Abstract
Background
Quercus infectoria gall extract is known to have broad spectrum anti-microbial activity in vitro. This study was conducted to determine the anti-microbial activity of Q. infectoria gall extract against pathogenic Leptospira and to evaluate the morphological changes of extract-treated cells using a scanning electron microscope (SEM).
Methods
A two-fold serial microdilution broth assay was used to determine the minimum inhibitory concentration (MIC) of aqueous Q. infectoria gall extract against the L. interrogans serovar Javanica and the L. interrogans serovar Icterohaemorrhagiae, at concentrations ranging from 4.00 mg/mL to 0.0078 mg/mL. The minimum bactericidal concentration (MBC) was determined by sub-culturing the broth from the microtiter plate wells that showed no apparent growth or turbidity to the freshly prepared Ellinghausen-McCullough-Johnson-Harris (EMJH) broth media, and it was subsequently observed under a dark field microscope following three weeks of incubation for purposes of growth detection. The cell morphology of both extract-treated and untreated L. interrogans serovar Icterohaemorhagiae was analysed using the SEM.
Results
The results of the broth microdilution assay demonstrate that the aqueous Q. infectoria gall extract possesses anti-microbial activity against both of the L. interrogans serovars with MIC values of 0.125 mg/mL. The MBC values for the L. interrogans serovar Javanica and the L. interrogans serovar Icterohaemorhagiae are 0.125 mg/mL and 0.250 mg/mL, respectively. The SEM micrograph shows changes in shape and size of the extract-treated cells (at 8× MIC) in comparison to the untreated cells.
Conclusion
The Q. infectoria gall extract displays anti-microbial inhibition and killing activity against the pathogenic Leptospira isolates, and thus has the potential for further exploration of its efficacy and use in the treatment of leptospirosis.
Keywords: Quercus infectoria gall extract, pathogenic Leptospira, anti-microbial activity, cell morphology, scanning electron microscope (SEM)
Introduction
Leptospirosis is an infectious disease affecting animals and humans worldwide that is caused by the spiral-shaped bacteria known as the Leptospira species (1). Leptospirosis is reported to cause more than 500,000 cases of infection annually worldwide (2). Tropical and sub-tropical countries are ideal environments for the Leptospira to survive due to their high levels of humidity and warm temperatures (3). Currently, the data available for Malaysia are based on the Annual Report of Morbidity and Mortality from the Ministry of Health Hospitals, and the number of reported cases between the years 2004 and 2015 has increased from 263–5,370 (4). The disease is usually recognised only at its severe stage. During its early stages, the broad-spectrum symptoms of leptospirosis are often confused with other common bacterial infections (5). Thus, the antibiotic treatment administration is delayed for leptospirosis, which leads to a progressive and severe infection. Penicillin and doxycycline are used in the treatment, with intravenous penicillin G being mainly indicated for the severe form of leptospirosis. However, there is no conclusive evidence to show that penicillin or other antibiotics are effective in the prevention of complications and death arising from a severe infection (6).
Herbal alternatives to antibiotics for the treatment of infectious diseases are becoming more popular in our research culture and their promising treatment outcomes can be identified in the years to come. In addition, herbs may also be of great assistance in the prevention and control of certain infections. Quercus infectoria gall is one such herbal alternative and its use in traditional medicine for centuries is well known. There are several reports on the anti-microbial activity of the Q. infectoria gall extract against bacteria and yeast (7, 8). This extract is reported effective against multi-drug resistant (MDR) bacteria (8). A previous study indicates that there are several chemical components present in Q. infectoria gall, such as carbohydrates, lipids, mucilage, saponins and tannins (9). Another study reports that the tannins obtained from the gall serve as a natural defence against microbial infections and also have anti-inflammatory effects (10, 11). Tannins can be further classified into the hydrolysable and the condensed tannins. The Q. infectoria gall extract contains large amounts of tannic acid, which is considered to be a bioactive compound that leads to anti-microbial activity (12). Previously, pyrogallol (a type of hydrolysable tannin) is reported to be a major compound found in Q. infectoria galls (13).
Another plant extract that contains tannic acid, the Phyllunthus amarus plant extract, was tested positive for anti-leptospiral activity (14). However, the inhibition of the Leptospira spp. by a medicinal plant and its killing mechanisms are still poorly understood. The anti-leptospiral activity of the Q. infectoria galls is literally unknown. Based on its phytochemical substances, the gall extract might also possess anti-infective activity against pathogenic Leptospira. Therefore, this study was preliminarily conducted to determine the effects of the aqueous Q. infectoria gall extract against the L. interrogans serovars and to study the morphological changes of the Leptospira cells treated with the extract using a scanning electron microscope (SEM).
Materials and Methods
Preparation of the Q. infectoria Gall Extract
The Q. infectoria gall used in this study was obtained from Nur Saeida et al. (13) and its identification was previously performed based on its physical appearance and phytochemical composition (13, 15). The gall was first crushed in the mortar to small pieces and then grinded into a powder using an electrical grinder.
The extract was prepared by immersing the gall powder into a jar containing sterile distilled water with the weight to volume ratio of 1:5, which was then incubated at 50 °C for 72 h. Subsequently, the mixture was filtered using a coffee filter to separate the undissolved powder from the filtrate, and subsequent filtration was performed using the Whatmann filter paper. The filtrate product was then concentrated under reduced pressure at 80 °C using a rotary evaporator. The concentrated product was freeze-dried at −50 °C under a vacuum until a fine crystal-like crude extract was obtained, which was then stored in an air-tight container at 4 °C until further use.
Preparation of the Leptospira Inoculum
The L. interrogans serovar Javanica and the L. interrogans serovar Icterohaemorrhagiae were obtained from the Department of Medical Microbiology and Parasitology, School of Medical Sciences, Universiti Sains Malaysia (USM). The inoculum was prepared by diluting a seven-day old Leptospira culture with fresh Ellinghausen-McCullough-Johnson-Harris (EMJH) broth to reach a bacterial density of 2 × 106 CFU/mL by using a spectrophotometer at the 420 nm wavelength. The optical density (OD) of 0.32 at 420 nm approximately corresponded to 1 × 108 CFU/mL (16). The OD of 0.064 was approximately equal to 2 × 107 CFU/mL, and a 10-fold dilution was performed to reach a leptospiral density of 2 × 106 CFU/mL.
Determination of the Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC)
The extract’s MICs against both serovars were determined through a two-fold serial microdilution technique using 96 well-microplates (17), with extract concentrations ranging from 4.00 to 0.0078 mg/mL. Equal volumes of diluted inoculum suspensions (100 μL) were added to the designated test and control wells. The control wells were a positive (EMJH broth mixed with the organisms) and a negative (EMJH broth mixed with the extract) control groups. The test was performed in triplicates and the microplates were incubated at 30 °C for three days. The lowest concentration of the extract in the well-microplates that showed no turbidity after three days of incubation was recorded as the value of the MIC.
The MBC was determined by transferring 10 μL of broth from the wells without turbidity into a 15 mL falcon tube filled with 2 mL of a fresh EMJH broth medium. Then, the tubes were further incubated at 30 °C for three weeks. The MBC was documented if the lowest anti-microbial concentration yielded no growth of the L. interrogans serovars through observation under a dark field microscope.
Morphological Analysis Using a Scanning Electron Microscope (SEM)
The cell morphology of the extract-treated L. interrogans serovar Icterohaemorrhagiae was studied under the SEM and compared with the negative and positive controls. Following incubation at 30 °C for 24 h, 2 mL of both treated (extract and penicillin-treated) and untreated (negative control) cultures were centrifuged at 45,000 rpm for 10 min to observe the presence of cells indicated by pellet formation at the bottom of the tube, and the supernatant was discarded. The tube was fixed using the McDowel Trump fixative at 4 °C for two hours. Afterwards, the sample was washed using 0.1 M phosphate buffer saline (PBS) by centrifugation at 42,000 rpm for 10 min. The supernatant was discarded, and the washing process was repeated two more times.
Subsequently, the sample was post-fixed with 1% osmium tetroxide at 4 °C for one hour and dehydrated using an ascending concentration of acetone (50%, 75%, 95% and 100%). After each addition of different acetone concentrations, the sample was centrifuged at 42,000 rpm for 10 min and the supernatant was discarded. At 100% acetone, the sample was dehydrated two more times before 100% hexamethyldisilazane (HDMS) was added. The sample was dried using a critical point dryer, later mounted on the SEM sample carbon stub and gold coated. The mounted slide was viewed under the SEM and a micrograph of the Leptospira cells was taken with an accelerating voltage of 5.00 kV.
Results
MIC and MBC Determination
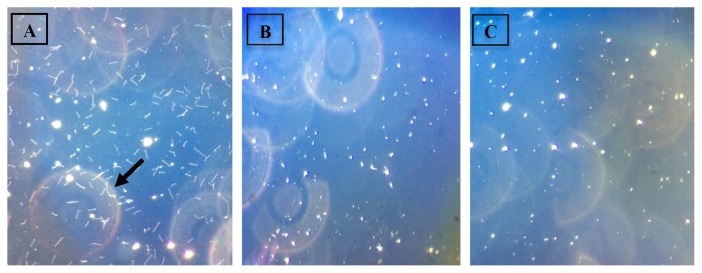
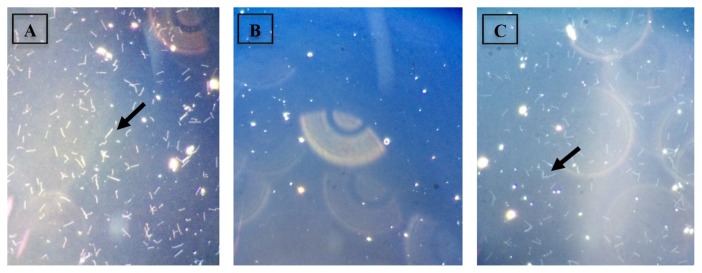
The MIC values of the aqueous Q. infectoria gall extract against both the L. interrogans serovar Javanica and the L. interrogans serovar Icterohaemorrhagiae were similar at 0.125 mg/mL, as shown in Table 1. The MBC for the L. interrogans serovar Javanica was similar to its MIC value, while the MBC value of the L. interrogans serovar Icterohaemorrhagiae was higher than its MIC value (Tables 2 and 3). The growth of the L. interrogans serovars was examined for MBC determination following three weeks of incubation, which was indicated by the absence or presence of motile spirochetes under the dark field microscope, as shown in Figures 1 and 2, respectively.
Table 1.
Microbroth dilution assay of aqueous Q. infectoria gall extract against L. interrogans serovars
| Q. infectoria gall extract concentration (mg/mL) | Leptospira interrogans serovars | |
|---|---|---|
|
| ||
| Javanica | Icterohaemorrhagiae | |
| 4.00 | − | − |
| 2.00 | − | − |
| 1.00 | − | − |
| 0.50 | − | − |
| 0.25 | − | − |
| 0.125 | − | − |
| 0.063 | + | + |
| 0.031 | + | + |
| 0.016 | + | + |
| 0.0078 | + | + |
| Positive control | + | + |
| Negative control | − | − |
No growth (−). Growth (+). Positive control: bacterial suspensions and broth; Negative control: extract and broth
Table 2.
MBC determination of aqueous Q. infectoria gall extract against L. interrogans serovars
| Q. infectoria gall extract concentration (mg/mL) | Leptospira interrogans serovars | |
|---|---|---|
|
| ||
| Javanica | Icterohaemorrhagiae | |
| 4.000 | − | − |
| 2.000 | − | − |
| 1.000 | − | − |
| 0.500 | − | − |
| 0.250 | − | − |
| 0.125 | − | + |
| 0.063 | + | + |
| 0.031 | ND | ND |
| 0.016 | ND | ND |
| 0.0078 | ND | ND |
| Positive control | + | + |
| Negative control | − | − |
No growth (−). Growth (+). Positive control: bacterial suspensions and broth. Negative control: extract and broth. ND: not done because the well was turbid indicating the presence of the organisms within the well
Table 3.
MIC and MBC values of aqueous Q. infectoria gall extract against L. interrogans serovars
| L. interrogans serovars | Anti-microbial activity of aqueous Q. infectoria gall extract | ||
|---|---|---|---|
|
| |||
| MIC (mg/mL) | MBC (mg/mL) | MBC/MIC Ratio | |
| Javanica | 0.125 | 0.125 | 1 |
| Icterohaemorrhagiae | 0.125 | 0.250 | 2 |
Figure 1.
MBC determination of aqueous Q. infectoria gall extract against the L. interrogans serovars. These are the micrographs of the L. interrogans serovar Javanica cultures examined under the dark-field microscope (at 400× magnification) following three weeks incubation. A: The positive control (inoculum and broth) well shows a positive leptospiral growth indicated by the small white thread-like structures (arrow). B: The negative control (extract and broth) well shows the absence of growth or any intact cellular structure. C: Test well with MIC (0.125 mg/mL) shows the absence of growth or any intact cellular structure
Figure 2.
MBC determination of aqueous Q. infectoria gall extract against the L. interrogans serovars. These are the micrographs of the L. interrogans serovar Icterohaemorrhagiae cultures examined under the dark-field microscope (at 400× magnification) following three weeks incubation. A: The positive control (inoculum and broth) well shows a positive leptospiral growth indicated by the small white thread-like structures (arrow). B: The negative control (extract and broth) well shows the absence of growth or any intact cellular structure. C: Test well with MIC (0.125 mg/mL) shows the presence of leptospiral growth (arrow)
Morphological Changes of the Treated Leptospira under the SEM
The morphology of the Leptospira during the logarithm growth phase was observed under the SEM. The untreated cells were spiral and helical in shape, with a smooth surface (Figure 3A). The organisms were clumped together, and a hook formation could not be observed. The exposure of the bacterial cells to the extract at 8× MIC (2 mg/mL) showed a slight deformation in the cell shape with mild elongation (Figure 3C). The positive control group showed elongated cells lacking a helical shape (Figure 3B). In addition to the affected shape of the bacterial cells, the size of the extract-treated (Figure 4C) and penicillin-treated cells (Figure 4B) was relatively smaller and shorter in comparison to the untreated cells (Figure 4A), as observed under the SEM at 60,000× magnification.
Figure 3.
Scanning electron micrographs of the treated and untreated L. interrogans serovar Icterohaemorrhagiae cultures observed at 30,000× magnification. A: The negative control (untreated) smear shows the spiral-shaped structures of cells. B: The positive control (penicillin-treated) smear shows an elongated cell lacking of the helical shape. C: The extract-treated (at 8× MIC) smear shows a slightly elongated and deformed spiral-shaped cell
Figure 4.
Scanning electron micrographs of the treated and untreated L. interrogans serovar Icterohaemorrhagiae cultures observed at 60,000× magnification. A: Negative control (untreated cell). B and C: Positive control (penicillin-treated) and extract-treated (at 8× MIC) smears show a relatively small size of cells in comparison to the one in the negative control (A)
Discussion
Currently, there is no standard method for assessing anti-microbial agents for anti-leptospiral activity. The broth microdilution technique (17) is used in our study to determine the anti-microbial activity of the aqueous Q. infectoria gall extract against the pathogenic Leptospira serogroups. The inhibition activities of the aqueous Q. infectoria gall extract against the L. interrogans serovars are observed after three days incubation at 30 °C, as is indicated through observation of the well without turbidity on the 96-well-microplate. In this study water is selected as a solvent, since water is a polar and universal solvent that is most commonly used to extract plant products with anti-microbial activity (18). Furthermore, another study reports that the water extract of a plant contains a high concentration of both gallic and tannic acids in comparison to other types of solvents (19). This study also uses the same plant source as the one by Nur Saeida et al. (13), which identifies pyrogallic acid (1,2,3–benzenetriol) as a major compound of its extraction. Pyrogallol, a hydrolysable tannin with three hydroxyl groups and alpha-beta double bonds, reportedly plays an important role for anti-bacterial activity (13).
The aqueous Q. infectoria gall extract displays significant inhibitory effect on the Leptospira serovars, which is of clinical significance. The MIC value (0.125 mg/mL) of the aqueous Q. infectoria gall extract against the L. interrogans serovars is slightly higher than the MIC value of the aqueous Q. infectoria gall extract tested against the methicillin resistant coagulase negative Staphylococcus (MRCoNS), the methicillin resistant Staphylococcus aureus (MRSA) and the Candida sp., which show MIC values of 0.08 mg/mL (8) and 0.06 mg/mL (7), respectively. The ratio of the MBC to the MIC for the aqueous Q. infectoria gall extract against the L. interrogans serovar Javanica and the L. interrogans serovar Icterohaemorrhagiae is found to be less than four. Hence, the gall extract affects both of the L. interrogans serovars through bactericidal activity, a finding that also proposes a possible concentration dependent killing of leptospires.
The presence of a hydrolysable tannin like pyrogallol, which crosses the cell wall polysaccharide network and proteins, is a likely mechanism responsible for the inhibitory or killing effects on the organism. In addition, the hydrolysed tannin can also prevent the adherence of the bacteria to the host cell, as they have a structure similar to the bacteria-binding receptors that are present in urinary tract cells (20). Thus, the effects seen in this study on the structural changes of the L. interrogans serovar Icterohaemorrhagiae may be the result of pyrogallol action on the outer membrane of the organism, which lead to an inhibition of its growth.
The morphological defects of the leptospires when exposed to the gall extract also support, to some extent, an explanation of the cell surface or membrane disruptive mechanisms. The role of penicillin against the L. interrogans involves the inhibition of peptidoglycan formation by binding to the transpeptidase (21–23). Peptidoglycan is the inner cell wall that contains the penicillin-binding proteins (PBPs) (24). Thus, the mechanisms of the anti-microbial activity of penicillin occur internally within the inner membrane of the Leptospira, which could not be clearly revealed here using the SEM. Therefore, ultrastructural analysis using a transmission electron microscope should be done in order to further study the killing mechanisms of penicillin and the extract against Leptospira sp.
Conclusions
The Q. infectoria gall extract has anti-microbial inhibition and killing activity against the Leptospira isolates. The findings presented in this research represent important data obtained regarding the anti-leptospiral activity of the Q. infectoria gall extract. Therefore, additional studies are needed to further evaluate the gall extract efficacy when used in combination with the currently available antibiotics and to assess its pre-clinical therapeutic use in more depth.
Acknowledgements
We would like to thank Mr Yusrin, research assistant at the Department of Medical Microbiology and Parasitology, School of Medical Sciences, USM and Mr Idris, project assistant at the School of Health Sciences, USM for their valuable guidance and assistance. We also would like to thank Mr Mohd Noor Mohamad Roze from the School of Health Sciences, USM for his technical assistance during SEM analysis. This study was funded by Universiti Sains Malaysia RUI grant (No: 1001/PPSK/812163).
Footnotes
Authors’ Contributions
Conception and design: NI, WNAWAW
Analysis and interpretation of the data: HM
Drafting of the article: HM
Critical revision of the article for important intellectual content: WNAWAW
Final approval of the article: WNAWAW
Provision of study materials or patients: NI
Obtaining of funding: WNAWAW
Administrative, technical, or logistic support: NI
This work is licensed under the terms of the Creative Commons Attribution (CC BY) (http://creativecommons.org/licenses/by/4.0/).
References
- 1.Tilahun Z, Reta D, Simenew K. Global epidemiological overview of leptospirosis. Int J Microbiol Res. 2013;4(1):9–5. doi: 10.5829/idosi.ijmr.2013.4.1.7134. [DOI] [Google Scholar]
- 2.Chadsuthi S, Modchang C, Lenbury Y, Iamsirithaworn S, Triampo W. Modeling seasonal leptospirosis transmission and its association with rainfall and temperature in Thailand using time-series and ARIMAX analyses. Asian Pac J Trop Med. 2012;5(7):539–546. doi: 10.1016/S1995-7645(12)60095-9. [DOI] [PubMed] [Google Scholar]
- 3.Benacer D, Who PY, Mohd Zain SN, Amran F, Thong KL. Pathogenic and saprophytic Leptospira species in water and soils from selected urban sites in peninsular Malaysia. Microbes Environ. 2013;28(1):135–140. doi: 10.1264/jsme2.ME12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zainudin AW. Epidemiology and current situation of leptospirosis in Malaysia. Persidangan Kesihatan Persekitaran Pihak Berkuasa Tempatan. Symposium conducted at Zoonoses Disease Control Division sector, Ministry of Health Malaysia; WP Labuan. 2015. [Google Scholar]
- 5.Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494–501. doi: 10.1111/j.1469-0691.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- 6.Charan J, Saxena D, Mulla S, Yadav P. Antibiotics for the treatment of leptospirosis: systematic review and meta-analysis of controlled trials. Int J Prev Med. 2013;4(5):501–510. [PMC free article] [PubMed] [Google Scholar]
- 7.Baharuddin NS, Abdullah H, Abdul Wahab WA. Anti-Candida activity of Quercus infectoria gall extracts against Candida species. J Pharm Bioallied Sci. 2015;7(1):15. doi: 10.4103/0975-7406.148742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan Nor Amilah WAW, Masrah M, Hasmah A, Noor Izani NJ. In vitro antibacterial activity of Quercus infectoria gall extracts against multi-drug resistant bacteria. Trop Biomed. 2014;31(4):680–688. [PubMed] [Google Scholar]
- 9.Vaidya V, Mahendrakumar C, Bhise K. Preliminary phytochemical screening of Quercus infectoria Oliv. for treatment of skin diseases. J Med Plant Res. 2013;7(27):2019–2027. doi: 10.5897/JMPR12.1026. [DOI] [Google Scholar]
- 10.Basri DF, Fan SH. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agent. Indian J Pharmacol. 2005;37(1):26–29. doi: 10.4103/0253-7613.13851. [DOI] [Google Scholar]
- 11.Khder AK, Muhammed SA. Potential of aqueous and alcohol extracts of Quercus infectoria, Linusm usitatissium and Cinnamomum zeylanicium as antimicrobials and curing of antibiotic resistance in E. coli. Curr Res J Biol Sci. 2010;2(5):333–337. [Google Scholar]
- 12.Nur Syukriah A, Liza M, Harisun Y, Fadzillah A. Effect of solvent extraction on antioxidant and antibacterial activities from Quercus infectoria (Manjakani) Int Food Res J. 2014;21(3):1031–1037. [Google Scholar]
- 13.Nur Saeida B, Hasmah A, Wan Nor Amilah W, Kerian K. Potential use of Quercus infectoria gall extracts against urinary tract pathogenic bacteria. Int J of Res in Pharmacology & Pharmacotherapeutics. 2014;3(3):184–191. [Google Scholar]
- 14.Verma S, Sharma H, Garg M. Phyllanthus Amarus: A review. J Pharmacogn Phytochem. 2014;3(2):18–22. [Google Scholar]
- 15.Shrestha S, Kaushik VS, Eshwarappa RSB, Subaramaihha SR, Ramanna LM, Lakkappa DB. Pharmacognostic studies of insect gall of Quercus infectoria Olivier (Fagaceae) Asian Pac J Trop Biomed. 2014;4(1):35–39. doi: 10.1016/S2221-1691(14)60205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miraglia F, Moraes ZMd, Melville PA, Dias RA, Vasconcellos SA. EMJH medium with 5-fluorouracil and nalidixic acid associated with serial dilution technique used to recover Leptospira spp from experimentally contaminated bovine semen. Braz J Microbiol. 2009;40(1):189–193. doi: 10.1590/S1517-83822009000100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CLSI. Methods for dilution anti-microbial susceptibility tests for bacteria that grow aerobically. Approved standard–ninth edition. Wayne PA: Clinical and Laboratory Standards Institute; 2012. (CLSI document M07-A9). [Google Scholar]
- 18.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: A review. Internationale Pharmaceutica Sciencia. 2011;1(1):98–106. [Google Scholar]
- 19.Harisun Y. Quantification of gallic acid and tannic acid from Quercus infectoria (manjakani) and their effects on antioxidant and antibacterial activities. Pertanika J Sci Technol. 2015;23(2):351–362. [Google Scholar]
- 20.Vasconcelos LCS, Sampaio FC, Sampaio MCC, Pereira MSV, Higino JS, Peixoto MHP. Minimum inhibitory concentration of adherence of Punicagranatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz Dent J. 2006;17(3):223–337. doi: 10.1590/S0103-64402006000300009. [DOI] [PubMed] [Google Scholar]
- 21.Haake DA, Walker E, Blanco D, Bolin C, Miller M, Lovett M. Changes in the surface of Leptospira interrogans serovar Grippotyphosa during in vitro cultivation. Infect Immun. 1991;59(3):1131–1140. doi: 10.1128/iai.59.3.1131-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenot A, Trott D, Saint Girons I, Zuerner R. Penicillin-binding proteins in Leptospira interrogans. Antimicrob Agents Chemother. 2001;45(3):870–877. doi: 10.1128/AAC.45.3.870-877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seesom W, Jaratrungtawee A, Suksamrarn S, Mekseepralard C, Ratananukul P, Sukhumsirichart W. Antileptospiral activity of xanthones from Garcinia mangostana and synergy of gamma-mangostin with penicillin G. BMC Complement Altern Med. 2013;13(182):1–6. doi: 10.1186/1472-6882-13-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736–747. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]