Abstract
Background
A better understanding of HIV transmission dynamics among populations at high risk is important for development of prevention strategies. We determined HIV transmission networks from infected individuals enrolled in the pre-exposure prophylaxis (PrEP) IPERGAY trial in combination with the ANRS PRIMO and Montreal PHI cohorts to identify and characterize active clusters of transmission in this high-risk population.
Methods
Genotypic resistance tests were performed on plasma samples from 31 IPERGAY participants. Reverse transcriptase sequences were analyzed in combination with unique HIV pol sequences from 1351 individuals enrolled in the PRIMO ANRS cohort (1999–2014) and 511 individuals enrolled in the Montreal PHI cohort (1996–2016). Network analyses were performed to infer putative relationships between all participants.
Results
Overall, 1893 participants were included. Transmission network analyses revealed that 14 individuals (45.2%) from the IPERGAY trial were involved in 13 clusters sampled over a median period (interquartile range) of 2 (0.3–7.8) years, including 7 dyads and 6 larger clusters ranging from 4 to 28 individuals. When comparing characteristics between clustering individuals enrolled in the PRIMO cohort (n = 377) and in IPERGAY (n = 14), we found that IPERGAY participants had a higher viral load (5.93 vs 5.20 log10 copies/mL, P = .032) and reported a higher number of partners in the last 2 months (P < .01).
Conclusions
These results demonstrate high rates of HIV transmission clustering among young high-risk MSM enrolled in the IPERGAY trial. In-depth sampling of high-risk populations may help to uncover unobserved transmission intermediaries and improve prevention efforts that could be targeted to the most active clusters.
Keywords: clustering, HIV, MSM, network, PrEP
Randomized trials have demonstrated that pre-exposure prophylaxis (PrEP) with oral tenofovir/emtricitabine is safe and effective for preventing HIV infection in uninfected men who have sex with men (MSM), transgender women who have sex with men, and heterosexual men and women [1–5]. The ANRS IPERGAY study was a double-blinded, randomized trial of PrEP for HIV-seronegative MSM with a high level of exposure to HIV conducted in France and Quebec [5]. Thirty-one individuals received a diagnosis of HIV-1 infection among 478 high-risk MSM who were screened in the IPERGAY trial. Ten (32.3%) HIV-1-positive individuals were diagnosed at the pre-enrollment visit; 4 acquired HIV-1 infection between pre-enrollment and inclusion, whereas 17 acquired HIV-1 infection during the follow-up period (16 during the blind phase and 1 during the open-label phase). Thus, regular HIV testing using antigen antibody immune assay during the PrEP program led not only to the diagnosis of HIV infection in exposed persons who were unaware of their HIV status, but also to very early diagnosis [6]. This is important, as nearly two-thirds of new infections are attributed to transmission from patients who are unaware of their status [7]. Moreover, the risk of transmission during primary infection could account for up to 30%–70% of new infections among MSM [8]. In a previous study, we characterized the HIV-1 transmission networks among identified primary HIV infection (PHI) individuals in France over 15 years and found that 28.5% of PHIs were virologically linked [9]. Beyond the cultural connections between Quebec and France, there has been a significant increase in migration from France to Quebec over the past years, particularly among students and workers seeking job opportunities. Nearly 70 000 French citizens are living in Montreal, double the number of a decade ago (https://www.economist.com/the-americas/2017/05/04/culture-shock-for-french-immigrants-in-french-canada). French nationals comprise 12.1% of MSM seen at the Montreal SPOT HIV rapid testing site (http://spotmontreal.com/). This influx could also raise questions about new HIV infections, as social and HIV networks are closely related [10]. A better understanding of HIV transmission dynamics among populations at high risk is important for development of prevention strategies and resource allocation for the implementation of the interventions. As the IPERGAY PreP trial was initiated in France and Quebec, we intended to investigate if there were transmission clusters involving both French and Canadian HIV-infected individuals enrolled in the 2 national PRIMO infection cohorts and in the IPERGAY trial.
Our objectives were to determine HIV transmission networks between infected individuals enrolled in the ANRS IPERGAY French Canadian trial and those identified in the French ANRS PRIMO and Montreal PHI cohorts and to characterize active clusters of transmission in this high-risk population.
METHODS
Thirty-one participants in the IPERGAY trial became infected between 2012 and 2014. From 1999 to 2014, 1351 individuals diagnosed with PHI were prospectively enrolled in the French PRIMO Cohort Study in France. PHI was considered when (1) an initially negative test for HIV antibody was followed within 6 months by a positive serology, (2) a negative or indeterminate HIV enzyme-linked immunosorbent assay was associated with a positive p24 antigen, or (3) there was an immunoblot profile compatible with ongoing seroconversion (incomplete immunoblot). All of the individuals were antiretroviral therapy (ART) naïve at enrollment. We estimated that the PRIMO ANRS cohort is representative of new HIV infections occurring in France. Indeed, based on the data recorded through the mandatory notification of new HIV infections, 12% of new HIV diagnoses are done at the time of primary infection, corresponding to 600–700 cases each year. Therefore, the cases included in the PRIMO ANRS cohort represent approximately 15% of all primary infections diagnosed in the country. In addition, the proportion of MSM included in our study (71.4%) is similar to that observed at the national level (73.6%). The Montreal cohort includes a partial sampling of recruited PHI-infected MSM in Montreal with primary infection (n = 474 MSM). Biological, treatment, and behavioral data for these cohorts were available.
Genotypic resistance tests were performed on plasma samples from all IPERGAY participants who acquired HIV before initiation of ART using the consensus technique of the ANRS Resistance Study Group (www.hivfrenchresistance.org). Reverse transcriptase (RT) sequences were analyzed in combination with unique HIV pol sequences from individuals enrolled in the PRIMO ANRS cohort (1999–2014) [9] and individuals enrolled in the Montreal PHI cohort [10] (1996–2016). All the sequences were analyzed with 5 recombination detection methods, implemented using RDP4 software, RDP, Geneconv, Bootscan, Maxchi, and Chimaera [11–16]. Sequences in which at least 1 method suggested recombination, with a P value <.05, were considered for exclusion. Network analyses were performed to infer putative relationships between all participants. A partial transmission network was inferred based on the pairwise nucleotide genetic distances between participants’ unique HIV-1 pol sequences collected at baseline before ART initiation. A putative transmission link was inferred between 2 individuals whenever their pol sequences were ≤0.015 substitutions/site distant (TN93 distance measure) for HIV subtype B and ≤0.01 substitutions/site for non-B sequences to identify recent putative transmission events and to prevent clusters from coalescing and networks from losing cluster resolution (Supplementary Figure 1) [17, 18]. Epidemiologically plausible genetic distance thresholds for HIV are usually between 0.01 and 0.02 substitutions/site. This genetic distance cutoff was selected because after a decade of longitudinal sampling, pol sequences in mono-infected patients typically do not diverge more than 1% from baseline sequences. Therefore, a cutoff of 1.5% is slightly conservative compared with the expected 2% divergence between 2 transmission partners after a decade but allows identification of more recent transmission events. Given that most of these studies were performed with HIV subtype B, we also refined the optimal threshold for non-B viruses by doing a sensitivity analysis, as presented in Supplementary Figure 1. We performed our initial analyses using a genetic distance threshold of 0.015 substitutions/site for B and 0.01 substitutions/site for non-B, because these distances allowed us to identify the maximum number of clusters in the genetic network. Above these cutoffs, networks lose resolution and clusters start to coalesce. More permissive thresholds can identify older putative transmission events but may also lead to spurious detection. A recent study by Wertheim et al. showed that the correlation between named partners and distance thresholds decreases dramatically above the plausible range of the threshold [18]. An additional analysis with all publicly available HIV polymerase sequences (n = 13 011 and n = 11 382 subtype B and non-B sequences, respectively) in the Los Alamos National Laboratory HIV sequence database with a known sampling location was performed.
Statistical Analysis
The groups were compared with the Fisher test for categorical variables and the Wilcoxon or Kruskal-Wallis test for continuous variables. All analyses were performed using R software (https://cran.r-project.org/).
RESULTS
Overall, 1893 HIV-infected participants were included in our study: 31 from the IPERGAY trial between 2012 and 2014, and 1351 and 511 enrolled in the PRIMO ANRS Cohort (1999–2014) and Montreal PHI Cohort (1996–2016), respectively. Baseline characteristics of the enrolled individuals are summarized in Supplementary Table 1.
All 31 participants from the IPERGAY trial were MSM; 29 were infected in France (25 in Paris area), and 2 in Montreal. Twenty-one of 31 (68%) individuals were infected with HIV subtype B, 7 (23%) with subtype CRF_02-AG, and 3 (9%) with other non-B subtypes. No significant signal for recombination was detected in our data set. At diagnosis, Fiebig stage I was observed in 2 individuals, stage II in 9, stage III in 3, stage IV in 6, stage V in 3, and stage VI in 7; the disease stage for 1 participant was undetermined [6]. The median age (interquartile range [IQR]) was 34 (26–43) years; median HIV RNA and CD4 counts were 5.3 (4.5–6.6) log10 copies/mL and 464 (402–657) cells/mL, respectively. A sexually transmitted infection (STI) was diagnosed in 13 (41.9%) participants, and 20 reported a median (range) of 13 (3–50) partners in the past 2 months. Substance use (ie, use of cocain, ecstasy, GHB, ketamine, LSD, crack, and/or speed in the past 12 months) was reported in 15/23 individuals (65.2%) with available data. Number of partners and use of recreational substances were not identified in 11 and 8 participants respectively, as some of them (10/31) were diagnosed at the pre-enrollment visit.
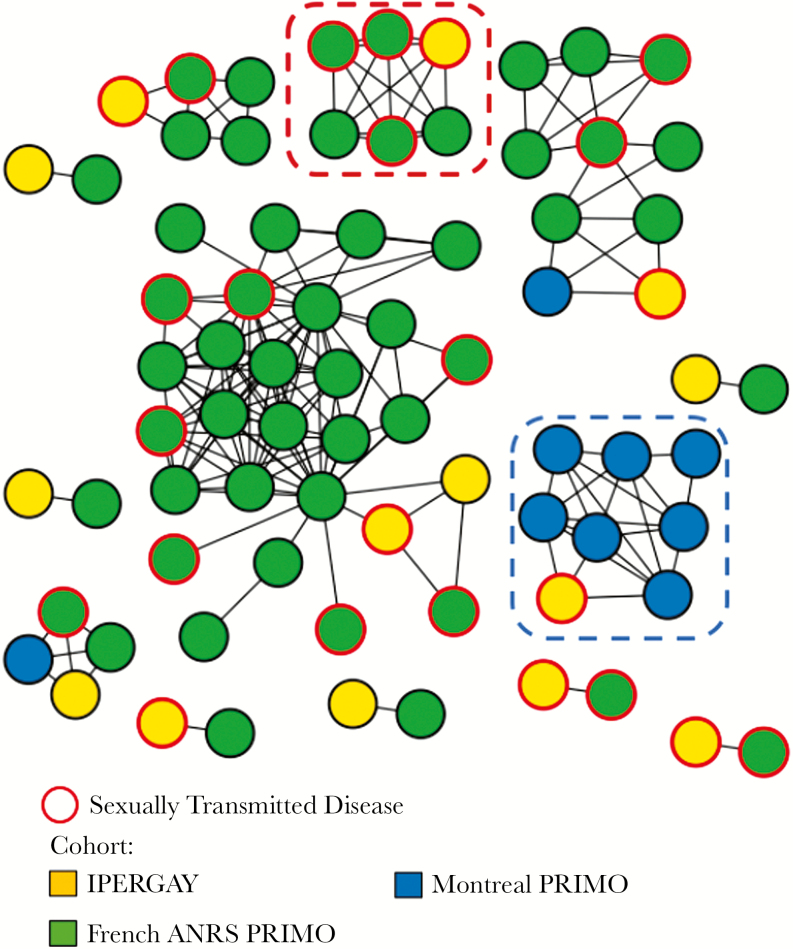
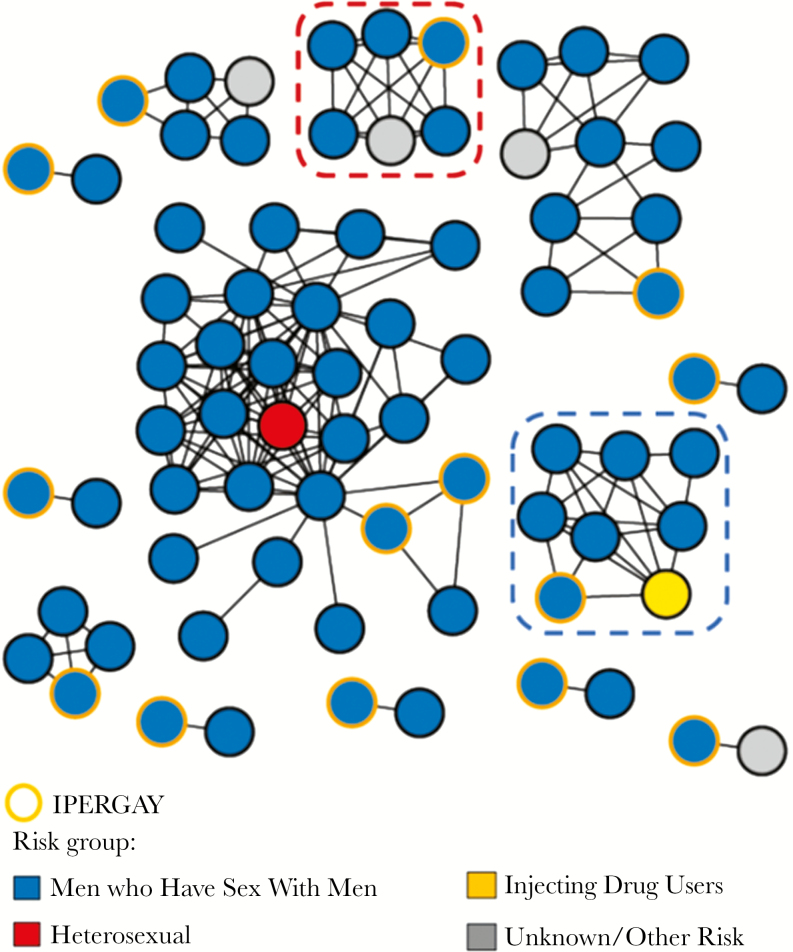
Transmission network analyses revealed that 14 individuals (45%) from the IPERGAY trial were involved in 13 clusters sampled over a median period (IQR) of 2 (0.3–7.8) years, including 7 dyads and 6 larger clusters ranging from 4 to 28 individuals (Figure 1). Among the 61 non-IPERGAY participants involved in these clusters, all but 6 were MSM (90%) (Figure 2).
Figure 1.
HIV transmission network for IPERGAY participants and the ANRS PRIMO ANRS and Montreal PHI cohorts. Genetic data mapped onto participant cohort. Edges indicate genetic linkage (<0.015 substitutions/site for B and <0.01 substitutions/site for non-B). Red dot circled cluster indicates cluster with 1 IPERGAY and 5 French ANRS PRIMO sequences harboring the same K103N mutation. Blue dot circled cluster indicates partial sampling of a larger previously reported cluster (>40 sequences) including sequences from other cities in Quebec (Sherbrooke, Quebec, and Montreal).
Figure 2.
HIV transmission network for IPERGAY participants and the ANRS PRIMO ANRS and Montreal PHI cohorts. Only clusters including participants from the ANRS IPERGAY trial are shown. Genetic data mapped onto participants’ reported main transmission risk. Red dot circled cluster indicates cluster with 1 IPERGAY and 5 French ANRS PRIMO sequences harboring the same K103N mutation. Blue dot circled cluster indicates partial sampling of a larger previously reported cluster (>40 sequences) including sequences from other cities in Quebec (Sherbrooke, Quebec, and Montreal).
Nine (69%) of the 13 clusters included IPERGAY participants infected with HIV-1 subtype B. All but 2 clustering sequences were identified in France, including 9 (75%) in Paris, whereas 2 were from Quebec. For the latter, 1 cluster included 4 participants, 1 IPERGAY individual originating from Canada clustering with 1 patient from the PHI Montreal Cohort and 2 French patients from the PRIMO cohort. The second was a large Quebec cluster with 7 Montreal participants and 1 IPERGAY individual originating from Canada (Figure 1, blue dot circled cluster). This large cluster was part of a previously identified large MSM cluster (n = 49) including participants from Montreal, Sherbrooke, and Quebec diagnosed between 2012 and 2014 [10].
Of the 31 IPERGAY sequences, 1 harbored the NNRTI K103N mutation and was linked to 5 French sequences harboring the same drug resistance mutation diagnosed between 2009 and 2012 (Figure 1, red dot circled cluster).
The clinical, immunological, virological, and behavioral characteristics were compared between clustering and nonclustering subjects (Table 1). Overall, virologically linked individuals were more likely to be men (P < .01), infected through homosexual intercourse (P < .01), with higher viral load (P < .01), infected with subtype B (P < .01), with a higher number of casual sexual partnerships within the last 2 months (P < .01), and diagnosed with STI at the time of HIV diagnosis (P < .01). Specifically, in the Montreal PHI cohort, a higher viral load was significantly associated with being in a cluster (4.47 vs 4.81 log10 copies/mL, P < .001). In the ANRS PRIMO cohort, clustering individuals were significantly younger (33 vs 35 years, P < .01), had a higher number of partners in the last 2 months, and more frequently had a diagnosed STI at enrollment. For the IPERGAY trial, we found a trend toward having a higher number of sexual partners in the last 2 months in participants involved in a cluster (>10 partners: 50% vs 23.5%, P = .058). Clustering IPERGAY participants were also more frequently diagnosed with STI (57.1%) at the time of HIV-1 diagnosis compared with nonclustering individuals (29.4%, P = NS). When comparing characteristics between clustering individuals enrolled in the PRIMO cohort (n = 377) and in IPERGAY (n = 14), we found that IPERGAY participants had a higher viral load (5.93 vs 5.20 log10 copies/mL, P = .032) and reported a higher number of partners in the last 2 months (P < .01) (Supplementary Table 2). When the analysis was restricted to 13 clusters that included both IPERGAY and PRIMO participants (14 and 52 virologically linked individuals, respectively), the same trend was observed, with a higher viral load (5.93 vs 5.45 log copies/mL, P = .09) and a number of reported partners above 10 in the last 2 months being higher (38% vs 50%, P = .04) for the IPERGAY participants compared with the ANRS PRIMO participants (Supplementary Table 3). When comparing characteristics between clustering IPERGAY individuals (n = 14), we did not find any differences between patients in dyads (n = 7) or in a cluster >2 (n = 7) (Supplementary Table 4).
Table 1.
Clinical, Biological, and Behavioral Characteristics of the Study Participants According to Clustering
| Montreal PHI Cohort | ANRS PRIMO Cohort | ANRS IPERGAY Trial | All | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Not Clustering (n = 263) | Clustering (n = 248) | P Value | Not Clustering (n = 974) | Clustering (n = 377) | P Value | Not Clustering (n = 17) | Clustering (n = 14) | P Value | ||
| Age, median [IQR], y | 36 [30–42] | 34 [28–42] | .069b | 35 [29–43] | 33 [27–39] | <.01b | 37 [28–45] | 31 [26–36] | .146b | 0.614c |
| Sex, No. (%) | .870a | <.01a | 1a | <0.01a | ||||||
| Male | 251 (95.4) | 235 (94.8) | 810 (83.2) | 362 (96.0) | 17 (100) | 14 (100) | ||||
| Female | 11 (4.2) | 11 (4.4) | 160 (16.4) | 14 (3.7) | 0 (0.0) | 0 (0.0) | ||||
| NA | 1 (0.4) | 2 (0.8) | 4 (0.4) | 1 (0.3) | 0 (0.0) | 0 (0.0) | ||||
| Risk group, No. (%) | .342a | <.01a | 1a | <.01a | ||||||
| MSM | 204 (77.6) | 193 (77.8) | 642 (65.9) | 323 (85.7) | 17 (100) | 14 (100) | ||||
| Heterosexual | 22 (8.4) | 12 (4.8) | 265 (27.2) | 29 (7.7) | 0 (0.0) | 0 (0.0) | ||||
| IDU | 32 (12.2) | 36 (14.5) | 3 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Others/NA | 5 (1.9) | 7 (2.8) | 64 (6.6) | 25 (6.6) | 0 (0.0) | 0 (0.0) | ||||
| Subtype, No. (%) | .561a | <.01a | .860a | <.01a | ||||||
| B | 251 (95.4) | 240 (96.8) | 681 (69.9) | 298 (79) | 12 (70.6) | 9 (64.3) | ||||
| A/G/AG | 8 (3) | 4 (1.6) | 224 (23) | 66 (17.5) | 3 (17.6) | 4 (28.6) | ||||
| Others | 4 (1.5) | 4 (1.6) | 69 (7.1) | 13 (3.4) | 2 (11.8) | 1 (7.1) | ||||
| CD4/mm3, median [IQR] | 498 [382–649] | 480 [363–620] | .318b | 503 [364–660] | 530 [392–664] | .063b | 445 [402–689] | 478 [409–640] | .943b | .269c |
| VL log10 copies/mL, median [IQR] | 4.47 [3.73–5.14] | 4.81 [4.16–5.3] | <.01b | 5.15 [4.46–5.81] | 5.2 [4.63–5.78] | .274b | 5.04 [4.35–6.36] | 5.93 [5.26–6.63] | .126b | <.01c |
| Cluster size, median [IQR] | 5 [2–7] | 3 [2–5] | 3 [2–8] | <.01c | ||||||
| Cluster category, No. (%) | <.01c | |||||||||
| Dyad | 71 (28.6) | 166 (44.0) | 7 (50.0) | |||||||
| Medium | 143 (57.7) | 176 (46.7) | 4 (28.6) | |||||||
| Large | 34 (13.7) | 35 (9.3) | 3 (21.4) | |||||||
| No. of casual partners in the last 2 mo, No. (%) | .88a | <.01a | .058a | <.01a | ||||||
| None | 1 (0.4) | 2 (0.8) | 4 (0.4) | 2 (0.5) | 0 (0) | 0 (0) | ||||
| <4 | 199 (75.7) | 188 (75.8) | 424 (43.5) | 135 (35.8) | 2 (11.8) | 1 (7.1) | ||||
| 5–9 | 18 (6.8) | 18 (7.3) | 112 (11.5) | 55 (14.6) | 6 (35.3) | 0 (0) | ||||
| >10 | 30 (11.4) | 30 (12.1) | 252 (25.9) | 137 (36.3) | 4 (23.5) | 7 (50) | ||||
| NA | 15 (5.7) | 10 (4) | 182 (18.7) | 48 (12.7) | 5 (29.4) | 6 (42.9) | ||||
| STI at time of HIV diagnosis, No. (%) | .013a | .157a | <.01a | |||||||
| Yes | NA | NA | 253 (26) | 128 (34) | 5 (29.4) | 8 (57.1) | ||||
| No | NA | NA | 529 (54.3) | 178 (47.2) | 12 (70.6) | 6 (42.9) | ||||
| NA | NA | NA | 192 (19.7) | 71 (18.8) | 0 (0) | 0 (0) |
Abbreviations: IDU, intravenous drug user; IQR, interquartile range; MSM, men who have sex with men; NA, not available; STI, sexually transmitted infection; VL, viral load.
aFisher test.
bWilcoxon test.
cKruskal-Wallis test.
Additional analysis combining 13 011 subtype B sequences and 11 382 non-B sequences from LANL with our B and non-B data set identified a limited number of genetic links. For subtype B, 170 LANL sequences were part of clusters including individuals from the French PRIMO cohort (n = 333), the Montreal PRIMO cohort (n = 259), and IPERGAY (n = 10), forming a total of 71 mixed clusters of sizes ranging from 2 to 128 (Supplementary Table 5, Supplementary Figures 2 and 3). Most of the LANL sequences were collected in North America (126/170, including 51 from Canada). The largest cluster of 128 individuals included 69 sequences from the United States and 7 from Canada. These results support our findings of linkage between the epidemics in North America and Europe but are also influenced by the depth of sampling in these countries.
For non-B, 46 LANL sequences were part of clusters including individuals from the French PRIMO cohort (n = 3) and the Montreal PRIMO cohort (n = 3), forming a total of 5 mixed clusters ranging in size from 2 to 23 (Supplementary Table 5, Supplementary Figures 2 and 3). One individual from the Montreal PRIMO cohort infected with HIV subtype F was linked to 22 individuals from Spain, whereas 1 individual from the French PRIMO cohort infected with CRF07_BC was linked 17 individuals diagnosed in China.
DISCUSSION
Here, we investigated the transmission network of the HIV-1 epidemic in 31 participants with a diagnosis of HIV-1 in the IPERGAY PrEP trial between 2012 and 2014. We particularly focused on this population to provide a better understanding of the dynamics of these epidemics in high-risk MSM in France and Montreal. In this preventive trial, a high incidence rate of HIV among MSM, up to 9 per 100 person-years in Paris, was described [5]. We observed a high proportion of participants (45%) involved in transmission clusters. This is higher than what was observed in the French ANRS PRIMO cohort (28%) and in previous studies in France (12.7% to 27%) [19, 20]. This could be explained by a higher viral load and a high number of partners in the last 2 months for the IPERGAY participants, reflecting both early diagnosis, as regular HIV testing was done every 2 months during the PrEP program, and risky sexual practices. On the other hand, the rate of 45% is close to the reported clustering rate in the Montreal cohort (48.5%). These differences might be the consequence of various sampling depths between cohorts. Individuals enrolled in the Montreal PHI cohort are more likely to report recent injecting drug use (13.3% vs 0.2%, P < .01) and MSM exposure (77.7% vs 71.4%, P ≤ .01) compared with patients enrolled in the French ANRS PRIMO cohort. Moreover, the Montreal PHI cohort and the IPERGAY trial enrolled patients living in densely populated but quite restricted areas, which could have facilitated the identification of clustered events in the studied population. The setting is different in the PRIMO cohort; participants are scattered throughout the French territory, and half of them are living outside the Paris area.
Identifying and monitoring HIV clusters is crucial in tracking the leading edge of HIV transmission in epidemics. Here, the small sample size of our study (n = 31 IPERGAY participants) and the limited sampling density prevent us from capturing the global dynamics of the HIV epidemics among high-risk HIV-infected individuals and their role in driving the epidemics. However, considering that it is not likely that viral sequence data from routine genotyping can ever completely represent the population of interest [21], the partial transmission network reported here provides valuable information about the central role of high-risk behavior in sustaining epidemics. Combining data sets from various sites, our results also illustrate the role of human migration in spreading the HIV epidemic, raising new concerns such as the recent upsurge of the HIV-1 CRF02_AG epidemic among young MSM in Quebec [8]. HIV clustering and network inferences are directly affected by sampling density; studies with low sampling density have shown minimal HIV clustering, whereas in-depth sampling allows more accurate characterization of HIV transmission networks [21]. The Montreal PHI cohort and the IPERGAY trial enrolled HIV-infected individuals living in densely populated but restricted areas, which could have facilitated the identification of putative transmission events in the studied population. IPERGAY participants are also screened for HIV repeatedly (every 2 months), and repeated screening in this high-risk population increased the risk of cluster identification. Participants in the PRIMO cohort are scattered throughout the French territory, and half of them are living outside the Paris area.
Molecular analyses are used to clarify dynamics of local transmission networks. These results can be used to target HIV prevention and intervention strategies. Previous studies have demonstrated that network-guided interventions targeting transmission hubs (eg, high-risk individuals, core transmitters) can successfully impact local transmission [22–24]. By identifying past growing clusters, we may also better determine which clusters are likely to grow in the future. Recent work from Poon et al. has also demonstrated that clustering analysis of routinely collected HIV genotypes can become an effective and cost-effective resource for public health intervention in localized outbreaks of HIV transmission [25]. HIV transmission network methods can also be used to examine patterns of HIV transmission, identify factors associated with HIV transmission, and assess the impact of targeted HIV prevention interventions, focusing on highly affected communities. Understanding the dynamic of HIV transmission is crucial in the design of effective interventions, and recently individuals have contributed disproportionately to the spread of the HIV epidemic. Considering the limited time frame of HIV transmission, targeted prevention strategies focusing on PHI may have a significant impact on the HIV epidemic. In addition to clustering analysis, it is important to understand the role of social networks in HIV transmission and consequently to identify critical parameters of the epidemic (eg, populations at higher risk to transmit) [18, 26–28]. Molecular analyses can be used to trace past transmission events and, when combined with social data, can be valuable for public health interventions as implementation of PrEP.
CONCLUSIONS
These results demonstrate high rates of HIV transmission clustering among young high-risk MSM enrolled in the IPERGAY trial. In-depth sampling of high-risk populations may help to uncover unobserved transmission intermediaries and improve prevention efforts that could be targeted to the most active clusters.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We acknowledge the ANRS IPERGAY Study Group.
Prior presentations. This study was presented in part at the annual meeting of the International AIDS Society; 23–26 July 2017; Paris, France.
Financial support. This study was funded by the French Research Agency ANRS as part of the IPERGAY trial and the ANRS PRIMO cohort. The Montreal PHI cohort is funded by the FRQS-Réseau SIDA.
Potential conflicts of interest. We declare that there are no conflicts of interest in relation to the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Site Investigators. Paris St-Louis: C. Pintado, B. Loze, C. Delaugerre, P. Charbonneau, C. Gatey, D. Ponscarme, P. Penot, L. Niedbalski, R. Veron, J. Delgado, E. Dalle, S. Parlier, I. Madelaine, J. Fonsart, M. Danet, N. Mahjoub, N. Mezreb, K. Moudachirou, S. Morel, G. Conort, F. Lorho, M. Meunier, W. Rozenbaum, J.M. Molina. Paris Tenon: J. Chas, C. Monfort, J. Foucoin, B. Boissavy, S. Cousseau, S. Huon, M. Danet, A. Djessima, V. Berrebi, A. Adda, S. le Nagat, L. Zarka, J. Berdougo, G. Pialoux. Lyon: C. Chidiac, N. Mzoughi, F. Clement, A. Decouty, C. Chapolard, M. Godinot, C. Adouard-groslafeige, J. Koffi, A. Pansu, A. Becker, S. Pailhes, F. Bonnet, F. Jeanblanc, C. Brochier, X. Teruin, S. Rouby, L. Gilly, L. Cotte. Nice: C. Etienne, F. Tolonin, S. Breaud, V. Péchenot, S. Bagge, T. Cepitelli, P.M. Roger, E. Rosenthal, E. Cua. Tourcoing: A. Cheret, P. Cornavin, S. Vandamme, J. Lambec, N. Dumon, O. Leclanche, T. Huleux, R. Biekre, O. Robineau, H. Melliez, H. Bazus, A. Pasquet. Nantes: C. Bernaud, M. Besnier, B. Bonnet, N. Hall, M. Cavellec, H. Hue, L. Larmet, M. Colas, R. Choquet, F. Raffi. Montréal: C. Beauvais, P. Arlotto, C. Fortin, A. Talbot, A. Chamberland, A. McKenzie, M. Blanchette, R. Rousseau, K. Montheuth, J. Otis, D. Thompson, M. Morin, M. Wainberg, C. Tremblay. INSERM SC10-US19: L. Meyer, C. Capitant, I. Charreau, B. Guillon, E. Netzer, N. Leturque, J. Binesse, V. Foubert, M. Saouzanet, F. Euphrasie, D. Carette, J.P. Aboulker. INSERM UMR 912 SESSTIM: B. Spire, L. Sagaon-Teyssier, M. Suzan, L. Fressard, B. Demoulin, G. Cattin, N. Lorente. ANRS: V. Doré, L. Marchand, S. Le Mestre, A. Mennecier, E. Choucair, N. Etien, A. Diallo, S. Gibowski, M.C. Simon, J.F. Delfraissy. AIDES community advocacy group and community peer counselors: J.M. Le Gall, S. Morel, V. Pechenot, S. Bagge, A. Djessima Taba, M. Danet, K. Moudachirou, B. Dos Santos, J. Lambec, S. Rouby, X. Teruin, N. Dumon, V. Coquelin, P. Brunet, L. Gilly, T. Cepitelli, R. Porion, D. Rojas Castro, B. Spire.
Members of the Scientific Committee. Jean-Michel Molina (Chair), Mark Wainberg, Benoit Trottier, Cécile Tremblay, Jean-Guy Baril, Gilles Pialoux, Laurent Cotte, Antoine Chéret, Armelle Pasquet, Eric Cua, Michel Besnier, Willy Rozenbaum, Christian Chidiac, Constance Delaugerre, Nathalie Bajos, Julie Timsit, Gilles Peytavin, Julien Fonsart, Isabelle Durand-Zaleski, Laurence Meyer, Jean-Pierre Aboulker, Bruno Spire, Marie Suzan-Monti, Gabriel Girard, Daniela Rojas Castro, Marie Préau, Michel Morin, David Thompson, Catherine Capitant, Lucie Marchand, Véronique Doré, Marie-Christine Simon, Isabelle Charreau, Joanne Otis, France Lert, Alpha Diallo, Séverine Gibowski, and Cecile Rabian.
References
- 1. Grant RM, Lama JR, Anderson PL, et al. iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baeten JM, Donnell D, Ndase P, et al. Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choopanya K, Martin M, Suntharasamai P, et al. Bangkok Tenofovir Study Group Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 4. Thigpen MC, Kebaabetswe PM, Paxton LA, et al. TDF2 Study Group Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 5. Molina JM, Capitant C, Spire B, et al. ANRS IPERGAY Study Group On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 6. Delaugerre C, Antoni G, Mahjoub N, et al. IPERGAY Study Group Assessment of HIV screening tests for use in preexposure prophylaxis programs. J Infect Dis 2017; 216:382–6. [DOI] [PubMed] [Google Scholar]
- 7. Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med 2015; 175:588–96. [DOI] [PubMed] [Google Scholar]
- 8. Brenner BG, Ibanescu RI, Hardy I, Roger M. Genotypic and phylogenetic insights on prevention of the spread of HIV-1 and drug resistance in “real-world” settings. Viruses 2017; 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaillon A, Essat A, Frange P, et al. Spatiotemporal dynamics of HIV-1 transmission in France (1999–2014) and impact of targeted prevention strategies. Retrovirology 2017; 14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brenner BG, Ibanescu RI, Hardy I, et al. the Montreal PHI, SPOT cohorts Large cluster outbreaks sustain the HIV epidemic among MSM in Quebec. AIDS 2017; 31:707–17. [DOI] [PubMed] [Google Scholar]
- 11. Smith JM. Analyzing the mosaic structure of genes. J Mol Evol 1992; 34:126–9. [DOI] [PubMed] [Google Scholar]
- 12. Padidam M, Sawyer S, Fauquet CM. Possible emergence of new geminiviruses by frequent recombination. Virology 1999; 265:218–25. [DOI] [PubMed] [Google Scholar]
- 13. Martin D, Rybicki E. RDP: detection of recombination amongst aligned sequences. Bioinformatics 2000; 16:562–3. [DOI] [PubMed] [Google Scholar]
- 14. Posada D. Evaluation of methods for detecting recombination from DNA sequences: empirical data. Mol Biol Evol 2002; 19:708–17. [DOI] [PubMed] [Google Scholar]
- 15. Martin DP, Posada D, Crandall KA, Williamson C. A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Res Hum Retroviruses 2005; 21:98–102. [DOI] [PubMed] [Google Scholar]
- 16. Martin DP, Williamson C, Posada D. RDP2: recombination detection and analysis from sequence alignments. Bioinformatics 2005; 21:260–2. [DOI] [PubMed] [Google Scholar]
- 17. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 1993; 10:512–26. [DOI] [PubMed] [Google Scholar]
- 18. Wertheim JO, Kosakovsky Pond SL, Forgione LA, et al. Social and genetic networks of HIV-1 transmission in New York city. PLoS Pathog 2017; 13:e1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frange P, Meyer L, Deveau C, et al. French ANRS CO6 PRIMO Cohort Study Group Recent HIV-1 infection contributes to the viral diffusion over the French territory with a recent increasing frequency. PLoS One 2012; 7:e31695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robineau O, Frange P, Barin F, et al. Combining the estimated date of HIV infection with a phylogenetic cluster study to better understand HIV spread: application in a Paris neighbourhood. PLoS One 2015; 10:e0135367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Novitsky V, Moyo S, Lei Q, et al. Impact of sampling density on the extent of HIV clustering. AIDS Res Hum Retroviruses 2014; 30:1226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Little SJ, Kosakovsky Pond SL, Anderson CM, et al. Using HIV networks to inform real time prevention interventions. PLoS One 2014; 9:e98443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leigh Brown AJ, Lycett SJ, Weinert L, et al. UK HIV Drug Resistance Collaboration Transmission network parameters estimated from HIV sequences for a nationwide epidemic. J Infect Dis 2011; 204:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones JH, Handcock MS. An assessment of preferential attachment as a mechanism for human sexual network formation. Proc Biol Sci 2003; 270:1123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poon AF, Gustafson R, Daly P, et al. Near real-time monitoring of HIV transmission hotspots from routine HIV genotyping: an implementation case study. Lancet HIV 2016; 3:e231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dennis AM, Murillo W, de Maria Hernandez F, et al. Social network-based recruitment successfully reveals HIV-1 transmission networks among high-risk individuals in El Salvador. J Acquir Immune Defic Syndr 2013; 63:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amirkhanian YA. Social networks, sexual networks and HIV risk in men who have sex with men. Curr HIV/AIDS Rep 2014; 11:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shushtari ZJ, Hosseini SA, Sajjadi H, et al. Social network and HIV risk behaviors in female sex workers: a systematic review. BMC Public Health 2018; 18:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.