Society The Japan Glaucoma Society Guidelines for Glaucoma (3rd Edition) Nippon Ganka Gakkai Zasshi. 2012;116(1):3–46. Japanese. [PubMed] [Google Scholar]
- 7.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition – chapter 3 Treatment principles and options supported by the EGS Foundation: part 1: foreword; introduction; glossary; chapter 3 treatment principles and options. Br J Ophthalmol. 2017;101(6):130–195. doi: 10.1136/bjophthalmol-2016-EGSguideline.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian glaucoma study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030–1036. doi: 10.1001/archopht.126.8.1030. [DOI] [PubMed] [Google Scholar]
- 9.Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi study. Ophthalmology. 2004;111(9):1641–1648. doi: 10.1016/j.ophtha.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Hollander DA, Volpe NJ, Moster ML, et al. Use of a portable head mounted perimetry system to assess bedside visual fields. Br J Ophthalmol. 2000;84(10):1185–1190. doi: 10.1136/bjo.84.10.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsapakis S, Papaconstantinou D, Diagourtas A, et al. Visual field examination method using virtual reality glasses compared with the Humphrey perimeter. Clin Ophthalmol. 2017;11:1431–1443. doi: 10.2147/OPTH.S131160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wroblewski D, Francis BA, Sadun A, Vakili G, Chopra V. Testing of visual field with virtual reality goggles in manual and visual GRASP modes. BioMed Research International. 2014;2014(8):1–10. doi: 10.1155/2014/206082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi M, Wang YT, Jung TP, et al. Detecting glaucoma with a portable brain-computer interface for objective assessment of visual function loss. JAMA Ophthalmol. 2017;135(6):550–557. doi: 10.1001/jamaophthalmol.2017.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto C, Yamao S, Nomoto H, et al. Visual Field Testing with Head-Mounted Perimeter ‘imo’. PLoS One. 2016;11(8):e0161974. doi: 10.1371/journal.pone.0161974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King-Smith PE, Grigsby SS, Vingrys AJ, Benes SC, Supowit A. Efficient and unbiased modifications of the quest threshold method: theory, simulations, experimental evaluation and practical implementation. Vision Res. 1994;34(7):885–912. doi: 10.1016/0042-6989(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 16.Goseki T, Ishikawa H, Shoji N. Bilateral concurrent eye examination with a head-mounted perimeter for diagnosing functional visual loss. Neuroophthalmology. 2016;40(6):281–285. doi: 10.1080/01658107.2016.1220593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- 18.Bengtsson B, Olsson J, Heijl A, Rootzén H. A new generation of algorithms for computerized threshold perimetry, SITA. Acta Ophthalmol Scand. 1997;75(4):368–375. doi: 10.1111/j.1600-0420.1997.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 19.Fuhr PS, Hershner TA, Daum KM. Ganzfeld blankout occurs in bowl perimetry and is eliminated by translucent occlusion. Arch Ophthalmol. 1990;108(7):983–988. doi: 10.1001/archopht.1990.01070090085045. [DOI] [PubMed] [Google Scholar]
- 20.Kogure S, Membrey WL, Fitzke FW, Tsukahara S. Effect of decreased retinal illumination on frequency doubling technology. Jpn J Ophthalmol. 2000;44(5):489–493. doi: 10.1016/s0021-5155(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 21.Spry PG, Furber JE, Harrad RA. The effect of ocular dominance on visual field testing. Optom Vis Sci. 2002;79(2):93–97. doi: 10.1097/00006324-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Gupta B, Paliga J, Laderman DJ, Szlyk JP. The effect of occlusive patching on visually-directed tasks. J AAPOS. 2005;9(5):485–492. doi: 10.1016/j.jaapos.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Clavagnier S, Hess RF. Short-term monocular deprivation strengthens the patched eye’s contribution to binocular combination. J Vis. 2013;13(5):12. doi: 10.1167/13.5.12. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Baker DH, Simard M, Saint-Amour D, Hess RF. Short-term monocular patching boosts the patched eye’s response in visual cortex. Restor Neurol Neurosci. 2015;33(3):381–387. doi: 10.3233/RNN-140472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamao S, Matsumoto C, Nomoto H, et al. Effects of head tilt on visual field testing with a head-mounted perimeter IMO. PLoS One. 2017;12(9):e0185240. doi: 10.1371/journal.pone.0185240. [DOI] [PMC free article] [PubMed] [Google Scholar]