Abstract
Retrospective evidence drawn from real‐world experience of a medicine's use outside its labelled indication is one of a number of techniques used in drug repurposing (DRP). Relying as it does on large numbers of real incidences of human experience, rather than individual case reports with limited statistical support, preclinical experiments with poor translatability or in silico associations, which are early‐stage hypotheses, it represents the best validated form of DRP. Cancer is the most frequent of such DRP examples (e.g. aspirin in pancreatic cancer, hazard ratio = 0.25). This approach can be combined with pathway analysis to provide first‐in‐class treatments for complex diseases. Alternatively, it can be combined with prospective preclinical studies to uncover a validated mechanism for a new indication, after which a repurposed molecule is chemically optimized.
Keywords: drug reprofiling, drug repurposing, drug rescue, retrospective data, therapeutic switching
Introduction
Drug repurposing, also called drug repositioning, reprofiling or therapeutic switching is an innovative way of finding new indications for existing drugs and is now considered to be one of the major strategies to improve productivity in pharmaceutical research and development 1, 2. The strategy offers shorter times for new medicine innovation, as well as reduced costs (most obviously the costs of the discovery phase) and lower rates of developmental attrition – which is unacceptably high 3, 4. In a recent analysis of the economics of pharmaceutical innovation, Grabowski and DiMasi calculated that out‐of‐pocket costs for a new chemical entity were $1.395bn, compared to the costs for a postapproval product of $466 m 5. Accordingly, it may be possible to obtain three repurposed medicines for the investment in one new chemical entity. This is important because high innovation costs are driving increasingly unaffordable drug costs for the healthcare systems of developed countries, not just in the publicly funded European systems, but also now in the USA. In addition, the public interest in new medicines to address unmet medical needs (particularly for the 95% of rare diseases for which there are no approved treatments) requires urgent solutions; such urgency is incompatible with innovation timescales than run slower than disease progression, as so vividly displayed by the debate about providing unapproved medicines for serious life‐threatening conditions 6.
Approaches
Typically, a drug purposing strategy consists of three steps before taking the candidate drug further through the late stages of product development and approval: (i) identification of a candidate molecule for a given indication or candidate indication for given molecule (hypothesis generation); (ii) mechanistic assessment of the drug effect in preclinical models (validation); and (iii) evaluation of efficacy in phase II clinical trials (assuming there is sufficient safety data from phase I studies undertaken as part of the original indication). Of these, the identification of the right drug for an indication of interest with a high level of confidence is critical, and this is where the modern approaches for hypothesis generation could be most useful.
The identification of a new use for an existing drug is not a new idea – it is a common feature of the pharmacopeia. Historically, however, discoveries of this kind have not generally involved a systematic approach: for example, the use of thalidomide for erythema leprosum nodosum was based on serendipity whereas the discovery of the use of sildenafil (Viagra) citrate for erectile dysfunction resulted from astute clinical observation in a trial where the effect was not preplanned. Recently, various systematic approaches have been increasingly employed for the association of new indications for candidate molecules, including computational and experimental methodologies.
The purpose of this review is not to re‐evaluate the world of drug repurposing as a whole, but to draw attention to the use of data from patients treated with Drug A for Indication A in order to discover, or underpin, the new use of Drug A for Indication B. This may be done to validate serendipitous clinical findings, as well as outputs from more systematic approaches to drug repurposing; but it can also be instantiated in a more purposeful way, to search for new uses from existing data absent such hypothetical foundations. Retrospective analysis is not a necessary component of the development of a new use for an existing drug. It was not, for example a component of the development of thalidomide for erythema leprosum nodosum: this arose from the case report of one doctor's prescription of thalidomide to a severely ill patient, and subsequent expansion by the same doctor of the evidence base to a case series, followed by clinical trials. Indeed, given the withdrawal of thalidomide from most markets in the 1960s, and the rarity of leprosy as a disease, retrospective analysis would be unlikely to be a fruitful approach.
Individual case reports, which often involve unexpected findings can often be the initiation point for drug repurposing developments, but are not statistically robust and, for the purposes of this review, are not included in the definition of retrospective human evidence. For example, the repurposed use of amantadine for Parkinson disease arose from a case report of one individual who reported an improvement in rigidity, tremor and akinesia while taking amantadine for flu. This observation was taken forward in a series of clinical trials of increasing size and power, resulting eventually in a regulatory approval of the use of amantadine for the treatment of Parkinson disease 7. Again, retrospective data were not a component in either the validation or the development of this repurposed medicine.
Although neither necessary nor sufficient for drug repurposing development, as shown in Figure 1, the use of data from human experience can nevertheless be of significant value in either discovering or validating new indications for a particular drug at various stages in the translation into a new medicine.
Figure 1.
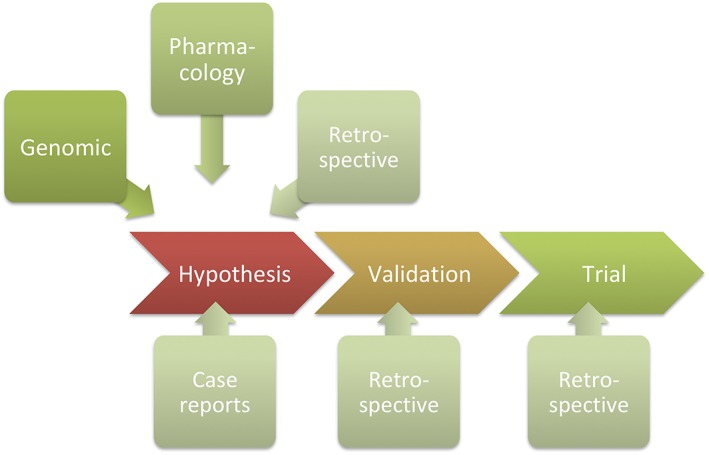
Purposeful involvement of retrospective analysis of human data at various stages in a drug repurposing development. Such analysis can be used for hypothesis generation, to validate hypotheses generated from genomic or pharmacological analysis, or can be used to refine indications and subgroups of patients in which medication is particularly beneficial from trial results. For example, the potential use of metformin in dementia can be evaluated: (i) as a hypothesis, from looking at cognitive performance of diabetic patients treated with metformin; (ii) to validate a hypothesis generated from genomic analysis associating genes most regulated in dementia with genes regulated by metformin; or (iii) from a trial in patients with cognitive dysfunction treated with metformin, to determine the relative benefit in situations involving vascular dementia relative to Alzheimer disease. Although case reports are often the bases for repurposing hypotheses, they lack statistical rigour and, for the purposes of this review, are not included in the definition of retrospective human evidence
Scientific complexities in translating repurposing hypotheses into marketed drugs
Despite the lower attrition rates, drug repurposing developments are not always successful. It is important to realise that the technology used for hypothesis generation is closely related to the confidence of its accuracy 8. As in pharmaceutical research and development as a whole, there is a hierarchy of reliability, with in silico methods generally delivering less predictive power than preclinical pharmacological experiments, and these in turn providing less confidence than observations in humans. Pharmacological results from in vitro experiments are less reliable than from in vivo experiments, and repurposing hypotheses based on clinical data are more persuasive than any other form. This order of reliability is inverse to the costs of each technology, and one of the advantages of repurposing hypotheses based on in silico technology is the scale over which they can be conducted. This breadth can give rise to more novel hypotheses, from which may derive more robust patents. However, in almost all cases, in silico predictions are of little value until they are validated. Some examples of drug repurposing failures are shown in Box 1.
Box 1 Problems in translating drug repurposing hypotheses.
Gene predictions: Several drug repurposing studies have been developed around an inverse signature methodology, including that of Sirota et al. 7, which tested for ‘oppositeness’ of gene expression signatures of disease to drug‐induced signatures. Dudley et al. 8 compared the gene expression signature of inflammatory bowel diseases (IBD) to the gene expression profile of 164 drugs. Gene expression signature of the anti‐epileptic drug topiramate anti‐correlated with the gene expression signature of IBD. The potential efficacy of topiramate in IBD was validated using a rodent model of IBD in which topiramate significantly reduced diarrhoea, visual manifestations of colitis on endoscopy, and microscopic manifestations of disease on colonic biopsy. However, a recent retrospective cohort study has failed to show a beneficial effect of topiramate 9, leading to much uncertainty about the eventual utility of this drug in IBD: an appropriately designed and powered randomized‐controlled trial would be required to definitively answer the question of whether topiramate can be used therapeutically in IBD.
In vivo pharmacology: latrepirdine (later trademarked as Dimebon) was an old nonselective antihistamine sold in Russia for the treatment of allergies. However, mechanistic studies also showed it had glutamatergic (NMDA) antagonistic and acetylcholinesterase inhibitory properties, both of which posited potential in neurodegenerative diseases such as Alzheimer and Huntington. Investigations in various animal models confirmed this potential utility and also indicated that the drug could affect the mitochondrial permeability transition and had neuroprotective effects against β‐amyloid toxicity 10. In a large Phase II clinical trial in mild–moderate Alzheimer's disease, 12 months of treatment showed a significant improvement in cognition relative to placebo 11. Unfortunately, Phase III clinical trials produced negative results, without any significant gains on any of the five efficacy endpoints vs. placebo after 6 months of treatment, including mini‐mental state examination, a standard measure of cognition. A further three Phase III trials also failed to show benefit in both Alzheimer and Huntington disease.
Clinical data: a variety of observational studies have been performed with respect to glioblastoma multiforme, the most lethal form of brain cancer, and the use of sodium valproate, an antiepileptic drug. Patients treated with sodium valproate for epilepsy have showed a improved outcome with respect to survival time and recurrence compared to patients treated with other antiepileptic drugs 12. However, a more recent meta‐analysis suggests this benefit is mainly found in older studies and in younger patients, indicating the need to exercise caution in assuming generalizability of the pooled effect. Overall, there is a considerable risk of bias in the current interpretation of the literature, and larger, prospective studies are required for validating these findings 13.
Despite much literature attention being paid to algorithmic approaches to drug repurposing, comparatively little attention has been paid to examples of new pharmaceutical uses deriving from retrospective clinical analysis. This is surprising because such analyses are validated in humans rather than in vivo, in vitro or in silico, generally using the same route of administration and dose as the marketed use, and they are therefore well positioned for rapid development to a new medicine with little or no experimental preamble and at low risk of technical failure. This comprehensive review attempts to correct this deficit.
Strategies for use of retrospective clinical evidence in repurposing development
Conventional medicinal innovation requires efficacy evidence to result from predeclared endpoints: these are prospective studies. This is the basis for randomized controlled trials upon which regulatory approval of pharmaceuticals is predicated. The prospective nature of the studies reduces the possibility of a chance finding. However, there are instances in which other things are found at the same time, which might be unexpected or unanticipated, and these observations can be taken further in subsequent prospective studies. In the much‐quoted example of the discovery of sildenafil for erectile dysfunction, the role of cyclic GMP phosphodiesterase in the control of smooth muscle relaxation within the corpus cavernosum was suggested from academic research in the early 1990s 9. In 1993, a pharmacokinetic study, which was conducted as part of a research programme designed to develop sildenafil for stable angina pectoris, reported sustained penile erections in volunteers treated with the drug. This led to the realization of the potential use of sildenafil for erectile dysfunction, and the initiation of a small efficacy trial in males with impotence. The significant success of this trial then led to further large scale Phase IIb and Phase III investigations, and the approval of sildenafil in 1998. The success of this innovation stemmed from the observation in the original pharmacokinetic trial, which was not designed for this purpose. There has been a significant debate about whether this result was expected or unexpected, given the status of understanding of the biochemistry of smooth muscle relaxation in the early 1990s 9, leading to an invalidity judgement for the method of use patent by the European Patent Office, but it was unanticipated in the sense that the trial was not designed to discover this effect. Moreover, there is no doubt of the important role of the clinical study staff in the pharmacokinetic trial in making the observation which led to the repurposing of the drug. Generally, the retrospective analysis of clinical trial data as a means of uncovering new indications has been dealt with before 10 and is analogous to the use of patient data, which is the main focus for this review.
Another more obvious example of a chance observation, this time after the product had been marketed, is that of latanoprost. This is a prostaglandin‐based drug, trade‐named Xalatan, an ophthalmic solution used to reduce pressure inside the eye for the treatment of ocular hypertension and open angle glaucoma. Soon after the product was marketed, a patient comment about the eyelashes of her latanoprost‐treated eye prompted unmasked examination of subsequent patients taking unilateral latanoprost. An observational study of patients reported increased thickness and length of eyelashes as a result of glaucoma treatment 11. This led to the understanding that prostaglandin F agonists could cause hypertrichosis of the eyelash, and ultimately to the development of a related prostaglandin, bimatoprost, as a cosmetic for incorporation in mascara and eyeliner products under the tradename Latisse. There are two points to make here: first, unlike the sildenafil example, latanaprost was a marketed drug, and so observational data could be used to validate the initial finding; and secondly, this is not a new pharmaceutical use, but a cosmetic one, and required separate regulatory approval. The serendipitous nature of the finding is suggested by the fact that, while the side effect of increased iris pigmentation was uncovered during the clinical trials that led to the approval of latanoprost, the effect of the drug on eyelash thickening was not revealed until the report from a patient who had applied it in one eye.
A third example is that of raloxifene, a selective oestrogen receptor modulator (SORM), which was launched in the UK under the trade name Evista for the prevention of osteoporosis in postmenopausal women in 1998.
SORMs mimic oestrogen in some tissues and have antioestrogenic activity in others; the first SORM was tamoxifen, which was discovered in the 1950s and used from 1973 for the treatment of breast cancer. The original purpose of the research programme that led to raloxifene was not to discover drugs for osteoporosis, but for the treatment of breast cancer patients who were either resistant to tamoxifen or became so after starting treatment. Raloxifene was tested in a small 14‐patient study, where it showed a poor response in the treatment of tamoxifen‐resistant breast cancer patients, and this indication was abandoned for further development 12. The developing company Lilly then made a deliberate decision to switch indications, bearing in mind the known involvement of oestrogen in osteoporosis, based on the high prevalence of osteoporosis in postmenopausal women, when oestrogen levels decline. In a randomized, double‐blind, placebo‐controlled trial, nearly 8000 women were treated for up to 2 years and effects on bone mineral density and reductions in vertebral fracture risk were assessed. The analysis showed that women taking raloxifene were 52% less likely to have a first spinal fracture. They used interim results from this study for the regulatory filing, which was granted a priority review by the Food and Drug Administration and subsequently approved for this indication in the USA in 1999. Slightly later, Lilly also evaluated it for the prevention (as opposed to treatment) of breast cancer. Looking at a continuation of the osteoporosis trial, they retrospectively analysed the risk of breast cancer in postmenopausal women receiving raloxifene for the treatment of osteoporosis. It appeared that there was a reduction of about 60% in the incidence of newly diagnosed breast cancer in this group of patients, and this led ultimately to the approval of raloxifene for reducing the risk of invasive breast cancer in postmenopausal women. In summary, while initially a failure in the treatment of tamoxifen‐resistant breast cancer, raloxifene had proven to be effective in both treating osteoporosis and secondarily in the prevention of breast cancer; in the latter, it had proven to be a superior treatment to tamoxifen, in terms of risk of uterine and ophthalmic side effects 13. This example of drug repurposing involves firstly the deliberate decision, based on scientific knowledge, to pursue osteoporosis when the initial development in tamoxifen‐resistant breast cancer was unsuccessful, and then the observationally‐based switch into prevention of breast cancer, as a result of posthoc analysis of patient data.
From the above examples, it is clear that observational analysis, whether during development or from measurements carried out later, represents a powerful way of finding associations in real patients. One promising way of finding new uses (as well as validating hypotheses derived from other repurposing methods) for drugs is to analyse outcomes from patients. Importantly, unlike in silico studies or pharmacological experiments, examinations of what happens to patients reveal effects that are relevant to the specific dose and by the specific route of administration relevant to the product for the primary indication. A particularly valuable UK resource in this regard is the Clinical Practice Research Datalink, based on the experiences of the National Health Service in the 70 years since it was established. The Clinical Practice Research Datalink is a rich source for retrospective analysis of this kind 13. Cancer protection is an area that is particularly likely to be identified as an unintended benefit of a treatment because the observation of malignancy is a possible indication of carcinogenicity of a drug and should therefore always be recorded as a potential adverse side effect. However, if the incidence of cancer for patients on a particular drug is less than would be anticipated by chance, the drug may be exerting a protective anticancer effect. A number of interesting anti‐cancer associations have been found, and drugs as diverse as metformin (an antidiabetic), propranolol (an antihypertensive) and clomipramine (an antidepressant) may have useful anticancer properties (see Table 1 below). Metformin in particular has raised hopes not just for preventing, but also for treating cancer, and a number of trials have begun for the use of this drug, including a large‐scale adjuvant study in breast cancer 14. It is important to differentiate the effect from this analysis as a cancer‐preventative agent with its utility as a cancer‐curative agent: prevention may relate to an effect against mutation or metastasis, whereas treatment of advanced cancer may not benefit from a drug confined in its effect to these early aspects of malignancy (similar to the situation described earlier with raloxifene); and secondly, the retrospective identification of an association is not proof positive from a prospective viewpoint. These two issues aside, this type of study is of significant power in drug repurposing.
Table 1.
Retrospective associations suggesting novel uses for existing pharmaceuticals
| Mechanism (where known) or drug | Indication | Reference |
|---|---|---|
| 5‐HT uptake inhibitor | Myocardial infarction | 26 |
| ACE inhibitor | Alzheimer disease | 27 |
| ACE inhibitor | Cachexia | 22 |
| ACE inhibitor | Immune downregulation | 28 |
| ACE inhibitor; Angiotensin II (AT1) antagonist | Nonalcoholic steatohepatitis | 29 |
| Acetylcholinesterase inhibitor | Autism | 30 |
| Angiotensin II (AT1) antagonists | Alzheimer disease | 31 |
| Beta‐2‐adrenergic antagonist | Parkinson disease | 32 |
| Beta‐adrenergic antagonist | Alzheimer disease | 27 |
| Beta‐adrenergic antagonist | Cancer | 33 |
| Beta‐adrenergic antagonist | Cancer metastasis; cancer, breast | 34 |
| Beta‐adrenergic antagonist | Cancer, liver | 35 |
| Beta‐adrenergic antagonist | Cancer, lung | 36 |
| Beta‐adrenergic antagonist | Cancer, ovarian | 37 |
| Beta‐adrenergic antagonist | Cancer, prostate | 38 |
| Beta‐adrenergic antagonist | Melanoma | 39 |
| Beta‐adrenergic antagonist | Osteoporosis | 40 |
| Bisphosphonate | Sepsis, ARDS | 41 |
| Calcium channel blockers | Alzheimer disease | 42 |
| Calcium channel blockers | Cancer, lung | 43 |
| Dipeptidyl peptidase (DPP‐IV) inhibitor | Inflammatory bowel disease | 44 |
| Gefitinib | Asthma | 45 |
| Glibenclamide | Sepsis | 46 |
| Glycogen synthase kinase 3 (GSK‐3) inhibitor | Cancer | 47 |
| Histamine H2 antagonist | Lung fibrosis | 48 |
| HMG‐CoA reductase inhibitor | Age‐related macular degeneration | 49 |
| HMG‐CoA reductase inhibitor | Alzheimer disease | 50 |
| HMG‐CoA reductase inhibitor | Asthma | 51 |
| HMG‐CoA reductase inhibitor | Bacterial diseases | 52 |
| HMG‐CoA reductase inhibitor | Burn injury | 53 |
| HMG‐CoA reductase inhibitor | Cancer | Oesophageal 54; liver 55; prostate 56 |
| HMG‐CoA reductase inhibitor | Cataracts | 57 |
| ACE inhibitor; HMG‐CoA reductase inhibitor | chronic obstructive pulmonary disease | 58 |
| HMG‐CoA reductase inhibitor | Depression | 59 |
| HMG‐CoA reductase inhibitor | Epilepsy | 60 |
| HMG‐CoA reductase inhibitor | Glaucoma | 61 |
| HMG‐CoA reductase inhibitor | Influenza, chronic obstructive pulmonary disease | 62 |
| HMG‐CoA reductase inhibitor | Osteoporosis | 63 |
| HMG‐CoA reductase inhibitor | Periodontitis | 64 |
| HMG‐CoA reductase inhibitor | Pneumonia | 65 |
| HMG‐CoA reductase inhibitor | Rheumatoid arthritis | 66 |
| HMG‐CoA reductase inhibitor | Sepsis | 67 |
| HMG‐CoA reductase inhibitor | Transplant rejection | 68 |
| Hydroxychloriquine | Diabetes (type II) | 69 |
| Ibuprofen | Parkinson disease | 70 |
| Ketamine | Fatigue | 71 |
| Metformin | Alzheimer disease | 72, 73, |
| Metformin | Cancer | Bladder 74, 75; colorectal 76; endometrial 77; liver 78; lung 79; pancreatic 80; prostate 81; |
| Metformin | Psoriasis | 82 |
| Modafinil | Depression | 83 |
| NSAIDs | Cancer | Breast 84; colorectal 85 |
| NSAIDs | Depression | 86 |
| NSAIDs | Sepsis, ARDS | 87 |
| Na+/K+ ATPase inhibitor (digoxin) | Cancer | Lung 88; prostate 89 |
| NMDA antagonist | Bipolar disorder | 90 |
| PPAR γ‐agonists | Cancer, colorectal; cancer, liver | 91 |
| PPAR γ‐agonists | Parkinson disease | 92 |
| PPAR γ‐agonists | Psoriasis | 82 |
| Quinolone antibiotic | Cancer | 93 |
| SORM (selective oestrogen receptor modulator) | Kidney disease, chronic (renal failure) | 94 |
| Sulfonylureas | Stroke | 19 |
| TNF antagonist | Cancer | 96 |
| TNF antagonist | Diabetes (type II) | 97 |
| TNF antagonist | Kawasaki disease | 98 |
| TNF antagonist | Stroke | 95 |
| TNF antagonist | Systemic vasculitis | 98 |
| Tricyclic antidepressants | Cancer | 99 |
| Valproic acid | Cancer, prostate | 100 |
| VEGF monoclonal antibody | Brain and spinal cord injury | 101 |
Abbreviations. ACE, angiotensin converting enzyme; HMG‐CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A; NMDA, N‐Methyl‐D‐aspartate; NSAID, non‐steroidal anti‐inflammatory drug; PPAR, peroxisome proliferator‐activated receptor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor
A survey of retrospective evidence in a variety of possible secondary indications is shown in Table 1 below. One of the most important, and best‐evidenced examples of retrospective data underpinning an additional use is that of aspirin in cancer. Aspirin's effects in oncology actually represent the third utility for this most widespread of medicines, since its use beyond inflammatory analgesia, as an antithrombotic, were revealed by mechanism‐based studies of cyclooxygenase inhibition by Sir John Vane in the 1960s. Its pharmacological effects in preventing platelet aggregation were translated, in a purposeful way, into a clinically substantiated use to prevent heart attacks and stroke in the 1980s, and specific low‐dose aspirin formulations are now sold for this effect. But the attribution of an anticancer effect relies mainly on a retrospective analysis of the incidence of various cancers in patients taking aspirin for long‐term cardiovascular purposes. In a very large meta‐analysis of 25 000 such patients and controls 15, daily treatment with aspirin was associated with a significant reduction in various gastrointestinal cancers. In the case of pancreatic cancer, the hazard ratio for death from the disease in patients having used aspirin for 5 years or more was 0.25 (P = 0.04), whereas that for colorectal cancer was 0.41 (P = 0.05). Despite this evidence, the translation of the associative relationship into an approved anticancer aspirin‐based product has not yet transpired; the differences between preventative and curative effects are salient in this regard, but so are the problems for a company seeking to commercialize such a development against a backdrop of widespread generic availability of aspirin for other uses. The other caveat for this third use of aspirin is its safety, since gastropathy is a risk in all patients who take such medication long‐term.
It is clear from Table 1, that retrospective effects have been identified in many areas in addition to cancer. A further example of the difference between prevention and treatment, is revealed by the example of the use of β‐hydroxy β‐methylglutaryl‐CoA reductase inhibitors (statins) in sepsis. In this case, rosuvastatin was not associated with an improvement in 60‐day mortality in sepsis‐associated acute respiratory distress syndrome (ARDS) 16, despite the various epidemiological associations of reduced mortality in statin users with sepsis. The difference between the two results can be attributed to the need for long‐term dosing with statins for the production of their benefits against vascular leak, through inhibition of RhoA prenylation, an off‐target effect of these drugs. A clinical benefit of statins in an emergency situation involving sepsis would require a much more immediate effect than can be produced by this mechanism. Similarly, the effect of aspirin in ARDS revealed from retrospective analysis 17 was not repeated in a prospective trial where no significant difference was found in newly developed ARDS 18.
Strategically, an effect revealed by retrospective analysis does not necessarily lead to development of that agent in a new indication. In some cases, it can provide excellent target validation for another discovery programme. For example, the antidiabetic sulfonylureas, which inhibit ATP‐sensitive K+ (KATP) channels in pancreatic β‐cells and stimulate insulin release in diabetes mellitus, mediate their effect on KATP channels via a high‐affinity sulfonylurea receptor (SUR). When looked at retrospectively in diabetic patients with ischaemic stroke, sulfonylurea drugs such as glibenclamide conferred protection against swelling and symptomatic haemorrhagic transformation. Analysis of the brain tissue obtained intraoperatively from such patients showed upregulation of the SUR1 subtype of the sulfonylurea receptor 19. Further cellular analysis of the SUR1 receptor in astrocytes showed that its effect on cytotoxic oedema is mediated via a nonselective cation channel, the NC (Ca‐ATP) channel 20, 21. This represents a potential new therapeutic approach to stroke.
An interesting example of retrospective analysis leading to a clinically effective agent is in the area of cachexia. Cachexia is formally defined as the loss of >5% of body weight over a period of <12 months, normally in association with a chronic disease. It occurs commonly with not only cancer, but also chronic heart failure, kidney disease and chronic obstructive pulmonary disease. A re‐examination of various clinical trials of therapeutic agents for chronic heart failure, specifically those of ACE inhibitors 22 and β‐blockers 23, looked at weight as a marker for cachexia. Both these classes of drug have been formally investigated for the treatment of chronic heart failure. Weight is, of course, routinely measured in any clinical trial protocol, so the re‐analysis could be done without the need for additional measurements. In the case of the β‐blockers, a stratification exercise to delineate the mechanistic basis indicated the involvement of β‐1 and β‐2 adrenergic as well as 5‐HT1A receptors for optimal activity, and identification of S‐pindolol (which possesses all three of these activities) as a preferred agent with greater efficacy than other similar agents, including its racemate 24. Interestingly, in the subsequent ACT‐ONE clinical trial, at therapeutic doses that do not decrease blood pressure, the product is not only highly effective for alleviation of catabolism, but also promotes anabolism and improves functional ability in cancer cachectic patients 25.
Conclusion
In conclusion, retrospective or observational analysis of human experience may prove of substantial benefit in identifying novel uses for existing drugs. The examples described herein involve such analysis in various stages in a drug's life, from early in development where a pharmacokinetic trial gave rise to the discovery of sildenafil for erectile dysfunction, through to an unexpected observation of increased eyelash growth in an antiglaucoma drug. In some cases, the secondary use might have been (almost) identified at the start of the project, as in the case of raloxifene, which was initially tested (unsuccessfully) for the treatment of breast cancer, and later became used for the prevention of breast cancer. The advantages of retrospective analysis include the fact that the dose and route of administration have been specified, in the biological species of interest, but nuances and uncertainties still persist. Most particularly, an association observed post facto does not necessarily imply a causal relationship, and prevention of a condition is not the same as treating it, since the latter implies a more stringent requirement to reverse the pathology.
Conflicts of Interest
The author is principal and director of Numedicus Limited, a drug repurposing consultancy.
Cavalla D. (2019) Using human experience to identify drug repurposing opportunities: Theory and practice, Br J Clin Pharmacol, 85, 680–689. 10.1111/bcp.13851.
References
- 1. Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019; 18: 41–58. [DOI] [PubMed] [Google Scholar]
- 2. Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 2004; 3: 673–683. [DOI] [PubMed] [Google Scholar]
- 3. Pammolli F, Magazzini L, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov 2011; 10: 428–438. [DOI] [PubMed] [Google Scholar]
- 4. Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, et al An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov 2015; 14: 475–486. [DOI] [PubMed] [Google Scholar]
- 5. DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ 2016; 47: 20–33. [DOI] [PubMed] [Google Scholar]
- 6. Wiley: Off‐label Prescribing: Justifying Unapproved Medicine ‐ David Cavalla [Internet]. [cited 2015 Sep 21]. Available at: http://eu.wiley.com/WileyCDA/WileyTitle/productCd‐1118912071,subjectCd‐MDM2.html (last accessed 21 September 2015).
- 7. Hubsher G, Haider M, Okun MS. Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology 2012; 78: 1096–1099. [DOI] [PubMed] [Google Scholar]
- 8. Cavalla D. APT drug R&D: the right active ingredient in the right presentation for the right therapeutic use. Nat Rev Drug Discov 2009; 8: 849–853. [DOI] [PubMed] [Google Scholar]
- 9. Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun 1990; 170: 843–850. [DOI] [PubMed] [Google Scholar]
- 10. Cavalla D, Singal C. Retrospective clinical analysis for drug rescue: for new indications or stratified patient groups. Drug Discov Today 2012; 17: 104–109. [DOI] [PubMed] [Google Scholar]
- 11. Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical Latanoprost. Am J Ophthalmol 1997; 124: 544–547. [DOI] [PubMed] [Google Scholar]
- 12. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of Ly156758 in metastatic breast cancer. Oncology 1988; 45: 344–345. [DOI] [PubMed] [Google Scholar]
- 13. Chlebowski RT, Schottinger JE, Shi J, Chung J, Haque R. Aromatase inhibitor, tamoxifen and endometrial cancer in breast cancer survivors. Cancer 2015; 121: 2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res 2014; 159: 355–376. [DOI] [PubMed] [Google Scholar]
- 15. Rothwell PM, Fowkes FGR, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long‐term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 2011; 377: 31–41. [DOI] [PubMed] [Google Scholar]
- 16. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network , Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, et al Rosuvastatin for sepsis‐associated acute respiratory distress syndrome. N Engl J Med 2014; 370: 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kor DJ, Carter RE, Park PK, Festic E, Banner‐Goodspeed VM, Hinds R, et al Effect of aspirin on development of ARDS in at‐risk patients presenting to the emergency department: the LIPS‐A randomized clinical trial. JAMA 2016; 315: 2406–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Zhong M, Wang Z, Song J, Wu W, Zhu D. The preventive effect of antiplatelet therapy in acute respiratory distress syndrome: a meta‐analysis. Crit Care Lond Engl 2018; 22: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kunte H, Busch MA, Trostdorf K, Vollnberg B, Harms L, Mehta RI, et al Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol 2012; 72: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, et al Newly expressed SUR1‐regulated NC (ca‐ATP) channel mediates cerebral edema after ischemic stroke. Nat Med 2006; 12: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen M, Dong Y, Simard JM. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J Neurosci 2003; 23: 8568–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anker SD, Negassa A, Coats AJS, Afzal R, Poole‐Wilson PA, Cohn JN, et al Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet Lond Engl 2003; 361: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 23. Lainscak M, Keber I, Anker SD. Body composition changes in patients with systolic heart failure treated with beta blockers: a pilot study. Int J Cardiol 2006; 106: 319–322. [DOI] [PubMed] [Google Scholar]
- 24. Pötsch MS, Tschirner A, Palus S, von Haehling S, Doehner W, Beadle J, et al The anabolic catabolic transforming agent (ACTA) espindolol increases muscle mass and decreases fat mass in old rats. J Cachexia Sarcopenia Muscle 2014; 5: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R, et al Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non‐small cell lung cancer or colorectal cancer: a randomized, double‐blind, placebo‐controlled, international multicentre phase II study (the ACT‐ONE trial). J Cachexia Sarcopenia Muscle 2016; 7: 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimmel SE, Schelleman H, Berlin JA, Oslin DW, Weinstein RB, Kinman JL, et al The effect of selective serotonin re‐uptake inhibitors on the risk of myocardial infarction in a cohort of patients with depression. Br J Clin Pharmacol 2011; 72: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wagner G, Icks A, Abholz H‐H, Schröder‐Bernhardi D, Rathmann W, Kostev K. Antihypertensive treatment and risk of dementia: a retrospective database study. Int J Clin Pharmacol Ther 2012; 50: 195–201. [DOI] [PubMed] [Google Scholar]
- 28. Gage JR, Fonarow G, Hamilton M, Widawski M, Martínez‐Maza O, Vredevoe DL. Beta blocker and angiotensin‐converting enzyme inhibitor therapy is associated with decreased Th1/Th2 cytokine ratios and inflammatory cytokine production in patients with chronic heart failure. Neuroimmunomodulation 2004; 11: 173–180. [DOI] [PubMed] [Google Scholar]
- 29. Pelusi S, Petta S, Rosso C, Borroni V, Fracanzani AL, Dongiovanni P, et al Renin‐angiotensin system inhibitors, type 2 diabetes and fibrosis progression: an observational study in patients with nonalcoholic fatty liver disease. PLoS ONE 2016; 11: PMC5029872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hardan AY, Handen BL. A retrospective open trial of adjunctive donepezil in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 2002; 12: 237–241. [DOI] [PubMed] [Google Scholar]
- 31. Hajjar I, Brown L, Mack WJ, Chui H. Impact of angiotensin receptor blockers on Alzheimer's disease neuropathology in a large brain autopsy series. Arch Neurol 2012; 69: 1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mittal S, Bjørnevik K, Im DS, Flierl A, Dong X, Locascio JJ, et al β2‐Adrenoreceptor is a regulator of the α‐synuclein gene driving risk of Parkinson's disease. Science 2017; 357: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cole SW, Sood AK. Molecular pathways: beta‐adrenergic signaling in cancer. Clin Cancer Res 2012; 18: 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, et al Beta‐blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010; 1: 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nkontchou G, Aout M, Mahmoudi A, Roulot D, Bourcier V, Grando‐Lemaire V, et al Effect of long‐term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV‐associated cirrhosis. Cancer Prev Res Phila Pa 2012; 5: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 36. Lin CS, Lin WS, Lin CL, Kao CH. Carvedilol use is associated with reduced cancer risk: a nationwide population‐based cohort study. Int J Cardiol 2015; 184: 9–13. [DOI] [PubMed] [Google Scholar]
- 37. Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, et al Clinical impact of selective and nonselective beta‐blockers on survival in patients with ovarian cancer. Cancer 2015; 121: 3444–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaapu KJ, Ahti J, Tammela TLJ, Auvinen A, Murtola TJ. Sotalol, but not digoxin is associated with decreased prostate cancer risk: a population‐based case‐control study. Int J Cancer 2015; 137: 1187–1195. [DOI] [PubMed] [Google Scholar]
- 39. Lemeshow S, Sørensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, et al β‐Blockers and survival among Danish patients with malignant melanoma: a population‐based cohort study. Cancer Epidemiol Biomarkers Prev 2011; 20: 2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonnet N, Pierroz DD, Ferrari SL. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact 2008; 8: 94–104. [PubMed] [Google Scholar]
- 41. Lee P, Ng C, Slattery A, Nair P, Eisman JA, Center JR. Preadmission bisphosphonate and mortality in critically ill patients. J Clin Endocrinol Metab 2016; 101: 1945–1953. [DOI] [PubMed] [Google Scholar]
- 42. Yasar S, Xia J, Yao W, Furberg CD, Xue QL, Mercado CI, et al Antihypertensive drugs decrease risk of Alzheimer disease ginkgo evaluation of memory study. Neurology 2013; 81: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zacharski LR, Moritz TE, Haakenson CM, O'Donnell JF, Ballard HS, Johnson GJ, et al Chronic calcium antagonist use in carcinoma of the lung and colon: a retrospective cohort observational study. Cancer Invest 1990; 8: 451–458. [DOI] [PubMed] [Google Scholar]
- 44. Abrahami D, Douros A, Yin H, Yu OHY, Renoux C, Bitton A, et al Dipeptidyl peptidase‐4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study. BMJ 2018; 360: k872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hur GY, Lee SY, Lee SH, Kim SJ, Lee KJ, Jung JY, et al Potential use of an anticancer drug gefinitib, an EGFR inhibitor, on allergic airway inflammation. Exp Mol Med 2007; 39: 367–375. [DOI] [PubMed] [Google Scholar]
- 46. Koh GCKW, Maude RR, Schreiber MF, Limmathurotsakul D, Wiersinga WJ, Wuthiekanun V, et al Glyburide is anti‐inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis 2011; 52: 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang RY, Hsieh KP, Huang WW, Yang YH. Use of lithium and cancer risk in patients with bipolar disorder: population‐based cohort study. Br J Psychiatry 2016; 209: 393–399. [DOI] [PubMed] [Google Scholar]
- 48. Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, et al Anti‐acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med 2013; 1: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McGwin G, Owsley C, Curcio CA, Crain RJ. The association between statin use and age related maculopathy. Br J Ophthalmol 2003; 87: 1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caballero J, Nahata M. Do statins slow down Alzheimer's disease? A review J Clin Pharm Ther 2004; 29: 209–213. [DOI] [PubMed] [Google Scholar]
- 51. Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, et al Statin use in patients with asthma: a nationwide population‐based study. Eur J Clin Invest 2011; 41: 507–512. [DOI] [PubMed] [Google Scholar]
- 52. Donnino MW, Cocchi MN, Howell M, Clardy P, Talmor D, Cataldo L, et al Statin therapy is associated with decreased mortality in patients with infection. Acad Emerg Med Off J Soc Acad Emerg Med 2009; 16: 230–234. [DOI] [PubMed] [Google Scholar]
- 53. Fogerty MD, Efron D, Morandi A, Guy JS, Abumrad NN, Barbul A. Effect of preinjury statin use on mortality and septic shock in elderly burn patients. J Trauma 2010; 69: 99–103. [DOI] [PubMed] [Google Scholar]
- 54. Hippisley‐Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010; 340: c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol Off J Am Soc Clin Oncol 2013; 31: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 56. Marcella SW, David A, Ohman‐Strickland PA, Carson J, Rhoads GG. Statin use and fatal prostate cancer. Cancer 2012; 118: 4046–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fong DS, Poon K‐YT. Recent statin use and cataract surgery. Am J Ophthalmol 2012; 153: 222–8.e1. [DOI] [PubMed] [Google Scholar]
- 58. Mortensen EM, Copeland LA, Pugh MJV, Restrepo MI, de Molina RM, Nakashima B, et al Impact of statins and ACE inhibitors on mortality after COPD exacerbations. Respir Res 2009; 10: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Otte C, Zhao S, Whooley MA. Statin use and risk of depression in patients with coronary heart disease: longitudinal data from the heart and soul study. J Clin Psychiatry 2012; 73: 610–615. [DOI] [PubMed] [Google Scholar]
- 60. Etminan M, Samii A, Brophy JM. Statin use and risk of epilepsy. Neurology 2010; 75: 1496–1500. [DOI] [PubMed] [Google Scholar]
- 61. Leung DYL, Li FCH, Kwong YYY, Tham CCY, Chi SCC, Lam DSC. Simvastatin and disease stabilization in normal tension glaucoma: a cohort study. Ophthalmology 2010; 117: 471–476. [DOI] [PubMed] [Google Scholar]
- 62. Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose‐dependent effects of statins. Chest 2007; 131: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 63. Scranton RE, Young M, Lawler E, Solomon D, Gagnon D, Gaziano JM. Statin use and fracture risk: study of a US veterans population. Arch Intern Med 2005; 165: 2007–2012. [DOI] [PubMed] [Google Scholar]
- 64. Cunha‐Cruz J, Saver B, Maupome G, Hujoel PP. Statin use and tooth loss in chronic periodontitis patients. J Periodontol 2006; 77: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 65. Vinogradova Y, Coupland C, Hippisley‐Cox J. Risk of pneumonia in patients taking statins: population‐based nested case‐control study. Br J Gen Pract 2011; 61: e742–e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chodick G, Amital H, Shalem Y, Kokia E, Heymann AD, Porath A, et al Persistence with statins and onset of rheumatoid arthritis: a population‐based cohort study. PLoS Med 2010; 7: e1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gupta R, Plantinga LC, Fink NE, Melamed ML, Coresh J, Fox CS, et al Statin use and sepsis events [corrected] in patients with chronic kidney disease. JAMA 2007; 297: 1455–1464. [DOI] [PubMed] [Google Scholar]
- 68. Li Y, Gottlieb J, Ma D, Kuehn C, Strueber M, Welte T, et al Graft‐protective effects of the HMG‐CoA reductase inhibitor pravastatin after lung transplantation – a propensity score analysis with 23 years of follow‐up. Transplantation 2011; 92: 486–492. [DOI] [PubMed] [Google Scholar]
- 69. Wasko MM, Hubert HB, Lingala V, et al HYdroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA 2007; 298: 187–193. [DOI] [PubMed] [Google Scholar]
- 70. Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, et al Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol 2005; 58: 963–967. [DOI] [PubMed] [Google Scholar]
- 71. Saligan LN, Luckenbaugh DA, Slonena EE, Machado‐Vieira R, Zarate CA. An assessment of the anti‐fatigue effects of ketamine from a double‐blind, placebo‐controlled, crossover study in bipolar disorder. J Affect Disord 2016; 194: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hsu CC, Wahlqvist ML, Lee MS, Tsai HN. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 2011; 24: 485–493. [DOI] [PubMed] [Google Scholar]
- 73. Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long‐term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 2014; 41: 61–68. [DOI] [PubMed] [Google Scholar]
- 74. Rieken M, Xylinas E, Kluth L, Crivelli JJ, Chrystal J, Faison T, et al Association of diabetes mellitus and metformin use with oncological outcomes of patients with non‐muscle‐invasive bladder cancer. BJU Int 2013; 112: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 75. Rieken M, Xylinas E, Kluth L, Crivelli JJ, Chrystal J, Faison T, et al Effect of diabetes mellitus and metformin use on oncologic outcomes of patients treated with radical cystectomy for urothelial carcinoma. Urol Oncol 2014; 32: 49.e7–14. [DOI] [PubMed] [Google Scholar]
- 76. Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011; 11: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ko EM, Walter P, Jackson A, Clark L, Franasiak J, Bolac C, et al Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol 2014; 132: 438–442. [DOI] [PubMed] [Google Scholar]
- 78. Pollak M, Gonzalez‐Angulo AM. Metformin and hepatic carcinogenesis. Cancer Prev Res Phila Pa 2012; 5: 500–502. [DOI] [PubMed] [Google Scholar]
- 79. Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DPH, Chen CC. Antidiabetes drugs correlate with decreased risk of lung cancer: a population‐based observation in Taiwan. Clin Lung Cancer 2012; 13: 143–148. [DOI] [PubMed] [Google Scholar]
- 80. Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, et al Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Res Clin Pract 2014; 106: 19–26. [DOI] [PubMed] [Google Scholar]
- 81. Yu H, Yin L, Jiang X, Sun X, Wu J, Tian H, et al Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta‐analysis of epidemiological observational studies. PloS One 2014; 9: e116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brauchli YB, Jick SS, Curtin F, Meier CR. Association between use of thiazolidinediones or other oral antidiabetics and psoriasis: a population based case‐control study. J Am Acad Dermatol 2008; 58: 421–429. [DOI] [PubMed] [Google Scholar]
- 83. Price CS, Taylor FB. A retrospective chart review of the effects of modafinil on depression as monotherapy and as adjunctive therapy. Depress Anxiety 2005; 21: 149–153. [DOI] [PubMed] [Google Scholar]
- 84. Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease‐free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 2014; 113 (Suppl. 1): i82–i87. [DOI] [PubMed] [Google Scholar]
- 85. Johnson CC, Jankowski M, Rolnick S, Yood MU, Alford SH. Influence of NSAID use among colorectal cancer survivors on cancer outcomes. Am J Clin Oncol 2014; 40: 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Iyengar RL, Gandhi S, Aneja A, Thorpe K, Razzouk L, Greenberg J, et al NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am J Med 2013; 126: 1017.e11–1017.e18. [DOI] [PubMed] [Google Scholar]
- 87. O'Neal HR, Koyama T, Koehler EAS, Siew E, Curtis BR, Fremont RD, et al Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med 2011; 39: 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lip S, Carlin C, McCallum L, Touyz RH, Dominiczak AF, Padmanabhan S. LB01.03: incidence and prognosis of cancer associated with digoxin and common antihypertensive drugs. J Hypertens 2015; 33 (Suppl. 1): e45. [Google Scholar]
- 89. Platz EA, Yegnasubramanian S, Liu JO, Chong CR, Shim JS, Kenfield SA, et al A novel two‐stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discov 2011; 1: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kelly TF, Lieberman DZ. The utility of the combination of dextromethorphan and quinidine in the treatment of bipolar II and bipolar NOS. J Affect Disord 2014; 167: 333–335. [DOI] [PubMed] [Google Scholar]
- 91. Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM, Chan KA. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatol Baltim Md 2012; 55: 1462–1472. [DOI] [PubMed] [Google Scholar]
- 92. Brakedal B, Flønes I, Reiter SF, Torkildsen Ø, Dölle C, Assmus J, et al Glitazone use associated with reduced risk of Parkinson's disease. Mov Disord 2017; 32: 1594–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Paul M, Gafter‐Gvili A, Fraser A, Leibovici L. The anti‐cancer effects of quinolone antibiotics? Eur J Clin Microbiol Infect Dis 2007; 26: 825–831. [DOI] [PubMed] [Google Scholar]
- 94. Melamed ML, Blackwell T, Neugarten J, Arnsten JH, Ensrud KE, Ishani A, et al Raloxifene, a selective estrogen receptor modulator, is renoprotective: a post‐hoc analysis. Kidney Int 2011; 79: 241–249. [DOI] [PubMed] [Google Scholar]
- 95. Westlake SL, Colebatch AN, Baird J, Curzen N, Kiely P, Quinn M, et al Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatol Oxf Engl 2011; 50: 518–531. [DOI] [PubMed] [Google Scholar]
- 96. Wu CY, Chen DY, Shen JL, Ho HJ, Chen CC, Kuo KN, et al The risk of cancer in patients with rheumatoid arthritis taking tumor necrosis factor antagonists: a nationwide cohort study. Arthritis Res Ther 2014; 16: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gupta‐Ganguli M, Cox K, Means B, Gerling I, Solomon SS. Does therapy with anti–TNF‐α improve glucose tolerance and control in patients with type 2 diabetes? Diabetes Care 2011; 34: e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, et al Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. J Pediatr 2011; 158: 644–9.e1. [DOI] [PubMed] [Google Scholar]
- 99. Walker AJ, Card T, Bates TE, Muir K. Tricyclic antidepressants and the incidence of certain cancers: a study using the GPRD. Br J Cancer 2011; 104: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Salminen JK, Tammela TLJ, Auvinen A, Murtola TJ. Antiepileptic drugs with histone deacetylase inhibition activity and prostate cancer risk: a population‐based case‐control study. Cancer Causes Control 2016; 27: 637–645. [DOI] [PubMed] [Google Scholar]
- 101. Li M, Jia Q, Chen T, Zhao Z, Chen J, Zhang J. The role of vascular endothelial growth factor and vascular endothelial growth inhibitor in clinical outcome of traumatic brain injury. Clin Neurol Neurosurg 2016; 144: 7–13. [DOI] [PubMed] [Google Scholar]


