ABSTRACT
Staphylococcus aureus (S. aureus) is a challenging bacterial pathogen which can cause a range of diseases, from mild skin infections, to more serious and invasive disease including deep or organ space surgical site infections, life-threatening bacteremia, and sepsis. S. aureus rapidly develops resistance to antibiotic treatments. Despite current infection control measures, the burden of disease remains high. The most advanced vaccine in clinical development is a 4 antigen S. aureus vaccine (SA4Ag) candidate that is being evaluated in a phase 2b/3 efficacy study in patients undergoing elective spinal fusion surgery (STaphylococcus aureus suRgical Inpatient Vaccine Efficacy [STRIVE]). SA4Ag has been shown in early phase clinical trials to be generally safe and well tolerated, and to induce high levels of bactericidal antibodies in healthy adults. In this review we discuss the design of SA4Ag, as well as the proposed clinical development plan supporting licensure of SA4Ag for the prevention of invasive disease caused by S. aureus in elective orthopedic surgical populations. We also explore the rationale for the generalizability of the results of the STRIVE efficacy study (patients undergoing elective open posterior multilevel instrumented spinal fusion surgery) to a broad elective orthopedic surgery population due to the common pathophysiology of invasive S. aureus disease and commonalties of patient and procedural risk factors for developing postoperative S. aureus surgical site infections.
KEYWORDS: staph, vaccine, orthopedic, surgery, infection
Staphylococcus aureus disease-an unmet medical need
S. aureus disease
S. aureus is a commensal Gram-positive coccus that colonizes the nares, axillae, pharynx, and other mucosal and skin surfaces of approximately 30% of humans at any given time.1–3 While S. aureus colonisation in healthy individuals generally does not lead to disease, the association between S. aureus nasal carriage and S. aureus infection risk at surgical sites is well established for cardiothoracic and orthopedic surgeries.4 Breaches in the skin or mucosa which allow bacteria to enter a normally sterile site can result in a wide range of infections, including invasive surgical site infections (SSIs).5 SSIs are the most common cause of healthcare-associated infections (HAI) in low-income settings and the second most common cause of HAI in high-income countries. S. aureus including both methicillin-resistant (MRSA) and methicillin-sensitive S. aureus (MSSA) is responsible for approximately 20% of all HAI in hospitalized patients and 30% of SSIs in the United States.6–10 S. aureus SSIs are associated with increased morbidity and mortality; sequelae include revision surgeries, poor quality of life, prolonged antibiotic treatment and rehabilitation, and associated lost work and productivity.3,11–15 Moreover, SSIs are associated with a substantial economic burden to the healthcare system as a result of increased length of hospital stay and increased risk of readmission.16,17
Current strategies aimed to prevent S. aureus SSIs include improved hygiene, aseptic surgical techniques, carrier screening, skin and nares decolonization, application of antibiotics to the surgical site prior to wound closure, and intravenous antibiotic prophylaxis.4,18–22
Some of these targeted preventative strategies have been shown to reduce SSIs in randomized controlled clinical trials and are often delivered as bundles. Strategies to prevent SSIs such as the Surgical Care Improvement Project (SCIP) initiative23-25, Epic Guidelines 1, 2, and 38726,27 and National Institute for Health and Clinical Excellence (NICE) SSI quality standards28 have been widely adopted. However, no consensus exists on the key components of a successful preventative bundle. Adherence to bundles is also resource intensive for clinical staff, and poor patient compliance has been implicated in lower than expected effectiveness.29 In addition, routine use of antibiotic prophylaxis and nasal decolonization agents such as mupirocin, have resulted in selection pressure on colonizing strains.30,31 Reports of increasing mupirocin resistance are of serious concern32,33 and the promotion of mupirocin resistance may also aid in the spread of multidrug resistance through co-selection with other resistance genes34 (e.g. high rates of clindamycin resistance in mupirocin resistant S. aureus isolates). Perioperative systemic antibiotic prophylaxis is a key preventative intervention shown to result in up to an 81% reduction in SSI incidence in orthopedic surgery.35 To be effective, systemic antibiotic prophylaxis has to achieve therapeutic tissue concentrations at the time of incision and while the wound is open.36,37 The effectiveness of intravenous antibiotics demonstrates that infections can be prevented at the time of surgery by systemic intervention strategies, which also supports a vaccine-based prevention strategy for S. aureus disease.38–41
Despite advancements in preventative strategies and improved adherence to infection control practices, S. aureus SSIs continue to occur, placing a substantial burden to the healthcare system. Thus, despite currently recommended prophylactic practices, there is a high unmet medical need for new strategies to prevent postoperative S. aureus infections including a safe and efficacious prophylactic vaccine.
The quest to develop a prophylactic vaccine to prevent S. aureus infections has been fraught with difficulties. Most notably two different monovalent vaccines were evaluated in phase 3 efficacy studies and failed. One contained two capsular polysaccharide (CP) conjugates serotypes (CP5 and CP8 linked to recombinant Pseudomonas aeruginosa exoprotein A; StaphVax [Nabi])42,43, an approach that has been highly successful for other pathogens.44 The second vaccine contained a single protein antigen (iron surface determinant B; IsdB) associated with iron acquisition.45 The potential reasons of the vaccine failures have been extensively reviewed.46 In addition to the IsdB vaccine not being efficacious, it also was associated with a safety signal of increased incidence of death and multiple organ failure in those who received vaccine and developed S. aureus infections.45 There are different theories as to what caused the safety signal for the IsdB-based vaccine.45,47 Of note though is that the design of the vaccine (single antigen without strong evidence of inducing a bacterial killing response and bacterial redundancy of iron acquisition mechanisms48) was different from other vaccines in clinical development. In addition, there is no established mechanism for the safety signal, and no such signal was observed with capsular polysaccharide-based StaphVax. Thus, there is no evidence to substantiate that the safety event is a class effect associated with all S. aureus vaccines.
SA4AG vaccine design and preclinical assessment
Learnings from previously unsuccessful vaccine development and pre-clinical research programs suggest that an effective vaccine against S. aureus should contain multiple antigens targeting different virulence mechanisms.49–51 An investigational four antigen vaccine (SA4Ag) targeting multiple virulence mechanisms is currently undergoing clinical development by Pfizer. SA4Ag contains four surface-expressed S. aureus antigens that target three virulence mechanisms deployed early in the infection process and are highly conserved, expressed in-vivo by the vast majority of global clinical isolates, and required by S. aureus to initiate and maintain infection.52–57 These antigens include CP5 and CP8, each conjugated to the nontoxic mutant form of diphtheria toxin (cross-reactive material 197 [CRM197]), (CP5-CRM197 and CP8-CRM197).58 The third antigen is a recombinant form of clumping factor A (ClfA) with a single amino acid substitution (Y338A) that prevents it from binding to its natural ligand fibrinogen.59 The fourth antigen is a recombinant non-lipidated form of the S. aureus manganese transporter C (MntC) protein called rP305A.60
Capsular polysaccharides (CP)
Expression of CP is a common mechanism by which pathogenic bacteria, including S. aureus, evade opsonophagocytosis (ie, complement-mediated uptake by neutrophils and macrophages).58 CP provide an effective immune evasion strategy by cloaking the bacteria and rendering them invisible to innate immune responses. Studies have shown that encapsulated S. aureus strains are more virulent in bacteremia models compared with capsule-defective isogenic mutants.52,53 Although 13 putative CPs have been described in S. aureus, all isolates have the genetic pathway for expression of CP5 or CP8.58,61 Preclinical animal studies using CP5 and CP8 antibodies or vaccinating with CP conjugates have shown evidence of protection against S. aureus infection in challenge studies.60,62–64 Moreover, vaccine induced anti-CP5 and anti-CP8 antibodies mediate opsonophagocytic killing activity as shown in preclinical and human clinical studies with the CP5-CRM197 and CP8-CRM197 conjugates or SA3Ag and SA4Ag vaccines.65–68 It is interesting to note that while StaphVAX (by Nabi Biopharmaceuticals), a bivalent vaccine containing capsular polysaccharide conjugates was found to be safe; it did not meet its efficacy endpoints in two studies to prevent S. aureus bacteraemia.43 Amongst possible explanations for the lack of efficacy were quoted manufacturing issues43, failure of consistently assessing vaccine immunogenicity with functional, bacterial killing responses and the challenge of protecting an immunocompromised end-stage renal disease population for a prolonged period of time. Furthermore, Scully et al. (2018) demonstrated that O-acetylation of the S. aureus capsular polysaccharide has to be maintained in the CP 5 and 8 conjugates for them to induce bacterial killing antibodies.69
Clumping factor a (CLFA)
ClfA is a surface adhesin that binds to the C-terminus of the plasma fibrinogen γ chain,70,71 and is present in 99% of S. aureus isolates including MRSA and MSSA.59 ClfA promotes fibrin cross-linking and mediates the binding of S. aureus to platelets, resulting in thrombus (blood clot) formation.72,73 It has also been shown to play a key role in the agglutination of staphylococci in the blood during infection, which leads to thromboembolic lesions in heart tissue and sepsis.54 The fibrinogen-binding activity of ClfA is linked to the ability of S. aureus to cause disease, as S. aureus strains with ClfA point mutations that prevent fibrinogen binding showed reduced virulence.74 This has also been shown in a Lactococcus lactis model that specifically demonstrated ClfA-attributed virulence, which was reversed by mutating the fibrinogen-binding domain of the protein (rClfAm). Virulence attributed to the native ClfA protein could only be prevented with antibodies that prevented ClfA from binding to fibrinogen.75 Preclinical studies evaluating ClfA as a vaccine antigen showed antibody-mediated protection in several animal models including osteomyelitis and septic arthritis.74 Furthermore, vaccination of mice with SA4Ag resulted in anti-ClfA antibodies that prevent S. aureus from binding to fibrinogen, which was in contrast to immunization with a vaccine comprised of ClfA expressing dead S. aureus cells.59,70 A fibrinogen binding inhibition (FBI) assay59 measuring anti-ClfA antibody-mediated inhibition of binding to fibrinogen of live S. aureus clinical isolates that express diverse ClfA variants as well as a competitive Luminex immunoassay (cLIA) were subsequently developed for clinical use.76 Humans naturally have ClfA binding antibodies through natural exposure; however, these antibodies are not potent enough to block the ability of ClfA to bind to fibrinogen and thus are not considered “functional”. It’s noteworthy that antibody infusions that were enriched for ClfA binding antibodies were not successful in phase 3 trial aimed at preventing S. aureus bacteremia among neonates77; the lack of potency and functionality of antibodies found naturally in unvaccinated humans may have contributed to this outcome. A ClfA monoclonal antibody (mAb) (tefibazumab, Aurexis; Inhibitex)78 was evaluated in phase 2 trial as an adjunct to standard therapy for adult patients with S. aureus bacteraemia also failed to demonstrate any significant differences in time to recovery with the mAb and antibiotic treatment compared to antibiotic treatment alone. It is noteworthy that tefibazumab was a humanized antibody derived from Ab12-9. The body of the preclinical data was demonstrated with Ab12-9 and so it cannot be conclusively assumed that the biological properties for Ab12-9 are the same as tefibaxumab. MAbs in general have the limitation that they only recognize a single epitope. The difference between a polyclonal antibody preparation and a monoclonal one was exemplified by Hawkins et al59 who demonstrated that polyclonal antibodies generated by an early formulation of SA4Ag in humans, were more potent than Ab12-9 at preventing S. aureus cells from binding to fibrinogen thus the lack of efficacy of the MAbs together with a lack of demonstrating bacterial killing in clinical studies may not be surprising retrospectively.
Manganese transporter c (MNTC)
A primary host defense mechanism against bacterial invasion is the sequestration of metal ions that are essential for bacterial survival. Like other bacteria, S. aureus has developed approaches to rapidly scavenge divalent cations like manganese and iron from the host when the bacterium establishes an infection. MntC is a highly conserved (>98% sequence identity) lipoprotein that is the surface-exposed metal binding subunit of MntABC, a heterotrimeric membrane transporter responsible for the acquisition of manganese.55–57 As a cofactor for a number of diverse enzymes, manganese plays important roles in bacterial metabolism, cell wall synthesis, and virulence. Most notably, it is the sole cofactor for superoxide dismutase enzymes, which inactivate reactive oxygen species generated during the oxidative burst in the phagosome of activated macrophages and neutrophils.79–81 Therefore, antibodies that target MntC have the potential to interfere with two critical S. aureus virulence mechanisms: nutrient acquisition and phagosome survival. MntC has been proposed as a potential vaccine candidate due to early expression in infection and its ability to provide protection in preclinical models of staphylococcal infection.55,82 In a study evaluating S. aureus antigens, IgG levels against 27 S. aureus antigens, including MntC (SA0688), were significantly elevated in bacteremia patients compared to controls indicating that MntC is an immunogenic and ubiquitously expressed antigen. The in vivo expression and antibody characterization data generated by our group and others provide a plausible mechanism of protection afforded by MntC antigen where anti-MntC antibodies deprive S. aureus of the ability to sequester manganese and thus make the bacteria more vulnerable to oxidative stress and killing by neutrophils (neutrophil respiratory burst) and macrophages.55–57,76
SA4AG clinical development
Phase 1/2a studies
The first clinical study in the SA4Ag program was initiated in January 2010, and demonstrated the safety and immunogenicity of a first-generation 3-antigen vaccine (SA3Ag) containing CP5 and CP8 conjugates and ClfA.83,84 Clinical development of SA4Ag including rP305A (MntC) began in August 2011 following preclinical studies demonstrating that the vaccine was efficacious in sepsis, bloodstream infection and implanted device animal models.55,69,75,85 A listing of completed and planned clinical studies is shown in Table 1. In February 2014, the FDA granted Fast Track designation for SA4Ag. Results from the completed Phase 1/Phase 2a clinical trials conducted in healthy volunteers in the United States confirmed that a single dose of SA4Ag elicited rapid and robust production of functional antibodies against the 4 vaccine antigens (CP5-CRM197, CP8-CRM197, ClfA, and MntC) and had an acceptable safety profile in healthy subjects 18 through 85 years of age.65,66,86 Both younger and older adults responded to the vaccine with an anamnestic-like response, which was anticipated given that humans are being exposed to S. aureus antigens since birth. Persistent immune responses were observed through 36 months after a single vaccination (data pending publication).87
Table 1.
SA4Ag clinical development plan.
| Study Number/Subject Age | Study Description | Study Design and Type of Control | Healthy Subjects Receiving SA4Ag Final Formulation | SA4Ag-Vaccinated Subjects Undergoing Surgery | Placebo | Study Status |
|---|---|---|---|---|---|---|
| B3451001 Healthy nonsurgical adults 18-< 65 years of age |
Dose escalation; safety and immunogenicity |
Phase 1/2a, multicenter, randomized, placebo-controlled, double-blind, sponsor-unblinded | 112 | 0 | 112 | Completed |
| B3451011 Healthy nonsurgical adults 65-< 86 years of age |
Dose escalation; safety and immunogenicity |
Phase 1/2a, multicenter, randomized, placebo-controlled, double-blind, sponsor-unblinded | 57 | 0 | 60 | Completed |
| B3451014 Healthy nonsurgical adults 18-< 86 years of age |
Antibody persistence up to 36 months after vaccination | Phase 2a, multicenter, open-label | Subjects from 1001/1011 | 0 | Subjects from 1001/1011 | Completed |
| B3451015 (CTM Resupply) Healthy nonsurgical adults 18-< 65 years of age |
Safety and immunogenicity of investigational CTM for STRIVE | Phase 1, single-arm, open-label | 100 | 0 | 0 | Completed |
| B3451003 (FIH in Japan); Clinicaltrials.gov: NCT02492958 Healthy nonsurgical adults 20-< 86 years of age |
Safety and immunogenicity in Japanese subjects | Phase 1/2a, placebo-controlled, randomized, double-blind, sponsor-unblinded | 68 | 0 | 68 | Completed |
| B3451002 (STRIVE)/ Adults 18-< 86 years of age scheduled to undergo elective, open posterior, spinal fusion procedures with multilevel instrumentation |
Efficacy and safety | Phase 2b/3a, randomized, placebo-controlled, double-blind | 0 | 3000 | 3000 | Ongoing |
| B3451006 Healthy nonsurgical adults 18-< 50 years of age |
Clinical lot consistency | Phase 3 | 2061 | 0 | 687 | Planned |
| Total Number | 2398 | 3000 | 3927 |
Abbreviations: CTM = clinical trial material (ie, investigational SA4Ag); FIH = first in human.
aPfizer plans to convert STRIVE to a pivotal Phase 3 study.
Selection of the population for initial efficacy evaluation
A population of patients undergoing elective open posterior multilevel instrumented spinal fusion was chosen for the clinical efficacy and safety evaluation of SA4Ag in the prevention of invasive S. aureus disease. This population was chosen as it is a stringent and well-defined orthopedic surgery subpopulation. Patients undergoing these procedures typically have a competent immune system (including those with comorbidities such as diabetes, obesity, vascular disease, and other non-immunocompromising conditions) who can be vaccinated prior to surgery with a known and defined time period of infection risk. Similar to patients undergoing other elective orthopedic surgeries, the period of risk for S. aureus infection is initiated by the surgical site incision and maintained while the wound is open. In addition, this population has a relatively high (~ 1.5%) and predictable incidence of invasive S. aureus disease, with the majority of SSIs occurring within 180 days of surgery, and 75–90% of infections occurring within 90 days of surgery.88–90 This allows for observation of invasive S. aureus clinical endpoints within a defined period of time.
Figure 1 illustrates that the optimal timing for vaccination with SA4Ag is 10 to 60 days before surgery based on the immune response profile elicited. The vaccination time window ensures the induction of robust functional antibodies to high levels at the time of incision and at the surgical site (ie, tissue, fascia, and joints). In healthy non-surgical subjects, antibody levels were shown to persist beyond the 180-day period of infection risk and remain elevated above baseline or placebo responses for up to 3 years after initial vaccination.
Figure 1.
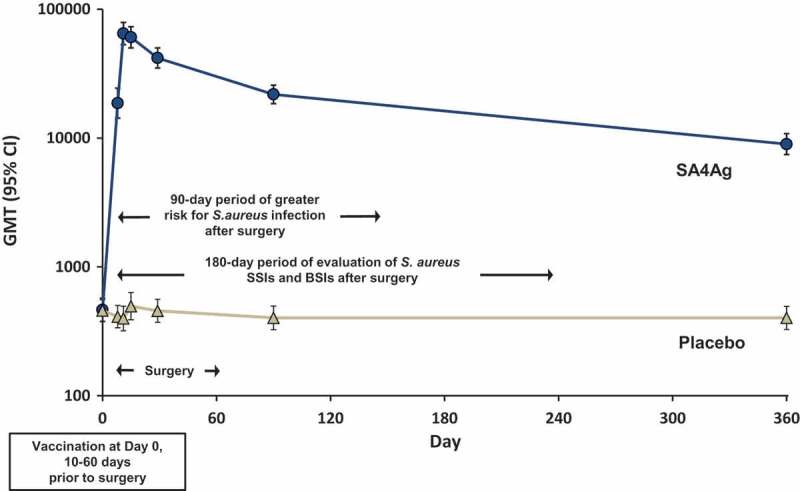
CP5 antibody levels measured by opsonophagocytic assay after SA4Ag vaccination and the risk period for S. aureus infection after surgery.
Abbreviations: BSIs = bloodstream infections; CP5 = S. aureus capsular polysaccharide serotype 5; GMT = geometric mean titer; SA4Ag = S. aureus 4-antigen vaccine; SSIs = surgical-site infections.Note: Graph represents GMTs (95% CI) for CP5 in healthy adult subjects 18 through 64 years of age. Arrows illustrate the window of time for vaccination, surgery, maximum risk of infection, and efficacy endpoint evaluation in patients included in STRIVE.Reprinted from “Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): Results from a first-in-human randomised, placebo-controlled phase 1/2 study”, Frenck RW, Creech CB, Sheldon EA, et al. Vaccine; 2017:375–384, with permission from Elsevier.
Sa4ag phase 2b/3 study design
Pfizer identified an elective orthopedic surgical subpopulation (patients undergoing elective open multilevel instrumented spinal fusion) that, while representative of other orthopedic surgical populations, has S. aureus infection rates at the higher end of the spectrum for orthopedic surgery populations.
The STaphylococcus aureus suRgical Inpatient Vaccine Efficacy (STRIVE) study (NCT 02388165) is a double-blind, placebo-controlled study evaluating the safety and efficacy of SA4Ag administered to adults 18 through 85 years of age undergoing elective open posterior multilevel instrumented spinal fusion surgery (index surgical procedure). Subjects are randomized in a 1:1 ratio to receive a single dose of SA4Ag or placebo 10 to 60 days prior to undergoing the index surgical procedure. From the time of consent, subjects are monitored for vaccine reactogenicity for 10 days after vaccination, all adverse events (AEs) through 6 weeks after the index surgery, and serious adverse events (SAEs) and newly diagnosed chronic medical disorders through Day 180 after the index surgery at 6 scheduled study visits. In addition, STRIVE includes pre-defined criteria to prospectively monitor and independently evaluate multiple organ failure and deaths following surgery. These comprehensive safety assessments have been included as a precaution due to the safety signal observed in the phase 3 study of the IsdB-based vaccine.45
STRIVE was initiated in July 2015, and enrollment and vaccination is ongoing at ~ 100 sites in the United States, United Kingdom, France, Spain, Germany, Hungary, Austria, Sweden, Canada, and Japan. The timing of the final efficacy assessment is based on case-accrual in STRIVE. It is estimated that approximately 6000 subjects will be needed to reach the number of cases required to evaluate vaccine efficacy.
To evaluate vaccine efficacy, subjects are monitored for occurrence of protocol-defined infections, including bloodstream infections (BSI), SSIs, and other invasive S. aureus infections, for 180 days after surgery, at each visit after the index surgical procedure. All protocol-defined infections undergo adjudication by an independent external event adjudication committee (EAC) that includes infectious disease physicians and surgeons with specialized expertise in SSIs. Protocol-defined infections caused by other organisms are also referred to the EAC and adjudicated. Subjects with EAC-confirmed postoperative S. aureus BSI and/or deep incisional or organ/space SSIs occurring within 90 days after the index surgical procedure contribute to the primary efficacy endpoint analysis. The STRIVE study design is outlined in Figure 2.
Figure 2.
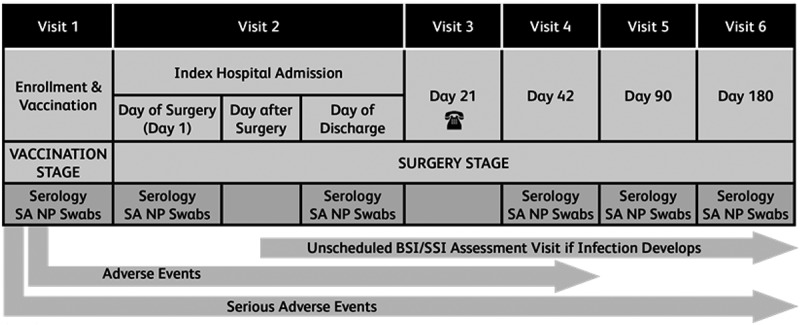
Summary of STRIVE study design.
Abbreviations: SA NP = Staphylococcus aureus Nasal and Pharyngeal
Generalizability of strive study results to all elective orthopedic surgical populations
The STRIVE study population is representative of other elective orthopedic surgical populations, with multiple commonalities in pathophysiology of infection, patient demographics, and surgical procedures. The risk factors for developing an infection are similar across elective orthopedic surgeries and include patient-related factors (eg, smoking, health status, and comorbidities) and procedure-related factors (eg, duration of surgery, involvement of similar anatomical structures, use of implanted instrumentation, and perioperative care).23,91–102 The advantage of evaluating vaccine efficacy in the STRIVE population is that the infection rates are at the higher end of the spectrum for elective orthopedic surgeries. This is primarily due to these surgeries being of longer duration93,97 with longer incisions compared to other elective orthopedic procedures.100,101
Similar pathophysiology of S. aureus ssis in elective orthopedic surgeries
For most elective orthopedic surgeries, the primary risk for establishing infection is during the surgical procedure itself (from the time of incision to wound closure), when bacteria can enter an otherwise normally sterile site.103,104 This is supported by data on the timely perioperative administration of prophylactic antibiotics that can significantly reduce SSIs across the surgical spectrum, whereas postoperative prophylactic antibiotic use has limited utility.105–107 The risk of wound inoculation is higher in patients colonized with S. aureus, yet the likelihood of colonization is independent of surgical procedure.
Additionally, the early pathophysiology of S. aureus SSI is similar across elective orthopedic surgical procedures, and specific strains are not linked to specific surgery types.4,108,109 Across all elective procedure types, early virulence factors, such as those targeted in SA4Ag, are required for S. aureus to initiate and maintain infection.60 Immediately upon entering the surgical site, S. aureus upregulate expression of genes to adapt to the wound microenvironment and avoid immune-mediated killing.110–112 Upregulation of capsular polysaccharides that help the bacteria to evade neutrophil-mediated killing (such as CP5 and CP8),52,53,58,111 tissue adhesion factors (such as ClfA), or bacterial proteins to obtain essential nutrients limited in the host microenvironment (such as MntC) are observed early in the infection process.76,111,112 Blocking these early virulence mechanisms should preclude the establishment of a productive infection and bacterial adhesion to host proteins on implant surface, subsequent biofilm formation and dissemination.
The association between S. aureus nasal carriage and S. aureus infection risk at surgical sites is well established.108,113–115 However, no evidence exists linking specific S. aureus strains to specific surgical procedures that would suggest a strain- or surgery-associated pathogenesis. Various S. aureus clonal types have been isolated from SSIs irrespective of surgery type. In situations where hospitals periodically have disease outbreaks caused by specific S. aureus isolates, these infections are not limited to particular surgical procedures.116,117
Rather, disease outbreaks are linked to a patient coming into contact with the outbreak strain either through carriage or from an exogenous source.4,108,109 Thus, both the accessibility to a previously sterile site during surgery and the presence of S. aureus during the surgery are prerequisites of SSIs, but not the type of elective surgery or the S. aureus strain.
Similar immune responses across elective orthopedic surgeries
The efficacy to be demonstrated in STRIVE is expected to be translatable to other elective orthopedic surgical sites including other anatomical joints and bone spaces, since these sites are accessible to immune responses elicited by SA4Ag. The various orthopedic surgical sites (eg, spine, knee, hip,) are sterile under normal circumstances (no exposure to pathogens) but have full access to the human immune repertoire. Vasculature is found in all bones throughout the body, and joints are drained by lymphatics. Both vasculature and lymph ensure that bones and joints are connected to and protected by the immune system. Therefore, the components required for the proposed mechanism of action of SA4Ag (induction of functional antibodies and host phagocytes that kill the bacteria) are available at these different anatomical sites.118
Similar risk factors across elective orthopedic surgeries
While the risk of postoperative invasive S. aureus disease is directly attributable to the surgical incision and duration of surgery, numerous preoperative, intraoperative, and postoperative patient and procedural risk factors influence risk of developing SSIs. The risk factors for SSI are common among elective open posterior multilevel instrumented spinal fusion surgical and other orthopedic surgical populations.88,92,119–121
Patient demographics for the spinal fusion population are broadly representative of other elective orthopedic surgical populations (Table 2).93,102 Patient risk factors are largely driven by the health status of the patient. The important patient-related risk factors for SSI are similar in patients undergoing open posterior multilevel instrumented spinal fusion surgery and other elective orthopedic surgeries and include age (>60 yrs), high BMI, diabetes, and smoking status.122 The percentage of patients with these risk factors and other comorbidities (ie, chronic obstructive pulmonary disease, congestive heart failure) are also similar across elective orthopedic surgical populations.93,100–102 Patients undergoing spinal surgery and other elective orthopedic surgeries have a similar Charlson Comorbidity Index (a validated prognostic indicator for factors that increase the risk of short-term mortality) affirming the similarity of the prevalence of comorbidities and the overall general health status among these populations.93,100–102 Additionally, rates of S. aureus nasal carriage, which has a well-established association with postoperative SSIs, are similar in patients undergoing spinal procedures and other orthopedic surgeries.22,52,123
Table 2.
Patient demographics and risk factors are similar across elective orthopedic surgical populations.
| Primary Total Knee Arthroplasty |
Primary Total Hip Arthroplasty |
Spinal Fusion |
|
|---|---|---|---|
| N = 15,157a | N = 7791a | N = 9719b | |
| Age (mean, years) | 67.3 | 65.4 | 56.7 |
| Male (%) | 35.5 | 44.3 | 46.2 |
| White (%) | 79.3 | 80.5 | 82.7 |
| Diabetes (%) | 18.2 | 11.6 | 15.1 |
| Smoking (%) | 8.6 | 13.8 | 26.4 |
| COPD (%) | 3.7 | 4.5 | NR |
| Congestive heart failure (%) | 0.2 | 0.5 | NR |
| Peripheral vascular disease (%) | 0.6e | 0.5f | NR |
| BMI (kg/m2) [SD] | 32.8 [7.3]e | 29.8 [6.5]f | NR |
| BMI >30 (%) | NR | NR | 42.9 |
| ASA: 1–2 (%) | 51.0e,g | 56.8f | 56.4h |
| ASA: 3–4 (%) | 48.9e,g | 43.2f | 43.6h |
Abbreviations: ACS NSQIP = The American College of Surgeons National Surgical Quality Improvement Program; ASA = American Society of Anesthesiologists (adopted a 5-category physical score); BMI = body mass index; COPD = chronic obstructive pulmonary disease; NR = not reported; SD = standard deviation.
aACS NSQIP: 2005–2010102
b70% lumbar spine; 66% posterior/posteriorlateral approach; 90% single level93
eThe total population was N = 27,745.128
fThe total population varied: BMI N = 17,514; peripheral vascular disease/ASA N = 17,628.100
Procedural risk factors for postoperative infection include duration of surgery, wound characteristics, involvement of similar anatomical structures (eg, bone, cartilage, and joint spaces with synovial fluid), use of implanted devices and blood transfusions, and perioperative care (Table 3).91–98 Surgical techniques and procedural characteristics for elective open posterior multilevel instrumented spinal fusion surgeries have numerous commonalities shared with other elective orthopedic surgeries. Each of these surgical procedures involves disruption of the dermis, soft tissue, fascial and muscle layers, and bone, allowing possible introduction of infection through the wound. The recommended perioperative care is the same for patients undergoing spinal surgery and other orthopedic surgeries.124 The wounds of >93% of these surgeries are classified as clean or clean/contaminated.125,126 Many of these surgeries have similar median durations, with spinal surgery having the longest median duration, which corresponds to infection rates at the higher end of the range for elective orthopedic surgeries.93,100,101 Many spinal surgeries and other major elective orthopedic procedures such as hip and knee arthroplasties commonly use implanted materials composed of titanium or cobalt chromium alloys, plastics, and stainless steel. In addition, the rate of postsurgical complications and mortality after these procedures is low (0.18 to 0.35%). Complications such as pulmonary embolism and myocardial infarction are comparable between hip and knee replacements and spinal surgery (Table 4).93,100,127
Table 3.
Procedural characteristics of spinal and other elective orthopedic surgeries.
| Total Knee Arthroplasty | Total Hip Arthroplasty | Instrumented Spinal Surgery | |
|---|---|---|---|
| OR time (mean minutes [SD]) | 96.9 (37.9)a | 97.6 (42.9)b | 196.6 (SD not provided)c |
| Incision length (mean [in]) |
~ 8–10 | ~ 8–12 | ~ 10–12 inches (larger incision if more vertebrae fused)d |
| Most common approaches | medial parapatella | anterior (lateral); posterior | PLIF, TLIF |
| Procedural overview | dermis → dissects between the muscles, tendons, and nerves to reach the joint | dermis → dissects between the muscles, tendons, and nerves to reach the joint | dermis → dissects between the muscles, tendons, and nerves to reach the vertebrae |
| Implant material | metal alloys (titanium or cobalt-chromium); plastics (ultra-high molecular weight polyethylene); ceramic; bone cement | plastic (polyethylene liner); metals (cobalt/chromium); ceramic; bone cement | plastics (PEEK), metals (titanium, stainless steel, cobalt); bone graft (autograft/allograft/BMP); bone cement |
Abbreviations: ACS NSQIP = The American College of Surgeons National Surgical Quality Improvement Program; BMP = bone morphogenetic protein; in = inches; OR = operating room; PEEK = polyetheretherketone; PLIF = posterior lumbar interbody fusion; SD = standard deviation; TLIF = transforaminal lumbar interbody fusion.
aACS NSQIP: 2005–2011128
bACS NSQIP: 2006–2011100
cACS NSQIP: 2005–201193
dIncision length calculated based on data available on the AAOS website regarding spinal surgery (http://orthoinfo.aaos.org/topic.cfm?topic=A00543) and adjusted for the mean vertebrae fused in STRIVE to date (5.1 vertebrae).
Table 4.
Similar incidence of 30-day postoperative complications across elective orthopedic surgeries.
| Primary Total Knee Arthroplasty |
Primary Total Hip Arthroplasty |
Spinal Fusion |
|
|---|---|---|---|
| N = 15,321a | N = 17,640b | N = 9719c | |
| Total events (n) | 1058 | 1074 | NR |
| Mortality (%) | 0.18 | 0.35 | 0.35 |
| UTI (%) | 1.49 | 1.45 | 1.96 |
| Superficial wound infection (%) | 0.79 | 0.83 | 1.24 |
| Deep venous thrombosis (%) | 1.34 | 0.51 | 0.90 |
| Postoperative sepsis (%) | 0.44 | 0.47 | 1.08 |
| Pneumonia (%) | 0.37 | 0.42 | 0.84 |
| Pulmonary embolism (%) | 0.78 | 0.31 | NR |
| Myocardial infarction (%) | NR | 0.24 | 0.20 |
| Septic shock (%) | 0.13 | 0.12 | 0.30 |
| Wound dehiscence (%) | 0.27 | 0.14 | 0.35 |
| Cardiac arrest requiring CPR (%) | 0.09 | 0.12 | 0.20 |
| Peripheral nerve injury (%) | 0.10 | 0.11 | 0.23 |
| Acute renal failure (%) | 0.12 | 0.07 | 0.5 |
Conclusions/future directions
As no immune correlate or threshold of protection has been established for S. aureus, a clinical endpoint efficacy study is required for S. aureus vaccine licensure. The ongoing STRIVE study is being conducted in a specific elective orthopedic surgical subpopulation of spinal fusion surgery recipients. Safety and efficacy of SA4Ag demonstrated in the STRIVE population is expected to be representative of the vaccine’s safety and efficacy in other elective orthopedic surgical populations because of the common pathophysiology of invasive S. aureus disease, similar immune function at the surgical incision, wound, and in synovial fluid across orthopedic surgical sites, and similar patient and procedural risk factors for developing postoperative SSIs across these elective surgical populations. Therefore, assuming STRIVE is successful at achieving its pre-specified safety and efficacy endpoints, the data should be representative of safety and efficacy in other orthopedic populations and support the licensure of SA4Ag for use in adults aged 18 years and older who are undergoing elective orthopedic surgery.
Following licensure, as with other vaccines, it is expected that national Vaccine Technical Committees will make recommendations for the use of SA4Ag to maximize the public health benefit of vaccine implementation. To provide an estimate of the potential public health impact of an effective S. aureus vaccine on the prevention of S. aureus infections after elective orthopedic surgeries, Pfizer conducted an analysis that incorporated epidemiological and clinical data to predict outcomes over a 10-year time horizon from 2021 to 2030. For this analysis, only major elective orthopedic surgeries were considered (projected US annual procedure volumes for major elective orthopedic surgeries are listed in Table 5). Pfizer estimates that if all eligible patients undergoing major elective orthopedic surgery in the 10 year time period received a 70% effective vaccine, vaccination could prevent 127,364 postoperative S. aureus infections, including 48,030 MRSA infections and 62,295 invasive infections (Table 6). Such a reduction in postoperative infections would also avert 2,243 deaths, 97,379 hospitalizations, and 68,742 disability-adjusted life years.
Table 5.
Projected US annual procedure volume for major elective orthopedic surgeries (2021–2030).
| 2021 | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spinal fusion | 549,625 | 561,807 | 574,260 | 586,989 | 599,999 | 613,299 | 626,893 | 640,788 | 654,991 | 669,509 | 6,078,160 |
| Spinal decompression | 329,702 | 337,010 | 344,480 | 352,115 | 359,920 | 367,898 | 376,052 | 384,387 | 392,907 | 401,616 | 3,646,087 |
| Inpatienta | 96,370 | 98,506 | 100,689 | 102,921 | 105,202 | 107,534 | 109,918 | 112,354 | 114,845 | 117,390 | 1,065,730 |
| Outpatienta | 233,332 | 238,504 | 243,790 | 249,194 | 254,717 | 260,363 | 266,134 | 272,033 | 278,063 | 284,226 | 2,580,357 |
| Hip arthroplasty | 534,674 | 546,525 | 558,639 | 571,021 | 583,678 | 596,615 | 609,839 | 623,357 | 637,173 | 651,297 | 5,912,817 |
| Knee arthroplasty | 1,008,230 | 1,030,578 | 1,053,421 | 1,076,770 | 1,100,637 | 1,125,033 | 1,149,970 | 1,175,459 | 1,201,514 | 1,228,145 | 11,149,757 |
| Other arthroplasty | 67,154 | 68,643 | 70,164 | 71,719 | 73,309 | 74,934 | 76,595 | 78,293 | 80,028 | 81,802 | 742,642 |
aSpinal decompressions are often conducted at the inpatient and outpatient settings, which could have an implication on the infection rate; therefore, they were also analyzed separately.
Source: Projected from Life Science Intelligence Report 2015, with adjustment based upon HCUP (2014) data analysis to eliminate overlapping multiple surgeries and emergent surgeries.
Table 6.
Potential US public health impact from a S. aureus vaccine assuming 70% efficacy on prevention of S. aureus infections following major elective orthopedic surgeries (2021–2030).
| Spinal Surgeries |
Arthroplasty |
Spinal Surgery/Arthroplasty Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimated 10-Year Vaccine Impact | Spinal Fusion | Spinal Decompression | Total | Hip Arthroplasty | Knee Arthroplasty | Other Arthroplasty | Total | |
| Surgical procedure volume | 6,078,160 | 3,646,087 | 9,724,247 | 5,912,817 | 11,149,757 | 742,642 | 17,805,216 | 27,529,463 |
| Total number of S. aureus infections averted | 39,569 | 17,214 | 56,783 | 31,870 | 37,463 | 1,248 | 70,581 | 127,364 |
| Total number of MRSA infections averted | 15,377 | 5764 | 21,141 | 12,659 | 13,771 | 459 | 26,889 | 48,030 |
| Total number of ISA infections averted | 19,146 | 8065 | 27,211 | 15,728 | 18,732 | 624 | 35,084 | 62,295 |
| Total number of deaths averted | 938 | 156 | 1094 | 845 | 300 | 5 | 1149 | 2243 |
| Total number of hospitalizations averted | 19,146 | 8065 | 27,211 | 31,456 | 37,464 | 1248 | 70,168 | 97,379 |
| Total number of disability-adjusted life years averted | 29,257 | 5889 | 35,146 | 21,000 | 12,277 | 319 | 33,596 | 68,742 |
Abbreviations: ISA = invasive S. aureus; MRSA = methicillin-resistant S. aureus.
Note: Formulas and data for these calculations are shown in Appendix 3.
Disclosure of potential conflicts of interest
The authors are employees of Pfizer, the company developing SA4Ag for commercial use.
Funding Statement
Funding for this work was provided by Pfizer, Inc.
Acknowledgments
Editorial assistance was provided by Scott Vuocolo Ph.D. (Pfizer).
References
- 1.Noble WC, Valkenburg HA, Wolters CH.. Carriage of Staphylococcus aureus in random samples of a normal population. J Hyg (Lond). 1967;65(4):567–573. PMC2130396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J Infect Dis. 2006;193(2):172–179. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 3.Control ECfDPa Antimicrobial resistance surveillance in Europe 2014. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm (Sweden): ECDC; 2015. [Google Scholar]
- 4.Levy PY, Ollivier M, Drancourt M, Raoult D, Argenson JN. Relation between nasal carriage of Staphylococcus aureus and surgical site infection in orthopedic surgery: the role of nasal contamination. A systematic literature review and meta-analysis. Orthopaedics Traumatology: Surg Res. 2013;99(6):645–651. doi: 10.1016/j.otsr.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Foster TJ. Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet Dermatol. 2009;20(5–6):456–470. doi: 10.1111/j.1365-3164.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DJ, Pyatt DG, Weber DJ, Rutala WA. North Carolina department of public health HAIAG. Statewide costs of health care-associated infections: estimates for acute care hospitals in North Carolina. Am J Infect Control. 2013;41(9):764–768. doi: 10.1016/j.ajic.2012.11.022 PMC3724767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis SS, Moehring RW, Chen LF, Sexton DJ, Anderson DJ. Assessing the relative burden of hospital-acquired infections in a network of community hospitals. Infect Control Hosp Epidemiol. 2013;34(11):1229–1230. doi: 10.1086/673443 PMC3977691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 9.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801 PMC4648343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson DJ, Podgorny K, Berrios-Torres SI, Bratzler DW, Dellinger EP, Greene L, Nyquist AC, Saiman L, Yokoe DS, Maragakis LL, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627. doi: 10.1086/676022 PMC4267723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suaya JA, Mera RM, Cassidy A, O’Hara P, Amrine-Madsen H, Burstin S, Miller LG. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis. 2014;14:296. doi: 10.1186/1471-2334-14-296 PMC4060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner J, Padley W, Davey S, Murphy K, Brown B. Patient narratives of surgical site infection: implications for practice. J Hosp Infect. 2013;83(1):41–45. doi: 10.1016/j.jhin.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Moore AJ, Blom AW, Whitehouse MR, Gooberman-Hill R. Deep prosthetic joint infection: a qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open. 2015;5(12):e00949510. doi: 10.1136/bmjopen-2015-009495 1136/bmjopen-2015-009495 PMC4679895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelhorn HL AS, Parvizi J, et al. The burden of illness associated with post-operative infections: the patient perspective. 26th Musculoskeletal Infection Society Annual Open Scientific Meeting; Autust 5-6, 2016; Charlotte, NC2016. [Google Scholar]
- 15.Kuhns BD, Lubelski D, Alvin MD, Taub JS, McGirt MJ, Benzel EC, Mroz TE. Cost and quality of life outcome analysis of postoperative infections after subaxial dorsal cervical fusions. J Neurosurg Spine. 2015;22(4):381–386. doi: 10.3171/2014.10.SPINE14228. [DOI] [PubMed] [Google Scholar]
- 16.Patel H, Khoury H, Girgenti D, Welner S, Yu H. Burden of surgical site infections associated with arthroplasty and the contribution of staphylococcus aureus. Surg Infect (Larchmt). 2016;17(1):78–88. doi: 10.1089/sur.2014.246. [DOI] [PubMed] [Google Scholar]
- 17.Patel H, Khoury H, Girgenti D, Welner S, Yu H. Burden of surgical site infections associated with select spine operations and involvement of staphylococcus aureus. Surg Infect (Larchmt). 2017;18(4):461–473. doi: 10.1089/sur.2016.186 PMC5466015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiser MC, Moucha CS. The current state of screening and decolonization for the prevention of staphylococcus aureus surgical site infection after total hip and knee arthroplasty. Jbjs. 2015;97(17):1449–1458. doi: 10.2106/jbjs.n.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster J, Osborne S. Pre-operative bathing or showering with skin antiseptics to reduce surgical site infection. Cochrane Database Syst Rev. 4;2004. doi: 10.1002/14651858.CD004985 [DOI] [Google Scholar]
- 20.Bode LGM, Kluytmans JAJW, Wertheim HFL, Bogaers D, Vandenbroucke-Grauls CMJE, Roosendaal R, Troelstra A, Box ATA, Voss A, van der Tweel I, et al. Preventing surgical-site infections in nasal carriers of staphylococcus aureus. New England J Med. 2010;362(1):9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 21.Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, Bogaers-Hofman D, de Baere GAJ, Stuurman A, van Belkum A, Kluytmans JAJW. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35(4):353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 22.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA, Loreen A. Intranasal mupirocin to prevent postoperative staphylococcus aureus infections. New England J Med. 2002;346(24):1871–1877.doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberger LH, Politano AD, Sawyer RG. The surgical care improvement project and prevention of post-operative infection, including surgical site infection. Surg Infect (Larchmt). 2011;12(3):163–168. doi: 10.1089/sur.2010.083 4702424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen N, Yegiyants S, Kaloostian C, Abbas MA, Difronzo LA. The surgical care improvement project (SCIP) initiative to reduce infection in elective colorectal surgery: which performance measures affect outcome? Am Surg. 2008;74(10):1012–1016. [DOI] [PubMed] [Google Scholar]
- 25.Cataife G, Weinberg DA, Wong HH, Kahn KL. The effect of surgical care improvement project (SCIP) compliance on surgical site infections (SSI). Med Care. 2014;52(2Suppl 1):S66–73. doi: 10.1097/MLR.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 26.Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SRLJ, McDougall C, Wilcox MH. epic2: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2007;65:.S1–S59. doi: 10.1016/S0195-6701(07)60002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, Browne J, Prieto J, Wilcox M. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86:.S1–S70. doi: 10.1016/S0195-6701(13)60012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez J W, Peyrani P, et al, editor Number of deaths in patients hospitalized with community-acquired pneumonia (CAP) in the Unites States: the University of Louisville Pneumonia Study. 10th International Symposium of Pneumococci and Pneumococcal Disease; 2016. June 26-30, 2016; Glasgow, Scotland. [Google Scholar]
- 29.Tanner J, Kiernan M, Hilliam R, Davey S, Collins E, Wood T, Ball J, Leaper D. Effectiveness of a care bundle to reduce surgical site infections in patients having open colorectal surgery. Ann R Coll Surg Engl. 2016;98(4):270–274. doi: 10.1308/rcsann.2016.0072 PMC5226025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 31.Bosco JA, Prince Rainier RT, Catanzano AJ, Stachel AG, Phillips MS. Expanded Gram-negative antimicrobial prophylaxis reduces surgical site infections in hip arthroplasty. J Arthroplasty. 2016;31(3):616–621. doi: 10.1016/j.arth.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 32.Fritz SA, Hogan PG, Camins BC, Ainsworth AJ, Patrick C, Martin MS, Krauss MJ, Rodriguez M, Burnham CA. Mupirocin and chlorhexidine resistance in Staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother. 2013;57(1):559–568. doi: 10.1128/AAC.01633-12 PMC3535967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caffrey AR, Quilliam BJ, LaPlante KL. Risk factors associated with mupirocin resistance in meticillin-resistant Staphylococcus aureus. J Hosp Infect. 2010;76(3):206–210. doi: 10.1016/j.jhin.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Fritz SA, Hogan PG, Camins BC, Ainsworth AJ, Patrick C, Martin MS, Krauss MJ, Rodriguez M, Burnham C-AD. Mupirocin and chlorhexidine resistance in staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother. 2013;57(1):559–6810. doi: 10.1128/AAC.01633-12 1128/AAC.01633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br. 2008;90(7):915–919. doi: 10.1302/0301-620X.90B7.20498. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg AD, Wambold D, Kraemer L, Begley-Keyes M, Zuckerman SL, Singh N, Cohen MM, Bennett MV. Ensuring appropriate timing of antimicrobial prophylaxis. J Bone Joint Surg Am. 2008;90(2):226–232. doi: 10.2106/JBJS.G.00297. [DOI] [PubMed] [Google Scholar]
- 37.Enzler MJ, Berbari E, Osmon DR. Antimicrobial prophylaxis in adults. Mayo Clin Proc. 2011;86(7):686–701. doi: 10.4065/mcp.2011.0012 PMC3127564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernard HR, Cole WR. The prophylaxis of surgical infection: the effect of prophylactic antimicrobial drugs on the incidence of infection following potentially contaminated operations. Surgery. 56;1964:151–157. [PubMed] [Google Scholar]
- 39.Campbell PC. Large doses of penicillin in the prevention of surgical wound infection. Lancet. 1965;2(7417):805–810. [DOI] [PubMed] [Google Scholar]
- 40.Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 50;1961:161–168. [PubMed] [Google Scholar]
- 41.Fogelberg EV, Zitzmann EK, Stinchfield FE. Prophylactic penicillin in orthopaedic surgery. J Bone Joint Surg Am. 1970;52(1):95–98. [PubMed] [Google Scholar]
- 42.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. Use of a staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. New England J Med. 2002;346(7):491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 43.Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: phase III randomized study. Hum Vaccin Immunother. 2015;11(3):632–641. doi: 10.4161/hv.34414 PMC4514248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durando P, Faust SN, Fletcher M, Krizova P, Torres A, Welte T. Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults* *Based on presentations given at the integrated symposium organized and funded by Pfizer international operations during the 22nd European congress of clinical microbiology and infectious diseases (ECCMID) 31 March to 3 April 2012, London, UK. Clin Microbiol Infect. 2013;19:.1–9. doi: 10.1111/1469-0691.12320. [DOI] [PubMed] [Google Scholar]
- 45.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309(13):1368–1378. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 46.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine. 2013;31(25):2723–2730. doi: 10.1016/j.vaccine.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Lu T, Porter AR, Kennedy AD, Kobayashi SD, DeLeo FR. Phagocytosis and killing of staphylococcus aureus by human neutrophils. J Innate Immun. 2014;6(5):639–649. doi: 10.1159/000360478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zorman JK, Esser M, Raedler M, Kreiswirth BN, Ala’aldeen DAA, Kartsonis N, Smugar SS, Anderson AS, McNeely T, Arduino JM. Naturally occurring IgG antibody levels to the Staphylococcus aureus protein IsdB in humans. Hum Vaccin Immunother. 2013;9(9):1857–1864. doi: 10.4161/hv.25253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Middleton JR. Staphylococcus aureus antigens and challenges in vaccine development. Expert Rev Vaccines. 2008;7(6):805–815. doi: 10.1586/14760584.7.6.805. [DOI] [PubMed] [Google Scholar]
- 50.Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012;30(19):2921–2927. doi: 10.1016/j.vaccine.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Schaffer AC, Lee JC. Staphylococcal Vaccines and Immunotherapies. Infect Dis Clin North Am. 2009;23(1):153–171. doi: 10.1016/j.idc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Thakker M, Park J-S, Carey V, Lee JC. Staphylococcus aureus Serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun. 1998;66(11):5183–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, Scully IL, McNeil LK, Aste-Amezaga JM, Cooper D, et al. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother. 2013;9(3):480–487. PMC3891703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAdow M, Kim HK, Dedent AC, Hendrickx AP, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011;7(10):e1002307. doi: 10.1371/journal.ppat.1002307 PMC3197598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, Nunez L, Carriere M, Singer C, Dilts DA, et al. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis. 2012;205(11):1688–1696. doi: 10.1093/infdis/jis272 PMC3348682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gribenko A, Mosyak L, Ghosh S, Parris K, Svenson K, Moran J, Chu L, Li S, Liu T, Woods VL Jr., et al. Three-dimensional structure and biophysical characterization of Staphylococcus aureus cell surface antigen-manganese transporter MntC. J Mol Biol. 2013;425(18):3429–3445. doi: 10.1016/j.jmb.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 57.Gribenko AV, Parris K, Mosyak L, Li S, Handke L, Hawkins JC, Severina E, Matsuka YV, Anderson AS. High resolution mapping of bactericidal monoclonal antibody binding epitopes on staphylococcus aureus antigen MntC. PLoS Pathog. 2016;12(9):e1005908. doi: 10.1371/journal.ppat.1005908 PMC5045189 following competing interests: AVG, KP, LM, LH, ES, YVM, JCH and ASA are current or former employees of Pfizer Inc and as such may own company stock. SL has no competing interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17(1):218–34321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawkins J, Kodali S, Matsuka YV, McNeil LK, Mininni T, Scully IL, Vernachio JH, Severina E, Girgenti D, Jansen KU, et al. A recombinant clumping factor A-containing vaccine induces functional antibodies to Staphylococcus aureus that are not observed after natural exposure. Clin Vaccine Immunol. 2012;19(10):1641–1650. doi: 10.1128/CVI.00354-12 PMC3485874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, Jansen KU. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother. 2012;8(11):1585–1594. doi: 10.4161/hv.21872 PMC3601133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nanra JS, Timofeyeva Y, Buitrago SM, Sellman BR, Dilts DA, Fink P, Nunez L, Hagen M, Matsuka YV, Mininni T, et al. Heterogeneous in vivo expression of clumping factor A and capsular polysaccharide by Staphylococcus aureus: implications for vaccine design. Vaccine. 2009;27(25–26):3276–3280. doi: 10.1016/j.vaccine.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 62.Lattar SM, Noto Llana M, Denoel P, Germain S, Buzzola FR, Lee JC, Sordelli DO. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun. 2014;82(1):83–91. doi: 10.1128/IAI.01050-13 PMC3911846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JC, Park JS, Shepherd SE, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65(10):4146–4151. PMC175596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fattom AI, Sarwar J, Ortiz A, Naso RA. Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64(5):1659–1665. PMC173976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Begier E, Seiden DJ, Patton M, Zito E, Severs J, Cooper D, Eiden J, Gruber WC, Jansen KU, Anderson AS, et al. SA4Ag, a 4-antigen Staphylococcus aureus vaccine, rapidly induces high levels of bacteria-killing antibodies. Vaccine. 2017;35(8):1132–1139. doi: 10.1016/j.vaccine.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 66.Frenck RW, Creech CB, Sheldon EA, Seiden DJ, Kankam MK, Baber J, Zito E, Hubler R, Eiden J, Severs JM, et al. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine. 2017;35(2):375–384. doi: 10.1016/j.vaccine.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Creech CB, Frenck RW, Sheldon EA, Seiden DJ, Kankam MK, Zito ET, Girgenti D, Severs JM, Immermann FW, McNeil LK, et al. Safety, tolerability, and immunogenicity of a single dose 4-antigen or 3-antigen Staphylococcus aureus vaccine in healthy older adults: results of a randomised trial. Vaccine. 2017;35(2):385–394. doi: 10.1016/j.vaccine.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 68.Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, Scully IL, McNeil LK, Aste-Amézaga JM, Cooper D, et al. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother. 2013;9(3):480–487. doi: 10.4161/hv.23223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scully IL, Pavliak V, Timofeyeva Y, Liu Y, Singer C, Anderson AS. O-Acetylation is essential for functional antibody generation against Staphylococcus aureus capsular polysaccharide. Hum Vaccin Immunother. 2018;14(1):81–84. doi: 10.1080/21645515.2017.1386360 PMC5791590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDevitt D, Nanavaty T, House‐Pompeo K, Bell E, Turner N, McIntire L, Foster T, Magnus H. Characterization of the interaction between the staphylococcus aureus clumping factor (ClfA) and Fibrinogen. Eur J Biochemistry. 2004;247(1):416–424. doi: 10.1111/j.1432-1033.1997.00416.x. [DOI] [PubMed] [Google Scholar]
- 71.Liu CZ, Shih MH, Tsai PJ. ClfA(221-550), a fibrinogen-binding segment of Staphylococcus aureus clumping factor A, disrupts fibrinogen function. Thromb Haemost. 2005;94(2):286–294. doi: 10.1160/TH05-03-0205. [DOI] [PubMed] [Google Scholar]
- 72.Bayer AS, Sullam PM, Ramos M, Li C, Cheung AL, Yeaman MR. Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect Immun. 1995;63(9):3634–3641. PMC173504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siboo IR, Cheung AL, Bayer AS, Sullam PM. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect Immun. 2001;69(5):3120–3127. doi: 10.1128/IAI.69.5.3120-3127.2001 PMC98267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Josefsson E, Hartford O, O’Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184(12):1572–1580. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 75.Scully IL, Timofeyeva Y, Keeney D, Matsuka YV, Severina E, McNeil LK, Nanra J, Hu G, Liberator PA, Jansen KU, et al. Demonstration of the preclinical correlate of protection for Staphylococcus aureus clumping factor A in a murine model of infection. Vaccine. 2015;33(41):5452–5457. doi: 10.1016/j.vaccine.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 76.Rozemeijer W, Fink P, Rojas E, Jones CH, Pavliakova D, Giardina P, Murphy E, Liberator P, Jiang Q, Girgenti D, et al. Evaluation of approaches to monitor Staphylococcus aureus virulence factor expression during human disease. PloS one. 2015;10(2):e0116945. doi: 10.1371/journal.pone.0116945 PMC4342157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaufman D. Veronate (Inhibitex). Curr Opin Investig Drugs. 2006;7(2):172–179. [PubMed] [Google Scholar]
- 78.Weems JJ Jr., Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, Winston L, John JF, Kubin CJ, Talwani R, et al. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50(8):2751–2755. doi: 10.1128/AAC.00096-06 PMC1538656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003;149(Pt10):2749–2758. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- 80.Clements MO, Watson SP, Foster SJ. Characterization of the major superoxide dismutase of Staphylococcus aureus and its role in starvation survival, stress resistance, and pathogenicity. J Bacteriol. 1999;181(13):3898–3903. PMC93877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10(2):158–164. doi: 10.1016/j.chom.2011.07.004 PMC3157011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect Immun. 2006;74(6):3415–3426. doi: 10.1128/IAI.00392-06 PMC1479260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nissen M, Marshall H, Richmond P, Shakib S, Jiang Q, Cooper D, Rill D, Baber J, Eiden J, Gruber W, et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine. 2015;33(15):1846–1854. doi: 10.1016/j.vaccine.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 84.Marshall H, Nissen M, Richmond P, Shakib S, Jiang Q, Cooper D, Rill D, Baber J, Eiden J, Gruber WC, et al. Safety and immunogenicity of a booster dose of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults: A randomized phase 1 study. J Infect. 2016;73(5):437–454. doi: 10.1016/j.jinf.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 85.Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, Nuñez L, Carriere M, Singer C, Dilts DA, et al. Staphylococcus aureus manganese transport protein C Is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis. 2012;205(11):1688–1696. doi: 10.1093/infdis/jis272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Creech CB, Jr RW F, Sheldon EA, Seiden DJ, Kankam MK, Zito ET, Girgenti D, Severs JM, Immermann FW, McNeil LK, et al. Safety, tolerability, and immunogenicity of a single dose 4-antigen or 3-antigen Staphylococcus aureus vaccine in healthy older adults: results of a randomised trial. Vaccine. 2017;35(2):385–394. doi: 10.1016/j.vaccine.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 87.Frenck RW CC, Fiquet A, et al, on behalf of the B3451014 Study Group. Persistence of immune responses through 36 months after vaccination with a novel Staphylococcus aureus 4-antigen vaccine (SA4Ag). 27th European Congress of Clinical Microbiology and Infectious Diseases; 2017. April 22-25; Vienna, Austria 2017. [Google Scholar]
- 88.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine. 2005;30(12):1460–1465. doi: 10.1097/01.brs.0000166532.58227.4f. [DOI] [PubMed] [Google Scholar]
- 89.Mok JM, Guillaume TJ, Talu U, Berven SH, Deviren V, Kroeber M, Bradford DS, Hu SS. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine (Phila Pa 1976). 2009;34(6):578–583. doi: 10.1097/BRS.0b013e31819a827c. [DOI] [PubMed] [Google Scholar]
- 90.Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, van Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010;19(10):1711–1719. doi: 10.1007/s00586-010-1421-y PMC2989231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for disease control and prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. 1999;27(2):97–132. quiz 3-4; discussion 96. [PubMed] [Google Scholar]
- 92.Lieber B, Han B, Strom RG, Mullin J, Frempong-Boadu AK, Agarwal N, Kazemi N, Tabbosha M. Preoperative predictors of spinal infection within the national surgical quality inpatient database. World Neurosurg. 2016;89:.517–524. doi: 10.1016/j.wneu.2015.12.085. [DOI] [PubMed] [Google Scholar]
- 93.McCutcheon BA, Ciacci JD, Marcus LP, Noorbakhsh A, Gonda DD, McCafferty R, Taylor W, Chen CC, Carter BS, Chang DC. Thirty-day perioperative outcomes in spinal fusion by specialty within the NSQIP database. Spine. 2015;40(14):1122–1131. doi: 10.1097/brs.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 94.Urquhart DM, Hanna FS, Brennan SL, Wluka AE, Leder K, Cameron PA, Graves SE, Cicuttini FM. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: A systematic review. J Arthroplasty. 2010;25(8):1216–22.e3. doi: 10.1016/j.arth.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 95.SM KD Patel, Patel SD, Gupta VM, Vegad VM. Study of risk factors including NNIS risk index in surgical site infections in abdominal surgeries. Gujarat Med J. 2011;66(1):42–45. [Google Scholar]
- 96.Song K-H, Kim ES, Kim YK, Jin HY, Jeong SY, Kwak YG, Cho YK, Sung J, Lee Y-S, Oh H-B, et al. Differences in the risk factors for surgical site infection between total hip arthroplasty and total knee arthroplasty in the korean nosocomial infections surveillance system (KONIS). Infect Control Hosp Epidemiol. 2012;33(11):1086–1093. doi: 10.1086/668020. [DOI] [PubMed] [Google Scholar]
- 97.Veeravagu A, Patil CG, Lad SP, Boakye M. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976). 2009;34(17):1869–1872. doi: 10.1097/BRS.0b013e3181adc989. [DOI] [PubMed] [Google Scholar]
- 98.Molinari RW, Khera OA, Molinari WJ 3rd.. Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J. 2012;21(Suppl 4):S476–82. doi: 10.1007/s00586-011-2104-z PMC3369056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound infection and temperature group. N Engl J Med. 1996;334(19):1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 100.Belmont PJ Jr, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty. 2014;29(10):2025–2030. doi: 10.1016/j.arth.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am. 2014;96(16):1387–1394. doi: 10.2106/JBJS.M.01048. [DOI] [PubMed] [Google Scholar]
- 102.Pugely AJ, Martin CT, Gao Y, Schweizer ML, Callaghan JJ. The incidence of and risk factors for 30-Day surgical site infections following primary and revision total joint arthroplasty. J Arthroplasty. 2015;30(9Suppl):47–50. doi: 10.1016/j.arth.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 103.Rubin RH. Surgical wound infection: epidemiology, pathogenesis, diagnosis and management. BMC Infect Dis. 2006;6:171. doi: 10.1186/1471-2334-6-171 PMC1687193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whyte W, Hambraeus A, Laurell G, Hoborn J. The relative importance of routes and sources of wound contamination during general surgery. I. Non-airborne. J Hosp Infect. 1991;18(2):93–107. [DOI] [PubMed] [Google Scholar]
- 105.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2014;5. doi: 10.1002/14651858.CD001181.pub4 CD001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lador A, Nasir H, Mansur N, Sharoni E, Biderman P, Leibovici L, Paul M. Antibiotic prophylaxis in cardiac surgery: systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(3):541–550. doi: 10.1093/jac/dkr470. [DOI] [PubMed] [Google Scholar]
- 107.Gillespie WJ, Walenkamp GH. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2010;3. doi: 10.1002/14651858.CD000244.pub2 CD000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Munoz P, Hortal J, Giannella M, Barrio JM, Rodriguez-Creixems M, Perez MJ, Rincon C, Bouza E. Nasal carriage of S. aureus increases the risk of surgical site infection after major heart surgery. J Hosp Infect. 2008;68(1):25–31. doi: 10.1016/j.jhin.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 109.McKinnell JA, Miller LG, Eells SJ, Cui E, Huang SS. A systematic literature review and meta-analysis of factors associated with methicillin-resistant Staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol. 2013;34(10):1077–1086. doi: 10.1086/673157 PMC3883507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3(12):948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 111.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48(6):1429–1449. [DOI] [PubMed] [Google Scholar]
- 112.Liu GY. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr Res. 2009;65(5 Pt 2):71R–7R. doi: 10.1203/PDR.0b013e31819dc44d 2919328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kluytmans JA, Mouton JW, Ijzerman EP, Vandenbroucke-Grauls CM, Maat AW, Wagenvoort JH, Verbrugh HA. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis. 1995;171(1):216–219. [DOI] [PubMed] [Google Scholar]
- 114.Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, de Baere GA. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000;21(5):319–323. doi: 10.1086/501763. [DOI] [PubMed] [Google Scholar]
- 115.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344(1):11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 116.Aamot HV, Blomfeldt A, Skramm I, Muller F, Monecke S. Molecular characterisation of methicillin-sensitive Staphylococcus aureus from deep surgical site infections in orthopaedic patients. Official Publ Eur Soc Clin Microbiol. 2012;31(8):1999–2004. doi: 10.1007/s10096-011-1532-3. [DOI] [PubMed] [Google Scholar]
- 117.Skramm I, Fossum Moen AE, Aroen A, Bukholm G. Surgical site infections in orthopaedic surgery demonstrate clones similar to those in orthopaedic staphylococcus aureus nasal carriers. J Bone Joint Surg Am. 2014;96(11):882–888. doi: 10.2106/JBJS.M.00919. [DOI] [PubMed] [Google Scholar]
- 118.Wilkinson LS, Edwards JC. Demonstration of lymphatics in human synovial tissue. Rheumatol Int. 1991;11(4–5):151–155. [DOI] [PubMed] [Google Scholar]
- 119.Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J, Fraser VJ. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Jt Surg. 2008;90(1):62–69. doi: 10.2106/jbjs.f.01515. [DOI] [PubMed] [Google Scholar]
- 120.Hikata T, Iwanami A, Hosogane N, Watanabe K, Ishii K, Nakamura M, Toyama Y, Matsumoto M, Kamata M. High preoperative hemoglobin A1c is a risk factor for surgical site infection after posterior thoracic and lumbar spinal instrumentation surgery. J Orthopaedic Sci. 2014;19(2):223–228. doi: 10.1007/s00776-013-0518-7. [DOI] [PubMed] [Google Scholar]
- 121.Ogihara S, Yamazaki T, Maruyama T, Oka H, Miyoshi K, Azuma S, Yamada T, Murakami M, Kawamura N, Hara N, et al. Prospective multicenter surveillance and risk factor analysis of deep surgical site infection after posterior thoracic and/or lumbar spinal surgery in adults. J Orthop Sci. 2015;20(1):71–77. doi: 10.1007/s00776-014-0669-1. [DOI] [PubMed] [Google Scholar]
- 122.Kaoutzanis C, Leichtle SW, Mouawad NJ, Welch KB, Lampman RM, Cleary RK. Postoperative surgical site infections after ventral/incisional hernia repair: a comparison of open and laparoscopic outcomes. Surg Endosc. 2013;27(6):2221–2230. doi: 10.1007/s00464-012-2743-0. [DOI] [PubMed] [Google Scholar]
- 123.Berthelot P, Grattard F, Cazorla C, Passot J-P, Fayard J-P, Meley R, Bejuy J, Farizon F, Pozzetto B, Lucht F. Is nasal carriage of Staphylococcus aureus the main acquisition pathway for surgical-site infection in orthopaedic surgery? Eur J Clin Microbiol Infect Dis. 2010;29(4):373–382. doi: 10.1007/s10096-009-0867-5. [DOI] [PubMed] [Google Scholar]
- 124.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43(3):322–330. doi: 10.1086/505220. [DOI] [PubMed] [Google Scholar]
- 125.Leekha S, Sampathkumar P, Berry DJ, Thompson RL. Should national standards for reporting surgical site infections distinguish between primary and revision orthopedic surgeries? Infect Control Hosp Epidemiol. 2010;31(5):503–508. doi: 10.1086/652156. [DOI] [PubMed] [Google Scholar]
- 126.Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ Jr.. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the national surgical quality improvement program. J Bone Joint Surg Am. 2011;93(17):1577–1582. doi: 10.2106/JBJS.J.01048. [DOI] [PubMed] [Google Scholar]
- 127.Belmont PJ, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty. Incidence and Risk Factors among a National Sample of 15,321 Patients. 2014;96(1):20–26. doi: 10.2106/jbjs.m.00018. [DOI] [PubMed] [Google Scholar]
- 128.Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: A propensity score matched analysis. Jbjs. 2014;96(16):1387–1394. doi: 10.2106/jbjs.m.01048. [DOI] [PubMed] [Google Scholar]


