Abstract
Background
Gene-mediated cytotoxic immunotherapy (GMCI) is a tumor-specific immune stimulatory strategy implemented through local delivery of aglatimagene besadenovec (AdV-tk) followed by anti-herpetic prodrug. GMCI induces T-cell dependent tumor immunity and synergizes with radiotherapy. Clinical trials in adult malignant gliomas demonstrated safety and potential efficacy. This is the first trial of GMCI in pediatric brain tumors.
Methods
This phase I dose escalation study was conducted to evaluate GMCI in patients 3 years of age or older with malignant glioma or recurrent ependymoma. AdV-tk at doses of 1 × 1011 and 3 × 1011 vector particles (vp) was injected into the tumor bed at the time of surgery followed by 14 days of valacyclovir. Radiation started within 8 days of surgery, and if indicated, chemotherapy began after completion of valacyclovir.
Results
Eight patients (6 glioblastoma, 1 anaplastic astrocytoma, 1 recurrent ependymoma) were enrolled and completed therapy: 3 on dose level 1 and 5 on dose level 2. Median age was 12.5 years (range 7–17) and Lansky/Karnofsky performance scores were 60–100. Five patients had multifocal/extensive tumors that could not be resected completely and 3 had gross total resection. There were no dose-limiting toxicities. The most common possibly GMCI-related adverse events included Common Terminology Criteria for Adverse Events grade 1–2 fever, fatigue, and nausea/vomiting. Three patients, in dose level 2, lived more than 24 months, with 2 alive without progression 37.3 and 47.7 months after AdV-tk injection.
Conclusions
GMCI can be safely combined with radiation therapy with or without temozolomide in pediatric patients with brain tumors and the present results strongly support further investigation.
Clinical trial registry
ClinicalTrials.gov NCT00634231
Keywords: gene therapy, glioblastoma, immunotherapy, immuno-oncology, viral therapy
Key Points.
Gene-mediated cytotoxic immunotherapy can be safely used in pediatric patients with brain tumors.
Durable progression-free responses are achievable in 2 of 5 patients at the highest dose level.
Importance of the Study.
The prognosis for pediatric malignant gliomas and recurrent ependymoma is very poor. Development of strategies that build on our most effective, albeit palliative treatment regimens offers the possibility to improve the outcome for these patients. Immunotherapy is generating considerable interest as a method of harnessing the patient’s immune system to identify and attack the tumor. This is especially important in tumors with invasive properties that limit the long-term efficacy of surgery and focal radiation therapy. Phase I studies of GMCI in adult malignant gliomas established a safe dose of AdV-tk for brain tumors, and a recent phase II demonstrated potential efficacy. Based on those results, we performed the first study of GMCI in pediatric patients with central nervous system tumors to evaluate the tolerability of this approach and establish a recommended AdV-tk dose for a planned upcoming phase II pediatric trial.
Malignant brain tumors are the most common malignancy of childhood for which treatment is usually unsuccessful.1 As in adults, the prognosis for high grade gliomas in children is particularly dismal despite combination therapy with surgery, radiation, and chemotherapy.2–4 Even tumors which are amenable to “gross total resection” frequently recur due to microscopic foci of tumor outside the main mass. For this reason, radiation therapy is used even after surgery in malignant gliomas, but efficacy is limited by the low radiation sensitivity of these tumor cells and the need to avoid the inclusion of normal brain tissue within the radiation field. Overall, results with chemotherapy have been disappointing. Temozolomide, an oral alkylating agent, was approved for use with radiation in adult malignant gliomas based on results of a randomized phase III trial comparing this regimen with radiation alone, which demonstrated a significant improvement in median survival from 12 to 15 months but may only be effective in patients with methylated MGMT promoter.5,6 Temozolomide has been shown to be well tolerated in children in combination with radiation for pediatric malignant gliomas but efficacy has not been demonstrated.2
Ependymomas are glial tumors arising from ependymal cells in the central nervous system. Primary therapy again consists of maximal surgical resection followed by radiation and, in some cases, chemotherapy.7 Treatment of relapse with repeat surgery and radiation has shown some improvement in survival,8 whereas chemotherapy was not associated with improved survival despite objective responses in 25% of patients.9 Expression of immune-related genes correlated with increased time to progression in relapsed ependymoma and with lack of recurrence for newly diagnosed disease, suggesting that immune surveillance may play a role in preventing or delaying recurrence.10
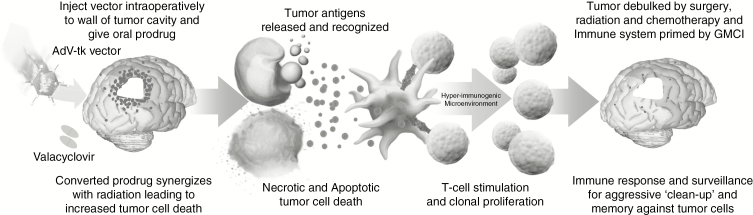
Immuno-oncology is revolutionizing treatment in many tumor types.11 An area of promise with encouraging clinical results in immuno-oncology is that of viral-based therapeutics designed to directly kill tumor cells locally and stimulate a systemic tumor-specific immune response.12,13 Gene-mediated cytotoxic immunotherapy (GMCI), which uses aglatimagene besadenovec (AdV-tk), an adenoviral vector expressing the herpes simplex virus (HSV) thymidine kinase (tk) gene, followed by an anti-herpetic prodrug, is one of these approaches (Fig. 1).14 AdV-tk is injected into the tumor site leading to local creation of nucleotide analogs that result in the death of dividing cancer cells and the consequent release of tumor neoantigens.14 GMCI stimulates an immune stimulatory milieu with STING (stimulator of interferon genes) pathway activation, immunostimulatory cytokine production, and super-antigen mediated T-cell activation.15–17 Thus, GMCI converts so-called cold tumor microenvironments into immune-active or “hot” microenvironments.14 This can be particularly difficult, and important, in highly immune-suppressive tumors such as malignant gliomas.11,18 Combining immunotherapy with standard of care (SOC) therapies, such as surgery, radiation, and chemotherapy, which can debulk the tumor and thus physically decrease the magnitude of immunosuppressive factors, may improve the potential to stimulate an effector immune response.15,19,20 Phase I/II clinical trials in multiple tumor types (brain, prostate, lung, pancreatic, ovarian) have demonstrated a good safety profile and encouraging efficacy results.21–29
Fig. 1.
Schematic of GMCI mechanism of action. GMCI synergizes with surgery and radiation, generating activated T cells that kill tumor cells left after tumor debulking by SOC. The GMCI mechanism includes 3 steps to induce an antitumor immune response: (1) AdV-tk (aglatimagene besadenovec) in tumor cells converts valacyclovir prodrug into nucleotide analogs that kill tumor cells, releasing tumor associated antigens; (2) the presence of the injected virions and the cell death through both necrosis and apoptosis generate “danger signals” which attract and stimulate antigen presenting cells (APCs) such as dendritic cells; and (3) the vector-expressed TK protein functions as a super-antigen that leads to a hyper-immunogenic microenvironment with STING pathway activation and production of proinflammatory cytokines, such as interleukin (IL)-2 and IL-12, with consequent antitumor T-cell stimulation and proliferation.
In adults, a phase I study in multiply recurrent malignant glioma demonstrated a safe dose range and encouraging results, with 3 of 13 patients surviving greater than 24 months.23 Based on animal studies showing synergy of GMCI with radiation30 and the hypothesis that immunotherapy would work best earlier in the disease, a 48-patient phase Ib/II trial was conducted in adults with newly diagnosed malignant glioma.21,22 AdV-tk was injected at the time of surgery and radiation was started within approximately 7 days to maximize synergy with GMCI. Standard temozolomide was started after completion of 14 days of valacyclovir. The approach was well tolerated with encouraging efficacy results. Median overall survival improved compared with an SOC control group (17.1 vs 13.5 mo, P = 0.0417) with most of the benefit seen in gross total resection patients (25 vs 16.9 mo, P = 0.0492). In the gross total resection group, 1-, 2-, and 3-year survival with GMCI was 90%, 53%, and 32% versus 64%, 28%, and 6% for SOC alone.
Based on the safety profile and promising phase II results in adults, this first-in-pediatric CNS phase I clinical trial was developed. We hypothesized that administration of AdV-tk to the surgical bed of malignant brain tumors at the time of surgery followed by 14 days of valacyclovir would be safe and effectively delivered without disturbing standard therapy and would have antitumor activity in pediatric patients. The primary objective of this dose-escalation study was to establish feasibility and the recommended dose for a phase II study of AdV-tk for pediatric patients with primary brain tumors. Secondary objectives included a descriptive assessment of overall survival (OS) and progression-free survival (PFS) and assessment of immunologic biomarkers in consenting patients.
Materials and Methods
Eligibility
The study was open for pediatric patients 3–18 years of age with newly diagnosed malignant glioma or recurrent ependymoma who were planning to have additional surgery and radiation therapy. Tumor accessible for injection and not located in the brainstem or deep midbrain was required. Additional eligibility criteria included Karnofsky score ≥60% if >10 years old or Lansky score ≥60% if <10 years old; absolute neutrophil count ≥1000/μL; platelets ≥100 000/μL (transfusion independent); hemoglobin ≥8.0 g/dL; serum creatinine ≤1.5 times the upper limit of institutional normal for age and/or glomerular filtration rate ≥70 mL/min/1.73 m2; bilirubin ≤1.5 times institutional normal; and serum glutamic pyruvic transaminase (alanine aminotransferase [ALT]) <3 times institutional normal. Signed informed consent, and patient assent when appropriate, was required.
Exclusion criteria included patients on immunosuppressive drugs (with the exception of corticosteroid), known history of HIV, and underlying immunodeficiency or acute infection (viral, bacterial, or fungal infections requiring therapy). Patients could not have other serious comorbid illnesses or compromised organ function and no other investigational antitumor agents within 30 days of study entry or during active participation in the study (defined as from study entry until tumor progression).
Ethics and Study Oversight
Institutional review board approval was obtained from all participating institutions. Patient management and assessments including adverse events (AEs) and progression assessments were done by the investigators at the institutions and reported to the sponsor, Advantagene. Reporting to the FDA and other regulatory bodies was performed as required. General oversight of the trial was by the principal investigator at Dana-Farber Cancer Institute.
Study Design and Therapy Administration
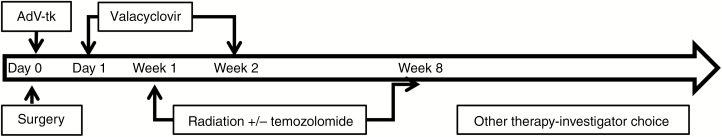
Two dose levels of AdV-tk in a ready-to-use formulation were evaluated: level 1 = 1 × 1011 vp per injection and level 2 = 3 × 1011 vp per injection in combination with standard of care (Fig. 2). The AdV-tk vector was injected at the time of surgery as previously described.21 Briefly, after resection, the neurosurgeon performed freehand injections of AdV-tk into 3–10 sites (median 5) of the infiltrating tumor bed using a total volume of 1 mL divided over the number of sites with the specific locations determined by the neurosurgeon at the time of surgery. Injections were not performed into adjacent motor or speech cortex to avoid the potential for causing acute neurologic deterioration. To avoid vector dispersing away from the tumor site, the cerebral ventricle or sites where spillage could occur into the subarachnoid space were also excluded. Valacyclovir prodrug dosing was 15 mg/kg (maximum 2 g) orally 3 times per day.31 A liquid formulation was used when needed. Valacyclovir was started 1–3 days after the AdV-tk injection and continued for 14 days total. Patients unable to take the oral prodrug for any reason could be prescribed intravenous acyclovir at 10 mg/kg 3 times per day. Standard radiotherapy began 3–8 days after AdV-tk injection. Temozolomide could be initiated after completion of the prodrug.
Fig. 2.
Study design. AdV-tk was injected at the time of surgery into the surgical wall. Valacyclovir was administered for 14 days starting on days 1–3. Radiation was started 3–8 days after AdV-tk injection to overlap with AdV-tk activity and valacyclovir administration. If indicated, temozolomide was administered after completing valacyclovir.
Monitoring
A history and physical exam were performed within 7 days of starting therapy, laboratory analyses included complete blood count with differential, platelets, prothrombin time, partial thromboplastin time, serum electrolytes (sodium, potassium, magnesium, calcium), creatinine, liver function tests (aspartate aminotransferase, ALT, and bilirubin), and optional blood draw for research. MRIs of the brain were required within 21 days of starting therapy, approximately 4 weeks after completion of radiation and then every 3 months for the first year and every 6–12 months after that. Clinical and laboratory assessments (including optional research blood) were performed at least once at the end of prodrug administration (wk 2–3) to assess for acute toxicity. After this, assessments were as per SOC, including approximately 4 weeks after completion of radiation, every 3 months for the first year, and then as per institutional standard practice for up to 5 years.
AEs were monitored and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. AEs not included in CTCAE v3.0 were reported and graded under “other adverse event” within the appropriate category. Dose-limiting toxicity (DLT) was defined as any GMCI-related grade 4 hematologic and non-hematologic toxicity or any treatment-related grade 3 toxicity requiring interruption in radiation therapy for more than 7 days. While grade 4 CNS hemorrhage was considered a DLT, grade 3 asymptomatic CNS hemorrhage based on radiologic imaging (commonly seen after surgery) was not. Accrual was staggered by 2 weeks between the first and second patient in each dose level and when advancing to the second dose level. The maximum tolerated dose or recommended phase II dose was defined as the maximal dose in which fewer than 2 of 6 patients had a DLT.
Statistical Considerations
Two dose levels were planned with a standard 3 + 3 design. Once 5 patients completed treatment at dose level 2 without DLT, the recommended phase II dose had already been determined and the study was closed to further enrollment. Progressive disease was defined as ≥25% increase in the product of 2 diameters on MR imaging. PFS was defined as the time from AdV-tk injection to disease progression, death, or last follow-up. OS was defined as the time from AdV-tk injection to death or last follow-up. PFS and OS were estimated using the Kaplan–Meier method. Participants without an event were censored at last contact.
Genomic Analysis and Immune Biomarker Studies
Tumor tissues from baseline resection were analyzed for mutations by targeted next-generation sequencing with the OncoPanel assay.32 Methylation of the cytosine-phosphate-guanine island of the O6-methylguanine-DNA methyltransferase (MGMT) gene was determined on DNA isolated from tumor paraffin blocks which underwent chemical (bisulfite) modification of unmethylated cytosines to uracil and subsequent PCR using primers specific for either methylated or modified unmethylated DNA. The PCR products were analyzed in duplicate parallel runs by capillary gel electrophoresis using standard methods. Peripheral blood samples were collected from consenting patients at baseline prior to AdV-tk injection and ~2 weeks later. The frequency of myeloid derived suppressor cells (MDSCs), defined as Lin−/Lo, human leukocyte antigen D related negative (HLA DR−), CD33+ CD11b+, was determined using flow cytometry on whole blood as previously described.33 For NanoString analysis, peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved using standard methods. Total RNA was isolated from PBMCs using standard TRIzol/chloroform purification methods, and the quality and concentration were assessed using a NanoDrop 2000 instrument (Thermo Scientific). NanoString gene expression profiling and analysis were conducted using approximately 200 ng of RNA per sample run on the nCounter PanCancer Immune Profiling Panel (NanoString Technologies) per manufacturer’s instructions. Differential gene expression was analyzed using N-solver software provided by NanoString. A normalization factor was calculated based on the average of the geometric mean of all of the housekeeping genes for each data point. The average of the geometric means across all data points was used as a reference against which each lane was normalized.
Results
Participants and Treatment
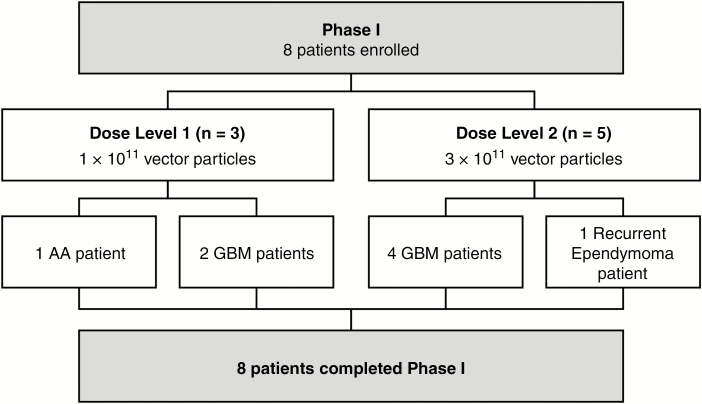
A total of 8 participants were enrolled and received AdV-tk injection from November 2010 through September 2015, with 3 patients on dose level 1 (1 × 1011 vp) and 5 patients on dose level 2 (3 × 1011 vp) (Fig. 3). Accrual was slow, as only patients with biopsy-proven malignant glioma that could benefit from reoperation prior to starting definitive treatment (radiation therapy) were eligible. In April and August of 2012, accrual was expanded to include Lurie Children’s Hospital and patients with recurrent ependymoma undergoing planned repeat surgical resection and re-radiation, respectively.
Fig. 3.
CONSORT diagram of patient enrollment. All 8 patients enrolled completed the study.
Patient characteristics are shown in Table 1. Median age was 12.5 years (range 7–17), 5 were female; 6 had glioblastoma, 1 anaplastic astrocytoma, and 1 recurrent ependymoma. Lansky/Karnofsky performance scores were 60–100. All tumors were supratentorial. Malignant glioma was recently diagnosed in patients, with the only prior treatment being surgery (n = 5) or biopsy (n = 2). The patient with ependymoma had been treated with surgery for grade II ependymoma 8 years prior to enrollment and had first recurrence, treated with resection and proton beam radiation, 2 years prior to enrollment. Tumor genomic profiling revealed that none of the evaluated patients had isocitrate dehydrogenase, H3, or BRAF mutations (Table 1).
Table 1.
Patient demographics and outcomes
| # | Dose Level | Age, y | PS | Diagnosis | Resection Day 0 | Tumor Location | Oncopanel Mutations | MGMT | RT Start, days | Other Treatment* | OS, mo | PFS, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 15 | 80 | AA | Minimal | Midline thalamic | N/A | U | 3 | No | 12.1 | 10.9 |
| 2 | 1 | 14 | 60 | Glioblastoma | None | Cortical, bilateral | N/A | U | 4 | No | 7.4 | 5.8 |
| 3 | 1 | 9 | 80 | Glioblastoma | Subtotal | Midline thalamic | N/A | U | 6 | No | 8.9 | 4.6 |
| 4 | 2 | 8 | 90 | Glioblastoma | Subtotal | Cortical, multifocal | p53+, IDH-, H3-, BRAF- | U | 7 | TMZ | 9.7 | 4.5 |
| 5 | 2 | 13 | 100 | Glioblastoma | Total | Cortical | p53wt, IDH-, H3-, BRAF- | N/A | 7 | TMZ, BV | 22.0 | 7.9 |
| 6 | 2 | 17 | 100 | Recurrent ependymoma | Total | Cortical | N/A | N/A | 5 | No | >47.7 | >47.7 |
| 7 | 2 | 12 | 80 | Glioblastoma | Subtotal | Cortical, bilateral | p53+, IDH-, H3-, BRAF- | M | 8 | TMZ, BV | 25.3 | 8.9 |
| 8 | 2 | 7 | 100 | Glioblastoma | Total | Cortical | p53wt, IDH-, H3-, BRAF- | U | 7 | cTMZ, BV, CCNU | >37.3 | >37.3 |
*Other treatment during and after radiation, prior to progression.
PS, performance score; AA, anaplastic astrocytoma; U, unmethylated; M, methylated; RT, radiation; TMZ, temozolomide (cTMZ, concomitant during radiation only); BV, bevacizumab; CCNU, lomustine; wt, wild type; N/A, not available.
Gross total resection was possible in only 3 participants; others had subtotal or no significant resection due to the extent of tumor invasion. Radiation was started 3–8 days after AdV-tk injection and consisted of 54–59.4 Gy over ~6 weeks, except for the patient with ependymoma, who received hypofractionated stereotactic radiation to 28 Gy in 5 days. If indicated, patients received temozolomide during radiation (Table 1) and then physician-choice SOC chemotherapy with or without bevacizumab.
Safety
GMCI was well tolerated in all 8 patients and there were no DLTs. Related AEs of grade >1 were seen only at dose level 2 and were predominantly related to local immune stimulation (Table 2). Toxicities within the first 21 days were CTCAE v3.0 grades 1–3 and were similar to those identified in the adult studies. The only possibly related grade 3 event and the only possibly related serious AE was hospitalization for headache on the second day of hypofractionated radiation therapy in the patient with recurrent ependymoma. MRI revealed some edema surrounding the resection cavity and symptoms improved after treatment with dexamethasone. Radiation was resumed and completed after a 3-day interruption. The most common related grade 1 AEs were gastrointestinal (nausea, vomiting, abdominal pain), which may have been related to valacyclovir but did not require discontinuation in any patient. Valacyclovir was well tolerated with only 3 patients missing 1–4 doses out of 42 due to nausea or vomiting. Only 2 patients required intravenous acyclovir instead of oral valacyclovir for 1–3 days after surgery and in one case when hospitalized for hydrocephalus. The only grade >2 lab abnormalities were transient lymphopenia, hyponatremia, and hypokalemia. These abnormalities resolved without complication and were considered possibly related to surgery, radiation, or anticonvulsant medications. Radiation was started 3–8 days after surgery and AdV-tk injection without complication and chemotherapy was given as indicated without delay. No unexpected or late AEs related to GMCI were identified.
Table 2.
Adverse events during the acute period (days 0–21) that were considered related to GMCI or any treatment emergent laboratory events. Grading is based on the National Cancer Institute CTCAE version 3.0
| Related Clinical Events | # of Patients | Adverse Event | Dose Level 1 (n = 3) | Dose Level 2 (n = 5) | ||||
|---|---|---|---|---|---|---|---|---|
| CTC 1 | CTC 2 | CTC 3 | CTC 1 | CTC 2 | CTC 3 | |||
| Cardiac | 1 | Sinus tachycardia | 1 | |||||
| Gastrointestinal | 3 | Abdominal pain | 1 | |||||
| Nausea | 2 | |||||||
| Vomiting | 1 | 1 | ||||||
| General disorders | 3 | Fatigue | 1 | 1 | 1 | |||
| Fever | 2 | |||||||
| Metabolism and nutrition disorders | 1 | Anorexia | 1 | |||||
| Nervous system disorders | 1 | Headache | 1 | |||||
| Hypoglossal nerve disorder | 1 | |||||||
| Numbness, face, arm, legs | 1 | |||||||
| Respiratory disorders | 1 | Dyspnea | 1 | |||||
| Laboratory events | ||||||||
| Metabolic | 6 | Hypocalcemia | 1 | |||||
| Hypokalemia | 1 | |||||||
| Hyponatremia | 1 | 2 | 1 | |||||
| Hematologic | 7 | Anemia | 1 | 1 | ||||
| Leukopenia | 2 | |||||||
| Lymphocytopenia | 1 | 1 | ||||||
| Thrombocytopenia | 1 | |||||||
Survival
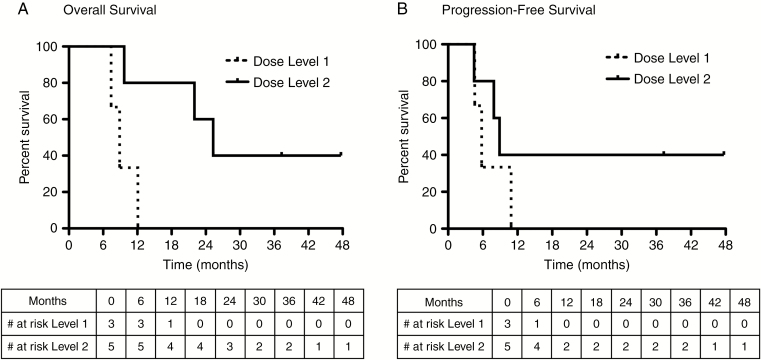
Three patients, 2 with glioblastoma and the patient with recurrent ependymoma, were alive more than 24 months after treatment, and 2 remain alive without progression at 37.3 and 47.7 months after AdV-tk injection (Table 1 and Fig. 4). All 3 of these patients were at dose level 2. Two patients with progression declared 8–9 months after AdV-tk injection survived for an additional 14–16 months, suggesting that the imaging changes may have been pseudoprogression.
Fig. 4.
Kaplan‒Meier analysis of overall survival and progression-free survival. (A) Median OS was 8.9 months for dose level 1 and 25.3 months for dose level 2. (B) Median PFS was 5.8 months for dose level 1 and 8.9 months for dose level 2.
Immunologic Correlates
The percentage of MDSCs defined as Lin−/Lo, HLA DR−, CD33+ CD11b+ from whole blood was determined in the 3 patients at dose level 1 before and 1–2 weeks after AdV-tk injection (Supplementary Figure 1). The level of circulating MDSCs was 2.66% (range 1.74‒3.31%) at baseline and did not show consistent change after treatment (decreased in 1, increased in the other 2 patients). The levels observed in these pediatric malignant glioma patients are comparable to the levels seen in adult cancer patients (mean 2.85%) and higher than seen in normal adult controls (mean 1.26%).33
NanoString PanCancer Immune Profiling was performed on PBMCs collected either before or 3 weeks after AdV-tk injection from 2 patients at dose level 2 (patients 4 and 5). In both patients, genes related to CD8 cytotoxic T-cell function had increased expression after compared with before treatment (Supplementary Figure 2). CD11b and CD33 were high in both patients at baseline and after injection increased in patient 4 and decreased in patient 5. Levels of programmed cell death 1, Forkhead box protein 3, and interleukin 10 were low before and after treatment. T-cell immunoglobulin and mucin-domain containing 3 and lymphocyte-activation gene 3 were stable and increased, respectively, after injection in patient 4 compared with baseline, whereas both went down in patient 5.
Discussion
Treatment outcomes for patients with malignant gliomas and recurrent ependymomas remain dismal despite therapeutic advancements.2–4,6,7 While maximal surgical resection and focal radiation remain the backbone of treatment, these modalities on their own are insufficient to prevent tumor progression and death for most patients. Immunotherapy has become recognized as a potential new weapon in the battle to control cancer by harnessing the patient’s immune system to better recognize and destroy tumor cells.11 GMCI is an approach that creates an immune-stimulatory microenvironment in the tumor to activate tumor-specific cytotoxic T cells and has been shown to synergize with SOC debulking therapies such as surgery, radiation, and chemotherapy. The combination with SOC may be particularly important for malignant gliomas due to their highly aggressive and immune suppressive nature. This was seen in the adult glioma phase II study22 and may be also reflected here, where 2 patients are still alive, at greater than 3 years out, both of whom had gross total resections.
In this first-in-pediatric brain tumor study of GMCI, the AdV-tk vector was injected into the tumor resection bed followed by oral administration of the anti-herpetic prodrug valacyclovir. Because this was the first clinical delivery of AdV-tk into the potentially smaller pediatric cranium and the potential differences in immune response, the study started with evaluation of a dose half-log lower than the current adult dose level, with the plan to escalate to the same dose used in the adult phase II study.22 Both dose levels were well tolerated without DLT. The feasibility and safety of starting radiation within 1 week of surgery and AdV-tk injection were also a question for this population, as it is typically started 3–4 weeks postsurgery. However, since there are preclinical data demonstrating synergy when GMCI overlaps with radiation and 1 week was the schedule in the adult phase II study, this was evaluated here. The feasibility for this schedule was demonstrated. Thus, this study successfully established safety of GMCI in this study design for pediatric brain tumors.
The neoadjuvant approach had unique challenges in the pediatric population. In the adult study, a diagnosis of malignant glioma could be made by frozen section, allowing AdV-tk injection during the initial surgery. By contrast, in pediatrics, this was not possible due to the multitude of potential diagnoses not distinguishable on frozen section. Thus, the trial was limited to patients with a known histologic diagnosis for which reoperation prior to starting radiation was indicated. This limited accrual and may have selected for patients with more extensive tumors.
Nevertheless, median OS was 8.9 months for dose level 1 and 25.3 months for dose level 2, with 3 of these patients surviving more than 2 years. Median PFS was 5.8 months for dose level 1 and 8.9 months for dose level 2, with 2 patients still progression free at 37.3 and 47.7 months. For the 4 glioblastoma patients in dose level 2, the median OS was 24 months with one still alive at 37.3 months. Although the numbers are too small to support any conclusions and the populations may not be equivalent, the outcomes compare favorably to expected outcomes with radiation and temozolomide with or without lomustine, where median OS was ~18–20 months.2,3 Most pediatric studies focus on PFS or event-free survival. However, due to pseudoprogression seen with immunotherapy, PFS endpoints can be difficult to assess. In fact, 2 of the glioblastoma patients at dose level 2 who had progression declared at 7.9 or 8.9 months, survived for 14.1 and 16.4 months after progression, respectively; given the unusually long postprogression survival interval, these may have been pseudoprogressions.
The study population was too small to evaluate prognostic factors that may impact efficacy. In the adult malignant glioma study, an important factor was extent of resection, with no difference seen in median survival between GMCI + SOC and SOC alone in the subtotal resection group, although there was indication of a potential tail effect, with sporadic long-term survivors in the subtotal resection group.22 Efficacy in adult malignant glioma did not seem to be influenced by MGMT methylation. In this pediatric study, the longer-term survivors included total and subtotal resection patients; the longest surviving glioblastoma patient had total resection and was MGMT unmethylated. None of the glioblastoma patients had IDH mutation or targetable mutations. A potential advantage of GMCI and polyclonal immunotherapy approaches is that they are mutation agnostic and have a low risk for resistance. This is particularly important for pediatric oncology, where each tumor type is rare and sequential therapies that are toxic and life-prolonging, but non-curative, can be particularly devastating for a growing child.
For immune-stimulatory approaches like GMCI, immune regulatory mechanisms can be a limiting factor. In this trial, immune studies on peripheral blood were piloted in an effort to evaluate potential biomarkers for future studies. However, interpretation of the results were limited by the inherent variability and small numbers of samples. MDSCs are immune-suppressive myeloid cells that are prominent in some tumors with high tumor burden and may explain part of the immune benefit of tumor debulking.15,33 MDSC levels were evaluated on whole unprocessed blood to preserve the phenotype, although this posed logistical challenges. Nevertheless, the levels of MDSCs in the 3 patients evaluated were found to be in the range of what is seen in adult cancer patients, which is higher than expected in adult normal volunteers, highlighting the immunosuppressive nature of pediatric gliomas. Evaluation of immune gene expression in peripheral blood using the NanoString immune panel provided the opportunity to look at many genes in a small sample size. This analysis provided evidence for immune stimulation and potential upregulation of some immune checkpoints, as previously observed in preclinical studies and clinical studies in other human tumor types.17,27 In pancreatic cancer, where AdV-tk could be injected prior to surgery and then the resected tumor evaluated for immune changes, the tumors had a greater than 20-fold average increase of CD8+ T-cell infiltrate; however, there was also upregulation of programmed cell death ligand 1.27 A similar study is ongoing in lung cancer, where peripheral blood and tumor are being evaluated before and after GMCI, and similar activation is being observed (personal communication). Hopefully these studies will identify blood-based biomarkers that may be used in a future pediatric brain study where it is not feasible to obtain tissue before and after GMCI.
Other immunotherapies that are being evaluated for adult and pediatric brain tumors include immune checkpoint inhibitors (ICIs) and chimeric antigen receptor (CAR) T-cell therapy. For CARs, the need for a tumor-specific homogeneously expressed target antigen and the immune-suppressive tumor microenvironment are some of the challenges for use in solid tumors.34,35 Clinical trials are ongoing for ICIs in adult gliomas and pediatric solid tumors, but to date the results have been disappointing in these less immunogenic tumors, where an immune-stimulatory priming may be required to jump-start the immune response.36 Based on preclinical data showing a dramatic benefit for GMCI combined with anti–programmed death 1 antibody therapy compared with either therapy alone,17 a combination trial in newly diagnosed adult malignant glioma was recently launched. This may provide another potential weapon for future pediatric brain tumor studies.
Based on the safety and potentially favorable survival outcomes observed in this study, a phase II study with dose level 2 is being planned.
Funding
This study was partially funded by a grant from the Matthew Larson Foundation and support from the Credit Unions Kids at Heart Program, the Joe Andruzzi Foundation, the CJ Buckley Brain Cancer Research Fund, The Stop and Shop Pediatric Brain Tumor Program, the Zach Carson Fund, the Ellie Kavalieros DIPG Fund, and Advantagene, Inc.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families for participation in this study.
Aspects of this manuscript were reported at the International Society for Pediatric Neuro-Oncology (ISPNO) meeting in Liverpool, England on June 15, 2016.
Conflict of interest statement.
AGM, MLSP, BWG, EAC, LKA are employees and shareholders of Advantagene, Inc.
Authorship statement.
MWK, LG, PM, SNC, KJM, AJD, TT, RL, LG, and SG ran the clinical trial at their respective sites, provided clinical samples, and reviewed and provided input on the manuscript. AGM, MLSP, BWG, and CMD-M performed experiments and analyzed data. MWK, LKA, EAC, and SG designed the study, supervised the overall project, and wrote the manuscript.
References
- 1. American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA; 2016. [Google Scholar]
- 2. Cohen KJ, Pollack IF, Zhou T, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13(3):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jakacki RI, Cohen KJ, Buxton A, et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol. 2016;18(10):1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grill J, Massimino M, Bouffet E, et al. Phase II, open-label, randomized, multicenter trial (HERBY) of bevacizumab in pediatric patients with newly diagnosed high-grade glioma. J Clin Oncol. 2018;36(10):951–958. [DOI] [PubMed] [Google Scholar]
- 5. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 6. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 7. Pejavar S, Polley MY, Rosenberg-Wohl S, et al. Pediatric intracranial ependymoma: the roles of surgery, radiation and chemotherapy. J Neurooncol. 2012;106(2):367–375. [DOI] [PubMed] [Google Scholar]
- 8. Bouffet E, Hawkins CE, Ballourah W, et al. Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys. 2012;83(5):1541–1548. [DOI] [PubMed] [Google Scholar]
- 9. Zacharoulis S, Ashley S, Moreno L, Gentet JC, Massimino M, Frappaz D. Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst. 2010;26(7):905–911. [DOI] [PubMed] [Google Scholar]
- 10. Donson AM, Birks DK, Barton VN, et al. Immune gene and cell enrichment is associated with a good prognosis in ependymoma. J Immunol. 2009;183(11):7428–7440. [DOI] [PubMed] [Google Scholar]
- 11. Reardon DA, Freeman G, Wu C, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16(11):1441–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aguilar LK, Arvizu M, Aguilar-Cordova E, Chiocca EA. The spectrum of vaccine therapies for patients with glioblastoma multiforme. Curr Treat Options Oncol. 2012;13(4):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112(8):1969–1977. [DOI] [PubMed] [Google Scholar]
- 15. Predina JD, Kapoor V, Judy BF, et al. Cytoreduction surgery reduces systemic myeloid suppressor cell populations and restores intratumoral immunotherapy effectiveness. J Hematol Oncol. 2012;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Predina JD, Judy B, Aliperti LA, et al. Neoadjuvant in situ gene-mediated cytotoxic immunotherapy improves postoperative outcomes in novel syngeneic esophageal carcinoma models. Cancer Gene Ther. 2011;18(12):871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Speranza MC, Passaro C, Ricklefs F, et al. Preclinical investigation of combined gene-mediated cytotoxic immunotherapy and immune checkpoint blockade in glioblastoma. Neuro Oncol. 2018;20(2): 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Plant AS, Koyama S, Sinai C, et al. Immunophenotyping of pediatric brain tumors: correlating immune infiltrate with histology, mutational load, and survival and assessing clonal T cell response. J Neurooncol. 2018;137(2):269–278. [DOI] [PubMed] [Google Scholar]
- 19. Fridlender ZG, Sun J, Singhal S, et al. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol Ther. 2010;18(11):1947–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heimberger AB, Sun W, Hussain SF, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiocca EA, Aguilar LK, Bell SD, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29(27):3611–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wheeler LA, Manzanera AG, Bell SD, et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18(8):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1(2):195–203. [DOI] [PubMed] [Google Scholar]
- 24. Herman JR, Adler HL, Aguilar-Cordova E, et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum Gene Ther. 1999;10(7):1239–1249. [DOI] [PubMed] [Google Scholar]
- 25. Miles BJ, Shalev M, Aguilar-Cordova E, et al. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum Gene Ther. 2001;12(16):1955–1967. [DOI] [PubMed] [Google Scholar]
- 26. Aggarwal C, Haas AR, Metzger S, et al. Phase I study of intrapleural gene-mediated cytotoxic immunotherapy in patients with malignant pleural effusion. Mol Ther. 2018;26(5):1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aguilar LK, Shirley LA, Chung VM, et al. Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. Cancer Immunol Immunother. 2015;64(6):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasenburg A, Tong XW, Fischer DC, et al. Adenovirus-mediated thymidine kinase gene therapy in combination with topotecan for patients with recurrent ovarian cancer: 2.5-year follow-up. Gynecol Oncol. 2001;83(3):549–554. [DOI] [PubMed] [Google Scholar]
- 29. Teh BS, Ayala G, Aguilar L, et al. Phase I-II trial evaluating combined intensity-modulated radiotherapy and in situ gene therapy with or without hormonal therapy in treatment of prostate cancer-interim report on PSA response and biopsy data. Int J Radiat Oncol Biol Phys. 2004;58(5):1520–1529. [DOI] [PubMed] [Google Scholar]
- 30. Chhikara M, Huang H, Vlachaki MT, et al. Enhanced therapeutic effect of HSV-tk+GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther. 2001;3(4):536–542. [DOI] [PubMed] [Google Scholar]
- 31. Bomgaars L, Thompson P, Berg S, Serabe B, Aleksic A, Blaney S. Valacyclovir and acyclovir pharmacokinetics in immunocompromised children. Pediatr Blood Cancer. 2008;51(4):504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris MH, DuBois SG, Bender JLG, et al. Multicenter feasibility study of tumor molecular profiling to inform therapeutic decisions in advanced pediatric solid tumors. JAMA Oncol. 2016;2(5):608–615. [DOI] [PubMed] [Google Scholar]
- 33. Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeRenzo C, Krenciute G, Gottschalk S. The landscape of CAR T cells beyond acute lymphoblastic leukemia for pediatric solid tumors. Am Soc Clin Oncol Educ Book. 2018;(38):830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bagley SJ, Desai AS, Linette GP, June CH, O’Rourke DM. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018;20(11):1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buerki RA, Chheda ZS, Okada H. Immunotherapy of primary brain tumors: facts and hopes. Clin Cancer Res. 2018; doi: 10.1158/1078-0432.CCR-17-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.