Summary
Merozoite surface proteins (MSPs) are critical for parasite invasion; they represent attractive targets for antibody‐based protection against clinical malaria. To identify protection‐associated target MSPs, the present study analysed antibody responses to whole merozoite extract (ME) and to defined MSP recombinant antigens in hospitalized patients from a low endemic urban area as a function of disease severity (mild versus cerebral malaria). Sera from 110 patients with confirmed severe cerebral malaria (CM) and 91 patients with mild malaria (MM) were analysed (mean age = 29 years) for total and subclass immunoglobulin (Ig)G to ME and total IgG to MSP1p19, MSP2, MSP3, MSP4 and MSP5 by enzyme‐linked immunosorbent assay (ELISA). Functional antibody responses were evaluated using the antibody‐dependent respiratory burst (ADRB) assay in a subset of sera. There was a trend towards higher IgG1 and IgG4 levels to ME in CM compared to MM; only ME IgM responses differed significantly between fatal and surviving CM patients. Increased prevalence of IgG to individual MSPs was found in the CM compared to the MM group, including significantly higher levels of IgG to MSP4 and MSP5 in the former. Sera from fatal (24·5%) versus surviving cases showed significantly lower IgG to MSP1p19 and MSP3 (P < 0·05). ADRB assay readouts correlated with high levels of anti‐MSP IgG, and trended higher in sera from patients with surviving compared to fatal CM outcome (P = 0·07). These results document strong differential antibody responses to MSP antigens as targets of protective immunity against CM and in particular MSP1p19 and MSP3 as prognostic indicators.
Keywords: antibodies, cerebral malaria, IgG subclasses, merozoite surface protein
Introduction
In the last 15 years, the malaria case burden has fallen from 262 to 219 million in 2017, a decline of 16·4%. Scaled‐up control measures have contributed to a 48% decline in fatalities from an estimated 839 000 to 435 000 from 2000 to 2017, including a 63% decrease for children aged under 5 years 1. However, this progress in many areas of Africa is threatened by parasite drug‐resistance and anopheles vector insecticide resistance 2, 3, 4, 5, 6. In addition, reduced exposure to the parasite results in waning natural immunity, increasing the risk of severe clinical outcomes in populations from endemic areas 7. New tools are needed to achieve the objectives of sustained control and elimination, including good prognostic markers of clinical outcome and identification of critical parasite antigenic targets for the development of efficient vaccines.
The protective role of antibodies against blood stage malaria has been clearly demonstrated using passive transfer immunization. Antibodies from hyperimmune African adults to Plasmodium falciparum patients were able to clear high levels of circulating parasites independently of their geographical origin 8, 9, 10. Merozoite surface proteins (MSPs) are considered attractive protective antigen candidates because they are exposed to circulating antibodies, and several appear to be critical for erythrocyte invasion 11.
In this study, the main goal was to identify promising protective MSP antigens by analysing antibody responses to a set of MSP antigens in individuals hospitalized for clinical malaria and living in a low endemic urban area. For this purpose, a 2‐year consecutive transversal recruitment was carried out at the military hospital of Dakar. A total of 110 patients with confirmed severe cerebral malaria (CM) hospitalized in the intensive care unit and 91 patients with mild malaria (MM) in the infectious disease unit of the Hôpital Principal of Dakar were included in the study.
Several studies have shown that natural antibody responses to MSPs are associated with clinical protection 12, 13, 14. This study focused on measuring antibody responses to several MSPs previously identified as potential vaccine candidates, including MSP1p19, MSP2, MSP3, MSP4 and MSP5. In addition, immunoglobulin (Ig)G and IgG subclass responses to whole merozoite extract (ME) and the functional antibody‐dependent respiratory burst (ADRB) assay were measured in a subset of sera.
The results obtained in this study implicate a protective role for antibodies to some of these antigens as a function of disease severity and fatal outcome.
Materials and methods
Study area and epidemiological context
The study was conducted in Dakar, which is characterized by seasonal low‐level transmission estimated at 0·5 to one infectious bites/person/year. The main vector is Anopheles arabiensis, and 98% of cases are due to P. falciparum 15, 16. Recent studies show that malaria transmission in this setting is highly unstable, with a marked heterogeneity of exposure depending on location (0·1 to nearly 250 bites per person per night during the rainy season), resulting in an entomological infection rate (EIR) of three to nine infective bites per individual per year during the rainy season in some locations 15, 17. A mean clinical incidence of 2·4% was observed at the time of the 2008 study, with no differences between adults and children 15. In 2015 the incidence of malaria in the region of Dakar was 0·85% 18 with a total of 23 431 confirmed clinical cases, including 2612 severe cases and 31 deaths (eight in < 5‐year‐old children).
Study design, ethics statements and procedures
The study was performed at the Hôpital Principal de Dakar, with two successive recruitments from September to December 2003 and 2004. Informed consent was obtained from each participant and/or their relatives prior to inclusion, after providing written or verbal information in their native language. The protocols were approved by the investigators' institutions, the National Ethic Committee and the Ministry of Health of Senegal.
The study involved 110 CM patients admitted to the intensive care unit for unarousable coma (Glasgow score < 9) with diagnosed severe P. falciparum infection according to World Health Organization criteria 19. The same medical staff managed all patients according to hospital treatment protocols based on national recommendations, and all patients with other infections were excluded as previously described 20, 21.
A group of 91 MM cases was enrolled as control in the infectious disease unit during the same time‐period. They were defined as having a positive blood smear for P. falciparum with fever and no symptoms of severe malaria, as previously reported 20, 22.
On the day of admission, biological parameters were determined by the hospital's clinical laboratory. Blood samples obtained after clinical biology processing were centrifuged, and the plasma was aliquoted and stored at −80oC until testing.
Antigens and enzyme‐linked immunosorbent assay (ELISA) procedure
The ME was prepared from synchronous FCR3 parasites cultivated on O + erythrocytes and 10% human serum in candle jars, as described elsewhere 21, 23. The extract was used to coat MaxiSorp plates (Nunc, Roskilde, Denmark) at an optimal dilution of 1 : 2000 after serial dilution for calibration.
MSP1p19 (Palo Alto allele), MSP4 (NF54 allele) recombinant proteins with varying ecto‐domain content and MSP5 (NF54 allele) were produced in Spodoptera frugiperda (Sf9) or Trichoplusia ni (High Five; Invitrogen, Carlsbad, CA, USA) insect cells infected with the recombinant baculovirus, and purified by metalloaffinity chromatography 24, 25.
Three MSP4 constructs were used: (i) the entire MSP4 ecto‐domain (MSP4p40); (ii) a deleted version lacking 30 amino acids (Gly‐45 to Asp‐74) in the variable region (MSP4p30); and (iii) the conserved, protease resistant C‐terminal half of 109 residues beginning at Lys‐132 (MSP4p20) 25. The baculovirus expression system has been shown to ensure optimal reproduction of conformational epitopes, including epidermal growth factor (EGF) domains 26. The MSP2 antigen was derived from the 3D7 serogroup A construct (46 kDa) and expressed in Escherichia coli 27 (kind gift from Dr Migot Nabias). The MSP3 antigen (clone T9/96) was an E. coli‐expressed DG‐210 purified protein 28 (kind gift from Dr C. Oeuvray).
Recombinant antigens were diluted in sterile phosphate‐buffered saline (PBS) to coat Immulon‐4 plates at a concentration of 0·5 µg ml–1. IgG/IgM responses were quantified by ELISA in duplicate plasma samples diluted 1 : 100, as previously described 21, 23, 29. IgG subclasses were determined using human subclass‐specific mouse monoclonal antibodies (mAbs) and peroxidase‐labelled goat anti‐mouse IgG (1 : 2000) from Sigma® Chemicals (St Louis, MO, USA) after calibration for optimal concentrations 29, 30, 31. Standards were included in each assay for plate comparability. Positive control was a pool of 25 sera from clinically immune adults living in Dielmo, a holoendemic village in Senegal; negative controls were a pool of non‐immune European and a pool of African non‐immune sera (individuals from Dakar selected for negativity against P. falciparum whole schizont extract antigen). Results were expressed as optical density (OD) ratio = OD sample/OD naive serum pool. Sera showing an OD ratio > 2 corresponding to the signal of naive controls + 3 standard deviations (s.d.) were considered seropositive for prevalence calculations 21, 23, 29, 32.
Measure of the ADRB activity
The ADRB activity was measured as described previously 33, 34. Briefly, polymorphonuclear (PMN) cells (pooled from six to seven healthy donors) were washed and suspended in PBS at 1–5 × 107 cells ml–1. Merozoites were incubated with 10 µl of test or control sera for 30 min at 37°C before addition of PMN (100 µl at a final ratio : effector/target = 10) and isoluminol before reading with a luminometer. Results were expressed as ADRB index = (rlu maximum sample/rlu maximum HIS) × 1000, where rlu maximum of hyperimmune serum (HIS) was an average of two measures 33.
Statistical analysis
Antibody levels and prevalence of responders in different groups were compared using the Mann–Whitney signed‐rank test, the Spearman's rank correlation test for non‐normally distributed paired data and Fisher's exact test. P‐values < 0·05 were considered significant.
To study associations between disease severity and antibody responses, coded gravity criteria were included in a generalized linear model adjusted for age to analyse gravity as a function of antibody responses to different targets.
Statistical analyses were performed with r and Statview version 5.0 (SAS Institute, Cary, NC, USA) software.
Results
Clinic and biological characteristics of the cohort of malaria patients
Study cohorts included 110 CM and 91 MM cases with no significant differences in age distribution. Characteristics and biological data for the two groups are summarized in Table 1. More than half the patients had received treatment with anti‐malarial before hospitalization, with a significantly higher proportion of CM patients (71 versus 48%; P = 0·004, Fisher's exact test).
Table 1.
Characteristics of the patient cohorts
| Patients | No. | Age1 | %H/F2 | TTt3 | Hb1 | ht1 | Thr1 | creat1 | bil1 | Glas1 | Pos4 | %pE1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All severe | 110 | 29·1 | 60% | 71% | 10·3 | 31 | 137 | 21 | 41 | 11 | 91% | 3·31 |
| MM | 91 | 29·1 | 45% | 48% | 11·1 | 36 | 227 | n.a. | n.a. | n.a. | 100% | 2·20 |
| Range | (1–77) | 7–16·3 | 24–49 | 137–512 | (0·01–16) | |||||||
| Comp. CM versus MM5 P = | n.s. | 0·01 | < 10–3 | < 10–3 | ||||||||
| surviving | 83 | 27·7 | 64% | 71% | 10·4 | 32 | 160 | 18 | 39 | 11 | 90% | 2·86 |
| Range | 8–73 | 2·1–15·9 | 10–46 | 11–800 | 6–71 | 2–145 | 6–15 | (0·01–13·1) | ||||
| fatal cases | 27 | 33·3 | 48% | 67% | 9·8 | 29 | 114 | 32 | 55 | 10 | 93% | 4·21 |
| Range | (11–74) | 7–13·4 | 20–40 | 9–467 | 5–80 | 5–240 | 4–15 | (0·01–9·9) | ||||
| Comp. surv versus fatal5 P = | 0·07 | 0·4 | 0·7 | 0·004 | 0·047 | n.a. | n.a. | |||||
Mean and range of values: haemoglobin (Hb gl–1); haematocrit (%); Thr = thrombocytes (×103 ml–1); creat = creatinine (mg ml–1); bil = total bilirubin (mg ml–1); Glasgow score (Glas); % of parasitized erythrocytes (%pE); CM = cerebral malaria; MM = mild malaria; n.s. = not significant.
Percentage of males.
Percentage of individuals who declared treatment before hospitalization.
Percentage of individuals with positive blood smear at recruitment.
Comparison of biological results (Mann–Whitney test).
Within the CM group, 25% had a fatal outcome. A large proportion of these patients were anaemic (43% with haematocrit < 30) with thrombocytopenia (50% with < 80 × 103 ml–1). However, anaemia and thrombocytopenia were not significantly different between surviving versus fatal cases. Marked signs of renal impairment were significantly associated with patients having a fatal outcome: 55% of patients had blood creatinine levels > 18 mg ml–1 compared to 20% in recovering individuals (see Table 1). Conversely, in MM cases, anaemia (haematocrit < 30) was less frequent and no thrombocytopenia < 80 × 103 ml–1 was observed.
Profile of IgG and IgG subclass responses to ME
IgG, IgM and IgG subclass levels and prevalence against ME are shown in Table 2 and illustrated as a box‐plot in Fig. 1. Comparison between MM and CM groups showed a trend for higher levels of antibody responses in CM compared to MM. Significantly higher levels and prevalence were found for total IgG, IgG1 and IgG4 to ME (Table 2, Fig.1a). CM antibody responses to ME generally trended to lower levels in fatal cases compared to survivors, and differences were significant for IgG3 (prevalence) and IgM (prevalence and levels) (Table 2, Fig.1b).
Table 2.
Levels and prevalence of antibody responses against merozoite extract in hospitalized patients
| Patients | No. | ME IgG1 | Max1 | Prev2 | IgG1 | Max | Prev | IgG2 | Max | Prev | IgG3 | Max | Prev | IgG4 | Max | Prev | ME IgM | Max | Prev |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All severe | 110 | 3·9 | 9·1 | 65% | 5·7 | 14·6 | 68% | 2·7 | 25·7 | 45% | 4·6 | 29·3 | 65% | 3·0 | 13·2 | 52% | 1·7 | 5·9 | 25% |
| MM | 91 | 2·9 | 9·8 | 51% | 4·4 | 13·6 | 54% | 2·7 | 11·4 | 42% | 4·4 | 26·7 | 54% | 2·2 | 21·3 | 36% | 1·8 | 7·8 | 27% |
| Comp. CM versus MM3 P = | < 10–2 | 0·04 | < 10–2 | 0·04 | 0·85 | 0·77 | 0·3 | 0·11 | < 10–2 | < 10–3 | 0·56 | 0·75 | |||||||
| Survival | 83 | 4·0 | 9·1 | 69% | 5·9 | 14·6 | 72% | 2·8 | 25·7 | 47% | 4·7 | 15·6 | 72% | 3·1 | 13·2 | 55% | 1·9 | 5·9 | 31% |
| Fatal cases | 27 | 3·4 | 7·8 | 56% | 5·1 | 12·0 | 63% | 2·3 | 7·1 | 37% | 4·1 | 29·3 | 44% | 2·5 | 6·8 | 41% | 1·2 | 2·5 | 7% |
| Comp. surv versus fatal4 P = | 0·23 | 0·24 | 0·36 | n.s. | 0·26 | 0·38 | 0·08 | 0·01 | 0·49 | 0·24 | < 10–3 | 0·01 | |||||||
Mean and maximum (Max) levels antibody responses to merozoite antigen expressed in optical density (OD) ratio.
Prevalence (Prev) of positive antibody responses, i.e. individuals with OD ratio > 2.
Comparison of antibody levels (Mann–Whitney test) and prevalence of responders (Fisher's exact test). ME = merozoite extract; Ig = immunoglobulin; CM = cerebral malaria; MM = mild malaria; n.s. = not significant.
Figure 1.
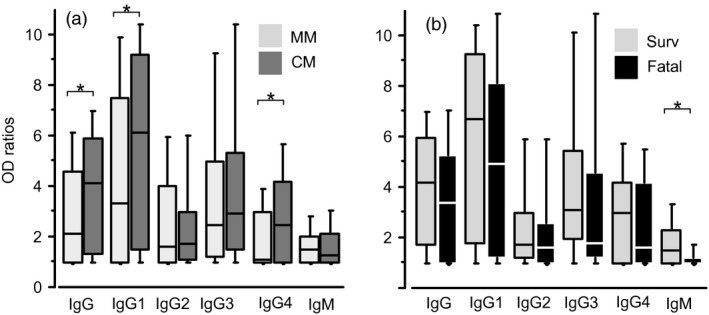
Antibody responses of patients to whole merozoite extract. Immunoglobulin (Ig)G, isotype‐specific IgG and IgM responses against whole merozoite extract in mild malaria (MM) and cerebral malaria (CM) patients are shown as box‐and‐whisker plots, representing the median with 25th and 75th percentile (boxes) and 10th and 90th percentiles (whiskers). Comparison of antibody responses in MM (light grey) versus CM (dark grey) and surviving (light grey) versus fatal (black) CM cases are shown in (a) and (b), respectively. Brackets with asterisk indicate significant different levels of IgG responses (P < 0·05).
Antibody responses did not correlate with age of individuals. There was a strong and significant association (P < 10–3) between total IgG levels and IgG subclass responses (rho from 0·66 to 0·97) for CM and MM. A graphical display of the correlation matrix for CM patients is shown in Fig. 2a (corrplot method from r).
Figure 2.
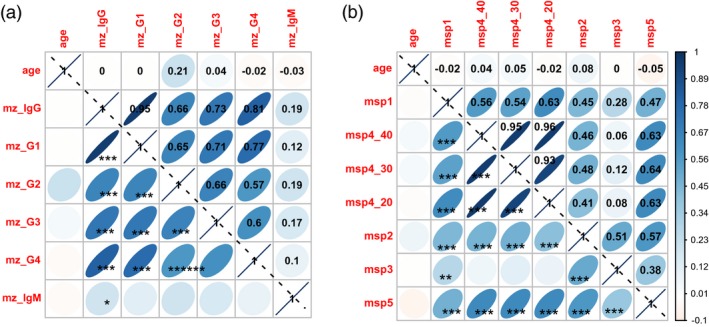
Intercorrelation display of antibody responses to ME and merozoite surface proteins (MSP) for cerebral malaria (CM) cases. The graphical displays of correlation matrix are shown for (a) immunoglobulin (Ig)G, IgG subclasses and IgM responses against whole merozoite extract, and (b) MSPs in CM patients. Positive correlations are displayed in blue and negative correlations in red. Colour intensity and the size of the ellipse are proportional to the correlation coefficients stated in each square from the upper triangular display. In the lower triangular display, P‐values are showed as asterisks (*P < 0·05; **P < 10–1; ***P < 10–3). Visualization is from the corrplot method of r.
Linear regression analysis showed that IgG responses were mainly of IgG1 subclass (estimates approximately 0·6, P < 10–4). There was a low but significant contribution of IgG3 and IgG4 in MM, and IgG2 and IgG4 in CM cases (estimates < 0·1, P < 10–2).
Profile of IgG responses to merozoite recombinant antigens
The magnitude and the prevalence of antibody responses against the seven MSP recombinant antigens analysed here are shown in Table 3 and comparisons of IgG levels are illustrated in Fig. 3.
Table 3.
Levels and prevalence of antibody responses against MSPs
| Patients | No. | MSP1p19 | MSP4p40 | MSP4p30 | MSP4p20 | MSP2 | MSP3 | MSP5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean1 | Prev2 | Mean | Prev | Mean | Prev | Mean | Prev | Mean | Prev | Mean | Prev | Mean | Prev | |||
| All severe | 110 | 7·8 | 81% | 6·1 | 65% | 8·4 | 73% | 5·5 | 67% | 3·3 | 59% | 2·0 | 26% | 3·7 | 66% | |
| MM | 91 | 6·5 | 53% | 4·9 | 45% | 4·5 | 40% | 4·0 | 41% | 3·0 | 32% | 2·0 | 18% | 2·6 | 33% | |
| Comp. CM versus MM3 P = | 0.16 | < 10–3 | 0·12 | < 10–2 | < 10–3 | < 10–4 | 0·01 | < 10–3 | 0·13 | < 10–4 | 0·4 | 0·17 | < 10–3 | < 10–4 | ||
| survival | 83 | 8·3 | 87% | 6·4 | 70% | 8·7 | 77% | 5·8 | 71% | 3·4 | 64% | 2·2 | 33% | 3·8 | 71% | |
| fatal cases | 27 | 6·2 | 63% | 5·3 | 52% | 7·4 | 59% | 4·5 | 56% | 2·7 | 44% | 1·6 | 7% | 3·6 | 52% | |
| Comp. surv versus fatal3 P = | 0.05 | 0·01 | 0·23 | 0·93 | 0·24 | 0·08 | 0·1 | 0·16 | 0·09 | 0·11 | 0·01 | 0·01 | 0·89 | 0·09 | ||
Mean and maximum (Max) levels antibody responses to merozoite antigen expressed in optical density (OD) ratio.
Prevalence (Prev) of positive antibody responses, i.e. individuals with OD ratio > 2.
Comparison of antibody levels (Mann–Whitney test) and prevalence of responders (Fisher's exact test). MSP = merozoite surface proteins; CM = cerebral malaria; MM = mild malaria.
Figure 3.
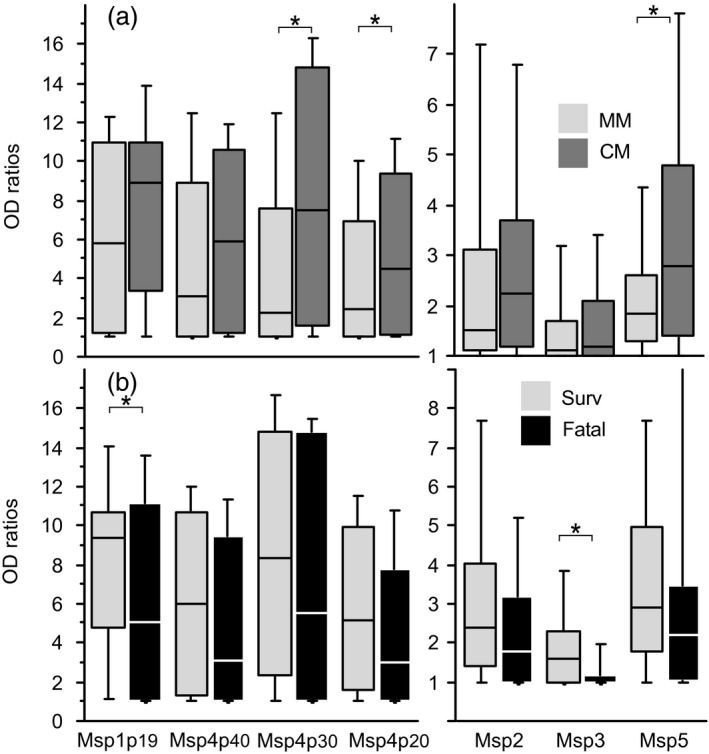
Antibody responses of patients to recombinant merozoite surface proteins. Levels of immunoglobulin (Ig)G antibody responses against merozoite surface proteins (MSP)1, MSP4, MSP2, MSP3 and MSP5 in MM and cerebral malaria (CM) patients are shown as box‐and whisker plots, representing median with 25th and 75th percentile (boxes) and 10th and 90th percentiles (whiskers). Comparison of antibody responses in MM (light grey) versus CM (dark grey) is shown in (a). Comparison of surviving (light grey) versus fatal (black) CM cases is shown in (b). Brackets with asterisk indicate significant different levels of IgG responses (P < 0·05).
When comparing MM to CM, the prevalence of responses to almost all antigens, except MSP3, was significantly lower in MM (Table 3, P < 10–2, Fisher's exact test). Antibody levels all trended lower in MM and were significant for MSP4p30, MSP4p20 and MSP5 (Fig. 3a).
Differences in antibody responses between surviving and fatal CM cases were much less pronounced, although general trends towards lower prevalence and lower levels in fatal outcome cases (Table 3, Fig.3b) were noted which were significant for MSP1p19 and MSP3 (P < 0·05, Fisher's exact test and Mann–Whitney test).
No correlation with age of patients was found for ME antigen. There was a variable interrelation between IgG levels to the different MSP (rho from 0·06 to 0·97) for CM and MM. A graphical display of the correlation matrix for CM patients is shown in Fig. 2b (corrplot method from r). The interrelation was significant for IgG to MSP1 and to all other MSP, including MSP3 (P < 10–2), with a weaker coefficient of correlation r = 0·28. IgG to MSP3 showed no significant correlation with MSP4.
Relationship between ADRB, antibody responses and outcome in severe malaria
The ADRB activity was measured in a representative subset of 57 sera (32 male/25 female; mean age = 28·5; range = 8–75), including 24 fatal cases. There was no difference in the age distribution between the whole cohort and the subset of sera tested for ADRB.
A mean level of 353 ADRB index units was found, ranging from 25 to 1588 (median = 257). Mean ADRB activities in fatal cases were lower than for survivors, with the 271 versus 413 difference closely approaching significance (P = 0.07; Mann–Whitney test). The prevalence of high ADRB responders (above median value) was higher in surviving (61%) than in fatal cases (33%), also approaching significance (P = 0·06, Fisher's exact test).
There was a significant correlation between ADRB activity and total IgG and IgG subclass responses to ME (P < 10–2, rho from 0·3 to 0·7). ADRB activity also correlated with antibody levels to all MSP antigens except MSP3 (P < 10–3, rho from 0·68 to 0·4; MSP4p20> MSP4p30> MSP4p40> MSp1p19> MSP5> MSP2).
Association between levels of antibody responses to the different antigens and ADRB are illustrated in Fig. 4. There was significantly higher ADRB activity in individuals with high levels to all antigens, except for IgG2 against ME and MSP3 (P < 0·05, Mann–Whitney test).
Figure 4.
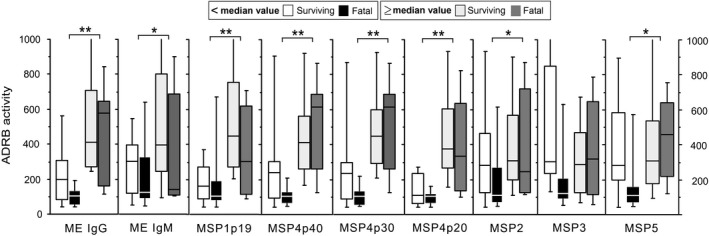
Relationship between antibody‐dependent respiratory burst (ADRB) and antibody responses in cerebral malaria cases. Immunoglobulin (Ig)G antibody responses against merozoite extract (ME), merozoite surface proteins (MSP)1, MSP4, MSP2, MSP3 and MSP5 and IgM response to ME are shown as box‐whisker plots, representing median with 25th and 75th percentile (boxes) and 10th and 90th percentiles (whiskers). ADRB levels in cerebral malaria (CM) are shown (i) as function of outcome: surviving (empty and light grey) versus fatal (black and dark grey) and (ii) as function of low versus high level of antibody responses (left versus right boxes = under or above median level of responses). Brackets with asterisk indicate significant different levels of IgG responses (**P < 0·01; *P < 0·05).
For high responders to all antigens, there was no significant difference in ADRB activity depending on surviving versus fatal issue. For low responders, responses to MSP3 and MSP5 showed significantly lower ADRB activity in fatal cases compared to survivors (P < 0·05, Mann–Whitney test).
Discussion
This study investigated anti‐P. falciparum IgG and IgM responses to whole ME and IgG responses to a set of recombinant MSPs as a function of disease severity in patients with mild and severe malaria, including surviving versus fatal outcomes in the latter. Patients were living in an urban area of low endemicity 15 and showed acute symptoms of illness requiring hospitalization.
It is commonly admitted that certain types of antibodies mediate protection against clinical symptoms and severe illness depending upon antigen recognition and IgG subclasses 10, 28, 35, 36, 37, 38, 39. In this study, IgG responses to whole ME showed significantly lower responses in MM compared to CM patients, differing from previous observations in Senegalese patients 40. Using a similar parasite extract, higher antibody responses were also reported in MM compared to CM for children aged under 5 years 38 and for older individuals in India 41.
No correlation was found between with age and antibody responses on the day of admission, as already observed in urban malaria 21. Such characteristics may be related to the symptomatic status of the patients and their variable and high parasite load when recruited and to their low immune background from an urban low endemic site.
Regarding IgG subclass responses, variable profiles of responses were observed depending upon clinical expressions of malaria 36. With regression analysis and data from Fig. 2a, we found a preferential contribution of IgG1 and IgG3 to whole IgG responses. These cytophilic subclasses are considered to be particularly important for protection against P. falciparum, as active defence mechanisms require cooperation between antibodies and cellular immune effectors such monocytes or polymorphonuclear neutrophils 8, 10, 28, 33, 35, 36, 37, 38, 39, 42. Here, two interesting points were underlined regarding differential contributions of class and subclass antibody responses to ME.
First, IgG4 responses were significantly higher in CM compared to MM 36 indicative of an inflammatory process possibly related to the well‐known association with IgE‐mediated allergy 43. In the complex antibody response to P. falciparum antigens, an allergic‐type inflammatory response may contribute to disease severity 44 as observed in individuals with subsequent IgE responses 45. In addition, excess IgG4 can compete with IgG1 and IgG3 for FcR binding, thus inhibiting opsonizing activity implicated in cell‐mediated protection mechanisms 10, 46.
Secondly, IgG3 and IgM responses did not differ between MM and CM in this study as opposed to higher levels associated with MM in other studies 36, 45. There was a trend towards lower levels of IgG3 against ME in surviving CM compared to fatal outcome, contrary to previously reported observations in the same setting 47, a study involving a lower number of individuals (n = 31) where IgG3 levels in fatal CM were markedly lower than in surviving patients.
It is important to note a significantly reduced IgM response in CM patients with fatal outcome compared to survivors. Such a difference can be interpreted as a lower individual capacity of response against parasite invasion. Importantly, the invasiveness and multiplication rates and of P. falciparum parasites from adult Thai patients was shown to be threefold higher in CM than MM cases 48 and could be imputed in part to the highly polymorphic parasites responsible for inducing CM 49. Thus, effective resistance by the host against emerging new parasite antigenic phenotypes requires a rapid, efficient antibody response against protection‐associated antigens, especially those with relatively conserved epitopes. The reason why such active responses associated with robust IgM production were not detected here in patients with fatal outcomes is unknown.
The IgG responses to the individual MSP targets in this study reflect the profiles found with ME antigen, i.e. a higher prevalence of antibody responses in CM compared to MM (except MSP3), together with a significantly higher level of antibodies to MSP4 and MSP5. Similar profiles have been reported for MSP1p19 and conserved epitopes of MSP2 antigens: in children aged under 5·5 years, IgG levels were found to increase significantly from severe anaemia to uncomplicated malaria and to severe malaria 35. Inversely, higher levels of IgG to MSP1 and MSP2 in MM compared to CM were observed in India in older children 41 and in all age groups in Sudan 50. In Sudanese children aged over 5 years, a lower prevalence of IgG to MSP2 and MSP3 was found in MM, but levels of antibodies were comparable 51. In this study, the antibody response data to MSP1p19 is in agreement with previous observations, especially the significantly lower IgG response in CM associated with fatal outcome compared to survivors 21.
The CM antibody responses to MSPs is highly variable in this study, possibly related to the urban setting with indeterminate individual episode chronology, the older (i.e. not children) individuals implicated and variable treatment before hospitalization. In this study, only antibodies to MSP1p19 and MSP3 showed significant associations with resistance to fatal outcome. These observations are in line with previous studies, underlining their strong association with natural protection. Antibody responses to MSP1p19 has repeatedly been shown to be associated with clinical protection 52, 53. The use of baculovirus‐expressed antigens which more faithfully reproduces conformational epitopes would appear to be an important factor for identifying relevant targets of protection‐associated immunity 13, 29, 32. Thus, the use of E. coli produced MSP1p19 by the Dodoo research team led to a negative association with protection 54 as opposed to more recent results using the baculovirus‐derived version 13. MSP3 has also been found to be associated with protection 55 and underwent early‐phase clinical studies 14. Importantly, these antigens were associated with anti‐parasite functional activity based on antibody‐dependent cellular effector mechanisms involving monocyte and polymorphonuclear neutrophils using the ADCI assay 8 for MSP3 28 and the ADRB assay 33 for MSP1p19 34.
Patients showed pre‐existing high levels of antibodies to MSPs at admission to hospital, both in MM and CM. However, two points deserve consideration: first, a threshold concentration of anti‐merozoite antibodies is required for protection from clinical episodes of malaria, with different levels required depending on the antigen 56. Secondly, antibodies to MSPs can confer protection against CM through multiple mechanisms either by direct blocking of the invasion process or by cell‐mediated mechanisms of parasite destruction such as ADRB and ADCI, which depend on initial merozoite opsonization by cytophilic IgG. As recently reported, the opsonic phagocytosis assay was shown to correlate with protective immunity 42, 57 while the magnitude of the anti‐merozoite antibody response correlated with the amount of reactive oxygen species production in the ADRB assay 37. Very importantly, it has been demonstrated that opsonization capacity in immune sera is not restricted to autologous parasite isolates or by the presence of different MSP gene when using transgenic parasites 58. These observations explain the efficacy of passive IgG transfer in curing malaria independently of the geographical origin of the IgG pools 8, 9. Indeed, functional activity probably relies on complex, unknown and variable combinations of multiple critical conformational conserved antigen epitopes, as demonstrated when using transgenic P. chabaudi/P. falciparum MSP1p19 constructs in in‐vivo and in‐vitro experiments 34, 59 or transgenic deficient parasites for MSP1p19, MSP3, MSP6, MSPDBL1 58.
In this study, ADRB was analysed in a limited subset of CM cases as a first step to determine potential associations with fatal outcomes. ADRB activity was indeed reduced in fatal cases compared to survivors, but the sample size was probably too small to reach statistical significance (P = 0·07), although ADRB activity was positively correlated with high levels of IgG to target MSPs, as shown in Fig. 3. Subsequent studies designed to relate functional antibody assays such as ADRB to disease severity and clinical outcome should include larger sample size with diverse clinical scenarios, and include complementary assays such as merozoite opsonization and antibody‐dependent phagocytosis.
Conclusion
Taken together, our results suggest that MSPs are targets of antibody‐based protective immunity against CM with antibodies to MSP1p19 and MSP3 implicated in surviving versus fatal outcomes. These results do not preclude the importance of antibody responses to other antigens not analysed here, which probably contribute to survivor outcomes. Future studies should address whether low antibody levels to these targets in fatal cases reflects antibody production mechanisms or consumption of antibodies in immune effector mechanisms such as that implicated in the ADRB assay.
Disclosures
None.
Author contributions
R. P., M. B. and A. D. designed the study. M. B., M. M. F., B. N., B. F., M. S. N. and M. N. were in charge of recruitment of patients. B. M., C. J., M. L. V. and R. P. performed the tests and were in charge of database management and the plasma database. C. L. conducted statistics analysis with B. M. and R. P. B. M. and R. P. drafted the manuscript with input from C. L., M. L. V. and A. D. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the patients and their relatives for the active participation and continued collaboration in this work. We thank Drs A. Toure and O. Puijalon for constant support. We are thankful to Dr S. Longacre for providing antigens and substantial contribution to this work. The work was supported by grants from the Institut Pasteur Foundation ACIP 2012 and grant IPP_Clayton Dedonder 2012.
References
- 1. WHO . WHO World Malaria Report 2018 ‐ Geneva: World Health Organisation. http://www.who.int/malaria/publications/world-malaria-report-2018/en/ 2018.
- 2. Dondorp AM, Nosten F, Yi P et al Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009;361:455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imwong M, Suwannasin K, Kunasol C et al The spread of artemisinin‐resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis 2017;17:491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mzilahowa T, Chiumia M, Mbewe RB et al Increasing insecticide resistance in Anopheles funestus and Anopheles arabiensis in Malawi, 2011–2015. Malar J 2016;15:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol 2016;32:187–96. [DOI] [PubMed] [Google Scholar]
- 6. Tun KM, Imwong M, Lwin KM et al Spread of artemisinin‐resistant Plasmodium falciparum in Myanmar: a cross‐sectional survey of the K13 molecular marker. Lancet Infect Dis 2015;15:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cotter C, Sturrock HJ, Hsiang MS et al The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 2013;382:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouharoun‐Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 1990;172:1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen S, McGregor IA, Carrington S. Gamma‐globulin and acquired immunity to human malaria. Nature 1961;192:733–7. [DOI] [PubMed] [Google Scholar]
- 10. Groux H, Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res Immunol 1990;141:529–42. [DOI] [PubMed] [Google Scholar]
- 11. Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell 2006;124:755–66. [DOI] [PubMed] [Google Scholar]
- 12. Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev 2016;40:343–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dodoo D, Aikins A, Kusi KA et al Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J 2008;7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sirima SB, Cousens S, Druilhe P. Protection against malaria by MSP3 candidate vaccine. N Engl J Med 2011;365:1062–4. [DOI] [PubMed] [Google Scholar]
- 15. Pages F, Texier G, Pradines B et al Malaria transmission in Dakar: a two‐year survey. Malar J 2008;7:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trape JF, Lefebvre‐Zante E, Legros F et al Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am J Trop Med Hyg 1992;47:181–9. [DOI] [PubMed] [Google Scholar]
- 17. Gadiaga L, Machault V, Pagès F et al Conditions of malaria transmission in Dakar from 2007 to 2010. Malar J 2011;10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. PNLP . Malaria Annual Epidemiology Bulletin. National Malaria Health Programme 2015:24. [Google Scholar]
- 19. World Health Organization (WHO) . Severe falciparum Malaria, World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med 2000;94:1–90. [PubMed] [Google Scholar]
- 20. Mbengue B, Niang B, Niang MS et al Inflammatory cytokine and humoral responses to Plasmodium falciparum glycosylphosphatidylinositols correlates with malaria immunity and pathogenesis. Immun Inflamm Dis 2016;4:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perraut R, Diatta B, Marrama L et al Differential antibody responses to Plasmodium falciparum glycosylphosphatidylinositol anchors in patients with cerebral and mild malaria. Microbes Infect 2005;7:682–7. [DOI] [PubMed] [Google Scholar]
- 22. Mbengue B, Fall MM, Sylla Niang M et al Relationship between antibody levels, IgG binding to Plasmodium falciparum‐infected erythrocytes, and disease outcome in hospitalized urban malaria patients from Dakar, Senegal. Biomed Res Int 2016;2016:5381956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perraut R, Marrama L, Diouf B et al Distinct surrogate markers for protection against Plasmodium falciparum infection and clinical malaria identified in a Senegalese community after radical drug cure. J Infect Dis 2003;188:1940–50. [DOI] [PubMed] [Google Scholar]
- 24. Bonnet S, Petres S, Holm I et al Soluble and glyco‐lipid modified baculovirus Plasmodium falciparum C‐terminal merozoite surface protein 1, two forms of a leading malaria vaccine candidate. Vaccine 2006;24:5997–6008. [DOI] [PubMed] [Google Scholar]
- 25. Polson HE, Conway DJ, Fandeur T, Mercereau‐Puijalon O, Longacre S. Gene polymorphism of Plasmodium falciparum merozoite surface proteins 4 and 5. Mol Biochem Parasitol 2005;142:110–5. [DOI] [PubMed] [Google Scholar]
- 26. Pizarro JC, Chitarra V, Verger D et al Crystal structure of a Fab complex formed with PfMSP1‐19, the C‐terminal fragment of merozoite surface protein 1 from Plasmodium falciparum: a malaria vaccine candidate. J Mol Biol 2003;328:1091–103. [DOI] [PubMed] [Google Scholar]
- 27. Taylor RR, Smith DB, Robinson VJ, McBride JS, Riley EM. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun 1995;63:4382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oeuvray C, Bouharoun‐Tayoun H, Gras‐Masse H et al Merozoite surface protein‐3: A malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 1994;84:1594–602. [PubMed] [Google Scholar]
- 29. Perraut R, Joos C, Sokhna C et al Association of antibody responses to the conserved Plasmodium falciparum merozoite surface protein 5 with protection against clinical malaria. PLOS ONE 2014;9:e101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aribot G, Rogier C, Sarthou JL et al Pattern of immunoglobulin isotype response to Plasmodium falciparum blood‐stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa). Am J Trop Med Hyg 1996;54:449–57. [DOI] [PubMed] [Google Scholar]
- 31. Nguer CM, Diallo TO, Diouf A et al Plasmodium falciparum‐ and merozoite surface protein 1‐specific antibody isotype balance in immune Senegalese adults. Infect Immun 1997;65:4873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perraut R, Varela ML, Joos C et al Association of antibodies to Plasmodium falciparum merozoite surface protein‐4 with protection against clinical malaria. Vaccine 2017;35:6720–6. [DOI] [PubMed] [Google Scholar]
- 33. Joos C, Marrama L, Polson HE et al Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLOS ONE 2010;5:e9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joos C, Varela ML, Mbengue B et al Antibodies to Plasmodium falciparum merozoite surface protein‐1p19 malaria vaccine candidate induce antibody‐dependent respiratory burst in human neutrophils. Malar J 2015;14:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dobano C, Rogerson SJ, Mackinnon MJ et al Differential antibody responses to Plasmodium falciparum merozoite proteins in Malawian children with severe malaria. J Infect Dis 2008;197:766–74. [DOI] [PubMed] [Google Scholar]
- 36. Leoratti FM, Durlacher RR, Lacerda MV et al Pattern of humoral immune response to Plasmodium falciparum blood stages in individuals presenting different clinical expressions of malaria. Malar J 2008;7:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murungi LM, Sonden K, Llewellyn D et al Targets and mechanisms associated with protection from severe Plasmodium falciparum malaria in Kenyan children. Infect Immun 2016;84:950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okech B, Mujuzi G, Ogwal A, Shirai H, Horii T, Egwang TG. High titers of IgG antibodies against Plasmodium falciparum serine repeat antigen 5 (SERA5) are associated with protection against severe malaria in Ugandan children. Am J Trop Med Hyg 2006;74:191–7. [PubMed] [Google Scholar]
- 39. Garraud O, Perraut R, Riveau G, Nutman TB. Class and subclass selection in parasite‐specific antibody responses. Trends Parasitol 2003;19:300–4. [DOI] [PubMed] [Google Scholar]
- 40. Mbengue B, Niang B, Diatta B et al The use of crude Plasmodium falciparum antigens for comparison of antibody responses in patients with mild malaria vs. cerebral malaria. Iran J Immunol 2010;7:150–61. [PubMed] [Google Scholar]
- 41. Lucchi NW, Tongren JE, Jain V et al Antibody responses to the merozoite surface protein‐1 complex in cerebral malaria patients in India. Malar J 2008;7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hill DL, Eriksson EM, Li Wai Suen CS et al Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLOS ONE 2013;8:e74627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van de Veen W, Akdis M. Role of IgG4 in IgE‐mediated allergic responses. J Allergy Clin Immunol 2016;138:1434–5. [DOI] [PubMed] [Google Scholar]
- 44. Blank U, Mecheri S. Duality and complexity of allergic type inflammatory mechanisms in determining the outcome of malaria disease. Front Immunol 2011;2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tangteerawatana P, Montgomery SM, Perlmann H, Looareesuwan S, Troye‐Blomberg M, Khusmith S. Differential regulation of IgG subclasses and IgE antimalarial antibody responses in complicated and uncomplicated Plasmodium falciparum malaria. Parasite Immunol 2007;29:475–83. [DOI] [PubMed] [Google Scholar]
- 46. Chaudhury S, Regules JA, Darko CA et al Delayed fractional dose regimen of the RTS, S/AS01 malaria vaccine candidate enhances an IgG4 response that inhibits serum opsonophagocytosis . Sci Rep 2017;7:7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarthou JL, Angel G, Aribot G et al Prognostic value of anti‐Plasmodium falciparum‐specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect Immun 1997;65:3271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chotivanich K, Udomsangpetch R, Simpson JA et al Parasite multiplication potential and the severity of Falciparum malaria. J Infect Dis 2000;181:1206–9. [DOI] [PubMed] [Google Scholar]
- 49. Bob NS, Diop BM, Renaud F et al Parasite polymorphism and severe malaria in Dakar (Senegal): a West African urban area. PLOS ONE 2010;5:e9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. A‐Elgadir T, Elbashir MI, Berzins K et al. The profile of IgG‐antibody response against merozoite surface proteins 1 and 2 in severe Plasmodium falciparum malaria in Eastern Sudan. Parasitol Res 2008;102:401–9. [DOI] [PubMed] [Google Scholar]
- 51. Iriemenam NC, Khirelsied AH, Nasr A et al Antibody responses to a panel of Plasmodium falciparum malaria blood‐stage antigens in relation to clinical disease outcome in Sudan. Vaccine 2009;27:62–71. [DOI] [PubMed] [Google Scholar]
- 52. Egan AF, Morris J, Barnish G et al Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19‐kDa C‐terminal fragment of the merozoite surface antigen, PfMSP‐1. J Infect Dis 1996;173:765–9. [DOI] [PubMed] [Google Scholar]
- 53. Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti‐merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta‐analysis. PLOS Med 2010;7:e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dodoo D, Theander TG, Kurtzhals JA et al Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect Immun 1999;67:2131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roussilhon C, Oeuvray C, Muller‐Graf C et al Long‐term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLOS Med 2007;4:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murungi LM, Kamuyu G, Lowe B et al A threshold concentration of anti‐merozoite antibodies is required for protection from clinical episodes of malaria. Vaccine 2013;31:3936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Osier FH, Feng G, Boyle MJ et al Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 2014;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hill DL, Wilson DW, Sampaio NG et al Merozoite antigens of Plasmodium falciparum elicit strain‐transcending opsonizing immunity. Infect Immun 2016;84:2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O'Donnell RA, Saul A, Cowman AF, Crabb BS. Functional conservation of the malaria vaccine antigen MSP‐119across distantly related Plasmodium species. Nat Med 2000;6:91–5. [DOI] [PubMed] [Google Scholar]


