Abstract
Objective
To investigate the association between maternal anemia and low/insufficient birth weight.
Design
A prospective cohort study of pregnant women who underwent prenatal care at the healthcare units in a municipality of northeast Brazil together with their newborn infants was carried out. The pregnant women were classified as having anemia when the hemoglobin level was below 11 g/dl. Infants who were born full term weighing less than 2500 grams were classified as low birth weight, and those weighing between 2500 and 2999 grams were classified as insufficient weight. The occurrence of maternal anemia and its association with birth weight was verified using crude and adjusted Relative Risk (RR) estimates with their corresponding 95% confidence intervals (95%CIs).
Results
The final sample was comprised of 622 women. Maternal anemia was considered a risk factor for low/insufficient birth weight, after adjusting the effect measurement for maternal age, family income, urinary infection, parity, alcoholic beverage consumption during pregnancy and gestational body mass index: RRadjusted = 1.38 [95% CI: 1.07 to 1.77].
Conclusions
Maternal anemia was associated with low/insufficient birth weight, representing a risk factor for the gestational outcomes studied.
Introduction
Low birth weight has been widely studied and is an important risk factor for infant morbidity and mortality [1–4]. However, insufficient weight has received little attention [5–8], even though three decades ago, children with birth weights less than 3000 grams were considered to have a risk of mortality that was three times higher during the first year of life than that of children whose weights were above or equal to this cutoff point [9,10]. In Brazil, 2017, 8.49% of the newborn infants had weight <2500g. In this same year, the frequency of children with insufficient weight, that is ≥ 2500g and <3000g, was almost three times more 22.41%, increasing the necessity to investigate this weight special group [7].
The classic risk factors for low birth weight are associated with unfavorable biological, social and environmental conditions that may occur before or during the pregnancy period [4, 11–13]. Nutritional determinants, such as pre-gestational weight and weight gain during pregnancy, influence birth weight. Thus, inadequate maternal caloric intake, which may be the result of a diet that is nutritionally poor, leads to lower absorption of essential micronutrients, such as vitamin B12 and iron, for fetal growth [14].
Although the determinants of both low and insufficient weight at birth are similar, the mechanism that links maternal anemia to insufficient birth weight is not fully known. Few prospective cohort studies have analyzed the association between maternal anemia and low birth weight [15–25]. Indeed, after a rigorous search of previous investigations on the topic, only two retrospective cohort studies, from Colombia and Finland, that addressed the relationship between nutritional exposure and insufficient birth weight were identified [5, 6].
Given the relevance of the theme, the high frequency of insufficient birth weight in the studied group, and the knowledge that the association between maternal anemia and birth weight is affected by demographic and socioeconomic factors, an investigation of this relationship in diverse populations is necessary to identify the groups at greatest risk. The objective of this study was to verify the frequency of maternal anemia and its association with low/insufficient birth weight in users of the public health service from a population in northeastern Brazil.
Materials and methods
Study design/population
A prospective analytical cohort study was carried out with pregnant women, who underwent prenatal follow-up at healthcare units in the urban area of Santo Antônio de Jesus, Bahia, Brazil, and their newborns. The data collection period was from January 2013 to March 2017.
Sample size
To calculate the sample size, the following parameters were used: an Odds Ratio of 2.36 [26], the frequency of the rarest outcome, a low-birth-weight incidence of 8.29% in the group of pregnant women without anemia [26] and a ratio of exposed versus not exposed to anemia of 1:3. A study power of 80%, alpha error of 5% and the 95% confidence interval were also considered. Thus, the minimum estimated sample size was 141 pregnant women in the group diagnosed with anemia and 421 in the non-anemic group. In addition, 10% was added to the sample size to correct for possible losses, with a minimum sample calculation of 618 pregnant women. The sample size was calculated using Epi Info (StatCalc), version 7 [27].
The present study was approved by the Research Ethics Committee of Feira de Santana State University. All pregnant women voluntarily participated in the study and signed the Free Consent Form.
Eligibility criteria
Inclusion criteria
The pregnant women in this study had the following: pregnancy with fetal gestational ages between 8 and 32 weeks, assistance from the public health system, prenatal care from the selected healthcare units, live births, and children available for the study.
Exclusion criteria
Women were excluded from the study if they had a twin pregnancy, preterm birth or a history of bleeding that required hospital treatment for at least 24 hours.
Data collection procedures
Information concerning the pregnant women was first obtained through interviews. Then, trained researchers performed blood collections, and oral examinations of all teeth were performed by a dentist according criteria defined previously [28, 29].
The data that could not be obtained during the interview were acquired from the patient's chart and/or pregnancy card. During the postpartum period, information on birth weight was collected from the birth registration document (Declaration of Live Births).
Data collection tools
The questionnaire form was divided into six sections: 1) identification, socioeconomic-demographic data and environmental data; 2) nutritional information; 3) gynecological-obstetric history; 4) drug information; 5) variables related to the anthropometry of the pregnant woman; and 6) information concerning childbirth.
Blood collection for laboratory tests
The blood collection followed the standard criteria of collection and storage [30] to obtain the complete blood count and ferritin dosage.
Birth weight
Birth weight was measured immediately after delivery on a precise scale and subsequently recorded in the Declaration of Live Birth by a health professional who participated in the birth [31].
Criteria for the definitions of exposure and outcome
Exposure: Maternal anemia
Study participants were diagnosed as having maternal anemia when the hemoglobin level was below 11 g/dl or when the hematocrit was less than 33% (32). Additionally, the participants were diagnosed with iron deficiency anemia when the reference value for serum ferritin was less than 15 femtoliters and the mean corpuscular volume (MCV) was less than 80 femtoliters [32]. Participants were diagnosed with anemia of chronic disease when the MCV was normal, from 80 to 96 femtoliters, and when they had the aforementioned hemoglobin level [32]. In addition, hemoglobin levels were evaluated in their continuous form.
Outcome: Low/Insufficient birth weight
The classification of birth weight was defined according to the criteria of the World Health Organization [2]. Children born with weights above or equal to 3000 grams were allocated to the group of newborns with satisfactory weights. Newborns with birth weights less than 2500 grams were classified as low birth weight, and those weighing between 2500 and 2999 grams were classified as insufficient weight. In addition, the birth weight was evaluated in its continuous form.
Procedure for analyzing the data
Descriptive analysis for all selected variables was performed, according to the relative and absolute frequency. The Kolmogorov-Smirnov test [33] was applied, and histogram inspection was performed to verify the normality of the continuous variables. Student's t-test [34] or the Mann-Whitney U test [35] was used, according to the normality test of the variable, employing the mean, median and standard deviation to verify the differences between the groups. Categorical covariables were also evaluated for distribution, the presence of maternal anemia and weight less than 3000 grams using the chi-square test [36] or Fisher's exact test [37], with a significance level of 5%.
The investigation of the association between maternal anemia and low/insufficient birth weight was performed using logistic regression analysis by estimating the crude and adjusted odds ratio (OR) with their 95% confidence intervals and a significance level of 5%. Poisson model was used to convert the association measurement in relative risk (RR) with it 95% confidence interval.
Initially, a conceptual framework was adopted to select the following covariables involved in the multicausality of the association between maternal anemia and low/insufficient birth weight: maternal age, family income, urinary infection, parity, alcoholic beverage consumption during pregnancy and gestational BMI.
Subsequently, potential confounding factors and effect modification were selected by stratified analysis. The interaction identification was performed using the maximum likelihood ratio test, according to the definition of the saturated and reduced models for each of the possible effect modifier variables. A covariable was considered confounding when, after being eliminated from the saturated model, the covariable promoted a variation in the effect measurement that was greater than 10%compared to the main association measurement. In addition, the criterion of epidemiological importance for the selection of confounding covariables was adopted during modeling. The diagnosis of the model was performed using the Hosmer-Lemeshow test [38].
To evaluate the relationship between hemoglobin levels and the outcome, the multicollinearity test was performed, followed by univariate and multiple linear regression analysis using the common minimum squares technique [39], after confirming the linearity between the main variables by means of the F test [40]. For the adjusted model, the covariables that were considered to be confounders were used. These covariables included those that altered the parameters of the null model based on the statistical criteria (p <0.20) and those of epidemiological relevance. Stata software, version 15, was used for data processing and analysis [41].
Results
The sample consisted of 622 pregnant (Fig 1) women who utilized the public health service of the municipality of Santo Antônio de Jesus, BA. The mean age of the participants was 25.5 years (± 6.5 SD), with a median of 25 years and a range of 13 to 46 years. The refusal rate was 2%.
Fig 1. Flowchart of the sample participant selection process.
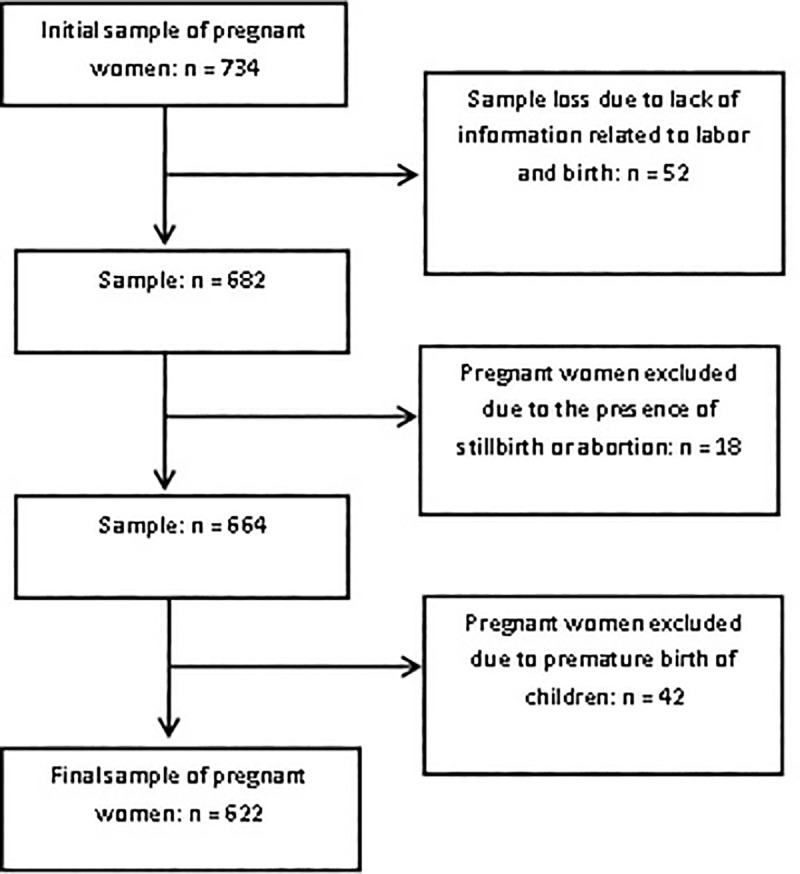
The pregnant women were classified into two groups per the maternal anemia diagnostic criteria: 24.9% (n = 155) with anemia and 75.1% (n = 467) without anemia. Regarding the severity of maternal anemia, 20.1% of the pregnant women were diagnosed with mild anemia, and 4.8% were diagnosed with moderate anemia; there was no record of severe maternal anemia. The frequency of iron deficiency anemia was 6.0% among the participants, whereas the frequency of anemia of chronic disease was 18.9%. Iron deficiency was present in 16.4% of the pregnant women.
The socioeconomic-demographic data are described in Table 1, and the data related to the maternal condition are shown in Table 2. Among the characteristics, only urinary infection, parity and the late onset of prenatal care showed statistically significant differences between the women with anemia and those without anemia, indicating that for most covariables, the comparison groups were homogeneous.
Table 1. Number (n) and percentage (%) of the socioeconomic-demographic characteristics of the sample, according to the presence of anemia.
Santo Antônio de Jesus, Bahia, Brazil, 2017 (n = 622).
| Maternal Anemia | |||
|---|---|---|---|
| CHARACTERISTICS | Yes | No | p† |
| n (%) | n (%) | ||
| 155 (24.9) | 467 (75.1) | ||
| AGE (years) | |||
| 18–35 | 120 (24.0) | 380 (76.0) | |
| <18 | 21 (33.9) | 41 (66.1) | 0.09 |
| >35 | 14 (23.3) | 46 (76.7) | 0.91 |
| EDUCATION LEVEL (years) | |||
| ≥8 | 108 (24.2) | 338 (75.8) | |
| < 8 | 47 (26.7) | 129 (73.3) | 0.52 |
| CONJUGAL STATUS | |||
| With partner | 140 (25.0) | 419 (75.0) | |
| Without partner | 15 (23.8) | 48 (76.2) | 0.83 |
| RACE/SKIN COLOR | |||
| Not black | 91 (24.0) | 288 (76.0) | |
| Black | 64 (26.3) | 179 (73.7) | 0.51 |
| CURRENT OCCUPATION | |||
| Paid | 65 (23.0) | 218 (77.0) | |
| Unpaid | 90 (26.6) | 249 (73.4) | 0.30 |
| FAMILY INCOME* | |||
| > 2 minimum wages | 51 (27.4) | 135 (72.6) | |
| ≤ 2 minimum wages | 104 (23.9) | 332 (76.1) | 0.35 |
| HOUSEHOLD DENSITY (number of people per household) | |||
| ≤ 4 | 132 (25.8) | 379 (74.2) | |
| >4 | 23 (20.7) | 88 (79.3) | 0.26 |
*Minimum wage values (per month) at the time of collection: 2013, R $ 678.00; 2014, R $ 724.00; 2015, R $ 788.00; 2016, R $ 880.00; 2017, R $ 937.00.
†p value: level of significance ≤ 0.05.
Table 2. Number (n) and percentage (%) of the characteristics related to the health and lifestyle of the sample, according to the presence of maternal anemia.
Santo Antônio de Jesus, Bahia, Brazil, 2017 (n = 622).
| Maternal Anemia | |||
|---|---|---|---|
| CHARACTERISTICS | Yes | No | p† |
| n (%) | n (%) | ||
| 155 (24.9) | 467 (75.1) | ||
| SEX OF THE NEWBORN | |||
| Male | 70 (22.9) | 236 (77.1) | 0.25 |
| Female | 85 (26.9) | 231 (73.1) | |
| URINARY INFECTION | |||
| No | 141 (24.1) | 445 (75.9) | |
| Yes | 14 (38.9) | 22 (61.1) | 0.05 |
| PERIODONTITIS** | |||
| No | 111 (23.5) | 361 (76.5) | 0.68 |
| Yes | 23 (25.6) | 67 (74.4) | |
| MATERNAL ARTERIAL HYPERTENSION | |||
| No | 151 (25.0) | 454 (75.0) | |
| Yes | 4 (23.5) | 13 (76.5) | 0.89 |
| ABORTION | |||
| No | 128 (25.2) | 380 (74.8) | |
| Yes | 27 (23.7) | 87 (76.3) | 0.74 |
| GESTATIONAL BMI* | |||
| Proper weight | 72 (25.3) | 213 (74.7) | |
| Low weight | 32 (27.6) | 84 (72.4) | 0.63 |
| Overweight | 40 (26.9) | 109 (73.1) | 0.72 |
| Obese | 11 (15.3) | 61 (84.7) | 0.08 |
| PARITY | |||
| > 2 children | 118 (33.5) | 234 (66.5) | 0.01 |
| ≤ 2 children | 65 (24.1) | 205 (75.9) | |
| BEGINNING OF THE PRENATAL ACCOMPANIMENT | |||
| ≤ 3 months | 128 (23.4) | 419 (76.6) | 0.02 |
| > 3 months | 27 (36.0) | 48 (64.0) | |
| MATERNAL SMOKING HABIT‡ | |||
| No | 140 (24.6) | 429 (75.4) | 0.89 |
| Yes | 38 (25.5) | 38 (74.5) | |
| ALCOHOLIC BEVERAGE CONSUMPTION DURING PREGNANCY | |||
| No | 124 (25.1) | 371 (74.9) | 0.54 |
| Yes | 24 (22.2) | 84 (77.8) | |
| FERROUS SALT SUPPLEMENTATION DURING PREGNANCY | |||
| No | 30 (20.0) | 120 (80.0) | |
| Yes | 125 (26.5) | 347 (73.5) | 0.11 |
* The Atalah curve was employed to calculate this covariable
** Gomes-Filho criteria for the definition of periodontitis28
† p value: level of significance ≤ 0.05
‡ There were deficits in this information.
Regarding birth weight, 29.4% (183) of the participants had children with birth weights less than 3000 g, with 3.4% of the live births being classified as low birth weight and 26% being classified as insufficient birth weight. The frequency of mothers who had children of satisfactory weight was 70.6% (439). The centile for birthweight were 2940 grams, 3272 grams and 3565 grams for 25%, 50% and 75%, respectively
The central tendency measures of the descriptors used for the diagnosis of maternal anemia, according to birth weight, are summarized in Table 3. Notably, statistically significant differences were evident only for the mean values of hemoglobin (p = 0.03) and hematocrit (p = 0.02).
Table 3. Central tendency and dispersion measurements of the descriptors used to evaluate maternal anemia, according to the newborn weight, in users of the public health system in Santo Antônio de Jesus, Bahia, Brazil, 2017 (n = 622).
| Weight < 3000 g | Weight ≥ 3000 g | ||||||
|---|---|---|---|---|---|---|---|
| Descriptors | Mean | ±SD* | Median | Mean | ±SD* | Median | p† |
| Hemoglobin level (g/dl) ‡ | 11.6 | ±1.1 | 11.6 | 11.8 | ±1.1 | 11.9 | 0.03 |
| Red blood cell count (millions) ‡ | 4.1 | ±0.4 | 4.1 | 4.6 | ±8.7 | 4.2 | 0.14 |
| Hematocrit (%)‡ | 35.1 | ±3.2 | 35.2 | 36.0 | ±4.2 | 36.3 | 0.02 |
| Ferritin level (femtoliters) ‡ | 44.8 | ±38.9 | 31.8 | 45.2 | ±40.2 | 32.2 | 0.89 |
| Mean Corpuscular Volume—MCV (femtoliters) ‡ | 85.9 | ±7.1 | 85.0 | 87.0 | ±5.5 | 86.4 | 0.06 |
* SD: Standard deviation
† p value: level of significance ≤ 0.05
‡ Reference value: Hemoglobin: ≥ 11 g / dl; Blood cell count: > 4 million; Hematocrit: ≥ 33%; Ferritin: ≥ 15 femtoliters; MCV = 80–96 femtoliters.
Women diagnosed with maternal anemia showed a significantly higher incidence of children with birth weights<3000 g than the women who were not exposed to anemia during pregnancy (RRcrude = 1.36; 95% CI: 1.06 to 1.76). According to the multiple-adjusted model, pregnant women with anemia had a 38% higher risk of having children with low/insufficient weight at birth than the women without anemia (RRadjusted = 1.38; 95% CI: 1.07 to 1.77; Table 4). The model was adjusted for the following confounders: maternal age, family income, urinary infection, parity, alcoholic beverage consumption during pregnancy and gestational body mass index. The quality of this model was considered good because the null hypothesis was rejected (p = 0.74).
Table 4. Crude and adjusted Relative Risk (RR) of the association between maternal anemia and low/insufficient birth weight with the corresponding 95% confidence intervals (95% CI).
| Maternal anemia | Birth weight | |||||||
|---|---|---|---|---|---|---|---|---|
| <3000 g | ≥3000 g | RRcrude | 95% CI p* | RRadjusted† | 95% CI p* | |||
| N | % | N | % | |||||
| Yes | 57 | 36.8 | 98 | 63.2 | 1.36 | 1.06 to 1.76 0.02 | 1.38 | 1.07 to 1.77 0.01 |
| No | 126 | 27.0 | 341 | 73.0 | ||||
* p value: level of significance ≤ 0.05
†Adjusted by maternal age, family income, urinary infection, parity, alcoholic beverage consumption during pregnancy and gestational BMI. Model fit test (Hosmer-Lemeshow): p = 0.74.
The linear regression analysis showed that on average, there was a 21-g decrease (p = 0.03) in the weight of the newborn per 1 g/dl of reduced maternal hemoglobin during the gestational period. In the saturated model, which was adjusted for the above-mentioned confounders, there was a 0.20-g decrease (p = 0.05) in birth weight per 1 g/dl of maternal hemoglobin lost during pregnancy.
Discussion
Despite a rigorous search of a large number of electronic databases, only two cohort studies were found that discussed the relationship between maternal anemia and insufficient birth weight [5, 6]. Studies addressing anemic exposure and low birth weight were more frequent. Regardless of the lack of previous investigations on the topic, the main results of the present investigation highlighted maternal anemia as a risk factor for low/insufficient birth weight, which was consistent with data from a previous study by Raisanen et al. (2014) [5]. Thus, these findings contribute to current knowledge concerning this important public health problem. Other findings from the present study using linear regression analysis confirmed the above association since women with hemoglobin depletion had children with significantly reduced birth weights. Only one investigation had contrasting findings [6], showing no association between maternal anemia and low/insufficient birth weight. In addition, the incidence of maternal anemia in the current sample was approximately 25%, corroborating the frequency found in other studies [17, 42–44]. Among these women, the incidence of children with low/insufficient birth weight was approximately 37%, whereas in children without anemia, the incidence was 27%.
The biological plausibility of the association between maternal anemia and low/insufficient birth weight is not fully understood [45, 46]. However, previous studies have argued that maternal anemia predisposes the fetus to intrauterine growth restriction and may consequently influence the birth weight [9, 47]. Physiologically, beginning during the middle of the second trimester of pregnancy, women produce an average of 30 to 40 ml of plasma per kilogram, corresponding to hypervolemia. However, when the number of hematological cells does not increase in parallel with this process, hemodilution occurs, and maternal anemia may develop [48].
Thus, low hemoglobin levels may stimulate changes in placental angiogenesis and favor fetal hypoxia. According to this theory, a reduction in nutrients and oxygen to the fetus due to deficits in placental transport may result from hemoglobin depletion. The potential framework of uterine growth restriction begins with a reduction in blood perfusion in the uterus, an elevation in vascular resistance and growth restriction of the trophoblastic surface, which is responsible for ejecting maternal arterial blood into the placenta. These events may result in the restriction of gas exchange within the maternal-fetal complex and, consequently, in low/insufficient birth weight [49].
Within the scope of the present investigation, the findings of only one study clearly point to the association between maternal anemia and a birth weight of less than 3000 g [5] since this exposure is often studied only in terms of its relationship with low birth weight [50–52]), without the inclusion of insufficient weight.
Multiple investigations have shown that maternal anemia is associated with low birth weight [53–55]. Conversely, several other studies that included only low birth weight as the outcome refuted the hypothesis under investigation [23, 56, 57], making the association controversial. Although discussions about the damage from insufficient birth weight have been carried out for more than 30 years, few studies have evaluated the relationship between insufficient weight and various undesirable gestational events [8–10, 58], emphasizing only the extreme ranges of birth weight, such as low weight and macrosomia [50–52].
A similarity does exist between low birth weight and insufficient weight, but the former is considered more serious than the latter for the newborn. However, the unfavorable effect produced by insufficient birth weight cannot be ignored since this condition may contribute to inadequate cognitive development and infant growth and increase the morbidity and mortality of this age group [3].
The findings of the present investigation are relevant in that they contribute to a better understanding of the importance of insufficient birth weight to pediatric health, and several method-related parameters, such as the sample size, diagnostic criteria for maternal anemia and treatment of confounders, should be carefully evaluated when comparing these results to those of previous studies on the topic.
The sample size of the present study exceeded the minimum calculated sample size to estimate the effect measurement. The total number of pregnant women involved in the study by Mesa et al. (2012) [6] was approximately half of that employed in the present investigation and may explain the non-association, which was probably due to the lack of power in the study, found between maternal anemia and low/insufficient birth weight. However, Raisanen et al. (2014) [5] evaluated a large sample comprising 290,622 pregnant women and found a positive association, corroborating the findings of the present study.
Regarding the diagnosis of anemia in the present investigation, to improve internal validity, a laboratory test was used to define maternal anemia, establishing a hemoglobin reference value <11 g/dl and confirming the result with a hematocrit <33%. These criteria are recommended by the World Health Organization [32]. Mesa et al. (2012) [6] used the same criteria, although the authors did not confirm the diagnosis of maternal anemia by hematocrit. However, Raisanen et al. (2014) [5] defined anemia using only the information contained in the hospital chart.
The subclassification of maternal anemia is highly valuable to ensure the adequate monitoring of pregnant women. For example, the diagnosis of iron deficiency anemia may facilitate therapy for the disease with iron supplementation. Increasingly, iron deficiency anemia has been classified as maternal anemia since multiple reports in the literature support the hypothesis that the primary cause of anemia in pregnant women is ferritin deficiency [17, 59–62]. This notion conflicts with the findings presented in this study because most women with the anemia were diagnosed with anemia of chronic disease (18.9%), with a low frequency of iron deficiency anemia (6.0%), thus corroborating other studies [63–65].
Regarding severity levels, the participants in this study had a higher frequency of mild anemia. This severity was expected because much of the research converges in this direction [60, 66–69].
In the present investigation, the effect measurement was adjusted by confounders, due to knowledge of the possible effects of these covariables on both the exposure factor and the outcome. This criterion was also used in the study by Raisanen et al. (2014) [5], which corroborated the findings of this study. In contrast, Mesa et al. (2012) [6] did not adjust for the confounding covariables, and this lack of adjustment may explain why their findings contrasted the association verified in the present research.
According to the conceptual framework adopted here and the multicausality involved in the association between maternal anemia and low/insufficient birth weight, the following covariables were considered in the adjustment of the final model: maternal age, family income, urinary infection, parity, alcoholic beverage consumption during pregnancy and gestational BMI.
Unfavorable socioeconomic-demographic factors can influence both the exposure and the gestational outcome of interest. Maternal age, in its extreme age ranges, is a classical confounding variable because younger women do not have complete biological maturity for gestation and because older women are more likely to have comorbidities, such as maternal anemia [25, 64]). Regarding family income, pregnant women who have lower purchasing power are more vulnerable in terms of poor living conditions and, consequently, health [43, 55]).
Infectious processes, such as urinary infection, influence the metabolism of new hemoglobin, leading to the development of maternal anemia [16], and intrauterine growth restriction, contributing to low/insufficient birth weight. Another relevant factor is parity since multiparity is a condition that can favor both low/insufficient birth weight and maternal anemia [25, 43].
Alcoholic beverage consumption during pregnancy can cause inflammatory disorders that restrict intrauterine growth, a contributing factor to low/insufficient birth weight [69, 70]. Alcohol consumption can also compromise caloric intake, making it insufficient and, consequently, favoring the development of maternal anemia [69, 70]. Women with inadequate nutrition are susceptible to both maternal anemia and having low/insufficient-birth-weight children, probably due to the poor dietary intake of essential micronutrients during pregnancy [43, 70].
Regarding the limitations of this investigation, the self-reported information may have produced calibration bias. Although the sample is representative of the urban population of the municipality investigated, caution should be exercised when interpreting the results by extrapolating them to other locations that do not have a population group that is similar to the one in this study. Other limitation is the exclusion of preterm births, since the source of information in Brazil about gestational age may be distorted, due to the absence of standardization of criteria used to define preterm birth. On the other hand, the low birth weight as outcome gave higher quality to the measurement due to its standardized form of collection [29, 71]. Regarding the definition of anemia, the criterion used was that standard, known worldwide and suggested by the WHO [32], which does not take into account the gestational trimester. As in any other investigation, the possibility of residual confounding remains since some factors may not have been measured in the present study.
Finally, this research can contribute information that has not previously been elucidated toward confirming the hypothesis. The temporality of the events, namely, the order of the exposure relative to the outcome, is established since the laboratory tests for the diagnosis of maternal anemia preceded delivery and birth. Possible sample losses were measured prior to the study, which assures the representativeness and power of the sample size and the reliability of the presented findings. The adoption of these criteria also minimizes the possibility of a spurious association. Furthermore, the use of validated instruments by the researchers, who were previously trained, strengthens the internal validity of this study.
Based on the method employed and the limitations described, the exposure investigated was confirmed as a risk factor for low/insufficient birth weight. Given the findings, a far-reaching measure in public health would be the implementation of healthcare actions for the prevention and control of maternal anemia, aiming to reduce unfavorable gestational outcomes.
Data Availability
The data of this research are confidential according to guidelines of the Ethics Committee in Research of the State University of Feira de Santana and Brazilian Law of Ethical Aspects 466/2012. The data can be requested to the main author of this research through the email: nesufrb@outlook.com. Upon re-submitting your revised manuscript, please upload your study’s minimal underlying data set as either Supporting Information files or to a stable, public repository and include the relevant URLs, DOIs, or accession numbers within your revised cover letter. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories. Any potentially identifying patient information must be fully anonymized. Important: If there are ethical or legal restrictions to sharing your data publicly, please explain these restrictions in detail. Please see our guidelines for more information on what we consider unacceptable restrictions to publicly sharing data: http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions. Note that it is not acceptable for the authors to be the sole named individuals responsible for ensuring data access. We will update your Data Availability statement to reflect the information you provide in your cover letter." Data can not be shared publicly because it is an original research conducted by a university. However, the data is available to researchers who meet the criteria for accessing confidential data through the principal investigator's email.
Funding Statement
This work was financial support from the Research Support Foundation of the State of Bahia (Fundação de Amparo à Pesquisa do Estado da Bahia-FAPESB)–PPSUS 0025/2014, Salvador, Bahia, Brazil; The National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq)–Universal 457809/2014-0, Brasília, Brazil and t his study was financed in part by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior)– Brasília, Brazil, (CAPES) – Finance Code 001”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Meeting of Advisory Group on Maternal Nutrition and Low Birthweight Genebra: WHO; 2002 Internet: http://www.who.int/nutrition/topics/lbw_strategy_background.pdf (accessed 23 august 2017).
- 3.Moraes IB. Risk factors for underweight at birth. Campinas, São Paulo: Universidade Estadual de Campinas, 2001. [Google Scholar]
- 4.UNICEF. Low Birthweight: Country,regional and global estimates. New York: UNICEF; 2004. [Google Scholar]
- 5.Raisanen S, Kancherla V, Gissler M, Kramer MR, Heinonen S. Adverse perinatal outcomes associated with moderate or severe maternal anaemia based on parity in Finland during 2006–10. Paediatr Perinat Epidemiol. 2014;28(5):372–80. 10.1111/ppe.12134 [DOI] [PubMed] [Google Scholar]
- 6.Mesa SLR, Sosa BEPS, Gómez JA, López NZ, Díaz CAG, Moreno CMR, Alarca NAC, Vásquez LEE. Maternal nutritional status and its relationship with birth weight of the newborn, pregnant women study Public Network of Medellín, Colombia Perspect Nutr Humana. 2012;14(201):199–208. [Google Scholar]
- 7.BRASIL. Ministério da Saúde. DATASUS. Informações relacionadas à saúde: indicadores de saúde (nascidos vivos), 2017.
- 8.Costa RSC, Caldevilla DE, Gallo PR, Sena BF, Leone C. Incidence and characteristics of insufficient birth weight Newborns from a cohort of neonates in a public regional Hospital of a metropolitan area. Journal of Human Growth and Development. 2013;23(2):238–44. [Google Scholar]
- 9.Puffer RR. Patterns of birthweights: a summary. Bull Pan Am Health Organ. 1987;21(2):185–95 (abrst). [PubMed] [Google Scholar]
- 10.Rees JM, Lederman SA, Kiely JL. Birth weight associated with lowest neonatal mortality: infants of adolescent and adult mothers. Pediatrics. 1996;98(6 Pt 1):1161–6. [PubMed] [Google Scholar]
- 11.Carniel EF, Zanolli ML, Antônio MRGM, Morcillo AM. Determinants for low birth weight according to Live Born Certificates. Rev. bras. epidemiol. 2008;11:169–79. 10.1590/S1415-790X2008000100016 [DOI] [Google Scholar]
- 12.Melo ASO, Assunção PL, Gondim SSR, Carvalho DF, Amorim MMR, Benicio MHDA, Cardoso MAA. Estado nutricional materno, ganho de peso gestacional e peso ao nascer. Rev. bras. epidemiol. 2007;10:249–57. 10.1590/S1415-790X2007000200012 [DOI] [Google Scholar]
- 13.Geib LTC, Fréu CM, Brandão M, Nunes ML. Social and biological determinants of infant mortality in population cohort in the city of Passo Fundo, Rio Grande do Sul State. Ciênc. saúde coletiva. 2010;15:363–70. 10.1590/S1413-81232010000200011. [DOI] [PubMed] [Google Scholar]
- 14.Bresani CC, Souza AId, Batista-Filho M, Figueiroa JN. Anemia and iron deficiency in pregnant women: disagreements among the results of a cross-sectional study. Rev Bras Saúde Matern Infant. 2007; Supl.1:S15–S22. 10.1590/S1519-38292007000600002. [DOI] [Google Scholar]
- 15.Yip R. Significance of an abnormally low or high hemoglobin concentration during pregnancy: special consideration of iron nutrition. Am J Clin Nutr 2000; 72: 272S–9S. 10.1093/ajcn/72.1.272S [DOI] [PubMed] [Google Scholar]
- 16.Donovan A, Roy C, Andrews N. The ins and outs of iron homeostasis. Physiology. 2006. 21:115–23. 10.1152/physiol.00052.2005 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015. [Google Scholar]
- 18.Batista Filho M, Souza AI, Bresani CC. Anemia as a public health problem: the current situation) Ciênc. saúde coletiva. 2008; 13:1917–22. 10.1590/S1413-8123200800060002. [DOI] [PubMed] [Google Scholar]
- 19.Peña-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2012(7):CD009997 10.1002/14651858.CD009997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, Ota E, Gilmour S, Shibuya K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495–504. 10.3945/ajcn.115.107896 [DOI] [PubMed] [Google Scholar]
- 21.Rocha DS, Netto MP, Priore SE, Lima NMM, Rosado LEFPL, Franceschini SCC. Nutritional status and iron-deficiency anemia in pregnant women: relationship with the weight of the child at birth. Rev Nutr. 2005;18(4):481–9. 10.1590/S1415-52732005000400004. [DOI] [Google Scholar]
- 22.Grotto HZ. Anaemia of cancer: an overview of mechanisms involved in its pathogenesis. Med Oncol. 2008;25(1):12–21. 10.1007/s12032-007-9000-8 [DOI] [PubMed] [Google Scholar]
- 23.Abeysena C, Jayawardana P, de A Seneviratne R. Maternal haemoglobin level at booking visit and its effect on adverse pregnancy outcome. Aust N Z J Obstet Gynaecol. 2010;50(5):423–7. 10.1111/j.1479-828X.2010.01220.x [DOI] [PubMed] [Google Scholar]
- 24.Bakacak M, Avci F, Ercan O, Köstü B, Serin S, Kiran G, Bostanci MS, Bakacak Z. The effect of maternal hemoglobin concentration on fetal birth weight according to trimesters. J Matern Fetal Neonatal Med. 2015;28(17):2106–10. 10.3109/14767058.2014.979149 [DOI] [PubMed] [Google Scholar]
- 25.Koura GK, Ouedraogo S, Le Port A, Watier L, Cottrell G, Guerra J, et al. Anaemia during pregnancy: impact on birth outcome and infant haemoglobin level during the first 18 months of life. Trop Med Int Health. 2012;17(3):283–91. 10.1111/j.1365-3156.2011.02932.x [DOI] [PubMed] [Google Scholar]
- 26.Padhi BK, Baker KK, Dutta A, Cumming O, Freeman MC, Satpathy R, Das BS, Panigrahi P. Risk of Adverse Pregnancy Outcomes among Women Practicing Poor Sanitation in Rural India: A Population-Based Prospective Cohort Study. PLoS Med. 2015;12(7):e1001851 10.1371/journal.pmed.1001851 eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CDC. Epi info, version 7. Centers for Disease Control and Prevention. 2017 Internet: https://www.cdc.gov/epiinfo/index.html. (accessed 02 august 2017).
- 28.Gomes Filho IS, Macedo TCN, Cruz SS, Soledade KR, Trindade SC, Sarmento VA. Comparison of clinical diagnosis criteria to establish periodontal disease. Rev odonto ciênc. 2006;21(51). [Google Scholar]
- 29.Gomes-Filho IS, Cruz SS, Rezende EJ, Dos Santos CA, Soledade KR, Magalhães MA, et al. Exposure measurement in the association between periodontal disease and prematurity/low birth weight. J Clin Periodontol. 2007;34(11):957–63. 10.1111/j.1600-051X.2007.01141.x [DOI] [PubMed] [Google Scholar]
- 30.Andriolo A, Ballarati CAF, Galoro CAO, Mendes ME, Melo MR, Sumita NM. Collection and Preparation of the Biological Sample. Barueri, São Paulo: Minha Editora, 2014. [Google Scholar]
- 31.Brasil. Manual de instruções para o preenchimento da declaração de nascido vivo Language in Portuguese. (Instruction manual for filling in the declaration of live birth) 3 ed Brasília: Ministério da Saúde, 2001. [Google Scholar]
- 32.World Health Organization. Iron Deficiency Anaemia:Assessment, Prevention, and Control: A guide for programme managers. Geneva: World Health Organization, 2001. [Google Scholar]
- 33.Ong LD, LeClare PC. The Kolmogorov-Smirnov test for the log-normality of sample cumulative frequency distributions. Health Phys. 1968;14(4):376. [PubMed] [Google Scholar]
- 34.Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics. 1946;2(6):110–4. [PubMed] [Google Scholar]
- 35.Mann H, Whitney D. On a test whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- 36.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philosophical Magazine Series 5. 1900;50(302):157–75. [Google Scholar]
- 37.Fisher RA. On the interpretation of χ 2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85(1):87–94. [Google Scholar]
- 38.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. New York: Wiley, 2013. [Google Scholar]
- 39.Davidson R, MacKinnon JG. Estimation and Inference in Econometrics. New York: Oxford University Press, 1993. [Google Scholar]
- 40.Box G. E. P. "Non-Normality and Tests on Variances. Biometrika. 1953; 40 (3/4): 318–335 [Google Scholar]
- 41.STATA. Data analysis and statistical software, version 15. Serial number: 401506208261. 2017.
- 42.Vural T, Toz E, Ozcan A, Biler A, Ileri A, Inan AH. Can anemia predict perinatal outcomes in different stages of pregnancy? Pak J Med Sci. 2016;32(6):1354–9. 10.12669/pjms.326.11199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bener A, Salameh KM, Yousafzai MT, Saleh NM. Pattern of Maternal Complications and Low Birth Weight: Associated Risk Factors among Highly Endogamous Women. ISRN Obstet Gynecol. 2012;2012:540495 10.5402/2012/540495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekhavat L, Davar R, Hosseinidezoki S. Relationship between maternal hemoglobin concentration and neonatal birth weight. Hematology. 2011;16(6):373–6. 10.1179/102453311X13085644680186 [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Domenico I, Vaughn MB, Paradkar PN, Lo E, Ward DM, Kaplan J. Decoupling ferritin synthesis from free cytosolic iron results in ferritin secretion. Cell Metab. 2011;13(1):57–67. 10.1016/j.cmet.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Harrison KA, Ibeziako PA. Maternal anaemia and fetal birthweight. J Obstet Gynaecol Br Commonw. 1973;80(9):798–804. [DOI] [PubMed] [Google Scholar]
- 48.Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr. 2010; 2010:401323 10.1155/2010/401323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stangret A, Wnuk A, Szewczyk G, Pyzlak M, Szukiewicz D. Maternal hemoglobin concentration and hematocrit values may affect fetus development by influencing placental angiogenesis. J Matern Fetal Neonatal Med. 2017; 30(2):199–204. 10.3109/14767058.2016.1168395 [DOI] [PubMed] [Google Scholar]
- 50.Ali AA, Rayis DA, Abdallah TM, Elbashir MI, Adam I. Severe anaemia is associated with a higher risk for preeclampsia and poor perinatal outcomes in Kassala hospital, eastern Sudan. BMC Res Notes. 2011;4:311 10.1186/1756-0500-4-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Misra A, Ray S, Patrikar S. A longitudinal study to determine association of various maternal factors with neonatal birth weight at a tertiary care hospital. Med J Armed Forces India. 2015;71(3):270–3. 10.1016/j.mjafi.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trojner Bregar A, Blickstein I, Bržan Šimenc G, Janša V, Verdenik I, Lučovnik M, Tul N. Perinatal Advantages and Disadvantages of Being Underweight before Pregnancy: A Population-Based Study. Gynecol Obstet Invest. 2017; 82(3):303–6. 10.1159/000447557 [DOI] [PubMed] [Google Scholar]
- 53.Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015. (10):CD009997 10.1002/14651858.CD009997.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lone FW, Qureshi RN, Emmanuel F. Maternal anaemia and its impact on perinatal outcome in a tertiary care hospital in Pakistan. East Mediterr Health J. 2004;10(6):801–7. [PubMed] [Google Scholar]
- 55.Msuya SE, Hussein TH, Uriyo J, Sam NE, Stray-Pedersen B. Anaemia among pregnant women in northern Tanzania: prevalence, risk factors and effect on perinatal outcomes. Tanzan J Health Res. 2011;13(1):33–9. [DOI] [PubMed] [Google Scholar]
- 56.Abioye AI, Aboud S, Premji Z, Etheredge AJ, Gunaratna NS, Sudfeld CR et al. Iron Supplementation Affects Hematologic Biomarker Concentrations and Pregnancy Outcomes among Iron-Deficient Tanzanian Women. J Nutr. 2016;146(6):1162–71. 10.3945/jn.115.225482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rukuni R, Bhattacharya S, Murphy MF, Roberts D, Stanworth SJ, Knight M. Maternal and neonatal outcomes of antenatal anemia in a Scottish population: a retrospective cohort study. Acta Obstet Gynecol Scand. 2016;95(5):555–64. 10.1111/aogs.12862 [DOI] [PubMed] [Google Scholar]
- 58.Viana KJ, Taddei JA, Cocetti M, Warkentin S. Birth weight in Brazilian children under two years of age. Cad Saude Publica. 2013;29(2):349–56. [DOI] [PubMed] [Google Scholar]
- 59.Banjari I, Kenjerić D, Mandić ML. What Is the Real Public Health Significance of Iron Deficiency and Iron Deficiency Anaemia in Croatia? A Population-Based Observational Study on Pregnant Women at Early Pregnancy from Eastern Croatia. Cent Eur J Public Health. 2015;23(2):98–103. [DOI] [PubMed] [Google Scholar]
- 60.Casanova BF, Sammel MD, Macones GA. Development of a clinical prediction rule for iron deficiency anemia in pregnancy. Am J Obstet Gynecol. 2005;193(2):460–6. 10.1016/j.ajog.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 61.Aikawa R, Ngyen CK, Sasaki S, Binns CW. Risk factors for iron-deficiency anaemia among pregnant women living in rural Vietnam. Public Health Nutr. 2006;9(4):443–8. [DOI] [PubMed] [Google Scholar]
- 62.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet. 2013; 382(9890):427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 63.Barroso F, Allard S, Kahan BC, Connolly C, Smethurst H, Choo L, Khan K, Stanworth S. Prevalence of maternal anaemia and its predictors: a multi-centre study. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):99–105. 10.1016/j.ejogrb.2011.07.041 [DOI] [PubMed] [Google Scholar]
- 64.Vandevijvere S, Amsalkhir S, Van Oyen H, Egli I, Ines E, Moreno-Reyes R. Iron status and its determinants in a nationally representative sample of pregnant women. J Acad Nutr Diet. 2013;113(5):659–66. 10.1016/j.jand.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 65.Bencaiova G, Breymann C. Mild anemia and pregnancy outcome in a Swiss collective. J Pregnancy. 2014;2014:307535 10.1155/2014/307535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pita GM, Jiménez S, Basabe B, García RG, Macías C, Selva L et al. Anemia in children under five years old in Eastern Cuba, 2005–2011. MEDICC Rev. 2014;16(1):16–23. [DOI] [PubMed] [Google Scholar]
- 67.Treister-Goltzman Y, Peleg R, Biderman A. Anemia among Muslim Bedouin and Jewish women of childbearing age in Southern Israel. Ann Hematol. 2015;94(11):1777–84. 10.1007/s00277-015-2459-z [DOI] [PubMed] [Google Scholar]
- 68.Pinho-Pompeu M, Surita FG, Pastore DA, Paulino DS, Pinto E Silva JL. Anemia in pregnant adolescents: impact of treatment on perinatal outcomes. J Matern Fetal Neonatal Med. 2016;1–5. 10.1080/14767058.2016.1205032 [DOI] [PubMed] [Google Scholar]
- 69.Smagulova IE, Sharmanov TSh, Balgimekov SA. The prevalence of anemia among children and women of reproductive age in Kazakhstan and basis of its prevention. Vopr Pitan. 2013;82(5):58–63. [PubMed] [Google Scholar]
- 70.Masukume G, Khashan AS, Kenny LC, Baker PN, Nelson G, Consortium S. Risk factors and birth outcomes of anaemia in early pregnancy in a nulliparous cohort. PLoS One. 2015;10(4):e0122729 10.1371/journal.pone.0122729 eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohamed MA, Ahmad T, Macri C, Aly H. Racial disparities in maternal hemoglobin concentrations and pregnancy outcomes. J Perinat Med. 2012;40(2):141–9. 10.1515/jpm.2011.137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this research are confidential according to guidelines of the Ethics Committee in Research of the State University of Feira de Santana and Brazilian Law of Ethical Aspects 466/2012. The data can be requested to the main author of this research through the email: nesufrb@outlook.com. Upon re-submitting your revised manuscript, please upload your study’s minimal underlying data set as either Supporting Information files or to a stable, public repository and include the relevant URLs, DOIs, or accession numbers within your revised cover letter. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories. Any potentially identifying patient information must be fully anonymized. Important: If there are ethical or legal restrictions to sharing your data publicly, please explain these restrictions in detail. Please see our guidelines for more information on what we consider unacceptable restrictions to publicly sharing data: http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions. Note that it is not acceptable for the authors to be the sole named individuals responsible for ensuring data access. We will update your Data Availability statement to reflect the information you provide in your cover letter." Data can not be shared publicly because it is an original research conducted by a university. However, the data is available to researchers who meet the criteria for accessing confidential data through the principal investigator's email.


