Abstract
Background
Cellular senescence and the senescence-associated secretory phenotype (SASP) may contribute to the development of radiation therapy–associated side effects in the lung and blood vessels by promoting chronic inflammation. In the brain, inflammation contributes to the development of neurologic disease, including Alzheimer’s disease. In this study, we investigated the roles of cellular senescence and Δ133p53, an inhibitory isoform of p53, in radiation-induced brain injury.
Methods
Senescent cell types in irradiated human brain were identified with immunohistochemical labeling of senescence-associated proteins p16INK4A and heterochromatin protein Hp1γ in 13 patient cases, including 7 irradiated samples. To investigate the impact of radiation on astrocytes specifically, primary human astrocytes were irradiated and examined for expression of Δ133p53 and induction of SASP. Lentiviral expression of ∆133p53 was performed to investigate its role in regulating radiation-induced cellular senescence and astrocyte-mediated neuroinflammation.
Results
Astrocytes expressing p16INK4A and Hp1γ were identified in all irradiated tissues, were increased in number in irradiated compared with untreated cancer patient tissues, and had higher labeling intensity in irradiated tissues compared with age-matched controls. Human astrocytes irradiated in vitro also experience induction of cellular senescence, have diminished Δ133p53, and adopt a neurotoxic phenotype as demonstrated by increased senescence-associated beta-galactosidase activity, p16INK4A, and interleukin (IL)-6. In human astrocytes, Δ133p53 inhibits radiation-induced senescence, promotes DNA double-strand break repair, and prevents astrocyte-mediated neuroinflammation and neurotoxicity.
Conclusions
Restoring expression of the endogenous p53 isoform, ∆133p53, protects astrocytes from radiation-induced senescence, promotes DNA repair, and inhibits astrocyte-mediated neuroinflammation.
Keywords: astrocytes, IL-6, p53 isoform, radiation-induced brain injury, senescence
Key Points.
Astrocyte senescence is increased in irradiated human brain tissue.
Radiation-induced astrocyte senescence induces neurotoxicity.
Δ133p53 inhibits radiation-induced astrocyte senescence to promote neuroprotection.
Importance of the Study.
With improvements in cancer therapies, an increasing number of patients survive long enough to experience late complications of radiotherapy, including progressive cognitive impairment that can escalate to severe memory loss and dementia. Astrocytes are ubiquitous brain cells that may promote neuroinflammation and neurotoxicity in neurodegenerative diseases through the adoption of SASP. This study identifies senescent astrocytes in irradiated patient brain tissues and demonstrates that ∆133p53 inhibits radiation-induced astrocyte senescence, promotes DNA repair, and prevents production of neurotoxic IL-6 from irradiated primary human astrocytes.
Cranial radiation therapy is used to effectively treat brain cancer in adult and pediatric patients.1,2 Since its development, protocols have evolved to incorporate methods to reduce side effects, such as shielding the hippocampus and fractioning the total radiation dose.3–5 However, even with improvements, over 40% of patients surviving >6 months experience late side effects. In up to 5% of these patients, neurocognitive impairment progresses from decreased attention and problem-solving ability to memory loss, ataxia, and dementia.6,7 Late effects may also develop in pediatric patients for whom radiation may be prescribed to treat the two most common cancer types: leukemia and glioma.8–10 Side effects in these patients include deficits in social functioning and vocational difficulty and poor performance in intelligence quotient testing and are most severe in the youngest patients receiving the highest radiation doses.4,9–13 As the number of cancer survivors increases, it becomes increasingly critical to understand the causes of these late effects and to develop strategies to prevent them.
Side effects of cancer therapy may be associated with injury to non-tumor cells.14 Following radiation exposure and accumulation of DNA damage, cells may adopt one of several cell type‒specific responses, including induction of cellular senescence.5,15 Importantly, although senescent cells do not replicate, they may avoid clearance and persist in tissues while continuing to produce inflammatory factors that contribute to tissue injury.16,17 In this way, radiation-induced cellular senescence is being recognized as an important mediator of tissue dysfunction promoting chronic inflammation and contributing to radiation-induced side effects, including pulmonary fibrosis and cerebrovascular dysfunction.18,19
To investigate the role of cellular senescence in cranial radiotherapy, this study examines brain tissue from patients who have undergone brain radiation treatment and identifies several senescent cell types, including astrocytes. Astrocytes perform many neuroprotective functions, including production of neurotrophic factors. However, astrocytes may also promote neurodegeneration in some diseases, including Alzheimer’s disease, which is thought to be related to induction of a senescence-associated secretory phenotype (SASP).17,20 The role of astrocytes and astrocyte senescence in radiation-induced brain injury has not been previously characterized.6
After identifying senescent astrocytes in irradiated tissues, this study investigates the potential functions of astrocyte senescence and SASP in promoting brain injury. Based on previous studies20 identifying regulation of replicative senescence by one of the p53 isoforms, ∆133p53, this study examines the role of ∆133p53 in regulating radiation-induced astrocyte senescence. These findings identify restoration of ∆133p53 as a potential therapeutic approach to inhibiting radiation-induced astrocyte senescence, promoting DNA repair in irradiated astrocytes, and preventing astrocyte-mediated neuroinflammation.
Methods
Human Patient Tissues
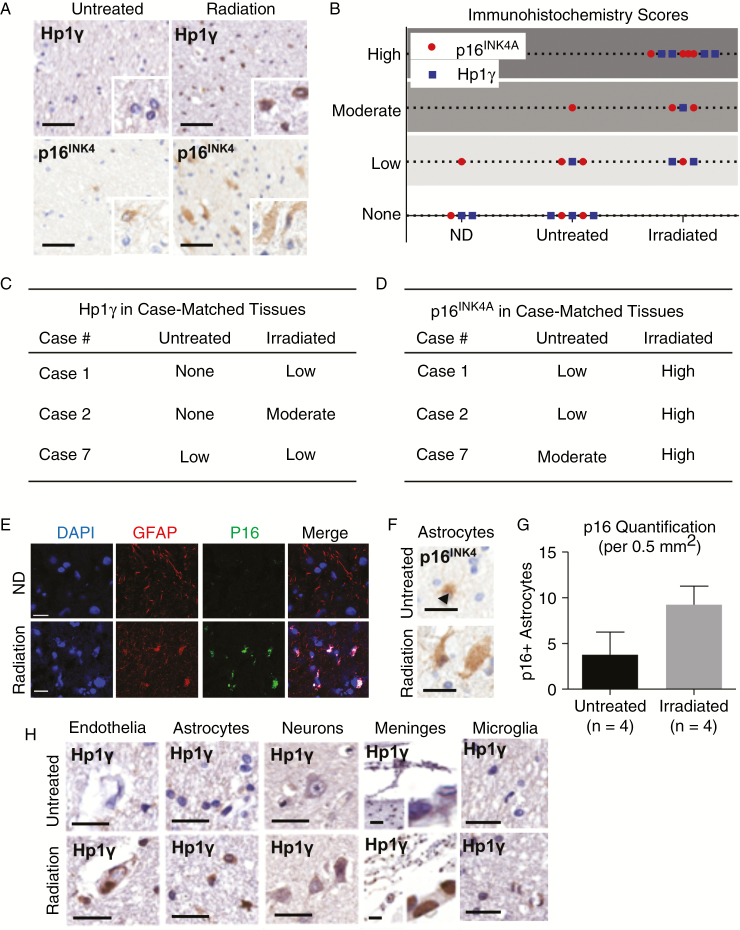
Case tissues were acquired with full institutional review board approval from the Georgetown Brain Bank, the Histopathology Tissue Shared Resource at Georgetown University, and Johns Hopkins Brain Bank and included non-tumor brain tissue from cancer patients with a history of cranial radiation treatment, with no history of treatment, or from non-disease, age-matched controls collected at autopsy (Supplementary Table 1). Patients receiving chemotherapy or immunotherapy were excluded.14 Tissues were anonymized, labeled with senescence-associated proteins (Supplementary Table 2), and examined by 3 pathologists (J.B., B.H., I.O.). Each control and radiation-treated tissue was assigned an immunoreactivity score in a blinded manner based on the intensity of immunohistochemical labeling (Fig. 1, Supplementary Table 3). Quantification of p16INK4A-positive astrocytes was completed in 20 microscopic fields (0.5 mm2) from untreated cancer patients (n = 4) and cancer patients receiving cranial radiation treatment (n = 4). In addition, 3 patients received stereotactic radiotherapy allowing for comparison of irradiated and untreated regions within the same patient (Fig. 1); these case-matched tissues were further reviewed to identify Hp1γ-positive cell types, to quantify percent of Hp1γ-positive microglia, and to quantify CD68-positive microglia per high-power field (40x) in irradiated and untreated brain tissue (Fig. 1, Supplementary Fig. 3).
Fig. 1.
Astrocyte senescence is increased in irradiated patient tissues. (A) Expression of senescence-associated proteins Hp1γ and p16INK4 in irradiated and untreated non-tumor brain tissues using immunohistochemistry. (B) Tissues were examined in a blinded fashion by 3 pathologists and scored from 0 (none) to 3 (high) based on intensity of cell labeling. (C) Hp1γ and (D) p16INK4A immunohistochemical labeling in 3 patients receiving stereotactic radiation with comparison of irradiated to untreated tissue in the same patient as an internal control. *Case 7 is from a patient with a previous diagnosis of Alzheimer’s disease in which astrocyte senescence is prominent and thought to promote neurodegeneration.20 (E) Immunocytochemistry of irradiated brain tissue demonstrating co-localization of p16INK4A and GFAP in astrocytes. (F) p16INK4A-positive astrocytes in irradiated human brain tissues using immunohistochemistry. (G) Quantification of p16INK4A-positive astrocytes in 20 microscopic fields (0.5 mm2) in non-tumor brain tissue from untreated cancer patients (n = 4) and cancer patients receiving radiation treatment (n = 4). (H) Representative images of cell types expressing senescence-associated Hp1γ in irradiated (stereotactic) and untreated brain tissue from the same patient, including endothelia, astrocytes, neurons, meninges, and microglia. Scale = 50 μm.
Cell Culture
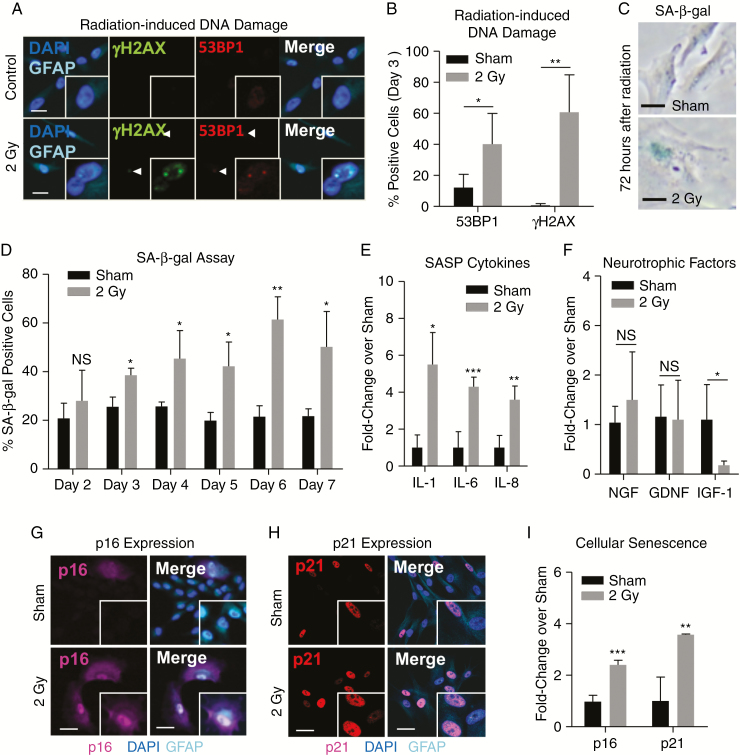
Primary human astrocytes were obtained from Sciencell and maintained in Astrocyte Medium supplemented with 2% fetal bovine serum, 1% astrocyte growth supplement from Sciencell, and 1% penicillin/streptomycin solution. Astrocytes expressed astrocyte-lineage marker (glial fibrillary acidic protein [GFAP]) (Fig. 2A, G–H, Supplementary Fig. 4N–P) were split at a ratio of 1:3 and continued to proliferate through passage 20. All experiments used proliferative, low passage astrocytes (<p10).
Fig. 2.
Radiation induces astrocyte senescence. (A) Representative image and (B) quantification of radiation-induced DNA damage identified by immunolabeling of double-stranded DNA breaks by 53BP1 and γH2AX in primary human astrocytes 3 days after radiation exposure (2 Gy). (C) Representative images of SA-β-gal staining in human astrocytes on day 3 after exposure to radiation (2 Gy). (D) Quantitative summary of the percent of astrocytes with SA-β-gal staining from 2 to 7 days after radiation (2 Gy). (E) Production of SASP-associated cytokine mRNAs (IL-1β, IL-6, and IL-8 mRNA) and (F) neurotrophic factor mRNAs (nerve growth factor [NGF], glial cell–derived neurotrophic factor [GDNF], insulin-like growth factor-1 [IGF-1]) in irradiated or sham-treated primary human astrocytes measured by qRT-PCR (Taqman). Representative images of (G) p16INK4A and (H) p21WAF1 immunolabeling in irradiated GFAP-positive human astrocytes on day 6 following exposure to radiation (2 Gy). (I) Quantitation of p16INK4A and p21WAF1 immunoreactivity in irradiated (2 Gy) and sham-treated primary human astrocytes on day 6. NS indicates P > 0.05, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 by unpaired 2-tailed Student’s t-test. Scale = 25 μm.
The induced pluripotent stem cell (iPSC) line, i20 (NIH stem cell bank), was differentiated to neural stem cells using Gibco Pluripotent Stem Cell Neural Induction (Life Technologies). Mature neurons were differentiated from a transgenic human control iPSC line by neurogenin 2 induction.21 The iPSCs were plated in Matrigel-coated 10 cm dishes at a density of 1.5 × 106 cells/dish with doxycycline (2 μg/mL) in Dulbecco’s modified Eagle’s medium/F12 containing N2 supplement (Invitrogen), non-essential amino acids (Invitrogen), L-glutamine (Invitrogen), and Y-27632 (10 μM; Tocris). After 3 days, cells were Accutase treated and plated onto poly-D-lysine/laminin coated 8-chamber slides (Corning) at a density of 300 000 cells in induction media without Y-27632, supplemented with mouse laminin (1 μg/mL; Invitrogen), B27 supplement (Invitrogen), and brain derived neurotrophic factor (10 ng/mL; R+D Systems). Neurons were analyzed after 7 days of differentiation.
Radiation Exposure
Human cells were exposed to ionizing radiation in an X-Rad 320 biologic irradiator (Precision X-ray), at a dose of 0.5 to 20 Gy as indicated.
IL-6 Treatment
Where indicated, recombinant interleukin (IL)-6 (InvivoGen) was incubated with iPSC-derived neural stem cells (NSCs) or mature neurons for 24 hours at a concentration of 5 ng/mL.
Senescence-Associated Beta Galactosidase Assay
SA-β-gal staining was performed with the Senescence Associated (SA)-β-Galactosidase Staining Kit (Cell Signaling Technology).
Transwell Experiments
Human astrocytes were irradiated in the top transwell chamber and co-cultured with untreated neural progenitor cells (NPCs) (ACS-5004) for 96 hours beginning on the third day after irradiation. Additional details are described in the Supplementary Material.
Statistical Analysis
Data are presented as mean and standard deviation of at least 3 independent experiments. Comparisons were made using 2-sided, unpaired Student’s t-test. Differences were considered significant at *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 or NS (not significant).
Additional methods—including antibodies, lentiviral vector transduction, cell viability assay, enzyme-linked immunosorbent assay, quantitative reverse transcriptase (qRT)-PCR, immunohistochemistry, immunofluorescence, western blot, RNA extraction, and cDNA preparation—can be found in the Supplementary Material.
Results
Astrocyte Senescence Is Increased in Irradiated Patient Tissues
Radiation-induced cellular senescence is a stress-induced cell cycle arrest that may contribute to the development of radiotherapy side effects.18,19 To characterize cellular senescence in the brain, tissue samples from patients with or without a history of radiation treatment were examined (Supplementary Table 1). Immunohistochemistry was performed using antibodies against senescence-associated proteins p16INK4A and Hp1γ22–24 (Fig. 1A) and scored based on the intensity of cellular labeling (Fig. 1B, Supplementary Table 3). Tissue immunoexpression of senescence proteins was lowest in brain tissue from non-disease, age-matched controls; was increased in untreated cancer patients; and was highest in irradiated tissues (Fig. 1B). Similar results were observed in a subset of patients with the same cancer type (metastatic melanoma; Supplementary Fig. 1A–C) and in patients receiving stereotactic radiotherapy (Fig. 1C–D), which in contrast to non-targeted whole brain radiotherapy allows for comparison of irradiated to untreated brain regions within the same patient as an internal control.
We next aimed to characterize senescent cell types in irradiated patient tissues. Hp1γ- and p16INK4A-positive cells were identified by 3 independent pathologists (J.B., B.H., I.O.). The majority of senescence-associated markers co-localized with GFAP-positive astrocytes (Fig. 1E), underscoring the potential importance of astrocyte senescence in the brain’s response to radiation. The mild increase in cellular senescence in untreated cancer patient tissues compared with non-disease controls (Fig. 1B) may indicate a role for the tumor microenvironment in promoting reactive astrocytosis and astrocyte senescence, which may be a general response of human astrocytes to injury. However, the number of p16INK4A-positive astrocytes was higher in patients receiving radiation treatment compared with untreated cancer patients (Fig. 1F–G), suggesting that radiotherapy may exacerbate this response. In addition, similar numbers of p16INK4A-positive astrocytes were observed in tissues irradiated between 3 months and 4 years prior to collection (Supplementary Fig. 2A–B), which is consistent with reports of increased numbers of reactive astrocytes in animal models of radiation-induced brain injury for at least 1 year following radiation exposure.25,26 Astrocyte senescence is also increased in Alzheimer’s disease (Case 7, Fig. 1C–D, Supplementary Fig. 2C–D) and may promote neurotoxicity, highlighting the potential importance of astrocyte senescence in neurodegenerative diseases.16,17,20 Finally, focal Hp1γ immunoreactivity was identified in several additional cell types, including microglia, which are important mediators of neuroinflammation27; however, this effect was less prominent than the described astrocyte senescence (Fig. 1H, Supplementary Fig. 3A–D).
Radiation Induces Cellular Senescence in Human Astrocytes
Radiation can induce DNA damage either directly through ionization or indirectly through the production of free radicals.5,14 Adult and pediatric patients with brain cancer may receive 30 to 60 Gy of radiation, which is administered in small doses or fractions of approximately 2 Gy per treatment until the total dose is achieved.1–3 After a single 2 Gy fraction, primary human astrocytes irradiated in vitro have significant increases in DNA double-strand breaks indicated by γH2AX (P = 0.013) and 53BP1 (P = 0.035) (Fig. 2A–B).28
Following accumulation of DNA damage, one of several cell type‒specific responses may occur, including induction of apoptosis, mitotic catastrophe, or cellular senescence.5,15 Our study has identified astrocytes as the major senescent cell type in irradiated brain tissues. In contrast to NSCs (Supplementary Fig. 4A),15,29 irradiated human astrocytes did not experience induction of apoptosis and maintained over 90% viability for up to 7 days following radiation exposure ((Supplementary Fig. 4B–D). To further characterize this, we next investigated astrocytes irradiated in vitro for the induction of cellular senescence, a response that may promote side effects of cancer treatment.18,19 Irradiated astrocytes experienced a significant, dose-dependent (Supplementary Fig. 4E–G) increase in SA-β-gal staining beginning 2 days after irradiation (P = 0.010, 1.5-fold) and persisting for up to 1 week (P = 0.03, 2.3-fold) (Fig. 2C–D). SASP-associated cytokines, including IL-1β and IL-6, are known to be upregulated in patients and animal models following radiation treatment.27,30–33 To determine whether astrocytes may contribute to radiation-induced inflammation, we examined several cytokines implicated in neurodegeneration20,34 and found a significant increase in IL-1β (P = 0.016), IL-6 (P = 0.0005), and IL-8 (P = 0.006) (Fig. 2E). The significant induction of SASP cytokines in irradiated astrocytes underscores their potential role in promoting neuroinflammation in radiation-induced brain injury. Similar induction of senescence and SASP-associated IL-6 was also observed in astrocytes irradiated at radiosurgical doses (10 Gy; Supplementary Fig. 4H–J). In addition, radiation-induced astrocyte senescence was accompanied by a significant loss of insulin-like growth factor 1 (IGF-1) (P = 0.015; Fig. 2F), which is reported to promote astrocyte-mediated neuroprotection and improve neurocognitive function in mouse models of brain injury.35,36 Finally, irradiated astrocytes demonstrated increased expression of senescence-associated p16INK4A (P < 0.0001) and p21 (P = 0.009) (Fig. 2G–I), reduced cell number (Supplementary Fig. 4K), enlarged cell size (Supplementary Fig. 4L), increased number of multinucleated cells15 (Supplementary Fig. 4M), and senescence-associated downregulation of GFAP37 (Supplementary Fig. 4N–P). Taken together, these in vitro results indicate that irradiated astrocytes undergo senescence, and are consistent with our findings in patient tissues and with animal models of radiation-induced brain injury.25–28
∆133p53 Is Decreased in Irradiated Astrocytes and Its Overexpression Protects Astrocytes from Radiation-Induced Cellular Senescence
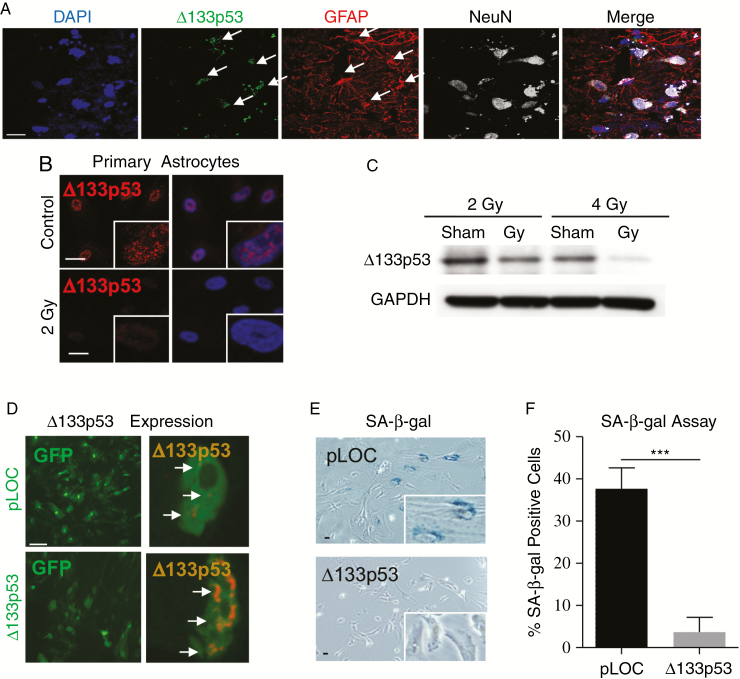
Senescent astrocytes are observed in patients with neurodegenerative diseases, including Alzheimer’s disease and amyotrophic lateral sclerosis, and have been shown to have reduced expression of the p53 isoform ∆133p53.20 To identify brain cells expressing ∆133p53 in human brain tissue, immunofluorescence was performed using a ∆133p53-specific antibody, MAP4,20,38 and cell type–specific antibodies for astrocytes (GFAP-positive20) or neurons (NeuN-positive39). The majority of ∆133p53 expression co-localizes with GFAP-positive astrocytes (Fig. 3A), indicating that astrocytes are the predominant source of ∆133p53. Following radiation exposure, primary human astrocytes have decreased ∆133p53 (Fig. 3B), which is further diminished after exposure to a second 2 Gy fraction (4 Gy total dose) (Fig. 3C), suggesting that loss of Δ133p53 may be associated with the induction of radiation-induced astrocyte senescence.
Fig. 3.
Δ133p53 is decreased in irradiated astrocytes and its overexpression protects astrocytes from cellular senescence. (A) Non-disease human brain tissue fluorescently labeled with antibodies to ∆133p53, astrocytic GFAP, and neuronal-specific nuclear protein (NeuN) to identify cellular sources of ∆133p53 (arrows). (B) Primary human astrocytes labeled with nuclear staining (4′,6′-diamidino-2-phenylindole [DAPI]) and ∆133p53 on day 6 following either sham or radiation treatment (2 Gy). (C) Western blot analysis of ∆133p53 on day 6 in sham-treated or irradiated primary human astrocytes irradiated one time at 2 Gy or twice at 2 Gy 24 hours apart (fractionated dose, 4 Gy total). (D) Nuclear Δ133p53 expression in human astrocytes transduced 3 days after radiation treatment (2 Gy) with either a green fluorescent protein lentiviral vector driving Δ133p53 expression or its control vector (pLOC). (E) Representative image of SA-β-gal staining in transduced, irradiated human astrocytes (2 Gy) on day 6. (F) Quantitative summary of SA-β-gal staining in primary human astrocytes with lentiviral pLOC and Δ133p53 following radiation exposure (2 Gy). Scale = 25 μm.
As ∆133p53 is diminished in irradiated senescent astrocytes, we investigated whether reconstitution of ∆133p53 expression would protect astrocytes from radiation-induced senescence. First, a lentiviral vector expressing ∆133p53 or pLOC control vector (Supplementary Material) was transduced in primary human astrocytes 3 days after radiation exposure (Fig. 3D). Irradiated astrocytes with reconstituted ∆133p53 had reduced SA-β-gal activity compared with control astrocytes (P = 0.0006) (Fig. 3E–F), indicating that Δ133p53 can rescue astrocytes from radiation-induced senescence. Finally, we examined the impact of transducing astrocytes with lentiviral vectors expressing ∆133p53 or pLOC control prior to radiation exposure and found that astrocytes with ∆133p53 had no increase in SA-β-gal staining (P = 0.483) compared with an increase of approximately 55% in irradiated pLOC control astrocytes (P < 0.0001) (Supplementary Fig. 5A–B), demonstrating that increasing ∆133p53 protects astrocytes from radiation-induced senescence when induced either prior to or after radiation exposure.
∆133p53 Promotes DNA Repair in Irradiated Astrocytes
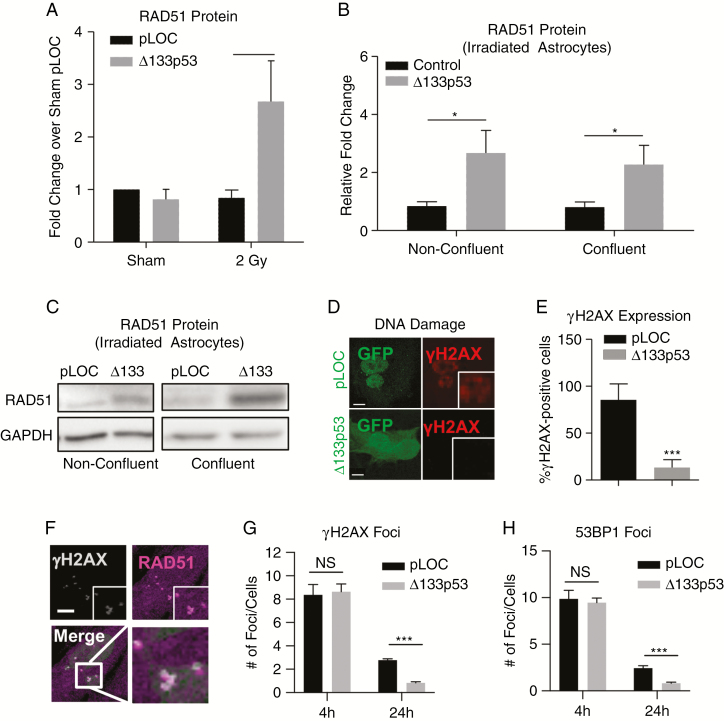
Recently, ∆133p53 has been shown to promote DNA repair in fibroblasts from patients with Hutchinson-Gilford progeria syndrome through the promotion of homologous recombination (HR) DNA repair protein RAD51.40 Following irradiation, RAD51 is significantly increased in astrocytes transduced with ∆133p53 (P = 0.016; Fig. 4A). Although this increase may be due to accelerated cell proliferation,41 confluent human astrocytes transduced with Δ133p53 maintained a 2- to 3-fold increase in RAD51 (Fig. 4B–C) despite an approximately 3.5-fold decrease in cellular proliferation (Ki-6742; Supplementary Fig. 6A–B). The sustained increase in RAD51 at confluency, which is associated with G1 arrest,43 suggests that the effect of Δ133p53 on HR may be at least in part due to an increased baseline expression of RAD51, although this finding does not rule out an S/G2 phase-specific regulation of HR machinery. To further examine the role of Δ133p53 in HR, DNA double-stranded breaks were labeled with γH2AX. Six days after irradiation, the percent of γH2AX-positive astrocytes was significantly reduced by ∆133p53 transduction after radiation exposure (P < 0.0001; Fig. 4D–E). A similar reduction in γH2AX was also observed in astrocytes transduced with Δ133p53 vector prior to radiation (Supplementary Fig. 6C). To examine DNA repair kinetics at earlier time points, astrocytes were transduced prior to irradiation and labeled at 4 and 24 hours post-irradiation with RAD51, γH2AX, and 53BP1 (Fig. 4F–H, Supplementary Fig. 6D–E). After 4 hours, the number of DNA damage foci labeled by γH2AX and 53BP1 was not significantly different (Fig. 4G–H), suggesting that both control and Δ133p53 transduced cells develop similar levels of radiation-induced DNA damage; however, after 24 hours, Δ133p53-transduced astrocytes had fewer γH2AX (P = 0.00002) and 53BP1 foci (P = 0.0006), suggesting that Δ133p53 promotes DNA repair in irradiated astrocytes.
Fig. 4.
133p53 promotes DNA repair. (A) RAD51 protein in sham and irradiated astrocytes expressing either control vector (pLOC) or Δ133p53. (B) Quantification and (C) representative western blots of RAD51 protein in irradiated astrocytes expressing pLOC or Δ133p53 at low and high confluency. (D) Labeling of DNA double-strand breaks with γH2AX in transduced, irradiated astrocytes. (E) Quantitative summary of γH2AX staining on day 6 in irradiated human astrocytes transduced with pLOC or Δ133p53 on day 3 after radiation exposure (2 Gy). (F) Representative image of RAD51 and γH2AX labeling 4 hours after radiation exposure. (G) Quantification of γH2AX- and (H) 53BP1-positive foci at 4 and 24 hours after radiation exposure in astrocytes transduced prior to radiation treatment. NS indicates P > 0.05, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 by unpaired 2-tailed Student’s t-test. Scale = 5 μm.
∆133p53 Inhibits Astrocyte-Mediated Neuroinflammation
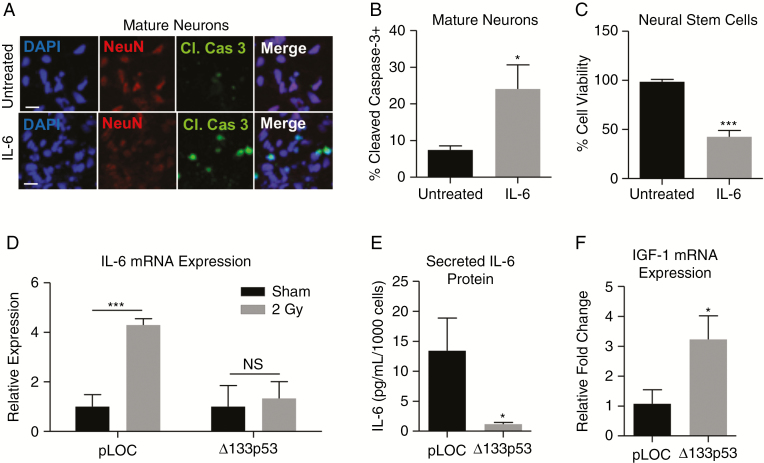
Because radiation-induced brain injury is associated with neurocognitive dysfunction, many studies focus on the effects of radiation on neurons and NPCs.6,29,44 Secretory factors derived from senescent astrocytes are known to impair astrocyte-mediated neuroprotection in animal models45 and may promote the late effects of radiation injury by contributing to chronic neuroinflammation. Of the SASP cytokines, IL-6 is most frequently upregulated in neurodegeneration.46 Following radiation exposure, human astrocytes secrete significantly more IL-6 (P = 0.018; Supplementary Fig. 5C), similar to replicatively senescent astrocytes, which are neurotoxic via IL-6 in neuron-astrocyte co-culture experiments.20 To examine whether radiation-induced senescent astrocytes are also neurotoxic, we cultured NPCs with irradiated or sham-treated astrocytes separated by a transwell membrane (Supplementary Fig. 5D). In co-culture, there was a significant loss of NPC viability (P = 0.009; Supplementary Fig. 5E) and a 2-fold induction of NPC apoptosis (P = 0.011; Supplementary Fig. 5F–G). These findings are not only consistent with our previous study of senescent astrocyte-mediated neurotoxicity but also demonstrate that astrocytes mediate neurotoxicity through secretory factors such as IL-6,20 rather than through direct cell-cell contact. This was further examined through direct exposure of NSCs and mature neurons to IL-6 (5 ng/mL). After 24 hours, there was an approximately 10% increase in the percent of mature neurons expressing the apoptotic marker cleaved caspase 3 (P = 0.013; Fig. 5A–B), and the viability of NSCs was reduced to less than 50% (P = 0.0001; Fig. 5C), suggesting that IL-6 plays a causative role in neuronal death mediated by senescent astrocytes.
Fig. 5.
Δ133p53 regulates radiation-induced, astrocyte-mediated neurotoxicity. (A–B) Immunopositivity of cleaved caspase 3 in mature neurons and (C) viability of neural stem cells following 24-hour IL-6 exposure (5 ng/mL). (D) IL-6 mRNA production in sham and irradiated astrocytes transduced with either Δ133p53 (P = 0.389) or the control vector on day 3 and examined on day 6 by qRT-PCR (Taqman). (E) IL-6 protein secreted by astrocytes transduced prior to radiation and examined by enzyme-linked immunosorbent assay, (F) IGF-1 mRNA expression in irradiated astrocytes transduced with pLOC, or Δ133p53 vector prior to radiation exposure (P = 0.015) and examined by qRT-PCR (Taqman). NS indicates P > 0.05, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 by unpaired 2-tailed Student’s t-test. Scale = 25 μm.
Because Δ133p53 was found to rescue irradiated astrocytes from senescence (Fig. 3), we next investigated whether ∆133p53 rescues astrocytes from radiation-induced production of neurotoxic IL-6. Irradiated control astrocytes experienced a significant 5-fold increase in IL-6 mRNA measured by qRT-PCR (P = 0.0005). In contrast, IL-6 mRNA was not significantly upregulated in irradiated astrocytes with restored ∆133p53 (P = 0.389; Fig. 5D), indicating that astrocyte-mediated neuroinflammation is repressed by reconstitution of ∆133p53 after radiation treatment. Similar findings were also observed in astrocytes transduced prior to radiation, including a significant reduction in secreted IL-6 (P = 0.017; Fig. 5E). In addition, astrocytes transduced with Δ133p53 demonstrated a partial rescue of neurotrophic IGF-1 mRNA expression (P = 0.015; Fig. 5F). Taken together, these findings suggest that radiation induces astrocyte senescence, thereby promoting astrocyte-mediated neurotoxicity through the production of neurotoxic cytokines. Critically, Δ133p53 has been identified as a potential therapeutic target for inhibiting radiation-induced astrocyte-mediated neurotoxicity (Fig. 6).
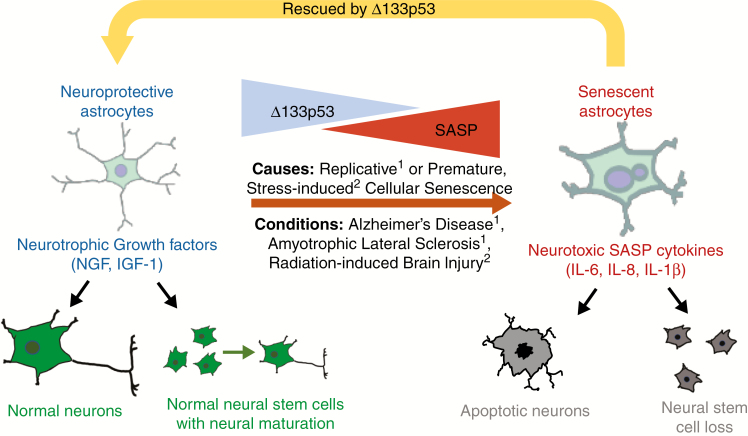
Fig. 6.
Proposed model of Δ133p53 regulation of astrocyte-mediated neuroprotection and neuroinflammation. Senescent astrocytes are increased in neurodegenerative diseases, including Alzheimer’s disease, and have diminished Δ133p53. Similarly, senescent astrocytes are observed in brain tissues from cancer patients receiving radiation treatment, suggesting that senescent astrocytes may contribute to chronic neuroinflammation in each of these pathologies. These findings are also reproduced in vitro where cellular senescence is induced in irradiated or replicatively exhausted astrocytes and is associated with loss of Δ133p53, adoption of the SASP, and diminished neurotrophic factor production, including IGF-1, which can each be rescued by enhanced expression of Δ133p53. 1Turnquist et al, 2016; 2current study.
Discussion
Radiation-induced brain injury may cause progressive cognitive deterioration, including dementia-like symptoms.6 It shares pathologic features with aging-associated neurodegeneration, including chronic oxidative stress, inflammation, and reduced neurogenesis.6,47,48 Current understanding of the pathogenesis of radiation-induced brain injury focuses on the acute loss of NSCs and its effect on hippocampus-dependent functions such as learning and memory.29,49 However, few studies have addressed the role of astrocytes. Our finding that astrocytes preferentially undergo senescence, while NPCs undergo cell death, indicates that astrocyte SASP may underlie the chronic nature of radiation-induced brain injury.
Animal models of radiation-induced brain injury have identified hypertrophied astrocytes that persist for at least 12 months following radiation treatment.25,26 Based on our findings in irradiated human tissues and our previous findings in Alzheimer’s disease and amyotrophic lateral sclerosis,20 many of these hypertrophied astrocytes are senescent, an important pathologic characterization that likely extends to other disease processes in the brain.
Following brain injury, astrocytes proliferate as part of reactive astrogliosis, which may lead to replicative senescence.20,50,51 In addition, direct injury including DNA injury or oxidative damage may induce premature cellular senescence.17,18,51 Both mechanisms of cellular senescence are controlled by p53 and its isoforms through p53-inducible cell cycle regulators, such as p21.20,38 In humans, TP53 has at least 12 isoforms through alternative promoters or splicing that may promote or inhibit full-length p53 activities or have independent functions. Of these isoforms, Δ133p53 is the best characterized as an endogenous inhibitor of cellular senescence.20,38,40 Based on this and previous studies,40 Δ133p53 enhances DNA repair in senescent cells by promoting HR; however, our study has also demonstrated that expression of Δ133p53 enhanced repair of foci positive for 53BP1, a component of non-homologous end-joining (NHEJ),52 suggesting that Δ133p53 may also regulate NHEJ in radiation injury by a currently unknown mechanism.
In addition to accumulating DNA damage, senescent cells may promote inflammation through induction of SASP.16,17 Increased release of the SASP cytokines IL-632,33 and IL-1β33 is reported in animal models of radiation-induced brain injury and may inhibit neurogenesis, contributing to cognitive impairment.30,53,54 Using anti-inflammatory drugs to target and reduce neuroinflammation in radiation injury improves neurogenesis,30 while IL-6 has been shown to reinforce radiation-induced senescence in animal models,55 underscoring the role of chronic neuroinflammation in promoting radiation-induced brain injury. Based on the findings outlined in this study, astrocyte senescence and astrocyte-derived neuroinflammation have been identified as potential contributors to radiation-induced brain injury.
This and previous studies have demonstrated that Δ133p53, through the inhibition of full-length p53, regulates p21,38,40 RAD51,40 and IL-6,20,40 each of which has been shown to be important in radiation-induced injury and neurotoxicity. Although the regulatory interactions between these factors have yet to be elucidated, our findings suggest that induction of the p53 isoform ∆133p53 may have potential therapeutic value by preventing astrocyte senescence and inhibiting astrocyte-mediated neuroinflammation (Fig. 6). Critically, this endogenous isoform is produced in human cells and has not been shown to be mutagenic or oncogenic.20,38,56 To study the role of Δ133p53 in other cell types and the tumor microenvironment in vivo, ongoing studies seek to establish an animal model and identify compounds which modulate ∆133p53. Future studies aim to reverse the senescence phenotype in diseases, such as radiation-induced brain injury, in which cellular senescence may initiate or worsen disease progression.20,38,40
Funding
This work was primarily funded by the National Cancer Institute (NCI), National Institutes of Health (NIH). C.T. was partially supported by the NCI’s Director’s Innovation Award. J.B. was partially supported by the Comparative Biomedical Scientist Training Program. B.V. is supported with projects CZ.02.1.01/0.0/0.0/16_019/0000868 from Regional development fund and Czech Science Foundation P206/12/G151. B.T.H. is partially supported as director and co-director of the Georgetown University Brain Bank and the Histopathology and Tissue Shared Resource, respectively. These cores are supported by Georgetown University and NCI-NIH through the Lombardi Comprehensive Cancer Center. Confocal microscopy was supported by NCI. We would like to thank the MedStar Georgetown University Hospital Department of Pathology and the Georgetown University Histopathology Tissue Shared Resource Core for preparing slides and performing the immunohistochemistry on human tissue sections. The content is solely the responsibility of the authors, and the funders had no role in the design, interpretation, or analysis of the data.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families for providing tissue samples with IRB approval.
Conflict of interest statement.
None.
Authorship statement.
C.T., J.B., I.H., B.T.H., and C.C.H. conceived and designed the project. C.T., J.B., I.O., and N.V.M. performed the experiments. C.T., J.B., I.H., B.T.H, and C.C.H. analyzed data. B.V., D.P.L, C.G., J.C., D.S., and H.M.A. provided reagents and guidance on the project. C.T., J.B., I.H., B.T.H, and C.C.H. wrote the manuscript.
References
- 1. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. [DOI] [PubMed] [Google Scholar]
- 2. Fisher BJ, Bauman GS, Leighton CE, Stitt L, Cairncross JG, Macdonald DR. Low-grade gliomas in children: tumor volume response to radiation. Neurosurg Focus. 1998;4(4):e5. [DOI] [PubMed] [Google Scholar]
- 3. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rowe LS, Krauze AV, Ning H, Camphausen KA, Kaushal A. Optimizing the benefit of CNS radiation therapy in the pediatric population-PART 1: understanding and managing acute and late toxicities. Oncology (Williston Park). 2017;31(3):182–188. [PubMed] [Google Scholar]
- 5. Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374(9701):1639–1651. [DOI] [PubMed] [Google Scholar]
- 8. Fischer C, Petriccione M, Donzelli M, Pottenger E. Improving care in pediatric neuro-oncology patients: an overview of the unique needs of children with brain tumors. J Child Neurol. 2016;31(4):488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schuitema I, de Sonneville L, Kaspers G, et al. Executive dysfunction 25 years after treatment with cranial radiotherapy for pediatric lymphoid malignancies. J Int Neuropsychol Soc. 2015;21(9):657–669. [DOI] [PubMed] [Google Scholar]
- 10. Agbahiwe H, Rashid A, Horska A, et al. A prospective study of cerebral, frontal lobe, and temporal lobe volumes and neuropsychological performance in children with primary brain tumors treated with cranial radiation. Cancer. 2017;123(1):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redmond KJ, Mahone EM, Terezakis S, et al. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro Oncol. 2013;15(3):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 1992;10(9):1390–1396. [DOI] [PubMed] [Google Scholar]
- 13. Maddrey AM, Bergeron JA, Lombardo ER, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72(3):245–253. [DOI] [PubMed] [Google Scholar]
- 14. Demaria M, O’Leary MN, Chang J, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31(4):363–372. [DOI] [PubMed] [Google Scholar]
- 16. Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–496. [DOI] [PubMed] [Google Scholar]
- 17. Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128(4):1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Citrin DE, Shankavaram U, Horton JA, et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst. 2013;105(19):1474–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Boerma M, Zhou D. ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat Res. 2016;186(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turnquist C, Horikawa I, Foran E, et al. p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration. Cell Death Differ. 2016;23(9):1515–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang C, Ward ME, Chen R, et al. Scalable production of iPSC-derived human neurons to identify Tau-lowering compounds by high-content screening. Stem Cell Reports. 2017;9(4):1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sohn JJ, Schetter AJ, Yfantis HG, et al. Macrophages, nitric oxide and microRNAs are associated with DNA damage response pathway and senescence in inflammatory bowel disease. PLoS One. 2012;7(9):e44156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130(8):1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price RE, Langford LA, Jackson EF, Stephens LC, Tinkey PT, Ang KK. Radiation-induced morphologic changes in the rhesus monkey (Macaca mulatta) brain. J Med Primatol. 2001;30(2):81–87. [DOI] [PubMed] [Google Scholar]
- 26. Suman S, Rodriguez OC, Winters TA, Fornace AJ Jr, Albanese C, Datta K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging (Albany NY). 2013;5(8):607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lumniczky K, Szatmári T, Sáfrány G. Ionizing radiation-induced immune and inflammatory reactions in the brain. Front Immunol. 2017;8:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. [DOI] [PubMed] [Google Scholar]
- 30. Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. [DOI] [PubMed] [Google Scholar]
- 31. Dong X, Luo M, Huang G, et al. Relationship between irradiation-induced neuro-inflammatory environments and impaired cognitive function in the developing brain of mice. Int J Radiat Biol. 2015;91(3):224–239. [DOI] [PubMed] [Google Scholar]
- 32. Haveman J, Geerdink AG, Rodermond HM. TNF, IL-1 and IL-6 in circulating blood after total-body and localized irradiation in rats. Oncol Rep. 1998;5(3):679–683. [DOI] [PubMed] [Google Scholar]
- 33. Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol. 2010;86(2):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34(1):3–11. [DOI] [PubMed] [Google Scholar]
- 35. Saatman KE, Contreras PC, Smith DH, et al. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147(2):418–427. [DOI] [PubMed] [Google Scholar]
- 36. Madathil SK, Carlson SW, Brelsfoard JM, Ye P, D’Ercole AJ, Saatman KE. Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS One. 2013;8(6):e67204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crowe EP, Tuzer F, Gregory BD, et al. Changes in the transcriptome of human astrocytes accompanying oxidative stress-induced senescence. Front Aging Neurosci. 2016;8:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujita K, Mondal AM, Horikawa I, et al. p53 isoforms delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11(9):1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarnat HB. Immunocytochemical markers of neuronal maturation in human diagnostic neuropathology. Cell Tissue Res. 2015;359(1):279–294. [DOI] [PubMed] [Google Scholar]
- 40. von Muhlinen N, Horikawa I, Alam F, et al. p53 isoforms regulate premature aging in human cells. Oncogene. 2018;37(18):2379–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamamoto A, Taki T, Yagi H, et al. Cell cycle-dependent expression of the mouse Rad51 gene in proliferating cells. Mol Gen Genet. 1996;251(1):1–12. [DOI] [PubMed] [Google Scholar]
- 42. Guillaud P, Vermont J, Seigneurin D. Automatic classification of cells in cell cycle phases based on Ki-67 antigen quantification by fluorescence microscopy. Cell Prolif. 1991;24(5):481–491. [DOI] [PubMed] [Google Scholar]
- 43. Davis PK, Ho A, Dowdy SF. Biological methods for cell-cycle synchronization of mammalian cells. Biotechniques. 2001;30(6):1322–6, 1328, 1330. [DOI] [PubMed] [Google Scholar]
- 44. Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 45. García-Matas S, Gutierrez-Cuesta J, Coto-Montes A, et al. Dysfunction of astrocytes in senescence-accelerated mice SAMP8 reduces their neuroprotective capacity. Aging Cell. 2008;7(5):630–640. [DOI] [PubMed] [Google Scholar]
- 46. Blum-Degena D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1β and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Letters. 1995;202(1–2):17–20. [DOI] [PubMed] [Google Scholar]
- 47. Mrak RE. Neuropathology and the neuroinflammation idea. J Alzheimers Dis. 2009;18(3):473–481. [DOI] [PubMed] [Google Scholar]
- 48. Wang B, Tanaka K, Ji B, et al. Low-dose total-body carbon-ion irradiations induce early transcriptional alteration without late Alzheimer’s disease-like pathogenesis and memory impairment in mice. J Neurosci Res. 2014;92(7):915–926. [DOI] [PubMed] [Google Scholar]
- 49. Abayomi OK. Pathogenesis of cognitive decline following therapeutic irradiation for head and neck tumors. Acta Oncol. 2002;41(4):346–351. [DOI] [PubMed] [Google Scholar]
- 50. Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev. 2014;94(4):1077–1098. [DOI] [PubMed] [Google Scholar]
- 51. Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128(4):1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stephanie P, Simon JB. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol. 2013;15(1):7. [DOI] [PubMed] [Google Scholar]
- 53. Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–330. [DOI] [PubMed] [Google Scholar]
- 54. Yang L, Yang J, Li G, et al. Pathophysiological responses in rat and mouse models of radiation-induced brain injury. Mol Neurobiol. 2017;54(2):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marmary Y, Adar R, Gaska S, et al. Radiation-induced loss of salivary gland function is driven by cellular senescence and prevented by IL6 modulation. Cancer Res. 2016;76(5):1170–1180. [DOI] [PubMed] [Google Scholar]
- 56. Horikawa I, Park KY, Isogaya K, et al. Δ133p53 represses p53-inducible senescence genes and enhances the generation of human induced pluripotent stem cells. Cell Death Differ. 2017;24(6):1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.