ABSTRACT
Background: Febrile seizure is the most common childhood neurological disorder, is an important health problem with potential short- and long-term complications, also leading to economic burden and increased parental anxiety about fevers and seizures occurring in their children. There are no routine recommendation to detect etiological causes of FS for neurological perspective, further knowledge about the etiological causes of FS in children will support preventive measures and follow-up strategies. The aim of this study is to evaluate the percentage of respiratory viruses in children with FS.
Methods: This prospective multicenter study, entitled “Viral etiological causes of febrile seizures for respiratory pathogens (EFES Study)” examined representative populations in eight different cities in Turkey between March 1, 2016 and April 1, 2017. Nasopharyngeal swabs were taken from all children at presentation. A respiratory multiplex array was performed to detect for influenza A and B; respiratory syncytial virus A and B; human parainfluenza virus 1-2-3 and 4; human coronavirus 229E and OC43; human rhinovirus; human enterovirus; human adenovirus; human bocavirus; human metapneumovirus.
Results: During the study period, at least one virus was detected in 82.7% (144/174) of children with FS. The most frequently detected virus was adenovirus, followed by influenza A and influenza B. Detection of more than one virus was present in 58.3% of the children with FS, and the most common co-existence was the presence of adenovirus and influenza B. In children younger than 12 months, Coronavirus OC43 was the most common, while influenza A was most frequently observed in children older than 48 months (p < 0.05). Human bocavirus was common in children who experienced complex FS, while respiratory syncytial virus (RSV) A was more common in children who experienced simple FS. Influenza B virus was the most common virus identified in children who were experiencing their first incidence of FS (p < 0.05).
Conclusions: This study indicates that respiratory viruses are important in the etiology of FS in children. The results show that antibiotics must be prescribed carefully in children with FS since the majority of cases are related to viral causes. Widespread use of the existing quadrivalent influenza vaccine might be useful for the prevention of FS related to the flu. Further vaccine candidates for potential respiratory pathogens, including RSV, might be helpful for the prevention of FS.
Keywords: children, febrile seizure, infection, respiratory virus, influenza, RSV
Introduction
Respiratory tract infections are the most common childhood infectious diseases worldwide and are often related to antibiotic prescriptions.1−3 Treatment requires hospitalization and can lead to lost time at day care centers or schools for the children, lost time at work for the parents, and high economic burdens.2−5 Due to these disease and economic burdens, respiratory tract infections are an important target for both new and current vaccines. Immunization could reduce the number of bacterial infections that need antibiotics, and reduce the number of viral infections for which antibiotics are unnecessarily given.6 After the widespread use of pneumococcal conjugate vaccines, invasive and mucosal pneumococcal infections decreased.7−9 Tri-valent and newly launched quadrivalent influenza vaccines are immunogenic in children and have a potential to reduce influenza infections and related complications in children as well as in adults.10−12 Respiratory syncytial virus (RSV) is an important cause of respiratory infections in children. RSV is also an important vaccine candidate; there are numerous vaccine studies available, but no licensed vaccine is available yet.13,14
Febrile seizure (FS), the most common childhood neurological disorder, is defined as a seizure event that is associated with fever in a child without a history of previous afebrile seizures, central nervous system (CNS) infection or inflammation, acute systemic metabolic abnomralities, and/or other acute symptomatic events.15−24 Viral infections are commonly identified in association with FS, and human herpes virus-6 (HHV-6) and influenza infections are mainly reported cause of FS due to high fever during these infections.19−21 Other viruses might be related with FS, however there are no relationship with viruses and complexity of the seizure course.15−24
Within the age range of FS, children are vulnerable to these common respiratory tract infections and are potential targets for new and current vaccines. With the development of new molecular laboratory methods, the aim of this study was to evaluate respiratory viruses in children who were admitted with FS and to compare positive results between age groups, hospitalized and ambulatory children and also between simple and complex FS.
Results
Patient’s demographics and clinical features of febrile seizure
Between March 1, 2016 and April 1, 2017, 192 consecutive children with FS who were admitted to emergency units from eight cities were evaluated. Of these, 174 (101 male and 73 female) children between 2 and 60 months were eligible for further analysis (median age was 25 months). Furthermore, 102 out of 174 (58.6%) children were experiencing their first FS episode, and 41.4% of children (74/174) had a history of recurrent seizures. In addition, 69.5% (121/174) of seizures were classified as simple FS and 30.5% (53/174) were described as complex FS. The presence of positive family history for febrile and/or afebrile seizures was 36.2% (63/174) and 10.3% (18/174), respectively. Eighteen children (10.3%) attended day care center. Peak body temperature varied between 36.2°C and 41.1°C. The mean interval between onset of fever and seizure was 5.70 ± 8.4 hours. The final diagnosis was upper respiratory tract infection in 140 children (80.4%) and lower respiratory tract infection (15%), with 40.8% of the 174 children requiring hospitalization. Demographic and clinical features are summarized in Table 1.
Table 1.
Demographic and clinical findings in children with febrile seizures.
| Gender n(%) | 101 boys, 73 girls (58/42) |
| Age (mean ± SD; min-max) | 27.5 ± 16.0 months (2–60 months) |
| Type of febrile seizure n(%) | |
| Simple | 121 (69.5) |
| Complex | 53 (30.5) |
| First febrile seizure n(%) | 102 (58.6) |
| Recurrent febrile seizures n(%) | 72 (41.4) |
| Family history of febrile seizures n(%) | 63 (36.2) |
| Family history of epilepsy n(%) | 18 (10.3) |
| Causes of fever | |
| Upper respiratory tract infections | 140 (80.4) |
| Lower respiratory tract infections | 26 (15) |
| Other infections | 8 (4,6) |
Microbiological analysis
During the study period, at least one virus was detected in 82.7% (144/174) of children with FS; further analysis was performed on this group. Among the positive samples for viruses, 41.6% of samples included one virus (n = 60), two coexisting viruses were detected in 31.3% (n = 45), three coexisting viruses were detected in 20.8% (n = 30), four co-existing viruses were detected in 4.8% (n = 7) and five co-existing viruses were detected in 1.4% (n = 2) (Please see Table 2).
Table 2.
Most frequent virus(es) detected in children with febrile seizure.
| Adenovirus | 11.1% (16) |
| Influenza A | 8.3% (12) |
| Enterovirus | 7.6% (11) |
| Adenovirus + Influenza B | 5.5% (8) |
| Influenza B | 4.8% (7) |
| Parainfluenza type 3 + Adenovirus + Bocavirus | 4.1% (6) |
| Rhinovirus | 3.4% (5) |
| RSV B | 2.7% (4) |
| Enterovirus + Adenovirus | |
| Adenovirus + Influenza B | |
| Adenovirus + Influenza A + Influenza B | |
| Adenovirus + Influenza A + RSV B | |
| Parainfluenza type 2 | 2.0% (3) |
| Parainfluenza type 2 + Adenovirus | 1.4% (2) |
| Influenza A + Influenza B | |
| Enterovirus + RSV A | |
| Adenovirus + Rhinovirus | |
| Adenovirus + RSV A | |
| Adenovirus + RSV B | |
| Adenovirus + Coronavirus OC43 | |
| Adenovirus + Coronavirus 229 | |
| Bocavirus + Rhinovirus | |
| Adenovirus + Rhinovirus + Coronavirus 229 | |
| Adenovirus + RSV A + RSV B | |
| RSV A | 0.7% (1) |
| Parainfluenza type 3 | |
| Coronavirus OC43 | |
| Parainfluenza type 2 + Parainfluenza type 4 | |
| Parainfluenza type 3 + Adenovirus | |
| Parainfluenza type 4 + Adenovirus | |
| Adenovirus + Bocavirus | |
| Influenza A + Bocavirus | |
| Parainfluenza type 3 + Human metapneumovirus | |
| Parainfluenza type 4 + Rhinovirus | |
| Enterovirus + Rhinovirus | |
| Influenza B + Rhinovirus | |
| Influenza B + Coronavirus 229 | |
| Parainfluenza type 3 + Adenovirus + Influenza B | |
| Adenovirus + Influenza A + Bocavirus | |
| Influenza A + Influenza B + Bocavirus | |
| Parainfluenza type 3 + Influenza B + Coronavirus OC43 | |
| Enterovirus + Adenovirus + Rhinovirus | |
| Adenovirus + Influenza A + Rhinovirus | |
| Adenovirus + Influenza B + RSV B | |
| Influenza B + Bocavirus + Rhinovirus | |
| Adenovirus + Rhinovirus + Coronavirus OC43 | |
| Adenovirus + RSV A + Human metapneumovirus | |
| Parainfluenza type 3+ Adenovirus + Influenza + Bocavirus | |
| Adenovirus + Influenza A + Influenza B + Bocavirus | |
| Parainfluenza type 3+ Adenovirus + Bocavirus + Rhinovirus | |
| Parainfluenza type 3+ Adenovirus + Bocavirus + Coronavirus OC43 | |
| Adenovirus + Influenza A + Influenza B + Coronavirus OC43 | |
| Adenovirus + Influenza B + Bocavirus + Rhinovirus | |
| Adenovirus + Influenza A + RSV A + Human Metapneumovirus | |
| Parainfluenza type 2 + Adenovirus + Influenza A + Rhinovirus + Human metapneumovirus | |
| Parainfluenza type 3 + Adenovirus + Bocavirus + Rhinovirus + Coronavirus OC43 |
Among positive samples for viruses, we detected 264 viruses in 144 nasopharyngeal samples; the most frequently detected virus was adenovirus (55.5%). Influenza was detected in 47.2% of samples: influenza A (24.3%) and influenza B (22.9%). RSV was detected in 16% of samples; RSV B was detected in 9.7% of all positive samples and RSV (A and B) was detected in 6.25%. The percentage of positive tests for each sample is shown in Figure 1. Influenza B was the most commonly identified virus in children who presented with their first febrile episode (p < 0.05). Although the results showed no significant difference in mean peak body temperature, enterovirus was the most common virus in children who experienced FS within 7–12 hours following the onset of fever (p < 0.05).
Figure 1.
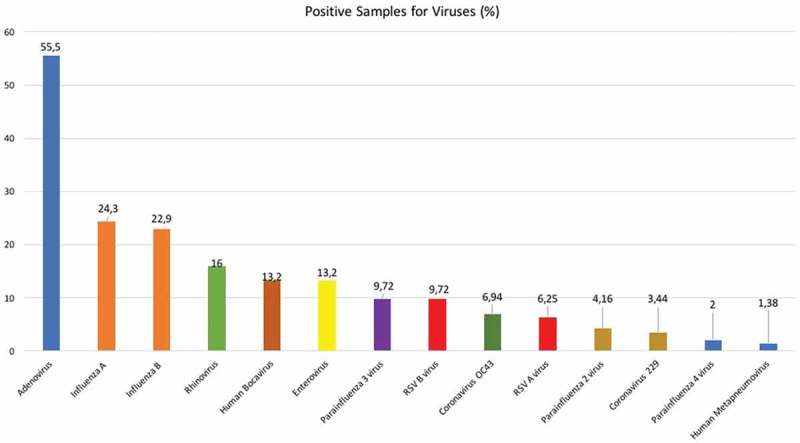
The percentage of positive tests for each virus in children with FS.
According to the data, 40.8% of the 174 children required hospitalization. The ratio of hospitalization was not different in children who had one or more viruses isolated (p > 0.05). None of the viruses were found more frequently among hospitalized children than ambulatory children (p > 0.05) (Figure 2).
Figure 2.
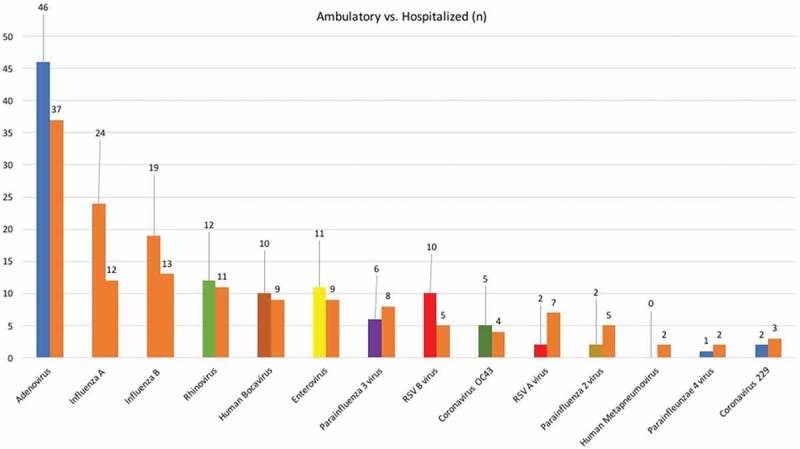
Comparison with the number of ambulatory and hospitalized children with FS for the respiratory viruses.
We evaluated respiratory viruses distributions according to the following age groups: 1–12 months, 13–24 months, 25–36 months, 37–48 months and 49–60 months. Coronavirus OC43 was more common in children younger than 12 months, and influenza A was more common in children older than 48 months (p < 0.05). For other viruses, there was no statistically significant difference between the age groups (Figure 3).
Figure 3.
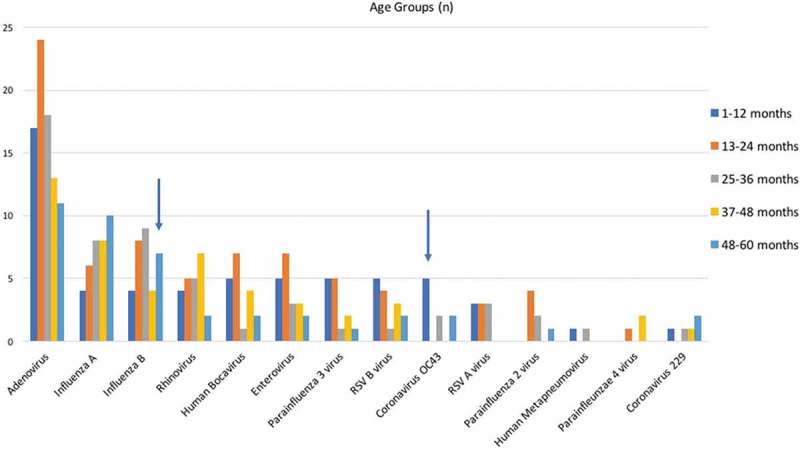
Respiratory viruses distributions according to the age groups. Blue arrows showed that Coronavirus OC43 was more common in children younger than 12 months, and influenza A was more common in children older than 48 months (p < 0.05).
The statistical analyses revealed that RSV A was more common in children with simple FS, and the human bocavirus (HBoV) was more common in children with complex FS (p < 0.05) (Figure 4). Influenza infection have been mainly seen in autumn/winter and RSV infection have been seen mainly in winter and spring. For other viruses there are no seasonal differences.
Figure 4.
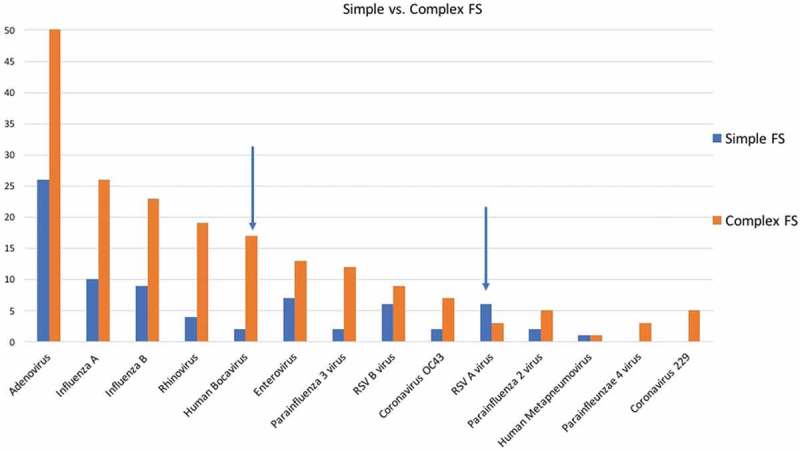
Respiratory viruses distributions between simple and complex FS (Blue arrow showed that RSV A was more common in children with simple FS, and the human bocavirus was more common in children with complex FS (p < 0.05); FS: febrile seizure, RSV: respiratory syncytial virus).
Discussion
In this prospective multicenter study in Turkey, at least one respiratory viral agent was found in 82.8% of children with FS; adenovirus, influenza virus A, and influenza virus B were the three most commonly found. There is no routine recommendation to detect etiological causes of FS for neurological perspective, further knowledge about the etiological causes of FS in children will support preventive measures and follow-up strategies. There are contradictory and limited results about the viruses, upper respiratory tract infections, and FS in children in developing countries, including Turkey. Previous studies showed a relationship between viral infection and FS, but reported different percentages of prevalence among the samples studied.20−25 Chiu et al.25 reported that the risk of developing FS following an influenza A infection was higher than after other respiratory viruses. Pokorn et al.24 studied respiratory viruses in 192 children with FS; respiratory virus was detected in 63.5%, with adenovirus, influenza, and rhinovirus the most commonly found viruses. A retrospective study done in Hong Kong with 923 FS patients showed that the influenza virus was detected in 17.6% of cases.22 Francis et al.’s26 study showed that viruses were detected in 71% of patients, with rhinovirus found to be the most common. A comparative study researched the relationship between the presence of five common viral infections and the relative risk of developing FS and seizure recurrence. The risk of developing FS was found to be similar among infections of influenza, adenovirus, or parainfluenza, but the risk of FS was lower with RSV or rotavirus infections.22 The differences among these studies could be explained by the different laboratory methods used in the research. The development of new laboratory techniques in recent years has advanced the detection of new respiratory pathogens, such as human metapneumovirus, human coronaviruses, human bocavirus; as a result, the rates of identified respiratory viruses have increased.27 In our study, with the multi array test, adenovirus was found in 55.5% of the children with FS, alone or combined with other respiratory viruses. Clinicians should kept in mind that majority of FS cases are due to viral causes, for this reason, routine antibiotic prescriptions is not necessary for all cases, and could be prescribed in selected cases with clinical findings.
In our study, influenza A and B were detected in 47.2% of all positive samples, alone or combined with other viruses. Influenza infection is an important public health problem worldwide among children and adults. It might be an important cause of admission to emergency care units and, due to the potential high fever complaints, FS might be seen during the course of the flu.12,20,21,25 Influenza infections might also be related to different neurological complaints.20,21 Influenza vaccines are recommended for all children aged 6 months to 18 years and should be taken every influenza season, preferably in October in northern hemisphere.12 Due to changing and dynamic patterns of influenza A and B subtypes, quadrivalent influenza vaccines (including two influenza A and two influenza B subtypes, according to the previous year flu activity) have been evaluated in infants and approved/recommended in infants.10−12 Trivalent and quadrivalent influenza vaccines are available in Turkey for private practices; however, vaccine coverage rate was low in this study period, especially in children with previous neurological conditions.28 In this study, influenza A and B were higher in children with FS, especially in the 48–60-month age group. Also, the influenza B virus was the most common virus identified in the children in the study who were experiencing their first incidence of FS. The age interval of children with potential risk for FS, 6 months to 5 years, is the appropriate age for the influenza vaccine which is recommended all children above 6 months. RSV is another important cause of respiratory tract infections and related morbidity, hospitalizations and mortality, mainly due to pneumonia.13,14 In our study, RSV was mainly related to simple FS. Influenza vaccines and potential RSV vaccines would have the chance to reduce the FS risk. In Turkey, after widespread use of live attenuated varicella vaccine in the national immunization program, varicella-related FS has been significantly decreased in vaccinated children, compared with the pre-vaccine era.29 Therefore, potential vaccines for respiratory pathogens related with FS might be helpful to control FS and related conditions.
We found human bocavirus in 13.2% of all positive samples, mainly combined with other viruses. Human bocavirus was first identified in 2005 and was previously detected in 9–10.4% of children with FS.24,26 In our study, human bocavirus is mainly detected in children with complex FS; however, human bocavirus has been detected with other respiratory viruses and it is difficult to explain complex FS with only this pathogen. Further studies on the neurological consequences of bocavirus in young children are needed.
In this study, in children younger than 12 months, Coronavirus OC43 was significantly common comparing the other age groups. A prospective study that observed the role of human coronavirus in children with FS showed that the largest proportionate relationship was found between coronavirus and FS. Coronavirus OC43 was found in 6.94% of patients in our study. Francis et al.26 also reported human coronavirus in 9% of children with FS and found that the OC43 type was the most commonly found strain, like our study.
Our study had some limitations. Children who applied with a complaint of fever but did not have a seizure were not taken as a control group. Our enrollment period includes only one year, and could not detect seasonal variability for viruses in years. Some viruses are postulated to be more neurotropic and more important in the causation of FS, studies in which both nasopharyngeal swabs and lumbar punctures (when available, lumbar puncture have been performed in 6.8% of cases in our study) are performed might provide additional valuable findings. In this multiplex respiratory assay, we did not evaluate whole pathogens and we did not perform standard culture method. HHV-6 has been previously described as an important cause of FS, however in this study, our aim is to to evaluate respiratory viruses, and we did not evaluate HHV-6 infection. Notwithstanding these limitations, our study is an observational field study and this assay is practical, less expensive and less complex, for this reason suitable for clinical condition like FS.
In conclusion, this study demonstrates that respiratory viruses are important in the etiology of FS in children and the results show that antibiotics must be prescribed carefully in children with FS. Adenovirus, influenza virus A, and influenza B were the three viruses most frequently found in our sample of children with FS. Widespread use of existing quadrivalent influenza vaccines might be useful for the prevention of FS as well as other complications related with influenza. FS is an important problem for families and the recurrent and complex nature might be related to long-term consequences, such as epilepsy.19,30 Further vaccine candidates for potential respiratory pathogens might also be helpful for the prevention of FS.
Material and method
This prospective multicenter study, entitled “Viral etiological causes of febrile seizures for respiratory pathogens (EFES Study)” focused on eight different cities between March 1, 2016 and April 1, 2017. We selected these cities as representative locations within Turkey. The study was approved by Eskisehir Osmangazi University’s Local Ethical Committee and has been supported by a research grant from Eskisehir Osmangazi University. Written informed consent was obtained from the parents of all children.
At each site, all children with seizures were evaluated and children with FS were enrolled over this one-year period. FS is defined as a seizure event that is associated with fever in a child without a history of unprovoked seizure, CNS infection, electrolyte imbalance, and/or other acute symptomatic events. FS is classified as simple if it was generalized, occurred only once within 24 hours, and lasted less than 15 minutes. FS is classified as complex if it persisted for more than 15 minutes, was focal, or recurred within 24 hours.16,17 Exclusion criteria were defined as age older than 60 months or below one month, children diagnosed with meningitis or acute symptomatic convulsions that presented with fever, children who received any childhood vaccine within 72 hours and children whose parents did not want to participate in the study.
Previous medical history and demographical features of all children were recorded. Previous seizure history and family history were evaluated. Detailed physical examinations have been performed, including neurological examinations. Day care attendance, were asked about and noted. The final diagnosis, whether hospitalization was required, and the duration between the onset of fever and seizures were also recorded.
To detect the presence of respiratory viruses, nasopharyngeal swabs of all children were taken at admission. Prior to nucleic acid extraction, swab samples were transported in a suitable transport container and were stored at −20°C, according to the manufacturer’s instructions. Nucleic acid (DNA and RNA) was extracted from nasopharyngeal samples using the Qiagen MineElute Virus Spin Kit (Manchester, UK), according to the manufacturer’s instructions. A 5 ml aliquot of each nucleic acid extraction was tested with the Respiratory Multiplex Array (Randox Laboratories, UK), based on a combination of multiplex PCR and biochip array hybridisation, for the detection of 22 viruses (influenza A and B; respiratory syncytial virus A and B; human parainfluenza virus 1-2-3 and 4; human coronavirus 229E and OC43; human rhinovirus A/B; human enterovirus A/B/C; human adenovirus B/C/E; human bocavirus 1/2/3; human metapneumovirus; and bacteria (Chlamydia pneumoniae, Legionella pneumophilia, Haemophilus influenzae, Bordatella pertussis, Streptococcus pneumoniae, Moraxella catarrhalis, and Mycoplasma pneumoniae). In this study, we evaluated positive results for viruses only.
Statistical analyses were performed using SPSS version 10.0 software (SPSS, Inc, Chicago, IL). The data of FS duration and body temperature that appeared to follow normal distribution was analyzed using One-Way ANOVA, and the results were expressed as mean and standard deviation (SD). The categorized variables were analyzed with the Chi-square test. A p value of less than 0.05 was considered significant.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Funding Statement
This study has been supported by a research grant from Eskisehir Osmangazi University.
Acknowledgement
Authors thanks to Huseyin KARACAN and Samet ECE from Diagen Laboratories Ltd, Ankara, Turkey; Stephen Peter FIZGERALD, Martin CROCKARD and Jason O’NEILL from Randox Laboratories, London, UK, for their kind support during laboratory analysis. We also thanks to our patients and their parents.
Ethical approval
This study was approved by Eskisehir Osmangazi University Ethics Committee (No:2016/80558721-82). The study was performed according to the principles of Helsinki Declaration.
Author Contributions
KBC and ECD participated in protocol development. MC, YK, SI, OK, AO, ASY, CN, CS, OK, MD, SLG, SY, AE, PP, AT, II and CY were collected the samples and performed clinical part of the study. DA analyzed the results statistically. KBC and ECD participated primary data analysis, interpretation and wrote the first version of the manuscript and also finalized the manuscript.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(Suppl 4):S284–9. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoa NQ, Thi Lan P, Phuc HD, Chuc NTK, Stalsby Lundborg C. Antibiotic prescribing and dispensing for acute respiratory infections in children: effectiveness of a multi-faceted intervention for health-care providers in Vietnam. Glob Health Action. 2017;10(1):1327638. doi: 10.1080/16549716.2017.1327638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacri AS, De Serres G, Quach C, Boulianne N, Valiquette L, Skowronski DM. Transmission of acute gastroenteritis and respiratory illness from children to parents. Pediatr Infect Dis J. 2014;33(6):583–588. doi: 10.1097/INF.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 5.Zoch B, Günther A, Karch A, Mikolajczyk R. Effect of disease definition on perceived burden of acute respiratory infections in children: a prospective cohort study based on symptom diaries. Pediatr Infect Dis J. 2017;36(10):956–961. doi: 10.1097/INF.0000000000001604. [DOI] [PubMed] [Google Scholar]
- 6.Atkins KE, Lipsitch M. Can antibiotic resistance be reduced by vaccinating against respiratory disease? Lancet Respir Med. 2018. pii: S2213-2600(18)30328-X. doi: 10.1016/S2213-2600(18)30328-X. [DOI] [PubMed] [Google Scholar]
- 7.Dinleyici EC. Current status of pneumococcal vaccines: lessons to be learned and new insights. Expert Rev Vaccines. 2010;9(9):1017–1022. doi: 10.1586/erv.10.86. [DOI] [PubMed] [Google Scholar]
- 8.Dinleyici EC, Yargic ZA. Current knowledge regarding the investigational 13-valent pneumococcal conjugate vaccine. Expert Rev Vaccines. 2009;8(8):977–986. doi: 10.1586/erv.09.68. [DOI] [PubMed] [Google Scholar]
- 9.Dinleyici EC, Yargic ZA. Pneumococcal conjugated vaccines: impact of PCV-7 and new achievements in the postvaccine era. Expert Rev Vaccines. 2008;7(9):11367–11394. doi: 10.1586/14760584.7.9.1367. [DOI] [PubMed] [Google Scholar]
- 10.Jain VK, Rivera L, Zaman K, Espos RA Jr, Sirivichayakul C, Quiambao BP, Rivera-Medina DM, Kerdpanich P, Ceyhan M, Dinleyici EC, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med. 2013;369(26):2481–2491. doi: 10.1056/NEJMoa1215817. [DOI] [PubMed] [Google Scholar]
- 11.Claeys C, Zaman K, Dbaibo G, Li P, Izu A, Kosalaraksa P, Rivera L, Acosta B, Arroba Basanta M. L, Aziz A, et al. Prevention of vaccine-matched and mismatched influenza in children 6−35 months of age: a multinational randomized trial across five influenza seasons. Lancet Child Adolesc Health. 2018;2(5):338–349. doi: 10.1016/S2352-4642(18)30062-2. [DOI] [PubMed] [Google Scholar]
- 12.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2017–18 influenza season. MMWR Recomm Rep. 2017;66(2):1–20. doi: 10.15585/mmwr.rr6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simões EAF, Bont L, Manzoni P, Fauroux B, Paes B, Figueras-Aloy J, Checchia PA, Carbonell-Estrany X. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infect Dis Ther. 2018;7(1):87–120. doi: 10.1007/s40121-018-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS, Englund JA, et al.; Respiratory Syncytial Virus Network (ReSViNET) Foundation . The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018. pii: S1473-3099(18)30292-5. doi: 10.1016/S1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- 15.Mittal R. Recent advances in febrile seizures. Indian J Pediatr. 2014;81(9):909–916. doi: 10.1007/s12098-014-1532-2. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for epidemiologic studies on epilepsy Commission on epidemiology and prognosis, international league against epilepsy. Epilepsia. 1993;34(4):592–596. [DOI] [PubMed] [Google Scholar]
- 17.Shinnar S, Glauser TA. Febrile seizures. J Child Neurol. 2002;17(Suppl 1):S44–52. doi: 10.1177/08830738020170010601. [DOI] [PubMed] [Google Scholar]
- 18.Carman KB, Ekici A, Yimenicioglu S, Yarar C, Arslantas D, Yakut A. The prevalence of febrile seizure and associated factors among Turkish children. Int J Clin Pediatr. 2014;3:1–4. [DOI] [PubMed] [Google Scholar]
- 19.Pavlidou E, Hagel C, Panteliadis C. Febrile seizures: recent developments and unanswered questions. Childs Nerv Syst. 2013;29(11):2011–2017. doi: 10.1007/s00381-013-2224-3. [DOI] [PubMed] [Google Scholar]
- 20.Çiftçi E, Tuygun N, Özdemir H, Tezer H, Şensoy G, Devrim İ, Dalgiç N, Kara A, Turgut M, Tapisiz A, et al. Clinical and epidemiological features of Turkish children with 2009 pandemic influenza A (H1N1) infection: experience from multiple tertiary paediatric centres in Turkey. Scand J Infect Dis. 2011;43(11–12):923–929. doi: 10.3109/00365548.2011.598872. [DOI] [PubMed] [Google Scholar]
- 21.Britton PN, Blyth CC, Macartney K, Dale RC, Li-Kim-Moy J, Khandaker G, Crawford NW, Marshall H, Clark JE, Elliott EJ, et al.; Australian Childhood Encephalitis (ACE) Study Investigators, Influenza Complications Alert Network (FluCAN) Investigators, and Paediatric Active Enhanced Disease Surveillance (PAEDS) Network . The spectrum and burden of influenza-associated neurological disease in children: combined encephalitis and influenza sentinel site surveillance from Australia, 2013–2015. Clin Infect Dis. 2017;65(4):653–660. doi: 10.1093/cid/cix412. [DOI] [PubMed] [Google Scholar]
- 22.Chung B, Wong V. Relationship between five common viruses and febrile seizure in children. Arch Dis Child. 2007;92(7):589–593. doi: 10.1136/adc.2006.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang J, Yan W, Li Y, Zhang B, Gu Q. Relationship between common viral upper respiratory tract infections and febrile seizures in children from Suzhou, China. J Child Neurol. 2014;29(10):1327–1332. doi: 10.1177/0883073813515074. [DOI] [PubMed] [Google Scholar]
- 24.Pokorn M, Jevšnik M, Petrovec M, Steyer A, Mrvič T, Grosek Š, Lusa L, Strle F. Respiratory and enteric virus detection in children. J Child Neurol. 2017;32(1):84–93. doi: 10.1177/0883073816670820. [DOI] [PubMed] [Google Scholar]
- 25.Chiu SS, Tse CY, Lau YL, Peiris M. Influenza A infection is an important cause of febrile seizures. Pediatrics. 2001;108(4):E63. [DOI] [PubMed] [Google Scholar]
- 26.Francis JR, Richmond P, Robins C, Lindsay K, Levy A, Effler PV, Borland M, Blyth CC. An observational study of febrile seizures: the importance of viral infection and immunization. BMC Pediatr. 2016;16(1):202. doi: 10.1186/s12887-016-0740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson KE, Couturier MR. Multiplexed molecular diagnostics for respiratory, gastrointestinal, and central nervous system infections. Clin Infect Dis. 2016;63(10):1361–1367. doi: 10.1093/cid/ciw494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinleyici M, Carman KB, Kilic O, Laciner Gurlevik S, Yarar C, Dinleyici EC. The immunization status of children with chronic neurological disease and serological assessment of vaccine-preventable diseases. Hum Vaccin Immunother. 2018;14(8):1970–1976. doi: 10.1080/21645515.2018.1460986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinleyici EC, Kurugol Z, Carman KB; VARICOMP Study Group . Neurologic causes of varicella related hospitalizations in Turkey (VARICOMP study 2008–2015). Eur J Paediatric Neurol. 2017;21(Suppl 1):e91. doi: 10.1016/j.ejpn.2017.04.670. [DOI] [Google Scholar]
- 30.Shinnar RC, Shinnar S, Hesdorffer DC, O’Hara K, Conklin T, Cornett KM, Miazga D, Sun S; FEBSTAT Study Team . Parental stress, pediatric quality of life, and behavior at baseline and one-year follow-up: results from the FEBSTAT study. Epilepsy Behav. 2017;69:95–99. doi: 10.1016/j.yebeh.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


