Summary
The aim of this study was to determine the expressions of Toll‐like receptors (TLRs) 7–9 and type I interferon (IFN) signal in labial salivary glands (LSGs) and cultured salivary gland epithelial cells (SGECs) from primary Sjögren's syndrome (pSS) patients. We performed an immunohistochemistry analysis of LSGs from 11 patients with pSS as defined by American–European Consensus Group classification criteria and five healthy subjects. The pSS patients' SGECs were analyzed by immunofluorescence and western blotting. IFN‐α expression was examined by immunosorbent assay and flow cytometry. Mononuclear cells (MNCs) from pSS patients' LSGs showed TLR‐7‐dominant expression. B cells, plasma cells and plasmacytoid dendritic cells (pDCs) co‐expressed with TLR‐7. Myeloid differentiation primary response gene 88 (MyD88), tumor necrosis factor receptor‐associated factor 6 (TRAF6) and interferon regulatory factor 7 (IRF7) co‐expressed with the pDC marker CD303 in LSGs. Ducts from pSS patients dominantly expressed TLR‐7, and TLR‐7 in the ducts co‐expressed with MyD88, TRAF6 and IRF7. Type I IFNs including IFN‐α and IFN‐β were detected in MNCs and ducts in pSS patients' LSGs. Increased TRAF6 expression and the nuclear translocation of IRF7 in SGECs were detected by immunofluorescence following loxoribine (a TLR‐7 ligand) stimulation despite IFN‐β pretreatment. Western blotting showed increased TRAF6 expression in SGECs following IFN‐β and loxoribine stimulation. Although no increase in IFN‐α was detected in supernatant from stimulated SGECs, the IFN‐α in supernatant from stimulated peripheral blood pDCs from pSS patients was significantly increased. Our findings suggest that TLR‐7 is dominantly expressed in both MNCs and ducts with downstream signals for type I IFNs, indicating that TLR7‐dominant innate immunity is related to the development of sialadenitis in pSS.
Keywords: plasmacytoid dendritic cells, Sjögren's syndrome, TLR‐7, type I interferons
Introduction
Primary Sjögren's syndrome (pSS) is a systemic autoimmune disease characterized by periductal lymphocytic infiltration of the salivary and lacrimal glands, which results in reduced secretory functions and oral and ocular dryness 1. Although the pathogenesis of pSS is not yet established, innate immune responses including type I interferon (IFN) activity were shown to be associated with the pathogenesis of pSS 2, 3, as were the conventional acquired immunity responses, including major histocompatibility class II‐mediated antigen presentation 4. Toll‐like receptors (TLRs), especially TLRs 7–9, are important innate immune receptors that recognize a range of RNA and DNA molecules from viruses and self‐antigens, and lead to the production of type I IFN via downstream molecules such as myeloid differentiation primary response gene 88 (MyD88), tumor necrosis factor receptor‐associated factor 6 (TRAF6) and interferon regulatory factor 7 (IRF7) signaling 5. The expressions of TLR‐7 and TLR‐9 were high in the peripheral blood mononuclear cells (PBMCs) of pSS patients 6. However, little is known regarding the expression pattern and the roles of TLR‐7–9 in the salivary glands. In this study, we analyzed the expressions of TLR‐7–9 in labial salivary glands (LSGs) of pSS patients. Based on the TLR‐7‐dominant expression observed, we also analyzed TLR‐7 downstream signals and function in an in vitro examination.
Materials and methods
Patients
For the immunohistochemical analysis, we retrospectively analyzed materials from 11 patients with pSS and five control subjects who visited Nagasaki University Hospital during the period from 2008 to 2013. The patients' pSS classification was based on the 2002 American–European Consensus Group (AECG) Sjögren's Syndrome (SS) classification criteria 7. The control subjects had sicca symptoms but did not fulfill the AECG SS classification criteria (non‐SS sicca control subjects). LSG biopsies specimens were obtained from all participants for our assessment of the pathological findings of pSS.
For the determination of focus scores (i.e. the number of foci per 4 mm2) in LSGs, the number of foci in a section from LSGs was counted and the surface area of the section was measured by a hybrid cell count system mounted on a microscope (BZ‐X700; Keyence, Osaka, Japan) 8. The clinical and serological characteristics of the pSS patients and control subjects are summarized in Table 1. All patients gave their informed consent to be subjected to the protocol, which was approved by the Institutional Ethics Committee of Nagasaki University Hospital (approval no. 09102822‐3).
Table 1.
Background information of enrolled subjects
| Variables | pSS (n = 11) | Non‐SS (n = 5) | P‐value |
|---|---|---|---|
| Age (years), median (IQR) | 61 (57–65) | 52 (40–68) | 0·65a |
| Female, n (%) | 11 (100%) | 5 (100%) | 1b |
| Xerostomia, n (%) | 10 (90·9%) | 3 (60%) | 0·21b |
| Xerophthalmia, n (%) | 6 (54·5%) | 3 (60%) | 1b |
| Schirmer test positivity, n (%) | 4 (36·4%) | 2 (40%) | 0·84b |
| Saxon test positivity, n (%) | 9 (81·8%) | 3 (60%) | 0·35b |
| Anti‐SS‐A/Ro antibody positivity, n (%) | 11 (100%) | 0 (0%) | <0·001b |
| Anti‐SS‐B/La antibody positivity, n (%) | 4 (36·3%) | 0 (0%) | 0·12b |
| ANA positivity, n (%) | 11 (100%) | 0 (0%) | <0·001b |
| RF positivity, n (%) | 7 (63·6%) | 4 (80%) | 0·51b |
| Serum IgG, mg/dl, median (IQR) | 2140 (1550–2550) | 1047 (656–1246) | 0·009a |
| LSG biopsy, focus score | 6·9 (4–19·2) | 0 | 0·0019a |
| ESSDAI score, median (IQR) | 6 (4–6) | n.a. | n.a. |
P‐values < 0·05 were considered significant.
Non‐SS = these subjects were classified as non‐SS sicca control subjects based on the American–European Consensus Group (AECG) classification criteria.
ANA = anti‐nuclear antibody; ESSDAI = European League Against Rheumatism Sjögren's Syndrome Disease Activity Index; IgG = immunoglobulin G; IQR = interquartile range; LSG = labial salivary gland; n.a. = not assessed; pSS = primary Sjögren's syndrome; RF = rheumatoid factor.
Mann–Whitney U‐test.
Fisher's exact test.
Culture of salivary gland epithelial cells (SGECs)
We performed the culturing of SGECs as described previously 9. Briefly, the LSG tissues were cut with fine needles and scalpels and placed in six‐well plates coated with type‐I collagen (Iwaki, Tokyo, Japan) with culture medium consisting of defined keratinocyte–serum‐free media (SFM) (Invitrogen Life Technologies, Carlsbad, CA, USA), 0·4 μg/ml hydrocortisone (Sigma‐Aldrich, St Louis, MO, USA), 25 μg/ml bovine pituitary extract (Kurabo, Osaka, Japan), 100 U/ml penicillin and 100 μg/ml streptomycin (gibco, Grand Island, NY, USA). When an outgrowth of SGECs was observed, the cells were transferred into 100‐mm2 plates coated with type‐I collagen (Iwaki) after the cells reached confluence. After the SGECs reached confluence on the 100‐mm2 plates, the cells were cultured onto 60‐mm2 plates coated with type‐I collagen (Iwaki) for Western blotting and an enzyme‐linked immunosorbent assay (ELISA).
When the SGECs reached confluence, the cells were treated with 1 mM loxoribine (InvivoGen, San Diego, CA, USA), a TLR‐7 ligand, for 10 min, 60 min or 6 h, and/or 1000 U/ml interferon (IFN)‐β (Betaferon®; Bayer Pharma, Berlin, Germany) for 12 h. For immunofluorescence, SGECs were distributed onto 12‐mm2 cover slips coated with a type I collagen, Cellmatrix (Nitta Gelatin, Osaka, Japan) in 24‐well plates (Corning, New York, NY, USA) after the SGECs reached confluence on the 100‐mm2 plates. Subsequently, the SGECs were treated with 1 mM loxoribine for various lengths of time between 0 and 60 min and/or 1000 U/ml IFN‐β for 12 h.
Isolation and culture of peripheral blood mononuclear cells (PBMCs)
Heparinized whole blood (7 ml) was obtained from each pSS patient and control subjects (patients other than those described in Table 1). PBMCs were isolated from whole blood by centrifugation using Ficoll‐Paque™ Plus (GE Healthcare Bio‐Sciences, Uppsala, Sweden); the cells were subsequently seeded on six‐well plates (Iwaki) and cultured in RPMI‐1640 medium (Wako, Osaka, Japan) supplemented with 10% heat‐inactivated fetal bovine serum (gibco, Paisley, Scotland, UK), 0·25 μg/ml amphotericin B (Sigma‐Aldrich), 100 U/ml penicillin and 100 μg/ml streptomycin (gibco). The cells were then treated with 1 mM loxoribine for 6 h and/or 1000 U/ml IFN‐β for 12 h for the ELISA and flow cytometry.
Immunohistochemistry (IHC)
We performed IHC to examine the expressions of TLR‐7–9 and TLR‐7 downstream molecules in LSGs. A cancer tissue array including four types of cancer – colon, breast, lung and prostate – and normal tissue (US Biomax, Derwood, MD, USA) was used as the positive control for TLR‐7–9 expression. The primary antibodies used were rabbit anti‐TLR‐7 polyclonal (working dilution 1 : 100; Enzo Life Sciences, Farmingdale, NY, USA), rabbit anti‐TLR‐8 polyclonal (working dilution 1 : 100; Sigma‐Aldrich), rabbit anti‐TLR‐9 polyclonal (working dilution 1 : 100; Bioss Antibodies, Woburn, MA, USA), mouse anti‐IFN‐α monoclonal (working dilution 1 : 100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and rabbit anti‐IFN‐β polyclonal antibody (working dilution 1 : 100; Abcam, Cambridge, MA, USA).
In summary, paraffin‐embedded sections from LSGs and the tissue array were incubated with 3% H2O2 solution for the inhibition of endogenous peroxidase activity after microwave epitope retrieval, and blocked with 5% normal horse serum. They were incubated with each primary antibody diluted with 5% normal horse serum for 60 min at room temperature (RT), followed by peroxidase‐conjugated secondary antibody (Histofine Simple Stain, Nichirei Biosciences, Tokyo, Japan) for 30 min at RT. After incubation, they were reacted with 3,3′‐diaminobenzidine solution for 10 min and counterstained with hematoxylin solution. Images were taken using a digital microscope color camera (DFC295; Leica Microsystems, Tokyo, Japan).
Immunofluorescence
We performed an immunofluorescence examination to determine the localization of TLR‐7, TLR‐8 and TLR‐7 downstream molecules in mononuclear cells (MNCs) of LSGs in vivo and the expression of TLR‐7 downstream molecules in SGECs in vitro. The primary antibodies used were rabbit anti‐TLR‐7 polyclonal (working dilution 1 : 100), mouse anti‐TLR‐7 monoclonal (working dilution 1 : 100; Thermo Fisher Scientific, Rockford, IL, USA), rabbit anti‐TLR‐8 polyclonal (working dilution 1 : 100), rabbit anti‐myeloid differentiation primary response gene 88 (MyD88) polyclonal (working dilution 1 : 100; Santa Cruz), rabbit anti‐TRAF6 monoclonal (working dilution 1 : 100; Abcam), rabbit anti‐IRF7 polyclonal (working dilution 1 : 100; Abnova, Taipei City, Taiwan), mouse anti‐CD4 monoclonal (working dilution 1 : 100; Agilent Technologies, Santa Clara, CA, USA), mouse anti‐CD8 monoclonal (working dilution 1 : 100; Agilent Technologies), mouse anti‐CD20 monoclonal (working dilution 1 : 1; Agilent Technologies), mouse anti‐CD68 monoclonal (working dilution 1 : 100, Agilent Technologies), mouse anti‐VS38c monoclonal (working dilution 1 : 100, Agilent Technologies) and mouse anti‐CD303 antibody (working dilution 1 : 100; Dendritics, Lyon, France) in vivo and rabbit anti‐TRAF6 monoclonal antibody (working dilution 1 : 100) and rabbit anti‐IRF7 polyclonal antibody (working dilution 1 : 100) in vitro. The procedure until incubation with primary antibodies was basically the same as that of the IHC in vivo.
After incubation with primary antibodies, the sections from LSGs were reacted with secondary antibodies including donkey anti‐mouse immunoglobulin (IgG) conjugated with fluorescein isothiocyanate (FITC) antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), donkey anti‐rabbit IgG conjugated with tetramethyl rhodamine isothiocyanate (TRITC) antibody (Jackson ImmunoResearch) and Hoechst dye 33258 (Sigma‐Aldrich) for 45 min at RT in the dark. The sections were then mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA).
In vitro, SGECs on 12‐mm2 coverslips were incubated in 4% paraformaldehyde for 10 min at 4°C and then immersed in methanol for 10 min at −20°C after loxoribine and/or IFN‐β stimulation. The cells were blocked with 5% normal horse serum and incubated in each primary antibody for 60 min at RT, followed by secondary antibodies including rabbit anti‐IgG conjugated with TRITC and Hoechst dye 33258 for 45 min at RT in the dark. The cells were subsequently mounted in Vectashield mounting medium. Images were captured by a microscope (BZ‐X700). The mean fluorescence intensity (MFI) of cells in a given area was calculated by the hybrid cell count system that was mounted on the BZ‐X700 microscope.
Terminal deoxynucleotidyl transferase‐mediated dUTP nick end‐labeling (TUNEL) staining
We performed TUNEL staining to examine apoptosis in SGECs, as described previously 10. Briefly, we used a Mebstain Apoptosis Kit Direct (MBL, Nagoya, Japan). After the SGECs were treated with loxoribine for 6 h and/or IFN‐β for 12 h, or 50 ng/ml recombinant tumor necrosis factor‐related apoptosis inducing ligand (TRAIL) (R&D Systems Minneapolis, MN, USA) for 3 h as a positive control, the cells were incubated with 50 μl of terminal deoxynucleotidyl transeferase (TdT) solution at 37°C for 1 h after incubation with TdT buffer for 10 min. The cells were subsequently mounted in Vectashield mounting medium. Images were captured by a microscope (BZ‐X700).
Western blotting
We performed Western blotting to detect the protein of TLR‐7, its downstream molecules and other molecules [B cell activating factor (BAFF), Ro52, and major histocompatibility (MHC) class I] after stimulation with loxoribine and/or IFN‐β. Western blotting was performed as described previously 9. After SGECs were lysed and the protein concentrations were measured, identical amounts of protein were subjected to 12·5% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE). The proteins were transferred to a polyvinylidene fluoride (PVDF) filter, and the filter was blocked using 5% non‐fat dried milk in Tris‐buffered saline containing 0.1% Tween 20 (TBS‐T) for 60 min, and incubated with rabbit anti‐TLR‐7 polyclonal antibody (working dilution 1 : 250), rabbit anti‐TRAF6 monoclonal antibody (working dilution 1 : 1000), mouse anti‐BAFF monoclonal antibody (working dilution 1 : 1000; Novus Biologicals, Littleton, CO, USA), rabbit anti‐MHC class I polyclonal antibody (working dilution 1 : 1000; Proteintech, Rosemont, IL, USA) or rabbit anti‐Ro52 polyclonal antibody (working dilution 1 : 1000; Cloud‐Clone Corp; Katy, TX, USA) at 4°C overnight, or mouse anti‐β actin monoclonal antibody (working dilution 1 : 5000; Santa Cruz) for 60 min at RT.
The filter was then washed with TBS‐T and incubated with mouse or rabbit anti‐IgG coupled with horseradish peroxidase (HRP) as secondary antibodies (working dilution 1 : 1000; MBL). After incubation, we used an Amersham Hyperfilm enhanced chemiluminescence system (GE Healthcare UK, Little Chalfont, UK) for detection of the bands. The density of each band was measured by Image J software developed at the US National Institutes of Health for the determination of the pixel intensity.
ELISA
The levels of IFN‐α in the culture supernatants of SGECs and PBMCs after stimulation with loxoribine and/or IFN‐β were measured using the VeriKine™ Human IFN‐α ELISA Kit (Product #41100; PBL Assay Science, Piscataway, NJ, USA) following the manufacturer's instructions. Briefly, the cells were seeded on 96‐well ELISA plates and incubated for 60 min at RT. They were incubated with detection antibody for 60 min at RT, followed by HRP‐conjugated secondary antibody for 60 min at RT. After that, they were incubated with substrate solution for 15 min at RT, and stop solution was added. Subsequently, the absorbance at 450 nm was determined by using a microplate reader.
Flow cytometry
After the isolated PBMCs from three pSS patients were stimulated with loxoribine and/or IFN‐β followed by treatment with 5 μg/ml of brefeldin A for 12 h to save the IFN‐α activity, the PBMCs were stained with phycoerythrin/cyanin 7 (PE/Cy7)‐conjugated mouse anti‐CD123 antibody (Biolegend, San Diego, CA, USA) and allophycocyanin (APC)‐conjugated mouse anti‐CD303 antibody (Biolegend) for 30 min at 4°C. After permeabilization, the PBMCs were stained with FITC‐conjugated mouse anti‐IFN‐α antibody (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) and phycoerythrin (PE)‐conjugated mouse anti‐TLR‐7 antibody (ThermoFisher Scientific) at 4°C overnight, and were then analyzed by flow cytometry using a BD FACS Verse (BD Biosciences, San Jose, CA, USA) and FlowJo version 10 software (Tree Star, Ashland, OR, USA). PE/Cy7‐conjugated mouse IgG1, APC‐conjugated mouse IgG1, FITC‐conjugated mouse IgG2a and PE‐conjugated mouse IgG1 (Biolegend) were used as isotype controls.
Statistical analysis
We used the Mann–Whitney U‐test or Fisher's exact test to compare the clinical and serological characteristics between the pSS and control groups, and a repeated‐measures analysis of variance (anova) to determine the differences in protein expressions detected by Western blotting and the percentages of IFN‐α+ cells measured by flow cytometry. Spearman's rank correlation coefficient was used to determine the correlation between the mean fluorescence intensity of IFN‐α or TLR‐7 stains in the mononuclear cells of LSGs and the percentage of IFN‐α+ TLR‐7+ cells among the PBMCs.
All statistical analyses in this study were performed using jmp software version 13 (SAS, Cary, NC, USA). P‐values < 0·05 were considered significant.
Results
Subject characteristics
Table 1 summarizes the characteristics of the 11 patients and the five control subjects. All the pSS patients were female. The group of pSS patients had significantly greater anti‐SS‐A/Ro antibody and anti‐nuclear antibody positivity, and significantly higher serum IgG levels and LSG biopsy focus scores compared to the control group (P < 0·001, P < 0·001, P = 0·009 and P = 0·0019).
The dominant expression of TLR‐7 in the MNCs and ducts of the pSS patients' LSGs
The expression levels of TLR‐7 were stronger in infiltrating MNCs and ducts of LSGs from the pSS patients compared to those of the control subjects (Fig. 1a,d). In contrast, TLR‐9 was weakly expressed in the MNCs and ducts from both the pSS patients and controls (Fig. 1c,f). TLR‐8 was expressed in the MNCs of several pSS patients (Fig. 1b,e). The immunostaining results for TLR‐7–9 are summarized in the Supporting information, Table S1.
Figure 1.
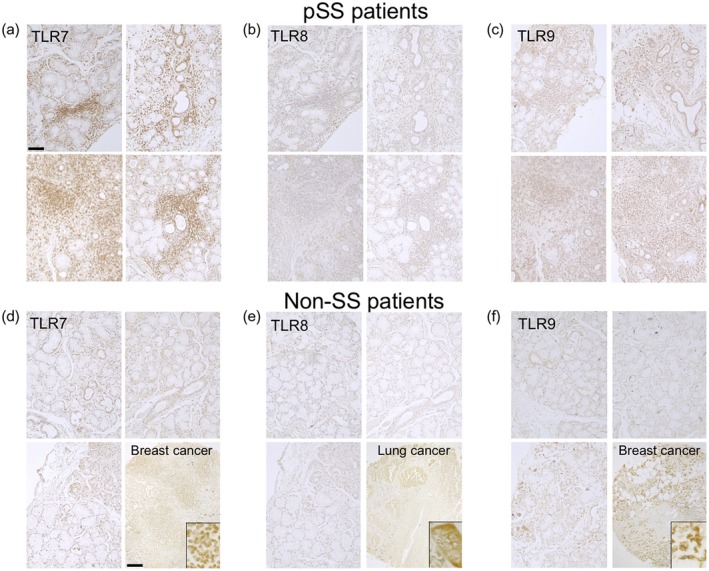
Expressions of Toll‐like receptors (TLRs) 7–9 in labial salivary glands (LSGs) from the primary Sjögren's syndrome (pSS) patients and non‐SS sicca control subjects. Representative samples of labial salivary glands (LSGs) stained with anti‐TLR‐7 (a), anti‐TLR‐8 (b) and anti‐TLR‐9 antibodies (c) from pSS patients (n = 10). Representative samples of LSGs from non‐SS patients (n = 5) and cancer tissue array (right lower panels) stained with anti‐TLR‐7 (d), anti‐TLR‐8 (e) and anti‐TLR‐9 antibody (f). Insets: representative staining in the cancer tissue array. Bar: 80 μM (LSGs), 200 μM (cancer tissue array). Non‐SS: these subjects were classified as non‐SS sicca control subjects based on the American–European Consensus Group (AECG) classification criteria.
The dominant expression of TLR‐7 in plasmacytoid dendritic cells (pDCs), CD8+ T cells, B cells and plasma cells of LSGs from the pSS patients
To clarify which cells expressed TLR‐7, we performed double immunofluorescence staining with TLR‐7 and several cell markers in LSGs from the pSS patients. The results showed that the TLR‐7‐positive cells were co‐localized mainly with plasmacytoid dendritic cells (pDCs; CD303‐positive cells). CD8+ T cells (CD8‐positive cells), B cells (CD20‐positive cells) and plasma cells (VS38c‐positive cells) were also co‐localized with TLR‐7‐positive cells, next to the co‐localization of pDCs, whereas other cell marker‐positive cells were partly co‐localized with TLR‐7‐positive cells (Fig. 2). We also performed double immunofluorescence staining with TLR‐8 and several cell markers in LSGs from the pSS patients, and the results demonstrated that TLR‐8‐positive cells were also co‐localized mainly with pDCs (CD303‐positive cells) (Supporting information, Fig. S1).
Figure 2.
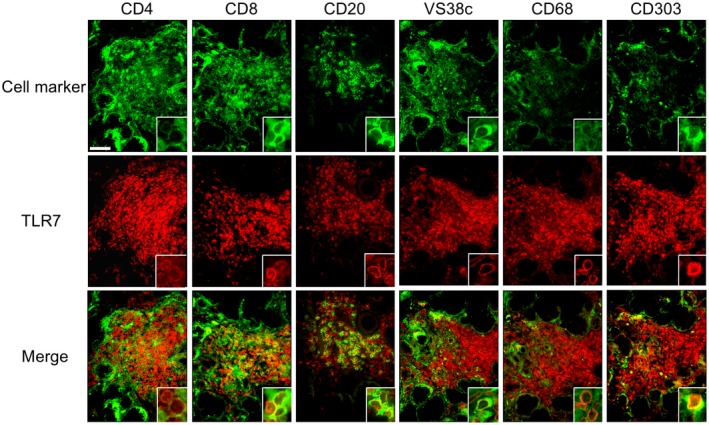
Co‐localization of Toll‐like receptor (TLR)‐7 and mononuclear cells (MNCs) in LSGs from the pSS patients. Representative samples of LSGs stained with cell markers for T cells (anti‐CD4 and anti‐CD8 antibodies), B cells (anti‐CD20 antibody), plasma cells (anti‐VS38c antibody), macrophages (anti‐CD68 antibody) or pDCs (anti‐CD303 antibody) (green), and anti‐TLR‐7 antibody (red) from pSS patients (n = 3). Hoechst (blue) was used for counterstaining the nuclei. Insets: representative staining for each panel. Bar: 40 μM.
The expression of TLR‐7 downstream molecules in pDCs from the pSS patients
We examined the results of the double immunofluorescence staining for TLR‐7 downstream molecules and CD303 in LSGs from the pSS patients, and observed that TLR‐7 was expressed in pDCs. TLR‐7 downstream molecule (MyD88, TRAF6 and IRF7)‐positive cells as well as TLR‐7‐positive cells were co‐localized mainly with pDCs (Fig. 3).
Figure 3.
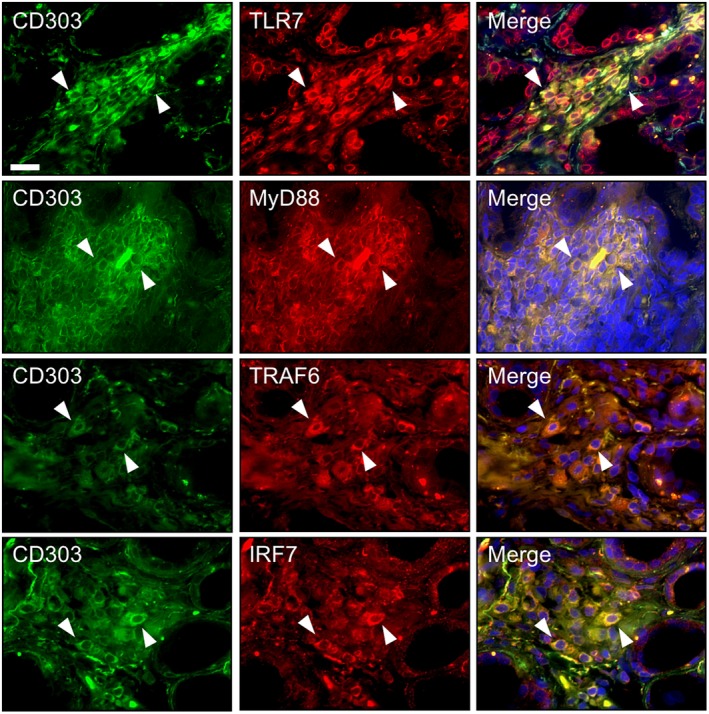
Expression of Toll‐like receptor (TLR)‐7 and its downstream molecules in plasmacytoid dendritic cells (pDCs) of labial salivary glands (LSGs) from the pSS patients. Representative samples of LSGs from pSS patients (n = 4) stained with a cell marker for pDCs (anti‐CD303 antibody) (green) and anti‐TLR‐7 (a), anti‐MyD88 (b), anti‐TRAF6 (c) or anti‐IRF7 antibody (d) (red). Hoechst (blue) was used for counterstaining the nuclei. White arrowheads: the expression of TLR‐7 or its downstream molecules in pDCs. Bar: 20 μM. IRF7 = interferon regulatory factor 7; MyD88 = myeloid differentiation primary response gene 88; TRAF6 = tumor necrosis factor receptor‐associated factor 6.
The expression of TLR‐7 and its downstream molecules in ducts from the pSS patients
We examined the results of the IHC staining with TLR‐7 downstream molecules in ducts of LSGs from the pSS patients. TLR‐7 was strongly expressed in ducts of LSGs from the pSS patients compared to the expressions of TLR‐8 and TLR‐9 (Fig. 4a). In the serial sections, immunofluorescence showed that the TLR‐7 downstream molecules MyD88, TRAF6 and IRF7 were also expressed in ducts of LSGs from the pSS patients (Fig. 4b). In addition, the expression levels of type I IFNs (IFN‐α and IFN‐β) were stronger in the MNCs and ducts of LSGs from the pSS patients compared to those of the control subjects (Fig. 5a).
Figure 4.
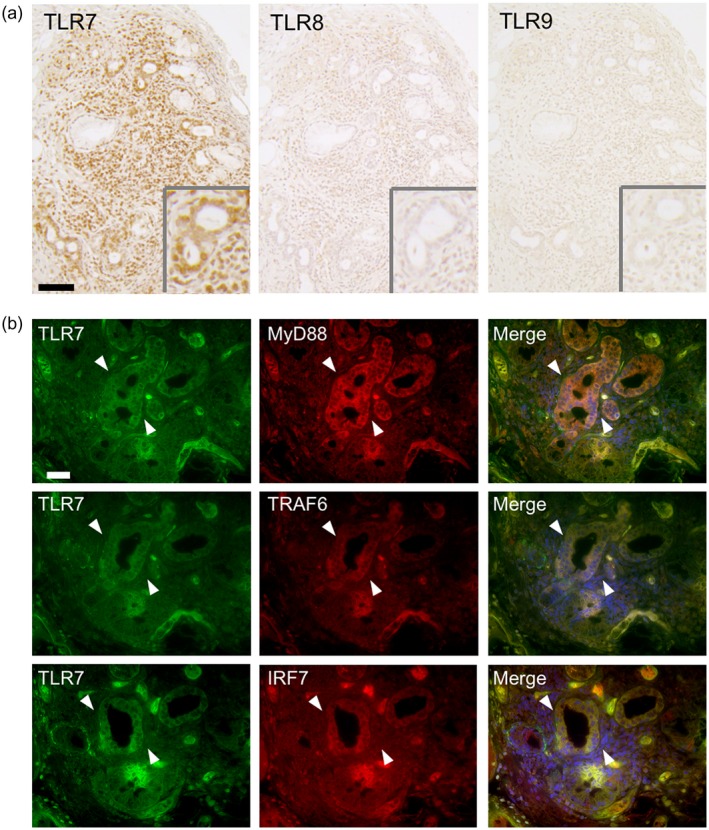
Expression of Toll‐like receptor (TLR)‐7 and its downstream molecules in ducts of labial salivary glands (LSGs) from the pSS patients. (a) Representative samples showing the expressions of TLR‐7–9 in LSGs from pSS patients. Insets: representative ductal expressions of TLR‐7–9. Bar: 80 μM. (b) Representative samples of LSGs from pSS patients (n = 5) stained with anti‐TLR‐7 (green), anti‐MyD88, anti‐TRAF6 and anti‐IRF7 antibody (red). Hoechst (blue) was used for counterstaining the nuclei. White arrowheads: the same ductal expression, using serial sections. Bar: 20 μM. IRF7 = interferon regulatory factor 7; MyD88 = myeloid differentiation primary response gene 88; TRAF6 = tumor necrosis factor receptor‐associated factor 6.
Figure 5.
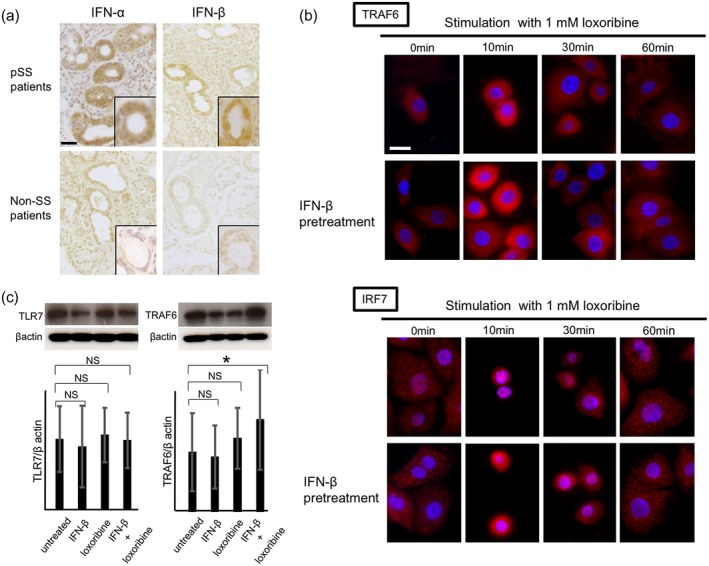
Expression of type I interferons (IFNs) in labial salivary glands (LSGs) and Toll‐like receptor (TLR)‐7 signal activation in salivary gland epithelial cells (SGECs). (a) Representative samples showing the expressions of IFN‐α and IFN‐β in LSGs from primary Sjögren's syndrome (pSS) patients (n = 4) and non‐SS patients (n = 4). Bar: 20 μM. Insets: representative expressions of IFN‐α and IFN‐β. (b) Representative images showing the expressions of TRAF6 and IRF7 (red) in SGECs from pSS patients (n = 3). Bar: 20 μM. (c) TLR‐7 and TRAF6 signal in SGECs from pSS patients (n = 5) stimulated with 1 mM loxoribine for 6 h and/or 1000 U/ml IFN‐β for 12 h analyzed by Western blotting. * P < 0·05 by analysis of variance (anova). Error bars: standard deviation; n.s. = not significant; IRF7 = interferon regulatory factor 7.
Activation of TLR‐7 signaling stimulated by TLR‐7 ligand in SGECs and PBMCs
We examined the expression of TLR‐7 signaling in SGECs after stimulation with TLR‐7 ligand, and we observed that TLR‐7 signaling was expressed in LSG ducts from the pSS patients. Our time–course experiments using immunofluorescence staining showed that TRAF6 expression was most strongly detected, and IRF7 expression was most aggregated around the nuclei, suggesting that IRF7 was translocated into the nucleus after stimulation with loxoribine for 10 min as well as by loxoribine for 10 min and IFN‐β for 12 h (Fig. 5b). In Western blotting, the expressions of TLR‐7 and TRAF6 were not changed by stimulation with loxoribine for 10–60 min and/or IFN‐β (data not shown). However, the TRAF6 expression was increased significantly by stimulation with IFN‐β coupled with loxoribine for 6 h. In contrast, the TLR‐7 expression was not changed by stimulation with IFN‐β and/or loxoribine for 6 h (Fig. 5c). IFN‐α was not detected in the supernatants of SGECs in the presence of IFN‐β and/or loxoribine by ELISA.
In contrast, the ELISAs showed that the levels of IFN‐α were increased after stimulation with loxoribine and IFN‐β in the supernatants of PBMCs from the pSS patients (Fig. 6a). Additionally, the flow cytometry revealed a significant increase in IFN‐α after stimulation with loxoribine and IFN‐β in TLR‐7+CD123/303+ pDCs from the pSS patients (Fig. 6b). ELISAs also showed a significant increase in IFN‐α in supernatants from the control subjects following stimulation with loxoribine and IFN‐β (data not shown).
Figure 6.
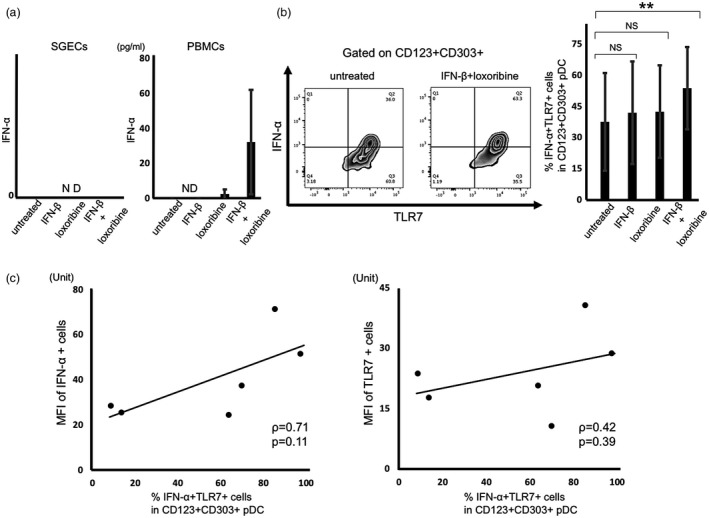
Toll‐like receptor (TLR)‐7 signal activation in salivary gland epithelial cells (SGECs) and peripheral blood mononuclear cells (PBMCs) from the primary Sjögren's syndrome (pSS) patients. (a) The levels of interferon (IFN)‐α in SGECs and PBMCs from pSS patients (n = 6) stimulated with 1 mM loxoribine for 6 h and/or 1000 U/ml IFN‐β for 12 h measured by enzyme‐linked immunosorbent assay (ELISA). (b) The expressions of IFN‐α and TLR‐7 in CD123+CD303+ pDCs from pSS patients (n = 6) stimulated with 1 mM loxoribine for 6 h and/or 1000 U/ml IFN‐β for 12 h, analyzed by flow cytometry. ** P < 0·01 by analysis of variance (anova). Error bars: standard deviation. (c) The correlation between the mean fluorescence intensity (MFI) of IFN‐α or TLR‐7 stains in mononuclear cells of LSGs and the percentage of IFN‐α+TLR‐7+ pDCs in flow cytometry from pSS patients (n = 6). The MFI of immunostaining was captured and calculated with a hybrid cell count system. The correlations were determined using Spearman's rank correlation coefficient. P‐values < 0·05 were considered significant; n.d. = not detected.
We also examined the correlation between the MFI of IFN‐α or TLR‐7 stains in mononuclear cells of LSGs revealed by immunofluorescence and the percentage of IFN‐α+ TLR‐7+ plasmacytoid dendritic cells among PBMCs after stimulation with loxoribine and IFN‐β, shown by flow cytometry from the same pSS patients. The results showed a tendency toward a positive correlation between IFN‐α or TLR‐7+ mononuclear cells of LSGs and IFN‐α+ TLR‐7+ plasmacytoid dendritic cells among PBMCs (Fig. 6c).
Apoptosis and activation of BAFF, Ro52 and MHC class I stimulated by TLR‐7 ligand in SGECs
We examined the induction of apoptosis and the activation of BAFF, Ro52 and MHC class I in SGECs after stimulation with a TLR‐7 ligand. TUNEL staining showed that apoptosis was not induced by stimulation with loxoribine and/or IFN‐β (Supporting information, Fig. S2a). Although neither apoptosis nor BAFF expression was induced by loxoribine and/or IFN‐β, both Ro52 and MHC class I were significantly up‐regulated with these stimulations in Western blotting (Supporting information, Fig. S2b–d).
Discussion
The findings revealed by our present analyses indicate that TLR‐7 and its downstream signaling are expressed strongly in ducts and MNCs (especially pDCs) of labial salivary glands from pSS patients in vivo, and we observed that TLR‐7 downstream molecules are expressed in SGECs from pSS patients after TLR‐7 ligand stimulation in vitro. A key contributor to the development of pSS is thought to be an adaptive immune response in which self‐reactive T cells recognize autoantigens such as M3 muscarinic acetylcholine receptor (M3R) 11 and alpha‐fodrin 12. The involvement of innate immune activity was recently reported to be related to the pathogenesis of many autoimmune diseases 13. TLR‐7–9 are representative pattern recognition receptors that recognize the nucleic acid sequences of viruses and self‐antigens, and these activities produce cytokines such as type I IFN or induce the activation of the nuclear factor kappa B (NF‐κB) pathway 5.
Previous studies have identified the over‐expression of type I IFN‐inducible genes such as IFN‐α‐inducible protein 27 (IFI27), IFN‐induced transmembrane protein 3 (IFITM3), IFN‐stimulated transcription factor 3 (ISGF3G) and IRF1 in the salivary glands and peripheral blood from pSS patients, suggesting that the type I IFN pathway is a key player in the pathogenesis of pSS 2, 3, 14. Our earlier investigation demonstrated the expression and function of TLR‐2–4 in patients with SS 15, and in the present study we focused on TLR‐7–9 from the viewpoint of receptors of nucleic acid sequences of viruses and/or self‐antigens.
Several studies showed that TLR‐7–9 are expressed in PBMCs from SS patients 6, 16, 17 and one study suggested that apoptosis induced TLR‐7 and ‐9 expressions in pDCs 18. However, the expression of TLR‐7–9 in salivary glands has not been well investigated. Zheng et al. reported that TLR‐7 and TLR‐9 were predominantly expressed in lymphocytes of the parotid glands from pSS patients compared to those of control subjects 6. In contrast, Maria et al. reported that TLR‐7 was expressed by few cells within the lymphocytic foci 16. Our present findings demonstrate that TLR‐7 was expressed more strongly than TLR‐9 in mononuclear cells of labial salivary glands. Because the expression of TLR‐7–9 was confirmed by positive control sections, the predominant expression of TLR‐7 in mononuclear cells was evident in our severely infiltrated samples from the pSS patients.
Fukui et al. reported that the multi‐transmembrane endoplasmic reticulum (ER)‐resident protein Unc93B1 predominantly up‐regulated TLR‐7 ligand‐induced activity due to a down‐regulation of TLR‐9 ligand‐induced activity 19. It was reported that single‐strand RNA (ssRNA) as a TLR‐7 ligand was recognized by TLR‐7 but not TLR‐8 and activated the transcription of TNF‐α in synovial tissues from rheumatoid arthritis patients, suggesting that the activation of TLR‐7 was more closely associated with inflammation compared to TLR‐8 activation 20. Similarly, TLR‐7‐mediated activation might predominantly occur in pDCs of labial salivary glands from pSS patients. Alveolar macrophages and dendritic cells produced interleukin (IL)‐33 induced by TLR‐7 activation in the respiratory tract of respiratory syncytial virus ssRNA‐infected mice 21. These findings strongly suggest the dominance of TLR‐7 with respect to the provocation of inflammation in LSGs.
Our present data demonstrated the strong expression of TLR‐7 in the ducts of labial salivary glands from pSS patients. We also evaluated the downstream signaling of TLR‐7 expressed in the ducts of labial salivary glands and TLR‐7‐mediated signal transduction in our in vitro experiments. Our findings suggest that the ducts as well as mononuclear cells are involved in TLR‐7‐mediated inflammation. Because TLR‐7 was expressed mainly in mononuclear cells (including pDCs and B cells), we speculate that the TLR‐7 signaling activation in the ducts is associated with mononuclear cells that expressed TLR‐7. However, the expression of TLR‐7 signaling in SGECs after stimulation with a TLR‐7 ligand in our in‐vitro analysis revealed that TLR‐7 signaling was activated in epithelial cells independently.
Another study showed that TLR‐7 was expressed in HSY cells (human salivary gland cells), whereas the levels of IL‐6 and IL‐8 in cultured supernatants did not increase after stimulation with ssPoly U as a TLR‐7 ligand 22. Spanchidou et al. reported that immunoregulatory molecules including intercellular adhesion molecule‐1, CD40 and MHC class I were up‐regulated after stimulation with TLR‐2–4 ligands in SGECs from pSS patients, suggesting that epithelial cells affected the role of the innate immune response 23. Additionally, an analysis of a murine model of SS revealed that epithelial cell apoptosis was essential for the development of inflammation 24. These data support the concept that epithelial cells have an important role in inflammation, including the activation of innate immunity.
Because it was reported that IFN‐β pretreatment with loxoribine led to an increased production of IFN‐α upon loxoribine stimulation in PBMCs from patients with multiple sclerosis 25, we analyzed SGECs and PBMCs stimulated with IFN‐β in addition to loxoribine. However, the level of IFN‐α in the cultured supernatant of SGECs was not detected by an ELISA. In contrast, the expression of IFN‐α in TLR‐7+CD123/303+ pDCs in cultured PBMCs from our pSS patients was significantly increased after stimulation with IFN‐β and loxoribine. In light of these findings, we suspected that the production of IFN‐α might depend upon a synergistic action of IFN‐β and TLR‐7 ligation. Eventually, we confirmed that compared with SGECs, the TLR‐7‐positive pDCs among PBMCs had a crucial role with respect to the IFN‐α production and secretion mechanism in pSS, considering that no IFN‐α was secreted by stimulated SGECs regardless of the positive expression of IFN‐α in the ducts of labial salivary glands. With regard to the secretion of soluble factors from SGECs in response to IFNs, there is a report 26 showing that the secretion of BAFF was up‐regulated by stimulation with IFNs such as IFN‐γ and IFN‐α. That report suggested that SGECs might have various functions to secrete some soluble factors in response to IFNs, although IFN‐α itself was not secreted into the culture medium.
As noted above, the increased type I IFN activity was observed in the salivary glands and peripheral bloods from pSS patients in previous studies. However, the relationship regarding TLR‐7 signaling between PBMCs and MNCs in salivary glands from pSS patients remains unclear. Our present results demonstrated only a tendency toward a positive correlation between IFN‐α+ or TLR‐7+ cells of LSGs and IFN‐α+ TLR‐7+ cells of PBMCs that were obtained from the same pSS patients, suggesting that the TLR‐7 signal activation was not always correlated between peripheral blood and salivary glands.
Our present findings demonstrated TLR‐7 expression in CD8+ T cells in labial salivary glands from the pSS patients. In general, TLR‐7 was weakly expressed in CD8+ T cells. Another study indicated that TLR‐7 signaling enhanced the cross‐priming of CD8+ T cells, suggesting that this signaling may have influenced the TLR‐7 expression itself 27.
An earlier investigation showed that apoptotic particles induced TLR‐7 and TLR‐9 expression in pDCs in vitro 18. Although our prior study demonstrated that TLR‐3 signal activation induced apoptosis in SGECs from individuals with pSS 9, it is not yet known whether stimulation of the TLR‐7 signaling pathway induces apoptosis. However, our present experiments demonstrated that TLR‐7 signal activation induced no apoptosis, regardless of type I IFN activation in SGECs from the pSS patients.
We also investigated the expression of relevant molecules including Ro52 and MHC class I (Supporting information, Fig. S2). As we observed a significant increase of Ro52 with loxoribine stimulation with IFN‐β, we speculated that a TLR‐7‐mediated augmentation of SS‐related antigen exists. Because Higgs et al. reported that TLR‐7 stimulation promoted the association of Ro52 with IRF7 28, TLR‐7 might have a pathogenic function with regard to the control of the expression of Ro52. In, addition, we observed that stimulation with loxoribine and IFN‐β augmented MHC class I expression in SGECs. Although it has been shown that another TLR‐7 agonist, i.e. imiquimod, weakly up‐regulated MHC class I in vaginal epithelial cells 29, our present findings might demonstrate a specific effect of TLR7 stimulation on SGECs regarding the association of endogenous antigen.
Several limitations of this study should be mentioned. First, we analyzed the expression of TLRs in mainly pDCs among mononuclear cells. In future analyses, we should analyze the expression of TLR‐7 in another cell type, such as B cells. Because TLR‐7 has the potential to induce autoantibody production through B cell activation, a TLR‐7‐mediated autoantibody production system in SS should be investigated in future studies. Secondly, a recent study showed that retinoic acid‐inducible gene‐I (RIG‐I) and melanoma differentiation‐associated gene‐5 (MDA5) (as innate sensors associated with the production of type I IFNs other than TLRs) were up‐regulated in pDCs and monocytes and highly expressed in mononuclear cells of LSGs from IFN‐positive pSS patients 13. Because it has been reported that the expressions of RIG‐I and MDA5 are induced by TLR‐7 signal activation, we should analyze these functions in LSGs in future studies. Because the up‐regulation of cyclic GMP‐AMP synthase (cGAS), a stimulator of interferon genes (STING) pathway, was shown in monocytes of patients with systemic lupus erythematosus 30, the pathogenic characteristics of these molecules in pSS should also be investigated. As a third limitation of the present study, we did not perform peripheral blood transcriptomics or gene expression analysis for the 11 pSS patients.
In conclusion, the results of our analyses showed that TLR‐7 signaling for type I IFNs is strongly expressed in ducts and mononuclear cells – especially the plasmacytoid dendritic cells of labial salivary glands – from pSS patients. Our findings suggest that TLR‐7‐mediated downstream signals might be activated due to stimulation with ssRNA. In addition, ductal epithelial cells as non‐immune cells as well as mononuclear cells, expressed TLR‐7, indicating that it is possible that ductal epithelial cells per se act as receptors of adventive microbes and/or endogenous antigens. To investigate these speculations, it is desirable to determine whether or not the direct activation of microbes and/or self‐antigens with ssRNA activates epithelial cells from patients with pSS. By elucidating these mechanisms, the role of TLR‐7 signaling activation in the pathogenesis of pSS will be clarified.
Disclosures
The authors declare that they have no conflicts of interest.
Author contributions
Study conception and design: H. N. Salivary gland biopsy: T. S., H. N., A. T., Y. H., S. K., T. M. and Y. N. Acquisition of data: T. S. and H. N. Analysis and interpretation of data: T. S., H. N., M. U. and A. K.
Supporting information
Fig. S1. Co‐localization of TLR8 and MNCs in LSGs from the pSS patients. Representative samples of LSGs stained with cell markers for T cells (anti‐CD4 and anti‐CD8 antibodies), B cells (anti‐CD20 antibody), plasma cells (anti‐VS38c antibody), macrophages (anti‐CD68 antibody), or pDCs (anti‐CD303 antibody) (green), and anti‐TLR8 antibody (red) from pSS patients (n = 2). Hoechst (blue) was used for counterstaining the nuclei. Insets: Representative staining for each panel. Bar: 40 μM.
Fig. S2. The apoptosis and expressions of BAFF, Ro52, and MHC class I in SGECs from the pSS patients. (a) Representative images showing the TUNEL staining (green) in SGECs from pSS patients (n = 3). Bar: 20 μM. Hoechst (blue) was used for counterstaining of the nuclei. (b) BAFF, (c) Ro52, (d) MHC class I signal in SGECs from pSS patients (n = 5) in which the SGECs were stimulated with 1 mM loxoribine for 6 hr and/or 1000 U/ml IFN‐β for 12 hr, analyzed by western blotting. ** P < 0·01 by ANOVA. Error bars: std. dev. BAFF: B‐cell activating factor, MHC: major histocompatibility complex.
Acknowledgements
This work was supported in part by JSPS KAKENHI (grant no. JP16K09899). We thank Ms Yoshiko Seike‐Takahashi and Ms Kaori Furukawa (Nagasaki University) for their excellent technical assistance and Dr Tomohiro Koga and Dr Kunihiro Ichinose (Nagasaki University) for collection of the patients' blood samples.
References
- 1. Ramos‐Casals M, Tzioufas AG, Font J. Primary Sjogren's syndrome: new clinical and therapeutic concepts. Ann Rheum Dis 2005;64:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kimoto O, Sawada J, Shimoyama K et al Activation of the interferon pathway in peripheral blood of patients with Sjogren's syndrome. J Rheumatol 2011;38:310–6. [DOI] [PubMed] [Google Scholar]
- 3. Vakaloglou KM, Mavragani CP. Activation of the type I interferon pathway in primary Sjogren's syndrome: an update. Curr Opin Rheumatol 2011;23:459–64. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura H, Kawakami A, Eguchi K. Mechanisms of autoantibody production and the relationship between autoantibodies and the clinical manifestations in Sjogren's syndrome. Transl Res 2006;148:281–8. [DOI] [PubMed] [Google Scholar]
- 5. Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 2008;8:594–606. [DOI] [PubMed] [Google Scholar]
- 6. Zheng L, Zhang Z, Yu C, Yang C. Expression of toll‐like receptors 7, 8, and 9 in primary Sjogren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:844–50. [DOI] [PubMed] [Google Scholar]
- 7. Vitali C, Bombardieri S, Jonsson R et al Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjogren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol 1974;37:217–29. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura H, Kawakami A, Ida H, Koji T, Eguchi K. EGF activates PI3K‐Akt and NF‐kappaB via distinct pathways in salivary epithelial cells in Sjogren's syndrome. Rheumatol Int 2007;28:127–36. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura H, Kawakami A, Iwamoto N, Ida H, Koji T, Eguchi K. Rapid and significant induction of TRAIL‐mediated type II cells in apoptosis of primary salivary epithelial cells in primary Sjogren's syndrome. Apoptosis 2008;13:1322–30. [DOI] [PubMed] [Google Scholar]
- 11. Sumida T, Tsuboi H, Iizuka M, Nakamura Y, Matsumoto I. Functional role of M3 muscarinic acetylcholine receptor (M3R) reactive T cells and anti‐M3R autoantibodies in patients with Sjogren's syndrome. Autoimmun Rev 2010;9:615–7. [DOI] [PubMed] [Google Scholar]
- 12. Haneji N, Nakamura T, Takio K et al Identification of alpha‐fodrin as a candidate autoantigen in primary Sjogren's syndrome. Science (NY) 1997;276:604–7. [DOI] [PubMed] [Google Scholar]
- 13. Chen JQ, Szodoray P, Zeher M. Toll‐like receptor pathways in autoimmune diseases. Clin Rev Allergy Immunol 2016;50:1–17. [DOI] [PubMed] [Google Scholar]
- 14. Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum 2005;52:1534–44. [DOI] [PubMed] [Google Scholar]
- 15. Kawakami A, Nakashima K, Tamai M et al Toll‐like receptor in salivary glands from patients with Sjogren's syndrome: functional analysis by human salivary gland cell line. J Rheumatol 2007;34:1019–26. [PubMed] [Google Scholar]
- 16. Maria NI, Steenwijk EC, AS IJ et al Contrasting expression pattern of RNA‐sensing receptors TLR7, RIG‐I and MDA5 in interferon‐positive and interferon‐negative patients with primary Sjogren's syndrome. Ann Rheum Dis 2016;6:721–73. [DOI] [PubMed] [Google Scholar]
- 17. Karlsen M, Jakobsen K, Jonsson R, Hammenfors D, Hansen T, Appel S. Expression of toll‐like receptors in peripheral blood mononuclear cells of patients with primary Sjogren's syndrome. Scand J Immunol 2016;85:220–6. [DOI] [PubMed] [Google Scholar]
- 18. Ainola M, Porola P, Takakubo Y et al Activation of plasmacytoid dendritic cells by apoptotic particles – mechanism for the loss of immunological tolerance in Sjögren's syndrome. Clin Exp Immunol 2018;191:301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukui R, Saitoh S, Matsumoto F et al Unc93B1 biases toll‐like receptor responses to nucleic acid in dendritic cells toward DNA‐ but against RNA‐sensing. J Exp Med 2009;206:1339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chamberlain ND, Kim SJ, Vila OM et al Ligation of TLR7 by rheumatoid arthritis synovial fluid single strand RNA induces transcription of TNFalpha in monocytes. Ann Rheum Dis 2013;72:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qi F, Wang D, Liu J et al Respiratory macrophages and dendritic cells mediate respiratory syncytial virus‐induced IL‐33 production in TLR3‐ or TLR7‐dependent manner. Int Immunopharmacol 2015;29:408–15. [DOI] [PubMed] [Google Scholar]
- 22. Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional toll‐like receptors, NOD1 and NOD2 to produce anti‐microbial peptides, but not proinflammatory cytokines. Mol Immunol 2007;44:3100–11. [DOI] [PubMed] [Google Scholar]
- 23. Spachidou MP, Bourazopoulou E, Maratheftis CI et al Expression of functional toll‐like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjogren's syndrome. Clin Exp Immunol 2007;147:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okuma A, Hoshino K, Ohba T et al Enhanced apoptosis by disruption of the STAT3‐IkappaB‐zeta signaling pathway in epithelial cells induces Sjogren's syndrome‐like autoimmune disease. Immunity 2013;38:450–60. [DOI] [PubMed] [Google Scholar]
- 25. Derkow K, Bauer JM, Hecker M et al Multiple sclerosis: modulation of toll‐like receptor (TLR) expression by interferon‐beta includes upregulation of TLR7 in plasmacytoid dendritic cells. PLoS ONE 2013;8:e70626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ittah M, Miceli‐Richard C, Eric Gottenberg J et al B cell‐activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren's syndrome. Arthritis Res Ther 2006;8:R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei J, Waithman J, Lata R et al Influenza A infection enhances cross‐priming of CD8+ T cells to cell‐associated antigens in a TLR7‐ and type I IFN‐dependent fashion. J Immunol 2010;185:6013–22. [DOI] [PubMed] [Google Scholar]
- 28. Unutmaz D, Higgs R, Lazzari E et al Self protection from anti‐viral responses – Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral toll‐like receptors. PLOS ONE 2010;5:e11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nurkkala M, Nordstrom I, Telemo E, Eriksson K. MHC expression and chemokine production in the murine vagina following intra‐vaginal administration of ligands to toll‐like receptors 3, 7 and 9. J Reprod Immunol 2007;73:148–57. [DOI] [PubMed] [Google Scholar]
- 30. Wang J, Dai M, Cui Y et al Elevated IFIT3 contributes to abnormal overactive cGAS‐STING signaling in human systemic lupus erythematosus monocytes. Arthritis Rheumatol 2018. Epub. 10.1002/art.40576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Co‐localization of TLR8 and MNCs in LSGs from the pSS patients. Representative samples of LSGs stained with cell markers for T cells (anti‐CD4 and anti‐CD8 antibodies), B cells (anti‐CD20 antibody), plasma cells (anti‐VS38c antibody), macrophages (anti‐CD68 antibody), or pDCs (anti‐CD303 antibody) (green), and anti‐TLR8 antibody (red) from pSS patients (n = 2). Hoechst (blue) was used for counterstaining the nuclei. Insets: Representative staining for each panel. Bar: 40 μM.
Fig. S2. The apoptosis and expressions of BAFF, Ro52, and MHC class I in SGECs from the pSS patients. (a) Representative images showing the TUNEL staining (green) in SGECs from pSS patients (n = 3). Bar: 20 μM. Hoechst (blue) was used for counterstaining of the nuclei. (b) BAFF, (c) Ro52, (d) MHC class I signal in SGECs from pSS patients (n = 5) in which the SGECs were stimulated with 1 mM loxoribine for 6 hr and/or 1000 U/ml IFN‐β for 12 hr, analyzed by western blotting. ** P < 0·01 by ANOVA. Error bars: std. dev. BAFF: B‐cell activating factor, MHC: major histocompatibility complex.


