ABSTRACT
Background: Extracranial metastasis is a rare phenomenon of anaplastic oligoastrocytoma. When patients progress after comprehensive treatment, there is often no effective treatment. Rapid development of gene detection technology makes precision treatment of glioma possible.
Patient and methods: A 22-year-old girl was firstly diagnosed with anaplastic oligoastrocytoma WHO grade III-IV in 2014, and progressed rapidly after chemoradiotherapy in multiple extraneural lesions in 2016. She was expected to have a short life and Next-Generation Sequencing (NGS) was applied.
Results: Mutation of BRAF (V600E) was reported by 1st NGS and oral vemurafenib stabilized her disease for 6 months. PIK3CA was reported by 2nd NGS after her progression of vemurafenib. The oral administration of everolimus together with vemurafenib stabilized her disease for another 6 months. However, the patient died due to the rapid progression of the disease on 24 February 2018.
Conclusion: We successfully treated a BRAF V600E-mutated anaplastic oligoastrocytoma with multiple extraneural metastases with vemurafenib and everolimus. For late-staged patients who have no clear and effective treatment plan, NGS may serve as an effective option.
Keywords: Oligoastrocytoma, Vemurafenib, everolimus, BRAF, mTOR, PIK3CA, next-generation sequencing, cancer genomics, gene therapy, tumor metastases
1. Introduction
Oligoastrocytoma is a “mixed glioma” that contains both abnormal astrocytoma and oligodendroglioma cells. Primary oligoastrocytomas account for 1% of all brain tumors and 5–10% of gliomas. Extracranial metastasis is a rare phenomenon of anaplastic oligoastrocytoma. The highest frequency of central nervous system tumors harboring BRAF V600E mutation is found in pediatric patients.1 BRAF mutations have not been reported in anaplastic oligoastrocytoma samples. Vemurafenib, the BRAF V600E inhibitor, has been approved by FDA for use in melanoma. It was also reported that a 51-year-old man with BRAF V600E–mutated anaplastic thyroid cancer showed a dramatic response to vemurafenib.2 On the other hand, some mutations of PIK3CA could activate PI3K signaling in the majority of glioblastoma patients.3The downstream effectors of PI3K including mTOR and AKT, play key roles in cell survival. mTOR inhibitors such as everolimus have shown excellent effect for tuberous sclerosis complex (TSC) patients with sub-ependymal giant cell astrocytomas.4 We present a case of a patient who was firstly diagnosed with anaplastic oligoastrocytoma WHO grade III-IV at the age of 22 in 2014, and rapid progression after chemoradiotherapy in multiple extraneural lesions was validated in 2016. Extracranial metastasis of anaplastic oligoastrocytoma is rare and these patients were reported with poor prognosis and short survival. She was expected to have a very short survival time, but she managed to live for more than one year with a good quality of life with Next-Generation Sequencing (NGS) and matched targeted therapy applied. Mutations of BRAF (V600E) and PIK3CA were reported by NGS. The patient benefited from the matched therapy vemurafenib and everolimus and got a long period of tumor control.
2. Case report
A 22-year-old Chinese girl developed headaches and dizziness at age of 20 (2012). An MRI scan performed at that time revealed a mass in the left temporal lobe. The patient underwent a tumor resection in April 2014, which revealed histological evidence of a WHO grade III-IV anaplastic oligoastrocytoma (AO). The patient underwent adjvuant radiotherapy concurrent with temozolomide, completed in Jun 2014. The dose delivered to the tumor bed was 60Gy in 30 fractions. She then received 23 cycles of adjuvant chemotherapy with temozolomide, repeated every 28 days, ended in March 2016 (Figure 1).
Figure 1.
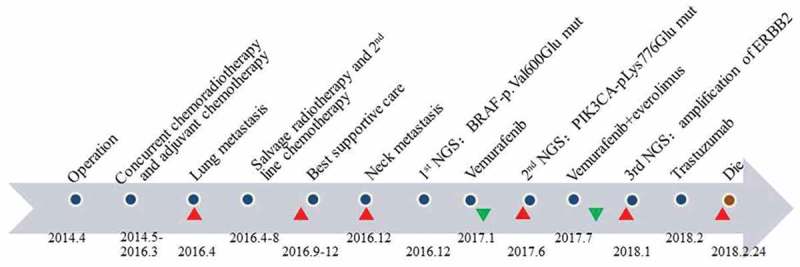
Time chart of treatment. Chemoradiotherapy was not effective in the patient after recurrence. However, NGS alleviated the patient’s disease twice and extended the patient’s survival by almost one year. The red triangles represented the progression of disease, while green triangles represented disease remission.
The patient accidentally got a hacking cough in March 2016. On 4 December 2016, a CT scan demonstrated a mass in the middle lobe of the right lung, multiple lymph node metastasis at right pulmonary hilar and mediastinal followed by lymphatic invasion in upper middle lobe of right lung. The biopsy was undertaken through bronchofiberscope. The confirmed diagnosis of lung metastasis from AO was based on immunohistochemical staining. The irradiation therapy alone was given to pulmonary lesions with total planning dose of 60Gy started in April 2016. The radiotherapy was terminated at final dose of 46Gy, because her CT scan of the chest, obtained on 25 May 2016, showed the lesions in lung and mediastinal lymph node progression than before, with right pleural effusion. On 3 Jun 2016, the hydrothorax exfoliative cytologic examination found a large number of lymphocytes, a few mesothelial cells and some atypical cell with red dry of cytoplasmic membranes, which was considered to be AO metastasis.
Then, she received 3 cycles of chemotherapy with CPT-11 combined with bevacizumab every two weeks from 7 Jun 2016. The imaging evaluation after chemotherapy demonstrated progressive disease in both lung and right parietal lobe. The patient developed progressive symptom of respiratory distress. She couldn’t suffer chemo- or radio-therapy, and best supportive care (BSC) was given.
Four months later, the patient developed the symptom of high fever and respiratory distress, and right supraclavicular lymph nodes enlarged. A staging fluorodeoxyglucose positron emission tomography (FDG PET) scan noted abnormal uptake within several right hilar lymph nodes (SUV 6.4 and 5.1), right supraclavicular lymph nodes(SUV 11.5), right lung (SUV 5.0)and left ilium(SUV 5.0). Additionally a biopsy of right supraclavicular lymph nodes was taken on 5 December 2016. The confirmed diagnosis of AO metastasis to right supraclavicular lymph nodes was based on immunohistochemical staining, GFAP(+), Olig-2(partial+), Vimentin(+), Ki-67(index 5%), CD31(vessel +), syn(-), NeuN(-), S100(+), P53(+++), CK(+), NF(several +), IDH1(-). Meanwhile, the molecular characteristics of the original tumor detected by NGS method demonstrated the presence of TERT (C228T), BRAF-p.Val600Glu, BLM-p.Asp684Gly, IGF2R-pIle889Thr, MET-p.Val969Ala and PTCH1-p.Gln466X mutations, without IDH1/2 mutation, MGMT methylation, 1p19q deletion, ATRX loss, and TP53 mutation. Her treatment with vemurafenib 960 mg orally twice per day started from January 2017. The patient recovered from fever one month later. A pulmonary CT scan showed a partial reduction of pulmonary lesion; arthralgias, keratosis pilaris or other adverse reactions did not occur. But four months later, the patient felt difficult to breathe gradually. A pulmonary CT scan on June 19th revealed an increase in the size of the lesion, suggesting the progression of disease. Then the ctDNA detection showed the existence of ALK-p.Leu600Pro, BRAF-p.Val600Glu, HRAS-p.Glu143Lys and PIK3CA-pLys776Glu mutations. Therefore, she took everolimus 5mg once a day along with vemurafenib 960 mg twice per day from 7/12/2017. Two weeks after initiation of therapy, CT examination on 27 July 2017 suggested that lung lesions shrank again. The patient continued to take combination of everolimus and vemurafenib for 6 more months with stable control of disease.
The patient re-caught cough and respiratory distress in January 2018, and was diagnosed with rapid progress in lung and brain lesions, considering drug resistance in targeted therapy. Vemurafenib and everolimus were stopped due to swallow obstruction and BSC was given.
The third genetic test of ctDNA by NGS in January 2018 demonstrated amplification of ERBB2 and new mutations NOTCH1-p.Val1676lle, APC-p.Asn81Lys, CSF1R-p.Gln915Lys, LRP1B-p.Val4107Ala, PTPRT-p.Asp882Gly and SMARCB1-p.Arg201Gln. Mutations BRAF-p.Val600Glu and PIK3CA-pLys776Glu still existed with similar mutation rates. So the patient was treated with trastuzumab weekly since February 2018. However, trastuzumab failed to work this time and she died on 24 February 2018 due to the rapid progress of the disease.
3. Discussion
To our knowledge, this is the first report of combination use of vemurafenib and everolimus in the treatment of a BRAF V600E-mutated anaplastic oligoastrocytoma with multiple extraneural metastases. The BRAF V600E-specific inhibitors vemurafenib and dabrafenib have been approved by FDA for use in melanoma.5–7 It was reported that a complete response in a BRAF V600E-mutated glioblastoma to vemurafenib therapy was observed after 4 months of therapy and this effect sustained through 6 months.8 A case report also demonstrated a brainstem ganglioglioma patient with the BRAF V600E mutation was successfully treated with vemurafenib and vinblastine after experiencing treatment failure with conventional therapy.9 Similarly, a recent case report described an excellent radiographic response of a progressive BRAF V600E-mutated anaplastic pleomorphic xanthoastrocytoma (PXA) treated with vemurafenib.10
The genotype of our patient was IDH wild-type, 1p/19q non-deleted, ATRX intact and TP53 intact. Thus, according to the 2016 World Health Organization Classification of Tumors of the Central Nervous System,11 the diagnosis of this patient should be anaplastic oligoastrocytoma, NOS, WHO III-IV. She developed rare multiple extraneural metastases despite surgery, radiation and treatment with 23 cycles of temozolomide. Then, she was treated with cisplatin, irinotecan in combination with bevacizumab, but disease progressed rapidly. Review of the molecular characteristics of the biopsy tissue of right supraclavicular lymph nodes on Dec 5 2016 demonstrated the presence of a BRAF V600E mutation. She was therefore treated with vemurafenib since January 2017 and the symptom of panting was relieved one month after the therapy started.
A CT scan obtained 4 weeks after the initiation of therapy revealed shrinkage of lung metastases. However, the lesions in pulmonary was progressive again three months after starting therapy while the mass in brain was obviously shrunk. The tumor heterogeneity of different metastatic lesions may be the main cause of this phenomenon. Because there was not sufficient tissue left for NGS test and the patient refused to have another biopsy, peripheral ctDNA was tested in June 2017. The mutation of BRAF V600E still existed and a few new genetic mutations appeared, including the mutation of PIK3CA-pLys776Glu. The mutation of PIK3CA could lead to activation of PI3K-AKT-mTOR signalling pathway, which is associated with poor prognosis in glioblastoma.12
Everolimus, a mTOR inhibitor, has been studied in multiple trials.13–16 A phase I study of temozolomide and everolimus in combination with radiation therapy in newly diagnosed glioblastoma showed acceptable tolerance, and imaging with FDG-PET showed decreased tumor metabolic activity in a subset of patients.13 Given the patient’s decline, in July 2017, she started with everolimus 5mg once a day along with vemurafenib 960 mg twice per day. Fortunately, the symptom of panting alleviated and the lung lesions shrank again and she got stable control of disease for the next 6 months (Figure 2). The patient began to develop dyspnea and persistent cough due to the enlargement of the mass in her neck and pulmonary hilar in January 2018. A third-time NGS indicated trastuzumab as a potentially effective drug. However, trastuzumab failed to work after 2 cycle infusion and she died in February 2018 due to the rapid progression of the lesions.
Figure 2.
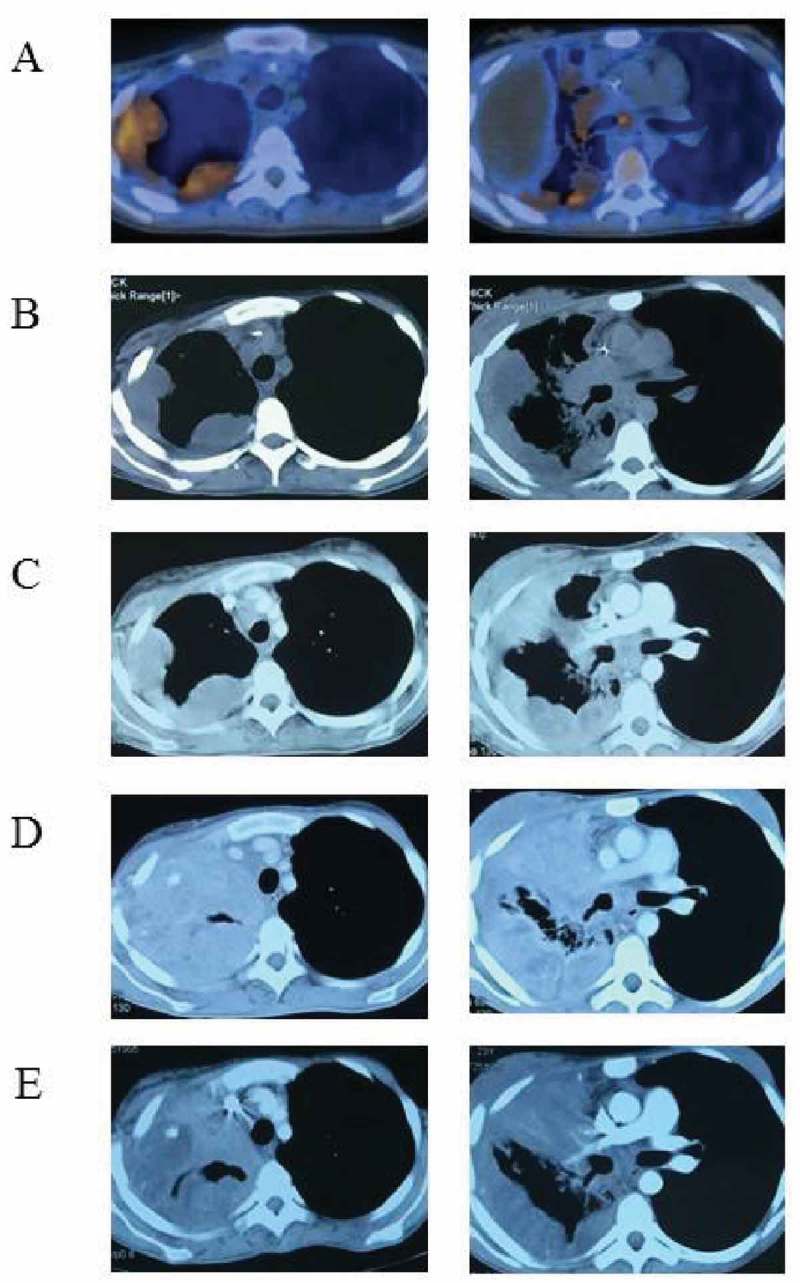
Imaging changes of targeted therapy. The patient developed extensively after radiotherapy and chemotherapy and performed a PET-CT examination (A) on 16 November 2016. Vemurafenib was indicated as a potential benefit drug after NGS. CT scans 2 (B) and 4 (C) months later confirmed its effectiveness. However, CT examination on 19 Jun 2017 (D) found extensive progress in the lung and a second NGS was carried out. Everolimus might be another beneficial drug and the patient took everolimus along with vemurafenib. CT examination on 27 July 2017 (E) suggested lung lesions shrank again.
We also performed further molecular analysis of the biopsy tissue from lung metastasis. Vemurafenib is a RAF kinase inhibitor and the braf protein is upstream of the MAPK/ERK pathway.17The phosphorylation of ERK1/2 was detected with a low expression level. This suggests that vemurafenib might work through ERK1/2 phosphorylation. Everolimus is an mTOR kinase inhibitor, while p-ULK1 (S757) and p-AKT (S473) are substrates for mTORC1 and mTORC2, respectively.18 The phosphorylation level of p-ULK1 (S757) was stronger, while the phosphorylation level of p-AKT (S473) was lower (Figure 3A). The above suggests that everolimus might work through the p-ULK1(S757) pathway rather than the p-AKT (S473) pathway. We also performed immunohistochemical analysis of the expression of PDL1, and the results showed that PDL-1 was not significantly expressed in this patient. This suggests that the patient may not benefit from immunotherapy (Figure 3B).
Figure 3.
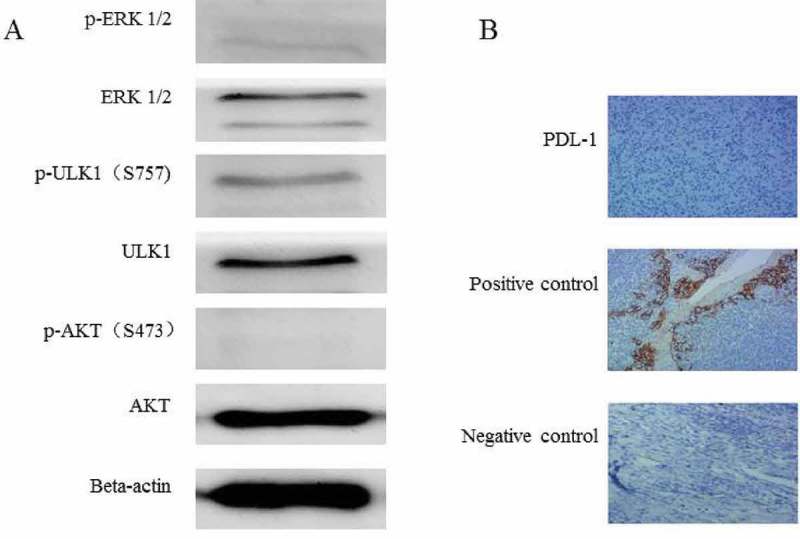
Molecular analysis of the biopsy tissue from lung metastasis. (A) The phosphorylation of ERK1/2 and AKT(S473)was weak in the metastatic tissue,and the phosphorylation of ULK-1 S757 was relatively strong. (B) The PDL-1 expression was negative in the biopsy tissue of lung metastases (IHC 10 × 10).
In summary, we successfully treated a BRAF V600E-mutated anaplastic oligoastrocytoma with multiple extraneural metastases with vemurafenib and everolimus. The Cancer Genome Atlas (TCGA) has unveiled the complexity of tumor heterogeneity and provides new insights into the precision treatment targeted to the mutated genes.19–22
Based on this rare case, it is suggested that for late-staged patients who have no clear and effective treatment plan, precision medicine or NGS may serve as an effective option. In consideration of the complexity of tumor heterogeneity, genetic mutations may change in the course of treatment, and therefore repeated genomic tests might provide more promising therapeutic strategies. Besides, even when one targeted therapy is no longer effective, it may not be completely replaced. Use of this once-worked drug in combination with another matched therapy may provide a more effective option.
Disclosures of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 2.Rosove MH, Peddi PF, Glaspy JA.. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med. 2013;368:684–685. doi: 10.1056/NEJMc1215697. [DOI] [PubMed] [Google Scholar]
- 3.Wen PY, Lee EQ, Reardon DA, Ligon KL, Alfred Yung WK. Current clinical development of PI3K pathway inhibitors in glioblastoma. Neuro Oncol. 2012;14:819–829. doi: 10.1093/neuonc/nos117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner SG, Peters KB, Vredenburgh JJ, Desjardins A, Friedman HS, Reardon DA. Everolimus tablets for patients with subependymal giant cell astrocytoma. Expert Opin Pharmacother. 2011;12:2265–2269. doi: 10.1517/14656566.2011.601742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, etal. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 8.Robinson GW, Orr BA, Gajjar A.. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer. 2014;14:258. doi: 10.1186/1471-2407-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rush S, Foreman N, Liu A. Brainstem ganglioglioma successfully treated with vemurafenib. J Clin Oncol. 2013;31:e159–160. doi: 10.1200/JCO.2012.44.1568. [DOI] [PubMed] [Google Scholar]
- 10.Lee EQ, Ruland S, Leboeuf NR, Wen PY, Santagata S. Successful treatment of a progressive BRAF V600E-mutated anaplastic pleomorphic xanthoastrocytoma with vemurafenib monotherapy. J Clin Oncol. 2016;34:e87–89. doi: 10.1200/JCO.2013.51.1766. [DOI] [PubMed] [Google Scholar]
- 11.Louis DN, Perry A, Reifenberger G, von DeimlingA, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, Loeffler JS. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 13.Sarkaria JN, Galanis E, Wu W, Peller PJ, Giannini C, Brown PD, Uhm JH, McGraw S, Jaeckle KA, Buckner JC. North central cancer treatment group phase I trial N057K of everolimus (RAD001) and temozolomide in combination with radiation therapy in patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;81:468–475. doi: 10.1016/j.ijrobp.2010.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason WP, Macneil M, Kavan P, Easaw J, Macdonald D, Thiessen B, Urva S, Lwin Z, McIntosh L, Eisenhauer E. A phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: an NCIC CTG study. Invest New Drugs. 2012;30:2344–2351. doi: 10.1007/s10637-011-9775-5. [DOI] [PubMed] [Google Scholar]
- 15.Chinnaiyan P, Won M, Wen PY, Rojiani AM, Wendland M, Dipetrillo TA, Corn BW, Mehta MP. RTOG 0913: a phase 1 study of daily everolimus (RAD001) in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2013;86:880–884. doi: 10.1016/j.ijrobp.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hainsworth JD, Shih KC, Shepard GC, Tillinghast GW, Brinker BT, Spigel DR. Phase II study of concurrent radiation therapy, temozolomide, and bevacizumab followed by bevacizumab/everolimus as first-line treatment for patients with glioblastoma. Clin Adv Hematol Oncol. 2012;10:240–246. [PubMed] [Google Scholar]
- 17.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006. January 19;439(7074):358–362. Epub 2005 Nov 6. doi: 10.1038/nature04304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Nicolantonio F, Arena S, Tabernero J, Grosso S, Molinari F, Macarulla T, Russo M, Cancelliere C, Zecchin D, Mazzucchelli L, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010. August;120(8):2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan CW, Verhaak RG, Mckenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al. The Somatic Genomic Landscape of Glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavaré S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abou-El-Ardat K, Seifert M, Becker K, Eisenreich S, Lehmann M, Hackmann K, Rump A, Meijer G, Carvalho B, Temme A, et al. Comprehensive molecular characterization of multifocal glioblastoma proves its monoclonal origin and reveals novel insights into clonal evolution and heterogeneity of glioblastomas. Neuro Oncol. 2017;19:546–557. doi: 10.1093/neuonc/now231. [DOI] [PMC free article] [PubMed] [Google Scholar]


