Abstract
Objective
The aim of the present study was to demonstrate the effects of misoprostol in ovalbumin-induced allergic rhinitis (AR). The second purpose was to compare the effect profile of the combination of an antihistamine with misoprostol during treatment of AR.
Materials and Methods
Twenty-five adult male rats were used and were randomly classified into five groups (n=5): healthy+saline, AR, AR and desloratadine (D)-treated group, AR and misoprostol (M)-treated group, and AR and combined-treated group.
Results
Desloratadine administration had significantly lower nasal symptoms than the AR group, but nasal symptoms in the AR+M group were better than those in the AR+D group. The best improvement in serum IgE levels was seen in the misoprostol alone and combination treatment groups.
Conclusion
We suggest that prostaglandins should be considered in the treatment of AR, and that the effects of these types of drugs should be tested clinically in patients.
Keywords: Allergy, desloratadine, misoprostol, rat, rhinitis
Introduction
Allergic rhinitis (AR) is defined as the presence of at least one of the following: nasal discharge, sneezing, nasal itchiness, and nasal congestion [1]. Patients with AR may also be disturbed by sleep disorders, mood disorders, impaired daily activities, and deterioration in social relationships [2]. Concurrently, it is stated that AR causes significant morbidity and has a significant effect on the quality of life. According to epidemiological evidence, the prevalence of AR, as well as all atopic diseases, is increasing worldwide [3]. AR is a common disease that affects 10%–40% of the world’s population [4].
Allergic rhinitis (AR) treatment consists of allergen prevention, patient education, pharmacological treatment, and allergen immunotherapy. Preventing contact between the allergen and the nasal mucosa is the main prevention method, but in most cases, this is insufficient, and appropriate drug treatment for symptom control is also required. Currently, the most commonly used treatment agents are nasal steroids, antihistamines, leukotriene receptor antagonists, and immunotherapy. The aim of the treatment is to increase the patient’s quality of life and adaptation to their current life by controlling the symptoms [5, 6]. However, because of the frequency of side effects and the chronic use of the drugs used in the treatment of AR, most patients stop using the drugs and cannot be treated. Therefore, the production of drugs that are more effective, especially those with fewer central side effects, plays an important role in increasing patient compliance.
Prostaglandin E (PGE) is a metabolite of the arachidonic acid produced by the cyclooxygenase enzyme, which is known to prevent inflammation developed in the airways [7]. PGE performs its physiological function through four PGE receptors (EP1-4), which play a pharmacologically important role [7]. Studies have shown that PGE receptors mediate many physiological events, such as pain, fever, platelet aggregation, gastric cytoprotection, airway protection, ovulation, fertilization, bronchodilation, vasodilatation, angiogenesis, cytokine, and chemokine modulation. PGE is synthesized in the body in two forms, PGE1 and PGE2, but it is also among the most important prostaglandins, having ligands at four different receptors. However, while endogenous PGE2 acts as an inflammatory, PGE1 acts as an anti-inflammatory agent [8, 9]. Studies of our subject have shown that the EP1, 2, 3, and 4 receptor analogs may have beneficial effects on antigen-induced hypersensitivity and mucus secretion in the nasal epithelium [10]. It is shown here that the EP2 and 3 analogs prevent mucus production, and the EP3 analog prevents neutrophil migration, thus reducing inflammation in the airways. However, the selective EP1 analog has been shown to have no effect. Additionally, the EP4 analog has been shown to affect the effector phase of the antigen. In a clinical study, it has been shown that the local administration of PGE1 and PGE2 analogs is beneficial for AR and improves symptoms [11]. However, the local use of PGE1 has been reported to cause nasal irritation.
Misoprostol is a PGE1 analog drug used in the treatment of peptic ulcers due to nonsteroidal anti-inflammatory drugs’ resistance to destructive enzymes, such as aspirin [12]. The effect of misoprostol on prostaglandin EP2 receptors is attributed to the prostaglandin EP3 receptor and prostaglandin EP4 receptors. However, it has no effect on the prostaglandin EP1 receptor [13]. In 1992, misoprostol was patented to treat allergic diseases in the late phase, but its effect on AR was not directly demonstrated [14].
Therefore, the aim of the present study was to demonstrate the effects of misoprostol in ovalbumin (OVA)-induced AR. The second purpose of the study was to compare the effect profile of the combination of an antihistamine with misoprostol during treatment of AR.
Materials and Methods
Animals
Male Sprague-Dawley rats were supplied by the Ataturk University’s Experimental Medical and Research Center. The study was approved by the Ataturk University National Animal Ethics Committee (2018/207). Rats were housed in standard plastic cages with sawdust bedding. The cages were placed in an air-conditioned room at 22 °C under lighting controls (14 h light/10 h dark cycle). All rats had free access to standard rat food and tap water. Rats were fed a standard rat diet.
A total of 25 adult male Sprague-Dawley rats (220–250 g) were used. Rats were randomly classified into five groups (n=5): healthy+saline, AR, AR and desloratadine (D)-treated group, AR and misoprostol (M)-treated group, and AR and combined-treated group.
Preparation of OVA-induced AR rat model
The AR model was established according to literature-based standard protocols [15, 16]. Specifically, 20 rats belonging to the AR, AR+D, AR+M, and AR+DM groups were sensitized with a 1 mL intraperitoneal injection of 30 mg Al (OH)3 and 0.3 mg OVA (Sigma, USA) solutions once every other day over a period of 14 days (total of seven injections). Then, sensitization continued via nasal challenge (by putting drops in bilateral nasal cavities) with 20 mL 10% OVA once a day from day 15 to day 21. All rats in the AR+D group received 10 mg/kg desloratadine orally (dissolved in saline solution), the AR+M group received 100 μg/kg misoprostol administered orally (dissolved in saline solution), and the AR+DM group received combined therapy with the nasal challenge once every 7 days at the same schedule. The healthy group and the AR group were given a saline solution alone according to the same schedule.
Evaluation of nasal symptoms
On day 21 of the study, rats were placed in an observation cage for approximately 10 min for acclimatization before the experiment started. After nasal instillation of 20 mL 10% OVA into the bilateral nasal cavities, the animals were placed in observation cages (one animal/cage), and the numbers of sneezes and nasal rubbing movements were counted for 30 min by four blinded observers who were unaware of the sample’s identity. Thereafter, blood samples were collected from each rat via cardiac puncture under anesthesia using isoflurane; the rats were then killed. Serum was prepared and frozen at −80 °C prior to biochemical analysis. Nasal tissue and submandibular glands were immediately removed from all the rats at the end of the study for histopathological changes.
Immunoglobulin E measurements in serum
OVA-specific IgE levels in serum were measured in duplicate using a highly sensitive enzyme-linked immunosorbent assay (Sunred, China) according to the manufacturer’s instructions (201-11-4050) for kits designed specifically for rats.
Histopathological examination
The tissues reserved for histopathological examination were rapidly fixed in a 10% buffered formalin solution for 24 h. After fixation, a tissue sample was routinely processed and embedded in paraffin. For routine histological examination, 5 μm-thick sections were cut as paraffin-embedded tissue samples. After 5 μm-thick sections of the tissue were mounted onto positively charged slides and then underwent deparaffinization and rehydration, two sections of each rat were stained with Mayer’s hematoxylin and eosin and Toluidine blue. All sections were examined and photographed using a light photomicroscope (Nikon Eclipse E600; USA).
Statistical analysis
The Statistical Package for the Social Sciences (SPSS), version 20.0 software (IBM Corp., Armonk, NY, USA) was used for molecular analysis. The results are presented as mean±standard deviation. Between-group comparisons for biochemical analyses and nasal symptoms were performed using a one-way ANOVA and Tukey’s multiple comparison tests. A p<0.05 was considered significant.
Results
Nasal symptom findings
In AR studies, the gold standard that is closest to the clinic is the evaluation of nasal symptoms. As shown in Table 1 and Figure 1, nasal symptoms were significantly higher in the AR group than in the healthy group (p=0.04). Desloratadine administration had significantly lower nasal symptoms than the AR group, but nasal symptoms in the AR+M group were better than those in the AR+D group (p=0.026). In our study, if we evaluate the nasal symptoms in the AR+D group, the best improvement is seen after combined treatment (p=0.000). In this case, misoprostol significantly inhibits the symptoms both alone and when combined with an antihistamine.
Table 1.
Demonstration of serum IgE levels and nasal symptom scores
| Mean | SD | Min | Max | p | ||
|---|---|---|---|---|---|---|
| IgE | H | 96.77 | 12.09 | 77.30 | 113.37 | 0.000 |
| AR | 241.01 | 12.63 | 222.83 | 263.39 | ||
| AR+D | 173.67 | 14.25 | 149.11 | 199.50 | ||
| AR+M | 158.61 | 12.44 | 138.03 | 176.85 | ||
| AR+DM | 149.33 | 14.25 | 118.79 | 172.18 | ||
| Nasal symptoms | H | 29.80 | 8.93 | 19.00 | 40.00 | 0.000 |
| AR | 303.60 | 15.63 | 285.00 | 322.00 | ||
| AR+D | 144.20 | 7.60 | 133.00 | 151.00 | ||
| AR+M | 125.60 | 7.92 | 118.00 | 138.00 | ||
| AR+DM | 105.60 | 3.65 | 101.00 | 110.00 |
p<0.05 significant, test: post hoc Tukey. H: healthy; AR: allergic rhinitis; D: desloratadine; M: misoprostol.
Figure 1.
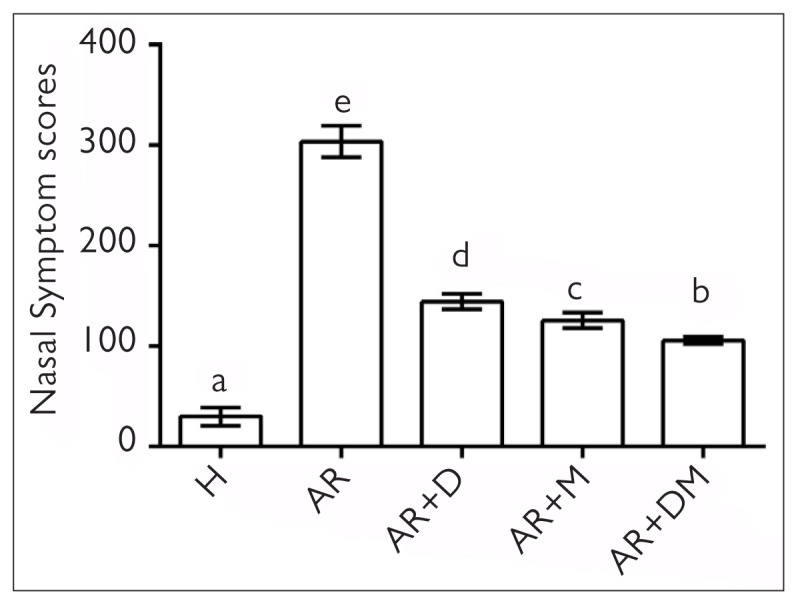
Nasal symptom scores after OVA-induced allergic rhinitis model. The same letters in the columns were not statistically significant. Different letters in the columns were statistically significant (p<0.05).
H: healthy; AR: allergic rhinitis; D: desloratadine; M: misoprostol.
Serum IgE results
Since AR is IgE dependent, it is important to show serum IgE levels. In Figure 2, the level of IgE is significantly higher in the AR group than in the healthy group (p=0.001). Desloratadine significantly improved serum IgE levels when compared with the AR group. However, the best improvement was seen in the misoprostol alone and combination treatment groups (p=0.000).
Figure 2.
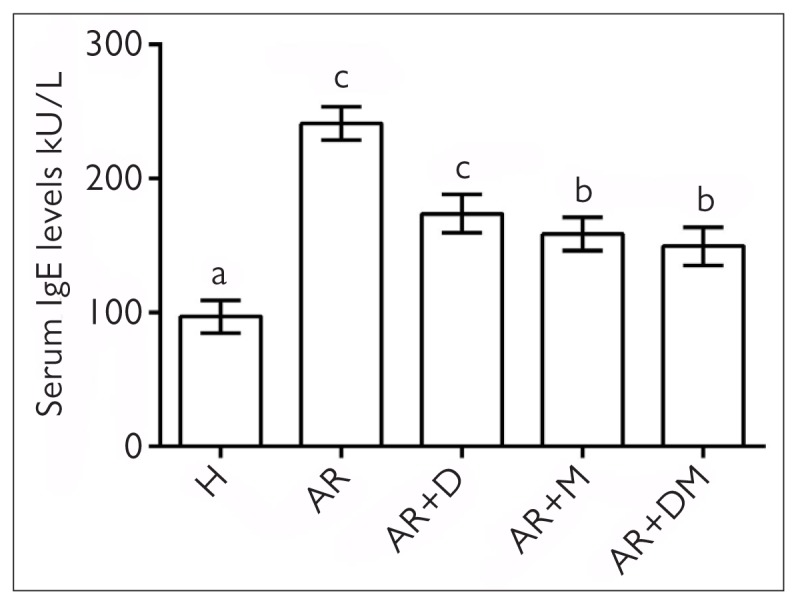
Serum IgE levels of the groups. The same letters in the columns were not statistically significant. Different letters in the columns were statistically significant (p<0.05).
H: healthy; AR: allergic rhinitis; D: desloratadine; M: misoprostol.
Histopathological results
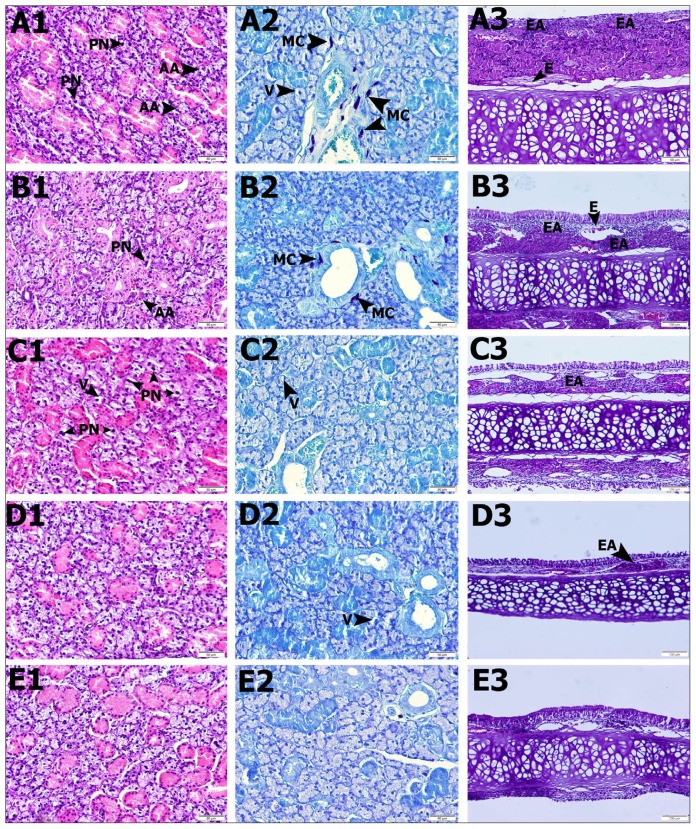
Finally, we evaluated the nasal septum and submandibular gland histopathologically. In the healthy group, the nasal septum epithelium and connective tissue were normal, and no histopathological findings were found (Figure 3-E3). Serous and mucous sinuses were histologically normal in the submandibular gland (Figure 3-E1). No mast cells were observed in this group (Figure 3-E2). In the nasal septum of the AR group, intense inflammatory cell areas in the epithelium and connective tissue, edema areas within the connective tissue, and a significant thickness increase in the connective tissue were observed (Figure 3-A3). In the submandibular glands, changes in the atrophic sinuses, from serous acinus and pyknotic nuclei, were significant in some gland epithelial cells. However, it has been observed that they had lost their prominent limits (Figure 3-A1). Mast cell increase was observed (Figure 3-A2). In the AR+D group, inflammatory cell areas were observed in the nasal septum connective tissue, whereas the connective tissue increase was relatively lower than in the AR group (Table 1, Figure 3-B3). In the submandibular gland, the serous acinus was normal, but atrophic acinus and pyknotic nuclei were partly found in epithelial cells (Figure 3-B1). However, fewer mast cells were observed than in the AR group (Figure 3-B2). There were markedly higher inflammatory cell areas in the epithelial and connective tissues in the nasal septum of the AR+M group than those of the AR group, but no edema areas were observed (Table 1, Figure 3-C3). In the submandibular glands, serous and mucous membranes were similar to that of healthy tissues. However, some vacuolization and pyknotic nuclei were observed (Figure 3-C2). No further mast cells were observed (Figure 3-C2). In the AR+DM group, relatively fewer inflammatory cell areas were seen in the nasal septum epithelium and connective tissue, but edema areas were not observed. However, the increase of goblet secretion cells in the epithelium was noteworthy (Table 2, Figure 3-D3). Serous and mucous acini were the most similar healthy tissue in the submandibular gland. However, rarely, pyknotic nucleus gland epithelium and vacuation were seen (Figure 3-D1). No mast cells were observed in this group (Figure 3-D2).
Figure 3.
Light photomicroscope results of the submandibular gland and nasal septum. First column (1) is hematoxylin and eosin staining of the submandibular gland. Second column (2) is Toluidine blue staining of the submandibular gland. Third column (3) is hematoxylin and eosin staining of the nasal septum.
E: healthy; A: allergic rhinitis; B: AR+desloratadine; C: AR+misoprostol; D: AR+DM groups.
Table 2.
Histopathological scoring
| Groups | AA | E | EA | V | PN | MC |
|---|---|---|---|---|---|---|
| Healthy | 0 | 0 | 0 | 0 | 0 | 0 |
| AR | 3 | 2 | 3 | 0 | 3 | 3 |
| AR+desloratadine | 2 | 1 | 2 | 0 | 2 | 2 |
| AR+misoprostol | 1 | 0 | 1 | 1 | 2 | 0 |
| AR+desloratadine+misoprostol | 0 | 0 | 1 | 1 | 1 | 0 |
E: edema; AA: acinar atrophy; EA: inflammation area; V: vacuolization; PN: pyknotic nucleus; MC: mast cell. Scoring: 0 (none), 1 (mild), 2 (moderate), and 3 (severe).
Discussion
In our study, we have shown that misoprostol may have beneficial effects alone and in combination in the OVA-induced allergic model.
Allergic rhinitis (AR) is characterized by nasal mucosal inflammation that develops because of an IgE-mediated reaction. Cells, mediators, cytokines, chemokines, neuropeptides, and various adhesion molecules involved in inflammation interact with one another in a complex structure and contribute to the emergence of specific symptoms and nonspecific nasal hyperreactivity [17]. In experimental AR model studies, nasal symptoms are evaluated clinically. Observable nasal discharge and nasal itching scores were observed to be significantly higher in the AR model than in the healthy group [18]. Significant improvements are observed in these symptoms due to treatment [19]. In our study, it was shown that nasal symptoms increased significantly due to hyperreactivity in the AR group. Concurrently, it was reported that serum IgE levels were significantly higher after AR, and serum IgE levels approached normal levels [20]. In the present study, serum IgE levels were significantly higher in the AR group than in the healthy group, thus supporting the appropriateness of our model.
The inflammatory process in AR begins when the nasal mucosa meets the allergen and continues with inflammatory cell infiltration. IgE production occurs as a result of complex interactions between B cells, T cells, mast cells, and basophils [21]. In this process, cytokines, such as interleukin (IL)-4, IL-13, and IL-18, as well as surface and adhesion molecules, which provide a physical interaction between T and B cells, also play an important role [22]. T helper cell type 2-type cytokines induce allergen-specific IgE production by inducing an IgE isotype shift in IL-4 and IL-13 B cells and lead to the maturation and aggregation of cells, such as eosinophils, basophils, and mast cells [23]. Allergens associated with bridging to IgEs after allergen exposure cause the release of histamine; leukotrienes C4, D4, and E4; prostaglandin D2; and cytokines from mast cells and basophils, causing early and late phase inflammation [24, 25]. However, the pathophysiology of AR is still not fully resolved. Therefore, the development of new treatment strategies and the clarification of physiopathology are still up for discussion.
Prostaglandin E (PGE) is a metabolite of the arachidonic acid produced by the cyclooxygenase enzyme and is known to have a physiological effect in the prevention of inflammation in the airways [7]. It dilates the bronchi, inhibits the proliferation of lymphocytes, and inhibits the production of important inflammatory cytokines, such as tumor necrosis factor-α, IL-1β, IL-8, and IL-12, and the production of important chemokines released from macrophages, neutrophils, and dendritic cells, such as chemokine ligand (CCL) 3 and CCL4 [26–28]. It performs its physiological effects with four types of receptors (EP1-4), which are also pharmacologically important [7]. PGE receptors have been shown to mediate many physiological events, such as pain, fever, platelet aggregation, gastric cytoprotection, bone health, airway protection, ovulation, fertilization, bronchodilation, vasodilatation, angiogenesis, cytokine, and chemokine modulation. Endogenous PGE1 acts as anti-inflammatory [8, 9]. Therefore, in our study, we evaluated misoprostol, which is the PGE1 analog and has the effect of binding to prostaglandin EP2, EP3, and EP4 receptors. In the present study, we found the best IgE recovery in the combination group at serum IgE level when compared with misoprostol and desloratadine. This suggests that misoprostol may play an important role in the treatment of AR, which is another important finding in combination with existing antihistamine therapy. Previous studies have shown that misoprostol decreases IgE dependent histamine release, supporting our findings [29]. Furthermore, EP1, 2, 3, and 4 analogs have been shown to have beneficial effects on antigen-induced hypersensitivity and mucus secretion in the nasal epithelium [10]. It is shown here that EP2 and EP3 analogs prevent mucus production, and EP3 analog also reduces neutrophil migration and inflammation in the airways. However, the selective EP1 analog had no effect. Furthermore, the EP4 analog was shown to act on the effector phase of the antigen. A clinical study also showed that the local administration of PGE1 and PGE2 analogs was beneficial in AR and improved symptoms [11]. In our study, misoprostol showed a significant effect on both the AR group and the AR+D group. However, the combination of desloratadine and misoprostol has been more effective in relieving the symptoms.
In our study, nasal septum and submandibular glands were evaluated histopathologically. Pathological changes in the nasal tissue due to AR are usually as follows: thickening of the nasal respiratory epithelium, edema, and degeneration of the mucous membrane, mast cell increase, and vascular congestion [16, 18]. In addition, significant histopathological changes occur in the salivary glands of patients with AR. Significant changes, such as reduction of salivary secretion, decreased contents, and number of secretory cells, have been recorded [15]. It is thought that the acinar cells did not recover despite treatment with vacuolization, apoptosis, and irradiation, and this situation is associated with decreased salivation [30]. In our study, intense inflammatory cell areas in the AR group and edema areas within the connective tissue, as well as a significant thickness increase in the connective tissue, were observed, whereas atrophic acini and some of the gland epithelial cells were observed in the serous mucins of the submandibular glands. Inflammatory cell areas of the epithelium and connective tissue were significantly lower in the nasal septum of the AR+M group than that of the AR+D group. Nevertheless, some vacuolization and pyknotic nuclei were observed. In the AR+DM group, relatively fewer inflammatory cell areas were observed in the nasal septum epithelium and connective tissue, but edema areas were not observed. Serous and mucous acini were determined as the closest group to healthy tissue in submandibular glands.
As a result of our study, we have shown that the pathophysiology of AR, which has become an important disease in modern society, has not yet been clarified. Many cytokines, as well as immunological cells of these cells and systems, can be important in products, and we have determined that it is important for new treatment methods to be taken into consideration. In conclusion, we suggest that prostaglandins should be considered in the treatment of AR, and that the effects of these types of drugs should be tested clinically in patients.
Footnotes
This study was an oral presentation at Turkish Society of Anaesthesiology and Reanimation 51st National Congress 2017.
Ethics Committee Approval: Ethics Committee approval was received for this study from the Ethics Committee of Atatürk University (2018/207).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.Y., Z.K.; Design – A.B., N.E.A.; Supervision – H.U., I.C.; Data Collection and/or Processing – S.O., E.T.; Analysis and/or Interpretation – M.Y., V.M.; Literature Search – Z.K., V.M.; Writing – Z.K., M.Y.; Critical Reviews – H.U., Z.K, M.Y.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res. 2011;3:148–56. doi: 10.4168/aair.2011.3.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousquet J, Neukirch F, Bousquet PJ, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care. J Allergy Clin Immunol. 2006;117:158–62. doi: 10.1016/j.jaci.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 3.Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9. doi: 10.2500/aap.2007.28.2934. [DOI] [PubMed] [Google Scholar]
- 4.Bauchau V, Durham SR. Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy. 2005;60:350–3. doi: 10.1111/j.1398-9995.2005.00751.x. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Arnavielhe S, Bedbrook A, et al. The Allergic Rhinitis and its Impact on Asthma (ARIA) score of allergic rhinitis using mobile technology correlates with quality of life: The MASK study. Allergy. 2018;73:505–10. doi: 10.1111/all.13307. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Schunemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130:1049–62. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu THR, Shimizu S, Kouzaki H, Majima Y. Role of prostaglandin E2 as a protective mediator for allergic inflammation. Clin Exp Allergy Rev. 2007;7:36–40. doi: 10.1111/j.1365-2222.2007.00123.x. [DOI] [Google Scholar]
- 8.Murota H, Kotobuki Y, Umegaki N, Tani M, Katayama I. New aspect of anti-inflammatory action of lipo-prostaglandinE1 in the management of collagen diseases-related skin ulcer. Rheumatol Int. 2008;28:1127–35. doi: 10.1007/s00296-008-0589-5. [DOI] [PubMed] [Google Scholar]
- 9.Sobota RM, Muller PJ, Heinrich PC, Schaper F. Prostaglandin E1 inhibits IL-6-induced MCP-1 expression by interfering specifically in IL-6-dependent ERK1/2, but not STAT3, activation. Biochem J. 2008;412:65–72. doi: 10.1042/BJ20071572. [DOI] [PubMed] [Google Scholar]
- 10.Hattori R, Shimizu S, Majima Y, Shimizu T. Prostaglandin E2 receptor EP2, EP3, and EP4 agonists inhibit antigen-induced mucus hypersecretion in the nasal epithelium of sensitized rats. Ann Otol Rhinol Laryngol. 2009;118:536–41. doi: 10.1177/000348940911800714. [DOI] [PubMed] [Google Scholar]
- 11.Karim SM, Adaikan PG, Kunaratnam N. Effect of topical prostaglandins on nasal patency in man. Prostaglandins. 1978;15:457–62. doi: 10.1016/0090-6980(78)90129-6. [DOI] [PubMed] [Google Scholar]
- 12.Graham DY, White RH, Moreland LW, et al. Duodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Misoprostol Study Group. Ann Intern Med. 1993;119:257–62. doi: 10.7326/0003-4819-119-4-199308150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Moreno JJ. Eicosanoid receptors: Targets for the treatment of disrupted intestinal epithelial homeostasis. Eur J Pharmacol. 2017;796:7–19. doi: 10.1016/j.ejphar.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Rafeul Alam J, Grant Andrew, inventors. Use of misoprostol for the treatment of allergy. WO1993006831A1. US patent. 1991
- 15.Tatar A, Parlak SN, Yayla M, Ugan RA, Polat E, Halici Z. Effects of allergic rhinitis and desloratadine on the submandibular gland in a rat allergy model. Int Forum Allergy Rhinol. 2015;5:1164–9. doi: 10.1002/alr.21589. [DOI] [PubMed] [Google Scholar]
- 16.Tatar A, Yayla M, Kose D, Halici Z, Yoruk O, Polat E. The role of endothelin-1 and endothelin receptor antagonists in allergic rhinitis inflammation: ovalbumin-induced rat model. Rhinology. 2016;54:266–72. doi: 10.4193/Rhin15.059. [DOI] [PubMed] [Google Scholar]
- 17.Shinmei Y, Yano H, Kagawa Y, et al. Effect of Brazilian propolis on sneezing and nasal rubbing in experimental allergic rhinitis of mice. Immunopharmacol Immunotoxicol. 2009;31:688–93. doi: 10.3109/08923970903078443. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Feng J, Sun J, et al. H2-Eb1 expression is upregulated in the nasal mucosa of allergic rhinitis. Asian Pac J Allergy Immunol. 2014;32:308–15. doi: 10.12932/AP0478.32.4.2014. [DOI] [PubMed] [Google Scholar]
- 19.Jung HW, Jung JK, Park YK. Antiallergic effect of Ostericum koreanum root extract on ovalbumin-induced allergic rhinitis mouse model and mast cells. Asian Pac J Allergy Immunol. 2011;29:338–48. [PubMed] [Google Scholar]
- 20.Kim M, Kim KE, Jung HY, et al. Recombinant erythroid differentiation regulator 1 inhibits both inflammation and angiogenesis in a mouse model of rosacea. Exp Dermatol. 2015;24:680–5. doi: 10.1111/exd.12745. [DOI] [PubMed] [Google Scholar]
- 21.Small P, Keith PK, Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018;14:51. doi: 10.1186/s13223-018-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Small PFS, Becker A, Boisvert P, et al. Rhinitis: a practical and comprehensive approach to assessment and therapy. J Otolaryngol. 2007;36:5–27. doi: 10.2310/7070.2006.X002. [DOI] [Google Scholar]
- 23.Campo P, Rondon C, Gould HJ, Barrionuevo E, Gevaert P, Blanca M. Local IgE in non-allergic rhinitis. Clin Exp Allergy. 2015;45:872–81. doi: 10.1111/cea.12476. [DOI] [PubMed] [Google Scholar]
- 24.Pullerits T, Praks L, Ristioja V, Lotvall J. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109:949–55. doi: 10.1067/mai.2002.124467. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AM, O’Byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysis. Am J Med. 2004;116:338–44. doi: 10.1016/j.amjmed.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Takayama KG-CG, Sukhova GK, Comander J, Gimbrone MA, Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–54. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 27.Yamane H, Sugimoto Y, Tanaka S, Ichikawa A. Prostaglandin E(2) receptors, EP2 and EP4, differentially modulate TNF-alpha and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem Biophys Res Commun. 2000;278:224–8. doi: 10.1006/bbrc.2000.3779. [DOI] [PubMed] [Google Scholar]
- 28.Vassiliou E, Jing H, Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223:120–32. doi: 10.1016/S0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 29.Babakhin AA, Nolte H, DuBuske LM. Effect of misoprostol on the secretion of histamine from basophils of whole blood. Ann Allergy Asthma Immunol. 2000;84:361–5. doi: 10.1016/S1081-1206(10)62787-1. [DOI] [PubMed] [Google Scholar]
- 30.Muhvic-Urek M, Bralic M, Curic S, Pezelj-Ribaric S, Borcic J, Tomac J. Imbalance between apoptosis and proliferation causes late radiation damage of salivary gland in mouse. Physiol Res. 2006;55:89–95. doi: 10.33549/physiolres.930739. [DOI] [PubMed] [Google Scholar]