ABSTRACT
Aberrant expression of CUL4B was identified in various types of solid cancers. Cumulative evidences support the oncogenic role of CUL4B in cancers, including regulation of cell proliferation and signal transduction. However, its clinical value and potential pathogenic mechanism in diffuse large B-cell lymphoma (DLBCL) have not been described previously. Therefore, we hypothesize that overexpressed CUL4B may contribute to the pathogenesis of DLBCL. The aim of this study is to assess the expression and the biological function of CUL4B in DLBCL progression. In our study, CUL4B overexpression was observed in DLBCL tissues, and its upregulation was closely associated with poor prognosis in patients. Furthermore, the functional roles of CUL4B was detected both in vitro and in vivo. We demonstrated that silencing CUL4B could not only induce cell proliferation inhibition, cell cycle arrest, and motility attenuation of DLBCL cells in vitro, but also decrease tumor growth in DLBCL xenografts mice. In addition, we identified that CUL4B may act as a potent inductor of JNK phosphorylation in regulation of autophagy. Our findings demonstrated a significant role of CUL4B in the development and progression of DLBCL. CUL4B may act as a useful biomarker and a novel therapeutic target in DLBCL.
KEYWORDS: Cullin4b, diffuse large B-cell lymphoma, cell proliferation, autophagy, signaling pathway
Introduction
Diffuse large B-cell lymphoma (DLBCL), a clinically and genetically heterogeneous tumor, is the most common histologic subtype of non-Hodgkin lymphoma. With recent advancement of novel targeted therapy, such as monoclonal anti-CD20 antibody, the outcome of DLBCL patients have been significantly improved [1]. However, there are still 40 ~ 50% of DLBCL patients suffering relapse and eventually die from disease progression [2]. Therefore, searching more effective chemotherapeutic regimens for therapeutic management of DLBCL is warranted.
Cullin4B (CUL4B), a scaffold protein of the CUL4B-RING E3 ubiquitin ligase complex, participates in a wide array of biological processes, including embryonic development, cell cycle progression, DNA damage response, chromatin remodeling, and signaling transduction [3–6]. Recent studies have revealed that CUL4B contributed to the development of various tumors, including non-small-cell lung cancer, hepatocellular carcinoma and osteosarcoma [6–9]. Although CUL4B has been demonstrated to regulate the survival of several solid neoplasms cells, its biological functions in DLBCL have not yet been validated. Aneel Paulus et al. [10] performed a comparative analysis and reported treatment response or overall survival in multiple myeloma (MM) patients. They also found that CUL4B was significantly decreased in the responder group versus non-responder control, after treatment with proteasome inhibitor and immunomodulatory [10]. Therefore, CUL4B may serve as a prognostic biomarker in MM. In accordance with MM, DLBCL also belongs to the B-cell malignancies, which gives us a hint that CUL4B might also act as a potential biomarker for DLBCL.
Several studies have demonstrated that CUL4B plays significant roles in the invasion, differentiation, and metastasis of carcinogenesis [11–13], the involved mechanisms may be related to signaling dysregulation. However, the expression and potential function of CUL4B in DLBCL is unclear. Nevertheless, the relationship between CUL4B and autophagy is still undefined. Autophagy is defined as a highly conserved process that promotes homeostasis maintenance, stress adaptation, and intracellular recycling [14–17] in cancer cells, by capturing cytoplasmic components (damaged or superfluous proteins and organelles, etc.) in autophagosomes and delivering them to lysosomes for degradation and recycle [18]. Increased basal level of autophagy in DLBCL cells has been demonstrated [19], but related mechanisms remains unclear. Therefore, exploring the mechanism between CUL4B and autophagy will be of great significance in DLBCL.
Our present study aimed to explore the expression and function of CUL4B in DLBCL, including the demonstration of molecular mechanisms through loss-of-function and gain-of-function assays in DLBCL. Enhanced CUL4B expression was discovered in DLBCL tissues and cells. CUL4B promoted the growth of DLBCL cells both in vitro and in vivo, which was by autophagy mediated by JNK signaling. This study illuminated the potential therapeutic value of CUL4B in the therapeutic management of DLBCL.
Materials and methods
Patient samples and cell lines
This study was approved by the Medical Ethical Committee of Shandong Provincial Hospital affiliated to Shandong University (Jinan, China). Paraffin-embedded archived samples were obtained from 48 newly diagnosed DLBCL patients (31 males and 17 females; age range 21–80 y, median 51 y) from 2010 to 2016 and 30 reactive hyperplasia patients (RHP, controls) in Shandong Provincial Hospital. The patients with low-grade B-cell lymphoma or another type of indolent lymphoma with subsequent transformation into DLBCL, AIDS/HIV infection, primary cutaneous DLBCL, primary central nervous system DLBCL, Epstein-Barr virus-positive DLBCL, were excluded from the study. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors. LY1 and LY8 cells were maintained in Iscove’s Modified Dulbecco’s Medium (IMDM, Gibco, Life Technologies, Carlsbad, CA, USA) enriched with 10% fetal bovine serum (HyClone, Logan, UT, USA) [20]. The medium contained 1% penicillin/streptomycin mixture and 2 mmol/L glutamine. All cells were incubated in a humidified atmosphere of 5% CO2 at 37°C.
Reagents
Propidium iodide (PI) and Annexin V-PE/7-aminoactinomycin D (7AAD) apoptosis detection kit were purchased from BD Biosciences (Bedford, MA, USA). SP600125 (SP) and Anisomycin (AN) were purchased from Selleck (Houston, TX, USA). Anti-CUL4B antibody was bought from Sigma Aldrich (St Louis, MO, USA). Anti-Beclin 1 and LC3B were purchased from Abcam (Cambridge, MA, USA). Anti-phospho-AKT, and total pan-AKT, phospho-mTOR, and total pan-mTOR, phospho-c-JUN, and total pan-c-JUN, phospho-JNK, and total pan-JNK were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-GAPDH, purchased from Zhongshan (Golden Bridge, Beijing, China) was served as the internal reference.
Cell transfection
The green fluorescent protein (GFP)-tagged lentivirus vectors either encoding CUL4B (shCUL4B, lvCUL4B) or control were purchased from Genechem (Shanghai, China). Stable cell lines expressing the lvCUL4B or shCUL4B were generated by transfection of LV-CUL4B or CUL4B-RNAi lentivirus vectors into LY1 and LY8 cells according to the manufacturers’ instruction, and screened for expression with 5 μg/mL puromycin (AMRESCO, USA). Infection efficiencies were assessed using GFP through flow cytometry and validated using qRT-PCR and western blot assays.
qRT-PCR
Total RNA was extracted using the RNAiso Plus (TaKaRa, Dalian, China) and processed to cDNA with the reverse transcription reagents (TaKaRa, Dalian, China). The mRNA expression level was determined by qRT-PCR using SYBR Green Master Mix (TaKaRa, Dalian, China) in LightCycler 480II (Roche, Basel, Swizerland). The CUL4B primers were as follows: forward, 5′-TGCTGCTCAGGAGGTCAGATC-3′ and reverse, 5′-TGGAATCAAAGTCTTCTCTCTCGTT-3′. Gene expression was normalized to GAPDH.
Western blotting
Total protein was isolated using radio-immunoprecipitation assay buffer (ShenergyBiocolor, Shanghai, China) together with protease inhibitor cocktail protease inhibitor cocktail tablets (ShenergyBiocolor, Shanghai, China) and 1× phosphatase inhibitor cocktail (bosterbio, Wuhan, China). Equal amount of 40μg total protein was electrophoretically separated on an 7.5–12% polyacrylamide gel made by TGXTM FastcastTM Acrylamide Kit (Bio-Rad, USA) and blotted onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Membranes were blocked for 1 hour with 5% defatted milk dissolved in tris-buffered saline containing 0.05% Tween-20, and then incubated overnight at 4°C with primary antibodies. PVDF membranes were washed with TBST, followed by hybridized at room temperature for 1 hour with the HRP-conjugated secondary antibodies (Zhongshan Goldenbridge, Beijing, China). Finally, protein bands were detected using the electro-chemi-luminescence kit (Millipore, Billerica, MA, USA) with the Amersham Imager 600 imaging system (General Electric, USA). Protein bands intensities were quantified by ImageJ software. GAPDH was used as an endogenous control (Zhongshan Goldenbridge, Beijing, China).
Transmission electron microscopy
Briefly, the cells were collected by centrifugation, fixed with 4% glutaraldehyde in media for 4 h at 4°C, and washed three times in 0.1 M PBS. Cell monolayers were post-fixed in aqueous 1% OsO4 and K3 [Fe (CN)6] for 1 h. After washing three times with 0.1 M PBS, the cells were dehydrated in a graded series of 30% to 100% ethanol, infiltrated, and embedded. Then, ultrathin sections (70 nm) were stained with 3% uranyl acetate/lead citrate. Ultrastructural examination was conducted using a JEM-1200EX transmission electron microscope (Japan).
Immunohistochemistry (IHC)
Sections from tissue blocks from patients were taken to paraffin imbedding and cut, and stained by hematoxylin. 4μm thick sections of tissues were sliced. After deparaffinization and rehydration, samples were blocked from endogenous peroxidases with 3% solution of hydrogen peroxide. Following this, IHC staining was performed using the specific primary antibodies against CUL4B according to standard protocols. After 1× PBS rinses for 15min, tissue sections were incubated with the rabbit anti-goat biotinylated secondary antibody, and then followed by incubation with strept avidin-horseradish peroxidase complex (SABC) and stained with 3,3′-diaminobenzidine tetrachlorhydrate dihydrate (DAB). Sections were counterstained with hematoxylin. The staining results were evaluated by two independent observers who were blinded to clinical data at two different time points, and were scored by the percentage of positive tumor cells. Uniform staining of more than 30% of tumor cells with CUL4B staining cells was considered as positive. Pictures of the highest immunoreactivity field were taken at ×400 magnification. Staining intensity was classified using a 0-to-3 scale: 0 (negative), 1 (weak), 2 (moderate), 3 (strong). Specimens of mice tumor tissues were dissected fixed in 4% paraformaldehyde and embedded with paraffin. IHC of mice subcutaneous tumors was performed as described above.
Cell proliferation assay
Cell proliferation assay was conducted using the Cell Counting Kit-8 (CCK-8) assay kit (Dojindo, Japan). DLBCL cells were seeded at a density of 5000 cells/well in 96-well plates for 24 h and subsequently incubated with 10 μL/well CCK-8 for 4 h at 37°C. The optical density was detected at 450 nm by Multiskan GO Microplate Reader (Thermo Scientific, Rockford, IL, USA).
Flow cytometry analysis
Cell cycle distribution and percentage of apoptosis were determined by flow cytometry on FACS-Navios Flow Cytometer (Beckman Coulter, CA, USA) according to the manufacturer’s instructions. PI staining was employed to measure all phases of the cell cycle. Cell apoptosis was detected using the double-staining method of the Annexin V-PE/7AAD apoptosis detection kit (BD Biosciences, Bedford, MA, USA).
Cell invasion and migration assay
Cell invasion and migration assays for LY8 cells were conducted using 24-well transwell chambers (8.0 μm, Corning, USA). A total of 2 × 10^5 cells in 200 µL serum-free IMDM were seeded into the upper chamber coated with matrigel and 600µL IMDM containing 10% FBS was used as the chemotactic factor in the lower chamber. After incubation for 24 hours at 37 ℃ in an incubator supplemented with 5% CO2, cells that invaded onto the lower surface of 24-well transwell chambers were fixed in 4% paraformaldehyde solution and stained with hematoxylin. Finally, the invasive cells were counted at ×200 microscope under a light on five random fields. Except for when coated with matrigel, cell migration assays were similar to cell invasion assays. Cells that migrated into IMDM in the lower chamber were counted at ×400 magnification on five random fields. Each cell group was plated in three duplicate wells.
In vivo xenograft tumor models
All animal experiments were performed in accordance with the rules of the Animal Care and Use Ethics Committee of Shandong Provincial Hospital. Tumor xenograft models were established using 6-week-old female severe combined immunodeficiency (SCID) beige mice (WeitongLihua Laboratory Animal Center, Beijing, China). The mice were randomly divided into two groups and injected subcutaneously with 5 × 106 cells (stably CUL4B-depleted vector transfected and empty control vector transfected) suspended in 0.2 mL PBS. Tumor volume was measured manually using digital caliper every 2 d. Tumor volume was estimated using the equation V = (a × b 2)/2, where “a” is the largest dimension and “b” is the perpendicular diameter. Tumor tissues were harvested for weight and subsequent pathological analyzes.
Statistical analysis
All experiments were performed at least in triplicate. All data were presented as mean ± standard deviation and analyzed using SPSS23.0 software (SPSS Inc., Chicago, IL, USA). Statistical analysis was performed using a two-tailed unpaired t-test (GraphPad Software, San Diego, CA, USA). Kaplan-Meier analysis was performed to evaluate the survival functions. Chi-square test was used to analyze the correlation between the clinical parameters of DLBCL patients and CUL4B staining levels in tissue sections. Statistical significance was considered at P < 0.05.
Results
CUL4B expression was increased in human DLBCL tissues and associated with tumor progression
Firstly, to explore the pathological functions of CUL4B in DLBCL, we analyzed the microarray datasets [21] obtained from Oncomine database (www.oncomine.com) to explore the mRNA expression of CUL4B in B-lymphocyte, germinal center B-lymphocyte and DLBCL samples. It was observed that DLBCL tissues exhibited a higher expression of CUL4B compared to B-Lymphocyte and Germinal Center B-Lymphocyte tissues (Figure 1(a), P = 0.008). To investigate the protein expression of CUL4B in DLBCL, IHC staining was performed in 48 DLBCL tissues and 30 reactive hyperplasia lymphoid (RHL) tissues. CUL4B protein was located in the nucleus of DLBCL cells. The number of CUL4B-positive cells was higher in DBLCL tissues than in RHL tissues (Figure 1(b)). Positive expression of CUL4B was observed in 70.8% (34/48) of DLBCL samples and in 3.3% (1/30) of control tissues.
Figure 1.
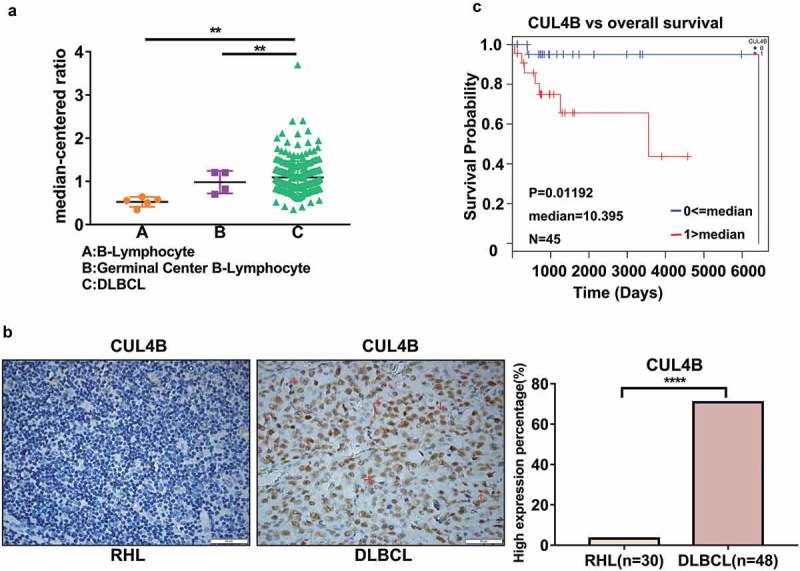
CUL4B was upregulated in DLBCL tissues and correlated with poor prognosis of DLBCL. (a) Expression of CUL4B in public database. Analysis of datasets from Oncomine database showed CUL4B mRNA expression was upregulated in DLBCL, compared to B-Lymphocyte and Germinal Center B-Lymphocyte (**P < 0.01). (b) Paraffin-embedded sections obtained from DLBCL tissues and RHP tissues (tissues from reactive nodes, controls) were evaluated by immunohistochemistry for CUL4B expressions (original magnification, ×400). Scale bar = 50μm. The presence of more CUL4B+ DLBCL cells (brown cells, shown by red arrows) was found in DLBCL tissues compared to RHP tissues. (c) Kaplan-Meier curves of OS in high or low CUL4B expressing groups of DLBCL patients (n = 45) from TCGA database showed worse outcome for patients exhibiting high CUL4B positivity (P < 0.05).
We further analyzed the relationship between CUL4B expression and clinical characteristics of DLBCL patients (Table 1). CUL4B overexpression was found to be positively associated with higher Ann Arbor stage (III/IV, P = 0.049), presence of B symptoms (P = 0.043), elevated serum lactate dehydrogenase (LDH, P = 0.019), and high international prognostic index (IPI) score (P = 0.041) of DLBCL patients. By analyzing previously published gene expression data of 48 DLBCL patients from the Cancer Genome Atlas (TCGA) [22], Kaplan-Meier survival analysis indicated that patients with high expression level of CUL4B presented a shorter overall survival (OS) than those without CUL4B overexpression (Figure 1(c), P = 0.01192, n = 45). Above data suggested that CUL4B may serve as a biomarker of DLBCL progression.
Table 1.
Correlation between CUL4B expression and clinical characteristics of DLBCL patients.
| Characteristics | No. of patients | CUL4B expression |
p value | |
|---|---|---|---|---|
| Negative | Positive (%) | |||
| Age (years) | 0.071 | |||
| <60 | 34 | 13 | 21 (61.8%) | |
| ≥60 | 14 | 1 | 13 (92.9%) | |
| Gender | 1 | |||
| Male | 31 | 9 | 22 (71.0%) | |
| Female | 17 | 5 | 12 (70.6%) | |
| Ann Arbor stage | 0.049 | |||
| I or II | 33 | 13 | 20 (60.6%) | |
| III or IV | 15 | 1 | 14 (93.3%) | |
| B symptoms | 0.043 | |||
| Present | 38 | 8 | 30 (78.9%) | |
| Absent | 10 | 6 | 4 (40.0%) | |
| Subtype | 0.471 | |||
| GCB | 21 | 5 | 16 (76.2%) | |
| Non-GCB | 27 | 9 | 18 (66.7%) | |
| Serum LDH | 0.019 | |||
| Normal | 17 | 9 | 8 (47.1%) | |
| Elevated | 31 | 5 | 26 (83.9%) | |
| Extranodal involvement | 0.549 | |||
| Present | 33 | 11 | 22 (66.7%) | |
| Absent | 15 | 3 | 12 (80.0%) | |
| IPI score | 0.041 | |||
| 0–2 | 20 | 9 | 11(55.0%) | |
| 3–5 | 28 | 5 | 23 (82.1%) | |
LDH: lactate dehydrogenase, IPI: international prognostic index
Role of CUL4B in DLBCL cell proliferation and apoptosis
We next validated the expression of CUL4B in DLBCL cell lines (LY1 and LY8) by western blot. As expected, expression of CUL4B was upregulated in DLBCL cell lines compared to the normal PBMCs (Figure 2). To investigate the role of CUL4B in DLBCL cells, stable knockdown and overexpression of CUL4B (designated as shCUL4B and lvCUL4B, respectively) in LY1 and LY8 cells were established. Effective silencing or overexpression of CUL4B in LY1 and LY8 cells were confirmed by qRT-PCR and western blot (Supplementary Figures 1 and 2), and shCUL4B#3, one of the three lentivirus-mediated RNA interference vectors against CUL4B, exhibited highest efficacy of CUL4B-depletion. Cell proliferation of LY1 and LY8 cells were evaluated by CCK-8 assay. Silencing of CUL4B resulted in growth suppression of LY1 and LY8 cells (Figure 3(a,b)), whereas CUL4B overexpression promoted the growth of LY1 and LY8 cells (Figure 3(c,d)).
Figure 2.

CUL4B was upregulated in DLBCL cell lines. (a.) Western blotting assays indicated high levels of CUL4B expression in LY1, LY8 cells and controls (N1 and N2). (b) Relative CUL4B protein expression in each group. Representative results from triplicate experiments are shown as the mean ± SD. **P < 0.01, ***P < 0.001, using GAPDH as a loading control.
Figure 3.

CUL4B promoted DLBCL cell growth. a and b Cell proliferation was assayed by CCK8. CUL4B depletion markedly decreased cell viability (mean ± SD, n = 3, *P < 0.05, **P < 0.01). c and d Enforced CUL4B expression promoted cell viability in DLBCL cell lines (mean ± SD, n = 3, *P < 0.05, **P < 0.01). e and f CUL4B-deletion induced cell cycle arrest at G0/G1 phase in LY1 and LY8 cells. Cell cycle distribution was detected by flow cytometry (mean ± SD, n = 3, *P < 0.05, **P < 0.01, ***P < 0.001).
Furthermore, cell cycle analysis was performed with PI staining. The result revealed that shCUL4B increased accumulation of cells in the G1 phase of the cell cycle, with concomitant decreases in the proportion of cells in the G2 and S phases (Figure 3(e,f)). Regulatory effect of CUL4B on cell apoptosis was also investigated. However, no significant difference of cell apoptosis was observed in either shCUL4B groups or lvCUL4B groups compared to control groups (Supplementary Figure 3).
CUL4B promoted DLBCL invasion and migration in vitro
To determine the role of CUL4B in the invasion and migration of DLBCL cells, transwell assays were performed. Significant reduction in cell invasion, more than half decrease was observed in shCUL4B group compared with control group (Figure 4(a), P < 0.001). As shown in Figure 4(b), the migration ability of LY1 and LY8 cells was also impaired with approximately 50% reduction by CUL4B silencing. Conversely, CUL4B overexpression displayed the opposite effect in LY1 and LY8 cells. In CUL4B overexpression, a more than 2-fold increment in cell invasion and migration was seen, respectively (Figure 4(c,d)). Taken together, these results indicated that CUL4B played an important role in DLBCL cell invasion and migration.
Figure 4.
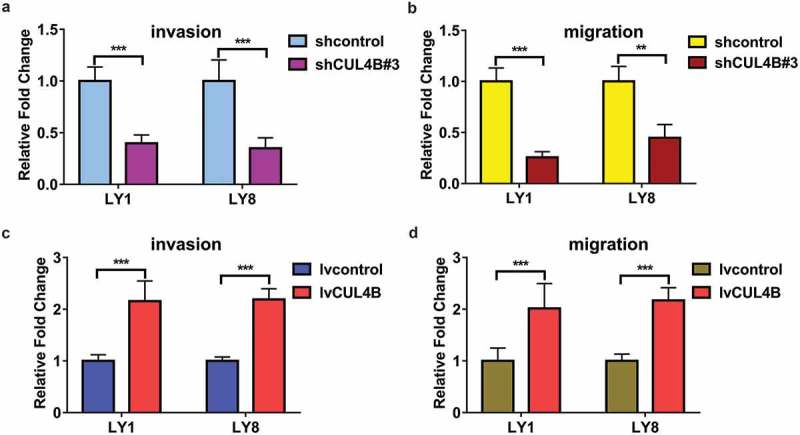
Modulation of CUL4B expression in invasion and migration of DLBCL cells. (a) Cell invasion and migration were assayed in CUL4B-silencing LY1 and LY8 cells by transwell assays. Matrigel invasion assay, ***P < 0.001. (b) Transwell migration assay was used to assess the ability of migration in CUL4B-depletion LY1 and LY8 cells, **P < 0.01, *** P < 0.001. c and d. Enforced CUL4B expression promoted cell invasion in LY1 and LY8 cells, ***P < 0.001. (d) Enforced CUL4B expression promoted cell migration in LY1 and LY8 cells, ***P < 0.001.
CUL4B deletion suppressed tumor growth in vivo
To further explore the role of CUL4B on tumor growth in vivo, we established a xenograft mice model with human DLBCL cells. ShCUL4B or control cells were injected subcutaneously into the right axilla of SCID beige mice. Consistent with the results in vitro, CUL4B knockdown greatly decreased tumor growth in DLBCL xenograft models (Figure 5(a), P < 0.01). IHC assay confirmed that CUL4B expression in the xenograft tumor sections derived from shCUL4B cells was significantly lower than that in controls (Figure 5(b)). In addition, the Ki67 and c-MYC percentage score of tumor cells in shCUL4B group was relatively reduced compared with those in control group (Figure 5(b)). In all, these results supported CUL4B as an important regulator of tumor proliferation in DLBCL.
Figure 5.

CUL4B-deletion suppressed DLBCL tumor growth in vivo. (a) Tumor xenograft models were established by 6-week-old female Severe Combined Immunodeficiency (SCID) beige mice. LY8 cells stably expressing CUL4B shRNA or control (shcontrol) were transplanted into SCID mice. Tumors were measured every 2 d using a vernier calliper and the volume was calculated according to the formula: 1/2× length×width^2. SCID mice with CUL4B-knockdown LY8 cells revealed lower tumor volume than controls (n = 6 per group, **P < 0.01). (b) IHC staining results confirmed the downregulation of CUL4B, Ki-67 and c-MYC in DLBCL xenograft tumors. Original magnification, ×400. Scale bar = 50μm. The presence of more CUL4B+ DLBCL cells (brown cells, shown by red arrows) was found in shcontrol tissues compared to shCUL4B tissues.
Role of CUL4B in autophagy and related mechanism
LC3B and Beclin 1 are the most widely recognized biomarkers of autophagy [23,24]. Thus, we detected the expression of LC3B (LC3I and II) and Beclin1 in DLBCL cells transfected with shCUL4B and lvCUL4B. Downregulated CUL4B resulted in significant decrease of the expression of LC3B and Beclin1 in DLBCL cells (Figure 6(a)). Whereas, the expression level of LC3B protein was upregulated when CUL4B was overexpressed (Figure 6(b)). Neither CUL4B-silence nor overexpression affected the mRNA level of LC3B and Beclin1 (Supplementary Figure 4). These suggested that CUL4B might potentially play a regulatory role of autophagy modulation at the post translational level. Furthermore, the autophagic vesicles and double-membrane autophagosomes in cytoplasm of shCUL4B, lvCUL4B and control cells were visualized by transmission electron microscopy. As shown in Figure 6(c), the number of autophagosomes (another golden hallmark of autophagy) with engulfed bulk cytoplasm and cytoplasmic organelles in shCUL4B cells was lower than that in control cells. Contrarily, CUL4B overexpression exhibited the opposite effect (Figure 6(d)). These results indicated that CUL4B contributed to the autophagy induction in DLBCL cells.
Figure 6.
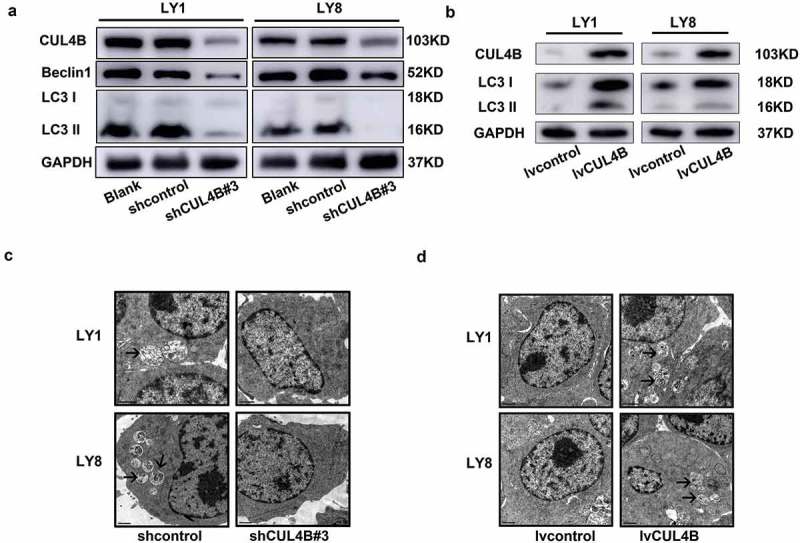
CUL4B activated autophagy in DLBCL cells. a and b. CUL4B and LC3B were analyzed by western blotting from CUL4B-knockdown (a) and CUL4B-overexpression (b) and control LY1 and LY8 cells. (c) Autophagosomes were observed using transmission electron microscope. The presence of fewer autophagosomes (shown by black arrows) was found in CUL4B-silicing cells compared to controls. (d) More autophagosomes (shown by black arrows) were observed in CUL4B-overexpression cells compared to controls.
To unravel the mechanisms of CUL4B regulating autophagy in DLBCL cells, possible regulatory pathways were evaluated. Several signaling pathways have been demonstrated to participate in autophagy regulation in solid cancers [25–28], but the mediated mechanisms in DLBCL still remains unknown. Previous research regarded the regulating pathways, mainly PI3K/AKT/mTOR and JNK signaling pathways, as the targeted treatment for DLBCL due to their positive regulation in further promotion of cell survival, proliferation, and drug resistance [2,29,30]. Hence, we hypothesized that CUL4B might be able to modulate autophagy via PI3K/AKT/mTOR or JNK pathway. Phosphorylation of AKT, mTOR and JNK was assessed upon lentiviral vectors mediated inhibition or elevation of CUL4B expression in LY1 and LY8 cells. As shown in Figure 7(a), the phosphorylation of AKT protein was significantly inhibited in DLBCL cells with CUL4B knockdown but increased with CUL4B overexpression. However, neither of CUL4B depletion nor overexpression exerted a substantial effect on mTOR phosphorylation. Despite all this, western blot assay further revealed that phosphorylation within JNK and c-JUN was downregulated in shCUL4B cells (Figure 7(b)) but elevated in lvCUL4B cells (Figure 7(c)). We then investigated whether JNK signaling had a functional impact on autophagy induced by the changed expression of CUL4B. We combined a pan-JNK activator (Anisomycin) with CUL4B knockdown and evaluated the autophagy. As shown in Figure 8(a), treatment of shCUL4B cells with Anisomycin increased the LC3B protein expression. Meanwhile, autophagosomes were observed by transmission electron microscopy. CUL4B-knockdown cells within Anisomycin treatment showed more autophagosomes than control cells (Figure 8(b)). Combination of SP600125 with lvCUL4B cells reduced the expression of LC3B (Figure 8(c)). Autophagosomes was fewer in CUL4B-overexpression cells within SP600125 treatment than in cells without SP600125 treatment (Figure 8(d)). In conclusion, our results suggested that CUL4B possibly affected autophagy mainly by regulating the JNK signaling pathway in DLBCL cells, but not the PI3K/AKT/mTOR signaling pathway. However, the specific mechanism remained to be further studied.
Figure 7.
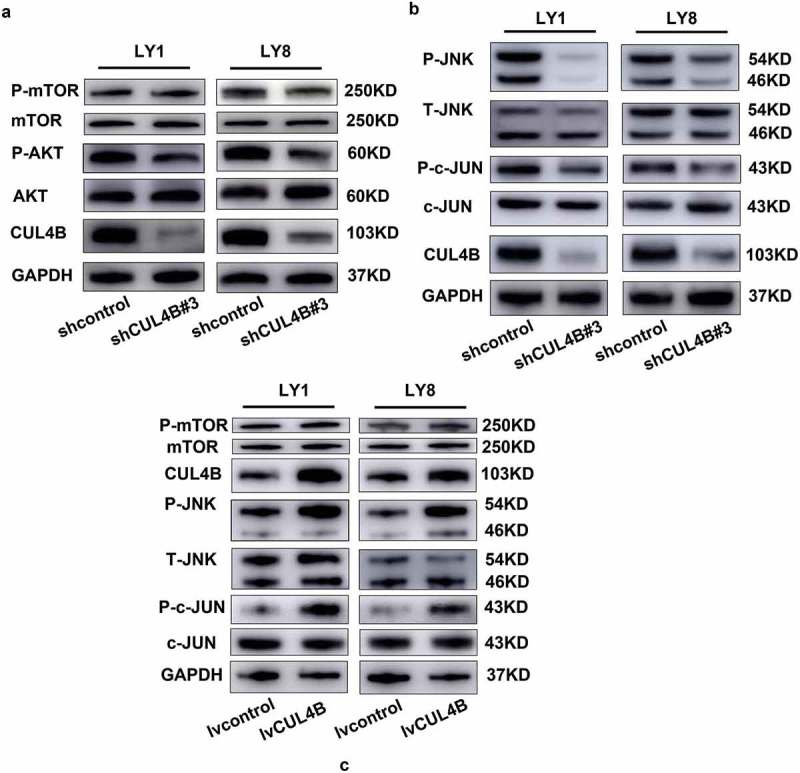
CUL4B regulated autophagy by activating JNK signaling pathway. (a) Western blot analysis of phosphorylated AKT, total pan-AKT, phosphorylated mTOR and total pan-mTOR in CUL4B-knockdown LY1 and LY8 cells. GAPDH protein was served as the internal reference. (b) Phospho-c-JUN, total pan-c-JUN, phospho-JNK, and total pan-JNK were analyzed by western blotting from CUL4B-knockdown and control cells. GAPDH protein was served as the internal reference. (c) CUL4B, phosphorylated mTOR and total pan-mTOR, phospho-c-JUN, total pan-c-JUN, phospho-JNK, and total pan-JNK were analyzed by western blotting from enforced CUL4B expression cells and control cells. GAPDH protein was served as the internal reference.
Figure 8.
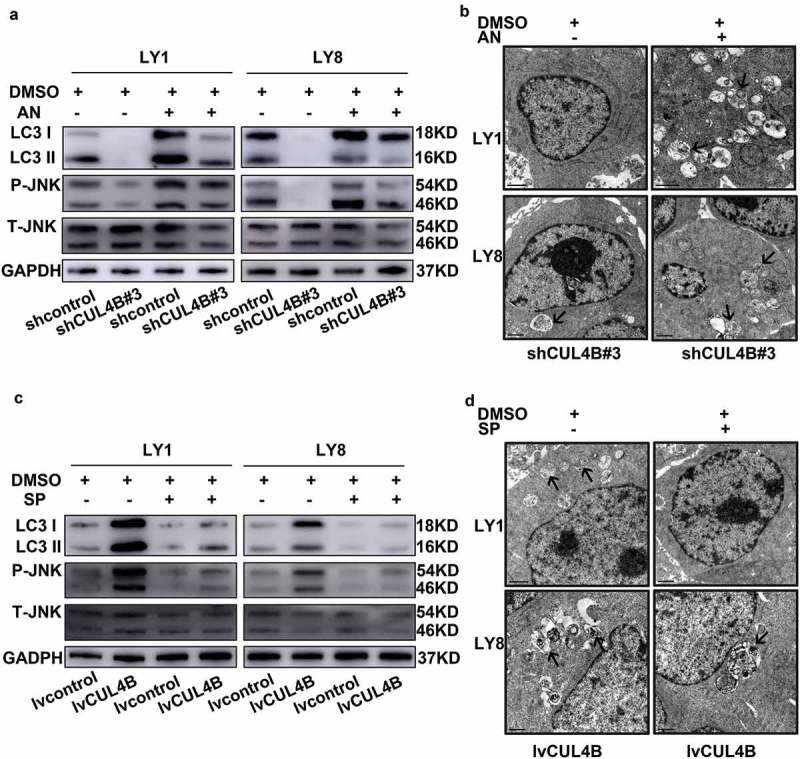
JNK signaling had a functional impact on autophagy induced by the changed expression of CUL4B. (a) Cells were treated with 4μM Anisomycin for 6 hours. The expression of LC3B protein and the phosphorylation of JNK protein were evaluated by western blot assay. (b) Transmission electron microscopy analysis was performed on CUL4B-silence cells treated with 4μM of Anisomycin for 6 hours. Black arrows indicate autophagosomes. (c) Western blot assay was used to evaluated the expression of LC3B protein and the phosphorylation of JNK protein within SP600125 treated (40 μM, 24 hours). (d) Autophagosomes were detected in CUL4B-overexpression cells treated with 40μM SP600125 for 24 hours by transmission electron microscopy.
Discussion
In the present study, we elucidated for the first time that CUL4B was upregulated in DLBCL tissues and cell lines. CUL4B played a stimulus role in the proliferation, cell cycle arrest, invasion and migration, and autophagy of DLBCL cell lines. In terms of a functional mechanism, our investigations identified that CUL4B could modulate the autophagy level of DLBCL cells via regulating JNK signaling. In short, these findings suggested CUL4B overexpression was a frequent cancer-specific event and CUL4B functioned as an oncogene in DLBCL progression. Furthermore, overexpressed CUL4B was potentially associated with poor prognosis of DLBCL patients. These inspiring discoveries may help to develop a novel therapeutic strategy in DLBCL management to promote long term survival.
As we alluded to above, emerging evidence had identified that CUL4B was highly expressed in different human malignancies, including carcinoma of the cervix, esophagus, liver, gastric and pancreatic cancer, and had a positive correlation with tumor progression [3,8,12,13,31]. Furthermore, CUL4B overexpression was significantly correlated with tumor size, advanced tumor stage, vascular and lymphatic invasion, distant metastasis, and histological differentiation, and was positively correlated with undesirable outcomes [3,32–34]. In hematological malignancies, Aneel Paulus et al. reported that CUL4B expression was closely associated with diseases response or survival in MM patients. These data was extracted from the CoMM pass trial at the 59th American Society of Hematology (ASH) Annual Meeting [10]. Disappointingly, relatively systematic reports about the expression and biological functions of CUL4B in DLBCL were still lacking. In order to solve this problem, we first searched and analyzed the microarray datasets [21] obtained from Oncomine database (www.oncomine.com). Data shown that the CUL4B mRNA expression was higher in DLBCL samples than B-Lymphocyte and Germinal Center B-Lymphocyte samples. Then, we collected 48 newly diagnosed DLBCL patient samples and observed elevated CUL4B expression in DLBCL tissues compared with reactive hyperplasia lymphoid tissues by IHC assay. Similarly, the result was confirmed in the DLBCL cell lines. Through correlation analysis, we identified a closely relationship of CUL4B and the clinicopathological features of DLBCL patients. Enhanced CUL4B expression was significantly correlated with the higher Ann Arbor stage, the presence of B symptoms, higher serum LDH, and IPI scores of DLBCL patients. In addition, survival analysis in 48 DLBCL patients from TCGA [22] shown that CUL4B overexpression might be associated with poor outcome of DLBCL patients. Our findings suggested that CUL4B might serve as a potential biomarker for predicting the prognosis of DLBCL patients. Continued studies with more patients are urgently needed to further confirm the independent prognostic value of CUL4B in DLBCL.
We explored the potentially biological significance of CUL4B in DLBCL by loss-of-function assay. CUL4B inhibition by shRNA interference resulted in S phase cell cycle arrest in human osteosarcoma cells [35] or G1 phase cell cycle arrest in glioma cell lines via down-regulation of cyclin D1 and up-regulation of p16 [36]. Available studies came to the conclusion that silencing of CUL4B inhibited cell proliferation and decreased tumor formation. Accordingly, these data supports our previous arguments that CUL4B deletion showed the adverse effects of DLBCL cells via suppressing cell proliferation, inducing G0/G1 cell cycle arrest, inhibiting cell invasion and migration, and attenuating autophagy, instead of inducing apoptosis. However, the function of CUL4B in cell cycle regulation remained controversial. Ji et al. identified that loss of CUL4B resulted in G1 cell cycle arrest [5]. Liu et al. put forward a different opinion that CUL4B downregulation arrested the cell cycle at the G2/M phase in neural progenitor cells [37]. Several studies have demonstrated that RNA interference silencing of CUL4B led to a prolonged S phase [38,39]. Therefore, regulation mechanisms of CUL4B in mitotic progression deserved further elucidation. In addition, silencing of CUL4B is also involved in cell growth. Different cell experiments had confirmed that CUL4B activates the expression of mRNA and protein levels of c-Myc, promoting Wnt/β-catenin signaling activation and tumor progression ultimately [40,41]. Investigation of DLBCL consistently illustrated the connection between Ki-67, c-Myc and aggressive clinical behavior [42–44]. Ki-67 and c-Myc were regarded as proliferative markers but also poor prognostic markers in DLBCL [45]. Using xenograft model of DLBCL, we showed that CUL4B knockdown significantly inhibited staining positive rate of Ki-67 and c-Myc, and in turn demonstrated that knockdown of CUL4B could significantly suppress tumor growth. Taken together, our findings suggested that CUL4B functioned as an important regulator in DLBCL progression.
On the other hand, our findings showed that CUL4B expression was involved in autophagy. Autophagy plays complex roles in human malignancies, which depend on tumor subtypes, stages, stimuli or drugs used, driving oncogenes, and tumor suppressor genes [15,46,47]. Although autophagy played certain roles in tumor suppression, some studies showed that autophagy promoted cancer cell survival, especially in response to chemotherapy [48,49]. Based on the relationship with pathogenesis and drug resistance [50–52], recent investigations unveiled the multiple regulation pathways of autophagy but also considered autophagy as a novel regulated mechanism of DLBCL. Yuan et al. demonstrated that Tenovin-6, a small molecular compound which can activate p53 and inhibit the protein deacetylase activity of purified SIRT1/2/3, can inhibit cell survival of DLBCL via blocking autophagy [50]. With respect to cancer, autophagy is often associated with a pro-survival phenotype [53,54]. In our study, we found that CUL4B could promote cell growth in DLBCL. Although autophagy and CUL4B could both promote cancer cell survival in DLBCL; rarely research underlined the relationship between CUL4B and autophagy. There might be a link between CUL4B and autophagy in the regulation of DLBCL progression. In our study, we observed that CUL4B knockdown could significantly reduce the level of autophagy in DLBCL cells, and lentiviral vectors mediated elevation of CUL4B expression could upregulated the level of autophagy to some extent. The inhibition of proliferation induced by CUL4B deletion may be attributed to the blocking of the pro-survival ability mediated by autophagy.
Extensive number of genes and proteins were confirmed to interference autophagy and further promote tumorigenesis through regulation of different mechanisms and signal pathways, including PI3K/AKT/mTOR and JNK signaling pathways [55–58]. Aberrant activation of signaling pathways led to activation of multiple downstream effectors, including those involved in growth, survival, and autophagy [20,54]. Previously studies showed that blocking of the PI3K/AKT/mTOR and JNK signaling could reduce cell survival and function of lymphocytes [59–62], potentially regarded as a therapeutic method for DLBCL. Except in tumor proliferation, mTOR played a negative role in the regulation of autophagy [63,64]. Our data showed that knockdown of CUL4B in DLBCL cells resulted in attenuation of cell autophagy, significant inhibition of the phosphorylation of AKT, JNK, and c-JUN protein. On the other hand, CUL4B silencing had no effect on mTOR protein expression, which was consistent with previous results from Hussain, S et al [65]. With JNK activator treatment, we observed increased expression of LC3B protein and increased autophagosomes in CUL4B-knockdown cells. Conversely, the protein level of LC3B and the number of autophagosomes in CUL4B-overexpression cells were both reduced with JNK inhibitor treatment. Observations above provided a strong rationale for CUL4B regulating autophagy primarily through JNK signaling but not PI3K/AKT/mTOR signaling in DLBCL cells. Moreover, CUL4B has been reported to play an oncogenic role by epigenetically repressing tumor suppressors, including PTEN (an inhibitor of AKT signaling), by catalyzing H2AK119 mono-ubiquitination and H3K27 trimethylation on their promoters [3]. Qian, Y et al identified that CUL4B sustained AKT activity by transcriptionally repressing AKT phosphatases through H2AK119 mono-ubiquitination [66]. Based on the evidence from previous other studies [3,66,67], it stands to reason that it is loss of CUL4B on DNA level that may lead to the inhibition of phosphorylation of AKT protein in DLBCL. Further investigation is required to determine the molecular mechanism of CUL4B in regulating AKT and JNK signaling in DLBCL.
In addition, CUL4B shares approximately 82% identity with CUL4A in their protein sequences [68]. CUL4A has been found to be a putative oncogene and overexpressed in different types of carcinomas [69,70]. However, the effects of CUL4A in DLBCL remain poorly understood. Further studies are still needed to clarify whether CUL4A plays an important role in DLBCL progression, or a redundant role in the regulation of CUL4B in DLBCL. Taken together, the exploration of the mechanisms of CUL4B in the tumorigenesis and progression of DLBCL may help us find new therapeutic targets.
Conclusions
We found that CUL4B was highly expressed in both DLBCL tissues and cell lines. CUL4B overexpression was positively associated with an advanced Ann Arbor stage, the presence of B symptoms, elevated serum LDH, and high IPI scores of DLBCL patients. CUL4B may be a prognostic index in DLBCL. We postulated an intriguing mechanism where CUL4B generates a tumor-promoting effect by remodeling DLBCL proliferation and the cell cycle. CUL4B could induce autophagy to enhance pro-survival ability through the regulation of the JNK signaling pathway. In addition to DLBCL, a number of tumor models had also documented the oncogene function of CUL4B. Taken together, targeting the expression of CUL4B may provide a novel approach to cancer therapy.
Funding Statement
This work was supported by the National Natural Science Foundation of China [81473486];National Natural Science Foundation of China [81270598];National Natural Science Foundation of China [81770210];Program of Shandong Medical Leading Talent;Key Research and Development Program of Shandong Province [2018CXGC1213];Technology Development Projects of Shandong Province [2017GSF18189];Taishan Scholar Foundation of Shandong Province
Disclosure statement
No potential conflict of interest was reported by the authors..
Supplementary material
Supplementary data for this article can be accessed here.
References
- [1].Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366:2008–2016. [DOI] [PubMed] [Google Scholar]
- [2].Matthews JM, Bhatt S, Patricelli MP, et al. Pathophysiological significance and therapeutic targeting of germinal center kinase in diffuse large B-cell lymphoma. Blood. 2016;128:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hu H, Yang Y, Ji Q, et al. CRL4B catalyzes H2AK119 monoubiquitination and coordinates with PRC2 to promote tumorigenesis. Cancer Cell. 2012;22:781–795. [DOI] [PubMed] [Google Scholar]
- [4].Zou Y, Mi J, Wang W, et al. CUL4B promotes replication licensing by up-regulating the CDK2-CDC6 cascade. J Cell Biol. 2013;200:743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ji Q, Hu H, Yang F, et al. CRL4B interacts with and coordinates the SIN3A-HDAC complex to repress CDKN1A and drive cell cycle progression. J Cell Sci. 2014;127:4679–4691. [DOI] [PubMed] [Google Scholar]
- [6].Yang Y, Liu R, Qiu R, et al. CRL4B promotes tumorigenesis by coordinating with SUV39H1/HP1/DNMT3A in DNA methylation-based epigenetic silencing. Oncogene. 2015;34:104–118. [DOI] [PubMed] [Google Scholar]
- [7].Wang X, Chen Z.. Knockdown of CUL4B suppresses the proliferation and invasion in non-small cell lung cancer cells. Oncol Res. 2016;24:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yuan J, Han B, Hu H, et al. CUL4B activates Wnt/beta-catenin signalling in hepatocellular carcinoma by repressing Wnt antagonists. J Pathol. 2015;235:784–795. [DOI] [PubMed] [Google Scholar]
- [9].Chen Z, Shen BL, Fu QG, et al. CUL4B promotes proliferation and inhibits apoptosis of human osteosarcoma cells. Oncol Rep. 2014;32:2047–2053. [DOI] [PubMed] [Google Scholar]
- [10].Paulus A, Coignet M, Manna A, et al. Comparative analysis of gene expression status to predict response to initial multiple myeloma treatment: utility of the Mmrf commpass database in validating historical findings. Blood. 2017;130:2178. [Google Scholar]
- [11].He F, Cheng XM, Gu WL. Effects of cullin 4B on the proliferation and invasion of human gastric cancer cells. Mol Med Rep. 2018;17:4973–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mi J, Zou Y, Lin X, et al. Dysregulation of the miR-194-CUL4B negative feedback loop drives tumorigenesis in non-small-cell lung carcinoma. Mol Oncol. 2017;11:305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qi M, Jiao M, Li X, et al. CUL4B promotes gastric cancer invasion and metastasis-involvement of upregulation of HER2. Oncogene. 2018;37:1075–1085. [DOI] [PubMed] [Google Scholar]
- [14].Endo S, Nakata K, Ohuchida K, et al. Autophagy is required for activation of pancreatic stellate cells, associated with pancreatic cancer progression and promotes growth of pancreatic tumors in mice. Gastroenterology. 2017;152:1492–506 e24. [DOI] [PubMed] [Google Scholar]
- [15].White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Galluzzi L, Bravo-San Pedro JM, Levine B, et al. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cheong H, Lu C, Lindsten T, et al. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alinari L. Toward autophagy-targeted therapy in lymphoma. Blood. 2017;129:1740–1742. [DOI] [PubMed] [Google Scholar]
- [20].Zhou X, Fang X, Jiang Y, et al. Klotho, an anti-aging gene, acts as a tumor suppressor and inhibitor of IGF-1R signaling in diffuse large B cell lymphoma. J Hematol Oncol. 2017;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. [DOI] [PubMed] [Google Scholar]
- [22].Reddy A, Zhang J, Davis NS, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171:481–94 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen S, Jiang YZ, Huang L, et al. The residual tumor autophagy marker LC3B serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Clin Cancer Res. 2013;19:6853–6862. [DOI] [PubMed] [Google Scholar]
- [24].Zhao H, Yang M, Zhao B. Beclin 1 and LC3 as predictive biomarkers for metastatic colorectal carcinoma. Oncotarget. 2017;8:59058–59067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xu J, Xia Y, Zhang H, et al. Overexpression of long non-coding RNA H19 promotes invasion and autophagy via the PI3K/AKT/mTOR pathways in trophoblast cells. Biomed Pharmacothe. 2018;101:691–697. [DOI] [PubMed] [Google Scholar]
- [26].Xue L, Zhang WJ, Fan QX, et al. Licochalcone a inhibits PI3K/Akt/mTOR signaling pathway activation and promotes autophagy in breast cancer cells. Oncol Lett. 2018;15:1869–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodriguez-Hernandez MA, Gonzalez R, de la Rosa AJ, et al. Molecular characterization of autophagic and apoptotic signaling induced by sorafenib in liver cancer cells. J Cell Physiol. 2018;234(1):692–708. [DOI] [PubMed] [Google Scholar]
- [28].Liu Y, Wang N, Zhang S, et al. Autophagy protects bone marrow mesenchymal stem cells from palmitateinduced apoptosis through the ROSJNK/p38 MAPK signaling pathways. Mol Med Rep. 2018;18:1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Go H, Jang JY, Kim PJ, et al. MicroRNA-21 plays an oncogenic role by targeting FOXO1 and activating the PI3K/AKT pathway in diffuse large B-cell lymphoma. Oncotarget. 2015;6:15035–15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang J, Xu-Monette ZY, Jabbar KJ, et al. AKT hyperactivation and the potential of AKT-targeted therapy in diffuse large B-cell lymphoma. Am J Pathol. 2017;187:1700–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang JQ, Chen S, Gu JN, et al. MicroRNA-300 promotes apoptosis and inhibits proliferation, migration, invasion and epithelial-mesenchymal transition via the Wnt/beta-catenin signaling pathway by targeting CUL4B in pancreatic cancer cells. J Cell Biochem. 2018;119:1027–1040. [DOI] [PubMed] [Google Scholar]
- [32].Jiang T, Tang HM, Wu ZH, et al. Cullin 4B is a novel prognostic marker that correlates with colon cancer progression and pathogenesis. Med Oncol. 2013;30:534. [DOI] [PubMed] [Google Scholar]
- [33].Li P, Zhang L, Yang M, et al. Cul4B is a novel prognostic marker in cholangiocarcinoma. Oncol Lett. 2017;14:1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jia L, Yan F, Cao W, et al. Dysregulation of CUL4A and CUL4B ubiquitin ligases in lung cancer. J Biol Chem. 2017;292:2966–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhang C, Chen B, Jiang K, et al. Activation of TNF-alpha/NF-kappaB axis enhances CRL4B(DCAF)(11) E3 ligase activity and regulates cell cycle progression in human osteosarcoma cells. Mol Oncol. 2018;12:476–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dong J, Wang XQ, Yao JJ, et al. Decreased CUL4B expression inhibits malignant proliferation of glioma in vitro and in vivo. Eur Rev Med Pharmacol Sci. 2015;19:1013–1021. [PubMed] [Google Scholar]
- [37].Liu HC, Enikolopov G, Chen Y. Cul4B regulates neural progenitor cell growth. BMC Neurosci. 2012;13:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zou Y, Mi J, Cui J, et al. Characterization of nuclear localization signal in the N terminus of CUL4B and its essential role in cyclin E degradation and cell cycle progression. J Biol Chem. 2009;284:33320–33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen Z, Wang K, Hou C, et al. CRL4BDCAF11 E3 ligase targets p21 for degradation to control cell cycle progression in human osteosarcoma cells. Sci Rep. 2017;7:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Song B, Zhan H, Bian Q, et al. Knockdown of CUL4B inhibits proliferation and promotes apoptosis of colorectal cancer cells through suppressing the Wnt/beta-catenin signaling pathway. Int J Clin Exp Pathol. 2015;8:10394–10402. [PMC free article] [PubMed] [Google Scholar]
- [41].Mao XW, Xiao JQ, Xu G, et al. CUL4B promotes bladder cancer metastasis and induces epithelial-to-mesenchymal transition by activating the Wnt/beta-catenin signaling pathway. Oncotarget. 2017;8:77241–77253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [42].Tang YL, Zhou Y, Cheng LL, et al. BCL2/Ki-67 index predict survival in germinal center B-cell-like diffuse large B-cell lymphoma. Oncol Lett. 2017;14:3767–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Patrascu AM, Rotaru I, Olar L, et al. The prognostic role of Bcl-2, Ki67, c-MYC and p53 in diffuse large B-cell lymphoma. Rom J Morphol Embryol. 2017;58:837–843. [PubMed] [Google Scholar]
- [44].Lai C, Roschewski M, Melani C, et al. MYC gene rearrangement in diffuse large B-cell lymphoma does not confer a worse prognosis following dose-adjusted EPOCH-R. Leuk Lymphoma. 2018;59:505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Broyde A, Boycov O, Strenov Y, et al. Role and prognostic significance of the Ki-67 index in non-Hodgkin’s lymphoma. Am J Hematol. 2009;84:338–343. [DOI] [PubMed] [Google Scholar]
- [46].Nyfeler B, Eng CH. Revisiting autophagy addiction of tumor cells. Autophagy. 2016;12:1206–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Amaravadi R, Kimmelman AC, White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25:1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yuan H, He M, Cheng F, et al. Tenovin-6 inhibits proliferation and survival of diffuse large B-cell lymphoma cells by blocking autophagy. Oncotarget. 2017;8:14912–14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu X, Wang A, Liang X, et al. Simultaneous inhibition of Vps34 kinase would enhance PI3Kdelta inhibitor cytotoxicity in the B-cell malignancies. Oncotarget. 2016;7:53515–53525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sehgal AR, Konig H, Johnson DE, et al. You eat what you are: autophagy inhibition as a therapeutic strategy in leukemia. Leukemia. 2015;29:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–1518. [DOI] [PubMed] [Google Scholar]
- [54].Bhalla S, Evens AM, Prachand S, et al. Paradoxical regulation of hypoxia inducible factor-1alpha (HIF-1alpha) by histone deacetylase inhibitor in diffuse large B-cell lymphoma. PLoS One. 2013;8:e81333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lu TX, Young KH, Xu W, et al. TP53 dysfunction in diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2016;97:47–55. [DOI] [PubMed] [Google Scholar]
- [56].Liu J, Wang H, Gu J, et al. BECN1-dependent CASP2 incomplete autophagy induction by binding to rabies virus phosphoprotein. Autophagy. 2017;13:739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang P, Liu X, Li H, et al. TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKbeta/AMPKalpha/mTOR pathway. Sci Rep. 2017;7:3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mahli A, Saugspier M, Koch A, et al. ERK activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut. 2018;67:746–756. [DOI] [PubMed] [Google Scholar]
- [59].Herrero-Sanchez MC, Rodriguez-Serrano C, Almeida J, et al. Targeting of PI3K/AKT/mTOR pathway to inhibit T cell activation and prevent graft-versus-host disease development. J Hematol Oncol. 2016;9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lonetti A, Cappellini A, Bertaina A, et al. Improving nelarabine efficacy in T cell acute lymphoblastic leukemia by targeting aberrant PI3K/AKT/mTOR signaling pathway. J Hematol Oncol. 2016;9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zang C, Eucker J, Liu H, et al. Concurrent inhibition of PI3-kinase and mTOR induces cell death in diffuse large B cell lymphomas, a mechanism involving down regulation of Mcl-1. Cancer Lett. 2013;339:288–297. [DOI] [PubMed] [Google Scholar]
- [62].Knies N, Alankus B, Weilemann A, et al. Lymphomagenic CARD11/BCL10/MALT1 signaling drives malignant B-cell proliferation via cooperative NF-κB and JNK activation. Proc Nat Acad Sci. 2015;112:E7230–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sheng Z, Ma L, Sun JE, et al. BCR-ABL suppresses autophagy through ATF5-mediated regulation of mTOR transcription. Blood. 2011;118:2840–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hu B, Zhang Y, Jia L, et al. Binding of the pathogen receptor HSP90AA1 to avibirnavirus VP2 induces autophagy by inactivating the AKT-MTOR pathway. Autophagy. 2015;11:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hussain S, Feldman AL, Das C, et al. Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Mol Cell Biol. 2013;33:1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Qian Y, Yuan J, Hu H, et al. The CUL4B/AKT/beta-catenin axis restricts the accumulation of myeloid-derived suppressor cells to prohibit the establishment of a tumor-permissive microenvironment. Cancer Res. 2015;75:5070–5083. [DOI] [PubMed] [Google Scholar]
- [67].Yuan L, Lv Y, Li H, et al. Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat Cell Biol. 2015;17:1169–1181. [DOI] [PubMed] [Google Scholar]
- [68].Zhao Y, Sun Y. CUL4B ubiquitin ligase in mouse development: a model for human X-linked mental retardation syndrome? Cell Res. 2012;22:1224–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Huang G, Liu TT, Weng SW, et al. CUL4A overexpression as an independent adverse prognosticator in intrahepatic cholangiocarcinoma. BMC Cancer. 2017;17:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang Y, Wen M, Kwon Y, et al. CUL4A induces epithelial-mesenchymal transition and promotes cancer metastasis by regulating ZEB1 expression. Cancer Res. 2014;74:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


