ABSTRACT
Debilitating recurrent urinary tract infections (UTIs) are often associated with gastrointestinal colonisation by uropathogens, such as uropathogenic Escherichia coli (UPEC), suggesting that these populations might be a suitable target for the treatment and prevention of recurrent UTI. However, antimicrobial treatment is generally unable to prevent recurrent UTI, and often selects for multidrug resistant uropathogens in the gut, and causes dysbiosis of the gut, vaginal, and urinary microbiota. Of note, the globally-disseminated multi drug resistant UPEC lineage, ST131, is known to both persistently colonise the gut and the urinary tract, and is associated with antibiotic treatment failure, indicating the need for novel non-antibiotic therapeutics for the treatment of UTI. This study therefore presents hyperimmune bovine colostrum (HBC) as a suitable therapy for the treatment of UPEC gastrointestinal colonisation. This work demonstrates that the vaccination of pregnant cows with inactivated cells from a ST131 UPEC isolate results in a highly specific anti-UPEC HBC, and that this product is able to disrupt the gastrointestinal colonisation of ST131 UPEC in mice.
Keywords: uropathogenic Escherichia coli, urinary tract infection, antibiotic resistance, hyperimmune bovine colostrum, immunotherapeutic, gastrointestinal colonisation
Introduction
Urinary tract infections (UTI) affect up to 50% of women in their lifetime, with 20–30% of women going on to develop recurrent UTI.1 The most common aetiology of UTI is uropathogenic Escherichia coli (UPEC), causing more than 80% of community-acquired UTI.1 UPEC typically originates from the gut;2 therefore this intestinal population might act as a reservoir for re-infection, and might be a suitable target for the treatment and prevention of recurrent UTI. The multidrug resistant clonal group E. coli ST131 is currently the most predominant lineage of extra-intestinal pathogenic E. coli, and is often associated with UTI.3 ST131 is a diverse clonal group, encompassing three major sub-lineages, with the fluoroquinolone resistant H30 sub-lineage (also known as clade C) reported to be most dominant.4,5 Although it has been acknowledged that the reported prevalence of ST131 isolates may reflect a bias towards the collection of multidrug resistant pathogens,4,6,7 ST131 remains an important global cause of difficult-to-treat UTI.7,8 Importantly, ST131 isolates are also known to persistently colonise both the urinary tract and the gut with no apparent fitness trade-off,9 with this ability being recognised as one of the lineage’s most important factors for transmission between hosts.10 With diagnosis of a ST131 UTI strongly associated with treatment failure,8 alternative therapeutics need to be explored, including the development of novel non-antibiotic therapies for treatment and prevention.
Hyperimmune bovine colostrum (HBC) can be produced by the immunisation of cows during pregnancy, resulting in a product with a high level of antigen-specific IgG. Oral immunotherapy using HBC has been successfully used to treat infection with several enteric pathogens.11–13 Importantly, HBC does not disrupt the normal microbiota,13 and the antimicrobials, prebiotics, and growth factors it contains can stimulate the growth and repair of host tissues.14 In this study, we aimed to test whether passive immunotherapy using HBC targeting the ST131-H30 isolate EC958 could reduce gastrointestinal carriage of UPEC in mice. This work presents HBC as a novel therapeutic targeting UPEC gastrointestinal colonisation as a potential means to prevent recurrent UTI, and reflects the current need for novel non-antibiotic therapies against multidrug resistant uropathogens.
Results
Anti-UPEC colostrum contains a high level of specific IgG and binds to UPEC proteins in vitro
Platform technology developed by Immuron Ltd was used for the production of HBC containing antibodies that target UPEC (UPEC-HBC). UPEC-specific IgG titres were higher in UPEC-HBC compared to anti-enterotoxigenic E. coli (ETEC) colostrum (ETEC-HBC; Travelan) (Figure 1a), suggesting that the vaccination of cows with UPEC resulted in a targeted immune response. Western blot analysis showed that IgG from each colostrum product tested bound UPEC proteins, with each showing a differing binding profile (Figure 1b). ETEC-HBC demonstrated some cross-reactivity, however there was overall less binding when compared to UPEC-HBC, except for one predominant band at approximately 37 kDa. These results show that IgG purified from the ETEC-HBC binds to UPEC proteins in vitro but with less efficiency than IgG purified from UPEC-HBC.
Figure 1.
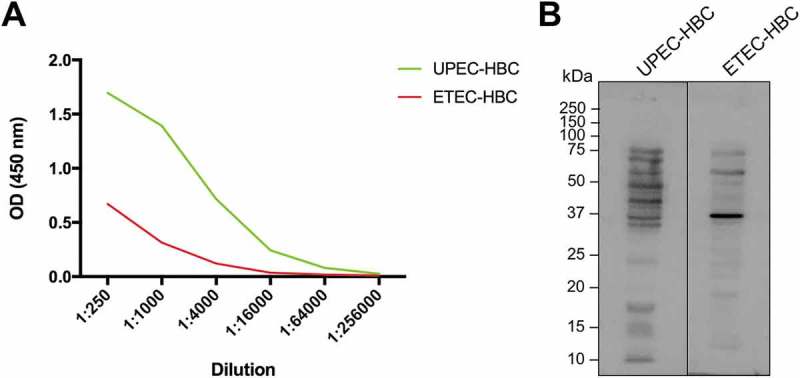
Comparative in vitro analysis of anti-uropathogenic Escherichia coli (UPEC) and anti-enterotoxigenic E. coli (ETEC) hyperimmune bovine colostrum (HBC). (A) UPEC-specific IgG antibodies in colostrum from cows vaccinated with UPEC or ETEC antigens. ELISA plates were coated with E. coli EC958 to determine the UPEC-specific antibody titres of colostrum from cows immunised with UPEC whole cells (UPEC-HBC) compared with colostrum from cows immunised with ETEC antigens (ETEC-HBC). (B) The in vitro binding affinity of IgG purified from colostrum from cows immunised with UPEC or ETEC antigens. IgG purified from UPEC-HBC and ETEC-HBC was used to detect antigens from whole cell lysates of E. coli EC958.
Anti-UPEC colostrum is unable to prevent UPEC colonisation of the gut
Prophylactic studies in mice showed that neither UPEC-HBC nor ETEC-HBC prevented the colonisation of UPEC in the gut, with all colostrum-treated and untreated groups showing high levels of UPEC shedding in the faeces at 24 hours post-inoculation (Figure 2a). However, at four days post-inoculation (five days following the start of colostrum treatment), both the UPEC-HBC and ETEC-HBC-treated groups showed a reduction in UPEC gut colonisation that is significantly different from that of the untreated group at the same time point (p = 0.0159 and p = 0.0238, respectively). Thus, although UPEC-HBC did not prevent UPEC gut colonisation, this data suggests that these products might contribute to the clearance of UPEC in the gut over time.
Figure 2.
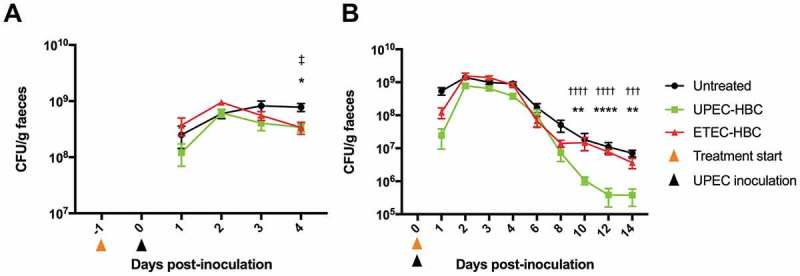
Faecal shedding of uropathogenic Escherichia coli (UPEC) in hyperimmune bovine colostrum (HBC)-treated and untreated mice. Mice inoculated with E. coli EC958 were either untreated or given UPEC-HBC or enterotoxigenic E. coli (ETEC)-HBC from (A) 24 hours before inoculation, and monitored for faecal shedding for four days, or (B) 24 hours post-inoculation, and monitored for faecal shedding for 14 days. Faecal shedding of UPEC is calculated as CFU/g of faeces. Error bars = Mean ± SEM; A, n = 5 mice per group; B, n = 9–15 mice per group (untreated = 15 mice; UPEC-HBC = 9 mice; ETEC-HBC = 10 mice). Statistical significance determined by Mann-Whitney U test is denoted as follows: UPEC-HBC vs. untreated = *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001; UPEC-HBC vs. ETEC-HBC = †††p ≤ 0.001, ††††p ≤ 0.0001; ETEC-HBC vs. untreated = ‡p ≤ 0.05.
Anti-UPEC colostrum disrupts UPEC gut colonisation after prolonged use
Treatment studies in mice, where colostrum was administered after UPEC inoculation, showed that UPEC-HBC was able to significantly displace UPEC gut colonisation in comparison to the untreated group (Figure 2b). This reduction was initially observed at 10 days post-inoculation (p = 0.0041), with a further reduction at 12 days post-inoculation (p < 0.0001), and remaining statistically significant at 14 days post-inoculation (p = 0.0041). One mouse belonging to the UPEC-HBC-treated group was excluded from analysis after being identified as a statistical outlier in five of the nine time points, however, the colonisation data at 10, 12, and 14 days post-inoculation remains statistically significant with the inclusion of this data (data not shown). Levels of UPEC gut colonisation at days 10, 12, and 14 post-inoculation in the UPEC-HBC-treated group were also significantly lower than the ETEC-HBC-treated groups (p < 0.0001, < 0.0001, and = 0.0003, respectively), suggesting that UPEC-HBC contributes to the clearance of UPEC in the gut during an established colonisation, potentially using a targeted mechanism.
Discussion
The maintenance of intestinal populations of UPEC is likely to be an important reservoir for re-infection, and therefore this population was postulated as a target for oral passive immunotherapy to reduce UPEC gut colonisation. In this study, we produced HBC with a high level of UPEC-specific IgG and tested it for efficacy in preventing and displacing UPEC gut colonisation in mice. This work was carried out in comparison to HBC targeting ETEC (Travelan), which was chosen in order to observe the specificity of UPEC-HBC in comparison to HBC targeting another pathotype of E. coli, as opposed to non-immune colostrum.
The UPEC strain chosen for this study is the prototypical clade C isolate, EC958, which is notably one of the best characterised and widely-used ST131 isolates.4,15 Importantly in the context of this study, EC958 is a multidrug resistant, clinically relevant cause of UTI,3,15 and its ability to adhere to and invade intestinal cells in vitro is representative of isolates from other ST131 sub-lineages.16 Thus, E. coli EC958 was deemed an appropriate representative strain for the study of non-antibiotic therapeutics targeting multidrug resistant UPEC in the gut, and was used in this study for the production of UPEC-HBC, and as the infecting strain in the mouse gut colonisation model for the analysis of this product.
As a treatment, UPEC-HBC was able to displace UPEC gut colonisation following approximately nine days of treatment (Figure 2b), resulting in an ~18-fold difference in colonisation levels between untreated and UPEC-HBC-treated mice at 14 days post-inoculation. When given prophylactically, UPEC-HBC was not able to prevent UPEC gut colonisation, but again showed the potential to displace UPEC after five days of treatment (Figure 2a), with a ~2-fold difference seen in colonisation levels at this time point between untreated and UPEC-HBC-treated mice. The delay observed in each trial from the start of colostrum administration until the initial significant drop in UPEC gut colonisation might suggest that a prolonged exposure to the antibodies is required before a protective effect can be observed. This delay, however, was shorter when UPEC-HBC was given prophylactically (five days), compared to when it was given as a treatment (nine days). In this case, the existing presence of colostrum antibodies at mucosal surfaces in the gut might assist with an accelerated clearance of UPEC, however, as the prophylaxis trial was terminated at the time point this clearance was observed, it is unknown if this initial drop in UPEC gut colonisation was the beginning of a more significant displacement, with further work required to explore this possibility.
The mechanism of the disruption to UPEC gut colonisation observed is unknown, but can potentially be attributed to the neutralisation of essential colonisation factors. The type 1 fimbriae expressed by UPEC strains including E. coli EC958 are required for urinary tract colonisation, which is mediated by the tip adhesin, FimH.2 Type 1 fimbriae also facilitate the binding of UPEC to the intestinal epithelium,16,17 and are therefore likely to play a role in the adherence and maintenance of intestinal UPEC populations. Several treatment methods targeting FimH have been shown to protect against UPEC colonisation of the urinary and gastrointestinal tracts,18,19 including that of E. coli EC958,17 supporting the importance of type 1 fimbriae in the gut colonisation of ST131 strains. It is therefore possible that the protective nature of UPEC-HBC can be attributed in part to antibodies against type 1 fimbriae, since their presence was confirmed during vaccine preparation, and the same culturing method was used to prepare the inoculum for mouse colonisation trials. However, the colonisation levels achieved from ten days post-inoculation in this study following prolonged treatment with UPEC-HBC are lower than those seen in an EC958 type 1 fimbriae null mutant tested in the same model, at the same time point.16 This is in agreement with the observation that although type 1 fimbriae enhance the gastrointestinal colonisation of EC958, they are not required for persistent colonisation,16 suggesting the presence of other factors involved in the gastrointestinal colonisation of this strain, which might also be targeted by UPEC-HBC – an important consideration for the ongoing development of HBC as a therapeutic against ST131 gut colonisation. These data also suggest that Travelan might be protective against UPEC when given prophylactically (Figure 2a), which is in line with the cross-reactivity observed via Western blot (Figure 1b), and might be explained by the cross-reactivity of FimH,20,21 as well as antigens found in the gram-negative outer membrane and cell wall, which are components of the Travelan vaccine.11 Further work is required to confirm these hypotheses and fully elucidate the mechanisms of UPEC clearance by HBC antibodies.
For the future development of HBC for UPEC gut decolonisation, there are several considerations that need to be made in regards to vaccine optimisation, as this represents a major weakness in the current study. Rather than vaccinate with whole cells as was done here, it is preferred to immunise cows with a single antigen to maximise the specific immune response. The repeated vaccination of separate cows with a single antigen should yield highly targeted HBC that can be combined to produce a final product with a high concentration of antibodies targeting multiple antigens.22 The identification of these antigens may be facilitated by mechanistic studies of the protective effect of UPEC-HBC, as these were not performed in the work presented. The final product should also confer protection against various UPEC strain types, including emerging multidrug resistant UPEC lineages such as ST131 and ST1193,10,23 as well as strains of various O-antigen and H-antigen subtypes, which might also contribute to cross-reactivity with other gram-negative pathogens, as discussed previously for Travelan. Testing against various UPEC lineages and other uropathogens in a gut colonisation mouse model should be performed, as only the UPEC strain used to formulate the vaccine was tested in the current study. It is unknown whether a reduction of UPEC in the gut will lead to a reduction in UTI occurrence, representing a further limitation to this work, and our ability to test this is limited as no animal model currently exists to reflect the faecal-perineal-urethral route of infection, with current models of UTI requiring inoculation directly into the bladder.24,25 However, the eradication of multidrug resistant E. coli from the gut using faecal microbiota transplant has been shown to prevent urinary tract infection in a patient with recurrent pyelonephritis,26 and gut decolonisation using antibiotics is often used as a means to prevent infection in susceptible patients,27 however this is often associated with re-colonisation and the development of antibiotic resistance.28,29
Overall, this study has shown for the first time that gastrointestinal UPEC colonisation can be disrupted using targeted HBC. It is likely that with further development, a more potent and targeted product can be produced that could to eliminate UPEC from the gut microbiota. We therefore propose that HBC can be used as a safe and economical adjunctive therapeutic in treating and preventing gastrointestinal carriage of UPEC, with a reduced impact on normal microbiota in comparison to conventional antibiotic therapies.
Methods
Bacterial culture
E. coli EC958 was grown statically in Luria broth (LB) at 37°C for 18 hours to facilitate type 1 fimbriae expression. Following incubation, cells were washed with PBS, and adjusted to 1 × 109 CFU/ml. For immunisations, type 1 fimbriae production was confirmed by yeast cell agglutination,16 prior to fixation in 10% formalin in PBS overnight at 4°C and washing (3xPBS).
Immunisation
Animal handling and experimentation was performed in accordance with Victorian Government guidelines (Department of Economic Development, Jobs, Transport & Resources). All experimental protocols were approved by the Immuron Ltd animal ethics committee (application A17). A pregnant Holstein dairy cow was vaccinated with 1 × 109 E. coli EC958 cells, as described previously.22 Vaccine was prepared by emulsifying 1 ml of fixed E. coli EC958 cells with 1 ml of Montanide ISA206VG adjuvant (Tall Bennett).
Colostrum preparation and purification of IgG antibody
Colostrum was collected within 24 hours after calving and processed by de-fatting, pasteurisation, concentration, and freeze drying, as described previously.22 Travelan, a powdered HBC targeting enterotoxigenic E. coli (ETEC) was provided by Immuron Ltd. For use, colostrum was resuspended at 10% (w/v) in dH2O. IgG antibody was purified from colostrum using a protein G column (GE Healthcare) as per the manufacturer’s instructions.
Quantification of UPEC-specific IgG
UPEC-specific IgG was quantified by ELISA as described previously,22 except that wells were coated with 105 E. coli EC958 cells per well. Dilutions of each HBC product were applied to the wells, followed by a goat anti-bovine IgG peroxidase conjugate (1:2000; Sigma) prior to measuring the absorbance.
SDS-PAGE and western blot
E. coli EC958 cells were lysed using a Precellys 24 tissue homogeniser (Bertin Technologies) and the protein concentration of the lysate was determined by BCA assay (Thermo Fisher Scientific). Proteins (10 μg) were separated by 12% SDS-PAGE and transferred onto a nitrocellulose membrane. Proteins were detected using IgG purified from UPEC- or ETEC-specific colostrum (1:500 dilution of a 30 mg/ml stock), followed by a goat anti-bovine IgG peroxidase conjugate (Sigma; 1:2000 dilution). Bound antibody complexes were detected using a Western Lightning Chemiluminescence reagent kit (Perkin-Elmer) and imaged using a Chemidoc system (Biorad).
Murine model for the prevention and treatment of UPEC gut colonisation
Animal handling and experimentation was performed in accordance with Victorian State Government regulations and approved by the Monash University Animal Ethics Committee (AEC no. MARP/2013/117). Female 6–7 week old C57BL/6J mice (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) were pre-treated with 5 mg/ml streptomycin prior to oral gavage with E. coli EC958 (108 CFU), as described previously.16 Mice were provided with plain water (untreated) or 10% w/v HBC in the drinking water daily during the trial, beginning one day prior to inoculation for prophylaxis studies, and one day following inoculation for treatment studies. Faeces were collected daily from individual mice for 4 days, and then every second day thereafter. To enumerate E. coli EC958 shedding, faeces were resuspended in PBS (100 mg/ml), serially diluted and plated on LB agar supplemented with 100 μg/ml ampicillin, 50 μg/ml kanamycin, and 5 μg/ml tetracycline, and incubated overnight at 37°C. Faeces collected from mice prior to inoculation with E. coli EC958 (before and after antibiotic pre-treatment) were screened on this selection to ensure no growth of the resident microbiota. Mice were euthanised by CO2 overdose at defined endpoints. Treatment studies were conducted across three trials, one of which also included prophylaxis studies. Five mice per treatment group were tested in each trial, with each trial also including one untreated control group. The untreated controls presented with the prophylaxis trial data were conducted alongside both prophylaxis and treatment studies, and are therefore included in both sets of data (faecal shedding data from days 1–4 post-inoculation presented with prophylaxis data, and days 1–14 post-inoculation presented with treatment data).
Statistical analysis
Statistical significance of mouse colonisation data was determined by Mann-Whitney U test, performed using GraphPad Prism version 7. Statistical outliers were identified by Grubbs’ test (significance level = 0.05), performed using GraphPad QuickCalcs (www.graphpad.com/quickcalcs/grubbs1). Mice were excluded from analysis if they were identified as an outlier at a majority of time points.
Funding Statement
This work was supported by an Australian Government Research Training Program Scholarship to SL, an Australian Research Council Future Fellowship [FT120100779] to DL, and an industry partner, Immuron Ltd.
Acknowledgments
The authors thank Professor Mark Schembri and Dr Makrina Totsika for the provision of E. coli EC958. We also acknowledge the facilities, scientific and technical assistance of Immuron Ltd.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28(1):1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Totsika M, Beatson SA, Sarkar S, Phan MD, Petty NK, Bachmann N, Szubert M, Sidjabat HE, Paterson DL, Upton M, et al. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: genome analysis and virulence mechanisms. PLoS One. 2011;6(10): e26578. doi: 10.1371/journal.pone.0026578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petty NK, Ben Zakour NL, Stanton-Cook M, Skippington E, Totsika M, Forde BM, Phan MD, Gomes Moriel D, Peters KM, Davies M, et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci U S A. 2014;111(15): 5694–5699. doi: 10.1073/pnas.1322678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, et al. The epidemic of extended-spectrum-beta-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio. 2013;4(6): e00377–13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamed M, Clabots C, Porter SB, Thuras P, Johnson JR. Isolation and characterization of Escherichia coli Sequence Type 131 and other antimicrobial-resistant gram-negative bacilli from clinical stool samples from veterans. Antimicrob Agents Chemother. 2016;60(8):4638–4645. doi: 10.1128/AAC.00383-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Reijer PM, van Burgh S, Burggraaf A, Ossewaarde JM, van der Zee A. The widespread presence of a multidrug-resistant Escherichia coli ST131 clade among community-associated and hospitalized patients. PLoS One. 2016;11(3):e0150420. doi: 10.1371/journal.pone.0150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Can F, Azap OK, Seref C, Ispir P, Arslan H, Ergonul O. Emerging Escherichia coli O25b/ST131 clone predicts treatment failure in urinary tract infections. Clin Infect Dis. 2015;60(4):523–527. doi: 10.1093/cid/ciu864. [DOI] [PubMed] [Google Scholar]
- 9.Chen SL, Wu M, Henderson JP, Hooton TM, Hibbing ME, Hultgren SJ, Gordon JI. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci Transl Med. 2013;5(184):184ra60. doi: 10.1126/scitranslmed.3005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27(3):543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otto W, Najnigier B, Stelmasiak T, Robins-Browne RM. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand J Gastroenterol. 2011;46(7–8):862–868. doi: 10.3109/00365521.2011.574726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg PD, Cello JP. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(4):348–354. PMID: 8948373. [DOI] [PubMed] [Google Scholar]
- 13.Sponseller JK, Steele JA, Schmidt DJ, Kim HB, Beamer G, Sun X, Tzipori S. Hyperimmune bovine colostrum as a novel therapy to combat Clostridium difficile infection. J Infect Dis. 2015;211(8):1334–1341. doi: 10.1093/infdis/jiu605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menchetti L, Traina G, Tomasello G, Casagrande-Proietti P, Leonardi L, Barbato O, Brecchia G. Potential benefits of colostrum in gastrointestinal diseases. Front Biosci. 2016;8:331–351. PMID: 27100711. doi: 10.2741/s467. [DOI] [PubMed] [Google Scholar]
- 15.Forde BM, Ben Zakour NL, Stanton-Cook M, Phan MD, Totsika M, Peters KM, Chan KG, Schembri MA, Upton M, Beatson SA. The complete genome sequence of Escherichia coli EC958: a high quality reference sequence for the globally disseminated multidrug resistant E. coli O25b: H4-ST131clone. PLoS One. 2014;9(8):e104400. doi: 10.1371/journal.pone.0104400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar S, Hutton ML, Vagenas D, Ruter R, Schüller S, Lyras D, Schembri MA, Totsika M. Intestinal colonisation traits of pandemic multidrug resistant Escherichia coli ST131. J Infect Dis. 2018. doi: 10.1093/infdis/jiy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaulding CN, Klein RD, Ruer S, Kau AL, Schreiber HL, Cusumano ZT, Dodson KW, Pinkner JS, Fremont DH, Janetka JW, et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature. 2017;546(7659): 528–532. doi: 10.1038/nature22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langermann S, MöLlby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A, Strouse R, Schenerman MA, Hultgren SJ, Pinkner JS, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181(2):774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 19.Totsika M, Kostakioti M, Hannan TJ, Upton M, Beatson SA, Janetka JW, Hultgren SJ, Schembri MA. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis. 2013;208(6):921–928. doi: 10.1093/infdis/jit245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thankavel K, Madison B, Ikeda T, Malaviya R, Shah AH, Arumugam PM, Abraham SN. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J Clin Invest. 1997;100(5):1123–1136. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham SN, Sun D, Dale JB, Beachey EH. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988;336(6200):682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- 22.Hutton ML, Cunningham BA, Mackin KE, Lyon SA, James ML, Rood JI, Lyras D. Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative. Sci Rep. 2017;7(1):3665. doi: 10.1038/s41598-017-03982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Lan F, Lu Y, He Q, Li B. Molecular characteristics of ST1193 clone among phylogenetic group B2 non-ST131 fluoroquinolone-resistant Escherichia coli. Front Microbiol. 2017;8:2294. doi: 10.3389/fmicb.2017.02294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4(8):1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hvidberg H, Struve C, Krogfelt KA, Christensen N, Rasmussen SN, Frimodt-Møller N. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob Agents Chemother. 2000;44(1):156–163. PMID: 10602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh R, van Nood E, Nieuwdorp M, van Dam B, Ten Berge IJ, Geerlings SE, Bemelman FJ. Donor feces infusion for eradication of extended spectrum beta-lactamase producing Escherichia coli in a patient with end stage renal disease. Clin Microbiol Infect. 2014;20(11):O977–8. doi: 10.1111/1469-0691.12683. [DOI] [PubMed] [Google Scholar]
- 27.Septimus EJ, Schweizer ML. Decolonization in prevention of health care-associated infections. Clin Microbiol Rev. 2016;29(2):201–222. doi: 10.1128/cmr.00049-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieg S, Küpper MF, de With K, Serr A, Bohnert JA, Kern WV. Intestinal decolonization of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBL): a retrospective observational study in patients at risk for infection and a brief review of the literature. BMC Infect Dis. 2015;15(475). doi: 10.1186/s12879-015-1225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeb MB, Main C, Eady A, Walker-Dilks C. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340. doi: 10.1002/14651858.CD003340. [DOI] [PubMed] [Google Scholar]


