Abstract
Aims
The prevalence of dementia is rising as life expectancy increases globally. Behavioural and psychological symptoms of dementia (BPSD), including agitation and aggression, are common, presenting a challenge to clinicians and caregivers.
Methods
Following PRISMA guidelines, we systematically reviewed evidence for gabapentin and pregabalin against BPSD symptoms of agitation or aggression in any dementia, using six databases (Pubmed, CINHL, PsychINFO, HealthStar, Embase, and Web of Science). Complementing this formal systematic review, an illustrative case of a patient with BPSD in mixed Alzheimer's/vascular dementia, who appeared to derive benefits in terms of symptom control and functioning from the introduction of gabapentin titrated up to 3600 mg day−1 alongside other interventions, is presented.
Results
Twenty‐four relevant articles were identified in the systematic review. There were no randomized trials. Fifteen papers were original case series/case reports of patients treated with these compounds, encompassing 87 patients given gabapentin and six given pregabalin. In 12 of 15 papers, drug treatment was effective in the majority of cases. The remaining nine papers were solely reviews, of which two were described as systematic but predated PRISMA guidelines. Preliminary low‐grade evidence based on case series and case reviews suggests possible benefit of gabapentin and pregabalin in patients with BPSD in Alzheimer's disease. These benefits cannot be confirmed until well‐powered randomized controlled trials are undertaken. Evidence in frontotemporal dementia is lacking.
Conclusion
Gabapentin and pregabalin could be considered for BPSD when medications having stronger evidence bases (risperidone, other antipsychotics, carbamazepine and citalopram) have been ineffective or present unacceptable risks of adverse outcomes.
Keywords: Clinical pharmacology, dementia, neurology, pharmacotherapy, statistics and study design, systematic review
Introduction
The World Health Organization estimates that around 50 million people worldwide are suffering from dementia with 10 million new cases diagnosed every year 1. Approximately 75% of patients diagnosed with dementia will exhibit at least one behavioural and psychological symptom such as agitation, lethargy, oppositional behaviour or aggression in a given month 2, 3. Episodes of aggressivity in dementia can appear spontaneously or be triggered by physical illness or pain, unfamiliar surroundings or medical examinations. These episodes pose risks to individuals with dementia themselves and also to their relatives or caregivers, and, when they occur outside an individual's own home, to staff and other people with whom they share their residential setting. High distress among caregivers of patients with behavioural and psychological symptoms of dementia (BPSD) has been associated with excess health care utilization, including increased visits to emergency services, more hospitalizations and higher medical costs 4.
Non‐pharmacological approaches are usually the first option when addressing BPSD but when these are unsuccessful and risk of self‐harm or harm to others persists, other approaches are needed 5. Pharmacological treatments for aggression and related symptoms in dementia include antipsychotics, certain antidepressants, mood stabilizers and other medications. Antipsychotics are the most commonly prescribed drugs for these symptoms 6 and the most effective, at least in the short term 7, with risperidone being licensed or approved for short‐term treatment of BPSD in Alzheimer's disease in both Canada and the United Kingdom on the basis of several randomized placebo‐controlled trials 6, 8, 9, 10, 11.
Several other antipsychotic drugs also have evidence of effectiveness for BPSD in Alzheimer's disease such as aripiprazole 12, quetiapine 13, olanzapine 14 and haloperidol 15. However, antipsychotics carry risks including increased mortality and increased risk of cardiovascular events 16, 17. Some antidepressants have an evidence base for treating BPSD in Alzheimer's disease, most notably citalopram 18, 19, 20, 21. However, use of citalopram is associated with two concerns: firstly its link with QTc prolongation and cardiac arrhythmia 22, and secondly the suggestion that the time required for its onset of action 23 limits its utility for treating individuals considered to be at imminent risk of harming themselves or others. Other antidepressants with evidence of efficacy in BPSD include sertraline, which has one small randomized trial in Alzheimer's disease 24, and trazodone, which has evidence in frontotemporal dementia (FTD) 25. There is also randomized trial evidence in Alzheimer's disease for a small number of other drugs, including the mood stabilizer carbamazepine 26, memantine 27, prazosin 28, dextromethorphan‐quinidine 29, diphenhydramine 30, cyproterone acetate 31 and estrogen 32. However, in addition to having a limited evidence base, many of these drugs are associated with risks of unacceptable side effects, pharmacokinetic and pharmacodynamic drug interactions (which may be especially problematic in a patient group which is often exposed to multiple medications) or slow onset of action. Benzodiazepines are sometimes used for treatment of BPSD due to their anxiolytic and sedating properties, but are associated with an increase in risk of falls 33 as well as the possibility of tolerance 34.
In this context, clinicians are faced with the dilemma of what to prescribe for agitation when drugs with the strongest evidence base have not been effective or cannot be prescribed because of possible interactions or other risks.
In this paper we systematically review the evidence for two pharmacologically related compounds that might be alternative drug treatments for agitation in the elderly with dementia: gabapentin and pregabalin. Both substances were originally marketed as anticonvulsants and have subsequently been studied, and in some cases, licensed, for other indications including anxiety disorders 35, pain control 36, 37, 38 and substance abuse 39, 40. They have similar mechanisms of action and share common pharmacokinetic properties.
We describe a case where gabapentin was used to treat a patient with mixed Alzheimer's/vascular dementia presenting with severe aggression requiring hospitalization. The case is followed by a systematic review of current literature of the use of these drugs in aggression in patients with dementia.
Pharmacodynamics of pregabalin and gabapentin
Gabapentin and pregabalin (known as ‘gabapentinoids’ and in NBN‐2 terminology 41 as ‘voltage gated calcium channel blockers’) are γ‐aminobutyric acid (GABA) analogues but exert their action by binding to the α2‐δ protein, an auxiliary subunit of presynaptic voltage‐gated calcium channels 42, 43 illustrated in Figure 1. The decrease in presynaptic calcium is linked to a reduction in the liberation of excitatory neurotransmitters such as glutamate and substance P. It is hypothesized that this reduction attenuates network hyperexcitability leading potentially to anxiolytic, antiepileptic and analgesic effects 43. The overexpression of α2‐δ proteins in brain areas associated with the processing of emotions, sensitivity to pain and seizure control, such as pyriform and insular cortex, amygdala, and hippocampus, supports this hypothesis 43.
Figure 1.
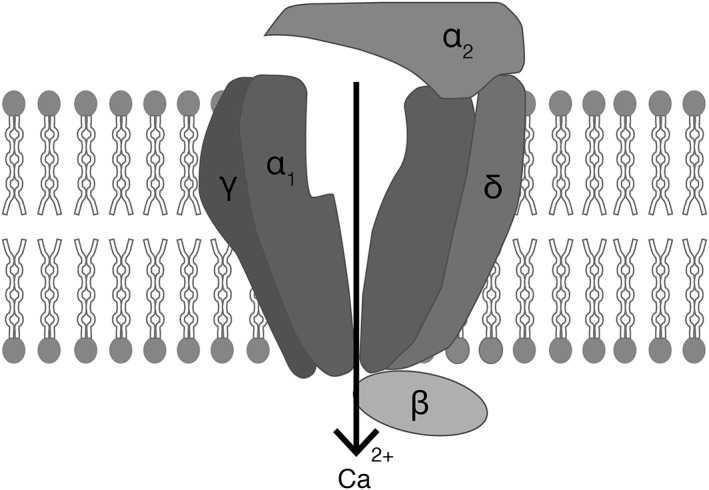
Schematic diagram of presynaptic voltage‐gated calcium channel, composed of subunits as indicated, in the lipid bilayer. Gabapentin and pregabalin are thought to bind a site associated with the auxiliary α2 and δ subunits (labelled ‘α2’ and ‘δ’)
Case report
Mr. R, a 77‐year‐old married man living in Canada and of Afghan origin, had a 10‐year history of progressive cognitive decline with a working diagnosis of Alzheimer's dementia (Folstein Mini Mental State Examination (MMSE) score = 7/26). He was prescribed donepezil 5 mg day−1, and mirtazapine 15 mg nightly. Prior to admission, he was taking amlodipine 5 mg daily, lansoprazole 30 mg daily, and once daily multivitamins. He was noted to be using zopiclone, acetaminophen and ibuprofen as needed, though doses could not be confirmed at the time of admission. He was known to exhibit emotional lability, with physical and verbal agitation and intermittent episodes of sustained aggression towards others and was therefore prescribed haloperidol 0.5 mg PRN which was administered at home up to 1.5 mg day−1. Increasingly, he exhibited self‐harm, hitting himself and biting his hands when distressed as well as prolonged restlessness, confusion and physical aggression towards caregivers. Concern from his children led to admission from his home to a Geriatric Psychiatry inpatient unit.
Rating scale scores on admission are summarized in Table 1. He presented as disoriented, restless and physically threatening. The haloperidol was replaced with risperidone 0.25 mg PRN (up to 2 doses per day) to target agitation with good effect. Acetaminophen 500 mg TID was started for management of intermittent thoracic pain with good response. Physical examination and laboratory tests revealed no acute infection or other secondary cause for potential delirium, and no reversible cause of dementia. Pharmacological management was initiated with substitute consent. Our management followed the principles of the hospital's Integrated Care Pathway (ICP) for Agitation and Aggression in Dementia, which includes a published sequential drug treatment algorithm for agitation/aggression in Alzheimer's or mixed Alzheimer's/vascular dementia 44. This algorithmic approach allows trials of evidence‐based treatments to be undertaken using predetermined dosing schedules and operationalized decision‐making at prespecified time points 44. In accordance with the provisions of the ICP, prior to starting any new medications to attempt to control BPSD symptoms, ten days were allocated for observation, non‐pharmacological interventions and for discontinuing psychotropic medications started for management of neuropsychiatric symptoms (although cholinesterase inhibitors need only be discontinued if they are judged to have contributed to worsening behaviours). In Mr. R's case, mirtazapine was discontinued but donepezil was continued, later being titrated to 10 mg day−1.
Table 1.
NPI‐Q, CGI, and MOCA scores recorded at admission and 12 months after admission (4 weeks after supplementing Gabapentin 1200 mg TID with Trazodone 25 mg BID + 75 mg QHS + up to three × 25 mg PRN trazodone doses per day)
| NPI‐Qa | CGIb | ||||
|---|---|---|---|---|---|
| Severity | Distress | Illness severity | Global improvement | MOCAc | |
| Baseline scores at admission | 7/36 | 11/60 | 5/7 | n/a | 5/30 |
| Scores 12 months after admission (on gabapentin 1200 mg TID + trazodone 25 mg BID + 75 mg QHS + up to three × 25 mg PRN trazodone doses per day and acetaminophen 1000 mg TID. | 2/36 | 3/60 | 3/7 | 1/7d | 4/30 |
NPI‐Q, The Neuropsychiatric Inventory Questionnaire
CGI, Clinical Global Impression Scale
MOCA, Montreal Cognitive Assessment
CGI Global improvement score of 1 represents ‘Very much improved’ in comparison to baseline (i.e. time of admission to hospital)
BID, twice daily; QHS, at bedtime; TID, three times daily
Following this washout period, risperidone monotherapy was initiated as the first trial, with trazodone (12.5 mg, maximum 150 mg day−1) available PRN for severe agitation. During the first day of this trial, he received three trazodone doses over a period of less than 24 h, but experienced a sudden decrease in level of consciousness, leading to a fall and head injury. A computed tomography (CT) scan revealed no acute bleed, but indicated chronic microangiopathic changes and lacunar infarcts. His working diagnosis was revised to major neurocognitive disorder with mixed aetiology (Alzheimer's disease: AD, and vascular dementia: VaD) and both the risperidone and the PRN trazodone were continued, the PRN trazodone initially being given sparingly from 0 to 25 mg day−1. Anxiety was reduced and persecutory ideation improved with the risperidone dose at 1 mg day−1, but restlessness and pacing continued. Gradual titration up to 2 mg day−1 provoked rigidity and intermittent involuntary movements. Risperidone was then tapered off, and aripiprazole was initiated as a second trial, with a gradual titration to 12.5 mg day−1. This led to mild worsening of agitation, and increased unsteadiness in gait. On discussion with the family, risperidone was retrialled with a lower target dose (1.25 mg day−1). Again, after an initial response, agitation and self‐directed aggression recurred. His PRN trazodone usage had gradually increased while having the trials of risperidone and aripiprazole, being in the range of 0–62.5 mg day−1 during this time. After risperidone discontinuation he continued to exhibit intermittent involuntary tremor of his right hand, suggesting tardive dyskinesia. Next carbamazepine, the third ICP drug, was titrated to 100 mg BID but without discernible benefit, the dose being limited by unsteadiness and falls. As a fourth trial, citalopram was titrated to 20 mg daily, but again no benefit emerged. Throughout these trials he had intermittent falls, self‐harm behaviours and aggression towards others, but there were no serious injuries. He was typically given between 25 mg and 75 mg day−1 (and on one occasion 100 mg day−1) of PRN trazodone (in 12.5 mg doses) while taking carbamazepine and citalopram. Acetaminophen was increased to 1000 mg TID as untreated musculoskeletal pain was thought to be contributing to residual aggression, though this change was not thought to be effective. The only medical complication was intermittent uncomplicated urinary tract infections throughout the hospitalization.
Finally, with the BPSD symptoms still prevalent approximately 6 months after admission, gabapentin, was introduced, titrating to 1800 mg day−1 (the maximum dose envisaged in the ICP). His renal function was normal and gabapentin provoked no side effects. There was marked improvement in sleep, agitation and affect. He began expressing himself in English and was more verbally communicative with the nursing staff. Pacing and restlessness diminished, and he was observed to be sitting calmly throughout the day, interacting appropriately with staff and patients. Episodes of agitation and self‐harm had decreased significantly in frequency, duration and severity. Aggression towards patients and staff became rare, occurring only on unwanted invasion of his personal space, and he was granted short periods of leave from the unit with family.
In line with the limited evidence discussed below, consent was obtained to increase gabapentin gradually to 3600 mg day−1 in 600 mg day−1 increments. A trial lasting 21 days was planned at each dose level, aiming for further reductions in frequency and severity of agitation and aggressive episodes. Continued partial improvement was noted at 2400 mg day−1 and 3000 mg day−1. After titration to 3600 mg day−1, with serum level recorded as 73.7 μmol l−1 (normal therapeutic range: 12.0–120.0 μmol l−1), the patient was noted to be sleeping better during the night. However, he initially developed worsening agitation during daytime, necessitating frequent physical restraint. This was thought to be caused by neck pain, which was treated with additional acetaminophen doses as needed and resolved within a few days. Acute infection, haematological and endocrine abnormalities were ruled out as potential causes. His PRN trazodone dose was increased to 25 mg (max 150 mg day−1) with response often being observed only after three or four consecutive 25 mg doses. Gabapentin was continued at 3600 mg day−1 (1200 mg three times daily) for 21 days. In view of the PRN doses being required regularly, the trazodone was changed to regularly scheduled doses (25 mg two times daily + 75 mg before bedtime) with up to three further 25 mg PRN doses as required to a grand total (regular + PRN) of up to 200 mg day−1. With this regimen of 3600 mg day−1 of gabapentin and 125 mg day−1 of trazodone, and up to three further trazodone 25 mg doses on a PRN basis (although no PRN trazodone was required on about 40% of subsequent days), control of his neuropsychiatric symptoms was restored, with further reductions in frequency and severity of agitated episodes. Approximately 12 months after admission (and 6 months from the start of the gabapentin trial), Mr. R was noted to be calm, pleasant, and cooperative on most days for most of the day. He was observed by staff to be spending more time out of his room and participating in group recreational activities. Episodes of agitation were rare, and were described as ‘verbal outcries’. Instances of violent behaviour were now short‐lived and limited to rare occasions involving direct provocation from other agitated patients. Non‐pharmacological interventions were successful in ensuring that there was no escalation into more serious incidents. Self‐harm behaviours resolved completely. Mr. R's sleep was regular. He was effective in communicating his needs in English, whereas in the first 6 months of his admission he had not spoken English at all. These benefits were confirmed by marked improvements on the relevant neuropsychiatric rating scales (Table 1). Compared to admission, the Neuropsychiatric Inventory Questionnaire (NPI‐Q) severity scores 45 decreased from 7/36 to 2/36, and NPI‐Q distress scores decreased from 11/60 to 3/60. Clinical Global Impression (CGI) scores suggested marked improvement in illness severity, decreasing from ‘markedly ill’ (score of 5/7) to ‘mildly ill’ (score of 3/7), with global improvement showing the best possible score of ‘very much improved’ (score of 1/7). Finally, Montreal Cognitive Assessment (MOCA) scores decreased slightly from 5/30 to 4/30 at 4 weeks after, and remained at 4/30 one year from, his date of admission. Furthermore, he was able to tolerate several day‐long periods of leave with family members without difficulty over the following 2 months. At the time of writing, arrangements were being made for Mr. R to be transferred to long‐term care. The combination of high dose gabapentin with trazodone and optimized pain relief through acetaminophen therefore coincided with a definitive improvement in quality of life for Mr. R and his caregivers, which could not be achieved through non‐pharmacological approaches nor through prescription of any of the earlier drug treatments (atypical antipsychotics, carbamazepine and citalopram), all of which have a better developed evidence base for behavioural and psychological symptoms of Alzheimer's or mixed vascular dementia.
Systematic review methods
Data search
A systematic review was performed using six databases (PubMed, CINHL, PsychINFO, HealthStar, Embase, and Web of Science). Articles in English, Spanish, French, Italian and Japanese published from 1983 until 2017 were considered. Keywords used in the search included: ‘pregabalin’, ‘gabapentin’, several terms for ‘geriatric’ and ‘aging’, terms for ‘dementia’, and terms for ‘aggression’, ‘agitation’ and ‘violence’. Mesh terms were also included: pregabalin, gabapentin, dementia and other relevant terms. The full list of search terms employed was registered and recorded on the Prospero website (reg. no.: CRD42017067119). Grey literature was also searched. The review was performed following the guidelines detailed in the PRISMA statement 46.
Studies were included if they met all of the following criteria: dementia (any type) with agitation/aggression, treated with gabapentin or pregabalin or any other known ‘gabalin’ agent such as imagabalin. Subjects could be in any setting (e.g. hospital, home, care home) and were included if fulfilling the above criteria regardless of psychiatric comorbidity. Studies were excluded if they met any of the following criteria: agitation secondary to acute organic illness, delirium, pain, agitation attributed to substance abuse or withdrawal, or agitation solely attributed to anxiety, depression or psychosis.
After initial searching identified papers of potential relevance, abstracts were assessed to confirm whether each paper might meet the search criteria. Those papers that were considered to possibly meet the criteria for inclusion were then assessed by two clinical reviewers (T.S. and B.B.), with experience in geriatric psychiatry, with a geriatric psychiatry faculty member (S.D.) being employed to resolve disagreements. All articles were hand searched for additional papers. Studies were evaluated according to the Oxford Centre of Evidence Based Medicine level of evidence criteria 47. The search strategy and systematic review protocol can be obtained from the authors.
Results
The search identified 158 articles with three additional records found by hand search. There were 116 articles after duplicates were removed, 114 articles in English and two articles in French. Further examination of abstracts excluded 87 that did not fulfil inclusion criteria; 29 articles were assessed for eligibility by examining the full‐text. Of these, five were excluded as they did not fulfil inclusion criteria. Thus 24 relevant articles were found. Figure 2 presents a diagram illustrating the systematic search selection process.
Figure 2.
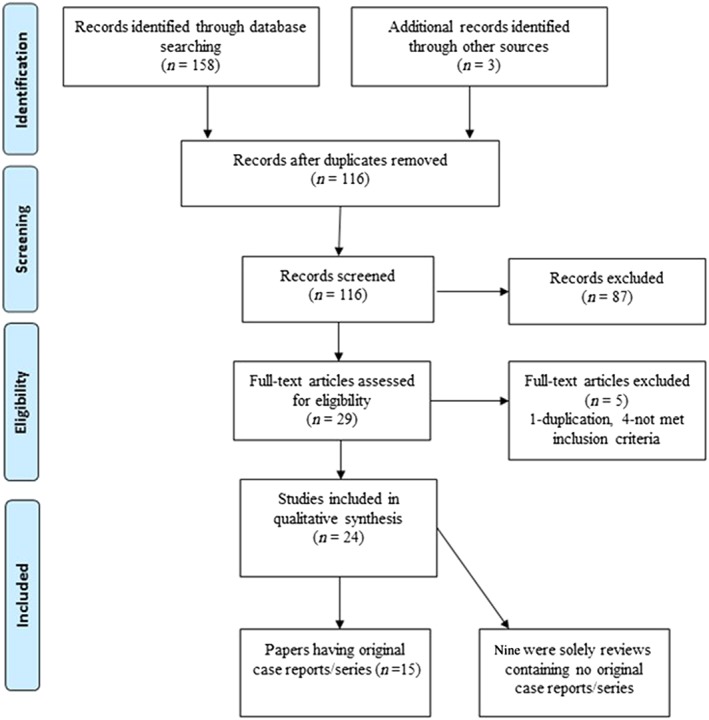
PRISMA diagram illustrating the selection of 24 articles by systematic search
Of the 24 publications that met our criteria for inclusion 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 15 contained original data (case reports or case series) 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, whereas nine were solely reviews of the literature with no original data 48, 49, 65, 66, 67, 68, 69, 70, 71. One of the 15 papers that contained an original report additionally included a narrative review. Among the ten papers that were reviews, none were fully systematic; eight were scoping or narrative reviews. One of these eight 71 outlined a search strategy but searches were limited to one database only (in contrast to the present systematic review which employed multiple databases, in line with current guidance). The remaining two 48, 49, neither of which included pregabalin, employed systematic searches but having been published in 2008 which predates the publication of the current PRISMA guidelines, fall short of current methodological expectations, for example in only searching for and including studies published in English.
Among the 15 papers that contained original reports, 10 were case series and five were single case reports. Fourteen studies examined gabapentin and one examined pregabalin. The total number of patients included across the studies was 87 for gabapentin and six for pregabalin. Studies differed in the scales and measures used. A summary of all the studies that contained original case descriptions is shown in Table 2; in addition the 10 review papers identified (including the one that also contained an original case report) are listed in Table 3.
Table 2.
Summary of all the individual studies found. Fourteen studies examined gabapentin only, and one study pregabalin only (total number of patients across studies 91 for gabapentin and 6 for pregabalin)
| Author/year | Type of study | n (Female) | Type of dementia | Drug | Average daily dose | Measure of agitation scale/score | Results | Side effects, and number discontinuing (where stated) | LOE |
|---|---|---|---|---|---|---|---|---|---|
| Regan and Gordon 1997 50 | Case report | 1 (1) | AD | Gabapentin | 600 mg day−1 (300 mg BID) | Nursing report | Improved | N/A | 5 |
| Goldenberg et al. 1998 51 | Case report | 1 (1) | AD | Gabapentin | 600 mg day−1 (200 mg TID) | Nursing report | Control of the behavioral symptoms at follow up period (2 months) | none | 5 |
| Sheldon et al. 1998 55 | Case series | 2 (1) | AD, AD+FTD | Gabapentin | 600 mg day−1 (300 mg BID) | Nursing report | Improved | none | 4 |
| Low and Brandes 1999 52 | Case report | 1 (0) | Unspecified | Gabapentin | 900 mg day−1 (300 mg TID) | Nursing report | Improved | N/A | 5 |
| Dallocchio et al. 2000 56 | Case series | 2 (0) | AD | Gabapentin | 300 mg day−1 (300 mg qHS) | NPIe | Improved | Drowsiness | 4 |
| Hawkins et al. 2000 60 | Case series | 24(0) | AD, VaD, dementia of traumatic brain injury, anoxic brain damage dementia, dementia NOS, alcoholic dementia, Parkinson's dementia | Gabapentin | 1318 mg day−1 average (300–3600 mg day−1) | OASa, OASSYb, CMAIc, CGI‐Id | 5‐very much improved 12‐ much Improved 4‐Minimally Improved 1‐Unchanged 2‐Dropout | Excessive sedation, 2 | 4 |
| Herrmann et al. 2000 61 | Case series | 12(1) | AD, VaD, FTD, alcoholic dementia | Gabapentin | 900 mg day−1 average (200–1200 mg day−1) | NPIe, CGI‐Id, CMAIc | 2‐Much improved 3‐Minimally improved 6‐Unchanged 1‐Minimally worse 2‐Dropout | Sedation, unsteady gait, 2 | 4 |
| Roane et al. 2000 58 | Case series | 4(3) | AD, VaD | Gabapentin | 2400 mg day−1 | OASa | Improved | Headache, dizziness, reduced ambulation, sedation, disorientation | 4 |
| Miller 2001 54 | Case report | 1 | VaD | Gabapentin | 600–900 mg day−1 | N/A | Improved | None | 5 |
| Rossi et al. 2002 57 | Case series | 2(1) | DLB | Gabapentin | 300–900 mg day−1 | N/A | Worsen | Confusion, agitation, worsening of hallucinations | 4 |
| Moretti et al. 2003 59 | Case series/open label | 20(7) | AD | Gabapentin | 980 ± 154 mg day−1 | NPIe, CMAIc, CGI‐Id | Improved | None | 4 |
| Raudino et al. 2004 62 | Case series | 9(4) | AD | Gabapentin | 600–1200 mg day−1 | NPIe |
2 – Good 5 – Worse 2 – Discontinued |
Excessive Sedation | 4 |
| Buskova et al. 2011 53 | Case report | 1(1) | VaD | Gabapentin | 400 mg day−1 | CMAIc | 0 agitation/ wandering (CMAI was 39 after intervention but unclear as to whether this was improvement) | N/A | 5 |
| Cooney et al. 2013 63 | Case series | 7(1) | VaD, Mixed (AD+VaD) | Gabapentin | 100–200 mg BID | Nursing report | Improved | N/A | 4 |
| Bardet 2015 64 | Case series | 6(N/A) | Unspecified | Pregabalin |
Case 1: Acutely: 200 mg TID, later 350 mg OD. Case 2, 3, 4: 300–600 mg OD. Case 5: 125 mg OD. Case 6: 75 mg OD. |
Reports from nurse assistant | Decrease in aggressive behaviours | None | 4 |
Overt Aggression Scale (OAS)
Overt Agitation Severity Scale of Yudofsky and Silver (OASSY)
Cohen‐Mansfield Agitation Inventory (CMAI)
Clinical Global Impression, Improvement (CGI‐I)
Neuropsychiatric Inventory (NPI)
AD, Alzheimer's disease; BID, twice daily; DLB, lewy body dementia; FTD, frontotemporal dementia; LOE, levels of evidence; N/A, not stated; NOS, not otherwise specified; OD, once daily; QHS, at bedtime; TID, three times daily; VaD, vascular dementia
Table 3.
Summary of all the reviews identified and the relevant studies they included
| Author/year | Number of relevant studies included | Regan and Gordon 1997 50 | Goldenberg et al. 1998 51 | Sheldon et al. 1998 55 | Low and Brandes 1999 52 | Dallocchio et al. 2000 56 | Hawkins et al. 2000 60 | Hermann et al. 2000 61 | Roane et al. 2000 58 | Miller 2001 54 | Moretti et al. 2001 b | Rossi et al. 2002 57 | Moretti et al. 2003 59 | Raudino et al. 2004 [ 64] | Buskova et al. 2011 53 | Cooney et al. 2013 63 | Bardet 2015 64 | Systematic or narrative | Meta‐analysis Yes/No |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Letterman et al. 1999 65 | 2 | ● | ● | N | No | ||||||||||||||
| Miller 2001 54 | 7c | ● | ● | ● | ● | ● | ● | ● | N | No | |||||||||
| Guay et al. 2007 66 | 6 | ● | ● | ● | ● | ● | ● | N | No | ||||||||||
| Sommer et al. 2007 67 | 4 | ● | ● | ● | ● | N | No | ||||||||||||
| Konovalov et al. 2008 a 49 | 9 | ● | ● | ● | ● | ● | ● | ● | ● | ● | Sa | No | |||||||
| Kim et al. 2008 a 48 | 12 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | Sa | No | ||||
| Pinheiro 2008 68 | 9 | ● | ● | ● | ● | ● | ● | ● | ● | N | No | ||||||||
| Amann et al. 2009 69 | 2d | ● | ● | N | No | ||||||||||||||
| Yeh et al. 2012 70 | 11 | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | N | No | |||||
| Gallagher et al. 2014 71 | 2d | ● | ● | N | No |
These reviews contained a systematic search but did not follow current PRISMA recommendations
Moretti et al. (2001) 72 was not included in our synthesis as the four cases outlined appear to be duplicated in the series described in Moretti et al. (2003) 59
Miller (2001) 54 included one new case report in addition to reviewing seven earlier studies
These reviews included discussion of the earlier systematic review of Kim et al. (2008) 48
The first case report published about the use of gabapentin in agitation in dementia was by Regan and Gordon 50. In this case, the average dose used was 600 mg day−1. The patient, a woman aged 68 with Alzheimer's dementia (AD) had less agitation as per nursing observational reports after 12 weeks of treatment. Goldenberg et al. reported using gabapentin at the same dose for 8 weeks to control disruptive behaviour in a 92‐year‐old woman with AD who had multiple medical comorbidities 51 and who improved according to nursing reports. Low and Brandes described using gabapentin at a dose of 900 mg day−1 for the management of agitation in a 62‐year‐old man with dementia (type not specified); improvement by nursing report was apparent within 10 days 52. In 2011, Buskova et al. reported the use of gabapentin at a dose of 400 mg day−1 for dementia‐associated nocturnal agitation in a 77‐year‐old woman with vascular dementia (VaD) with nocturnal episodes of confusion and multiple medical comorbidities 53; the patient was assessed using the Cohen–Mansfield Agitation Inventory (CMAI) with a score change to 39 (score at baseline not reported).
Miller reported a case presenting with confusion, anxiety, depressive mood, insomnia and verbal and physical aggressiveness in a 62‐year‐old man who had underlying vascular dementia, major depression and alcohol abuse. Gabapentin 300 mg twice daily was administered at |Day 28 and had reduced his aggression by Day 30 of hospitalization. He was discharged 6 days later on gabapentin 300 mg three times daily, by which time his Folstein MMSE score had returned to 30/30, having been as low as 14/30 on admission 54.
Sheldon et al. reported two cases of gabapentin use for the treatment of primary agitation at a dose of 600 mg day−1. The first described an 87‐year‐old man with AD whose symptoms reduced in 2 weeks and was then stable for a year according to nursing report. The second was a 74‐year‐old woman with AD and frontal lobe dementia who was stable at the 6‐week follow‐up consultation 55. Dallochio et al. published a case series with a combination of donepezil 5 mg day−1 and gabapentin 300 mg day−1 for behavioural disorder in two male patients with AD. Their NPI‐Q score improved but mild drowsiness was reported 56.
Rossi et al. reported two cases of dementia with lewy bodies (DLB). Patients were prescribed 300–900 mg day−1 of gabapentin. Worsening of neuropsychiatric symptoms, including confusion, agitation and increase in hallucinations, were reported 57. Roane et al. reported three cases in which higher doses of gabapentin were used for the treatment of agitation in AD and one case of mixed dementia. The average dose of gabapentin in this study was 2400 mg day−1. All patients improved as measured by the Overt Agitation Severity Scale (OASS) but several side effects were reported, including headache, dizziness, reduced ambulation, sedation and disorientation 58.
Moretti et al. published an open label case series (n = 20) where patients received an average of 980 ± 154 mg day−1 of gabapentin. The only side effect reported was excessive sedation 59. We noted that the same authors had written up a small number of cases with similar attributes and treatment in an earlier paper 72 which was excluded on the grounds of presumed duplication. Hawkins et al. published a retrospective study of 24 cases with different types of dementia in 2000. The average dose of gabapentin used was 1318 mg day−1 and the dose range varied from 300 mg to 3600 mg day−1. Disruptive and aggressive behaviours were measured with OASS, OAS, CMAI and CGI. Two patients dropped out, one did not improve and the rest all showed some benefit 60. Herrmann et al. reported a case series of 12 patients with heterogeneous diagnoses of dementia (Alzheimer's disease, vascular dementia, frontal lobe dementia and alcoholic dementia) with mixed results. They used gabapentin at an average dose of 900 mg day−1. Aggression and agitation were measured with NPI, CGI and CMAI. Two patients dropped out because of sedation and unsteady gait respectively, one patient worsened, six did not report any benefits, three obtained minimum benefit and two improved 61.
Raudino et al. published a case series of nine patients treated with gabapentin 600–1600 mg day−1 in the context of behavioural disorder in severe Alzheimer's disease. Two patients improved, five worsened and two dropped out because of excessive sedation 62. Cooney et al. reported seven patients who had behavioural problems subsequent to vascular dementia mixed with Alzheimer disease. A low dose of gabapentin was used (100–200 mg twice daily). There was improvement measured by nursing report. No adverse effects were reported 63. In the most recent series identified, Bardet 64 described six patients prescribed pregabalin (dose range 75–600 mg day−1). Nursing reports showed a decrease in aggressive behaviour in all patients. No adverse events were reported. This was the only report found for pregabalin.
Discussion
Our systematic review found 15 case reports/case series relating to the use of gabapentin or pregabalin for aggression in dementia of all types. Of these, as many as 14 suggested that the introduction of these drugs was associated with positive outcomes in at least some of the cases described, although in two of these studies patients who improved were outnumbered by those who continued to worsen. The only study in which no individual was reported to benefit from the medication was of a single patient who had lewy body dementia and experienced worsening of symptoms on gabapentin. However, agitation in lewy body dementia is known to be especially difficult to treat with pharmacological approaches. While most reports were in Alzheimer's, vascular or mixed Alzheimer's/vascular dementia, there were a small number with other diagnoses, including two who had frontotemporal dementia (one pure FTD and one FTD in combination with Alzheimer's). Drug treatments for BPSD in FTD have been studied much less extensively with only the antidepressant trazodone having positive evidence in a randomized placebo‐controlled trial 73. Unfortunately, neither of the two papers which included a patient with FTD among those with other diagnoses specified whether this individual improved with gabapentin.
Overall, the quality of the evidence is low, and although these reports provide preliminary evidence to suggest that these drugs might be useful for aggression in dementia, without randomized controlled trials no firm conclusions can be drawn. We also identified nine review papers (and one of the case report papers contained a further review); however, only two of these reviews purported to be systematic 48, 49, but both were ten years old and used search strategies less comprehensive than would currently be expected.
Regarding dosage, for gabapentin this varied from 200 mg day−1 to 3600 mg day−1. While this indicates that gabapentin has been used safely across a wide dose range, the evidence base is not sufficiently developed for a target dosage to be recommended. The most common side effects of gabapentin and pregabalin are dizziness, somnolence and diminished motor coordination. A total of four patients out of 87 on gabapentin stopped the drug due to excessive sedation (Table 2). Sedative effects with gabapentinoids can range from mild drowsiness to overt sedation leading to unsteady gait and poor coordination followed by increase risks of falls 74. Other side effects described in our review include a patient with DLB with worsening of hallucinations, confusion and agitation after treatment with gabapentin.
Formularies recommend reducing the maximum dose of gabapentin or pregabalin in patients with poor renal function: eGFR below 80 ml min−1 1.73m−2, and more so if eGFR is under 50 ml min−1 1.73m−2 75. However, we did not find any examples of doses being limited because of kidney problems in any of the papers reviewed.
Gabapentin and pregabalin can be abused 76, although the abuse potential is thought to be less than that associated with benzodiazepines. However, this risk is increased in patients with a past history of substance misuse and concomitant use of opioids 76, 77. The population with BPSD is not at higher risk for substance use disorders relative to other diagnostic groups, but they are often dependent on caregivers who have access to their medications. The possibility of diversion of medications by caregivers is an issue which should be considered before prescribing these drugs. Pregabalin has a greater propensity for addiction 78 for several reasons. First, pregabalin has six‐fold greater potency than gabapentin with respect to effect on the calcium channel 79. Second, the pharmacokinetic profiles of gabapentin/pregabalin are similar, but the absorption of pregabalin is faster, reaching its peak plasma concentration in 1 h 80. Euphoria has been reported with pregabalin in about 4–5% of patients 81. Pregabalin was described in a review of online reports as an ‘ideal psychotropic drug’ for recreational purposes with alcohol‐like effects mixed with euphoria 76, 78, 81, 82. It has been suggested that euphoric mood may be more common when pregabalin is administered twice a day than when it is only used at night 83. Sustained release formulations do not reduce the euphoric effect 84, 85.
There are some limitations to this study. First, although the literature search was systematic and thorough (in contrast to earlier reviews, six databases were searched for articles in five languages, employing mesh terms and non‐mesh terms), the evidence found was limited to case reports and case series which are considered to be substantially weaker than the gold standard of randomized controlled trials (RCTs) and cannot be taken to provide definitive evidence that these drugs would be effective when examined in RCTs. Despite finding 15 articles fulfilling our criteria, with the evidence being limited to case series and case reports, meta‐analysis could not be performed and it was not possible to implement a detailed analysis of bias. It can be inferred that in general only successful cases are reported, and therefore this evidence may be overoptimistic. In fact, only one report described worsening of symptoms with gabapentin 57 in all cases of their sample, while a further two studies reported that individuals who experienced worsening outnumbered those who responded positively. Some authors have suggested that the predilection to introduce a trial of gabapentin for a variety of off‐label indications has been orchestrated by aggressive drug company marketing. In fact, in 2004 a US court established that the marketing of gabapentin for off‐label indications was illegal leading to a class action lawsuit 86 and it remains possible that the cases that have reached the literature and are described here may be both unrepresentative of the full spectrum of experience with the drug due to publication bias and the enthusiasm to report positive findings having some relation to the impact of marketing 86, 87, 88. Nevertheless, both gabapentin and pregabalin were relatively well tolerated in the elderly patients with BPSD described in the papers identified. This mirrors the acceptability of pregabalin for generalized anxiety disorder described in an earlier large randomized placebo‐controlled trial undertaken exclusively in elderly patients 89. In the case we described, improvement in BPSD symptoms was dose related, which supports the putative role of gabapentin in diminishing symptoms of aggression; however, it is difficult to ascertain direct causality as fluctuations in these symptoms may be attributable to a variety of causes, not least the presence or absence of inadequately treated pain which appeared to have some impact on the BPSD symptoms in this case. A further limitation is that the patient received PRN trazodone throughout his inpatient admission. While the marked improvement we described appeared to coincide with the introduction of gabapentin, the daily intake of trazodone was increased after his period of relapse and it remains possible that the higher daily dose of trazodone accounted for some or even most of the response after this adjustment.
Conclusion
Behavioural and psychological symptoms of dementia have an important impact on quality of life for patients with cognitive disorders. As the population ages and the prevalence of dementia increases, it is likely that the number of cases of agitation attributable to dementia will rise. As existing drug treatments have limitations through side effects, increased risk of mortality and drug interactions, or have limited evidence of efficacy, alternatives are required to ensure effective treatment in people with dementia who experience these problems.
Gabapentinoids may have a role to play in patients who are resistant to, or cannot tolerate, the few drugs that have randomized trial evidence of efficacy in BPSD. Their onset of action is relatively rapid (at least in comparison to some drugs such as SSRI antidepressants) and the side effect profile is relatively mild. Unfortunately, although these drugs have good quality evidence of efficacy in related conditions such as anxiety disorders, the lack of randomized controlled trials in agitation or aggression in the context of dementia means that evidence for the use of these drugs in the elderly for this indication is currently of low quality. Taking this into account, gabapentin and pregabalin can be useful drugs to treat BPSD in patients who have adequate renal function and minimal risk of misuse in the caregiving environment. Wide dose ranges can be employed as reports were identified illustrating benefits with gabapentin at doses from 200 mg day−1 to a maximum of 3600 mg day−1, with the main side effect being excessive sedation.
Until better evidence from randomized controlled trials is available, these drugs should only be considered for BPSD symptoms in Alzheimer's or mixed Alzheimer's/vascular dementia when other compounds with a stronger evidence base at the level of randomized controlled trials, such as risperidone and other antipsychotics, carbamazepine and citalopram, have not been effective or present sufficient risks of toxicity, interactions or other adverse outcomes that their use is judged to be undesirable. In frontotemporal dementia, with the exception of the antidepressant trazodone, there is very limited randomized trial evidence for drug treatment of BPSD. The two papers that included single individuals with FTD did not state whether gabapentin was beneficial specifically for these patients. We are therefore unable to offer any opinion on whether gabapentin could be considered as an alternative treatment for BPSD in the context of this diagnosis; again, more rigorous study is required.
At present, BPSD remains a widespread clinical problem among the ever‐increasing number of individuals who have dementia and causes suffering and risk both to the individuals themselves and to their carers and others in their immediate environment. There is a clear‐cut need for high quality treatment trials to be undertaken with drugs that have been reported to have preliminary evidence of beneficial effects, especially when such medications are effective in related conditions (e.g. anxiety disorders), are generally well tolerated, and are relatively rapid in their onset of action and associated with few pharmacokinetic interactions. Gabapentin and pregabalin meet these criteria and are logical targets for evaluation in future randomized trials in BPSD.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 90, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 91.
Competing Interests
There are no competing interests to declare. Within the past five years, S.J.C.D. has received grant support from the Centre for Addiction and Mental Health (CAMH)/University of Toronto, NIHR (UK), Canadian Centre for Ageing and Brain Health Innovation, Canadian Consortium for Neurodegeneration in Aging (CCNA) and Medical‐Psychiatry Alliance.
Contributors
All authors contributed to the writing of the paper with relevant intellectual content. The case study was written and edited by S.A., V.L.W. and S.J.C.D. The systematic review was conducted by T.S., B.M.B.‐A. and S.J.C.D. All authors revised and approved the final draft.
Supasitthumrong T., Bolea‐Alamanac B. M., Asmer S., Woo V. L., Abdool P. S., and Davies S. J. C. (2019) Gabapentin and pregabalin to treat aggressivity in dementia: a systematic review and illustrative case report, Br J Clin Pharmacol. 85, 690–703, 10.1111/bcp.13844.
References
- 1. World Health Organization . Dementia, 12 December 2017. [online]. Available at http://www.who.int/mediacentre/factsheets/fs362/en/ (last accessed 12 January 2019).
- 2. Watt J, Goodarzi Z, Tricco AC, Veroniki AA, Straus SE. Comparative safety and efficacy of pharmacological and non‐pharmacological interventions for the behavioral and psychological symptoms of dementia: protocol for a systematic review and network meta‐analysis. Syst Rev 2017; 6: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002; 288: 1475–1483. [DOI] [PubMed] [Google Scholar]
- 4. Maust DT, Kales HC, McCammon RJ, Blow FC, Leggett A, Langa KM. Distress associated with dementia‐related psychosis and agitation in relation to healthcare utilization and costs. Am J Geriatr Psychiatry 2017; 25: 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore A, Patterson C, Lee L, Vedel I, Bergman H. Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia: recommendations for family physicians. Can Fam Physician 2014; 60: 433–438. [PMC free article] [PubMed] [Google Scholar]
- 6. Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med 2006; 355: 1525–1538. [DOI] [PubMed] [Google Scholar]
- 7. Gareri P, Segura‐García C, Manfredi VG, Bruni A, Ciambrone P, Cerminara G, et al Use of atypical antipsychotics in the elderly: a clinical review. Clin Interv Aging 2014; 9: 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, et al A randomized placebo‐controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry 2003; 64: 134–143. [DOI] [PubMed] [Google Scholar]
- 9. De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PL, Eriksson S, et al A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999; 53: 946–955. [DOI] [PubMed] [Google Scholar]
- 10. Duran JC, Greenspan A, Diago JI, Gallego R, Martinez G. Evaluation of risperidone in the treatment of behavioral and psychological symptoms and sleep disturbances associated with dementia. Int Psychogeriatr 2005; 17: 591–604. [DOI] [PubMed] [Google Scholar]
- 11. Suh GH, Greenspan AJ, Choi SK. Comparative efficacy of risperidone versus haloperidol on behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry 2006; 21: 654–660. [DOI] [PubMed] [Google Scholar]
- 12. Mintzer JE, Tune LE, Breder CD, Swanink R, Marcus RN, McQuade RD, et al Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double‐blind, placebo‐controlled assessment of three fixed doses. Am J Geriatr Psychiatry 2007; 15: 918–931. [DOI] [PubMed] [Google Scholar]
- 13. Cheung G, Stapelberg J. Quetiapine for the treatment of behavioural and psychological symptoms of dementia (BPSD): a meta‐analysis of randomised placebo‐controlled trials. N Z Med J 2011; 124: 39–50. [PubMed] [Google Scholar]
- 14. Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, et al Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double‐blind, randomized, placebo‐controlled trial. The HGEU Study Group. Arch Gen Psychiatry 2000; 57: 968–976. [DOI] [PubMed] [Google Scholar]
- 15. Verhey FR, Verkaaik M, Lousberg R, Olanzapine‐Haloperidol in Dementia Study Group . Olanzapine versus haloperidol in the treatment of agitation in elderly patients with dementia: results of a randomized controlled double‐blind trial. Dement Geriatr Cogn Disord 2006; 21: 1–8. [DOI] [PubMed] [Google Scholar]
- 16. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials. JAMA 2005; 294: 1934–1943. [DOI] [PubMed] [Google Scholar]
- 17. Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, et al Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiat 2015; 72: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollock BG, Mulsant BH, Sweet R, Burgio LD, Kirshner MA, Shuster K, et al An open pilot study of citalopram for behavioral disturbances of dementia: plasma levels and real‐time observations. Am J Geriatr Psychiatry 1997; 5: 70–78. [PubMed] [Google Scholar]
- 19. Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, et al Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry 2002; 159: 460–465. [DOI] [PubMed] [Google Scholar]
- 20. Pollock BG, Mulsant BH, Rosen J, Mazumdar S, Blakesley RE, Houck PR, et al A double‐blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007; 15: 942–952. [DOI] [PubMed] [Google Scholar]
- 21. Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014; 311: 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qirjazi E, McArthur E, Nash DM, Dixon SN, Weir MA, Vasudev A, et al Risk of ventricular arrhythmia with citalopram and escitalopram: a population‐based study. PLoS One 2016; 11: e0160768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weintraub D, Drye LT, Porsteinsson AP, Rosenberg PB, Pollock BG, Devanand DP, et al Time to response to citalopram treatment for agitation in Alzheimer disease. Am J Geriatr Psychiatry 2015; 23: 1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaber S, Ronzoli S, Bruno A, Biagi A. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl 2001; 7: 159–162. [DOI] [PubMed] [Google Scholar]
- 25. Martinon‐Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev 2004; 4: CD004990. [DOI] [PubMed] [Google Scholar]
- 26. Olin JT, Fox LS, Pawluczyk S, Taggart NA, Schneider LS. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment‐resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry 2001; 9: 400–405. [PubMed] [Google Scholar]
- 27. Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer's disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69: 341–348. [DOI] [PubMed] [Google Scholar]
- 28. Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH, et al Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry 2009; 17: 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW, et al Effect of dextromethorphan‐quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 30. Coccaro EF, Kramer E, Zemishlany Z, Thorne A, Rice CM 3rd, Giordani B, et al Pharmacologic treatment of noncognitive behavioral disturbances in elderly demented patients. Am J Psychiatry 1990; 147: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 31. Huertas D, Lopez‐Ibor Alino JJ, Molina JD, Chamorro L, Balanza J, Jimenez MP, et al Antiaggressive effect of cyproterone versus haloperidol in Alzheimer's disease: a randomized double‐blind pilot study. J Clin Psychiatry 2007; 68: 439–444. [DOI] [PubMed] [Google Scholar]
- 32. Kyomen HH, Satlin A, Hennen J, Wei JY. Estrogen therapy and aggressive behavior in elderly patients with moderate‐to‐severe dementia: results from a short‐term, randomized, double‐blind trial. Am J Geriatr Psychiatry 1999; 7: 339–348. [PubMed] [Google Scholar]
- 33. Díaz‐Gutiérrez MJ, Martínez‐Cengotitabengoa M, Sáez de Adana E, Cano AI, Martínez‐Cengotitabengoa MT, Besga A, et al Relationship between the use of benzodiazepines and falls in older adults: a systematic review. Maturitas 2017; 101: 17–22. [DOI] [PubMed] [Google Scholar]
- 34. Schroeck JL, Ford J, Conway EL, Kurtzhalts KE, Gee ME, Vollmer KA, et al Review of safety and efficacy of sleep medicines in older adults. Clin Ther 2016; 38: 2340–2372. [DOI] [PubMed] [Google Scholar]
- 35. Reinhold JA, Rickels K. Pharmacological treatment for generalized anxiety disorder in adults: an update. Expert Opin Pharmacother 2015; 16: 1669–1681. [DOI] [PubMed] [Google Scholar]
- 36. Calandre EP, Rico‐Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16: 1347–1368. [DOI] [PubMed] [Google Scholar]
- 37. Thomas B, Farquhar‐Smith P. Extended‐release gabapentin in post‐herpetic neuralgia. Expert Opin Pharmacother 2011; 12: 2565–2571. [DOI] [PubMed] [Google Scholar]
- 38. Smith MT, Moore BJ. Pregabalin for the treatment of fibromyalgia. Expert Opin Pharmacother 2012; 13: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 39. Soyka M, Müller CA. Pharmacotherapy of alcoholism – an update on approved and off‐label medications. Expert Opin Pharmacother 2017; 18: 1187–1199. [DOI] [PubMed] [Google Scholar]
- 40. Muller CA, Geisel O, Banas R, Heinz A. Current pharmacological treatment approaches for alcohol dependence. Expert Opin Pharmacother 2014; 15: 471–481. [DOI] [PubMed] [Google Scholar]
- 41. Nutt DJ, Blier P. Neuroscience‐based Nomenclature (NbN) for Journal of Psychopharmacology. J Psychopharmacol 2016; 30: 413–415. [DOI] [PubMed] [Google Scholar]
- 42. Altamura AC, Moliterno D, Paletta S, Maffini M, Mauri MC, Bareggi S. Understanding the pharmacokinetics of anxiolytic drugs. Expert Opin Drug Metab Toxicol 2013; 9: 423–440. [DOI] [PubMed] [Google Scholar]
- 43. Calandre EP, Rico‐Villademoros F, Slim M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert Rev Neurother 2016; 16: 1263–1277. [DOI] [PubMed] [Google Scholar]
- 44. Davies SJ, Burhan AM, Kim D, Gerretsen P, Graff‐Guerrero A, Woo VL, et al Sequential drug treatment algorithm for agitation and aggression in Alzheimer's and mixed dementia. J Psychopharmacol 2018; 32: 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–2314. [DOI] [PubMed] [Google Scholar]
- 46. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 47. The 2011 Oxford CEBM Levels of Evidence (Introductory Document) [online]. Oxford Centre for Evidence‐Based Medicine, 2011. Available at http://www.cebm.net/index.aspx?o=5653 (last accessed 12 January 2019).
- 48. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging 2008; 25: 187–196. [DOI] [PubMed] [Google Scholar]
- 49. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr 2008; 20: 293–308. [DOI] [PubMed] [Google Scholar]
- 50. Regan WM, Gordon SM. Gabapentin for behavioral agitation in Alzheimer's disease. J Clin Psychopharmacol 1997; 17: 59–60. [DOI] [PubMed] [Google Scholar]
- 51. Goldenberg G, Kahaner K, Basavaraju N, Rangu S. Gabapentin for disruptive behaviour in an elderly demented patient. Drugs Aging 1998; 13: 183–184. [DOI] [PubMed] [Google Scholar]
- 52. Low RA Jr, Brandes M. Gabapentin for the management of agitation. J Clin Psychopharmacol 1999; 19: 482–483. [DOI] [PubMed] [Google Scholar]
- 53. Buskova J, Busek P, Nevsimalova S. Gabapentin in the treatment of dementia‐associated nocturnal agitation. Med Sci Monit 2011; 17: CS149–CS151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miller LJ. Gabapentin for treatment of behavioral and psychological symptoms of dementia. Ann Pharmacother 2001; 35: 427–431. [DOI] [PubMed] [Google Scholar]
- 55. Sheldon LJ, Ancill RJ, Holliday SG. Gabapentin in geriatric psychiatry patients. Can J Psychiatry 1998; 43: 422–423. [PubMed] [Google Scholar]
- 56. Dallocchio C, Buffa C, Mazzarello P. Combination of donepezil and gabapentin for behavioral disorders in Alzheimer's disease. J Clin Psychiatry 2000; 61: 64. [DOI] [PubMed] [Google Scholar]
- 57. Rossi P, Serrao M, Pozzessere G. Gabapentin‐induced worsening of neuropsychiatric symptoms in dementia with lewy bodies: case reports. Eur Neurol 2002; 47: 56–57. [DOI] [PubMed] [Google Scholar]
- 58. Roane DM, Feinberg TE, Meckler L, Miner CR, Scicutella A, Rosenthal RN. Treatment of dementia‐associated agitation with gabapentin. J Neuropsychiatry Clin Neurosci 2000; 12: 40–43. [DOI] [PubMed] [Google Scholar]
- 59. Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Gabapentin for the treatment of behavioural alterations in dementia: preliminary 15‐month investigation. Drugs Aging 2003; 20: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 60. Hawkins JW, Tinklenberg JR, Sheikh JI, Peyser CE, Yesavage JA. A retrospective chart review of gabapentin for the treatment of aggressive and agitated behavior in patients with dementias. Am J Geriatr Psychiatry 2000; 8: 221–225. [PubMed] [Google Scholar]
- 61. Herrmann N, Lanctot K, Myszak M. Effectiveness of gabapentin for the treatment of behavioral disorders in dementia. J Clin Psychopharmacol 2000; 20: 90–93. [DOI] [PubMed] [Google Scholar]
- 62. Raudino F, Mascalzi MG, Zagami A. Gabapentin and behavioral disorders in severe Alzheimer disease. J Clin Psychopharmacol 2004; 24: 459–460. [DOI] [PubMed] [Google Scholar]
- 63. Cooney C, Murphy S, Tessema H, Freyne A. Use of low‐dose gabapentin for aggressive behavior in vascular and mixed vascular/Alzheimer dementia. J Neuropsychiatry Clin Neurosci 2013; 25: 120–125. [DOI] [PubMed] [Google Scholar]
- 64. Bardet R. [Opposition to health care in dementia: pregabalin can be useful to facilitate the management]. Opposition aux soins dans les demences: la pregabaline peut etre utile pour faciliter la prise en charge. Presse Med 2015; 44: 1279–1281. [DOI] [PubMed] [Google Scholar]
- 65. Letterman L, Markowitz JS. Gabapentin: a review of published experience in the treatment of bipolar disorder and other psychiatric conditions. Pharmacotherapy 1999; 19: 565–572. [DOI] [PubMed] [Google Scholar]
- 66. Guay DR. Newer antiepileptic drugs in the management of agitation/aggression in patients with dementia or developmental disability. Consult Pharm 2007; 22: 1004–1034. [DOI] [PubMed] [Google Scholar]
- 67. Sommer BR, Fenn HH, Ketter TA. Safety and efficacy of anticonvulsants in elderly patients with psychiatric disorders: oxcarbazepine, topiramate and gabapentin. Expert Opin Drug Saf 2007; 6: 133–145. [DOI] [PubMed] [Google Scholar]
- 68. Pinheiro D. [Anticonvulsant mood stabilizers in the treatment of behavioral and psychological symptoms of dementia (BPSD)]. Les antiepileptiques thymoregulateurs dans le traitement des symptomes comportementaux et psychologiques de la demence (SCPD). Encephale 2008; 34: 409–415. [DOI] [PubMed] [Google Scholar]
- 69. Amann B, Pantel J, Grunze H, Vieta E, Colom F, Gonzalez‐Pinto A, et al Anticonvulsants in the treatment of aggression in the demented elderly: an update. Clin Pract Epidemiol Ment Health 2009; 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yeh YC, Ouyang WC. Mood stabilizers for the treatment of behavioral and psychological symptoms of dementia: an update review. Kaohsiung J Med Sci 2012; 28: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gallagher D, Herrmann N. Antiepileptic drugs for the treatment of agitation and aggression in dementia: do they have a place in therapy? Drugs 2014; 74: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 72. Moretti R, Torre P, Antonello RM, Cazzato G. Gabapentin as a possible treatment of behavioral alterations in Alzheimer disease (AD) patients. Eur J Neurol 2001; 8: 501–502. [DOI] [PubMed] [Google Scholar]
- 73. Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord 2004; 17: 355–359. [DOI] [PubMed] [Google Scholar]
- 74. Gober JF, Ference T, Schnitzer O. Poster 358: Gabapentin causing neurologic dysfunction leading to falls. Phys Med Rehabil 2016; 8: S278. [DOI] [PubMed] [Google Scholar]
- 75. Joint Formulary Committee . British National Formulary, 71st edn. London: BMJ Group and Pharmaceutical Press, 2016. [Google Scholar]
- 76. Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol 2017; 27: 1185–1215. [DOI] [PubMed] [Google Scholar]
- 77. Peckham AM, Evoy KE, Covvey JR, Ochs L, Fairman KA, Sclar DA. Predictors of gabapentin overuse with or without concomitant opioids in a commercially‐insured US population. Pharmacotherapy 2018; 38: 436–443. [DOI] [PubMed] [Google Scholar]
- 78. Lyndon A, Audrey S, Wells C, Burnell ES, Ingle S, Hill R, et al Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction 2017; 112: 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schjerning O, Rosenzweig M, Pottegård A, Damkier P, Nielsen J. Abuse potential of pregabalin: a systematic review. CNS Drugs 2016; 30: 9–25. [DOI] [PubMed] [Google Scholar]
- 80. Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet 2010; 49: 661–669. [DOI] [PubMed] [Google Scholar]
- 81. Schifano F, D'Offizi S, Piccione M, Corazza O, Deluca P, Davey Z, et al Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom 2011; 80: 118–122. [DOI] [PubMed] [Google Scholar]
- 82. Schjerning O, Pottegard A, Damkier P, Rosenzweig M, Nielsen J. Use of pregabalin – a nationwide pharmacoepidemiological drug utilization study with focus on abuse potential. Pharmacopsychiatry 2016; 49: 155–161. [DOI] [PubMed] [Google Scholar]
- 83. Nasser K, Kivitz AJ, Maricic MJ, Silver DS, Silverman SL. Twice daily versus once nightly dosing of pregabalin for fibromyalgia: a double‐blind randomized clinical trial of efficacy and safety. Arthritis Care Res (Hoboken) 2014; 66: 293–300. [DOI] [PubMed] [Google Scholar]
- 84. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111: 1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Investig 2017; 37: 763–773. [DOI] [PubMed] [Google Scholar]
- 86. Landefeld CS, Steinman MA. The Neurontin legacy – marketing through misinformation and manipulation. N Engl J Med 2009; 360: 103–106. [DOI] [PubMed] [Google Scholar]
- 87. Ghinea N, Lipworth W, Kerridge I. Evidence, regulation and ‘rational’ prescribing: the case of gabapentin for neuropathic pain. J Eval Clin Pract 2015; 21: 28–33. [DOI] [PubMed] [Google Scholar]
- 88. Steinman MA, Bero LA, Chren MM, Landefeld CS. Narrative review: the promotion of gabapentin: an analysis of internal industry documents. Ann Intern Med 2006; 145: 284–293. [DOI] [PubMed] [Google Scholar]
- 89. Montgomery S, Chatamra K, Pauer L, Whalen E, Baldinetti F. Efficacy and safety of pregabalin in elderly people with generalised anxiety disorder. Br J Psychiatry 2008; 193: 389–394. [DOI] [PubMed] [Google Scholar]
- 90. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 2017; 174 (Suppl. 1): S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]


