Abstract
Background:
Pediatric bipolar disorder (PBD) (occurring prior to age 18) is a developmental brain disorder that is among the most severe and disabling psychiatric conditions affecting youth. Despite increasing evidence that brain connectivity is atypical in adults with bipolar disorder, it is not clear how brain connectivity may be altered in youths with PBD.
Methods:
This cross-sectional resting-state functional magnetic resonance imaging study included 80 participants recruited over 4 years: 32 youths with PBD, currently euthymic (13 males; 15.1 years old) and 48 healthy controls (HC) (27 males; 14.5 years old). Functional connectivity between 8 major intrinsic connectivity networks, along with connectivity measurements between 333 brain regions, was compared between PBD and HC. Additionally, connectivity differences were evaluated between PBD and HC samples in negatively correlated connections, as defined by 839 subjects of the Human Connectome Project dataset.
Results:
We found increased inter- but not intra-network functional connectivity in PBD between the default mode and salience networks (p = .0017). Throughout the brain, atypical connections showed failure to develop anticorrelation with age during adolescence in PBD but not control samples among connections that exhibit negative correlation in adulthood.
Conclusions:
Youths with PBD demonstrate reduced anticorrelation between default mode and salience networks. Further evaluation of the interaction between these networks is needed in development and with other mood states such as depression and mania to clarify if this atypical connectivity is a PBD trait biomarker.
Keywords: resting-state, bipolar, salience, default mode, attention, networks
Introduction
Pediatric bipolar disorder (PBD) (occurring prior to age 18) is a developmental brain disorder that is among the most severe and disabling psychiatric conditions affecting youth (1–4). Untreated bipolar disorder (BD), particularly during formative developmental years may be one of the causes of the significant morbidity and mortality seen with this disorder; therefore, early diagnosis and early-intervention may prevent the later sequelae of the illness (5, 6).
Current structural and functional neuroimaging studies have often focused on examining the fronto-limbic networks in bipolar illness in both youth and adult populations (7–15). However, recent studies in adults with BD have also identified aberrant functional connectivity in distributed, large-scale, resting-state networks. Among characterized intrinsic connectivity networks, abnormalities have most commonly been seen in association cortical networks, such as the default mode network (DMN), executive network (EN), and the salience network (salience) (16–20). The DMN is a large-scale network that inter- and intra-hemispherically connects spatially and functionally separate brain regions and has been found to be most active when an individual is not engaged in any externally driven tasks (21). The DMN is thought to be involved in introspection, theory of mind, and internally directed cognition (22, 23). Another large-scale network, the executive or task-positive network, appears to be active primarily during tasks requiring attention to internal stimuli (24). Interestingly, the DMN appears to have a reciprocal relationship with the executive network, in which its activation decreases as the executive network increases (22, 23, 25). Further, there is increasing evidence suggesting that the ability to attenuate the DMN during externally directed tasks has been linked to improved task performance in both adults and children (26, 27).
The salience network is another large-scale resting-state network that includes regions of the anterior cingulate cortex (ACC) and anterior insula cortex (24, 28, 29). The proposed role of the insula in the salience network is in the detection and segregation of important stimuli from insignificant stimuli, while the ACC is thought to modulate responses in the sensory, motor and association cortices based on the information provided by the anterior insula (24, 30, 31). Interestingly, the right anterior insula has been proposed to play a critical role in switching between the competitive DMN and the executive network, during cognitive information processing (24, 31). Due to the interplay between these 3 networks, it is possible that any inter- or intra-network communication abnormalities could lead to some of the core symptoms seen in BD and likely other mood and behavioral dysregulation disorders including mood instability, depressive or manic ruminations, poor attention, distractibility, increased goal-directed activity and impulsivity.
Several resting-state connectivity studies have been performed in youths with PBD (10, 12, 32–38), although most of these prior studies examined specific functional connectivity patterns related to affective and/or cognitive challenges (10, 12, 39), or examined regional connectivity (homogeneity) (35, 36). However, in a study by Ford and colleagues, DMN connectivity was specifically examined in a combined group of 15 youths with PBD and 15 with major depressive disorder (MDD) (34). This study explored DMN connectivity in association with the Bipolarity Index score. The authors reported DMN activation in the putamen and insula positively correlated with Bipolarity Index score. In addition, DMN activation in the post-central gyrus and posterior cingulate cortex (PCC) was negatively correlated with Bipolarity Index score (34). Although this was a mixed sample of youths with both MDD and PBD, it is one of the only studies to date that as specifically examined one of the large-scale networks in PBD. Given the paucity of studies in PBD and the growing evidence that intrinsic large-scale networks are impaired in adults with BD, further evaluation of large-scale brain networks in the resting-state is needed in children and adolescents with PBD.
For the current study, we first performed a priori evaluations of major resting-state networks, including the DMN, salience, and executive networks, to examine whether inter or intra-network functional connectivity differences could be detected in youths with PBD compared to HC. Based on findings in adults, we hypothesized that regions comprising the DMN, salience, and executive networks would have increased functional connectivity abnormalities as compared to a healthy population.
There has been a paucity of studies looking at functional connectivity patterns at rest in youth with PBD. This is unfortunate as resting-state connectivity data may elucidate key information regarding the basic functional architecture of the brain, and these findings may be particularly informative during development before longstanding brain changes associated with therapy and pathophysiology may complicate core brain phenotypes. In order to further explore functional connectivity patterns at rest, we performed two additional data-driven analyses to 1) clarify and extend our findings from the initial analyses and 2) to further assess the characteristics of positively vs. negatively correlated functional connections in PBD as compared to HC. The first of these analyses included a whole brain analysis in 333 ROIs to detect which brain regions had the highest number of aberrant connections in PBD as compared to HC. Again based on the adult literature, we hypothesized that brain regions that made up the DMN, salience, and attention networks would have the highest number of atypical connections in PBD as compared to HC. Secondly, we examined the differences in PBD as compared to HC connectivity associated with connection strength between brain regions. We previously reported on atypical connectivity patterns based on strength and distance in other developmental disorders, including autism (40, 41) and Down Syndrome (42). We predicted atypical connectivity between brain attentional networks would be present in association cortex, given the known behavioral abnormalities in bipolar disorder in attention and executive function with preservation of sensory perception.
Methods and Materials
Study Participants
The Institutional Review Board at the University of Utah approved this study. All subjects provided written informed assent or consent (if 18 years of age or older) prior to study participation. Parental consent was also acquired for all study participants under the age of 18. Recruitment efforts included the use of local advertisements and word of mouth. In addition, participants were also obtained via clinician referral from outpatient, residential and inpatient hospital settings. A total of 80 subjects, including 32 youths with PBD, currently euthymic (13 males; 15.1 ± 2.0 years old) and 48 healthy controls (HC) (27 males; 14.5 ± 2.4 years old), were included in this analysis (See Table 1 for demographic information). Inclusion criteria for study participants were: male or female, 10–19 years of age, and of any race or ethnicity. All adolescents, including HC, underwent a clinical and diagnostic semi-structured interview by a board-certified child psychiatrist (MLL). Adolescents were administered the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Episode (K-SADS-PL) (43) with additional mood onset and offset items derived from the WASH-U K-SADS (K-SADS-PL-W). Inclusion criteria for PBD subjects included a DSM-IV-TR diagnosis of bipolar disorder, currently euthymic.
Table 1.
Clinical Demographics for healthy control (HC) and pediatric bipolar disorder (PBD)
| HC (n = 48) | PBD (n = 32) | ||||
|---|---|---|---|---|---|
| n = | % | n = | % | X2 | |
| Sex (male) | 27 | 56.3 | 13 | 40.6 | ns |
| Attention Deficit Hyperactivity Disorder Diagnosis | - | - | 10 | 31.3 | - |
| Anxiety Disorder Diagnosis | - | - | 10 | 31.3 | - |
| History of Substance Abuse | - | - | 4 | 12.9 | - |
| Number of Unmedicated Participants | - | - | 10 | 31.3 | - |
| Family History of Bipolar Disorder in First Degree Relative | - | - | 17/29 | 58.6 | - |
| Family History of Bipolar Disorder in Any Family Member | - | - | 21/29 | 72.4 | - |
| Number of Participants taking an Antidepressant | - | - | 10 | 31.3 | - |
| Number of Participants Taking a Mood Stabilizer Other than Lithium | - | - | 15 | 46.9 | - |
| Number of Participants Taking Lithium | - | - | 3 | 0.09 | - |
| Number of Participants Taking an Atypical Antipsychotic | - | - | 14 | 43.8 | - |
| Number of Participants Taking a Stimulants | - | - | 6 | 18.8 | - |
| Mean | STD | Mean | STD | p = | |
| Age | 14.5 | 2.4 | 15.1 | 2.0 | ns |
| Full Scale Intelligence Quotient Score | 111.4 | 10.0 | 106.9 | 15.5 | ns |
| Young Mania Rating Scale Score | 0.3 | 1.1 | 6.6 | 4.6 | <.001 |
| Children’s Depression Rating Scale Score | 17.1 | 2.0 | 23.6 | 4.1 | <.001 |
| Child Behavior Checklist Internalizing Subscale Score | 3.6 | 3.6 | 19.3 | 10.7 | <.001 |
| Child Behavior Checklist Externalizing Subscale Score | 3.0 | 3.6 | 18.2 | 11.5 | <.001 |
Youths with PBD were allowed to continue currently prescribed medications; however, participants were asked to refrain from taking stimulant medications at least 24 hours prior to scanning. Unmedicated youths (Table 1) were not currently taking any psychotropic medication or recently (< 2 weeks) started on medication but on a sub-therapeutic dose. Healthy control participants had no current or past history of a DSM-IV-TR Axis I diagnosis and did not have a current or past history of using psychotropic medications. Exclusion criteria for both groups included: major sensorimotor handicaps, full scale IQ < 70, autism, schizophrenia, conduct disorder, anorexia nervosa or bulimia, drug or alcohol dependence, active neurological or medical disease, current pregnancy or lactation, metal fragments or implants, a history of claustrophobia, or any other MRI scan contraindications. Full scale intelligence quotient (FSIQ) was assessed using the Wechsler Abbreviated Scale of Intelligence (44). The Young Mania Rating Scale (YMRS) and Children’s Depression Rating Scale (CDRS) were used to assess mania and depressive symptoms (45, 46). A YMRS score less than 30 and a CDRS score less than or equal to 30 was used to define euthymia. Internalizing and externalizing dimensional symptoms, common in children and adolescents with PBD, were assessed using the Child Behavior Checklist (CBCL) (47).
Image Acquisition:
Images were acquired on Siemens 3 Tesla Trio scanner with 12-channel head coil. The scanning protocol consisted of initial 1 mm isotropic MPRAGE acquisition for an anatomic template. BOLD echoplanar images (240 volumes, 8 minutes, TR = 2.0 s, TE = 28 ms, GRAPPA parallel acquisition with acceleration factor = 2, 47 slices at 3 mm slice thickness, 64 × 64 matrix) were obtained during the resting state, where subjects were instructed to “Keep your eyes open and remain awake and try to let thoughts pass through your mind without focusing on any particular mental activity.” For all BOLD sequences, simultaneous plethysmograph (pulse oximeter) and chest excursion (respiratory belt) waveforms were recorded for offline analysis.
Image Processing:
Despiking (48) was performed using AFNI software package (49) (3dDespike) for initial correction of head motion displacement. Motion correction (realign), coregistration to MPRAGE, segmentation of MPRAGE, and normalization of MPRAGE and BOLD to MNI template was performed in SPM12b software (Wellcome Trust, London) for MATLAB (Mathworks, Natick MA). Phase-shifted soft tissue correction (PSTCor) (50) was used to regress physiological waveforms as well as regressors obtained from subject motion parameters, degraded white matter, degraded CSF, and soft tissues of the face and calvarium. No regression of the global signal was performed to avoid contamination of gray matter sources (50–52). Censoring of frames showing greater than 0.2 mm (motion scrubbing) was performed as a final step prior to analysis with concatenation of remaining frames (53). Groups showed no differences in head motion for individual parameters or root-mean-square aggregate motion estimates (PBD: 0.073 ± 0.068 mm; HC: 0.073 ± 0.052 mm; p = .99, two-tailed t-test). The number of scans removed during scrubbing did not differ between patient samples (PBD: 201.9 ± 40.5 volumes used; HC: 202.2 +/− 31.1 volumes used; p = .97, two-tailed t-test). Mean temporal signal to noise ratio (mean fMRI signal divided by standard deviation of the signal) (54) did not differ between patient groups (range for 8 networks: p = .31 to p = .94, two-tailed t-test).
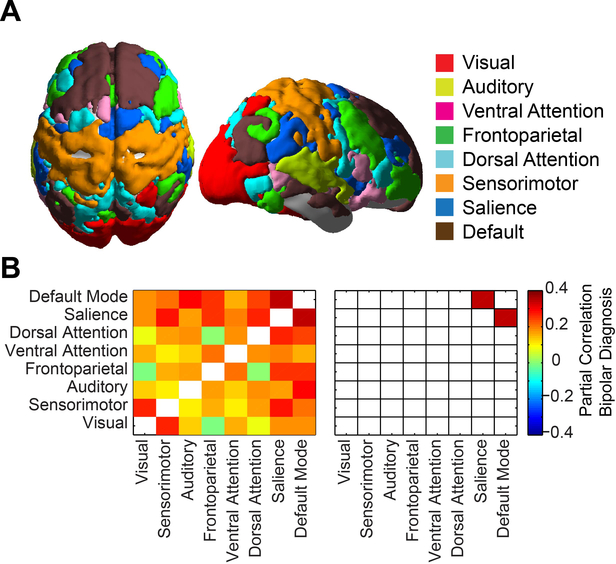
For functional connectivity analyses, 333 regions of interest comprising a parcellation of cortical gray matter were compared between PBD and HC, obtained from a functional network parcellation of the brain (55). The Gordon et al. parcellation, which is shared publicly (http://www.nil.wustl.edu/labs/petersen/Resources.html), includes 333 gray matter regions that cover the cortex and include 286 nodes that are ascribed to a functional brain network (47 regions were ascribed to “None” including regions in temporopolar and orbitofrontal cortex, detailed in Parcels.xlsx at the above resource). Community assignments showed stability over network density thresholds (Supplementary Figure 7) and improved network homogeneity to other functional brain network parcellations (Figure 4, Table 1) with high homology to existing functional network boundaries and excellent coregistration with task-derived functional network architecture (55). For functional network analysis, we used 8 networks, illustrated in Figure 1A, that combined the 12 Gordon et al networks as follows: Auditory, Salience (including Gordon et al. CinguloOpercular and Salience networks), FrontoParietal (including Gordon et al. FrontoParietal and CinguloParietal networks), Default (including Gordon et al. Default and RetrosplenialTemporal networks), Dorsal Attention, Ventral Attention, Sensorimotor (including Gordon et al. SMhand and SMmouth networks), and Visual. Some of the networks were combined due to functional similarity and because some of the networks (such as Gordon et al. Salience and CinguloParietal) comprised only a small number of nodes but had similar connectivity patterns to another network. Having networks more similar in size also facilitates comparisons of more similar signal to noise characteristics. Two levels of analysis were performed: one at the 8 network level, and one at the 333 ROI level to balance multiple comparisons and granularity of spatial information.
Figure 1:
Functional connectivity between resting-state networks. A) Parcellation of the brain into 8 resting state networks used for analysis. B) Partial correlation between bipolar diagnosis and functional connectivity between networks. Subject age and sex were also included in the model. Colored squares on the right satisfied acceptable false discovery rate q<0.05 across all network pairs.
For each pair of 8 networks, mean BOLD time series were extracted across the entire network and Fisher-transformed correlation coefficients were calculated and partial correlation was performed between these values and age, sex, and diagnosis across the 80-subject sample. An acceptable false discovery rate (q < .05) over all pairs of networks was used to denote significant partial correlation with diagnosis. For the PBD group and for all participants, partial correlation was also performed between connectivity measurements between each pair of 333 ROIs.
Next, we examined systematic differences in connectivity associated with positively vs. negatively correlated connections between ROIs. To evaluate which connections are negatively correlated (anticorrelated), we used an independent dataset for an unbiased estimate obtained from 839 subjects from the S900 Release of the Human Connectome Project (HCP) dataset. For each of these 839 subjects, time series were extracted for the same 333 ROIs FIX-ICA cleaned BOLD data, and functional connectivity was calculated between each pair of ROIs (Fisher-transformed Pearson correlation coefficient). As part of the HCP S900 Subjects Release, each of the 839 subjects has undergone 4 MRI sessions with these anatomic and functional sequences: diffusion imaging, resting-state fMRI, task-evoked fMRI, and T1- and T2-weighted MRI for structural and myelin mapping. BOLD fMRI images were performed using multiband technique with TR: 720 ms, TE: 33.1 ms, 72 slices, 2×2×2 mm spatial resolution, 1200 volumes for resting fMRI sequences. Detailed description of the exact MRI specifications can be found at http://www.humanconnectome.org/documentation/S900/HCP_S900_Release_Appendix_I.pdf. From mean functional connectivity measurements between each pair of ROIs, 1784 connections out of 55,278 total connections had mean functional connectivity less than 0. Additionally, all 55,278 connections were grouped into bins, regardless of spatial location in the brain, based on mean connectivity strength in the HCP sample, with incremental bin size of 0.02, ranging from mean functional connectivity of −0.2 to 0.6. Mean functional connectivity across the connections in each bin and for the set of negatively correlated connections were calculated for each subject and compared in PBD and HC samples using two-tailed t-tests.
Results
Functional Connectivity Results:
Boundaries of 8 major intrinsic connectivity networks were defined using the Gordon et al. functional parcellation of the brain (55). The cortico-cortical connectivity was evaluated between all pairs of the 8 networks, shown in Figure 1. A model was used that included functional connectivity, bipolar diagnosis, subject sex, and subject age. Partial correlation between functional connectivity and bipolar diagnosis was used to evaluate whether a relationship existed between functional connectivity in a pair of networks and diagnosis. Functional connectivity between PBD and HC samples showed significantly increased connectivity (reduced anticorrelation) in PBD between the DMN and salience networks (p = .0017, satisfying q < .05 False Discovery Rate). No significant assocations between age or sex and functional connectivity survived multiple comparison corrections.
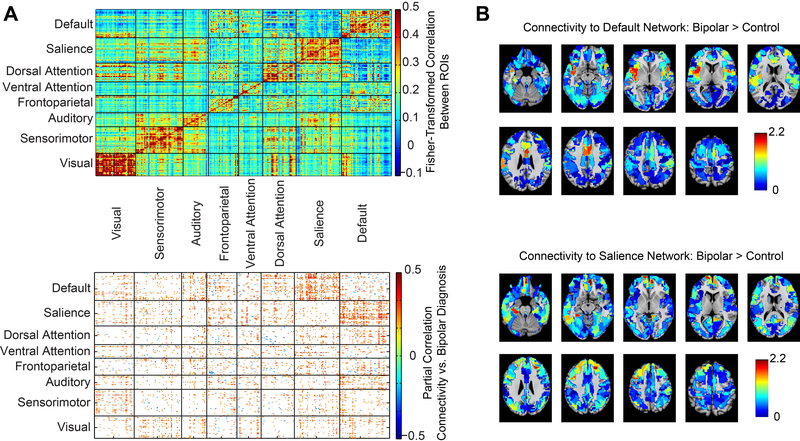
To assess the spatial specificity of reduced anticorrelations between the DMN and salience networks in PBD, a discovery analysis of 286 ROIs belonging to intrinsic connectivity networks was also performed. Mean connectivity for all 32 PBD and 48 HC subjects for each pair of ROIs is shown in Figure 2A, upper panel, with partial correlation of diagnosis and functional connectivity shown in Figure 2A, lower panel, for each pair of ROIs. Age and sex were again included as covariates in the model.
Figure 2:
Functional connectivity between 286 cortical regions. A) Plot above shows mean functional connectivity for all 80 participants between 286 regions covering the cortex and parcellated into 8 resting-state networks. Graph below shows differences between bipolar and control samples with partial correlation between functional connectivity and bipolar diagnosis, including subject age and sex in the model. B) Mean t-statistic between bipolar and control samples of functional connectivity for each ROI with all of the ROIs in the default mode network (above) or salience network (below).
Connectivity metrics were higher for bipolar disorder (possibly representing reduced anticorrelation) in connections between the DMN and salience networks than between other networks. Connections between the DMN and salience networks showed mean partial correlation to PBD diagnosis of 0.15 compared to the mean for all network pairs of 0.059 +/− 0.030 standard deviation. The percentage of atypical connections between the DMN and salience networks is thus 3.04 standard deviations greater than the mean over all network pairs and this network pair satisfies Grubb’s test as an outlier at alpha=0.01 (56). For each of the full 333 ROI sample in the Gordon et al. parcellation, t-statistic between PBD and HC samples were averaged across all of the ROI’s in the DMN and all of the ROI’s in the salience network. Results are shown graphically in Figure 2B. Mean t-statistic for greater connectivity to the DMN for PBD subjects is specifically higher in the salience network and vice versa.
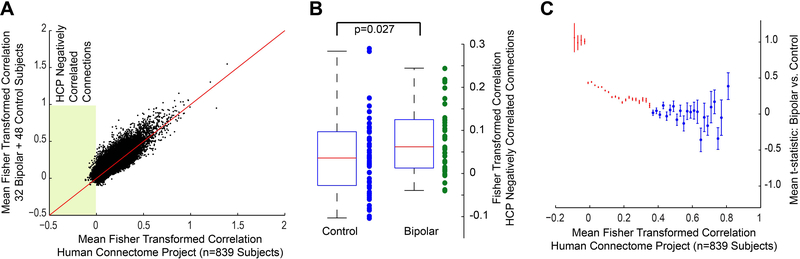
The salience network and DMN are known to exhibit anticorrelated connectivity (22). The specificity of abnormal connections in bipolar disorder between the DMN and salience network suggests that negatively correlated connections may be preferentially affected in bipolar disorder. To test this hypothesis, we used an independent dataset from the Human Connectome Project S900 subjects release containing 839 subjects to define which connections were anticorrelated across the population. For each pair of 333 × 333 ROIs, mean connectivity in our sample and in the Human Connectome Project sample is compared in Figure 3A, with “negative connections” or anticorrelated connections falling in the shaded region of the plot. Mean functional connectivity for this set of 1784 connections was calculated for each of the 32 PBD and 48 HC subjects, with significantly higher connectivity in the PBD sample among these connections (Figure 3B). When all connections were grouped into bins based on mean connectivity in the HCP sample, the mean PBD vs. HC t-statistic was significantly higher than zero (one-sample t-test for each bin) with Bonferroni correction for multiple comparisons for all bins containing connections where the HCP sample had connectivity less than 0.34 and none of the bins where population mean connectivity was greater than 0.34 (Figure 3C). This was particularly evident for bins with connections less than zero, where mean PBD vs. HC t-statistics were greatest. Mean connectivity for these “HCP Negative Connections” showed significant decrease with age in our control sample (r=−0.42, p=0.003, Figure 3D) but not for our PBD sample which showed a nonsignificant increase with age (r=0.297, p=0.099). The Human Connectome Project sample showed a nonsignificant decreased trend with age (r=−0.063, p=0.070), suggesting maturation of negatively correlated connections in adolescence in control subjects with relative stability after age 20, but with failure of such maturation with age in PBD. A two-way ANOVA analysis of “HCP Negative Connections” for each subject with age and diagnosis as factors showed a significant effect for diagnosis (F=7.5, p=0.0077) but not for age (F=0.20, p=0.65) and a significant interaction between age and diagnosis (F=10.1, p=0.0021). The connections in this “HCP Negative Connections” set were comprised primarily of connections between the DMN and Salience network and between the DMN and Dorsal Attention Network (Figure 3E).
Figure 3:
Differential functional connectivity in negatively correlated connections in bipolar disorder. A) Comparison of mean functional connectivity for 333 × 333 ROIs for the subjects in the study sample compared to 839 subjects of the Human Connectome Project. Shaded area represents connections with mean connectivity in HCP subjects less than zero (HCP Negatively Correlated Connections). B) Mean functional connectivity for HCP Negatively Correlated Connections for each bipolar and control subject. Boxplots show range and quartiles of each sample. C) 333 × 333 connections were divided into bins based on mean functional connectivity in the 839 HCP sample, with bin size 0.02 units of Fisher-transformed correlation. For each bin, the figure shows mean t-statistic for connections in the bin between bipolar and control samples. Error bars represent standard error of the mean for each bin. For red bins, the mean t-statistic between bipolar and control samples for connections in the bin were significantly greater than zero (Bonferroni corrected across all bins, two-tailed t-test). D) Mean functional connectivity for HCP Negatively Correlated Connections as a function of age in control, bipolar, and HCP subjects. E) Network distribution for HCP Negatively Correlated Connections. Colored squares show connections where mean functional connectivity was less than zero in the HCP sample.
Discussion
We found decreased anticorrelation in negatively correlated corticocortical connections in PBD, particularly in cortico-cortical connections between the DMN and salience networks. These findings were evident when considering functional network connectivity of the time series of the entire networks and on a more granular level between cortical regions in the DMN to cortical regions in the salience network. Finally, we found that atypical connectivity patterns included failure of development of anticorrelation with age during adolescence among these connections in PBD as compared to HC, with significant age by diagnosis interaction.
Our primary finding of decreased negative functional connectivity between the DMN and salience networks is supported by previous studies in adults with BD (16–20, 57). Our findings are also consistent with studies in PBD that reported increased functional connectivity of the DMN to the insula with higher bipolar index scores (34). In addition, structural and task-based fMRI studies in PBD have also found abnormalities in regions of DMN and salience including regions of the ACC (39, 58–62), medial prefrontal cortex (39, 59, 60, 62–64), and PCC/Precuneus (62). Furthermore, abnormalities in local connectivity or regional homogeneity in DMN regions have been reported in PBD in both the manic (35) and depressed (36) state.
Given the known interactive relationship between DMN and salience networks (22), our findings of decreased anticorrelation between these networks may suggest decreased segregation of the default mode and salience networks(65). There is evidence that brain connectivity matures during development through integration of distant brain regions into more coherent functional networks and separation or segregation of distinct distributed brain networks(66, 67) with development of anticorrelations between the DMN and brain attentional networks including the salience network(22) showing gradients of negative correlation between hubs of these respective networks(68). Interestingly, there is also increasing evidence suggesting that the ability to modulate the DMN during externally directed tasks is linked to improved task performance in both adults and children (26, 27). In light of our findings of reduced anticorrelation between these networks in PBD, further evaluation of the inter-network dynamics between these two networks appears warranted in PBD, including further evaluation of the developmental trajectories of DMN and salience networks across the life span.
On the data-driven, whole-brain functional connectivity analysis, we found that brain regions with the most atypical connections in PBD as compared to HC were in the salience and DMN, including medial prefrontal, anterior insula, and dorsal ACC. Of note, connections involving the executive network did not exhibit atypical connectivity similar to the DMN and salience networks. Furthermore, we also found that the patterns of increased functional connectivity metrics found in PBD was primarily due to reduced anticorrelation among connections in PBD as compared to HC. We hypothesize that failed development of negative connections between spatially segregated brain regions may represent failure of maturation of a specific type of brain connection. Nevertheless, “negative connections” could arise either from inhibition or decreased excitation between regions, or decreased shared activation from other brain regions. Hypotheses about underlying pathophysiology could be constrained by considering data from other modalities such as electrophysiological and diffusion imaging.
There is emerging evidence in the literature that anticorrelated connectivity between brain networks is relevant to pathophysiology of a number of neurodevelopmental and neuropsychiatric conditions. Decreased anticorrelation in functional MRI connectivity has been observed among negatively correlated connections, particularly between the DMN and brain attentional networks (22), in autism (69), Down Syndrome (70), ADHD (71), and schizophrenia (72). Such negatively correlated connections become more robust between childhood and adulthood (73), and are associated with improved performance on working memory and executive function (74). The interaction between decreased anticorrelation of such connections in bipolar disorder and development of psychopathology requires further study, as this observation could emerge as a sequelae of altered executive function in bipolar disorder or play a role in development of clinical symptoms.
Our current findings should be interpreted with care given the modest sample size and cross-sectional nature of the study. Furthermore, the inclusion of youths with past and current comorbidities and concurrent medication use were also limitations of the study. However, our comorbidity and medication use of our participants is lower than most studies examining bipolar youth in a euthymic state. Furthermore, all participants were off of their stimulant medication for at least 24 hours prior to scanning and 31 percent of our bipolar youths were unmedicated. Unfortunately, the number of unmedicated bipolar youths is too small to perform additional analyses. Finally, even though we did use both sex and age as covariates in our analyses, it is possible that these factors are still influencing our results. Further examination of both sex and age in the developmental trajectories of these intrinsic networks is needed.
To our knowledge, this is the first study to apply a region of interest and whole brain, data driven approach to examine and identify abnormal functional connectivity using resting-state techniques in PBD youth during euthymia. This pattern of atypical functional connectivity may be associated with the pathophysiology PBD, or may be epiphenomenal, associated with symptomatic behaviors of PBD. On whole brain analysis, we found that brain regions of the DMN and salience networks had the highest number of atypical connections. Finally, we found that abnormal connections in PBD were primarily due to reduced development of anticorrelation during adolescence among connections in PBD as compared to HC. Further studies examining the developmental dynamics between large-scale networks such as the DMN, attention, executive and salience, both at rest and during tasks, is essential in the search for neural correlates of BD. Furthermore, evaluation of the interaction between the DMN and salience networks is needed in other mood states such as depression and mania and in at risk youth to clarify if reduced anticorrelation is specific to BD and whether or not the dysconnectivity patterns are related to state or trait pathology.
Acknowledgments and Disclosures:
Research was funded by NIH grants: 5K23MH087831 (MLL), and MH092697 (JSA). Data were also provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. All authors report no financial or potential conflicts of interest.
References
- 1.Faedda GL, Baldessarini RJ, Suppes T, Tondo L, Becker I, Lipschitz DS (1995): Pediatric-onset bipolar disorder: a neglected clinical and public health problem. Harv Rev Psychiatry. 3:171–195. [DOI] [PubMed] [Google Scholar]
- 2.Wozniak J, Bierderman J (1995): Childhood mania exists (and coexists) with ADHD. 6:4–5. [Google Scholar]
- 3.Ahn MS, Frazier JA (2004): Diagnostic and treatment issues in childhood-onset bipolar disorder. Essent Psychopharmacol. 6:25–44. [PubMed] [Google Scholar]
- 4.Leibenluft E (2008): Pediatric bipolar disorder comes of age. Arch Gen Psychiatry. 65:1122–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, et al. (2004): Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry. 55:875–881. [DOI] [PubMed] [Google Scholar]
- 6.Demeter CA, Townsend LD, Wilson M, Findling RL (2008): Current research in child and adolescent bipolar disorder. Dialogues Clin Neurosci. 10:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR, et al. (2010): Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 67:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, et al. (2009): Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 66:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, et al. (2013): Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 73:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passarotti AM, Ellis J, Wegbreit E, Stevens MC, Pavuluri MN (2012): Reduced functional connectivity of prefrontal regions and amygdala within affect and working memory networks in pediatric bipolar disorder. Brain connectivity. 2:320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlman SB, Almeida JR, Kronhaus DM, Versace A, Labarbara EJ, Klein CR, et al. (2012): Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar disorders. 14:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Bobrow L, Liu J, Spencer L, Blumberg HP (2012): Corticolimbic functional connectivity in adolescents with bipolar disorder. PloS one. 7:e50177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wegbreit E, Passarotti AM, Ellis JA, Wu M, Witowski N, Fitzgerald JM, et al. (2013): Where, when, how high, and how long? The hemodynamics of emotional response in psychotropic-naive patients with adolescent bipolar disorder. Journal of affective disorders. 147:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Houenou J, d’Albis MA, Vederine FE, Henry C, Leboyer M, Wessa M (2012): Neuroimaging biomarkers in bipolar disorder. Frontiers in bioscience. 4:593–606. [DOI] [PubMed] [Google Scholar]
- 15.Torrisi S, Moody TD, Vizueta N, Thomason ME, Monti MM, Townsend JD, et al. (2013): Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar disorders. 15:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA (2008): Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Human brain mapping. 29:1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, et al. (2010): Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 183:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, et al. (2012): Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biological psychiatry. 71:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, et al. (2011): Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 36:2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamah D, Barch DM, Repovs G (2013): Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J Affect Disord. 150:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. (2007): Resting-state networks in the infant brain. Proceedings of the National Academy of Sciences of the United States of America. 104:15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greicius MD, Menon V (2004): Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 16:1484–1492. [DOI] [PubMed] [Google Scholar]
- 24.Menon V, Uddin LQ (2010): Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gusnard DA, Raichle ME (2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2:685–694. [DOI] [PubMed] [Google Scholar]
- 26.Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS (2005): Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proceedings of the National Academy of Sciences of the United States of America. 102:15700–15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomason ME, Chang CE, Glover GH, Gabrieli JD, Greicius MD, Gotlib IH (2008): Default-mode function and task-induced deactivation have overlapping brain substrates in children. NeuroImage. 41:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig AD (2009): Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philosophical Transactions of the Royal Society B: Biological Sciences. 364:1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medford N, Critchley HD (2010): Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function. 214:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickstein DP, Gorrostieta C, Ombao H, Goldberg LD, Brazel AC, Gable CJ, et al. (2010): Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biological psychiatry. 68:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavuluri MN, Ellis JA, Wegbreit E, Passarotti AM, Stevens MC (2012): Pharmacotherapy impacts functional connectivity among affective circuits during response inhibition in pediatric mania. Behavioural brain research. 226:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford KA, Theberge J, Neufeld RJ, Williamson PC, Osuch EA (2013): Correlation of brain default mode network activation with bipolarity index in youth with mood disorders. J Affect Disord. 150:1174–1178. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Q, Zhong Y, Lu D, Gao W, Jiao Q, Lu G, et al. (2013): Altered regional homogeneity in pediatric bipolar disorder during manic state: a resting-state fMRI study. PloS one. 8:e57978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao W, Jiao Q, Lu S, Zhong Y, Qi R, Lu D, et al. (2014): Alterations of regional homogeneity in pediatric bipolar depression: a resting-state fMRI study. BMC psychiatry. 14:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu D, Jiao Q, Zhong Y, Gao W, Xiao Q, Liu X, et al. (2014): Altered baseline brain activity in children with bipolar disorder during mania state: a resting-state study. Neuropsychiatric disease and treatment. 10:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoddard J, Hsu D, Reynolds RC, Brotman MA, Ernst M, Pine DS, et al. (2015): Aberrant amygdala intrinsic functional connectivity distinguishes youths with bipolar disorder from those with severe mood dysregulation. Psychiatry Res. 231:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA (2007): Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 62:158–167. [DOI] [PubMed] [Google Scholar]
- 40.Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, et al. (2011): Functional connectivity magnetic resonance imaging classification of autism. Brain. 134:3742–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen JA, Zielinski BA, Fletcher PT, Alexander AL, Lange N, Bigler ED, et al. (2013): Multisite functional connectivity MRI classification of autism: ABIDE results. Frontiers in human neuroscience. 7:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson JS, Nielsen JA, Ferguson MA, Burback MC, Cox ET, Dai L, et al. (2013): Abnormal brain synchrony in Down Syndrome. NeuroImage Clinical. 2:703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D (1999): WASI: Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 45.Young RC, Biggs JT, Ziegler VE, Meyer DA (1978): A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science. 133:429–435. [DOI] [PubMed] [Google Scholar]
- 46.Poznanski E, Mokros H (1996): Children’s Depression Rating Scale–Revised (CDRS-R). Los Angeles: WPS. [Google Scholar]
- 47.Achenbach TM, Rescoria L (2001): Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: ASEBA. [Google Scholar]
- 48.Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, et al. (2013): Effective Preprocessing Procedures Virtually Eliminate Distance-Dependent Motion Artifacts in Resting State FMRI. Journal of applied mathematics. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- 50.Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D (2011): Network anticorrelations, global regression, and phase-shifted soft tissue correction. Human brain mapping. 32:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, et al. (2012): Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welvaert M, Rosseel Y (2013): On the definition of signal-to-noise ratio and contrast-to-noise ratio for FMRI data. PloS one. 8:e77089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE (2016): Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 26:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grubbs F (1969): Procedures for Detecting Outlying Observations in Samples. Technometrics. 11:1–21. [Google Scholar]
- 57.Goya-Maldonado R, Brodmann K, Keil M, Trost S, Dechent P, Gruber O (2016): Differentiating unipolar and bipolar depression by alterations in large-scale brain networks. Hum Brain Mapp. 37:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Monkul ES, et al. (2005): Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 162:1637–1643. [DOI] [PubMed] [Google Scholar]
- 59.Wilke M, Kowatch RA, DelBello MP, Mills NP, Holland SK (2004): Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry research. 131:57–69. [DOI] [PubMed] [Google Scholar]
- 60.Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, et al. (2007): Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar disorders. 9:679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW (2005): Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 58:713–723. [DOI] [PubMed] [Google Scholar]
- 62.Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, et al. (2007): Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 48:852–862. [DOI] [PubMed] [Google Scholar]
- 63.Najt P, Nicoletti M, Chen HH, Hatch JP, Caetano SC, Sassi RB, et al. (2007): Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neurosci Lett. 413:183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, et al. (2006): Regional cerebral cortical thinning in bipolar disorder. Bipolar disorders. 8:65–74. [DOI] [PubMed] [Google Scholar]
- 65.Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. (2007): Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 104:13507–13512.17679691 [Google Scholar]
- 66.Power JD, Fair DA, Schlaggar BL, Petersen SE (2010): The development of human functional brain networks. Neuron. 67:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. (2009): Functional brain networks develop from a “local to distributed” organization. PLoS computational biology. 5:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D (2011): Connectivity gradients between the default mode and attention control networks. Brain connectivity. 1:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nielsen JA, Zielinski BA, Fletcher PT, Alexander AL, Lange N, Bigler ED, et al. (2013): Multisite functional ocnnectivity MRI classification of autism: ABIDE results. Front Hum Neurosci. 7:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson JS, Nielsen JA, Ferguson MA, Burback MC, Cox ET, Dai L, et al. (2013): Abnormal Brain Synchrony in Down Syndrome. NeuroImage: Clinical. 2:703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castellanos FX, Proal E (2012): Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends in cognitive sciences. 16:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S (2014): Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of cognitive neuroscience. 26:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keller JB, Hedden T, Thompson TW, Anteraper SA, Gabrieli JD, Whitfield-Gabrieli S (2015): Resting-state anticorrelations between medial and lateral prefrontal cortex: association with working memory, aging, and individual differences. Cortex; a journal devoted to the study of the nervous system and behavior. 64:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]