Abstract
Background
We sought to determine the value of diffusion-weighted (DW) magnetic resonance imaging (MRI) for characterization of benign and malignant peripheral nerve sheath tumors (PNSTs) in patients with neurofibromatosis type 1 (NF1).
Methods
Twenty-six patients with NF1 and suspicion of malignant transformation of PNSTs were prospectively enrolled and underwent DW MRI at 3T. For a set of benign (n = 55) and malignant (n = 12) PNSTs, functional MRI parameters were derived from both biexponential intravoxel incoherent motion (diffusion coefficient D and perfusion fraction f) and monoexponential data analysis (apparent diffusion coefficients [ADCs]). A panel of morphological MRI features was evaluated using T1- and T2-weighted imaging. Mann–Whitney U-test, Fisher’s exact test, and receiver operating characteristic (ROC) analyses were applied to assess the diagnostic accuracy of quantitative and qualitative MRI. Cohen’s kappa was used to determine interrater reliability.
Results
Malignant PNSTs demonstrated significantly lower diffusivity (P < 0.0001) compared with benign PNSTs. The perfusion fraction f was significantly higher in malignant PNSTs (P < 0.001). In ROC analysis, functional MRI parameters showed high diagnostic accuracy for differentiation of PNSTs (eg, ADCmean, 92% sensitivity with 98% specificity, AUC 0.98; Dmean, 92% sensitivity with 98% specificity, AUC 0.98). By contrast, morphological imaging features had only limited sensitivity (18–94%) and specificity (18–82%) for identification of malignancy. Interrater reliability was higher for monoexponential data analysis.
Conclusion
DW imaging shows better diagnostic performance than morphological features and allows accurate differentiation of benign and malignant peripheral nerve sheath tumors in NF1.
Keywords: diffusion-weighted imaging, intravoxel incoherent motion analysis, morphological features, neurofibromatosis type 1, peripheral nerve sheath tumor
Key Points.
DWI allows sensitive and specific identification of malignancy in NF1.
Morphological features have limited accuracy for identifying malignant transformation.
Importance of the Study.
Malignant transformation of PNSTs is the leading cause of mortality in patients with NF1. The only curative approach is early detection followed by radical resection. Morphological features of tumors as determined by MRI have limited diagnostic accuracy. We aimed to assess the value of DW MRI for characterization of PNSTs in NF1 as malignant or benign. Our results show that DW imaging is better suited for identification of malignant transformation. Functional MRI should therefore be implemented as a complementary diagnostic tool when screening for malignant PNSTs in NF1 patients.
Neurofibromatosis type 1 (NF1) is an autosomal-dominantly inherited tumor predisposition syndrome with an incidence of 1 in 2500 newborns.1 A key feature of NF1 is the development of peripheral nerve sheath tumors (PNSTs).2 PNSTs undergo transformation into malignant peripheral nerve sheath tumors (MPNSTs) in 8–13% of NF1 patients.3 Malignant PNSTs are a leading cause of death in NF1 (up to 55%)4 and contribute to the increased mortality of these patients.5 The only curative approach to malignant PNSTs is early detection and resection.2,6 Symptoms suggestive of malignant transformation such as tumor growth, neurological deficits, or pain are nonspecific, not observed in all cases, and often signs of advanced disease.7
Therefore, NF1 patients are repeatedly imaged with whole-body magnetic resonance imaging (MRI), allowing evaluation of tumor burden8–10 and thus identification of patients at risk of developing malignant PNSTs.11,12 Morphological features of PNSTs at MRI such as intratumoral heterogeneity, intratumoral cystic changes, and ill-defined tumor margins cannot reliably discriminate between benign and malignant PNSTs,13,14 advocating a role for functional parameters for detection of malignancy, such as increased 18F-fluorodeoxyglucose (18F-FDG) consumption at positron emission tomography (PET).15–17 However, PET is associated with radiation exposure, and false positive results in plexiform neurofibromas have been described in 5%.7
Functional MRI with diffusion-weighted imaging (DWI) has been suggested for differentiation between malignant and benign soft-tissue tumors to increase the diagnostic accuracy.18 A recent retrospective study demonstrated that DWI-derived parameters may hold promise to distinguish malignant from benign PNSTs.14 However, that study excluded many tumors in NF1 patients (eg, tumors <5 cm), leaving the applicability for early detection of malignant transformation unanswered. Furthermore, only a monoexponential apparent diffusion coefficient (ADC) model based on a limited number of b-values was applied. However, monoexponential ADC models do not consider the physiological process of microcapillary perfusion. By contrast, the biexponential intravoxel incoherent motion (IVIM) model enables further separation of tissue perfusion and diffusivity.19,20 IVIM has shown promise for distinguishing malignant from benign pediatric abdominal tumors21 and in the differentiation of high- from low-grade hepatocellular carcinoma.22
Therefore, we aimed to assess the value of DW MRI for characterization of benign and malignant PNSTs in patients with NF1, and to compare the diagnostic performance of biexponential IVIM with that of monoexponential data analysis and established morphological features of malignant transformation.
Materials and Methods
This prospective study is compliant with the Health Insurance Portability and Accountability Act and the Declaration of Helsinki and has been approved by the local institutional review board. Written informed consent was obtained from all patients.
Patient Cohort
Patients who were referred for MRI screening for malignant PNSTs between August 2014 and September 2017 were eligible for inclusion in this study. Inclusion criteria were fulfillment of the National Institutes of Health diagnostic criteria for NF1,23 changes in texture or size of known tumors in screening whole-body MRI scans or onset of clinical symptoms, and minimum tumor size of ≥20 mm. Patients were consecutively and prospectively enrolled if none of the following exclusion criteria were present: unwillingness or disability to give informed consent.
Histopathological evaluation after tumor resection and/or radiological follow-up served as the reference standard. Resected tumors were classified according to the grading systems of the World Health Organization (WHO) and the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC).24,25 Tumors were considered benign when no changes in size or appearance were present in follow-up examinations within ≥12 months.
Image Acquisition
Imaging was performed at 3T (Philips Ingenia). MRI sequences consisted of a T1-weighted gradient recalled echo localizer, a coronal T2-weighted turbo spin echo sequence, an axial T2-weighted turbo spin echo sequence with fat suppression, and a 3D T1-weighted multipoint Dixon (mDixon) turbo spin echo sequence with in- and opposed phase (Supplementary Table 1). DWI consisted of axial respiratory-triggered echo-planar sequences with 11 diffusion gradient b-values (0, 10, 20, 30, 50, 70, 100, 300, 400, 600, 800 s/mm2). Intravenous contrast material was not administered. Scan time was 35 minutes.
Image Analysis
Images were independently analyzed in a randomized blinded fashion by 2 radiologists with 7 (J.S.) and 4 years (L.W.) of experience in MR imaging. Image analyses were undertaken on a workstation with a picture archiving and communication system (Centricity Universal Viewer, v6.0, GE Healthcare).
Image Processing and Data Analysis Using DWI
Data were processed with a self-developed image-analysis framework (qMapIt)26 extending the open source software ImageJ (National Institute of Mental Health). Quantitative parametric maps were calculated by nonlinear regression with pixelwise fitting of signal intensities over the spectrum of b-values to the corresponding model. A monoexponential function was applied for ADC determination. The IVIM model was fitted in 2 steps to a biexponential function. First, the tail of the curve with b-values larger than 150 s/mm2 was fitted to a monoexponential function and the diffusion coefficient D was extracted. Next, D was kept constant when fitting for the determination of the perfusion fraction f and the pseudo perfusion D*. The value of D* was determined in an initial study performing the analysis over regions averaging pixel values improving the signal-to-noise ratio. D* was consequently fixed to 20000 µm2/s to further increase numerical stability.
ImageJ was used for analysis of parameter maps (Fig. 1A). For all parameters, regions of interest (ROIs) were placed in diffusion images (b-value 50 s/mm2). ROIs were placed along tumor margins in 3 adjacent slices with largest axial tumor diameter (Supplementary Fig. 1). After manual placement of ROIs along tumor margins, the software automatically reduced the radius of ROIs by 3 mm to eliminate the risk of exceeding tumor margins. ImageJ then automatically extracted values from the previously created ADC and IVIM parameter maps. Averaged values from the 3 adjacent ROIs were calculated to determine ADCmean, ADCmin, Dmean, and Dmin. Perfusion fraction f was determined likewise, and termed fcenter. Additionally, a single ROI was placed along tumor margins at lowest signal intensity, to determine ADCdark and Ddark. The rationale for this approach was that malignant PNSTs arise within benign PNSTs and are associated with foci of high cellularity.2 Therefore, minimum ADC values or areas of low signal intensity might improve diagnostic yield.14,27 Finally, ring-like ROIs—containing the area between tumor margin ROIs and ROIs with automatically reduced radius—were used to obtain fmargin and Dmargin, respectively (Supplementary Fig. 1).
Fig. 1.
(A) MR images of a 49-year-old male patient with a malignant (M)PNST, left column) and of a 39-year-old female patient with a benign (B)PNST, right column). T2-weighted images (upper row) and parametric maps (ADC, D, and f, respectively) calculated from DWI data. The thoracic MPNST is characterized by lower signal intensity in T2w imaging as well as lower values in ADC and D parameter maps, and higher perfusion fraction f compared with the BPNST. (B–E) Box-and-whisker plots of ADC (B) and IVIM-derived parameters (C–E) for characterization of MPNSTs and BPNSTs. Significant differences between MPNSTs and BPNSTs were observed for all approaches. (F) T2-weighted MR images of different patients with malignant (upper row) and benign (lower row) PNSTs demonstrating the evaluated morphological features.
Evaluation of Morphological Features
Morphological evaluation was performed using T1- and T2-weighted sequences. After measurement of tumor diameter, each PNST was evaluated for the presence or absence of 7 distinct morphological features (Fig. 1F): shape (round vs irregular), margin definition (well vs ill-defined), intratumoral lobulation, intratumoral heterogeneity, cystic changes (ie, high homogeneous T2w- and low homogeneous T1w signal), target sign (ie, hyperintense margin with hypointense center on T2-weighted images), and peritumoral edema.13,28,29
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation (SD). Categorical variables are presented with absolute and relative frequencies. For comparison of continuous data, P-values were calculated from Mann–Whitney U-tests. For categorical variables, P-values were computed from contingency tables using Fisher’s exact test. Receiver operating characteristics (ROC) analyses were performed to determine the area under the ROC curve (AUC) and to determine optimal cutoff values for differentiation of benign from malignant PNSTs. Spearman correlation analysis was used to correlate findings between IVIM-derived values D and f. Cohen’s kappa was used to evaluate interrater reliability. P-values <0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism 5.0 for Windows.
Results
Study Population and Reference Standard
Twenty-six patients (13 men and 13 women) with a mean age of 34.2 years (17–54 y) were imaged, and a total of 67 PNSTs were analyzed.
Histopathological evaluation following resection was available in 42 of 67 PNSTs (62.7%). All (12 of 12) malignant PNSTs were verified by histopathology (6 WHO grade III/FNCLCC grade II and 6 WHO grade IV/FNCLCC grade III tumors). Thirty of 55 benign PNSTs (54.5%) were verified by histopathology (29 plexiform neurofibromas WHO grade I and one neurofibroma), whereas the remaining 25 benign PNSTs (45.5%) were confirmed by follow-up.
PNSTs were more frequently observed in female patients (40 tumors; 60%) than in male patients (27 tumors; 40%). Among these, malignant PNSTs were also more frequently found in female patients (8 tumors; 67%) than in male patients (4 tumors; 33%). Considering the topographic distribution of tumors, we observed 9 cervical tumors (13%; 1 MPNST/8 benign PNSTs), 23 thoracic tumors (34%; 8 MPNSTs/15 benign PNSTs), 31 abdominal tumors (46%; 2 MPNSTs/29 benign PNSTs), and 4 tumors located within the lower extremities (6%; 1 MPNST/3 benign PNSTs).
Diffusion-Weighted Imaging of Peripheral Nerve Sheath Tumors
ADC and D values were significantly lower in malignant PNSTs (ADCmean, 1.23 ± 0.25 × 10−6 mm2/s; Dmean, 1.03 ± 0.28 × 10−6 mm2/s) (all P < 0.0001) compared with benign PNSTs (ADCmean, 2.09 ± 0.30 × 10−6 mm2/s; Dmean, 1.88 ± 0.27 × 10−6 mm2/s) (Fig. 1, Table 1). The average ADC, D, and f values of malignant and benign PNSTs are shown in Table 1.
Table 1.
ADC and IVIM-derived parameters of malignant (M) and benign (B)PNSTs
| Parameter | MPNSTs | BPNSTs | P |
|---|---|---|---|
| ADC mean | 1.23 ± 0.25 | 2.09 ± 0.30 | <0.0001 |
| ADC dark | 1.21 ± 0.26 | 2.03 ± 0.30 | <0.0001 |
| ADC min | 0.60 ± 0.33 | 1.60 ± 0.38 | <0.0001 |
| D mean | 1.03 ± 0.28 | 1.88 ± 0.27 | <0.0001 |
| D dark | 0.99 ± 0.30 | 1.82 ± 0.30 | <0.0001 |
| D min | 0.32 ± 0.33 | 1.40 ± 0.31 | <0.0001 |
| f center | 21 ± 11 | 11 ± 5 | 0.0003 |
| f margin | 24 ± 9 | 14 ± 6 | <0.0001 |
| D margin | 0.92 ± 0.23 | 1.77 ± 0.25 | <0.0001 |
ADC and D values are shown as (value) × 10–6 mm2/s ± standard deviation; f values are shown as %.
Both malignant and benign PNSTs demonstrated a significantly higher perfusion fraction in the tumor periphery than in the tumor center (MPNSTs, fcenter 21 ± 11% vs fmargin 24 ± 9%; P = 0.0038; benign PNSTs, fcenter 11 ± 5% vs fmargin 14 ± 6%; P < 0.0001). Perfusion fraction f was significantly higher in malignant PNSTs, which was observed both in the center (21 ± 11% vs 11 ± 5%; P = 0.0003) and in the periphery of tumors (24 ± 9% vs 14 ± 6%; P < 0.0001) (Table 1). There were no significant ADC, D, or f differences between WHO grade IV and grade III malignant PNSTs (Supplementary Table 2).
There was an inverse correlation between diffusion coefficient D and perfusion fraction f (Dmin vs fcenter; r = −0.488, P < 0.0001).
Interrater reliability
Interrater reliability was “very good” for all ADC-derived parameters (kappa ADCmean, 0.898 − ADCdark, 0.906), whereas interrater reliability was “good to very good” for IVIM-derived diffusion coefficients (kappa Dmean, 0.646 − Ddark, 0.831), and “moderate” for perfusion fraction (kappa fcenter, 0.467 − fmargin, 0.468) (Supplementary Table 3).
Morphological Features of Peripheral Nerve Sheath Tumors
Malignant PNSTs were significantly associated with irregular shape (P = 0.0133), ill-defined margins (P < 0.0001), intratumoral lobulation (P = 0.0293), intratumoral heterogeneity (P < 0.0001), intratumoral cystic changes (P = 0.0006), and peritumoral edema (P < 0.0001) (Fig. 1 and Table 2). The target sign was absent in all malignant PNSTs, but also in a substantial number of benign PNSTs (P = 0.055). Morphological features were not significantly different between WHO grade IV and grade III malignant PNSTs (P > 0.05 in all cases) (Supplementary Table 4). The most common morphological features in both WHO grade IV and grade III tumors were peritumoral edema and intratumoral heterogeneity (grade IV: 6/6 [100%]; grade III: 5/6 [83%]).
Table 2.
Morphological features of MPNSTs (n = 12) versus BPNSTs (n = 55) on MRI
| Parameter | MPNSTs (%) | BPNSTs (%) | P |
|---|---|---|---|
| n | 12 (18) | 55 (82) | ‒ |
| Largest diameter, mm | 0.1407 | ||
| Mean ± SD | 72 ± 75 | 35 ± 15 | |
| Range | 20–290 | 20–103 | |
| Shape | 0.0133 | ||
| Round shape | 6 (50) | 47 (85) | |
| Irregular shape | 6 (50) | 8 (15) | |
| Margin definition | <0.0001 | ||
| Well-defined | 6 (50) | 55 (100) | |
| Ill-defined | 6 (50) | 0 (0) | |
| Intratumoral lobulation | 0.0293 | ||
| Present | 4 (33) | 4 (7) | |
| Absent | 8 (67) | 51 (93) | |
| Intratumoral heterogeneity | <0.0001 | ||
| Heterogeneous | 11 (92) | 5 (9) | |
| Homogeneous | 1 (8) | 50 (91) | |
| Intratumoral cystic changes | 0.0006 | ||
| Present | 4 (33) | 1 (2) | |
| Absent | 8 (67) | 54 (98) | |
| Target sign | 0.055 | ||
| Present | 0 (0) | 23 (42) | |
| Absent | 12 (100) | 32 (58) | |
| Peritumoral edema | <0.0001 | ||
| Present | 11 (92) | 1 (2) | |
| Absent | 1 (8) | 54 (98) |
Diagnostic Performance of Different Imaging Approaches for Differentiation of Malignant from Benign PNSTs
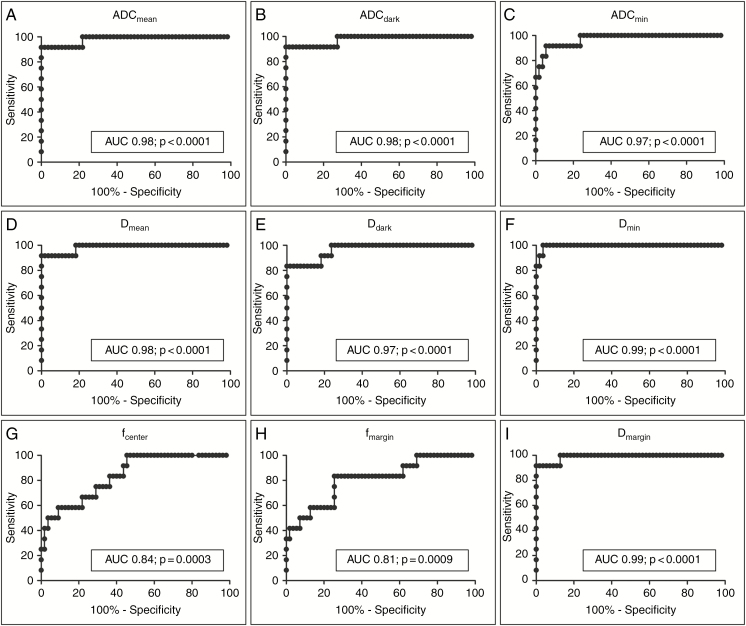
Morphological features demonstrated a sensitivity of 18‒94%, and a specificity of 18‒82% to differentiate between malignant and benign PNSTs (Table 3). The highest sensitivity (94%) was achieved using intratumoral cystic changes as a criterion, albeit associated with low specificity (18%). By contrast, ADC and IVIM-derived parameters demonstrated both high sensitivity and specificity for identification of malignant PNSTs (Fig. 2, Table 4). At ROC analysis, the largest AUC was obtained for Dmin (sensitivity 100% and specificity 96% at a cutoff value of 0.96 × 10−6 mm2/s, AUC, 0.99; P < 0.0001), followed by ADCmean and ADCdark (both AUC, 0.98, P < 0.0001). In general, large AUCs were obtained for both, all ADC and all D values (Fig. 2, Table 4).
Table 3.
Diagnostic performance of morphological features discriminating malignant from benign PNSTs
| Morphological Feature | AUC | 95% CI | Sensitivity | Specificity |
|---|---|---|---|---|
| Shape | 0.52 | 0.42–0.61 | 21% (12–33) | 82% (71–90) |
| Margin definition | 0.55 | 0.45–0.64 | 91% (82–97) | 18% (10–29) |
| Intratumoral lobulation | 0.53 | 0.43–0.63 | 88% (78–95) | 18% (10–29) |
| Intratumoral heterogeneity | 0.53 | 0.43–0.63 | 24% (14–36) | 71% (71–90) |
| Intratumoral cystic changes | 0.56 | 0.46–0.66 | 94% (85–98) | 18% (10–29) |
| Target sign | 0.58 | 0.49–0.68 | 34% (23–47) | 82% (71–90) |
| Peritumoral edema | 0.50 | 0.40–0.60 | 18% (10–29) | 82% (71–90) |
Values in parentheses are 95% CIs.
Fig. 2.
ROC curves of ADC and IVIM-derived parameters for differentiation of benign from malignant PNSTs.
Table 4.
Diagnostic performance of ADC and IVIM-derived parameters for discriminating malignant from benign PNSTs
| Parameters | AUC | 95% CI | Optimal Cutoff Value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| ADCmean | 0.98 | 0.95–1.00 | 1.60 | 92% | 98% |
| ADCdark | 0.98 | 0.93–1.00 | 1.54 | 92% | 98% |
| ADCmin | 0.97 | 0.93–1.00 | 0.95 | 92% | 95% |
| Dmean | 0.98 | 0.95–1.00 | 1.41 | 92% | 98% |
| Ddark | 0.97 | 0.92–1.00 | 1.30 | 83% | 100% |
| Dmin | 0.99 | 0.99–1.00 | 0.96 | 100% | 96% |
| fcenter | 0.84 | 0.73–0.95 | 10 | 100% | 55% |
| fmargin | 0.81 | 0.67–0.95 | 16 | 83% | 75% |
| Dmargin | 0.99 | 0.97–1.00 | 1.15 | 92% | 100% |
AUC with 95% CI and sensitivity/specificity for optimum cutoff values. Cutoff values are shown as (value × 10–6 mm2/s) for ADC and D, or % for f, respectively.
Discussion
In this study, we demonstrated that utilization of DWI-derived parameters for differentiation of malignant from benign PNSTs resulted in higher levels of sensitivity and specificity compared with morphological features.
Both the ADC and the IVIM model improved diagnostic accuracy compared with morphological MR features, and both models demonstrated that malignant PNSTs display significantly higher diffusion restriction than benign PNSTs. This is in line with the results of histopathologic evaluation of PNSTs showing a higher cellularity in cases of malignancy.30 In our study, DWI-derived values of malignant PNSTs (ADCmean, 1.23 ± 0.25 × 10−6 mm2/s) matched previously published data on malignant soft tissue tumors.30 The highest levels of sensitivity and specificity were achieved by determining minimal values of the diffusion coefficient D in tumors using the IVIM model, resulting in a sensitivity of 100% and a specificity of 96%. This finding is in line with results of Demehri et al, who suggested that the minimum ADC value is a better predictor of malignancy than the mean ADC value.14
However, we observed excellent AUCs for all ADC measures, with only minor differences (eg, between ADCmean and ADCmin). Diagnostic accuracy using the simplified ADCdark and Ddark approach was similar to the more complex method using 3 ROIs only in case of ADCdark, whereas Ddark showed lower sensitivity at optimal cutoff values. For ADCdark, this simplified approach of determining DWI-derived values in areas of highest cellularity by placement of a single ROI might be better suited for use in the clinical setting. Simple visual inspection of DWI sequences did not allow for reliable discrimination between malignant and benign PNSTs in all cases (Supplementary Fig. 2), advocating quantification.
Regarding the IVIM model, best results were obtained for Dmin to differentiate between malignant and benign PNSTs. Dmean and Ddark measurements of diffusion coefficient D also resulted in high levels of diagnostic accuracy, confirming previous results for the IVIM model in other entities.21 The diagnostic performance of ADC-derived values was similar to that of IVIM-derived values with high levels of sensitivity (ADCmean 92%; Dmean 92%) and specificity (ADCmean 98%; Dmean 98%).
However, interrater reliability analysis demonstrated that the monoexponential data analysis was more robust. The highest agreement was observed for ADC-derived parameters (kappa, 0.898–0.906), whereas Cohen’s kappa was substantially lower for IVIM-derived parameters (kappa D, 0.646–0.831; and f, 0.467–0.468). The lower interrater reliability of IVIM-derived parameters might be explained by the more complex calculation and fitting procedure in which minor differences in ROI placement between readers may sum to greater variability in final values, indicating that monoexponential data analysis is a more robust method and should be preferentially applied.
Perfusion fraction f was significantly increased in malignant PNSTs and inversely correlated with diffusion coefficient D. The inverse correlation between D and f can be explained by the fact that malignant transformation may cause an increase in both vascularity and cellularity, thus increasing perfusion fraction f and decreasing diffusion coefficient D within the IVIM model. Perfusion fraction f was of limited usefulness regarding tumor discrimination due to moderate levels of sensitivity (83–100%) and specificity (55–75%). Nevertheless, perfusion fraction might add relevant information regarding tumor aggressiveness or treatment response.31,32
Morphological imaging features demonstrated limited diagnostic accuracy for differentiation of malignant and benign PNSTs with sensitivities of 18–94% and specificities of 18–82%, similar to previous observations.13 Malignant PNSTs were significantly associated with distinct morphological features, such as irregular shape, ill-defined margins, intratumoral heterogeneity, and peritumoral edema. Peritumoral edema was the most common feature of malignant PNSTs (92% of cases), which is in line with previous studies.14,28 Intriguingly, the maximum tumor diameter was not a useful indicator of malignancy in this study. By contrast, other studies have suggested tumor size as suggestive of malignancy.15,28 This discrepancy may be explained by exclusion of tumors <2 cm in this study, since other morphological features cannot be reliably evaluated in these. Regarding other established imaging techniques for screening of NF1 patients such as PET/CT, functional DWI-derived parameters may improve the diagnostic accuracy to a considerable extent. DWI may be particularly useful as a complementary method in cases of borderline glucose metabolism of tumors in PET imaging. Additionally, DWI can support planning of image-guided biopsy, since targeting areas of minimal ADC/D values can improve diagnostic yield.27 Finally, functional DWI might be applied to monitor therapeutic effects of mitogen-activated protein kinase inhibitors (MEK inhibitors).32,33 Previous studies demonstrated that DWI, and the IVIM model, can contribute to monitor therapeutic effects in pharmacological therapy.34,35
Some limitations of this study should be acknowledged. The number of malignant tumors is relatively small, but syndromal malignant PNSTs in the context of NF1 are rare tumors, and the number of malignant PNSTs analyzed in this study is comparable to other studies.17,36 The higher prevalence of benign PNSTs compared with MPNSTs in our study might have affected the results of the statistical analysis. However, robust and significant differences were observed between both groups, and the higher prevalence of benign tumors in NF1 patients is related to the nature of the disease. Due to the non-invasive nature of our study, we did not histopathologically confirm the diagnosis of benign PNSTs in all cases for both practical and ethical reasons. However, all tumors that were categorized as benign showed stable imaging results over at least 12 months, virtually excluding malignant transformation. We did not administer intravenous contrast material in this study. Contrast enhancement has previously been suggested to represent an indicator of malignancy.13,15,28 However, contrast-enhanced MRI examinations have not been recommended in the International Consensus Statement on Malignant Peripheral Nerve Sheath Tumors,6 and a recent work demonstrated that contrast enhancement is not an independent feature of malignancy.13 Furthermore, considering the ongoing discussion regarding gadolinium deposits in subcortical structures, an intravenous administration of gadolinium-based contrast agents was not permitted by the institutional review board in this patient cohort, which will typically undergo multiple MR studies over time. Moreover, despite good to very good interrater reliability, the precise cutoffs obtained in our study should not be used at other institutions due to intersystem and intervendor variability of ADC measurements, which is usually minor and lies within the range of test-retest variability.37,38 Site-specific cutoffs should be established taking into account differences between the equipment and imaging protocols. This is particularly important for biexponential data analysis, which was less robust in this study. Finally, a selection bias due to clinical evaluation of patients cannot be excluded, since our center represents a national referral center for NF1 patients, potentially contributing to the excellent diagnostic performance of functional MRI.
In conclusion, DW MRI with intravoxel incoherent motion or monoexponential data analysis improves accuracy compared with morphological MRI in the differentiation of benign and malignant PNSTs in NF1. Diffusion-weighted MRI may aid the diagnosis of malignant transformation in PNSTs. This study provides a rationale for further prospective studies elucidating the comparative diagnostic performance of DWI techniques and other imaging modalities such as 18F-FDG PET, or integration of both techniques to improve the diagnosis of malignant transformation in NF1.
Funding
This study was supported by a grant from the “Bundesverband Neurofibromatose e.V.” to J.S.
Conflict of interest statement.
Nothing to declare.
Authorship statement.
All authors were involved in the writing of the manuscript at draft and revision, and have read and approved the final version. In addition, LW and JS performed imaging studies, data analysis, and statistical analysis. MGK developed the software used to perform analysis of DWI data, and supported the statistical analysis. SF has contributed in patient acquisition and clinical evaluation of examined patients. JH contributed by planning of imaging sequences and data analysis due to his experience with previously performed DWI/IVIM projects. KIG contributed in patient imaging and preparation of data for evaluation. CH performed histopathological evaluation of resected tumors. MB was the surgeon in charge for tumor resection. PB and GA contributed in writing and revising the manuscript. VFM and TD conceptualized this study, and have revised the manuscript for important intellectual content.
Supplementary Material
References
- 1. Huson SM, Compston DA, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. J Med Genet. 1989;26(11):712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57(10):2006–2021. [DOI] [PubMed] [Google Scholar]
- 3. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zöller M, Rembeck B, Akesson HO, Angervall L. Life expectancy, mortality and prognostic factors in neurofibromatosis type 1. A twelve-year follow-up of an epidemiological study in Göteborg, Sweden. Acta Derm Venereol. 1995;75(2):136–140. [DOI] [PubMed] [Google Scholar]
- 5. Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68(5):1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62(5):1573–1577. [PubMed] [Google Scholar]
- 7. Ferner RE, Golding JF, Smith M, et al. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol. 2008;19(2):390–394. [DOI] [PubMed] [Google Scholar]
- 8. Cai W, Kassarjian A, Bredella MA, et al. Tumor burden in patients with neurofibromatosis types 1 and 2 and schwannomatosis: determination on whole-body MR images. Radiology. 2009;250(3):665–673. [DOI] [PubMed] [Google Scholar]
- 9. Plotkin SR, Bredella MA, Cai W, et al. Quantitative assessment of whole-body tumor burden in adult patients with neurofibromatosis. PLoS One. 2012;7(4):e35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kluwe L, Nguyen R, Vogt J, et al. Internal tumor burden in neurofibromatosis type I patients with large NF1 deletions. Genes Chromosomes Cancer. 2012;51(5):447–451. [DOI] [PubMed] [Google Scholar]
- 11. Tucker T, Friedman JM, Friedrich RE, Wenzel R, Fünsterer C, Mautner VF. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J Med Genet. 2009;46(2):81–85. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen R, Jett K, Harris GJ, Cai W, Friedman JM, Mautner VF. Benign whole body tumor volume is a risk factor for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. J Neurooncol. 2014;116(2):307–313. [DOI] [PubMed] [Google Scholar]
- 13. Matsumine A, Kusuzaki K, Nakamura T, et al. Differentiation between neurofibromas and malignant peripheral nerve sheath tumors in neurofibromatosis 1 evaluated by MRI. J Cancer Res Clin Oncol. 2009;135(7):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demehri S, Belzberg A, Blakeley J, Fayad LM. Conventional and functional MR imaging of peripheral nerve sheath tumors: initial experience. AJNR Am J Neuroradiol. 2014;35(8):1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Derlin T, Tornquist K, Münster S, et al. Comparative effectiveness of 18F-FDG PET/CT versus whole-body MRI for detection of malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Clin Nucl Med. 2013;38(1):e19–e25. [DOI] [PubMed] [Google Scholar]
- 16. Salamon J, Derlin T, Bannas P, et al. Evaluation of intratumoural heterogeneity on ¹⁸F-FDG PET/CT for characterization of peripheral nerve sheath tumours in neurofibromatosis type 1. Eur J Nucl Med Mol Imaging. 2013;40(5):685–692. [DOI] [PubMed] [Google Scholar]
- 17. Salamon J, Veldhoen S, Apostolova I, et al. 18F-FDG PET/CT for detection of malignant peripheral nerve sheath tumours in neurofibromatosis type 1: tumour-to-liver ratio is superior to an SUVmax cut-off. Eur Radiol. 2014;24(2):405–412. [DOI] [PubMed] [Google Scholar]
- 18. Lee SY, Jee WH, Jung JY, et al. Differentiation of malignant from benign soft tissue tumours: use of additive qualitative and quantitative diffusion-weighted MR imaging to standard MR imaging at 3.0 T. Eur Radiol. 2016;26(3):743–754. [DOI] [PubMed] [Google Scholar]
- 19. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. [DOI] [PubMed] [Google Scholar]
- 20. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407. [DOI] [PubMed] [Google Scholar]
- 21. Meeus EM, Zarinabad N, Manias KA, et al. Diffusion-weighted MRI and intravoxel incoherent motion model for diagnosis of pediatric solid abdominal tumors. J Magn Reson Imaging. 2018;47(6):1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woo S, Lee JM, Yoon JH, Joo I, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology. 2014;270(3):758–767. [DOI] [PubMed] [Google Scholar]
- 23. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, Maryland, USA, July 13–15, 1987. Neurofibromatosis. 1988;1(3):172–178. [PubMed] [Google Scholar]
- 24. Fletcher CD, Sundaram M, Rydholm A, Coindre J, Singer S. WHO classification of soft tissue tumours. World Heal Organ Classif Tumours Pathol Genet Tumours Soft Tissue Bone. 2002;139(9):9–18. [Google Scholar]
- 25. Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37–42. [DOI] [PubMed] [Google Scholar]
- 26. Herrmann J, Ittrich H, Kaul MG, et al. Functional assessment of the kidneys in a 10month-old child with renal artery stenosis by intravoxel incoherent motion. Nephrology (Carlton). 2017;22(3):257–260. [DOI] [PubMed] [Google Scholar]
- 27. Rosenkrantz AB, Kim S, Lim RP, et al. Prostate cancer localization using multiparametric MR imaging: comparison of prostate imaging reporting and data system (PI-RADS) and Likert scales. Radiology. 2013;269(2):482–492. [DOI] [PubMed] [Google Scholar]
- 28. Wasa J, Nishida Y, Tsukushi S, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194(6):1568–1574. [DOI] [PubMed] [Google Scholar]
- 29. Friedrich RE, Kluwe L, Fünsterer C, Mautner VF. Malignant peripheral nerve sheath tumors (MPNST) in neurofibromatosis type 1 (NF1): diagnostic findings on magnetic resonance images and mutation analysis of the NF1 gene. Anticancer Res. 2005;25(3A):1699–1702. [PubMed] [Google Scholar]
- 30. Maeda M, Matsumine A, Kato H, et al. Soft-tissue tumors evaluated by line-scan diffusion-weighted imaging: influence of myxoid matrix on the apparent diffusion coefficient. J Magn Reson Imaging. 2007;25(6):1199–1204. [DOI] [PubMed] [Google Scholar]
- 31. Huang YC, Chen TW, Zhang XM, et al. Intravoxel incoherent motion diffusion-weighted imaging of resectable oesophageal squamous cell carcinoma: association with tumour stage. Br J Radiol. 2018;91(1084):20170421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen X, Ma Z, Huang Y, et al. Multiparametric MR diffusion-weighted imaging for monitoring the ultra-early treatment effect of sorafenib in human hepatocellular carcinoma xenografts. J Magn Reson Imaging. 2017;46(1):248–256. [DOI] [PubMed] [Google Scholar]
- 33. Fischer-Huchzermeyer S, Dombrowski A, Wilke G, et al. MEK inhibitors enhance therapeutic response towards ATRA in NF1 associated malignant peripheral nerve sheath tumors (MPNST) in-vitro. PLoS One. 2017;12(11):e0187700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galla N, Chiang G, Chakraborty S, et al. Apparent diffusion coefficient changes predict survival after intra-arterial bevacizumab treatment in recurrent glioblastoma. Neuroradiology. 2017;59(5):499–505. [DOI] [PubMed] [Google Scholar]
- 35. Xiao-ping Y, Jing H, Fei-ping L, et al. Intravoxel incoherent motion MRI for predicting early response to induction chemotherapy and chemoradiotherapy in patients with nasopharyngeal carcinoma. J Magn Reson Imaging. 2016;43(5):1179–1190. [DOI] [PubMed] [Google Scholar]
- 36. Broski SM, Johnson GB, Howe BM, et al. Evaluation of (18)F-FDG PET and MRI in differentiating benign and malignant peripheral nerve sheath tumors. Skeletal Radiol. 2016;45(8):1097–1105. [DOI] [PubMed] [Google Scholar]
- 37. Donati OF, Chong D, Nanz D, et al. Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology. 2014;270(2):454–463. [DOI] [PubMed] [Google Scholar]
- 38. Corona-Villalobos CP, Pan L, Halappa VG, et al. Agreement and reproducibility of apparent diffusion coefficient measurements of dual-b-value and multi-b-value diffusion-weighted magnetic resonance imaging at 1.5 Tesla in phantom and in soft tissues of the abdomen. J Comput Assist Tomogr. 2013;37(1):46–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.