Abstract
Background
The goal of this study was to evaluate outcomes in children with relapsed, molecularly characterized intracranial ependymoma treated with or without craniospinal irradiation (CSI) as part of a course of repeat radiation therapy (re-RT).
Methods
This was a retrospective cohort study of 31 children. Patients with distant relapse received CSI as part of re-RT. For patients with locally recurrent ependymoma, those treated before 2012 were re-irradiated with focal re-RT. In 2012, institutional practice changed to offer CSI, followed by boost re-RT to the site of resected or gross disease.
Results
Median follow-up was 5.5 years. Of 9 patients with distant relapse after initial RT, 2-year freedom from progression (FFP) and overall survival (OS) were 12.5% and 62.5%, respectively. There were 22 patients with local failure after initial RT. In these patients, use of CSI during re-RT was associated with improvement in 5-year FFP (83.3% with CSI vs 15.2% with focal re-RT only, P = 0.030). In the subgroup of patients with infratentorial primary disease, CSI during re-RT also improved 5-year FFP (100% with CSI, 10.0% with focal re-RT only, P = 0.036). Twenty-three patients had known molecular status; all had posterior fossa group A tumors (n = 17) or tumors with a RELA (v-rel avian reticuloendotheliosis viral oncogene homolog A) fusion (n = 6). No patient developed radiation necrosis after fractionated re-RT, though almost all survivors required assistance throughout formal schooling. Five out of 10 long-term survivors have not developed neuroendocrine deficits.
Conclusions
Re-irradiation with CSI is a safe and effective treatment for children with locally recurrent ependymoma and improves disease control compared with focal re-irradiation, with the benefit most apparent for those with infratentorial primary tumors.
Keywords: ependymoma, pediatrics, re-irradiation, recurrence
Key Points.
Re-RT for recurrent ependymoma is safe and provides disease control in some patients.
CSI re-RT improves disease control in locally recurrent ependymoma.
No case of necrosis solely attributable to fractionated re-RT was observed.
Importance of the study.
This study reports clinical and toxicity outcomes of a cohort of children treated with repeat irradiation for recurrent ependymoma. In 2012, our institution changed practice to offer CSI for patients with locally recurrent ependymoma, with focal re-irradiation being routine prior to this date. This provided an ideal before-and-after cohort to evaluate the role of CSI. We found that CSI for locally recurrent ependymoma offered a freedom-from-progression benefit, especially for those with infratentorial tumors. No cases of radiation necrosis (in the absence of disease progression) were seen after fractionated re-irradiation, though patients treated with re-RT are at risk of long-term cognitive and endocrine deficits. Given the improved disease control seen with comprehensive neuraxis re-irradiation, CSI should be discussed as an option for patients with locally recurrent ependymoma.
Ependymoma accounts for 9% of pediatric central nervous system (CNS) tumors, with 90% arising intracranially.1,2 At diagnosis, patients with localized disease typically undergo a maximal safe resection, followed by adjuvant postoperative radiotherapy (RT1) to the tumor bed.3,4 Challenges arise in the setting of recurrent disease, which occurs in approximately 30–50% of patients.4 Maximum safe resection is appropriate, and in the absence of effective systemic therapy,3,5,6 a second course of radiation (RT2) may be undertaken which overlaps with the first.7–9 Despite traditional concerns surrounding the potential side effects of re-irradiating the pediatric brain to cumulative doses above what would usually be considered normal tissue tolerance, this has been found to be well tolerated, with low rates of radiation necrosis.8,9
It has previously been demonstrated that re-irradiation for children with recurrent ependymoma results in improved survival compared with historical controls who were not treated with RT2.8 In the setting of disseminated relapse, patients should receive craniospinal irradiation (CSI) as a component of RT2, with subsequent boost RT to sites of gross or resected disease. However, the optimal radiation field for localized recurrence is controversial and not well defined. For localized relapse without distant dissemination, patients have been typically re-irradiated with focal RT2 to the recurrent tumor bed.7–9
Since our institution adopted focal re-irradiation for locally recurrent ependymoma, it has been observed that a large proportion of patients who relapse following focal RT2 subsequently develop distant disease elsewhere within the neuraxis. This raised the question as to whether CSI as part of RT2 could improve disease control compared with focal RT2. Since 2012 and onward, CSI has been offered as a standard treatment in the setting of locally recurrent ependymoma. This change created an ideal before-and-after cohort to study the role of CSI for recurrent intracranial ependymoma in children. Thus, the objectives of this study were to evaluate freedom from progression (FFP) and overall survival (OS) between patients who were treated with and without CSI as a component of RT2, in a molecularly characterized study cohort.
Materials and Methods
Pediatric patients age 18 or under (at the time of initial ependymoma diagnosis) who were treated at the Hospital for Sick Children and Princess Margaret Cancer Centre in Toronto, Canada between 1999 and 2018 were retrospectively reviewed. Eligible patients had a pathological diagnosis of intracranial ependymoma and received focal RT1 for localized disease at initial diagnosis. Those who subsequently relapsed and received at least one course of re-irradiation were identified from neuro-oncology and radiotherapy databases. Patients underwent repeat surgery at recurrence, when possible.
Patients with recurrence at solely the site of the original disease, within the RT1 field, were considered to have local relapse. Patients with relapse distant to the site of original disease and/or malignant cells within the CSF were considered to have disseminated disease. Those with synchronous local and distant failure were included in the “disseminated” group due to the small number of combined failures (n = 3). For the infratentorial tumors, molecular subgroup characterization was performed on initial resection specimens using a combination of genome-wide methylation arrays10 and H3K27me3 immunohistochemistry staining.11,12 Two cases had tissue that could not be characterized due to weak H3K27me3 staining and lack of further tissue for methylation profiling. In the supratentorial tumors, a genome-wide methylation array was used to characterize fusions of v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA).13
Radiotherapy
At the time of initial presentation (RT1), children aged <18 months or ≥18 months received 54 Gy or 59.4 Gy, respectively, in 1.8 Gy daily fractions to the postoperative tumor bed. For patients who relapsed with localized disease, our institutional practice had been to offer focal re-irradiation (RT2) until 2011. Focal RT2 dose was adapted to RT1 dose; those who had received RT1 59.4 Gy were treated with RT2 54 Gy, while those who had received RT1 54 Gy were treated with RT2 59.4 Gy (Supplementary Figure S1). The gross tumor volume (GTV) included the recurrent, postoperative tumor bed and all macroscopic disease with a 0–1 cm clinical target volume (CTV) margin and a 0–0.5 cm planning target volume (PTV) margin, depending on proximity to organs at risk. Where the RT2 prescription dose was 59.4 Gy and the PTV overlapped with brainstem, cord, or optic structures, a two-phase plan was adopted: the PTV was treated to 54 Gy, followed by a boost treatment (5.4 Gy in 3 fractions) with reduced margins to minimize overlap with critical structures. Two patients received cobalt-based stereotactic radiosurgery (SRS; 15–24 Gy) using a rigid stereotactic frame (Elekta AB) for small, isolated local recurrences.
From 2012 onward, CSI as a component of RT2 (CSI-RT2) was adopted as a standard option for patients with isolated focal relapse (Supplementary Figure S1). CSI was delivered to the whole neuraxis at a dose of 23.4–36 Gy in 1.8 Gy daily fractions, with lower doses reserved for selected younger patients (age 8 or under, see Supplementary Table S1). Focal boosts of 54–59.4 Gy were delivered to the recurrent postoperative tumor bed and any residual macroscopic disease as described above. Similarly, patients who relapsed with disseminated disease received 36 Gy CSI, followed by boost RT2 to 54–59.4 Gy.
All patients were treated with photon external beam RT. The shortest RT1-to-RT2 time interval in the study was 6 months, with all remaining patients having at least a 10-month gap between the two treatments to allow for potential normal tissue recovery. At re-irradiation, brainstem, cord, and optic doses were limited to a point maximum of 54 Gy, aiming for ≥95% PTV coverage with ≥95% of the RT2 prescription dose; no cumulative dose limits were applied. No doses >100% of the prescription dose were permitted in brainstem, optics, or cord for RT2. CSI included all structures in the brain and spinal cord, without shielding applied to optic structures, brainstem, or cervical spinal cord. All re-irradiated patients received real-time image guidance with daily cone beam computed tomography. Posttreatment surveillance MR imaging was used to detect tumor recurrences or radiation necrosis. When available, MR spectroscopy was used to help differentiate between necrosis and recurrence. In cases of diagnostic uncertainty, short-interval repeat imaging was used to clarify the true diagnosis.
Analysis
Clinical characteristics were reported descriptively; proportions and continuous variables were compared using Fisher’s exact test and the Wilcoxon rank-sum test, respectively. The Kaplan–Meier method was used to calculate OS and FFP (defined as time to disease recurrence) from the first day of RT2. Living patients were censored at last follow-up. Median follow-up was calculated by reversing the censoring variable in the Kaplan–Meier analysis for death. OS and FFP, stratified by clinicopathologic factors, were compared using log-rank tests. Cox regression was used to explore univariate factors associated with OS and FFP and to evaluate statistical interaction between variables. Multivariable analysis was not performed due to the small sample size. Analyses were performed using SAS version 9.4. The study was approved by the research ethics boards of both the University Health Network and the Hospital for Sick Children.
Results
We identified 31 patients who were re-irradiated for relapsed ependymoma. Baseline characteristics are summarized in Table 1, and individual patient data are listed in Supplementary Table S2. All patients underwent surgery at initial presentation prior to RT1. For all but 1 patient, RT1 was delivered at initial presentation, and the remaining patient received surgery and chemotherapy initially and RT1 at first recurrence. In total, 10 patients received chemotherapy at initial presentation, either to delay radiotherapy due to young age (n = 2), to facilitate more complete surgery (n = 5, one patient eventually proceeded to second surgery), as adjuvant therapy (n = 2), or as an alternative to radiotherapy (in the 1 patient who received RT1 at first relapse, chemotherapy was given at first presentation to facilitate further surgery). Twenty-three patients (74%) had complete molecular subgroup characterization; all the patients with infratentorial tumors had group A tumors, while all the supratentorial tumors had RELA fusions. There were no patients with posterior fossa group B tumors.
Table 1.
Baseline characteristics
| Characteristic | Local Failure | Distant Failure | All (n = 31) | |
|---|---|---|---|---|
| Focal RT2 (n = 15) | CSI RT2 (n = 7) | CSI RT2 (n = 9) | ||
| N (%) | ||||
| Sex (female) | 7 (47) | 0 (0) | 4 (44.4) | 11 (35.5) |
| Age at diagnosis, y, median (range) | 4.8 (0.8–12.9) | 4.9 (1.7–12.6) | 8.3 (4.7–17.2) | 6.1 (0.8–17.2) |
| Site of original disease | ||||
| Infratentorial | 12 (20.0) | 4 (57.1) | 6 (66.7) | 22 (80.0) |
| PF-A | 7 (100) | 4 (100) | 6 (100) | 17 (100) |
| Not known | 5 | 5 | ||
| Supratentorial | 3 (80.0) | 3 (42.9) | 3 (33.3) | 9 (29.0) |
| RELA fusion | 1 (100) | 2 (100) | 3 (100) | 6 (100) |
| Not known | 2 | 1 | 3 | |
| Histological grade | ||||
| II | 7 (46.7) | 4 (57.1) | 1 (11.1) | 12 (38.7) |
| III | 8 (53.3) | 3 (42.9) | 8 (88.9) | 19 (61.3) |
| Initial surgery | ||||
| GTR | 3 (20) | 3 (42.9) | 6 (66.7) | 12 (38.7) |
| NTR | 7 (46.7) | 1 (14.3) | 2 (22.2) | 10 (32.3) |
| STR | 5 (33.3) | 3 (42.9) | 1 (11.1) | 9 (29.0) |
| Age at RT1, y (range) | 5.0 (1.2–13.0) | 5.1 (1.8–12.7) | 8.3 (4.8–17.5) | 6.2 (1.2–17.5) |
| Chemotherapy at initial presentation | ||||
| Yes* | 6 (40) | 3 (42.9) | 1 (11.1) | 10 (32.3) |
| No | 9 (60) | 4 (57.1) | 8 (88.9) | 21 (67.7) |
| Time to first relapse, mo, median (range) | 23.6 (8.5–105.5) | 29.4 (3.7–138.3) | 14.8 (8.6–97.3) | 19.4 (3.7–138.3) |
| Surgery at relapse | ||||
| GTR | 9 (60) | 6 (85.7) | 5 (55.6) | 20 (64.5) |
| NTR | 1 (6.7) | 1 (11.1) | 2 (6.5) | |
| STR | 3 (20) | 1 (14.3) | 4 (12.9) | |
| Biopsy only | 1 (11.1) | 1 (3.2) | ||
| None | 2 (13.3) | 2 (22.2) | 4 (12.9) | |
| Time between RT1 and RT2, y, median (range) | 2.4 (0.9–10.0) | 2.6 (0.5–11.7) | 1.4 (0.9–8.2) | 1.9 (0.5–11.7) |
| Age at RT2, y, median (range) | 7.1 (2.4–16.9) | 8.4 (5.3–22.3) | 10.2 (6.5–18.4) | 8.4 (2.4–22.3) |
| CSI RT2 treatment technique | ||||
| 2D | 6 (85.7) | 7 (87.5) | ||
| IMRT | 1 (14.3) | 1 (12.5) | ||
| Not known** | 1 | |||
| Focal or boost RT2 treatment technique | ||||
| 3DCRT | 2 (25.0) | 2 (6.7) | ||
| IMRT or VMAT | 13 (86.7) | 7 (100) | 6 (75.0) | 26 (86.7) |
| SRS | 2 (13.3) | 2 (6.7) | ||
| Not known** | 1 | 1 | ||
*Chemotherapy consisted of (i) vincristine, carboplatin, cyclophosphamide, and etoposide, or (ii) carboplatin, procarbazine, etoposide, cisplatin, vincristine, and cyclophosphamide, or (iii) cyclophosphamide, vincristine, cisplatin, and etoposide, or (iv) temozolomide.
**One individual received RT2 at an outside institution.
2D = two-dimensional; 3DCRT = three-dimensional conformal radiation therapy; IMRT = intensity modulated radiation therapy; GTR = gross total resection; NTR = near total resection; PF-A = posterior fossa group A tumor; STR = subtotal resection; VMAT = volumetric arc radiation therapy
Surgery was recommended to reduce the burden of disease as well as to obtain pathologic confirmation of recurrence prior to embarking on RT2. A majority of patients underwent surgery for relapsed ependymoma, with 65% achieving gross total resection (GTR). Of 16 patients without GTR at first surgery and subsequent local recurrence, 10 achieved GTR at subsequent surgery for recurrence because: (i) first surgery was done by a different surgeon at an outside institution (n = 5); (ii) more aggressive surgery near critical structures was performed at recurrence (n = 4); or (iii) the pathologic diagnosis was revised from glioma to ependymoma after first surgery (n = 1). Nine patients relapsed with macroscopic disseminated disease, including 3 with concurrent local relapse. Of these nine, 6 underwent tumor resection and 3 had more extensive recurrences; of these three, 1 underwent biopsy, and 2 had no surgery.
Of all patients, 22 relapsed locally, without radiologic or pathologic evidence of dissemination. Among these children, 15 underwent focal RT2 (13 with fractionated RT2, and 2 using single fraction SRS). The 2 patients who received SRS for small recurrences in the original tumor bed did not have surgery prior to RT2. Seven patients with local relapse received CSI-RT2, with a further boost to the local site of recurrence. One patient among these 7 (patient #14) had suspicious cerebrospinal fluid cytology prior to RT2 without macroscopic evidence of distant disease. There was no statistically significant difference in the proportion of locally recurrent patients who attained a GTR versus not before RT2, between the focal or CSI-RT2 groups (P = 0.35). The median age at RT2 was 6.4 years (range, 5.3–8.4) and 13.2 years (range, 7.3–22.3) among those who received 23.4 Gy (n = 4) and 36 Gy (n = 3) CSI, respectively, for locally recurrent disease (P = 0.057). The shortest RT1-to-RT2 time interval in the study was 6 months, with a median interval of 23 months.
Of all re-irradiated patients, 27 received re-irradiation at the time of first recurrence and 3 received re-irradiation at second recurrence (with surgery and/or chemotherapy at first recurrence). One patient received RT at third recurrence. The mean and median combined RT1 and RT2 physical prescriptions were 106 Gy and 113 Gy, respectively.
Survival
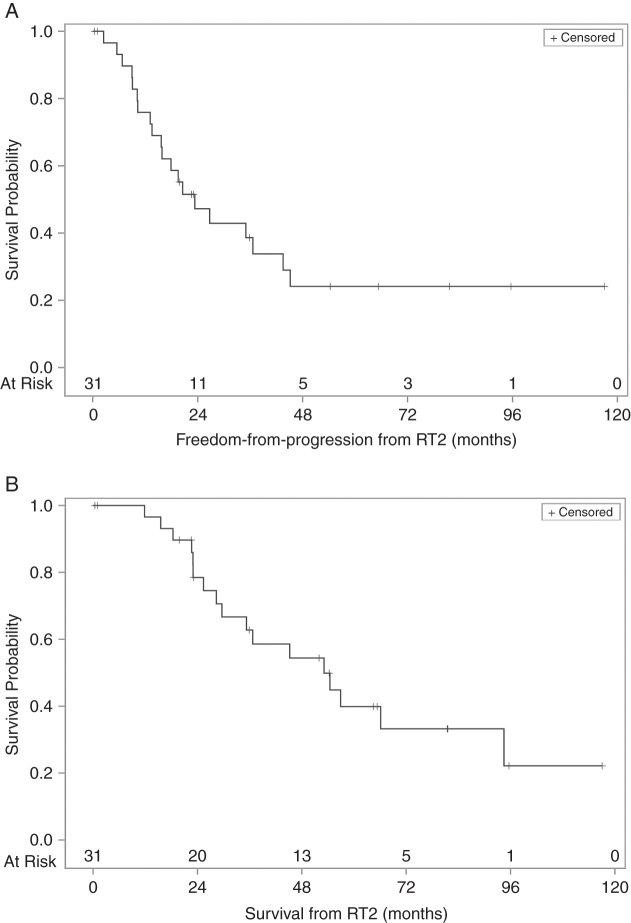
Median follow-up was 65.3 months. Median FFP was 23.3 months (95% CI: 13.6–43.5), with 3- and 5-year FFP of 38.6% (95% CI: 20.6–56.4) and 24.1% (95% CI: 9.4–42.5; Fig. 1). Because no patient died without the presence of progressive ependymoma, FFP was equal to progression-free survival. The median survival for the entire cohort was 53.1 months (95% CI: 28.4–94.5), with 3- and 5-year OS of 62.8% (95% CI: 41.7–78.0) and 39.9% (95% CI: 20.5–58.7).
Fig. 1.
(A) Freedom from progression and (B) overall survival from RT2.
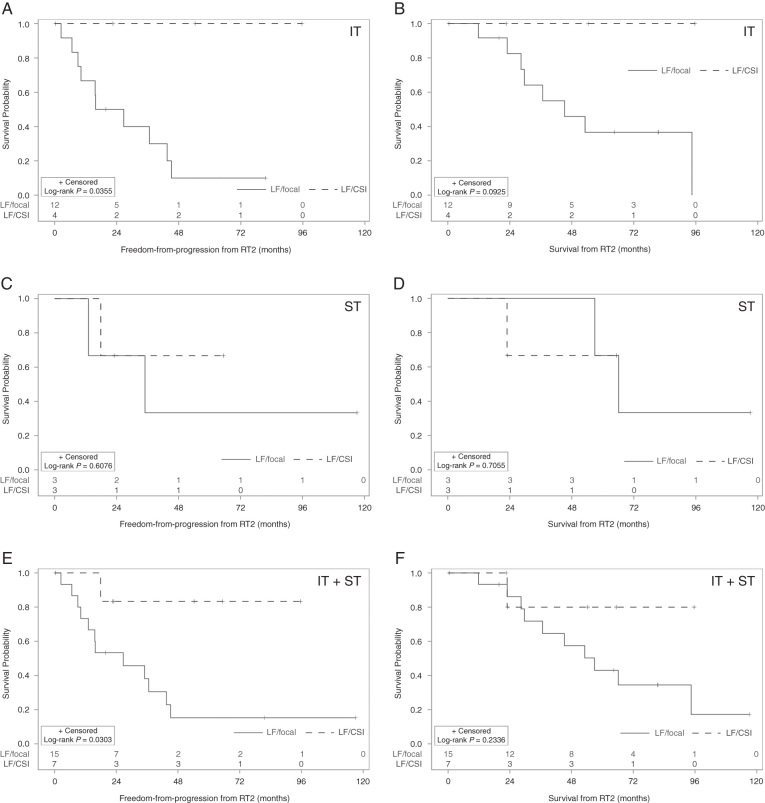
Among those with local failures after RT1, use of CSI-RT2 was associated with a statistically significant improvement in FFP. The median FFP was 26.7 months (95% CI: 9.1–43.5) in those who did not receive CSI-RT2, versus not reached for those who received CSI-RT2 (log-rank P = 0.030; Fig. 2). The 5-year FFP was 15.2% (95% CI: 2.5–38.2) versus 83.3% (95% CI: 27.3–97.5) for no CSI versus CSI-RT2, respectively. However, this difference did not translate into a statistically significant difference in OS, with a 5-year OS of 43.1% (95% CI: 17.9–66.2) versus 80.0% (95% CI: 20.4–96.9; log-rank P = 0.23) for no CSI versus CSI-RT2, respectively.
Fig. 2.
Outcomes for re-irradiated patients with local failure, by RT2 field and site of disease. (A) Freedom from progression in patients with infratentorial (IT) primaries. (B) Overall survival in patients with IT primaries. (C) Freedom from progression in patients with supratentorial (ST) primaries. (D) Overall survival in patients with ST primaries. (E) Freedom from progression in all patients. (F) Overall survival in all patients.
Among 22 patients with infratentorial ependymoma, 16 experienced local failures after RT1. Of those, four received CSI as part of RT2 while 12 received focal RT2. In an exploratory subgroup analysis of patients with infratentorial ependymoma and local relapse, CSI-RT2 was associated with improvement in 5-year FFP (100% [95% CI: 100‒100]) compared with those treated with focal RT2 only (10.0% [95% CI: 0.6–35.5], log-rank P = 0.036; Fig. 2). There may be an associated OS improvement (5-year survival of 100% [95% CI: 100‒100] vs 36.7% [95% CI: 11.3–63.0], respectively) but this did not reach statistical significance (log-rank = 0.093).
Among 9 patients with supratentorial ependymoma, 6 experienced local failures after RT1; of these, half received CSI as part of RT2. In an exploratory subgroup analysis of patients with supratentorial ependymoma and local relapse, CSI-RT2 was not associated with differences in FFP or OS, likely due to a small sample size (Fig. 2).
Because the radiation treatment technique differed between fractionated RT2 and SRS RT2, analyses were repeated after excluding all children treated with a second course of radiation using SRS (n = 2). Results are shown in Supplementary Fig. S2. Use of CSI-RT2 continued to be associated with a statistically significant FFP benefit among the entire cohort (log-rank P = 0.016) and in the subgroup of patients with infratentorial primary tumors (log-rank P = 0.039).
Among all patients with distant failures after RT1, CSI was always given. Despite this treatment, there were no long-term survivors beyond 5 years. Median survival was 30.3 months; 2-year and 4-year OS probabilities were 62.5% (95% CI: 22.9–86.1) and 37.5% (95% CI: 8.7–67.4), respectively. Median FFP was 16.6 months, with a 2-year FFP probability of 12.5% (95% CI: 0.6–42.3).
Factors associated with OS and FFP are listed in Table 2. On univariate analysis, no factor was significantly associated with OS. There was a strong trend to improved FFP with use of CSI for local failures after RT1. There was no interaction between use of CSI and the extent of surgery before RT1 (P = 0.81) or before RT2 (P = 0.64) for the FFP endpoint. Sex was excluded from this analysis because all patients with locally recurrent ependymoma treated after 2011 with CSI-RT2 were males. Few females, who have a better prognosis overall,4,14 recurred after 2011; thus far, none had yet been treated with CSI for a locally recurrent ependymoma.
Table 2.
Clinicopathologic factors associated with OS and FFP after RT2 on univariate analysis
| Variable | Overall Survival | Freedom from Progression | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Supratentorial (vs infratentorial) | 1.1 (0.41–3.0) | 0.84 | 0.98 (0.37–2.5) | 0.98 |
| Less than GTR before RT1 (vs GTR) | 2.0 (0.65–6.2) | 0.22 | 2.6 (0.92–7.1) | 0.071 |
| Less than GTR before RT2 (vs GTR) | 1.9 (0.74–5.0) | 0.18 | 1.7 (0.69–4.0) | 0.26 |
| Treatment group | ||||
| LF/focal RT2 | Reference | Reference | ||
| LF/CSI RT2 | 0.30 (0.04–2.4) | 0.26 | 0.14 (0.02–1.1) | 0.061 |
| dissem/CSI RT2 | 2.5 (0.82–7.4) | 0.11 | 1.5 (0.57–4.1) | 0.40 |
| Time between RT1 and RT2 (per year) | 0.93 (0.77–1.1) | 0.52 | 0.91 (0.75–1.1) | 0.35 |
dissem = disseminated or combined (distant and local) failure after RT1; HR = hazard ratio; LF = local failure after RT1.
The patterns of failure after RT2, stratified by type of RT1 failure and treatment received, are listed in Table 3. Most patients who received CSI-RT2 for local failure after RT1 did not recur; 1 patient in this group developed recurrent disease outside the RT2 boost volume (but within the CSI-RT2 treated volume).
Table 3.
Patterns of failure after RT2
| Pattern of Failure after RT2 | Local Failure after RT1 | Distant Failure after RT1 | |
|---|---|---|---|
| Focal RT2 (n = 15) | CSI RT2 (n = 7) | CSI RT2 (n = 9) | |
| n (%) | |||
| No recurrence | 3 (20) | 6 (86) | 2 (20) |
| Local failure | 6 (40) | ||
| Disseminated failure | 6 (40) | 1 (14) | 7 (70) |
Toxicities and Functional Outcomes After RT2
Patients who were treated with RT2 tolerated re-treatment well, with no high-grade acute toxicities. All patients completed the planned course of RT2 re-irradiation.
One patient developed radiation necrosis attributable to RT2; patient #7 developed grade 3 necrosis after SRS RT2 that was treated with dexamethasone alone. The patient had some neurologic recovery but remained ataxic and wheelchair dependent; this individual eventually developed local disease progression and died. Two patients developed tumor progression with evidence of necrosis after RT2 (patients #3 and #24). Three other patients developed necrosis: one after a third course of radiotherapy (RT3) (21 Gy SRS; patient #1), one after fractionated RT3 (36 Gy CSI followed by in-field supratentorial boost to 54 Gy with RT1 and RT2 overlap; patient #6), and one after a fifth course of RT (SRS, patient #12). Treatments for necrosis included observation (patients #3 and #12), dexamethasone only (patients #1 and #7), bevacizumab (patients #6 and #24), and hyperbaric oxygen (patient #6).
Functional outcomes among 10 long-term survivors without evidence of active disease (median follow-up: 59.8 months) are listed in Table 4. Most individuals had mild or moderate difficulties with formalized education, requiring additional assistance in the form of an individualized education plan.15 Two patients—including one who received CSI—were able to enroll in a post-secondary education program. Half of all surviving patients did not, or have not yet, developed neuroendocrine deficits; of the 5 who developed such deficits, 3 received 23.4 Gy CSI and 2 received 36 Gy.
Table 4.
Functional outcomes among survivors without active disease*
| Patient no. | Age at RT2 | RT2 Field | Follow-up Time Since RT2 (mo) | School Performance at Last Follow-Up | Neuroendocrine Deficits |
|---|---|---|---|---|---|
| 2 | 5.4 | focal (RT3 CSI 23.4 Gy) | 81.4 | Attends regular school with extra help in mathematics and language | Hypothyroidism and GH deficiency |
| 5 | 11.2 | SRS | 117.1 | Attended post-secondary education (university) | None |
| 9 | 22.3 | CSI 36 Gy | 65.3 | Gainfully employed but with some neurocognitive deficits | None |
| 11 | 3.3 | focal | 81.6 | Developmental delay with delayed speech | None |
| 13 | 5.3 | CSI 23.4 Gy | 54.3 | Attends regular school with extra help with language | GH deficiency |
| 14 | 9.8 | CSI 36 Gy | 95.6 | Attends post-secondary education (university) | Hypothyroidism and GH deficiency |
| 16 | 13.2 | CSI 36 Gy | 35.9 | Attends regular school with extra help for mathematics and science | Irregular menses |
| 26 | 6.6 | focal | 19.8 | Attends regular school with extra help for mathematics; has grade 2 ataxia | None |
| 27 | 8.4 | CSI 23.4 Gy | 23.1 | Attends regular school with extra help for language (2 grades behind) and mathematics (1 grade behind) | None |
| 28 | 6.1 | CSI 23.4 Gy | 22.6 | Attends regular school | GH deficiency |
*Two individuals with <18 months of follow-up are excluded from this table.
Third Course of Radiation
Following RT2, eight patients went on to receive at least one more course of radiotherapy, with overlap with RT1 or RT2 in 7 individuals. Three patients received whole spine RT3 followed by focal spine boosts, 2 received fractionated focal RT3, 2 received CSI followed by boost RT, and 1 received SRS. Of all 8 patients, 2 had received prior CSI as part of RT2 (36 Gy, patients #18 and #21). Individual cases are discussed in detail in the Supplementary Material. Median survival after RT3 was 19.3 months, and median FFP was 8.8 months. In patient #2, RT3 led to long-term survival (>5 y from RT3); this individual was treated with focal RT2 but suffered distant failure; RT3 consisting of 23.4 Gy CSI and boost to all sites of disease led to long-term disease control.
Discussion
We present 31 pediatric patients treated with a repeat course of radiation for relapsed intracranial ependymoma. Most patients had near total resection or GTR prior to RT2. Our institutional practice to systematically offer CSI to patients with recurrent local disease from 2012 onward created an ideal quasi-experimental before-and-after cohort to study the role of CSI in treatment of locally recurrent intracranial ependymoma. This study found that CSI was associated with a statistically significant improvement in FFP, particularly in the setting of local recurrence of infratentorial (posterior fossa) ependymoma. There was also a trend to improved OS with use of CSI-RT2 for locally recurrent infratentorial ependymoma.
A majority of patients (n = 23) had successful molecular subgroup characterization. This is the first known study of recurrent intracranial ependymoma to include molecular and methylation subgroup information. It is well known that posterior fossa group A and RELA-fused tumors carry a worse prognosis13,16; thus, it is not surprising that our cohort comprised exclusively these tumors. Gain of chromosome 1q is also known as a negative prognostic factor17,18; in a large cohort of patients treated at St Jude Children’s Research Hospital, patients with recurrent intracranial ependymoma treated with repeat RT were enriched for both 1q gain and RELA fusions.9
Prior Studies
Merchant et al from St Jude were the first to report detailed outcomes for pediatric patients re-irradiated for ependymoma,7 with a recent update by Tsang et al.9 There were 46 locally recurrent patients who received focal RT2 and 10 patients who received CSI as part of RT2. In a multivariable analysis, use of CSI for locally recurrent disease was not associated with a statistically significant improvement in OS or FFP. CSI did eliminate disseminated failures after RT2, whereas 44% of recurrences after focal RT2 were distant-only failures. Patients selected for RT2-CSI with locally recurrent ependymoma in this study likely had attributes of more aggressive disease.
A survey of the management of 108 children with relapsed ependymoma was reported by Messahel et al.19 Sixty-nine percent of these children had local-only relapse. In a comparison of all patients, including those with distant recurrence, children who received CSI at relapse (n = 10) had improved survival compared with those irradiated focally at relapse (n = 17) and those who did not receive radiotherapy at relapse (n = 45). Only 14 of these 27 irradiated patients had RT as re-irradiation; nonetheless, as with this current study, CSI as a component of salvage therapy was associated with improved outcomes.
Few other studies have reported specifically upon the use of CSI for locally recurrent ependymoma. In a series by Lobon et al, 15 patients with local recurrences were treated with focal irradiation, with a crude disease control rate of 40%.20 Distant failures represented about half of post-RT2 recurrences. In a study by Eaton et al, 11 patients with locally recurrent ependymoma were re-treated with focal proton therapy.21 In this subgroup, 5 experienced further local failures after RT2 without any cases of distant failures. Outcomes for up to 12 re-irradiated patients are included within other series.22–24 For example, in the study by Combs et al, 3-year progression-free survival and OS for 7 patients focally re-irradiated for local recurrence were 64% and 83%, respectively, following a median dose of 36 Gy (range 20–60 Gy) in 1.8–2.0 Gy fractions.23 Survival outcomes were comparable to those observed in this current study for focally re-irradiated patients.
Radiation Necrosis
Some caution may be required if using a hypofractionated schedule for re-irradiation in young patients: Hoffman et al described hypofractionated RT2 in 11 pediatric patients with locally recurrent ependymoma using 24 Gy in 3 fractions with excellent 3-year local control (89%).25 However, radiation necrosis occurred in 45% of re-irradiated patients (n = 5), two of whom required treatment with bevacizumab.25 Merchant et al reported low rates of disease control and high rates of necrosis (crude rate 83%) in 6 patients re-irradiated with SRS.7 In the present study, there were 2 individuals who developed evidence of tumor progression synchronously with necrosis after RT2. The remaining 4 instances of necrosis occurred after SRS or irradiation after RT2. Therefore, our contemporary practice is to deliver RT2 using daily fractions of 1.8 Gy per day.
To our knowledge, this is one of the 3 largest series examining re-irradiation in children with relapsed ependymoma. It is also the only known study to specifically evaluate CSI as part of RT2 in the setting of isolated focal relapse, and to report the molecular subgrouping of recurrent pediatric ependymoma. However, there are some important differences between these data and the cases previously reported in the literature.
First, in the present study, those with disseminated relapse after RT1 had poor outcomes, with no long-term survivors, despite treatment with CSI as part of RT2. This is in contrast to the data from St Jude9 and from France,20 which showed that those with distant failures treated with CSI did well. In the present study, the interval between RT1 and failure was 1.4 years among those with disseminated relapse, comparable to the French data (1.2 y for all patients) and the St Jude cohort (1.8 y for all patients). In our dataset, those with combined local and distant failure, a recognized poor prognostic factor,9 were categorized together in one “disseminated” group due to the small numbers of patients with combined failures; this may have negatively affected the overall outcome of this cohort. Second, all but one of these patients had anaplastic histology at diagnosis, which may be challenging to control, even with RT2.9 There can also be a referral bias among studies in the literature, whereby patients with a better prognostic profile may be included, resulting in improved outcomes reported by other research groups as compared with our present study, which included an unselected group of patients. Overall, longer-term follow-up is needed to better elucidate the true nature of this high-risk subgroup of children.
This study has some limitations. Although associated with a large FFP benefit, the number of patients with local recurrence after RT1 treated with CSI-RT2 was small (n = 7). Detailed tests of neuropsychological function after RT2 were available for only 4 patients and are not reported here due to small numbers. Furthermore, infratentorial and supratentorial ependymoma are 2 different disease entities with different molecular alterations; as such, they should be considered separately.17 To date, re-irradiation studies have not handled ependymoma in this manner. Although our subgroup analysis of infratentorial tumors demonstrated an FFP benefit to CSI-RT2, greater patient numbers are needed to validate these findings. There were insufficient patient numbers to rigorously evaluate the role of CSI-RT2 in supratentorial tumors, in neither local nor disseminated recurrence; these are subgroups deserving of further study. There were also insufficient events to perform a multivariable analysis. Testing for 1q chromosome gain was not performed. It was not possible to stratify results by molecular subgroup because all the supratentorial tumors had RELA fusions and no infratentorial patient had posterior fossa group B pathology; therefore, molecular pathology was reported descriptively only. Most—though not all—of our patients were treated with modern, inverse-planned radiotherapy. It is possible that more modern techniques have resulted in improved tumor targeting and/or better organ-at-risk sparing compared with older techniques, but this current dataset does not have sufficient numbers of patients treated with older techniques to allow such an analysis. None of the patients in this series received proton therapy, which may add additional benefits. Radiotherapy techniques, in particular the impact of protons, should therefore be the focus of further study. Finally, our data are not able to determine whether 23.4 Gy or 36 Gy CSI is optimal for locally recurrent ependymoma.
There is published evidence supporting use of proton beam therapy to deliver CSI as definitive treatment for primary brain tumors in adults26 and children.27 Proton CSI is associated with reduced acute toxicities26,27 as well as an expectation of reduced late toxicities due to dosimetric sparing of normal tissues in the torso.28–32 Given the high rates of disease control with CSI for locally recurrent ependymoma, proton CSI could be considered in this setting. There is an ongoing prospective study (ClinicalTrials.gov identifier NCT02125786) that allows use of proton CSI for recurrent ependymoma.
Conclusion
In a molecularly characterized cohort of pediatric patients, a statistically significant FFP benefit was observed among individuals with locally recurrent ependymoma treated with CSI as a component of re-irradiation, compared with those treated with focal RT2 alone. This FFP benefit was also observed in the subgroup of patients with locally recurrent infratentorial tumors. Long-term follow-up is needed to see if CSI-RT2 results in an OS benefit with time. Our findings warrant further prospective investigation and validation in a larger multi-institutional collaborative setting. Until these data become available, patients with locally recurrent ependymoma should be offered the option of repeat surgery and CSI as part of re-irradiation to maximize the likelihood of disease control, though the potential benefits should be weighed against the long-term side effects of CSI.
Funding
LM was previously supported by the Princess Margaret Cancer Foundation and is currently a University Clinical Academic Fellow funded by Yorkshire Cancer Research (award number L389LM). MZ was supported by a Meagan’s Walk Fellowship in Paediatric Neuro-Oncology and fellowships from the Garron Family Cancer Centre and Restracomp.
Supplementary Material
Conflict of interest statement
None.
Authorship statement
Collected data. DST, LM, VR, MZ, MDT, CH, EB, NL
Data analysis. DST, LM, EB
Interpreted the data. All authors
Wrote the manuscript. All authors
References
- 1. Ries LAG, Smith MA, J.G. G, et al. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995, National Cancer Institute, SEER Program. NIH Publication Number 99–4649 Bethesda, MD: National Cancer Institute, SEER Program; 1999. [Google Scholar]
- 2. CBTRUS. Statistical Report: Primary Brain Tumors in the United States, 1997–2001. Hinsdale, IL: Published by the Central Brain Tumor Registry of the United States; 2004. [Google Scholar]
- 3. Tamburrini G, D’Ercole M, Pettorini BL, Caldarelli M, Massimi L, Di Rocco C. Survival following treatment for intracranial ependymoma: a review. Childs Nerv Syst. 2009;25(10):1303–1312. [DOI] [PubMed] [Google Scholar]
- 4. Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reni M, Gatta G, Mazza E, Vecht C. Ependymoma. Crit Rev Oncol Hematol. 2007;63(1):81–89. [DOI] [PubMed] [Google Scholar]
- 6. Sangra M, Thorp N, May P, Pizer B, Mallucci C. Management strategies for recurrent ependymoma in the paediatric population. Childs Nerv Syst. 2009;25(10):1283–1291. [DOI] [PubMed] [Google Scholar]
- 7. Merchant TE, Boop FA, Kun LE, Sanford RA. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys. 2008;71(1):87–97. [DOI] [PubMed] [Google Scholar]
- 8. Bouffet E, Hawkins CE, Ballourah W, et al. Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys. 2012;83(5):1541–1548. [DOI] [PubMed] [Google Scholar]
- 9. Tsang DS, Burghen E, Klimo P Jr, Boop FA, Ellison DW, Merchant TE. Outcomes after reirradiation for recurrent pediatric intracranial ependymoma. Int J Radiat Oncol Biol Phys. 2018;100(2):507–515. [DOI] [PubMed] [Google Scholar]
- 10. Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bayliss J, Mukherjee P, Lu C, et al. Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci Transl Med. 2016;8(366):366ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panwalkar P, Clark J, Ramaswamy V, et al. Immunohistochemical analysis of H3K27me3 demonstrates global reduction in group-A childhood posterior fossa ependymoma and is a powerful predictor of outcome. Acta Neuropathol. 2017;134(5):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massimino M, Miceli R, Giangaspero F, et al. Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro Oncol. 2016;18(10):1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The pediatrician’s role in development and implementation of an Individual Education Plan (IEP) and/or an Individual Family Service Plan (IFSP). American Academy of Pediatrics. Committee on children with disabilities. Pediatrics. 1999;104(1 Pt 1):124–127. [DOI] [PubMed] [Google Scholar]
- 16. Ramaswamy V, Hielscher T, Mack SC, et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: a retrospective multicohort analysis. J Clin Oncol. 2016;34(21):2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Godfraind C, Kaczmarska JM, Kocak M, et al. Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol. 2012;124(2):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendrzyk F, Korshunov A, Benner A, et al. Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res. 2006;12(7 Pt 1):2070–2079. [DOI] [PubMed] [Google Scholar]
- 19. Messahel B, Ashley S, Saran F, et al. ; Children’s Cancer Leukaemia Group Brain Tumour Committee Relapsed intracranial ependymoma in children in the UK: patterns of relapse, survival and therapeutic outcome. Eur J Cancer. 2009;45(10):1815–1823. [DOI] [PubMed] [Google Scholar]
- 20. Lobón M, Bautista F, Riet F, et al. Re-irradiation of recurrent pediatric ependymoma: modalities and outcomes: a twenty-year survey. SpringerPlus. 2016;5(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eaton BR, Chowdhry V, Weaver K, et al. Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol. 2015;116(2):301–308. [DOI] [PubMed] [Google Scholar]
- 22. Antony R, Wong KE, Patel M, et al. A retrospective analysis of recurrent intracranial ependymoma. Pediatr Blood Cancer. 2014;61(7):1195–1201. [DOI] [PubMed] [Google Scholar]
- 23. Combs SE, Thilmann C, Debus J, Schulz-Ertner D. Local radiotherapeutic management of ependymomas with fractionated stereotactic radiotherapy (FSRT). BMC Cancer. 2006;6:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zacharoulis S, Ashley S, Moreno L, Gentet JC, Massimino M, Frappaz D. Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst. 2010;26(7):905–911. [DOI] [PubMed] [Google Scholar]
- 25. Hoffman LM, Plimpton SR, Foreman NK, et al. Fractionated stereotactic radiosurgery for recurrent ependymoma in children. J Neurooncol. 2014;116(1):107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown AP, Barney CL, Grosshans DR, et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86(2):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song S, Park HJ, Yoon JH, et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol. 2014;53(9):1158–1164. [DOI] [PubMed] [Google Scholar]
- 28. Zhang R, Howell RM, Giebeler A, Taddei PJ, Mahajan A, Newhauser WD. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys Med Biol. 2013;58(4):807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58(3):727–734. [DOI] [PubMed] [Google Scholar]
- 30. Seravalli E, Bosman M, Lassen-Ramshad Y, et al. Dosimetric comparison of five different techniques for craniospinal irradiation across 15 European centers: analysis on behalf of the SIOP-E-BTG (radiotherapy working group). Acta Oncol. 2018;57(9):1240–1249. [DOI] [PubMed] [Google Scholar]
- 31. Brodin NP, Munck Af Rosenschöld P, Aznar MC, et al. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol. 2011;50(6):806–816. [DOI] [PubMed] [Google Scholar]
- 32. Mailhot Vega RB, Kim J, Bussière M, et al. Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer. 2013;119(24):4299–4307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.