Abstract
Aims
To evaluate the availability of pharmacokinetic, safety and efficacy analyses specifically targeted at elderly, prior to the authorization of drugs.
Methods
A cross‐sectional, structured review of publicly available initial approval documents of Food and Drug Administration‐approved drugs was performed. The 10 most frequently on‐label prescribed drug classes, drugs with known pharmacokinetic differences in the elderly or drugs that are relatively contraindicated in elderly (e.g. anticholinergics or benzodiazepines) were included in the analyses.
Results
In total, 1129 unique active pharmaceutical ingredients were found eligible for the analyses, of these, 506 were found in the Food and Drug Administration database (45%). The initial approval documents were available for 182 drugs. For the majority of the drugs, the initial approval documents in the database showed information on pharmacokinetics in elderly (n = 113; 62%). Furthermore, over time, the availability of information with regard to elderly increased statistically significantly from 0% in the period 1970–1979 to 76% for the period 2010–2018. Information on safety and efficacy was less frequently present, i.e. 42% and 45%, respectively and, moreover, the availability of information did not improve over time.
Conclusion
The under‐representation of elderly in clinical trials thereby challenging the external validity of benefit/risk assessments of launched drugs was confirmed. Priority should be given to a study population that is representative for the target population.
Keywords: clinical trials, efficacy, elderly, pharmacokinetics, safety
What is already known about this subject
Under‐representation of elderly in clinical trials has been described earlier, thereby challenging the external validity of benefit/risk assessments of launched drugs.
Pharmacokinetic differences in the elderly may give rise to differences in safety and efficacy. Therefore, it is pivotal include geriatric patients in clinical trials of medical substances.
What this study adds
This is the first cross sectional, structured research on availability of pharmacokinetic, safety and efficacy analyses of the publicly available initial approval documents of Food and Drug Administration‐approved drugs.
For the majority of the drugs, the initial approval documents showed information on pharmacokinetics in elderly, but information on safety and efficacy was missing.
1. INTRODUCTION
The elderly represent a fast‐growing majority of the population in the Netherlands and worldwide.1, 2 In Europe, 25% of the population is aged 60 years or over and is expected to grow to 35% in 2050.3 Importantly, the representation of older people in clinical drug trials requires special attention, as it is known that pharmacokinetics and pharmacodynamics (and hence efficacy and safety) substantially change after the age of 75 years; albeit, not all drugs are similarly affected leading to increased variability in drug levels.4 In literature, different physiological parameters are discussed to affect absorption, distribution, metabolism and excretion of drugs during aging. For example, sarcopenia and increased percentage of fat tissue results in a different distribution volume.5 With regard to metabolism, the total liver mass reduces with age and there is a lower capacity for phase 1 reactions through the cytochrome P‐450 enzymes.6, 7 In contrast, conjugation reactions are not affected by ageing.5 Also, the hepatic blood flow is lower, which results in a reduced first pass effect.8 The renal function diminishes with age: there is reduced renal blood flow, diminished glomerular filtration rate and a reduced renal tubular secretory function.5 It is generally accepted that of the pharmacokinetic parameters, absorption is least affected by age.5 Pharmacodynamic changes in the elderly are the consequence of diminished reserve capacity or diseases of organ systems and changes in receptor number and affinity.5 In addition, comorbidity associated polypharmacy is more common among elderly and consequently the risk of interactions as well as adverse drug reactions is higher.9 These age‐related differences may give rise to age‐specific risk/benefit ratios for drugs in elderly.
The elderly consume the majority of prescribed medications and carry the largest burden of chronic diseases.10 Their representation in clinical trials should reflect this. For this reason, in 1993, the International Conference on Harmonization (ICH) of technical requirements for registration of pharmaceuticals for human use issued the ICH E7 guideline for the carrying out of studies in the geriatric population, stating that the trial population should represent the population that will consume the drug and should include a minimum percentage of older participants. The ICH E7 guideline was endorsed, among others, by the Food and Drug Administration (FDA) and pharmaceutical companies.11 Nevertheless, previous reports described that elderly are generally under‐represented in clinical studies in cardiology12, 13, 14, 15, 16, 17 and oncology18, 19, 20, 21 as they are excluded due to older age, multimorbidity or polypharmacy. Descriptive studies showed that in 30–40% of the original research papers in major medical journals, elderly people were excluded without justification.22, 23 Recent investigations carried out to evaluate the adherence to the ICH E7 guideline, showed that the proportion of the elderly in clinical trials is unacceptably low (1–9% in trials involving diseases not unique to old age).24 This was confirmed by an evaluation of the clinical trial database (clinicaltrials.gov).25 However, no investigation has systematically reviewed the available information on publicly available database of health care regulators.
In this study, we aimed to evaluate the availability of pharmacokinetic, safety and efficacy analyses specifically targeted at the elderly, prior to the authorization of the most frequently prescribed drug classes during the past years.
2. METHODS
2.1. Design
A cross‐sectional, structured assessment of publicly available initial approval documents of FDA‐approved drugs was performed. To obtain marketing authorization for newly developed drugs, companies are required to deliver quality, safety and efficacy information about the drug.
2.2. Drugs of interest
The following initial approval documents were assessed: those of frequently on‐label prescribed drug classes, those of drugs with known pharmacokinetic differences in the elderly, or those of drugs that are relatively contraindicated in elderly (e.g. anticholinergics or benzodiazepines). The following most frequently described drug classes were selected: antihypertensive drugs, medication for pain, drugs used for mental health or nervous system disorders, antibacterial drugs, lipid regulators, glucose lowering drugs, respiratory drugs, antiulcer drugs and thyroid therapies based on IQVIA.26 Furthermore, drugs with known large or small volumes of distribution and/or high or low hepatic clearance or renal excretion were included.4 Last, drugs with relative contraindications in elderly (e.g. anticholinergics or benzodiazepines).27
2.3. Assessment of information on pharmacokinetics, safety and efficacy
For these drug classes all available medical substances were extracted in December 2017 from the World Health Organization Anatomical Therapeutical Chemical Classification (ATC) index 2018.28 All drugs selected for the analyses were included in Table S1. Subsequently, using the FDA drug database, initial approval documents were retrieved for all selected drugs29 during the period December 2017–March 2018. When initial approval documents were available, these were evaluated for availability of data on pharmacokinetics, efficacy and safety analyses. The availability of analyses in the geriatric population was assessed as sufficient or insufficient, based on thorough assessment of the complete initial approval document. Information was deemed sufficient if information on pharmacokinetics, efficacy and safety was present in adequate numbers (e.g. representative of the target population). Adequate was assessed in concordance with the ICH7 guideline: “geriatric patients should be included in the Phase 3 database (and in Phase 2, at the sponsor's option) in meaningful numbers.” With regard to pharmacokinetic studies it is stated in the ICH7 guideline that “a pilot trial of limited size conducted under steady‐state conditions to look for sizable differences between older and younger subjects or patients” can be performed and “a larger, single‐dose pharmacokinetic study of sufficient size to permit statistical comparisons between geriatric and younger subjects’ or patients' pharmacokinetic profiles is also acceptable.” A pharmacokinetic screening approach as described in the ICH7 guideline was also deemed adequate.
Furthermore, the year of initial marketing approval was extracted. One researcher (Ri.R.) performed the inclusion and assessments. In case of uncertainty, a second researcher (Ro.R.) was consulted. A random sample of 10% (n = 18) was selected and double checked by the second researcher (Ro.R.), to ensure correct assessment of adequateness. Outcomes were numbers (%) of initial approval documents that contained adequate data on pharmacokinetics, efficacy and safety analyses.
2.4. Statistical analyses
To assess whether the percentage of available initial approval documents significantly increased during a certain time frame, χ2 test statistics were used, considering P < .05 statistically significant. Analyses were performed using SPSS statistics version 23.
3. RESULTS
In the ATC database, 1129 unique active pharmaceutical ingredients were found for the analyses (Table S1). Of these, 506 medical substances were found in the FDA database (45%). The majority consisted of drugs for mental health or nervous system disorders (n = 132; 26%); followed by antibacterial drugs (n = 101; 20%) and respiratory drugs (n = 86; 17%). Seventy‐one antihypertensive drugs were found (14%) and 39 glucose‐lowering drugs (8%). All other drugs each comprised <5% of the total amount of drugs found in the FDA database (Figure 1 ).
Figure 1.
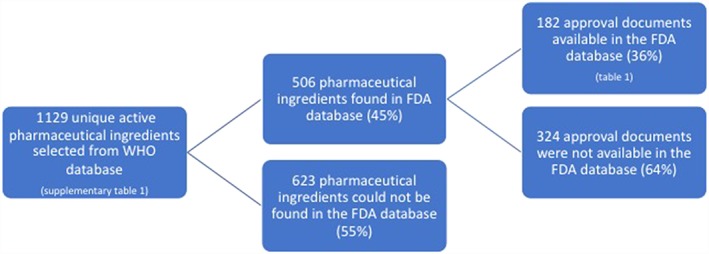
Flow diagram of the availability of initial approval documents in the Food and Drug Administration (FDA) database. WHO, World Health Organization
Of the 506 medical substances, 182 (36%) initial approval documents were available (Table 1). Of these, the majority were drugs for mental health or nervous system disorders (n = 51; 28%); followed by glucose‐lowering drugs (n = 32; 18%) and antibacterial drugs (n = 22; 12%). Twenty‐one antihypertensive drugs were found (12%), 16 lipid‐lowering drugs (9%) and 15 respiratory drugs (8%). Antiulcer drugs comprised 7% (n = 13) and all other drugs each comprised <5% of the total amount.
Table 1.
Overview of the 182 substances included in the analyses (sorted on ATC code)
| Number | ATC code | Generic product |
|---|---|---|
| 1 | A02BA01 | Cimetidine |
| 2 | A02BA02 | Ranitidine |
| 3 | A02BA03 | Famotidine |
| 4 | A02BA04 | Nizatidine |
| 5 | A02BB01 | Misoprostol |
| 6 | A02BC01 | Omeprazole |
| 7 | A02BC02 | Pantoprazole |
| 8 | A02BC03 | Lansoprazole |
| 9 | A02BC04 | Rabeprazole |
| 10 | A02BC05 | Esomeprazole |
| 11 | A02BC06 | Dexlansoprazole |
| 12 | A02BX02 | Sucralfate |
| 13 | A02BX05 | Bismuth subcitrate |
| 14 | A10AB01 | Insulin (inhalation) |
| 15 | A10AB02 | Insulin (glargine) |
| 16 | A10AB03 | Insulin (inhalation) |
| 17 | A10AB06 | Insulin glulisine |
| 18 | A10AD04 | Insulin lispro |
| 19 | A10AD06 | Insulin degludec and insulin aspart |
| 20 | A10AE04 | Insulin glargine |
| 21 | A10AE05 | Insulin detemir |
| 22 | A10AE06 | Insulin degludec |
| 23 | A10BA02 | Metformin |
| 24 | A10BB02 | Chlorpropamide |
| 25 | A10BB07 | Glipizide |
| 26 | A10BB12 | Glimepiride |
| 27 | A10BF01 | Acarbose |
| 28 | A10BG01 | Troglitazone |
| 29 | A10BG02 | Rosiglitazone |
| 30 | A10BG03 | Pioglitazone |
| 31 | A10BH01 | Sitagliptin |
| 32 | A10BH03 | Saxagliptin |
| 33 | A10BH04 | Alogliptin |
| 34 | A10BH05 | Linagliptin |
| 35 | A10BJ01 | Exenatide |
| 36 | A10BJ02 | Liraglutide |
| 37 | A10BJ03 | Lixisenatide |
| 38 | A10BJ04 | Albiglutide |
| 39 | A10BJ05 | Dulaglutide |
| 40 | A10BJ06 | Semaglutide |
| 41 | A10BK01 | Dapagliflozin |
| 42 | A10BK02 | Canagliflozin |
| 43 | A10BK03 | Empagliflozin |
| 44 | A10BX03 | Nateglinide |
| 45 | A10BX05 | Pramlintide |
| 46 | C03DA04 | Eplerenone |
| 47 | C03XA01 | Tolvaptan |
| 48 | C03XA02 | Conivaptan |
| 49 | C04AB01 | Phentolamine |
| 50 | C07AB09 | Esmolol |
| 51 | C07AB12 | Nebivolol |
| 52 | C07AG02 | Carvedilol |
| 53 | C08CA01 | Amlodipine |
| 54 | C08CA02 | Felodipine |
| 55 | C08CA03 | Isradipine |
| 56 | C08CA16 | Clevidipine |
| 57 | C09AA02 | Enalapril |
| 58 | C09AA03 | Lisinopril |
| 59 | C09AA10 | Trandolapril |
| 60 | C09AA13 | Moexipril |
| 61 | C09CA02 | Eprosartan |
| 62 | C09CA03 | Valsartan |
| 63 | C09CA04 | Irbesartan |
| 64 | C09CA06 | Candesartan |
| 65 | C09CA07 | Telmisartan |
| 66 | C09CA08 | Olmesartan medoxomil |
| 67 | C09CA09 | Azilsartan medoxomil |
| 68 | C09XA02 | Aliskiren |
| 69 | C10AA01 | Simvastatin |
| 70 | C10AA02 | Lovastatin |
| 71 | C10AA03 | Pravastatin |
| 72 | C10AA04 | Fluvastatin |
| 73 | C10AA05 | Atorvastatin |
| 74 | C10AA06 | Cerivastatin |
| 75 | C10AA07 | Rosuvastatin |
| 76 | C10AA08 | Pitavastatin |
| 77 | C10AB05 | Fenofibrate |
| 78 | C10AB11 | Choline fenofibrate |
| 79 | C10AC04 | Colesevelam |
| 80 | C10AX09 | Ezetimibe |
| 81 | C10AX11 | Mipomersen |
| 82 | C10AX12 | Lomitapide |
| 83 | C10AX13 | Evolocumab |
| 84 | C10AX14 | Alirocumab |
| 85 | G04BD07 | Tolterodine |
| 86 | H03AA01 | Levothyroxine sodium |
| 87 | J01AA12 | Tigecycline |
| 88 | J01DD15 | Cefdinir |
| 89 | J01DD16 | Cefditoren |
| 90 | J01DE01 | Cefepime |
| 91 | J01DH02 | Meropenem |
| 92 | J01DH03 | Ertapenem |
| 93 | J01DH04 | Doripenem |
| 94 | J01DI02 | Ceftaroline fosamil |
| 95 | J01FA13 | Dirithromycin |
| 96 | J01FA15 | Telithromycin |
| 97 | J01MA12 | Levofloxacin |
| 98 | J01MA13 | Trovafloxacin |
| 99 | J01MA14 | Moxifloxacin |
| 100 | J01MA15 | Gemifloxacin |
| 101 | J01MA16 | Gatifloxacin |
| 102 | J01XA03 | Telavancin |
| 103 | J01XA04 | Dalbavancin |
| 104 | J01XA05 | Oritavancin |
| 105 | J01XD02 | Tinidazole |
| 106 | J01XX08 | Linezolid |
| 107 | J01XX09 | Daptomycin |
| 108 | J01XX11 | Tedizolid |
| 109 | M01AH01 | Celecoxib |
| 110 | N02AA05 | Oxycodone |
| 111 | N02AB03 | Fentanyl |
| 112 | N02AX06 | Tapentadol |
| 113 | N02BG08 | Ziconotide |
| 114 | N02CC02 | Naratriptan |
| 115 | N02CC03 | Zolmitriptan |
| 116 | N02CC04 | Rizatriptan |
| 117 | N02CC05 | Almotriptan |
| 118 | N02CC06 | Eletriptan |
| 119 | N02CC07 | Frovatriptan |
| 120 | N03AF02 | Oxcarbazepine |
| 121 | N03AF03 | Rufinamide |
| 122 | N03AF04 | Eslicarbazepine |
| 123 | N03AG04 | Vigabatrin |
| 124 | N03AG06 | Tiagabine |
| 125 | N03AX11 | Topiramate |
| 126 | N03AX14 | Levetiracetam |
| 127 | N03AX15 | Zonisamide |
| 128 | N03AX16 | Pregabalin |
| 129 | N03AX18 | Lacosamide |
| 130 | N03AX22 | Perampanel |
| 131 | N03AX23 | Brivaracetam |
| 132 | N04 BC04 | Ropinirole |
| 133 | N04 BC05 | Pramipexole |
| 134 | N04 BC06 | Cabergoline |
| 135 | N04 BC07 | Apomorphine |
| 136 | N04 BC09 | Rotigotine |
| 137 | N04BD02 | Rasagiline |
| 138 | N04BD03 | Safinamide |
| 139 | N04BX01 | Tolcapone |
| 140 | N04BX02 | Entacapone |
| 141 | N05AE04 | Ziprasidone |
| 142 | N05AE05 | Lurasidone |
| 143 | N05AH03 | Olanzapine |
| 144 | N05AH04 | Quetiapine |
| 145 | N05AH05 | Asenapine |
| 146 | N05AX08 | Risperidone |
| 147 | N05AX12 | Aripiprazole |
| 148 | N05AX13 | Paliperidone |
| 149 | N05AX14 | Iloperidone |
| 150 | N05AX15 | Cariprazine |
| 151 | N05AX16 | Brexpiprazole |
| 152 | N05AX17 | Pimavanserin |
| 153 | N05BA09 | Clobazam |
| 154 | N05CF02 | Zolpidem |
| 155 | N05CF03 | Zaleplon |
| 156 | N05CF04 | Eszopiclone |
| 157 | N05CH02 | Ramelteon |
| 158 | N05CH03 | Tasimelteon |
| 159 | N05CM18 | Dexmedetomidine |
| 160 | N05CM19 | Suvorexant |
| 161 | N06AB04 | Citalopram |
| 162 | N06AB10 | Escitalopram |
| 163 | N06AX17 | Milnacipran |
| 164 | N06AX21 | Duloxetine |
| 165 | N06AX23 | Desvenlafaxine |
| 166 | N06AX24 | Vilazodone |
| 167 | N06AX26 | Vortioxetine |
| 168 | R01AA04 | Phenylephrine |
| 169 | R01AD13 | Ciclesonide |
| 170 | R03AC13 | Formoterol |
| 171 | R03AC18 | Indacaterol |
| 172 | R03AC19 | Olodaterol |
| 173 | R03BB04 | Tiotropium bromide |
| 174 | R03BB05 | Aclidinium bromide |
| 175 | R03BB07 | Umeclidinium bromide |
| 176 | R03DC03 | Montelukast |
| 177 | R03DX05 | Omalizumab |
| 178 | R06AX17 | Ketotifen |
| 179 | R06AX24 | Epinastine |
| 180 | R06AX27 | Desloratadine |
| 181 | R07AX01 | Nitric oxide |
| 182 | R07AX02 | Ivacaftor |
For the majority of the drugs, the initial approval documents in the database did show information on pharmacokinetics in elderly (n = 113; 62%). For 1 drug, it was explicitly stated that information on pharmacokinetics, safety and efficacy in elderly was not applicable (ivacaftor). Furthermore, over time, the availability of information on pharmacokinetics in elderly increased statistically significantly from zero in the period 1979–1979 to 76% (n = 32) in the period 2010–2018 (p = .02; Table 2). For safety and efficacy information in elderly, detailed information was present in respectively 77 and 81 documents (42% and 45%). In addition, the availability of information on safety and efficacy in elderly did not improve over time (p = 0.13 and 0.11, respectively).
Table 2.
Availability of information on pharmacokinetics, safety or efficacy with regard to elderly in the initial approval documents in the Food and Drug Administration database for the 10 most frequently prescribed drug classes, drugs with known large or small volumes of distribution, and/or high or low hepatic clearance or renal excretion, or which are relatively contraindicated in elderly per time period
| Before 1950 | 1950–1959 | 1960–1969 | 1970–1979 | 1980–1989 | 1990–1999 | 2000–2009 | 2010 onwards | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Number of available initial approval documents | 0 | 1 | 0 | 2 | 9 | 59 | 69 | 42 | 182 |
| Information on pharmacokinetics with regard to elderly sufficient, n (%) | NA | 0 (0) | NA | 0 (0) | 1 (11) | 36 (61) | 44 (64) | 32 (76) | 113 (62) |
| Information on safety with regard to elderly sufficient, n (%) | NA | 1 (100) | NA | 0 (0) | 4 (44) | 19 (32) | 30 (43) | 23 (55) | 77 (42) |
| Information on efficacy with regard to elderly sufficient, n (%) | NA | 1 (100) | NA | 0 (0) | 4 (44) | 20 (34) | 32 (46) | 24 (57) | 81 (45) |
NA, not available.
4. DISCUSSION
In this study the availability of pharmacokinetic, safety and efficacy analyses specifically targeted at the elderly, prior to the authorization of the most frequently prescribed drug classes was evaluated. Based on the available initial approval documents, it was concluded that 62% of the FDA documents included reports on pharmacokinetic analyses, and 42 and 45% on safety and efficacy analyses in the elderly. For the majority of the drugs, the initial approval documents were not available in the database; however, over time, the percentage of available initial approval documents, as well as the information on pharmacokinetics increased significantly. With regard to crucial data on safety and efficacy, presence of information specifically on elderly was insufficient and did not increase over time.
Our results are in line with earlier studies on under‐representation of the elderly in (pre‐authorization) trials and published reports.12, 13, 18, 22, 23, 24, 25 It was reported that only 3 of the 155 clinical trials on 4 widely prescribed drugs were exclusively designed for patients aged 65 years and older.30 Moreover, a recent assessment of all performed clinical trials in 2012 revealed that only 2% of the randomized controlled trials were designed for elderly aged 65 and over.31
Unfortunately, we have shown that despite efforts to include elderly patients in clinical drug trials, under‐representation of elderly patients is still present, which challenges the external validity of benefit/risk assessments of launched drugs and leads to the phenomenon of off label prescribing in old patients.32 With regard to elderly, adequate representation of the targeted population in clinical trials is of pivotal importance as pharmacokinetic differences may give rise to differences in safety and efficacy. However, there are differences in opinion on this issue between EU countries leading to differences in clinical trial regulations and practice in older people, further complicating the adequate inclusion of elderly in clinical trials.16 Efforts have been made to overcome the underrepresentation of the elderly in clinical research. The updated ICH E7 guideline emphasizes the need for additional short‐ and long‐term safety data, adapted age‐specific endpoints and subjective outcomes such as quality of life. Moreover, the population under research should reflect the population at which the drug under investigation is aimed. Unfortunately, no specific percentage can be given as the percentage of elderly in the target population differs per drug. Nevertheless, efforts should be made to include patients aged >80 years with different degrees of comorbidity and frailty.33 For example, it was recommended to include older people in phase 1 clinical trials on gynaecological cancers, as they have similar toxicity profiles compared to their younger counterpart.34 However, including elderly in clinical trials remains challenging: given the presence of protocol restrictions (e.g. exclusion criteria on age, polypharmacy and multimorbidity), many elderly must be screened before 1 study participant can be enrolled.30
One of the major drawbacks of this study is that it could not be verified which specific older patients in terms of age, ethnicity, sex and comorbidities were included in the clinical trials. This is important as the elderly population included in the assessed initial approval documents could have consisted of relatively healthy elderly with 1 disease, thus not being representative of the target population of elderly which the drugs are using namely those elderly with multiple comorbidities and polypharmacy.33 Furthermore, the presence of information on pharmacokinetic studies could only be quantitatively assessed and not qualitatively. The available information was highly variable per assessed molecular entity; for example, different definitions of elderly were used, and information on numbers of elderly in clinical trials was missing frequently. As noticed in the methods section, the ICH7 guideline was used to deem whether numbers of included elderly were adequate, a random sample (10%) of the assessed reports was double checked to verify correct assessment of adequateness. Also, pages of the assessed initial approval reports were regularly withdrawn for confidentiality reasons of the submitting company. Furthermore, there is no way to determine whether all data submitted to the FDA are available in the online published documents. Lastly, not all pharmaceutical ingredients were found as drugs in the FDA database, which probably leads to nondifferential misclassification.
To our knowledge, this is the first study to assess the presence of information on pharmacokinetics, safety and efficacy in initial approval documents accessible in the FDA database. During the period 1927–2013, a total of 1453 drugs obtained FDA approval and the FDA still approves dozens of drugs each year.35 However, an analysis on the availability of pre‐authorization information on pharmacokinetics, safety and efficacy in elderly in the FDA database has not yet been performed. In a methodological guideline on the use of FDA documents for evidence syntheses, the benefit of using aggregated clinical trial information from FDA documents for the interpretation of data was emphasized, as it is less biased than published trial information.36 Nevertheless, since 2010, there is still insufficient data available on safety and efficacy of the most frequently prescribed drugs in the elderly. Knowing that older people account for the majority of all drug consumers, priority should be given to clinical research with a study population that is representative for the actual patient population.10
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
Ri.R., J.B. and Ro.R. designed the study. Ri.R. and Ro.R. performed the analyses. Ri.R., J.B. and Ro.R. wrote the manuscript.
Supporting information
Table S1:
an overview of all selected medical substances from the World Health Organization database for the analyses.
ACKNOWLEDGEMENTS
We would like to express our gratitude to Ms Karen Broekhuizen, PhD, for her advice in writing the manuscript.
Ruiter R, Burggraaf J, Rissmann R. Under‐representation of elderly in clinical trials: An analysis of the initial approval documents in the Food and Drug Administration database. Br J Clin Pharmacol. 2019;85:838–844. 10.1111/bcp.13876
The authors confirm that the PI for this paper is Rikje Ruiter.
REFERENCES
- 1. European Commission Directorate‐General for Economic and Financial Affairs . The 2015 Ageing Report Economic and budgetary projections for the 28 EU Member States (2013–2060). 2015. 10.27658/77631 [DOI]
- 2. Fahy N, McKee M, Busse R, Grundy E. How to meet the challenge of ageing populations. BMJ. 2011;342(1):d3815. [DOI] [PubMed] [Google Scholar]
- 3. UN‐DESA ‐ The World Bank . World Population Prospects The 2017 Revision Key Findings and Advance Tables. World Popul Prospect 2017. 2017. 10.1017/CBO9781107415324.004 [DOI]
- 4. Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics concepts and applications. Pharm Sci. 2011;17(6):881‐887. [Google Scholar]
- 5. Kinirons M, Wood A. Pharmacokinetics. Drug Ther Old Age. 1998.
- 6. Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450‐linked drug metabolism in humans: An analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61(3):331‐339. [DOI] [PubMed] [Google Scholar]
- 7. Woodhouse KW, James OF. Hepatic drug metabolism and ageing. Br Med Bull. 1990;46(1):22‐35. [DOI] [PubMed] [Google Scholar]
- 8. Zoli M, Magalotti D, Bianchi G, et al. Total and functional hepatic blood flow decrease in parallel with ageing. Age Ageing. 1999;28(1):29‐33. [DOI] [PubMed] [Google Scholar]
- 9. Carbonin P, Pahor M, Bernabei R, Sgadari A. Is age an independent risk factor of adverse drug reactions in hospitalized medical patients? J Am Geriatr Soc. 1991;39(11):1093‐1099. [DOI] [PubMed] [Google Scholar]
- 10. Moore KL, Patel K, Boscardin WJ, Steinman MA, Ritchie C, Schwartz JB. Medication burden attributable to chronic comorbid conditions in the very old and vulnerable. PLoS One. 2018;13(4):e0196109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. US Food and Drug Administration . International Conference on Harmonisation; guidance on E7 studies in support of special populations; geriatrics; questions and answers; availability. Notice. Fed Regist. 2012;77:9948‐9949. [PubMed] [Google Scholar]
- 12. Vitale C, Fini M, Spoletini I, Lainscak M, Seferovic P, Rosano GM. Under‐representation of elderly and women in clinical trials. Int J Cardiol. 2017;232:216‐221. [DOI] [PubMed] [Google Scholar]
- 13. Gurwitz JH, Col NF, Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA. 1992;268(11):1417‐1422. [PubMed] [Google Scholar]
- 14. Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286(6):708‐713. [DOI] [PubMed] [Google Scholar]
- 15. Dodd KS, Saczynski JS, Zhao Y, Goldberg RJ, Gurwitz JH. Exclusion of older adults and women from recent trials of acute coronary syndromes. J Am Geriatr Soc. 2011;59(3):506‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crome P, Lally F, Cherubini A, et al. Exclusion of older people from clinical trials: professional views from nine European countries participating in the PREDICT study. Drugs Aging. 2011;28(8):667‐677. [DOI] [PubMed] [Google Scholar]
- 17. Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162(15). [DOI] [PubMed] [Google Scholar]
- 18. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race‐, sex‐, and age‐based disparities. JAMA. 2004;291(22):2720‐2726. [DOI] [PubMed] [Google Scholar]
- 19. Jennens RR, Giles GG, Fox RM. Increasing underrepresentation of elderly patients with advanced colorectal or non‐small‐cell lung cancer in chemotherapy trials. Intern Med J. 2006;36(4):216‐220. [DOI] [PubMed] [Google Scholar]
- 20. Trimble EL, Carter CL, Cain D, Freidlin B, Ungerleider RS, Friedman MA. Representation of older patients in cancer treatment trials. Cancer. 1994;74(S7):2208‐2214. [DOI] [PubMed] [Google Scholar]
- 21. Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer‐treatment trials. N Engl J Med. 1999;341(27):2061‐2067. [DOI] [PubMed] [Google Scholar]
- 22. Bugeja G, Kumar A, Banerjee AK. Exclusion of elderly people from clinical research: a descriptive study of published reports. Br Med J. 1997;315(7115):1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bayer A. Unjustified exclusion of elderly people from studies submitted to research ethics committee for approval: descriptive study. BMJ. 2000;321(7267):992‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beers E, Moerkerken DC, Leufkens HGM, Egberts TCG, Jansen PAF. Participation of older people in preauthorization trials of recently approved medicines. J Am Geriatr Soc. 2014;62(10):1883‐1890. [DOI] [PubMed] [Google Scholar]
- 25. Chien JY, Ho RJY. Drug delivery trends in clinical trials and translational medicine: evaluation of pharmacokinetic properties in special populations. J Pharm Sci. 2011;100(1):53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. IQVIA institute for human data science . Medicine use and spending in the U.S. A review of 2017 and outlook to 2022. 2018.
- 27. Samuel MJ. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227‐2246. [DOI] [PubMed] [Google Scholar]
- 28. WHO Collaborating Centre for Drug Statistics Methodology, Norwegian Institute of Public Health ATC/DDD Index 2014. 2014.
- 29. US Food and Drug Administration . Drugs@FDA: FDA approved drug products. WwwFdaGovCder/Orange/DefaultHtm 2008.
- 30. Konrat C, Boutron I, Trinquart L, Auleley GR, Ricordeau P, Ravaud P. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. PLoS One. 2012;7(3):e33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Broekhuizen K, Pothof A, de Craen AJM, Mooijaart SP. Characteristics of randomized controlled trials designed for elderly: a systematic review. PLoS One. 2015;10(5):e0126709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson SHD, Jansen PAF, Mangoni AA. Off‐label prescribing in older patients. Drugs Aging. 2012;29(6):427‐434. [DOI] [PubMed] [Google Scholar]
- 33. Mangoni AA, Jansen PA, Jackson SHD. Under‐representation of older adults in pharmacokinetic and pharmacodynamic studies: A solvable problem? Expert Rev Clin Pharmacol. 2013;6(1):35‐39. [DOI] [PubMed] [Google Scholar]
- 34. Buechel M, McGinnis A, Vesely SK, Wade KS, Moore KN, Gunderson CC. Consideration of older patients for enrollment in phase 1 clinical trials: Exploring treatment related toxicities and outcomes. Gynecol Oncol. 2018;149(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 35. Kinch MS, Haynesworth A, Kinch SL, Hoyer D. An overview of FDA‐approved new molecular entities: 1827‐2013. Drug Discov Today. 2014;19(8):1033‐1039. [DOI] [PubMed] [Google Scholar]
- 36. Ladanie A, Ewald H, Kasenda B, Hemkens L. How to use FDA drug approval documents for evidence syntheses. BMJ. 2018;362:k2815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1:
an overview of all selected medical substances from the World Health Organization database for the analyses.


