ABSTRACT
Introduction: Vaccination against various pathogens is recommended for HIV positive adults. There are not sufficient data either on vaccination coverage of HIV positive adults or the risk factors associated with poor adherence to routine vaccination.
Patients-Methods: During the period 2004–2014 vaccination coverage of a group of HIV infected adults against hepatitis A virus (HAV), hepatitis B virus (HBV), seasonal influenza virus and pneumococcal disease was recorded. Vaccination coverage was separated into two chronological periods, before and after 2010, as 2010 marks the start of the economic crisis in Greece.
Results: 1210 patients were included in our study. Vaccine coverage throughout the study for hepatitis B, hepatitis A, seasonal influenza and pneumococcal infection was 73.6%, 70.4%, 39% and 79%, respectively. The complete lack of insurance coverage was an independent factor of non-compliance in all proposed vaccines (vaccination against pneumococcal disease: OR: 0.82 95%CI: 0.49–1.35, vaccination against HBV: OR: 0.82, 95% CI: 0.45–1.49, vaccination against HAV OR: 0.54, 95%CI: 0.34–0.87, vaccination against influenza: OR: 1.27, 95% CI: 0.76–2.10). In addition, low educational level was associated with poor compliance to vaccination against pneumococcal disease, hepatitis A, hepatitis B, and influenza. Finally, the recommendation for vaccination after the onset of the economic crisis (2010) led to poor compliance to vaccination against HBV, HAV and pneumococcal disease, but not against influenza.
Conclusions: In our study, vaccination coverage for vaccine-preventable diseases was found to be insufficient for HIV positive adults in Northern Greece. Also, low educational level, lack of insurance coverage and economic distress have contributed to poor vaccine compliance, leading to poor protection of the HIV positive population and decreased immune coverage in the community.
Keywords: vaccination, adherence, HIV, economic crisis
Introduction
Vaccination is one of the cornerstones of public health, since it is one of the most cost-effective methods of preventing infectious diseases. In the setting of HIV infection, the significance of immunization rises due to susceptibility of HIV-positive individuals to several pathogens. Furthermore, HIV shares similar modes of transmission with other viruses like hepatitis A and B virus. In defiance of Highly Active Antiretroviral Therapy (HAART), ΗΙV patients remain at high risk for certain infections, such as pneumococcal infection, seasonal influenza and hepatitis viruses’ infections.1
Although immune responses to most vaccines have been assessed to be impaired in patients with HIV infection,2,3 vaccination against hepatitis B virus, hepatitis A virus, seasonal influenza and St. pneumoniae is currently recommended in HIV infected patients.4 Despite this fact, adherence to routine vaccination schedule is often assessed as suboptimal, leading to concern about herd immunity and prevention of infections in the community.5
The association between adherence to vaccination and HIV-infection parameters is interesting. Particularly, patients who had undetectable HIV viral load, higher CD4 T cell counts and lower nadir CD4 T cell counts were more adherent to HAV vaccination according to some studies.6 Some general risk factors for poor adherence or delay in vaccination in HIV patients are: the concern for decreased immunogenicity in patients with lower CD4 counts,7patient’s country of origin due to the difficulty in communication and compliance with doctors’ instructions,5 the absence of a vaccination card so as to remembering exactly which vaccinations and doses have been taken,8 and last but not least, the inadequate or even the nonexistent medical guidance.7
Additionally, poor adherence to vaccinations may be associated generally with poor retention to medical care and treatment, which has been linked to increased morbidity and mortality, drug resistance, and virological failure in HIV infection.1–4,9
Patient related determinants are demographic and behavioral characteristics as well as socioeconomic status (SES).1,10–13 Possible modes of association of SES with adherence to vaccine, are education’s effect on conforming a stable economic future, on acquiring health knowledge and literacy to visit and use health resources productively, while at the same time financial income plays a significant role in obtaining better housing conditions and earning time to access health care.14 Moreover, employment status affects the ongoing stress of the patients and their ability to use health care facilities and comply to care.14
It is noteworthy that even though SES is a commonly used term, it is rather difficult to define and measure it but it combines a set of variables like occupation, education, income, and place of residence.7,15 The effect of SES on adherence among HIV infected patients is considered a controversial issue.16–23
It is shown that, as far as HAV vaccination in HIV patients is concerned, education level was reported to be directly related to patients’ adherence and low vaccine coverage.6,24 For HBV vaccination, it is reported that only education level, HIV risk category and number of HIV clinician visits per year were found to be significant predictors of whether a patient received hepatitis B vaccine.25 Some countries manage to sustain high levels of vaccine coverage against HBV and HAV,8 while others not25
Finally, the experience of the physician taking care of patients affects vaccination coverage, especially for HBV and pneumococcal disease.26,27
There are several studies about factors that may have a negative impact on the compliance of HIV patients with antiretroviral therapy.28–31 Significant correlations were noted between education level, employment status, annual income, depression, treatment adherence self-efficacy, the age diagnosed with HIV, HIV symptom severity, the duration of ART and the adherence in HAART.8 While there are a lot of data about the adherence to HAART, the information about adherence to vaccination in HIV-population remain limited.32
In this retrospective cohort study, we document the adherence to vaccination against four distinct pathogens suggested to HIV patients served on the Infectious Diseases Unit of a tertiary University Hospital in Thessaloniki, Greece, as reflected by the completion of the routine schedule for preventing certain infections.
Results
Overall, 1210 HIV patients 18 years old or older were enrolled during the ten-year study period. Regarding vaccination against PD, the median age at infection of the total population was 34.12 years, and the median years of infection was 5.81 years. The cohort consisted mostly of male patients (85.1%) and Greek patients (93.4%). Most of the patients were receiving HAART (78.2%), and the most common HIV risk factor was male to male sex (75.4%). Most individuals had a CD4 count more than 350 (78.4%) and a plasma viral load less than 100,000 (79%). The number of adherent patients was 957 (79%) and of non-adherent patients was 253 (21%). About half of the population were vaccinated before 2010 (53.8%). It seems that age at infection, years of infection, sex, CDC staging, CD4 count and plasma viral load did not have an effect at the patients’ overall compliance. In contrast, other factors such as level of education, had an impact on the adherence to immunization.
With regards to vaccination against St. pneumoniae, patients with lower level of education were less likely to be adherent to pneumococcal disease vaccination. Similarly, individuals with no insurance coverage were less adherent than those having insurance coverage. Intravenous drug users were also less likely to be adherent. Additionally, patients vaccinated before the beginning of the financial crisis (2010) were more compliant than those vaccinated after 2010 (p < 0.0005). Factors tested for association with adherence to vaccine against St. pneumoniae are summarized in Table 1.
Table 1.
Unifactorial and multifactorial analysis for vaccination against pneumococcal disease.
| Non-adherent (N = 253, 21%) |
Adherent (N = 957,79%) | p-value | OR | 95%CI | |
|---|---|---|---|---|---|
| Age at infection [years] | |||||
| Median (range) | 34.49 (17–71) | 33.94 (15–75) | 0.585 | 1.01 | 1.00–1.03 |
| Mean (SD) | 35.35 (9.78) | 36.35 (11.05) | 0.160 | ||
| Years of infection [years] | |||||
| Median (range) | 2.59 (0.3–22.5) | 6.69 (0.3–25.2) | < 0.0005 | 1.07 | 1.01–1.13 |
| Mean (SD) | 4.42 (4.90) | 8.24 (5.87) | < 0.0005 | ||
| Sex [N (%)] | |||||
| Male | 222 (87.7%) | 808 (84.4%) | 0.198 | 1.00 | |
| Female | 31 (12.3%) | 149 (15.6%) | 1.29 | 0.62–2.68 | |
| Nationality[N (%)] | |||||
| Greek | 209 (82.6%) | 921 (96.2%) | < 0.0005 | 1.00 | |
| Other | 44 (17.4%) | 36 (3.8%) | 0.33 | 0.19–0.57 | |
| HAART [N (%)] | |||||
| No | 93 (36.8%) | 171 (17.9%) | < 0.0005 | 1.00 | |
| Yes | 160 (63.2%) | 786 (82.1%) | 1.88 | 1.29–2.75 | |
| HIV risk factor [N (%)] | |||||
| MSM | 177 (70.0%) | 735 (76.8%) a | < 0.0005 | 1.00 | |
| Heterosexual sex | 39 (15.4%) | 163 (17.0%) | 0.71 | 0.37–1.36 | |
| IVDU | 15 (5.9%) | 6 (0.6%) b | 0.12 | 0.04–0.42 | |
| Other | 22 (8.7%) | 53 (5.5%) | 1.33 | 0.72–2.47 | |
| Nadir CD4 cell count (log10) | |||||
| Mean (SD) | 2.37 (0.47) | 2.36 (0.45) | 0.941 | ||
| CD4 cell count (log10) | |||||
| Mean (SD) | 2.60 (0.36) | 2.71 (0.26) | < 0.0005 | ||
| Plasma viral load (log10) | |||||
| Mean (SD) | 4.44 (0.96) | 4.13 (1.17) | < 0.0005 | ||
| CDC [N (%)] | |||||
| A | 169 (66.8%) | 555 (58.0%) c | 0.035 | 1.00 | |
| B | 55 (21.7%) | 275 (28.7%) d | 0.91 | 0.61–1.36 | |
| C | 29 (11.5%) | 127 (13.3%) | 0.72 | 0.43–1.21 | |
| Insurance [N (%)] | |||||
| Yes | 157 (62.1%) | 631 (65.9%) | 0.001 | 1.00 | |
| No | 67 (26.5%) | 161 (16.8%) e | 0.82 | 0.49–1.35 | |
| Social Welfare | 29 (11.5%) | 165 (17.2%) f | 1.73 | 1.04–2.88 | |
| Education [N (%)] | |||||
| Primary | 122 (48.2%) | 362 (37.8%) g | 0.010 | 1.00 | |
| High School | 85 (33.6%) | 371 (38.8%) | 1.72 | 1.02–2.90 | |
| University | 46 (18.2%) | 224 (23.4%) | 1.43 | 0.92–2.23 | |
| CD4 cell count N(%) | |||||
| Less than 350 | 79 (33.5%) | 176 (18.7%) | < 0.0005 | 1.00 | |
| More than 350 | 157 (66.5%) | 767 (81.3%) | 2.00 | 1.37–2.93 | |
| Plasma viral load N(%) | |||||
| Less than 100,000 | 193 (76.3%) | 764 (79.8%) | 0.224 | 1.00 | |
| More than 100,000 | 60 (23.7%) | 193 (20.2%) | 1.03 | 0.68–1.54 | |
| Nadir CD4 cell count N(%) | |||||
| Less than 200 | 64 (29.6%) | 261 (28.3%) | 0.738 | ||
| More than 200 | 152 (70.4%) | 661 (71.7%) | |||
| Time of vaccination N(%) | |||||
| Before 2010 | 66 (26.1%) | 585 (61.1%) | < 0.0005 | 1.00 | |
| After 2010 | 187 (73.9%) | 372 (38.9%) | 0.52 | 0.30–0.89 | |
a: p = 0,024
b: p < 0,0005
c: p = 0,010
d: p = 0,026
e: p < 0,0005
f: p = 0,026
g: p = 0,003
Regarding vaccination against HBV, 328 patients had natural immunity and 264 had previous vaccination against HBV and 24 of them had unknown serological status. Of a total of 594 patients, most of them were adherent (437, 73.6%). Partially vaccination was recorded for 63 patients (receipt of one or two doses of vaccine). Data are illustrated in Figure 1. The median age at infection was 35.10 years, and the older the patient when infected, the less likely it was to be adherent (p < 0.0005). There was no significant difference in compliance according to sex, CDC staging or plasma viral load (Table 2). However, patients with lower education level and no insurance coverage were less likely to be adherent (p < 0.0005). Furthermore, intravenous drug users and those vaccinated after 2010 and individuals not receiving HAART were less compliant.
Figure 1.
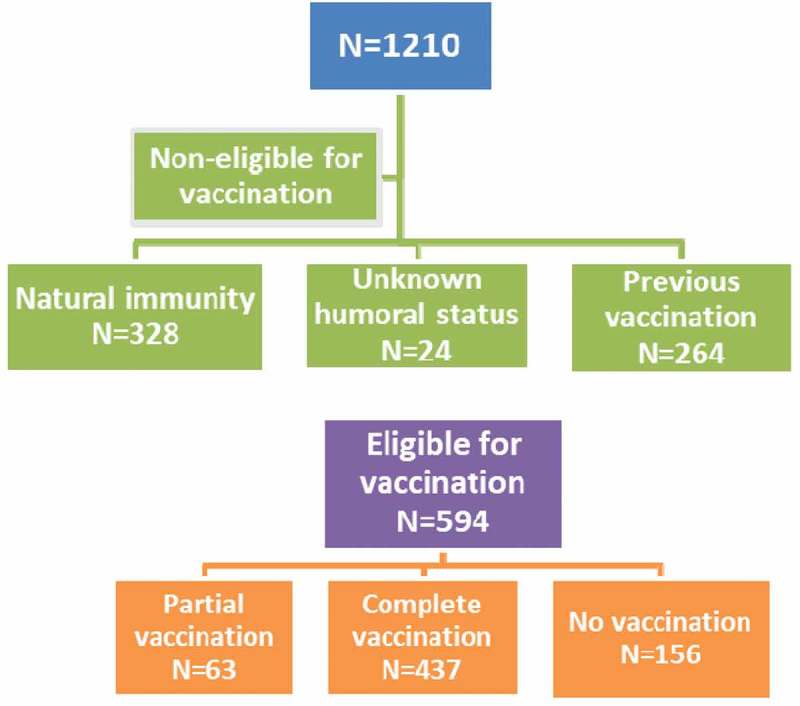
Flow chart of adherence to vaccination against HBV.
Table 2.
Unifactorial and multifactorial analysis for vaccination against hepatitis B virus.
| Non-adherent (N = 157, 26,4%) |
Adherent (N = 437, 73,6%) |
p-value | OR | 95%CI | |
|---|---|---|---|---|---|
| Age at infection [years] | |||||
| Median (range) | 37.00 (18–75) | 34.44 (15–72) | < 0.0005 | 0.96 | 0.94–0.98 |
| Mean (SD) | 39.43 (10.88) | 35.98 (10.12) | < 0.0005 | ||
| Years of infection [years] | |||||
| Median (range) | 4.52 (0.3–25.1) | 7.11 (0.3–25.1) | 0.001 | 0.97 | 0.91–1.02 |
| Mean (SD) | 7.14 (6.13) | 8.38 (5.79) | 0.023 | ||
| Sex [N (%)] | |||||
| Male | 126 (80.3%) | 371 (84.9%) | 0.208 | 1.00 | |
| Female | 31 (19.7%) | 66 (15.1%) | 0.69 | 0.39–1.25 | |
| Nationality[N (%)] | |||||
| Greek | 141 (89.8%) | 416 (95.2%) | 0,021 | 1.00 | |
| Other | 16 (10.2%) | 21 (64.8%) | 0.87 | 0.36–2.12 | |
| HAART [N (%)] | |||||
| No | 38 (24.2%) | 75 (17.2%) | 0.062 | 1.00 | |
| Yes | 119 (75.8%) | 362 (82.8%) | 1.87 | 1.03–3.39 | |
| HIV risk factor [N (%)] | |||||
| MSM | 108 (68.8%) | 334 (76.4%) | 0,307 | ||
| Heterosexual sex | 36 (22.9%) | 74 (16,9%) | |||
| IVDU | 2 (1,3%) | 4 (0.9%) | |||
| Other | 11 (7,0%) | 25 (5.7%) | |||
| Nadir CD4 cell count (log10) | |||||
| Mean (SD) | 2.27 (0.53) | 2.34 (0.48) | 0.153 | ||
| CD4 cell count (log10) | |||||
| Mean (SD) | 2.61 (0.35) | 2.72 (0.28) | < 0.0005 | ||
| Plasma viral load (log10) | |||||
| Mean (SD) | 4.24 (1.15) | 4.08 (1.20) | 0.167 | ||
| CDC [N (%)] | |||||
| A | 84 (53.5%) | 253 (57.9%) | 0.337 | ||
| B | 46 (29.3%) | 129 (29.5%) | |||
| C | 27 (17.2%) | 55 (12.6%) | |||
| Insurance [N (%)] | |||||
| Yes | 77 (49.0%) | 300 (68.6%) a | < 0.0005 | 1.00 | |
| No | 58 (36.9%) | 68 (15.6%) b | 0.82 | 0.45–1.49 | |
| Social welfare | 22 (14.0%) | 69 (15.8%) | 1.90 | 0.98–3.69 | |
| Education [N (%)] | |||||
| Primary | 111 (70.7%) | 162 (37.1%) c | < 0.0005 | 1.00 | |
| High School | 33 (21.0%) | 164 (37.5%) d | 5.87 | 2.74–12.55 | |
| University | 13 (8.3%) | 111 (25.4%) e | 3.34 | 1.86–25.98 | |
| CD4 cell count N(%) | |||||
| Less than 350 | 49 (32.5%) | 71 (16.5%) | < 0.0005 | 1.00 | |
| More than 350 | 102 (67.5%) | 360 (83.5%) | 2.23 | 1.32–3.77 | |
| Plasma viral load N(%) | |||||
| Less than 10,000 | 121 (77.1%) | 352 (80.5%) | 0.357 | ||
| More than 10,000 | 36 (22.9%) | 85 (19.5%) | |||
| Nadir CD4 cell count N(%) | |||||
| Less than 200 | 52 (36.4%) | 120 (28.3%) | 0.084 | 1.00 | |
| More than 200 | 91 (63.6%) | 304 (71.7%) | 1.39 | 0.85–2.28 | |
| Time of vaccination N(%) | |||||
| Before 2010 | 75 (47.8%) | 279 (63.8%) | 0.001 | 1.00 | |
| After 2010 | 82 (52.2%) | 158 (36.2%) | 0.48 | 0.25–0.92 | |
a: p < 0,0005
b: p < 0,0005
c: p < 0,0005
d: p < 0,0005
e: p < 0,0005
As far as vaccination against HAV is concerned, 338 of them had natural immunity and the serological status was unknown for 20 individuals. Of a total of 852 patients, 627 (73.6%) of them were adherent and 223 (26.1%) of them were not adherent (partial or no vaccination). Data are illustrated in Figure 2. Again, components such as sex, CD4 count, plasma viral load and CDC staging did not have an important influence in adherence. On the other hand, individuals who had lower education level and no insurance coverage were less adherent than the rest of the study group (Table 3). Patients who were vaccinated after the beginning of the financial crisis were less adherent compared to those previously vaccinated (p < 0.0005).
Figure 2.
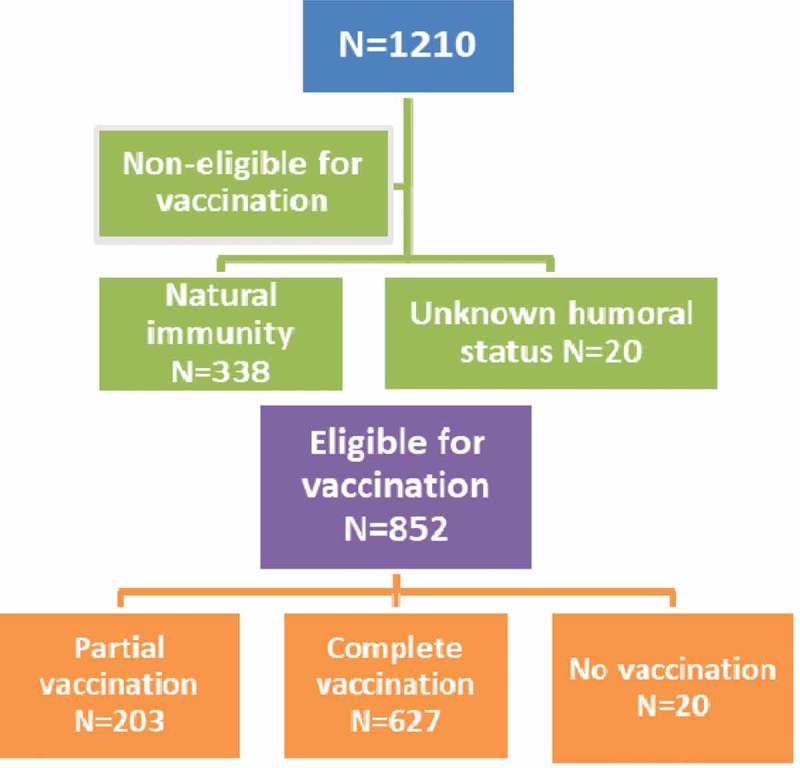
Flow chart of adherence to vaccination against HAV.
Table 3.
Unifactorial and multifactorial analysis for vaccination against hepatitis A virus.
| Non-adherent (N = 225, 26,4%) |
Adherent (N = 627, 73,6%) |
p-value | OR | 95%CI | |
|---|---|---|---|---|---|
| Age at infection [years] | |||||
| Median (range) | 35.69 (18–71) | 32.85 (15–75) | 0.002 | 0.98 | 0.97–1.00 |
| Mean (SD) | 36.78 (10.38) | 34.51 (10.02) | 0.004 | ||
| Years of infection [years] | |||||
| Median (range) | 4.45 (0.35–25.2) | 6.33 (0.3–24.54) | 0.003 | 0.99 | 0.94–1.03 |
| Mean (SD) | 6.85 (5.81) | 7.75 (5.66) | 0.041 | ||
| Sex [N (%)] | |||||
| Male | 194 (86,2%) | 534 (85,2%) | 0.742 | ||
| Female | 31 (13,8%) | 93 (14,8%) | |||
| Nationality[N (%)] | |||||
| Greek | 206 (91.6%) | 587 (93,6%) | 0,288 | ||
| Other | 19 (8.4%) | 40 (6,4%) | |||
| HAART [N (%)] | |||||
| No | 58 (25.8%) | 132 (21,1%) | 0.161 | 1.00 | |
| Yes | 167 (74.2%) | 495 (78,9%) | 1.29 | 0.86–1.95 | |
| HIV risk factor [N (%)] | |||||
| MSM | 168 (74,7%) | 481 (76.7%) | 0,672 | ||
| Heterosexual sex | 36 (16,0%) | 103 (16,4.0%) | |||
| IVDU | 4 (1,8%) | 7 (1,1%) | |||
| Other | 17 (7,6%) | 36 (5.7%) | |||
| Nadir CD4 cell count (log10) | |||||
| Mean (SD) | 2.36 (0.50) | 2.39 (0.43) | |||
| CD4 cell count (log10) | |||||
| Mean (SD) | 2.67 (0.31) | 2.70 (0.28) | 0.241 | ||
| Plasma viral load (log10) | |||||
| Mean (SD) | 4.31 (1.04) | 4.18 (1.13) | |||
| CDC [N (%)] | |||||
| A | 135 (60,0%) | 393 (62,7%) | 0.143 | 1.00 | |
| B | 54 (24,0%) | 165 (26,3%) | 0.92 | 0.61–1.39 | |
| C | 36 (16,0%) | 69 (11,0%) | 0.63 | 0.38–1.05 | |
| Insurance [N (%)] | |||||
| Yes | 100 (44,4%) | 395 (63,0%) a | < 0.0005 | 1.00 | |
| No | 96 (42,7%) | 113 (18,0%) b | 0.54 | 0.34–0.87 | |
| Social welfare | 29 (12,9%) | 119 (19,0%) | 1.41 | 0.84–2.37 | |
| Education [N (%)] | |||||
| Primary | 152 (67.6%) | 248 (39.6%) c | < 0.0005 | 1.00 | |
| High School | 40 (17.8%) | 242 (38.6%) d | 1.88 | 1.09–3.25 | |
| University | 33 (14.7%) | 137 (39.6%) e | 2.56 | 1.58–4.14 | |
| CD4 cell count N(%) | |||||
| Less than 350 | 47 (21.2%) | 129 (21.1%) | 1.000 | ||
| More than 350 | 175 (78.8%) | 482 (78.9%) | |||
| Plasma viral load N(%) | |||||
| Less than 10,000 | 174 (77.3%) | 494 (78.8%) | 0.638 | ||
| More than 10,000 | 51 (22.7%) | 133 (21.2%) | |||
| Nadir CD4 cell count N(%) | |||||
| Less than 200 | 59 (28.4%) | 159 (26.6%) | 0.651 | ||
| More than 200 | 149 (71.6%) | 438 (73.4%) | |||
| Time of vaccination N(%) | |||||
| Before 2010 | 103 (45.8%) | 367 (58.5%) | 0.001 | 1.00 | |
| After 2010 | 122 (54.2%) | 260 (41.5%) | 0.51 | 0.31–0.85 | |
a: p < 0,0005
b: p < 0,0005
c: p < 0,0005
d: p < 0,0005
e: p < 0,0005
Finally, regarding vaccination against seasonal influenza, most of the patients (61%) were not adherent (737 of a total population of 1210). Also, patients of an older age were more adherent than those younger patients (p < 0.0005). Sex, nationality, HAART, CD4 count, plasma viral load and CDC staging did not seem to affect compliance (Table 4). On the other hand, factors such as education level and insurance coverage did. Individuals with lower education level and lack of insurance coverage were less likely to be vaccinated (Table 4). Furthermore, patients vaccinated after 2010 and those who were using intravenous drugs were also less adherent.
Table 4.
Unifactorial and multifactorial analysis for vaccination against seasonal influenza.
| Non-adherent (N = 737, 61%) |
Adherent (N = 473,39%) | p-value | OR | 95%CI | |
|---|---|---|---|---|---|
| Age at infection [years] | |||||
| Median (range) | 30.27 (15–72.5) | 43.62 (22.3–75.5) | < 0.0005 | 1.29 | 1.25–1.33 |
| Mean (SD) | 30.57 (6.52) | 44.83 (10.42) | < 0.0005 | ||
| Years of infection [years] | |||||
| Median (range) | 5.85 (0.3–25.2) | 5.54 (0.3–25.0) | 0.052 | 1.00 | 0.96–1.03 |
| Mean (SD) | 7.80 (6.19) | 6.88 (5.35) | 0.008 | ||
| Sex [N (%)] | |||||
| Male | 620 (84.1%) | 410 (86.7%) | 0.247 | 1.00 | |
| Female | 117 (15.9%) | 63 (13.3%) | 1.01 | 0.60–1.71 | |
| Nationality[N (%)] | |||||
| Greek | 694 (92.8%) | 446 (84.3%) | 0.344 | ||
| Other | 53 (7.2%) | 27 (5.7%) | |||
| HAART [N (%)] | |||||
| No | 177 (24.0%) | 87 (18.4%) | 0.022 | 1.00 | |
| Yes | 560 (76.0%) | 386 (81.6%) | 1.07 | 0.66–1.73 | |
| HIV risk factor [N (%)] | |||||
| MSM | 561 (76.1%) | 351 (74.2%) | 0.301 | ||
| Heterosexual sex | 120 (16.3%) | 82 (17.3%) | |||
| IVDU | 16 (2.2%) | 5 (1.1%) | |||
| Other | 40 (5.4%) | 35 (7.4%) | |||
| Nadir CD4 cell count (log10) | |||||
| Mean (SD) | 2.40 (0.45) | 0.002 | |||
| CD4 cell count (log10) | |||||
| Mean (SD) | 2.71 (0.28) | 2.65 (0.29) | 0.001 | ||
| Plasma viral load (log10) | |||||
| Mean (SD) | 4.13 (1.14) | 0.008 | |||
| CDC [N (%)] | |||||
| A | 472 (64.0%) | 252 (53.3%) a | 0.001 | 1.00 | |
| B | 182 (24.7%) | 148 (31.3%) b | 1.02 | 0.67–1.57 | |
| C | 83 (11.3%) | 73 (15.4%) c | 1.33 | 0.74–2.39 | |
| Insurance [N (%)] | |||||
| Yes | 436 (59.2%) | 352 (74.4%) d | < 0.0005 | 1.00 | |
| No | 194 (26.3%) | 34 (7.2%) e | 0.04 | 0.02–0.09 | |
| Social welfare | 107 (14.5%) | 87 (18.4%) | 1.27 | 0.76–2.10 | |
| Education [N (%)] | |||||
| Primary | 328 (44.5%) | 156 (33.0%) f | < 0.0005 | 1.00 | |
| High School | 268 (36.4%) | 188 (39.7%) | 1.44 | 0.84–2.48 | |
| University | 141 (19.1%) | 129 (27.3%) g | 0.97 | 0.60–1.54 | |
| CD4 cell count N(%) | |||||
| Less than 350 | 141 (19.7%) | 114 (24.7%) | 0.043 | 1.00 | |
| More than 350 | 576 (80.3%) | 348 (75.3%) | 1.08 | 0.68–1.72 | |
| Plasma viral load N(%) | |||||
| Less than 100,000 | 605 (82.1%) | 352 (74.4%) | 0.002 | 1.00 | |
| More than 100,000 | 132 (17.9%) | 121 (25.6%) | 1.24 | 0.78–1.96 | |
| Nadir CD4 cell count N(%) | |||||
| Less than 200 | 168 (24.5%) | 157 (34.8%) | < 0.0005 | 1.00 | |
| More than 200 | 519 (75.5%) | 294 (65.2%) | 1.03 | 0.66–1.62 | |
| Time of vaccination N(%) | |||||
| Before 2010 | 402 (26.1%) | 249 (52.6%) | 0.555 | ||
| After 2010 | 335 (73.9%) | 224 (47.4%) | |||
a,d,e.f,g: p < 0,0005
b: p = 0,012
c: p = 0,035
Discussion
Vaccines are critical components for protecting HIV-infected adults from a certain number of preventable diseases. The national immunization program recommends the administration of four distinct vaccines to those patients against hepatitis A and hepatitis B viruses, influenza and St. pneumoniae.
St. pneumoniae is the most common cause of bacterial pneumonia in HIV infected individuals, despite the decrease of pneumococcal disease (PD) recorded since the introduction of HAART. The incidence is higher in patients with advanced HIV infection.2,3 There are some studies which dispute the clinical efficacy of the 23-valent PPV in HIV-infected patients, compared to general population, thus leading at a low rate of vaccination, in some cases.9,11 However, in our study, most of the patients were adherent to vaccination against PD. Risk factors associated with PD in HIV-infected patients in previous reports are: smoking, alcohol abuse, injection drug use, CD4 count < 200, previous pneumonia or previous hospitalization, or underlying conditions, such as liver cirrhosis, chronic obstructive pulmonary disease (COPD) or lymphoma.10 In our study the adherence to vaccination against S. pneumoniae is about 79%. Differentiation rises when comparing our results with other studies. Pneumococcal vaccine coverage has increased in HIV infected people since the 2009 A/H1N1 influenza pandemic and it reached nearly 65% in a patient cohort study conducted in 2011 in France5,27 In our study, it is noticeable that intravenous drug users, one of the vulnerable groups for developing PD, is not adherent to vaccination. Also, demographic groups with features of economic hardship such as low education level, and no insurance coverage are less adherent to vaccination, thus more susceptible to PD. One can assume that the total cost of providing vaccination to groups whose members are not able to vaccinate is lower than treating PD and hospitalization of those affected. The aftermath of the Greek financial crisis can be seen in adherence to vaccination against PD, because of the notable decline of compliance after 2010.
The high rates of HBV infection in HIV-infected patients, support the need for vaccination in this high-risk group.12,13 HBV infection in HIV infected individuals raises the risk of cirrhosis, end-stage liver disease, and death from liver disease, especially in patients with a low CD4 cell count or accompanying alcohol use.33,34 Prevention of HBV infection is proven both clinical imperative and cost effective. Additionally, HBV vaccination might benefit patients who have either lost HBsAb, and thus are at risk for reactivation or re-infection, or those who have a false-positive HBcAb test.14,18
In our study there was a high level of adherence in vaccination against HBV (73.6% of the study group). In vulnerable socioeconomic groups, e.g., those not having insurance coverage and those with a lower education level, the number of compliant individuals was significantly lower. Nearly half of HIV infected patients without insurance coverage were not adherent to HBV vaccination. Furthermore, intravenous drug users were less compliant, which is of great significance, considering the shared route of transmission between HIV and HBV. There are some studies that demonstrate better compliance in vaccination amongst individuals, if large-scale, multi-site hepatitis B vaccination programs are applied.35 On the contrary, in a large cohort study of HIV patients found only one-third had received at least one dose of the HBV vaccine.36 Some authors showed that factors significantly associated with vaccination in HIV infected people were younger, mainly men who have sex with men (MSM) and followed-up by an experienced physician. In another study, the immunization coverage against HBV was reported to be 61,8% (778 out of 1257), while the vaccination coverage for MSM was 75.5% but only 37% for intravenous drug users (IVDU) and 50% in cirrhotic patients.5 Other strategies have been used to raise the number of adherent patients, such an accelerated schedule of vaccination. An accelerated schedule has been proven better than a standard schedule.17
The occurence of HAV infection in HIV affected patients is high, ranging from 40% to 70%, even in resource-rich nations, and the fallout of HAV can vary, according to the stage of the HIV infection.15 For MSM, the risk of infection with hepatitis A is increased by higher numbers of sex partners related immune depression, and by certain sex practices such as oroanal contact. Vaccination of susceptible patients against HAV should be recommended early in HIV infection using the shorter course to encourage compliance. In our study adherence to vaccination against HAV was high (about 74% of the study cohort was adherent). Individuals with no insurance coverage and lower education level were less adherent, as less adherent were intravenous drug users. MSM were more likely to be adherent. Compared with results in other studies, HIV patients found to be less than 30% vaccinated against HAV.36 In another study, 47.4% of the patients were vaccinated against HAV.27 Patients with specific risk factors for HAV infection (62%) were significantly more often vaccinated than others. Vaccination coverage was 54.5% for MSM and 47.1% in cirrhotic patients.27 In a similar study in California, only 23.3% of 712 tested patients had received at least one dose against HAV.25
In our study adherence to vaccination against influenza was the lowest, with only about 39% of the patients being adherent. Several studies have also reported poor immune responses and poor compliance to conventional influenza vaccines in HIV-infected individuals. According to a study, vaccination coverage for seasonal influenza and A (H1N1) 2009 pandemic influenza, were 48.3% and 64.6%, respectively. Factors independently associated with vaccination were an older age, CD4 count > 200/mm (3) and HIV-RNA < 50 copies/mL and longer duration of HIV infection.37 Furthermore, in comparison with a study regarding influenza vaccinations among HIV-infected persons, in the US found that only 42% were vaccinated,36 while in a French hospital-based cohort analyzed in 2011 seasonal flu vaccine coverage was estimated to be 30.9%, compared to 37.5 to 48.5% in global high-risk populations.5 Strategies like different method of admission and increased antigen dose have not improved efficacy or compliance to vaccination against seasonal influenza.25 Despite lower immunogenicity in HIV-infected patients, the clinical efficacy of influenza vaccines is proved by a significant reduction in infectious respiratory diseases and in confirmed cases of influenza.38,39 Thus, it is essential that measures must be taken to raise adherence to vaccine against seasonal influenza amongst HIV infected individuals.
In a trial conducted in Brazil about vaccination coverage in HIV patients, specifically against HBV, HAV, influenza and S. pneumoniae, was ascertained that only 14.1% of patients completed the full vaccination schedule.8 There was no statistically significant difference in gender, skin color, marital status, family or occupational status. In another study, there was no difference between having or not having a complete vaccination schedule and age, years of education but CD4 + T-cells count of patients with incomplete immunization was lower than patients with complete immunization.40 Summarizing, many eligible HIV-positive adults do not receive vaccination against HBV, HAV Influenza and St. pneumoniae. Finally, given that SES affects the adherence to vaccine while SES depends on occupation status, education, income, and other factors analyzed above, this study points out that the financial crisis in Greece had a negative impact at vaccination adherence, as it is generally admitted that residents of Greece suffer from austerity, unemployment and reduced incomes.41 Therefore, health education strategies should be implemented to explain the importance of vaccination for patients at risk. Health education is an essential factor to ensure completion of the vaccination schedules.37
In all four suggested vaccines in our study, one can observe the reduction of percentage of adherence of vulnerable groups, such as those with lower educational level, those with no insurance coverage and intravenous drug users. These findings warrant further investigation. Although vaccines were offered free of charge, adherence was higher to HIV infected adults with higher socioeconomical status. This may reflect the gap of communication between the public health system and vulnerable groups and underlines the necessity of the eradication of said gap.
Our study has some limitations. First, we excluded from those patients who were lost to follow up (patients out of care for a year or more). This exclusion may restrict the number of patients enrolled in the study and diminish the true prevalence of adherent or not adherent patients to vaccination. This exclusion was decided to reassure that all relevant data were available for the analysis (initial humoral assessment and recording of vaccines administered). Second, our study is a retrospective cohort study, which relies on previous recordkeeping of medical history and thus it does not allow modification or clarification of previous data which is collected routinely. Furthermore, the H1N1 influenza outbreak in 2009 could have influenced the excess of vaccine recommendation to our patients since then. This fact although might have biased the frequency of adherent patients to this specific vaccine, could no have been handled particularly, since all necessary vaccinations are recommended pew protocol to all our HIV positive patients with the aim of herd protection against all vaccine preventable diseases.
Methods
We performed a ten-year (January 2004-January 2014) retrospective cohort study of all HIV infected adults followed in the Infectious Diseases Unit of the First Internal Medicine Department of the University General Hospital ‘AHEPA’ in Thessaloniki, to assess the immunization coverage of HIV adults with the recommended vaccines. Demographic data were collected, including age at infection, years of infection, sex, nationality (Greek or other), HIV mode of transmission (male to male sexual contact, heterosexual contact, injecting drug use, other), nadir CD4 count, plasma viral load, HIV stage of infection according to CDC (A,B,C), insurance coverage (insured, uninsured or receiving social welfare), educational level (primary, high school or university) and Highly Active Antiretroviral Therapy (HAART) intake. 2010 was the beginning of the economic crisis in Greece, which lead many patients to deteriotation of their SES, thus we also wanted to assess the potential impact of the country’s economic situation on adherence to vaccination.
Exclusion criteria for vaccine qualification contained patients seropositive for HBsAb, HBsAg, HBeAb and isolated HbcAb, patients with a very low CD4 count, patients who were lost to follow up and patients who had innate immunity or previous vaccination, proven with serological testing. Inclusion criteria were retention to care (defined as at least one visitation per year) and two years of follow up. A total of 1210 patients was documented and encouraged to get immunized against hepatitis B virus, hepatitis A virus, pneumococcal disease (Pneumococcal polysaccharide vaccine- PPSV23) and seasonal influenza. Patients were given vaccinations according to current guidelines for vaccination for HIV infected adults: One dose against PD every five years; three doses against HBV (0,1 and 6 months); two doses against HAV (0 and 6 months) and one dose against seasonal influenza annually. All doses of the vaccines listed above were free of charge, regardless patients’ insurance status, due to the fact that in Greece public hospitals provide necessary medicine for HIV infected adults. Patients’ follow up was performed every six months, as all HIV infected adults in our hospital are routinely monitored twice a year. Adherence to the suggested vaccination program was correlated to the aforementioned demographic data (age at infection, years of infection, sex, nationality, HIV mode of transmission, nadir CD4 count, plasma viral load, HIV stage of infection according to CDC, insurance coverage, educational level, HAART intake, and time of vaccination (before or after 2010)).
Data are expressed as mean± standard deviation (S.D.) or median (IQR) for continuous variables and as percentages for categorical data. The Kolmogorov – Smirnov test was utilized for normality analysis of the parameters. Univariate analyses were made by using the chi-square and Fisher exact test to analyse the relation between the outcome variable (non adherent vs adherent) and the qualitative variables, whereas the Student t-test or Mann-Whitney U-test and One-way ANOVA or Kruskal-Wallis were used to analyse the relation between the outcome variable and the quantitative measures respectively. Only variables with p-values < 0.20 in univariate analysis were entered into the multivariate models. Goodness of fit was evaluated using the Hosmer-Lemeshow statistic. The odds ratio (OR) of compliance was then estimated in a multifactorial logistic regression model, and ORs and their 95% confidence intervals (95% CI) are presented.
All tests are two-sided, a p-value of < 0.05 was used to denote statistical significance. All analyses were carried out using the statistical package SPSS vr 17.00 (Statistical Package for the Social Sciences, SPSS Inc, Chicago, Ill, USA).
Conclusions
Overall, the higher vaccination coverage in our study was observed against PD. This remark presents a successful sensitization of practitioners about the risk of PD in HIV-infected individuals and a good acceptability of this vaccine in this study group. There are many determinants of compliance in vaccination. According to one study, the most frequent reason for non-vaccination was that of not being suggested by the physician.42 In our study, it is made clear that vulnerable socioeconomic groups, such as intravenous drug users, individuals with no insurance coverage, should be approached and integrated in vaccination programmes. It has been proven with previous studies that when members of these groups are approached and informed about vaccination, they tend to be adherent.43 Also, the negative effect of Greek financial crisis is evident in vaccination adherence; after 2010 the number of HIV infected patients being adherent to vaccination has been reduced significantly.
Given the significant prevalence of hepatitis B, hepatitis A, influenza and St. pneumoniae infection in HIV-infected populations, failure to implement the vaccination guidelines represents a missed opportunity to prevent the morbidity and mortality associated with this disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, Khoshnood K, Holford TR, Schuchat A.. Declining incidence of invasive streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995-2000. J Infect Dis. 2005. June 15;191(12):2038–2045. doi: 10.1086/430356. [DOI] [PubMed] [Google Scholar]
- 2.Geretti AM, Doyle T. Immunization for HIV-positive individuals. Curr Infect Dis. 2010;23:32–38. doi: 10.1097/QCO.0b013e328334fec4. [DOI] [PubMed] [Google Scholar]
- 3.Abzug MJ. Vaccination in the immunocompromised child: a probe of immune reconstitution. Pediatr Infect Dis J. 2009;28:233–236. doi: 10.1097/INF.0b013e31819d31bc. [DOI] [PubMed] [Google Scholar]
- 4.European AIDS Clinical Society European guidelines for treatmentof HIV-infected adults in Europe—version 7.0. European AIDS Clinical Society; 2013. http://www.eacsociety.org/Portals/0/Guidelines. [Google Scholar]
- 5.Fresard A, Gagneux-Brunon A, Lucht F, Botelho-Nevers E, Launay O. Immunization of HIV-infected adult patients — french recommendations. Hum Vaccin Immunother. 2016;12(11):2729–2741. doi: 10.1080/21645515.2016.1207013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kourkounti S, Paparizos V, Leuow K, Paparizou E, Antoniou C. Adherence to hepatitis A virus vaccination in HIV-infected men who have sex with men. Int J STD AIDS. 2015 Oct;26(12):852–6. doi: 10.1177/0956462414560274. Epub 2014 Nov 18. [DOI] [PubMed] [Google Scholar]
- 7.Bailey CL, Smith V, Sands M. Hepatitis B vaccine: a seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults. Int J Infect Dis. 2008. November;12(6):e77–83. doi: 10.1016/j.ijid.2008.05.1226. [DOI] [PubMed] [Google Scholar]
- 8.Cunha GH, Galvão MT, Medeiros CM, Rocha RP, Lima MA, Fechine FV. Vaccination status of people living with HIV/AIDS in outpatient care in Fortaleza, Ceará, Brazil. Braz J Infect Dis. 2016. Sep-Oct;20(5):487–493. doi: 10.1016/j.bjid.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kernéis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boëlle P-Y. Long-term immune responses to vaccination inHIV-infected patients: A systematic review and meta-analysis. Clin Infect Dis. 2014;58(8):1130–1139. doi: 10.1093/cid/cit937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuorti JP, Butler JC, Gelling L, Kool JL, Reingold AL, Vugia DJ. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann Intern Med. 2000;132:182–190. doi: 10.7326/0003-4819-132-3-200002010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis. 2004;4:445–455. doi: 10.1016/S1473-3099(04)01060-6. [DOI] [PubMed] [Google Scholar]
- 12.Domınguez A, Salleras L, Fedson DS, Izquierdo C, Ruiz L, Ciruela P, Fenoll A, Casal J. Effectiveness of pneumococcal vaccination for elderly people in Catalonia, Spain: a casecontrol study. Clin Infect Dis. 2005;40:1250–1257. doi: 10.1086/429236. [DOI] [PubMed] [Google Scholar]
- 13.Guerrero M, Krueger S, Saitho A, Sorvillo F, Cheng K-J, French C, Beall G. Pneumonia in HIV infected patients: a case-control study of factors involved in risk and prevention. AIDS. 1999;13:1971–1975. doi: 10.1097/00002030-199910010-00021. [DOI] [PubMed] [Google Scholar]
- 14.Hadler SC, Judson FN, O’Malley PM, Altman NL, Penley K, Buchbinder S, Schable CA, Coleman PJ, Ostrow DN, Francis DP. Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis. 1991;163:454–459. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi RT, Wurcel A, Lee H, McGovern B, Shopis J, Geary M, Sivamurthy R, Sax PE, Ukomadu C. Response to hepatitis B vaccine in HIV-1–positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis. 2005. May 1;191(9):1435–1441. doi: 10.1086/429302. [DOI] [PubMed] [Google Scholar]
- 16.Quaglio G1, Talamini G, Lugoboni F, Lechi A, Venturini L, Jarlais DC, Mezzelani P, GruppoIntersert di CollaborazioneScientifica . Compliance with hepatitis B vaccination in 1175 heroin users and risk factors associated with lack of vaccine response. Addiction. 2002. August;97(8):985–992. [DOI] [PubMed] [Google Scholar]
- 17.Brook G. Prevention of viral hepatitis in HIV co-infection. J Hepatol. 2006;44(1 Suppl):S104–107. Epub 2005 Nov 28. doi: 10.1016/j.jhep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De CantuáriaTauil M, Sato APS, Waldman EA. Factors associated with incomplete or delayed vaccination across countries: A systematic review. Vaccine. 2016;34:2635–2643. doi: 10.1016/j.vaccine.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 20.De Waroux Le Polain O, Schellenberg JRA, Manzi F, Mrisho M, Shirima K, Mshinda H, Alonso P, Tanner M, Schellenberg DM. Timeliness and completeness of vaccination andrisk factors for low and late vaccine uptake in young children living in rural southern Tanzania. Int Health. 2013;5(2):139–147. doi: 10.1093/inthealth/iht006. [DOI] [PubMed] [Google Scholar]
- 21.Hoest C, Seidman JC, Lee G, Platts-Mills JA, Ali A, Olortegui MP, Bessong P, Chandyo R, Babji S, Mohan VR, the MAL-ED Network Investigators1, et al. Vaccine coverage and adherence to EPI schedules in eight resource poor settings in the MAL-ED cohort study. Vaccine. 2017;35:443–451. doi: 10.1016/j.vaccine.2016.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchidjou HK, Vescio MF, Sanou Sobze M, Souleyman A, Stefanelli P, Mbabia A, Moussa I, Gentile B, Colizzi V, Rezza G. Low vaccine coverage among children born to HIV infected women in Niamey, Niger. Hum Vaccin Immunother. 2016;12(2):540–544. doi: 10.1080/21645515.2015.1069451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burch LS, Smith CJ, Anderson J, Sherr L, Rodger AJ, O’Connell R, Geretti A-M, Gilson R, Fisher M *, Elford J, for the Antiretrovirals, Sexual Transmission Risk and Attitudes (ASTRA) Study Group et al. Socioeconomic status and treatment outcomes for individuals with HIV on antiretroviral treatment in the UK: cross-sectional and longitudinal analyses. Lancet Public Health. 2016;1:e26–36. doi: 10.1016/S2468-2667(16)30002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winnock M, Bani-Sadr F, Pambrun E, Loko M-A, Lascoux-Combe C, Garipuy D, Rosenthal E, Carrieri P, Dabis F, Salmon D. Prevalence of immunity to hepatitis viruses A and B in a large cohort of HIV/HCV-coinfected patients, and factors associated with HAV and HBV vaccination. Vaccine. 2011;29:8656–8660. doi: 10.1016/j.vaccine.2011.08.125. [DOI] [PubMed] [Google Scholar]
- 25.Tedaldi EM, Baker RK, Moorman AC, Wood KC, Fuhrer J, McCabe RE, Holmberg SD, the HIV Outpatient Study (HOPS) Investigators . Hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis. 2004. May 15;38(10):1478–1484. doi: 10.1086/420740. [DOI] [PubMed] [Google Scholar]
- 26.Mohseni-Zadeh M, Rey D, Batard ML, Beck Wirth G, Partisani ML, Lang JM, Hansmann Y, Christmann D, Martinot M. Inadequate vaccination coverage in a French cohort of HIV positive patients. Med Mal Infect. 2010;40:683–690. doi: 10.1016/j.medmal.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Valour F, Cotte L, Voirin N, Godinot M, Ader F, Ferry T, Vanhems P, Chidiac C. Vaccination coverage against hepatitis Aand B viruses, streptococcus pneumoniae, seasonal flu, and A(H1N1) 2009 pandemic influenza in HIV-infected patients. Vaccine. 2014. July 31;32(35):4558–4564. doi: 10.1016/j.vaccine.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18:S19–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 2004;99:361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 30.Hutton HE, Treisman G. The role of personality in HIV risk behaviors: implications for treatment En: Cohen MA, Gorman JM, editors. Comprehensive textbook of AIDS psychiatry. New York, NY: Oxford University Press; 2008. p. 141–149. [Google Scholar]
- 31.Bermudez LG, Jennings L, Ssewamala FM, Nabunya P, Mellins C, McKay M. Equity in adherence to antiretroviral therapy among economically vulnerable adolescents living with HIV in Uganda. AIDS Care. 2016;28:83–91. doi: 10.1080/09540121.2016.1176681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCoy K, Waldrop-Valverde D, Balderson BH, Mahoney C, Catz S. Correlates of antiretroviral therapy adherence among HIVinfected older adults. J Int Assoc Provid AIDS Care. 2016. May;15(3):248–255. doi: 10.1177/2325957416642019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penaranda M, Falco V, Payeras A, Jordano Q, Curran A, Pareja A, Samperiz G, Dalmau D, Ribera E, Riera M. Effectiveness of polysaccharide pneumococcal vaccine in HIV-infected patients. A Case-Control Study. 2007. October 1;45(7):e82–7. [DOI] [PubMed] [Google Scholar]
- 34.Wilson CM, Ellenberg JH, Sawyer MK, Belzer M, Crowley-Nowick PA, Puga A, Futterman DC, Peralta L. Adolescent medicine HIV/AIDS research network, serologic response to hepatitis B vaccine in HIV infected and high-risk HIV uninfected adolescents in the REACH cohort. J Adolesc Health. 2001. September;29(3 Suppl):123–129. [DOI] [PubMed] [Google Scholar]
- 35.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571–577. doi: 10.1086/jid.2003.188.issue-4. [DOI] [PubMed] [Google Scholar]
- 36.Crum-Cianflone NF, Wallace MR. Vaccination in HIV-infected adults. AIDS Patient Care STDS. 2014;28(8). doi: 10.1089/apc.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey CL, Smith V, Sands M. Hepatitis B vaccine: a seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults. Int J Infect Dis. 2008. doi: 10.1016/j.ijid.2008.05.1226. [DOI] [PubMed] [Google Scholar]
- 38.Soriano V, Puoti M, Peters M, Benhamou Y, Sulkowski M, Zoulim F, Mauss S, Rockstroh J. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-hepatitis B virus international panel. AIDS. 2008. July 31;22(12):1399–1410. doi: 10.1097/QAD.0b013e3282f8b46f. [DOI] [PubMed] [Google Scholar]
- 39.Neilsen GA, Bodsworth NJ, Watts N. Response to hepatitis A vaccination in human immunodeficiency virus: infected and uninfected homosexual men. J Infect Dis. 1997. October;176(4):1064–1067. [DOI] [PubMed] [Google Scholar]
- 40.Molloy A, Curtis H, Burns F, Freedman A. Routine monitoring and assessment of adults living with HIV: results of the British HIV association (BHIVA) national audit 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kentikelenis A, Karanikolos M, Papanicolas I, Basu S, McKee M, Stuckler D. Health effects of financial crisis: omens of a Greek tragedy. The Lancet. 2011;378(9801):1457–1458. doi: 10.1016/S0140-6736(11)61556-0. [DOI] [PubMed] [Google Scholar]
- 42.Seo YB, Lee J, Song JY, Choi HJ, Cheong HJ, Kim WJ. Safety and immunogenicity of influenza vaccine among HIV-infected adults: conventional vaccine vs. intradermal vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroon FP, Van Dissel JT, De Jong JC, Zwinderman K, Van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive3-year study. Vaccine. 2000;18:3040–3049. doi: 10.1016/S0264-410X(00)00079-7. [DOI] [PubMed] [Google Scholar]


