ABSTRACT
Aims: To evaluate the biological significance of dense vascular networks associated with low-grade NENs, we assessed the impact of PDGFRα tissue expression in 77 GEP/NEN patients, associating PDGFRα expression with the morphological characterization in low-grade tumors.
Methods and results: Paraffin-embedded specimens of 77 GEP- NEN tissues, collected from January 2006 to March 2018, were evaluated for PDGFRα tissue expression and correlations with clinicopathological characteristics. PDGFRα tissue expression was significantly correlated with grade and the NEN growth pattern (p < 0.001) but not with gender, primary site or lymph nodes metastatic status. PDGFRα staining was mainly localized in the vascular pole of the neuroendocrine cells and in Enterochromaffin (EC) cells. In particular PDGFRα tissue expression was significantly more expressed in G2 (p < 0.001) than G1 and G3 cases (p 0.004; p < 0.0002;) and correlated with an insular growth pattern. PDGFRα tissue expression was associated with the Ki67 index and we found a significant negative trend of association with the Ki67 proliferation index (P < 0.001): thus PDGFRα expression is referred to morphological and not to proliferative data.
Conclusions: PDGFRα represents an effective target for new anti-angiogenic treatment in WD- GEP-NENs, in particular in G2 cases, and in G3 cases only when there is a mixed insular-acinar pattern. In this context, it is important to carefully delineate those tumors that might better respond to this type of treatment alone or in combination. Further investigation of the relationship between PD-L1 and PDGFRa is warranted, and may contribute to optimize the therapeutic approach in patients with GEP-NENs.
Keywords: PDGFRα, NENs, immunotherapy, antiangiogenic therapies, epithelial–mesenchymal transition (EMT)
Introduction
Unlike traditional chemotherapy, biological therapy and immunotherapy represent a turning point and a revolutionary strategy in new oncology. In particular, rare tumors that are difficult to diagnose, such as gastroenteropancreatic neuroendocrine tumors (GEP-NEN), often defined as occult tumors, lack targeted markers and precise therapies.1 Despite their new classification (2010/2017 WHO), the fact remains that the clinical behavior of NEN is highly unpredictable, so low-grade cases can unexpectedly often present with advanced disease at diagnosis.2,3 At present it is not yet possible to define which biological, pathological or clinical characteristics could be able to characterize this ‘low risk’ subset of patients within the G1-G2 group. Current treatments for G1-2 well-differentiated (WD-NEN) tumors include somatostatin analogues (octreotide or lanreotide), platinum-based chemotherapy and targeted therapies but there is no precise definition of the best sequence.4–6 In most cases, these treatments have had poor responses. Therefore, in this era of personalized medicine, biomarkers predictive of response to therapy are central to treatment decision making. For this reason, differentiation, anaplasia, proliferative capacity and metastatic tendencies, angiogenesis and immunosuppression are hallmarks of cancer that have become the focus of close study.7 There is accumulating evidence that angiogenesis and immunosuppression are interconnected and facilitated by shared regulators not only during normal physiological processes, but also in cancer development.8 Notably, the vascular network, in terms of entity and typology, is an important regulator of the immune response because it controls lymphocyte trafficking9 and also acts as a mediator of localized immunosuppression within the tumor microenvironment (TME).10 The highly vascular nature of low-grade NETs, and hypoxia-dependent angiogenesis in high-grade NETs, led to an initial interest in angiogenesis inhibition as a treatment possibility in this disease.11–15 Whereas a high microvascular density is an adverse prognostic marker in most carcinomas, it seems to be a favorable parameter in pancreatic NENs: the intratumoral microvascular density is higher in low-grade than in high-grade tumors and is associated with a good prognosis, prolonged survival and a higher VEGF expression, a phenomenon called the “neuroendocrine paradox”.16,17 This paradox can be explained by the observation that cancer produces a poor imitation, like a monkey’s, of the tissue from which it derives. With increasing aggressiveness, WD NEN can have more vessels, as in normal tissue, compared to PD NEN. However, differences in the vascular architecture of pNENs and GI-NENs are likely associated with significant differences in the structural and functional relationships between tumor cells and vessels.18–20 Several pathways for tumor growth and tumor angiogenesis, including the VEGF, fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) pathways that have recently been identified, have attracted substantial interest due to their potential clinical applications.21,22 In particular, the PDGF family is known to be associated with tumor growth in different kinds of tissues, pathological conditions and angiogenesis through two cell-surface tyrosine kinase receptors, PDGFRα and PDGFRβ, and to induce angiogenesis by up-regulating VEGF production and modulating the proliferation and recruitment of perivascular cells.23 Furthermore, PDGFRα is believed to mediate multiple cellular behaviors such as migration, proliferation and cell survival via activation of several downstream pathways including MAPK (Erk1/Erk2), PI3K/Akt and PLCγ/PKC.24,25 A number of studies have illustrated the importance of PDGFα and PDGFβ in the recruitment of tumor stroma in skin cancer, melanoma, breast cancer, and lung cancer.26–28 Several studies highlighted that PDGFs/PDGFRs are often overexpressed in different tumors including colorectal cancer and their expression is associated with diagnosis,29,30 tumor growth,31 drug resistance,32 invasion, and poor survival.33 Clearly, GEP-NENs are capable of secreting a wide array of active growth factors which interact with tumour and its surrounding stroma in a complex autocrine and paracrine fashion. By identifying these key members of the growth factor pathway, novel therapeutic targets may be discovered. Many study have provide that PDGF ligands are expressed by cancer cells, whereas PDGF-Rs are expressed mainly by stromal cells.34,35 In particular PDGFR-α has been found on both tumour cell and its surrounding stroma, while PDGFR-β was only found in the stroma36-38 Moreover the distinction between PDGFR-A and PDGFR-B signaling is increasing with the discovery that PDGFR-A, not PDGFR-B, is localized and activated in a specialized structure on the cell membrane, the primary cilium, where PDGFR-A can activate MAPK and Akt signaling.39,40 Mutational activation in platelet-derived growth factor receptor alpha (PDGFRA), and stem cell factor receptor (KIT) has been assessed in neuroendocrine tumors to identify biomarkers that could potentially indicate the response of these neoplasms to chemotherapeutics developed against those targets. A good response was obtained in GIST and in other cancers.41,42 PDGF and its receptors (PDGFRs) are also expressed in 70% of carcinoid tumors37,43 and have been found on both tumor cells and stroma of carcinoid tumors, indicating a possible autocrine loop supporting tumor growth. Given the common neuro-oncogenic nature between GIST and NEN we deemed appropriate to evaluate the expression of PDGFRα in NENs. Moreover, apart from the autocrine mechanism, recent studies revealed a critical role for paracrine PDGF signaling in carcinogenesis through the regulation of epithelial-stromal interactions.44 Paracrine PDGF signaling is commonly observed in epithelial cancers, where it triggers stromal recruitment and may be involved in the epithelial–mesenchymal transition phenomenon, thereby affecting tumor growth, angiogenesis, invasion, and metastasis.45–47 Further systematic investigations in this promising field may lead to the discovery of novel therapeutic targets. Here we provide evidence of the impact of PDGFRα tissue expression on the grade and growth pattern of 77 GEP/NEN patients, associating PDGFRα expression with the morphological characterization and with low-grade neuroendocrine tumors and their vasculature pattern. Thus, anti-VEGF/PDGFRα drugs administration may become the pharmacological choice for WD-NENs, using a higher PDGFRα immunostaining pattern as a marker in tumors that show a morphologically bland appearance.
Patients and methods
Patients characteristics
Paraffin-embedded specimens of 77 GEP- NEN tissues were collected from January 2006 to March 2018 at the IRCCS ‘Saverio de Bellis’of Castellana Grotte (Ba, Italy). The following clinicopathological characteristics were collected for all patients: age, gender, primary site, tumor grade.
Pathological assessment
Histology was assessed in all tumors by two pathologists who reviewed FFPE tissue sections stained with hematoxylin and eosin (H&E), and a representative paraffin block from each specimen was chosen for IHC analysis. On H&E and PAS mucin-stained sections, the cytological characteristics of cells, growth patterns, the presence of ulcerations, perineural infiltration, vascular permeation and the presence of necrosis were evaluated. The specimens were classified according to the WHO 2010 and to the last WHO 2017 only for p-NENs. Morphologically, WD and PD- NENs were also distinguished separately according to the following growth pattern criteria to better analyze differences due to the vascular pattern. In detail, according to Soga and Tazawa,43 the architectural pattern of growth was subclassified as follows: insular solid, trabecular, acinar and poorly-differentiated type.
IHC and IHC evaluation
IHC analysis for PDGFRα was performed in the FFPE of 77 patients with NENs. Tumor sections of 4 μm were freshly cut and dried at 60 °C for 30 min. IHC analysis was carried out in sections after deparaffinization for 30 min and then rehydration in grades of alcohol. Antigen retrieval was performed at 90°C for 20 min with CitratoBuffer. To assess the PDGFRα staining employed for the present study, antibodies (clone C-20 Santa Cruz Biotechnology,Inc. CA, at 1:100 dilution) were evaluated on the NENs, using an automated autostainer (cat. K5007, Dako, Glostrup, Denmark). The Real Envision DAB Substrate Kit (DAKO) was used according to the manufacturer’s instructions. The staining pattern of PDGFRα antibodies was as expected: cellular membranous/submembranous and cytoplasmic. PDGFRα expression was scored for all staining patterns, according to both the staining intensity and the percentage of positively stained cells, by two independent, blinded pathologists; discrepancies in the interpretation of scoring were resolved by consensus. PDGFRa expression was scored: 0: (no staining) negative; 1: weak expression, but weaker than the positive control, staining in <5 % of tumor cells; 2: moderate expression in ≥ 5 % of tumor cells; and 3: strong; more than control membrane staining in >5 % of the tumor cells. For the data assessment, our cases were considered positive for PDGFRα expression only if they had scores of 2+ or 3+.
Statistical analysis
Correlations between PDGFRα expression and clinicopathological parameters were determined by Chi-square test. A p-value less than 0.05(*) was considered statistically significant. Associations of the PDGFRα score and and Ki67 index were analyzed with the Kruskal-Wallis rank test; Wilcoxon rank-sum (Mann-Whitney) test. All evaluations were performed using StataCorp. 2007 Stata Statistical Software: release 10 (StataCorp LP, College Station, TX, USA).
Results
Patient population
The characteristics of the 77 cases of NENs are summarized in Table 1. Patients median age was 68 years (range: 24–96): there were 37 females (48.05%) and 40 males (51.95%). Primary sites included the stomach (27.27%), small intestine (29.87%), liver (12.99%), gallbladder (2.60%), colon rectum (7.79%) appendix (6.49%) and pancreas (12.99%). The cases included are classified, according to 2010/2017 WHO, as follows: 50 grade 1 (64.94%), 12 grade 2 (15.58%) and 15 grade 3 (19.48%).
Table 1.
Clinicopathologic features of 77 patients with NEN.
| n = 77 | |
|---|---|
| Gender (M) (%) | 40 (51.95) |
| Age (yrs) (M ± SD) | 65.31 ± 14.69 |
| Median, (min-max) | 68 (24–96) |
| Localizzation | |
| Stomach | 21 (27,27) |
| Small Intetinal | 23 (29,87) |
| Colon- rectum | 6 (7,79) |
| Appendix vermiforme | 5 (6,49) |
| Pancreas | 10 (12,99) |
| Liver | 10 (12,99) |
| Gallbladder | 2 (2,60) |
| Grade (%) | |
| G1 | 50 (64.94) |
| G2 | 12 (15.58) |
| G3 | 15 (19.48) |
| Pattern Growth (%) | |
| Acinar | 17 (22.08) |
| Insular | 31 (40.26) |
| Trabecolar | 14 (18.18) |
| Poorly differentiated | 13 (19.48) |
| Angioinvasion | |
| Absent | 66 (86.00) |
| Present | 11 (14.00) |
| Lymphocytic infiltration | |
| Absent | 66 (86.00) |
| Present | 11 (14.00) |
The architectural growth pattern was subclassified as follows: 31 insular solid type (40.26%), 14 trabecular type (18.18%), 17 acinar type (22.08 %) and 13 poorly-differentiated type (16.88%). Among PD- NEN, we highlighted 2 (2.60%) patterns with a mixed insular-acinar form (Table 1). Although different architectural types may be related to the different site of origin, we highlighted a low association between type and origin (p 0.115; Table 2). In particular, in our cases, stomach neoplasms are mostly of trabecular type (50.00%), small intestine and colon rectum are frequently of insular type (41.94%; 9.68 %), whereas pancreas, liver and gallbladder neoplasms are mostly of acinic type (23.53%; 11.76%; 11.76 %) while the appendix has a mixed pattern between acinic (11.76%) and trabecular type (14.29%) (Table 2).
Table 2.
Different architectured types releted to site of origine.
| Growth Pattern |
|||||
|---|---|---|---|---|---|
| Acinar n(%) |
Insular n(%) |
Trabecolar n(%) |
Total n(%) |
||
| Localizzation | (n = 17) | (n = 31) | (n = 14) | (n = 62) | p-value* |
| Stomach | 2 (11.76) | 9 (29.03) | 7 (50.00) | 18 (29.03) | 0.115 |
| Small Intestinal | 5 (29.41) | 13 (41.94) | 3 (21.43) | 21 (33.87) | |
| Colo-rectum | 0 (–) | 3 (9.68) | 1 (7.14) | 4 (6.45) | |
| Appendix | 2 (11.76) | 1 (3.23) | 2 (14.29) | 5 (8.06) | |
| Pancreas | 4 (23.53) | 4 (12.90) | 1 (7.14) | 9 (14.52) | |
| Liver | 2 (11.76) | 1 (3.23) | 0 (–) | 3 (4.84) | |
| Gallbladder | 2 (11.76) | 0 (–) | 0 (–) | 2 (3.23) | |
PDGFRα expression in nens
To evaluate the biological significance of dense vascular networks associated with low-grade NENs, we analyzed the PDGFRα tissue expression in our 77 NENs. Among these, 18 cases resulted negative (23.4%) and 59 cases positive (76.6%). The relationship between PDGFRα expression and gender was not significant (P = 0.47). Our analysis showed that PDGFRα tissue expression was significantly correlated with grade (p < 0.001; (Table 3) but not gender, primary site or lymph nodes metastatic status. The PDGFRα staining intensity score on neoplastic cells was different among the three WHO grades. Based on the PDGFRα signal intensity, we created a score (Table 4) from 0 to 3+ (absent to strong). Importantly, PDGFRα expression was absent in 13 G3 patients (86.67%), while in G2 PDGFRα expression was present in all cases, although with a different intensity: medium staining in 3 (25%) cases and strong in 7 (58%) cases (Table 3). These data indicate that PDGFRα tissue expression was significantly stronger in G2 than G1 and G3 cases (p 0.004; p < 0.0002; Table 4). Furthermore, a statistically significant difference in PDGFRα expression was observed between the NEN growth pattern and grade, specifically in insular type G2 and G1 cases. G1 cases with an insular type pattern had a score of 2+ (60.71%) while the rest (6 patients) had a score of 3+ (100%). G2 patients showed a medium (2+) PDGFRα signal in 66.67 % and strong (3+) in 85.71 % of cases. In Insular pattern cases PDGFRα expression was higher than in acinar and trabecular patterns, and the insular type was significantly associated with a medium and strong PDGFRa (p < 0.001; Table 5; Figure 1). Among poorly-differentiated NEN (G3), only 2 (2.60%) cases expressed a pattern like that of the mixed insular-acinar form and had a score 2+, while in 97.40 % of G3 cases PDGFRα expression was absent. These data indicate that PDGFRα tissue expression is correlated with an insular growth pattern (Figure 2A–B) and that the PDGFRα signal is lost with the reduction and loss of differentiation. In particular, we highlighted PDGFRα staining mainly localized in the vascular pole of the neuroendocrine cell (Figure 2C–D), suggesting a peculiar functional role, as occurs for hormonal products. In insular type cases we observed emargination due to the polarization of the cells and a more intense positivity at the invasive tumor front than in the center of tumors where necrosis is often present (Figure 2B). Moreover, we detected a αhigh expression of PDGFRα in Enterochromaffin (EC) cells with a finely granular but intense positivity, with peripheral-membranous enhancement at the vascular pole of neuroendocrine cells (Figure 3). PDGFRα tissue expression was associated with Ki67 staining. We found a significant negative trend of association with the Ki67 proliferation index (P < 0.001): as the PDGFRα score increased, so the Ki67 index decreased, therefore PDGFRα is referred to morphological and non-proliferative data (Figure 4)
Table 3.
PDGFRα expression on different grade of NEN.
| Categories PDGFRα |
|||
|---|---|---|---|
| Absent (n = 18) |
Present (n = 59) |
p-value* | |
| Gender (M) (%) | 8 (44.44) | 32 (54.24) | 0.47 |
| Grade (%) | <0.001 | ||
| G1 | 5 (10.00) | 45 (90.00) | |
| G2 | 0 (0.00) | 12 (100) | |
| G3 | 13 (86.67) | 2 (13.33) | |
*Chisquare-test.
Table 4.
PDGFRa expression on different grade of NEN.
| Grade |
|||||||
|---|---|---|---|---|---|---|---|
| G1 |
G2 |
G3 |
Comparisons (p-value)§ |
||||
| (a) | (b) | (c) | p-value* | (b)vs(a) | (c)vs(a) | (c)vs(b) | |
| Score PDGFRα (%) | <0.001 | ||||||
| Absent | 5 (10.00) | 0 (0.00) | 13 (86.67) | 0.02 | <0.0001 | <0.0001 | |
| Weak | 11 (22.00) | 2 (16.67) | 0 (0.00) | 0.67 | 0.0004 | 0.14 | |
| Medium | 28 (56.00) | 3 (25.00) | 2 (13.33) | 0.04 | 0.0004 | 0.46 | |
| Strong | 6 (12.00) | 7 (58.33) | 0 (0.00) | 0.004 | 0.01 | 0.0002 | |
*Chisquare-test
^Fisher’s exact test
§Test z for proportions.
Table 5.
PDGFRα intensity score on neoplastic cells.
| Score PDGFRα |
|||||
|---|---|---|---|---|---|
| Absent (0) |
Weak (1+) |
Medium (2+) |
Strong (3+) |
p-value * | |
| Grade (G1) | |||||
| Pattern Growth (%) | <0.001 ^ | ||||
| Acinar | 1 (20.00) | 3 (27.27) | 11 (39.29) | 0 (0.00) | |
| Insular | 0 (0.00) | 0 (0.00) | 17 (60.71) | 6 (100) | |
| Trabecolar | 4 (80.00) | 8 (72.73) | 0 (0.00) | 0 (0.00) | |
| Grade (G2) | |||||
| Pattern Growth (%) | 0.05 ^ | ||||
| Acinar | 0 (0.00) | 0 (0.00) | 1 (33.33) | 1 (14.29) | |
| Insular | 0 (0.00) | 0 (0.00) | 2 (66.67) | 6 (85.71) | |
| Trabecolar | 0 (0.00) | 2 (100) | 0 (0.00) | (0.00) | |
| Grade (G3) | |||||
| Pattern Growth (%) | 0.01 ^ | ||||
| Poorly differentiated | 13 (100) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Pattern like insular-acinar | 0 (0.00) | 0 (0.00) | 2 (100) | 0 (0.00) | |
*Chisquare-test
^Fisher’s exact test
§Test z for proportions.
Figure 1.

Representative patterns of PDGFRα in different architectural types: A) insular solid type B) acinar type C) trabecular type D) poorly-differentiated type. (magnification 20×).
Figure 2.
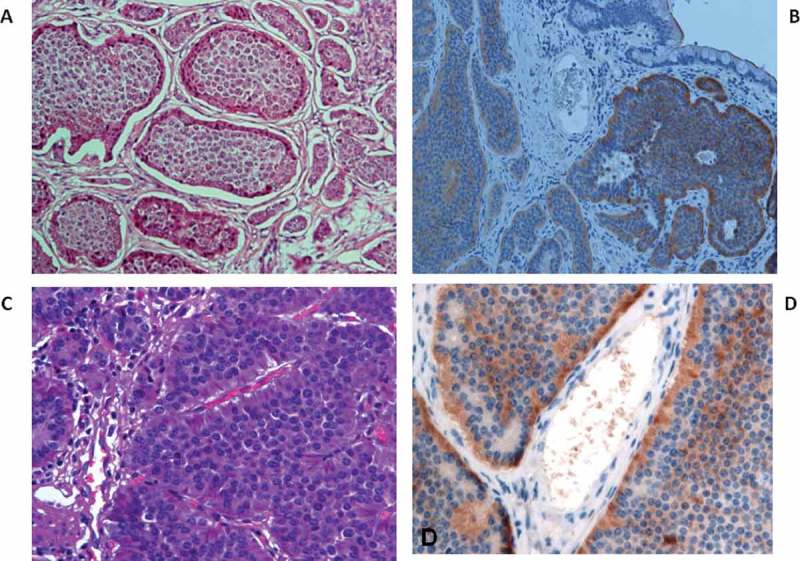
A-B) PDGFRα tissue expression in the insular solid growth pattern C-D) PDGFRα staining localized in the vascular pole of the neuroendocrine cell.
Figure 3.
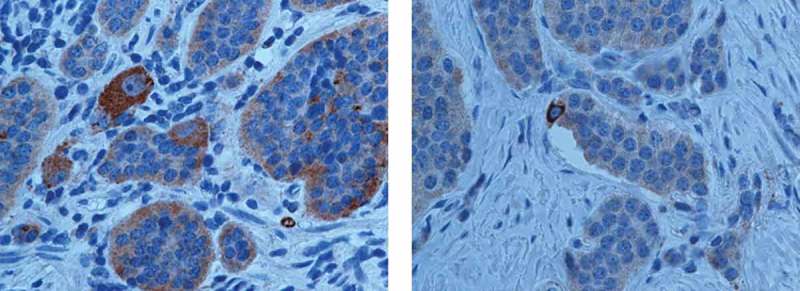
Representative PDGFRα staining in Enterochromaffin (EC) cells with a finely granular positivity with peripheral-membranous enhancement at the vascular pole of neuroendocrine cells.
Figure 4.

Association between the PDGFRα score and Ki67 index in NENs patients p < 0.001): Box plots of PDGFRα score and Ki67 index (expression %) *** = p < 0.001.
Discussion
Angiogenesis, like immunosuppression, is a crucial step in the development, invasiveness and malignant behavior of numerous solid tumors, and in many cases angiogenic regulation under the control of VEGF, FGF and PDGF plays a central role that may be strategically exploitable in the therapeutic setting. It has been widely demonstrated that VEGF and PDGF participate in changing the vascular permeability of endothelial cells and hence the tumor malignant potential expressed as metastatic power.48 As one of the peculiar features of GEP-NETs is a prominent hypervascularization of tissue, this offers an attractive target for anti-angiogenic treatment. The data available about gastrointestinal NENs are scarce and contradictory,18,20 and the relation between angiogenic factors (VEGF,PDGF, FGF), density, typology of the tumor vascular network and prognosis in GI-NENs cases is not straightforward. In the present study we investigated the impact of PDGFRa tissue expression on the grade and growth pattern of 77 GEP/NEN patients, associating PDGFRa expression with the morphological characterization of low-grade neuroendocrine tumors.
Low grade neuroendocrine tumors (G1-G2) have an extraordinary vascularization which is associated with the expression of many proangiogenic factors mimicking normal endocrine organ products. GI-NENs secrete a number of biologically active vascular endothelial growth factors that interact with the tumor cells and the surrounding stroma in a complex autocrine/paracrine fashion. In NENs, intratumor vessel density is associated with a good prognosis and prolonged survival, unlike in other digestive tumors. Recent studies revealed a critical role for paracrine PDGF signaling in carcinogenesis through the regulation of epithelial-stromal interactions, thereby affecting tumor growth, angiogenesis, invasion, and metastasis. Our results indicate, for the first time, that PDGFRα tissue expression was significantly correlated with grade and, in particular, was more expressed in G2 than G1 and G3 cases (p 0.004; p < 0.0002). NENs with stronger PDGFRα staining (G1-G2) were less aggressive than weaker PDGFRα staining NENs, confirming the “ neuroendocrine paradox” already observed for pNENs and their vascular pattern.16 Therefore, PDGFRα tissue expression is correlated with an insular growth pattern and the PDGFRα signal is lost with the reduction and loss of differentiation; in fact, in 97.40 % of our G3 cases PDGFRα expression was absent. The reduction in PDGFRα expression was associated with a reduction in microvessel density and tumor growth. To demonstrate that PDGFRα plays an important mediator role in the epithelial – stromal-interaction we highlighted PDGFRα staining mainly localized at the vascular pole of the neuroendocrine cell with a marked emargination due to the polarization of the cells along the vessels; we also evidenced a more intense positivity at the invasive tumor front than in the center of the tumors, where necrosis is more often present. Considering that currently the marker of choice to better classify these tumors remains the ki67 index, along with the morphological characterization, we evaluated the association between ki67 and PDGFRα. In particular, we found a negative trend of association of Ki67 with the proliferation index (P < 0.001). Thus demonstrates that PDGFRα is referred to morphological and not to proliferative data. The functional importance of anti-vascular endothelial growth factor in tumor angiogenesis and in immunosuppression has provided a convincing rationale for the development of inhibitors targeting the VEGF signaling pathway.49 Targeted therapy with the vascular endothelial growth factor pathway inhibitor sunitinib and the mTOR inhibitor everolimus has been shown to improve progression-free survival in patients with progressive pancreatic but not midgut NENs,50,51 although this success had only a limited impact on overall survival of cancer patients and rarely resulted in durable responses.52 WD-GEP-NEN are characterized by an unpredictable clinical course, thus the association between PDGFRα expression and the morphological characterization of low-grade neuroendocrine tumors defines PDGFRα as an active participant in tumorigenesis. This should drive the development of novel therapies aimed at reducing tumor growth, possibly suppressing PDGFRα production. Angiogenic agents as drugs targeting the stroma of different cancers will become very important in the future due to the recent observation and publication of data about a “ new organ”, the so- called “interstitium” that represents the ubiquitary stromal scaffold of all human systems, and plays an active role, not only offering passive support in carcinogenesis.53 We recognize that our 77 NENs cases, being rare tumors, are necessarily a small cohort, but the remarkable incidence of PDGFRα positivity obtained in our patients with grade G2 disease warrants larger future studies. Given the recent success of immunotherapies alone or combined in some tumors, antiangiogenic treatment associated with immune checkpoint blockers has become an attractive strategy in GEP- NEN. In our previous work we highlighted the role of programmed cell death ligand 1 (PD-L1) in GEP-NEN:54 PD-L1 expression was significantly associated with a high-grade WHO classification (G3), becoming the new gold standard for G3 NEN discrimination. Furthermore, pharmacological approaches using anti-PD-1 antibodies may become the logical choice for the treatment of G3 cases with a poor prognosis, while for G2 cases anti-angiogenic drugs could be an excellent therapeutic choice. A recent work highlighted the fact that targeted antiangiogenic and immune-based therapies have improved outcomes in advanced kidney cancer, yet novel strategies are needed to extend the duration of these benefits and extend them to more patients and tumor varieties.55 Therefore, targeting the tumor vasculature to induce vessel normalization may provide a promising strategy to optimize the efficacy of currently employed immunotherapies as it could reduce the level of immunosuppression in the tumor microenvironment (TME). In this regard, since NEN are notoriously clinically and biologically heterogeneous tumors resulting in unpredictable clinical outcomes, it is clear that to exploit the full potential of the immune system to cure cancer, we will have to act at multiple levels in order to ‘normalize’ the TME. Furthermore, based on these observations, treatment with a simultaneous combination of anti-PDGFRα and anti–PD-L1 antibodies may yield durable responses in all GEP-NEN cases. Moreover, PDGFRα might be a useful prognostic biomarker in WD- GEP-NENs. At present, it seems that G2 tumors might be the most suitable targets. In this context, it is important to carefully delineate those tumors that might better respond to this type of treatment. Further investigation of the relationship between PD-L1 and PDGFRα is warranted, and may contribute to optimize the therapeutic approach in patients with GEP-NENs.
Biography
Elisabetta Cavalcanti: study design, data interpretation, evaluation, writing and editing of the manuscript. A. Ignazzi: assembly of tissue and data,study design and immunohistological analysis. F De Michele histological evaluation. ML Caruso: evaluation and editing of the manuscript. All authors read the manuscript and approved its submission.
Funding Statement
The authors have not been funded for this work.
Conflict of Interest
The authors declare no conflict of interest related to this study.Compliance with ethical standards.
References
- 1.Klöppel G. Neuroendocrine neoplasms: dichotomy, origin and classifications. Visc Med. 2017;33(5):324–333. doi: 10.1159/000481390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO classification of tumours of endocrine organs (Vol. 10). Lyon: IARC Press; 2017. [Google Scholar]
- 3.Fazio N, Milione M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: new insights and treatment implications. Cancer Treat Rev. 50:61–67. doi: 10.1016/j.ctrv.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Müller -H-H, Arnold R.. 2017. Placebo-controlled, doubleblind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology. 104:26–32. doi: 10.1159/000443612. [DOI] [PubMed] [Google Scholar]
- 5.Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013. May;42(4):557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, et al. 2016. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 387:968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11(10):702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 9.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39(1):61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terme M, Colussi O, Marcheteau E, Tanchot C, Tartour E, Taieb J.. 2012. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol. 2012:492920. doi: 10.1155/2012/492920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner HE, Harris AL, Melmed S, Wass JAH. Angiogenesis in endocrine tumors. Endocr Rev. 2003;24(5):600–632. doi: 10.1210/er.2002-0008. [DOI] [PubMed] [Google Scholar]
- 12.Terris B, Scoazec J, Rubbia L, Bregeaud L, Pepper MS, Ruszniewski P, Belghiti J, Fléjou J, Degott C. Expression of vascular. endothelial growth factor in digestive neuroendocrine tumors. Histopathology. 1998;32(2):133–138. [DOI] [PubMed] [Google Scholar]
- 13.Schnirer II, Yao JC, Ajani JA. Carcinoid–a comprehensive review. Acta Oncol. 2003;42(7):672–692. [DOI] [PubMed] [Google Scholar]
- 14.Scoazec JY. Angiogenesis in neuroendocrine tumors: therapeutic applications. Neuroendocrinology. 2013;97(1):45–56. doi: 10.1159/000338371. [DOI] [PubMed] [Google Scholar]
- 15.Kulke MH. Clinical presentation and management of carcinoid tumors. Hematol Oncol Clin North Am. 2007;21(3):433–455. doi: 10.1016/j.hoc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K, et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92(1):94–101. doi: 10.1038/sj.bjc.6602245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marion-Audibert AM1, Barel C, Gouysse G, Dumortier J, Pilleul F, Pourreyron C, Hervieu V, Poncet G, Lombard-Bohas C, Chayvialle J-A, et al. Low microvessel density is an unfavorable histoprognostic factor in pancreatic endocrine tumors. Gastroenterology. 2003;125(4):1094–1104. [DOI] [PubMed] [Google Scholar]
- 18.La Rosa S, Uccella S, Finzi G, Albarello L, Sessa F, Capella C. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: correlation with microvessel density and clinicopathologic features. Hum Pathol. 2003;34(1):18–27. doi: 10.1053/hupa.2003.56. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J1, Jia Z, Li Q, Wang L, Rashid A, Zhu Z, Evans DB, Vauthey J-N, Xie K, Yao JC. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer. 2007;109(8):1478–1486. doi: 10.1002/cncr.22554. [DOI] [PubMed] [Google Scholar]
- 20.Besig S, Voland P, Baur DM, Perren A, Prinz C. Vascular endothelial growth factors, angiogenesis, and survival in human ileal enterochromaffin cell carcinoids. Neuroendocrinology. 2009;90(4):402–415. doi: 10.1159/000245900. [DOI] [PubMed] [Google Scholar]
- 21.Merla A, Goel S. Novel drugs targeting the epidermal growth factor receptor and its downstream pathways in the treatment of colorectal cancer: a systematic review. Chemother Res Pract. 2012;2012:387172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inanç M, Er O, Karaca H, Berk V, Ozkan M, Saraymen R, Elmalı F. factor levels during chemotherapy in patients with metastatic colorectal cancer. Med Oncol. 2012;29(5):3119–3124. doi: 10.1007/s12032-012-0250-8. [DOI] [PubMed] [Google Scholar]
- 23.Laschke MW, Elitzsch A, Vollmar B, Vajkoczy P, Menger MD. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006. January;21(1):262–268. doi: 10.1093/humrep/dei308. [DOI] [PubMed] [Google Scholar]
- 24.Choudhury GG, Grandaliano G, Jin DC, Katz MS, Abboud HE. Activation of PLC and PI 3 kinase by PDGF receptor alpha is not sufficient for mitogenesis and migration in mesangial cells. Kidney Int. 2000. March;57(3):908–917. doi: 10.1046/j.1523-1755.2000.00907.x. [DOI] [PubMed] [Google Scholar]
- 25.Mehrotra M, Krane SM, Walters K, Pilbeam C. Differential regulation of platelet-derived growth factor stimulated migration and proliferation in osteoblastic cells. J Cell Biochem. 2004. November 1;93(4):741–752. doi: 10.1002/jcb.20138. [DOI] [PubMed] [Google Scholar]
- 26.Seymour L, Dajee D, Bezwoda WR. Tissue platelet derived-growth factor (PDGF) predicts for shortened survival and treatment failure in advanced breast cancer. Breast Cancer Res Treat. 1993;26(3):247–252. [DOI] [PubMed] [Google Scholar]
- 27.Takanami I, Imamura T, Yamamoto Y, Kodaira S. Usefulness Usefulness of platelet-derived growth factor as a prognostic factor in pulmonary adenocarcinoma. J Surg Oncol. 1995;58(1):40–43. [DOI] [PubMed] [Google Scholar]
- 28.Herraiz C, Jiménez-Cervantes C, Sánchez-Laorden B, García-Borrón J. Functional interplay between secreted ligands and receptors in melanoma. Semin Cell Dev Biol. 2018;78:73–84. [DOI] [PubMed] [Google Scholar]
- 29.Manzat-Saplacan RM, Balacescu L, Gherman C, Visan S, Chira RI, Bintintan A, Nagy G, Popovici C, Valean SD, Anca C, et al. Is there a correlation between peripheral blood expression of angiogenic transcriptional factors/receptors and colorectal cancer? J BUON. 2015;20(5):1193–1200. [PubMed] [Google Scholar]
- 30.Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Hamilton SR, Fidler IJ. Expression of activated platelet-derived growth factor receptor in stromal cells of human colon carcinomas is associated with metastatic potential. Int J Cancer. 2006;119(11):2567–2574. doi: 10.1002/ijc.22229. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y. Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol Med. 2013;19(8):460–473. doi: 10.1016/j.molmed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Homsi J, Daud AI. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control. 2007;14(3):285–294. doi: 10.1177/107327480701400312. [DOI] [PubMed] [Google Scholar]
- 33.Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, Shiraga S, Bainbridge T, Morich J, Heinrich MC. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23(23):5357–5364. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- 34.Paulsson J, Sjoblom T, Micke P, Ponten F, Landberg G, Heldin CH, Bergh J, Brennan DJ, Jirstrom K, Ostman A. 2009. Prognostic Significance of stromal platelet-derived growth factorβ- receptor expression in human breast cancer. Am J Pathol. 175:334–341. doi: 10.2353/ajpath.2009.081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding W, Knox TR, Tschumper RC, Wu W, Schwager SM, Boysen JC, Jelinek DF, Kay NE. 2010. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 116:2984–2993. doi: 10.1182/blood-2010-02-269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauchamp D, Coffey RJ Jr., Lyons RM, Perkett EA, Townsend CM Jr., Moses HL. Human carcinoid cell production of paracrine growth factors that can stimulate fibroblast and endothelial cell growth. Cancer Res. 1991;51:5253–5260. [PubMed] [Google Scholar]
- 37.Chaudhry RA, Funa K, Oberg K. 1993. Expression of growth factor peptides and their receptors in neuroendocrine tumors of the digestive system. Acta Oncol. 32:107–114. doi: 10.3109/02841869309083898. [DOI] [PubMed] [Google Scholar]
- 38.Pietras K1, Sjöblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003. May;3(5):439–443. [DOI] [PubMed] [Google Scholar]
- 39.Bartoschek M, Pietras K. PDGF family function and prognostic value in tumor biology. Biochem Biophys Res Commun. 2018. June 22;S0006-291X(18)31418–9. doi: 10.1016/j.bbrc.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 40.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005. October 25;15(20):1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen C-J, Joseph N, Singer S, Griffith DJ, Haley A, Town A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 42.Knösel T, Chen Y, Altendorf-Hofmann A, Danielczok C, Freesmeyer M, Settmacher U, Wurst C, Schulz S, Yang LL, Petersen I. High High KIT and PDGFRA are associated with shorter patients survival in gastroenteropancreatic neuroendocrine tumors, but mutations are a rare event. J Cancer Res Clin Oncol. 2012;138(3):397–403. doi: 10.1007/s00432-011-1107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soga J, Tazawa K. Pathologic analysis of carcinoids. Histologic evaluation of 62 cases. Cancer. 1971;28(4):990–998. [DOI] [PubMed] [Google Scholar]
- 44.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008. January 29;5(1):e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishii Y, Hamashima T, Yamamoto S, Sasahara M. 2017. Pathogenetic significance and possibility as a therapeutic target of platelet derived growth factor. Pathol Int. 67:235–246. doi: 10.1111/pin.12530. [DOI] [PubMed] [Google Scholar]
- 46.Demoulin JB, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014;25(3):273–283. doi: 10.1016/j.cytogfr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Huijbers EJ, vanBeijnum JR, Thijssen VL, Sabrkhany S, Nowa Sliwinska P, Griffioen AW. 2016. Role of the tumor stroma in resistance to anti angiogenic therapy. Drug Resist Updates. 25:26–37. doi: 10.1016/j.drup.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15(6):385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 50.Chan JA, Kulke MH. Medical management of pancreatic neuroendocrine tumors: current and future therapy. Surg Oncol Clin N Am. 2016;25(2):423–437. doi: 10.1016/j.soc.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 52.Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy Angiogenesis. Angiogenesis. 2017;20(2):185–204. doi: 10.1007/s10456-017-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benias PC, Wells RG3, Sackey-Aboagye B, Klavan H, Reidy J, Buonocore D, Miranda M, Kornacki S, Wayne M, Carr-Locke DL, et al. Structure and distribution of an unrecognized interstitium in human tissues. Sci Rep. 2018. March 27;8(1):4947. doi: 10.1038/s41598-018-23062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavalcanti E, Armentano R, et al. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis. 2017;8(8):e3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Einstein DJ, McDermott DF. Combined blockade of vascular endothelial growth factor and programmed death 1 pathways in advanced kidney cancer. Clin Adv Hematol Oncol. 2017;15(6):478–488. [PubMed] [Google Scholar]


