Abstract
Cerebrovascular pressure autoregulation is the physiologic mechanism that holds cerebral blood flow (CBF) relatively constant across changes in cerebral perfusion pressure (CPP). Cerebral vasoreactivity refers to the vasoconstriction and vasodilation that occur during fluctuations in arterial blood pressure (ABP) to maintain autoregulation. These are vital protective mechanisms of the brain. Impairments in pressure autoregulation increase the risk of brain injury and persistent neurologic disability. Autoregulation may be impaired during various neonatal disease states including prematurity, hypoxic–ischemic encephalopathy (HIE), intraventricular hemorrhage, congenital cardiac disease, and infants requiring extracorporeal membrane oxygenation (ECMO). Because infants are exquisitely sensitive to changes in cerebral blood flow (CBF), both hypoperfusion and hyperperfusion can cause significant neurologic injury. We will review neonatal pressure autoregulation and autoregulation monitoring techniques with a focus on brain protection. Current clinical therapies have failed to fully prevent permanent brain injuries in neonates. Adjuvant treatments that support and optimize autoregulation may improve neurologic outcomes.
UNIQUE FEATURES OF INFANT CEREBRAL PHYSIOLOGY
CBF regulation in the premature and term infant’s brain is distinct from that of the fully matured brain. Term infants have functional autoregulation and vasoreactivity. Preterm infants, especially those born at the limits of viability, have anatomically incomplete and underdeveloped cerebral vasculature that cannot yet autoregulate fully. Studies in preterm lambs suggest that the cerebral artery and arteriole muscularis layers develop after 13 weeks gestation, which is equivalent to approximately 25 weeks of human gestation. Autoregulation is functional by 19 weeks gestation in lambs, which is approximately 36 weeks of human gestation.1 In clinical studies of premature neonates, autoregulatory function progressively improves between 23 and 33 weeks gestation.2 Therefore, the vasoreactive mechanisms that support autoregulation develop during the third trimester, which has implications for many infants who are born prematurely.
In addition to the on-going vascular development, anatomical features of the premature cerebral vasculature may place the premature infant at risk for injury. Preterm neonates have fragile periventricular vascular overgrowth in the germinal matrix that is prone to hemorrhage. White matter vascularization from penetrating pial arterial growth and collateral vascularization are also incomplete. Moreover, the newborn’s subarachnoid space contains a network of thin-walled vessels. These fragile and underdeveloped vascular networks place these infants at high risk of germinal matrix and subarachnoid hemorrhages, periventricular leukomalacia, leukoencephalopathy, corpus callosum gliosis, and multi-cystic encephalopathy.3
These unique aspects of preterm cerebral vasculature and anatomy are accompanied by gross differences in CBF between preterm and term neonates. CBF increases progressively during fetal life and infancy, peaking in the pre-teen years, and then declining steadily in adulthood. The infant brain consumes 17% of cardiac output and accounts for 17% of the body mass. In comparison, the adult brain comprises just over 2% of the total body mass, but utilizes 25% of the cardiac output. Low placental vascular resistance and low arterial oxygen content of fetal blood both influence fetal CBF. After birth, CBF increases during the first day of life due to decreasing cerebrovascular resistance, and then CBF continues to gradually increase over the first week of life.4 However, CBF in many infants hovers near the ischemic threshold of 20 cc/100 g/min. In healthy term and pre-term infants without respiratory distress syndrome, CBF at birth is roughly one-third of adult values.5–8 Rapid postnatal brain development then causes cerebral glucose consumption and blood flow to rapidly increase after birth and peak at approximately 5 years of age.9,10
Finally, the preterm neonate with hypotension can have CBF that is more influenced by cardiac cycle changes than term neonates. Premature neonates have very low blood pressure compared with term neonates. During diastole in a hypotensive preterm neonate, CBF is passive to diastolic blood pressure and often absent entirely. CBF autoregulation, when it occurs in this population, is most robust and present during the systolic phase cardiac cycle compared with CBF autoregulation of the mean and diastolic phases of the cardiac cycle. Further, pressure reactivity and autoregulation to systolic and mean ABP are not observed until 26 to 28 weeks gestation.2
NEONATAL ARTERIAL BLOOD PRESSURE MANAGEMENT
Hypotension with low CBF and post-ischemia reperfusion contribute to the pathogenesis of brain injury in human prematurity,11–14 HIE, and experimental animal models.15–20 Hypoperfusion, reperfusion, and hyperemia may promote neural injury through a variety of mechanisms that include oxygen–glucose deprivation, endoplasmic reticulum stress, disruptions in proteostasis, glutamate excitotoxicity, oxidative stress, inflammation, and ultimately activation of cell death pathways in gray and white matter.21–27 Ensuring appropriate CBF to the injured neonatal brain could block these injurious mechanisms and minimize secondary brain injury.
ABP is the most frequently monitored parameter to assess hemodynamic status in infants. However, ABP does not correlate to systemic blood flow as shock can occur during normotension.11,28 While the goal of neonatal ABP management is to adequately perfuse the brain and other vital organs, evidence-based ABP thresholds that define hypotension with cerebral hypoperfusion do not exist. Moreover, we do not know whether we should support ABP with vasopressors, inotropes, or fluid resuscitation and when to initiate these treatments. Defining optimal neonatal ABP management to support cerebral and other vital organ perfusion would significantly advance the clinical care of the neonate.29–33
Multiple observational cohort studies report “normative” ABP ranges based on birth weight, gestational age (GA), and postnatal age.12,34–38 Many neonatologists define hypotension on the first day of life as mean ABP less than or equal to the GA in weeks.39 However, outcomes are similar between neonates with mean ABP less than GA and those with normal blood pressure when perfusion is clinically adequate.40,41 The paucity of evidence showing improved outcomes after raising ABP to a “normal range” has prevented neonatologists from defining hypotension and setting ABP treatment thresholds. In fact, treating hypotension may be deleterious and could increase the risk of intraventricular hemorrhage.40,42
CEREBROVASCULAR PRESSURE AUTOREGULATION CURVES VARY AMONG INDIVIDUAL NEONATES
Cerebrovascular pressure autoregulation protects the brain from deleterious CBF fluctuations in the face of changing CPP. The classic mechanistic depiction is a sigmoidal curve with stable CBF across a range of CPP and pressure-passive CBF outside this range. (Fig. 1) The range of functional autoregulation, which is called the autoregulatory plateau, is bounded by the upper limit of autoregulation (ULA) and the lower limit of autoregulation (LLA). Cerebral vasoreactivity functions only when CPP is along this plateau. Above the ULA and below the LLA, CBF is passive to blood pressure due to impaired vasoreactivity and the brain is at risk of hyperemic or hypoperfusion injury.
Fig. 1.
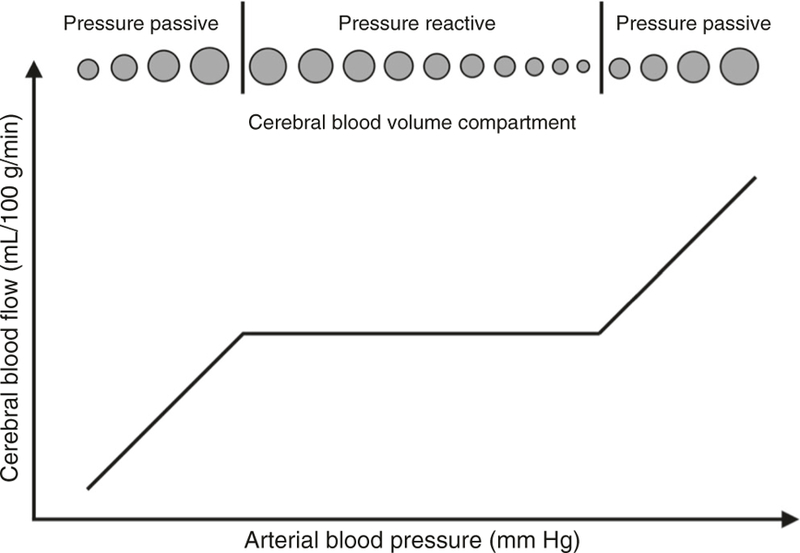
Cerebral blood flow autoregulation curve. Autoregulation is the physiologic mechanism that holds the blood flow to the brain constant across a range of blood pressure. The flat area of the curve is a state of pressure reactivity where blood pressure and blood flow go in opposite directions. However, outside the range of auto-regulation on the two ends is a state of pressure passivity where blood flow is purely dependent on blood pressure. There is no more reactivity in the vessels
The optimal ABP and CPP are conceptually located in the center of the autoregulatory plateau and represent the pressures at which vasoreactive responses are most robust. Optimizing ABP and CPP to maximize vasoreactivity may reduce the risk of secondary brain injury. Because neonatal intracranial pressure (ICP) monitoring is generally not feasible, neonatologists must rely upon ABP management to support autoregulation.
Pressure autoregulation is one of many cerebral servomechanisms that regulate the CBF. Other mechanisms include neurovascular coupling; the systemic vasoconstrictive response to shock with the activation of the sympathetic nervous system, renin–angiotensin–aldosterone axis, and vasopression release; and cerebral vasoreactivity to carbon dioxide, oxygen, and glucose.43–47 Pressure autoregulation can be measured independent of these other CBF regulators by collecting ABP and cerebral waveform data at the frequency of 0.004–0.05 Hz (20–250 s). This frequency is where slow wave changes in CBV occur during pressure autoregulatory vasoreactivity.48
Autoregulation can be characterized by static or dynamic metrics. Static autoregulation describes the steady-state changes of cerebrovascular resistance when ABP varies, and dynamic autoregulation metrics describe the changes in cerebrovascular resistance and flow in a faster time course. The results of these methods are related, but not necessarily interchangeable, and there is no gold standard measure of autoregulation. Autoregulation metrics must capture a change in ABP alongside a synchronous measurement of CBF, cerebral blood volume (CBV), or cerebral oxygenation. The relationship between the change in ABP and change in CBF, CBV, or oxygenation is mathematically estimated to provide an index of autoregulatory function. Vasoreactivity is measured by changes in CBV. Autoregulation is measured by changes in CBF or oxygenation.
Pressure autoregulation monitoring can define an individual patient’s unique cerebral autoregulation and vasoreactivity physiology to determine the optimal ABP with most-functional autoregulation in addition to the ULA and LLA. This could enable patient-oriented, neonatal precision medicine that is superior to generalized ABP goals based on gestational age or body weight.
An individual infant’s LLA, optimal ABP, ULA, and autoregulatory plateau may shift during evolving brain injury and treatment. In a piglet model of hydrocephalus, the LLA shifted to a higher CPP during progressive intracranial hypertension from artificial cerebrospinal fluid infusion or from cephalic venous outflow obstruction with intracranial venous congestion.49,50 Additional analysis from the piglet hydrocephalus data showed that optimal ABP increases with rising ICP. Intracranial hypertension can also move the ULA to a lower CPP.51 The combination of an increased LLA (rightward shift) and a decreased ULA (leftward shift) causes the autoregulatory plateau to narrow. Whole-body hypothermia after neonatal brain hypoxia acutely decreases the LLA, although this LLA shift was not sustained with longer durations of hypothermia.52,53 Further, autoregulatory responses to different vasopressors may vary by sex and age after brain injury.32,54–57 Milrinone may significantly decrease the blood pressure to below-optimal ABP in girls.32 Common neonatal clinical situations with the potential physiologic changes outlined above include progressive intracranial hypertension with hydrocephalus, cerebral venous outflow impediments with cavo-pulmonary shunts for cardiac anomalies, high ventilator airway pressures, therapeutic hypothermia for HIE, extracorporeal membrane oxygenation (ECMO), and vasopressor and afterload reduction therapies.
CRITICAL CLOSING PRESSURE
The critical closing pressure (CrCP) is a crucial component of neonatal cerebrovascular physiology that remains relatively understudied. CrCP is the ABP at which CBF ceases due to vascular collapse. It is posited to be the sum of vascular wall tension and ICP.58 CrCP normalizes the ABP to an “effective CPP” or “closing margin”, where the effective CPP = ABP–CrCP.59,60 In premature neonates, low diastolic blood pressures can approach or reach the CrCP with little evident CBF.2,61 CrCP in HIE and other neonatal neurologic diseases deserves further study. CrCP is distinct from and lower than the autoregulatory plateau. When ABP is below the lower limit of autoregulation, CBF is passive to ABP. When ABP is below the CrCP, CBF ceases altogether. The low diastolic blood pressure of the preterm neonate results in a CBF pattern that can occur only during systole as ABP cycles above and below the CrCP with each cardiac cycle. Intuitively, one might hypothesize that this state is a vulnerable to ischemia and white matter injury, but these associations have not yet been studied, as the tools to measure the CrCP are relatively new.
CrCP increases gradually from 23 to 31 weeks gestation at a rate of 1.4 mmHg per week and is ~22–33 mmHg in premature and term infants.61–63 Intersubject variability in the CrCP was high in that study, suggesting that CrCP cannot be estimated by age, but requires direct measurement (Fig. 2). The time courses of CrCP changes during transition to extrauterine life and during common comorbid conditions have not yet been elucidated. Small variances in CrCP cause large variances in effective CPP in preterm infants. For example, a 10 mmHg increase in CrCP from 20 to 30 mmHg would profoundly decrease CBF in a preterm infant with a mean ABP of 30 mmHg because the effective CPP would change from 10 mmHg to zero. An identical 10 mmHg CrCP increase would have trivial effect on CBF in an adult with a mean ABP of 70 mmHg.
Fig. 2.
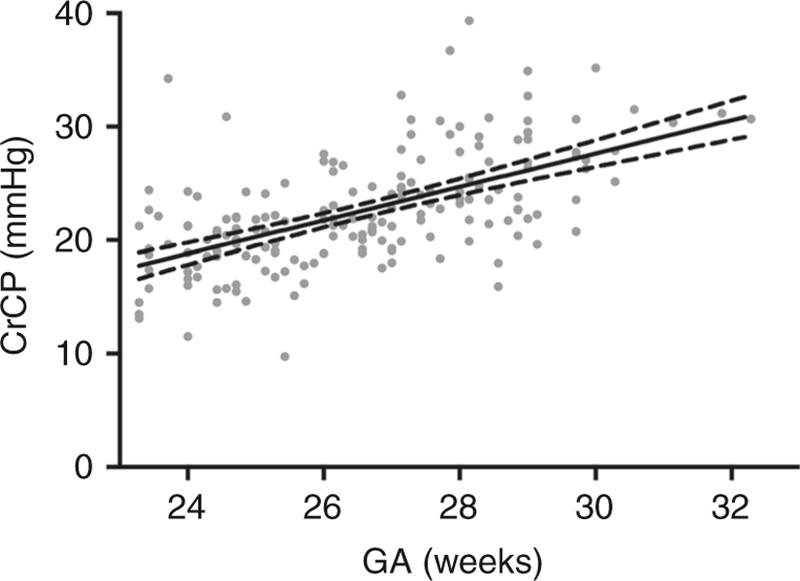
Changes in critical closing pressure across gestational age. Critical closing pressure increases with gestational age at a rate of 1.4 mmHg per week of gestation (P < 0.0001). From Rhee et al.61
The proximity of ABP to CrCP in preterm infants may make CrCP an effective cerebral perfusion metric in this population. Simply stated, the ABP- and CrCP-derived effective CPP may be more relevant to define brain perfusion in preterm neonates than ABP alone. Therefore, autoregulation monitoring in these infants should take into account CrCP and the effective CPP. Further, in very preterm neonates who may lack autoregulatory capacity entirely, the CrCP measurement may provide an alternate therapeutic target to manage cerebral hemodynamics in an “adequate but not hyperemic” range that has yet to be elucidated by wider study of this parameter in this population.
NON-INVASIVE AUTOREGULATION AND VASOREACTIVITY MONITORING IN NEONATES
ICP monitoring is frequently used to measure changes in CBV during cerebral vasoreactivity in adults and children. Because invasive cranial monitors are not routinely used in neonatal clinical practice, non-invasive autoregulation metrics have been developed and applied to neonates. All non-invasive autoregulation metrics are limited by the anatomic region. For instance, dysfunctional autoregulation in cerebellum will not be detected by measures in the frontal cortex or in a single cortical cerebral artery.1
Historically, neonatal cerebral autoregulation has been monitored most frequently with transcranial Doppler (TCD) ultrasound to measure the CBF velocity in a target artery, such as the middle or anterior cerebral artery.2,41,64–67 Prior to this technique, measurement of CBF in human subjects relied on indicator-dilution techniques. TCD allows for non-invasive estimates of CBF velocity and can easily be performed at bedside. However, TCD has its limitations; it is only capable of measuring velocity and not flow, and there are technical challenges of obtaining a stable long-term signal in very preterm infants.
Near-infrared spectroscopy (NIRS) has been widely used to study neonatal cerebral hemodynamics and oxygenation. NIRS takes advantage of the relative transparency of biological tissue to near-infrared light (700–1000 nm) and the wavelength-dependent absorption characteristics of hemoglobin, which varies with oxygenation, as well as changes in oxyhemoglobin (HbO2) and deoxyhemoglobin (HHb). In fact, changes in the sum of HbO2 and HHb can be calculated as a surrogate measure of CBV during autoregulatory vasoreactivity. Fluctuations in ICP and CBV during vasoreactive responses correlate to fluctuations in the NIRS relative total tissue hemoglobin.48 While absolute quantitative measurements of CBF and CBV can be made, they rely on the manipulation of FiO2 or injection of an optical tracer and provide only a static measurement.68 The difference between oxygenated hemoglobin and deoxyhemoglobin (HbD) has also been shown to reflect changes in CBF.69,70 More recently, NIRS-based metrics including oxyhemoglobin optical density have been used to estimate CBF.71 The regional oxyhemoglobin saturation (rSO2) is primarily a venous-weighted signal and reflects the hemoglobin-bound oxygen that remains after tissues have taken what they need. In other words, rSO2 demonstrates the changes in oxygen supply and demand and thus is affected by factors independent of pressure autoregulation, including hemoglobin level, inspired oxygen, seizures, sedation, and temperature. rSO2 may be a better surrogate of CBF in the premature infant because of the low and constant cerebral metabolic rate of oxygen in this population.
CALCULATING VASOREACTIVITY INDICES: SLOW WAVE PRESSURE MONITORING
Pressure autoregulation monitoring can identify the optimal ABP with most robust vasoreactivity in individual patients after pediatric and adult traumatic brain injury, during cardiopulmonary bypass, after pediatric cardiac arrest, and in neonatal HIE.72–74 The ability to accurately monitor pressure autoregulation depends on analyzing spontaneous and repetitive slow CBV and ICP waves. Lundgren first categorized these slow waves as “B waves”, which occur with a period between 20 and 200 s.75 These slow waves are generated from cerebral autoregulatory vasodilation and vasoconstriction that occur with fluctuations in CPP. As the vessels dilate, the CBV increases and the ICP correspondingly increases. When the vessels constrict, the CBV decreases and the ICP decreases. Autoregulatory vasoreactivity can then be measured by synchronously measuring ABP at the same frequency to analyze the relationship between the ABP and intracranial waveforms.
Multiple mathematical algorithms have been used to describe the relationship between ABP and CBV, ICP, or CBF to estimate autoregulation. These include simple correlation, and in the frequency domain, cross-correlation functions of coherence, phase, and gain have all been used. When vasoreactivity is impaired, CBF, CBV, and ICP are passive to ABP, and each of these functions quantifies passivity in a different way. The interchangeability of these myriad metrics is not known, and this proliferation of methods with minimal validation is a major limitation of the current literature of autoregulation. From controlled animal models with known states of intact and impaired autoregulation, it has been shown that fluctuations in ABP cause coherent and in-phase fluctuations in CBV and ICP when autoregulation is absent. This is equivalent to a positive correlation. When autoregulatory vasoreactivity is functional, CBV is pressure-reactive and the CBV and ICP waveforms are coherent with ABP, but have a 180° phase shift. This is equivalent to a negative correlation. (Fig. 3)
Fig. 3.
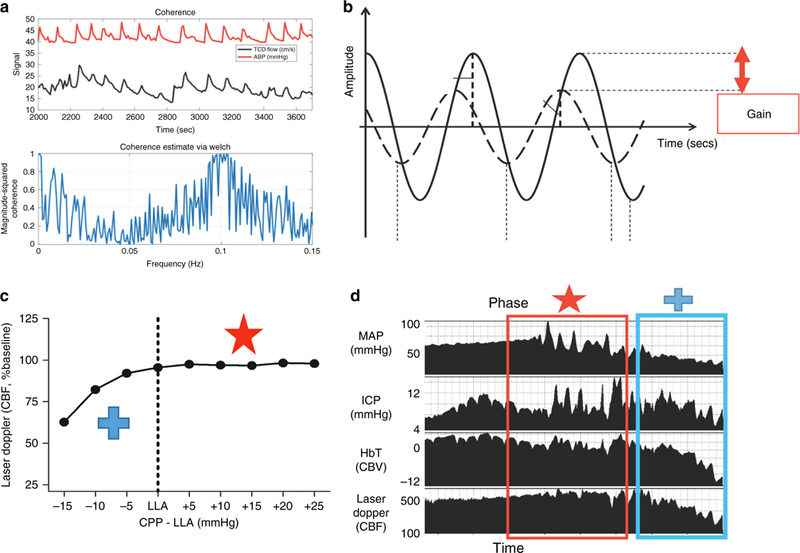
Graphical explanations of the autoregulation metrics. a Coherence analysis of CBF measured by transcranial Doppler (TCD) ultrasound in preterm neonate. Coherence is high between ABP (red line) and CBF (black line), indicating a state of dysautoregulation at slow wave frequencies. Bottom panel shows coherence as a function of frequency. b Waveform 1 (W1, solid line) and waveform 2 (W2, dotted line) are shown for concept. The gain is the damping effect and the phase shift is the time delay between the two waveforms. Lower gain indicates better autoregulatory function. c, d Blood pressure was decreased in a neonatal piglet with continuous mean arterial blood pressure (MAP), intracranial pressure (ICP), cerebral blood volume (CBV) NIRS tissue hemoglobin index, and cerebral blood flow (CBF) laser Doppler flow (LDF) measurements. When MAP exceeds the lower limit of autoregulation and CBF is relatively constant, slow MAP waves are out of phase with the ICP and CBV (red star and box). As MAP decreases below the lower limit of autoregulation and CBF falls, the MAP, ICP, and CBV waves are all in phase, indicating a state of dysautoregulation (blue cross and box). Adapted with permission from: SAGE Publications et al111, American Physiological Society et al112,, and Wolters Kuwer et al113
Continuous measurement of correlation between ABP and CBV or ICP can identify the optimal ABP with most-functional vasoreactivity. Optimal ABP was found at the nadir of the plot with correlation coefficients on the y-axis and ABP on the x-axis. (Fig. 4) A criticism of this technique is that optimal ABP is not identifiable in patients without a U-shaped curve and apparent nadir. Maintaining blood pressure within optimal ABP is associated with less brain injury in MRI and improved 2-year neurodevelopmental outcomes in neonatal HIE.76–80 Traumatic brain injury and preterm neonatal studies also support using optimal MAP as a hemodynamic goal.
Fig. 4.
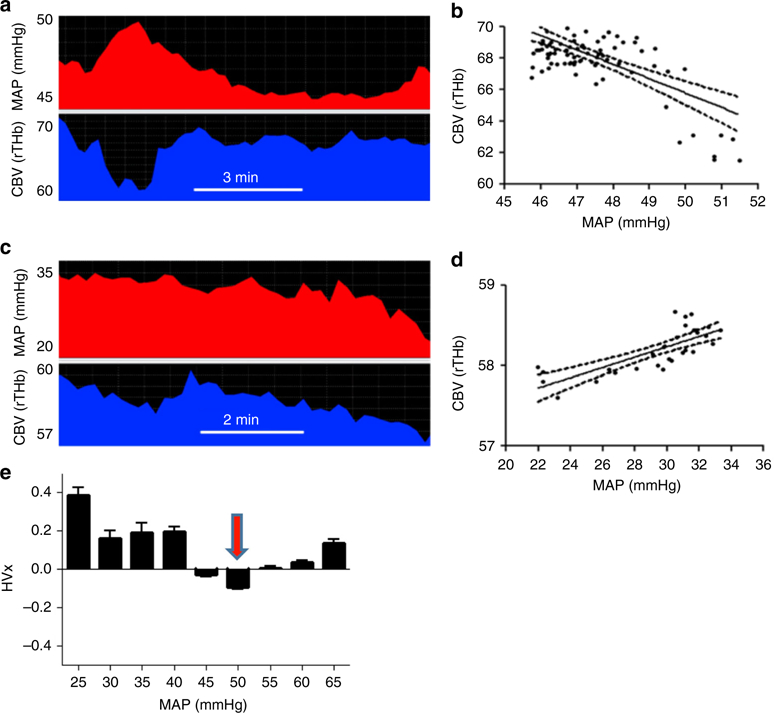
Determining optimal arterial blood pressure. An example of one patient using autoregulation monitoring to determine optimal mean arterial blood pressure (MAP). Slow waves of MAP (top) and cerebral blood volume (CBV, bottom) are depicted (a–c). Autoregulation monitoring during periods of intact and impaired autoregulation in this infant. When autoregulation is intact, MAP waves negatively correlate to changes in NIRS-measured CBV (a, b). The correlation of CBV and MAP is the hemoglobin volume index (HVx). When autoregulation is impaired, MAP and CBV become positively correlated, yielding a positive HVx (c, d). Thus, a more negative HVx indicates functional pressure reactivity and intact autoregulation, and a more positive HVx indicates decreased pressure reactivity and impaired autoregulation. When the measures of HVx are plotted as a function of MAP and placed in 5-mmHg bins, optimal MAP is identified as the nadir when the correlation coefficients are plotted against ABP (e). Here for this patient, optimal ABP is 50 mmHg, where autoregulation is intact and most robust
Spontaneous slow wave activity can have variable periodicity and amplitude, which generates imprecision in autoregulation monitoring. In order to minimize this effect, filters can be applied to the data to eliminate time epochs with inadequate slow wave power, and multiple measurements at the same ABP are commonly averaged together to mitigate noise in the measurements. This important limitation of continuous autoregulation monitoring is the subject of ongoing studies using advanced signal processing techniques. Limiting noise from this effect would result in a faster and more reliable determinant of optimal ABP for autoregulation.
METHODS TO MEASURE NEONATAL AUTOREGULATION
The above concepts have been applied to near-infrared measurements of HbD or rSO2 to estimate CBF autoregulation in neonates. Although slow waves of ABP are erratic and introduce noise in autoregulation measurements, they occur naturally and are therefore the safest way to measure autoregulation.81 In contrast, traditional autoregulation monitoring techniques that intentionally change the ABP with thigh cuff deflation or vasoconstrictor injections are not generally suitable for critically ill neonates.82
Time-domain analysis (correlation) has been used with filters to limit the analysis to the specific frequency range of slow waves. This method detected the LLA with high sensitivity and specificity in animal models.48,52,53,71 Maintaining the blood pressure within optimal ABP from this method is associated with survival and neurologic recovery in large adult studies of traumatic brain injury48,72,83,84 and in single-center studies of neonatal HIE, pediatric cardiac arrest, and pediatric traumatic brain injury.76–78,80,85,86 Blood pressure deviation from optimal ABP delineated in this way may also be associated with cardiopulmonary and renal injury in neonatal HIE and renal injury after cardiopulmonary bypass.31–33
Frequency-domain analyses including coherence, phase, and gain of transfer function have also been used to assess autoregulation in the preterm neonate.81 These analyses include assumptions of linearity and stationarity that are not always present in biologic systems.87 Limited animal data have validated these frequency domain cross-correlation functions for estimating cerebral autoregulation.88 Coherence and gain analyses of HbD and mean ABP identified an association between dysfunctional autoregulation and brain injury in MRI or death in neonatal HIE.70 Limitations include low precision that make monitoring for several hours essential to reliably measure coherence. In some studies coherence is found to be high when blood pressure is either above or below the LLA, and we suggest that coherence may be most useful as a filter to delineate adequate slow wave power to measure autoregulation with either phase or correlation.
Wavelet coherence analysis characterizes continuous or intermittent cross-correlations between two time series at multiple time scales, which makes no assumption about the stationarity of input signals. This technique has been performed in limited studies especially in preterm infants, and it can detect frequencies up to 0.017 Hz due to limitations of instruments and not by the analysis itself.89 Wavelet coherence analysis of ABP and cerebral oximetry at ultra-low frequencies <0.0002 Hz (80 min time scale) has been associated with neurologic outcome in HIE.90
Finally, an alternative assessment of autoregulation uses the relationship between heart rate changes and cerebral oxygenation. The tissue oxygenation heart rate reactivity index (TOHRx), an index of cerebral vascular reactivity, is derived from the correlation coefficient between slow waves of heart rate (HR) and tissue oxygenation index (TOI) measured with NIRS.91 Higher TOHRx values were observed in infants with worse clinical score of mortality and morbidity (Clinical Risk Index for Babies II). In addition, increased passivity between TOI and HR was observed with arterial hypotension. These TOHRx findings are unique to the preterm population and may be related to systemic hemodynamic control, as cardiac output in preterm infants is mainly regulated by heart rate due to the immature myocardium. In adults, CBF has no correlation with the output, however, in preterm infants, a positive correlation between CBF and left cardiac output has been described.92,93 Further, in preterm infants, optimal mean ABP has been defined using TOHRx in a cohort of infants born at ≤32 weeks gestational age. Absolute deviations away from optimal blood pressure were higher in those infants who died. Infants who developed severe IVH had deviations away from optimal values greater than 4 mmHg.94
LIMITATION OF NIRS-BASED AUTOREGULATION MONITORING
There are limitations of NIRS autoregulation monitoring. NIRS placed on the forehead or tempero-parietal region of the head measures the frontal lobe and tempero-parietal lobes, and cannot capture the vasoreactivity in other areas known to be vulnerable in HIE and prematurity, including many white matter regions (such as periventricular white matter), basal ganglia, thalamus, and other cortical regions. Thus, frontal optimal ABP may not reflect that of other anatomic regions. Autoregulation has a prominent macro-circulatory component from the involvement of large cerebral arteries and pial arterioles that are upstream from local parenchymal tissue. Autoregulatory vasoreactivity is primarily mediated by intrinsic myogenic responses to changes in transmural pressure. Global cerebral ischemia in HIE and vascular immaturity in preterm neonates would not be expected to cause large regional differences in optimal ABP. It is also likely that prolonged and large deviations in blood pressure from optimal MAP in frontal or tempero-parietal cortex will affect perfusion throughout much of the brain. In the absence of clinically available autoregulation metrics for regional autoregulatory function, identifying optimal MAP in the frontal lobe is a reasonable first approximation to guide hemodynamic management in critically ill neonates. NIRS also permits continuous monitoring over long periods of time that cannot be easily accomplished with transcranial Doppler.
NIRS AUTOREGULATION MONITORING IN SPECIFIC NEONATAL DISEASES
The purpose of this review of relevant studies was to examine those studies that were associated with specific outcome measures. The studies presented were found through PubMed search using “prematurity” or “premature” or “neonate” and “hypoxic ischemic encephalopathy” or “neonatal encephalopathy” with “autoregulation” and “cerebral blood flow.” Studies were then selected that focused on time and frequency domain or wavelet autoregulation methodologies using NIRS in this population.
PREMATURITY/INTRAVENTRICULAR HEMORRHAGE
Both time-domain and frequency-domain analyses have been used to measure autoregulation in premature infants. CBF autoregulation to systolic and mean ABP develops between 23 and 33 weeks gestation, and infants younger than 26 weeks GA are at high risk of pressure-passive CBF. However, CBF is overall passive to diastolic ABP before 33 weeks gestation.2 Dysfunctional autoregulation is common in premature infants, with many demonstrating fluctuating pressure passivity over the first few days of life.95 In addition, impairments in autoregulation increase the risk of severe intraventricular hemorrhage and death, and those infants with IVH often have cerebral hyperperfusion and pressure-passive cerebral perfusion with correlation of rSO2 and ABP.96–100 Further, sick infants are more prone to dysautoregulation and have a potentially narrower autoregulatory plateau, meaning those infants may be more sensitive to fluctuations in ABP.101 This is in agreement with our findings that CBF autoregulation to systolic and mean ABP develops between 23 and 33 weeks gestation, and infants younger than 26 weeks GA are at high risk of pressure-passive CBF. Dopamine therapy was associated with dysfunctional autoregulation, but it is unclear whether dopamine impaired autoregulation or if it was an indicator of severe illness.102 Finally, blood pressure divergence below optimal ABP was associated with death, and deviation above optimal ABP increased the risk of severe intraventricular hemorrhage.94
HIE
Frequency, time, and wavelet coherence analyses have been used to study HIE during spontaneous blood pressure fluctuations. Poorer autoregulatory function measured by coherence and gain between HbD and ABP were associated with brain injury in MRI or death.70 Blood pressure deviation from optimal ABP identified by time-domain analysis was associated with greater brain injury in MRI and worse 2-year neurodevelopmental outcomes.76–80 Finally, in-phase coherence of mean ABP and cerebral oximetry at time scales less than 80 min ( < 0.0002 Hz) and anti-phase coherence at time scales of approximately 2.5 h (~0.0001 Hz) were reported by wavelet coherence analysis. Both in-phase and anti-phase coherence were related to worse clinical outcomes.90
NEONATAL CARDIAC DISEASE AND CARDIOPULMONARY BYPASS
Autoregulation monitoring in neonates with congenital cardiac disease has been conducted in both the frequency and time domains. Hypercarbia and fluctuations in ABP were associated with dysfunctional autoregulation after cardiac surgery.103 Autoregulatory function worsens at lower blood pressures during cardiopulmonary bypass, and LLA can be identified in nearly 80% of patients during bypass.104 Hypothermia was thought to cause impaired autoregulation in this population, but further study demonstrated that neonates are managed with lower ABP during hypothermia, and the effect of hypothermia cannot be isolated in these data.105 Finally, term newborns with congenital heart disease displayed associations between abnormal cerebral autoregulation and greater fractional tissue oxygen extraction, lack of sedation, and lower hemoglobin, suggesting that impaired oxygen delivery and increased cerebral metabolic demand may overwhelm autoregulatory capacity in these infants.106
EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
Wavelet cross-correlation was calculated between mean ABP and NIRS oxyhemoglobin saturation in neonates receiving venoarterial ECMO. NIRS signals were measured in the frontal, parietal, and occipital cortices using multi-modal NIRS monitoring and a customized headcap. Decreasing the ECMO flow from 100 to 70% caused dysfunctional autoregulation at lower flow. Right parietal cortex had greater disruptions in autoregulation than the left parietal cortex, which may be related to right-sided cannulation of the common carotid artery and internal jugular vein.107 Obstructions in cephalic venous drainage may affect autoregulation by increasing the ICP.49 Further studies are needed on the effects of carotid and jugular ECMO cannulae on cerebrovascular hemodynamics.
FUTURE DIRECTIONS
The majority of neonatal autoregulation and vasoreactivity studies are limited to small and often single-center studies. Multi-center studies are needed to increase the sample size, account for variation in clinical practice, and accommodate the challenges of enrolling neonates, a medically fragile population, in clinical studies. Moreover, autoregulation monitoring requires an indwelling arterial catheter because non-invasive continuous ABP monitoring techniques, such as photoplethysmography, have not been well established in neonates. The requirement for an arterial catheter and reluctance of some families to enroll their critically ill infants in clinical research limits the sample sizes. If large-scale observational data support a link between functional autoregulation and improved neurologic outcomes, interventional studies to determine whether identifying and targeting optimal ABP should be considered. Ancillary studies to ongoing neonatal clinical trials, including erythropoietin and other adjuvant therapies, provide additional opportunities to test the effect of drug therapies on cerebral autoregulation.
Neonatal animal models could define the utility and limitations of non-invasive, regional autoregulation monitoring. For instance, regional variation in thalamic and cortical CBF after hypoxia in developing brain could be compared to clinically accessible NIRS and middle or anterior cerebral artery transcranial Doppler.108 This would require a large animal model that permits synchronized, multi-modal, cerebral measurements, such as piglets. Optimal ABP in discrete anatomic regions, including basal ganglia, thalamus, large white matter tracts, and different cortices, could be identified with laser Doppler flowmetry and local brain tissue oxygenation through correlation with ABP. Then, regional optimal ABP could be compared with that of frontal cortex over long monitoring periods to extrapolate information on clinical frontal NIRS autoregulation monitoring.
The ultimate goal of supporting autoregulation is to improve neurologic outcomes, and this can only be accomplished by preventing neural cell death. Animal models can help determine whether functional vasoreactivity blocks neuropathologic processes after hypoxia and reperfusion. These pathways include oxidative and endoplasmic reticulum stress, inflammation, disruptions in proteostasis and synaptogenesis, and activation of cell death pathways in white and gray matter. Premature swine and ovine models could also be used to test the interventions aimed at supporting autoregulation.109,110
CONCLUSION
Current clinical practice does not prevent neurologic injuries in critically ill neonates, and generalized blood pressure guidelines are inadequate. Cerebrovascular autoregulation and vasoreactivity monitoring can define the blood pressure range that best supports CBF to the developing brain. In particular, neonates affected by prematurity, hypoxia–ischemia, congenital heart disease, or infants requiring ECMO may benefit from autoregulation-directed care. Precision medicine applied to autoregulation and vasoreactivity monitoring can individualize neuroprotective treatments in critically ill neonates.
Acknowledgments
FUNDING
The authors disclosed the following financial support for the research, authorship, and/or publication of this article: NIH grants K23NS091382 (C.J.R.) and K08NS080984 (J.K.L.); SPARKS Charity (T.A.); Cambridge Trust and Coordencação de Aperfeiçoamento de Pessoal de Nível Superior (C.A.P.E.S.) scholarship (C.S.D.); and Evelyn Trust (T.A.).
ADDITIONAL INFORMATION
Competing interests: Dr. Brady is listed as a co-inventor of autoregulation monitoring technology that has been licensed to Medtronic Inc. Dr. Czosnyka has a financial interest in a part of the licensing fee for ICM+ software that is licensed by the University of Cambridge, Cambridge Enterprise Ltd. Dr. Lee is a paid advisory board member for Medtronic Inc. Dr. Lee also received a research grant from Medtronic for a separate clinical study on cerebrovascular autoregulation. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. Medtronic did not provide any support (financial or other) or have any input on the current project and manuscript. The remaining authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Helou S, Koehler RC, Gleason CA, Jones MD & Traystman RJ Cerebrovascular autoregulation during fetal development in sheep. Am. J. Physiol. Heart C 266, H1069–H1074 (1994). [DOI] [PubMed] [Google Scholar]
- 2.Rhee CJ et al. The ontogeny of cerebrovascular pressure autoregulation in human premature infants. J. Perinatol 34, 926–931 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rorke LB Anatomical features of the developing brain implicated in pathogenesis of hypoxic-ischemic injury. Brain. Pathol 2, 211–221 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Wladimiroff JW & van Bel F Fetal and neonatal cerebral blood flow. Semin. Perinatol 11, 335–346 (1987). [PubMed] [Google Scholar]
- 5.Pryds O & Edwards AD Cerebral blood flow in the newborn infant. Arch. Dis. Child. Fetal Neonatal Ed 74, F63–F69 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greisen G Cerebral blood flow in preterm infants during the first week of life. Acta Pædiatrica 75, 43–51 (1986). [DOI] [PubMed] [Google Scholar]
- 7.Lou H, Skov H & Pedersen H Low cerebral blood flow: a risk factor in the neonate. J. Pediatr 95, 606–609 (1979). [DOI] [PubMed] [Google Scholar]
- 8.Skov H, Lou H & Pedersen H Perinatal brain ischemia: impact at four years of age. Dev. Med. Child Neurol 26, 353–357 (1984). [DOI] [PubMed] [Google Scholar]
- 9.Kuzawa CW et al. Metabolic costs and evolutionary implications of human brain development. Proc. Natl Acad. Sci. USA 111, 13010–13015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiron C et al. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J. Nucl. Med 33, 696–703 (1992). [PubMed] [Google Scholar]
- 11.Osborn DA, Evans N & Kluckow M Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central-peripheral temperature difference. Arch. Dis. Child. Fetal Neonatal Ed 89, F168–F173 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins AM, West CR & Cooke RW Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum. Dev 19, 103–110 (1989). [DOI] [PubMed] [Google Scholar]
- 13.Kluckow M & Evans N Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch. Dis. Child. Fetal Neonatal Ed 82, F182–F187 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluckow M & Evans N Low systemic blood flow in the preterm infant. Semin. Neonatol 6, 75–84 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Perlman J & Volpe JJ Intraventricular hemorrhage in extremely small premature infants. Am. J. Dis. Child 140, 1122–1124 (1986). [DOI] [PubMed] [Google Scholar]
- 16.Volpe JJ Brain injury in the premature infant. Neuropathology, clinical aspects, pathogenesis, and prevention. Clin. Perinatol 24, 567–587 (1997). [PubMed] [Google Scholar]
- 17.Ment LR, Stewart WB, Duncan CC & Pitt BR Beagle puppy model of perinatal cerebral insults. J. Neurosurg 65, 847–850 (1986). [DOI] [PubMed] [Google Scholar]
- 18.Ment LR et al. Beagle puppy model of perinatal cerebral infarction. J. Neurosurg 63, 441–447 (1985). [DOI] [PubMed] [Google Scholar]
- 19.Lynch JK & Nelson KB Epidemiology of perinatal stroke. Curr. Opin. Pediatr 13, 499–505 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Del Toro J, Louis PT & Goddard-Finegold J Cerebrovascular regulation and neonatal brain injury. Pediatr. Neurol 7, 3–12 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Johnston M Excitotoxicity in neonatal hypoxia. Ment. Retard. Dev. Disabil. Res. Rev 7, 229–234 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Hua C, Ju W.-n., Jin H, Sun X & Zhao G Molecular chaperones and hypoxicischemic encephalopathy. Neural Regen. Res 12, 153–160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JK et al. Hypothermia and rewarming activate a macroglial unfolded protein response independent of hypoxic-ischemic brain injury in neonatal piglets. Dev. Neurosci 38, 277–294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B et al. Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic–ischemic encephalopathy. J. Cerebr Blood Flow Met 35, 781–793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B et al. White matter apoptosis is increased by delayed hypothermia and rewarming in a neonatal piglet model of hypoxic ischemic encephalopathy. Neuroscience 316, 296–310 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao M et al. Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies. Int. J. Mol. Sci 17, 2078 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler JH, et al. Dimethyl fumarate improves white matter function following severe hypoperfusion: involvement of microglia/macrophages and inflammatory mediators. J. Cerebr. Blood Flow Met 38, 1354–1370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soleymani S, Borzage M, Noori S & Seri I Neonatal hemodynamics: monitoring, data acquisition and analysis. Expert. Rev. Med. Devices 9, 501–511 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Laughon M et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics 119, 273–280 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Aweel I et al. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J. Perinatol 21, 272–278 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Lee JK et al. Relationships between cerebral autoregulation and markers of kidney and liver injury in neonatal encephalopathy and therapeutic hypothermia. J. Perinatol 37, 938–942 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavez-Valdez R et al. Sex-specific associations between cerebrovascular blood pressure autoregulation and cardiopulmonary injury in neonatal encephalopathy and therapeutic hypothermia. Pediatr. Res 81, 759–766 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hori D et al. Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interact. Cardiov Th 22, 445–451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Rajadurai VS & Tan KW Blood pressure standards for very low birthweight infants during the first day of life. Arch. Dis. Child. Fetal Neonatal Ed 81, F168–F170 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spinazzola R, Harper R, de Soler M & Lesser M Blood pressure values in 500-to 750-gram birthweight infants in the first week of life. J. Perinatol 11, 147–151 (1991). [PubMed] [Google Scholar]
- 36.Versmold H, Kitterman J, Phipps R, Gregory G & Tooley W Aortic blood pressure during the first 12 h of life in infants with birth weight 610 to 4,220 grams. Pediatrics 67, 607–613 (1981). [PubMed] [Google Scholar]
- 37.Hegyi T et al. Blood pressure ranges in premature infants: II. The first week of life. Pediatrics 97, 336–342 (1996). [PubMed] [Google Scholar]
- 38.Hegyi T et al. Blood pressure ranges in premature infants. I. The first hours of life. J. Pediatr 124, 627–633 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Report of a Joint Working Group of the British Association of Perinatal Medicine and the Research Unit of the Royal College of Physicians. Development of audit measures and guidelines for good practice in the management of neonatal respiratory distress syndrome. Arch. Dis. Child 67, 1221–1227 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dempsey E, Al Hazzani F & Barrington K Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch. Dis. Child. Fetal Neonatal Ed 94, F241–F244 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Lightburn MH, Gauss CH, Williams DK & Kaiser JR Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J. Pediatr 154, 824–828 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham S, Symon A, Elton R, Zhu C & McIntosh N Intra-arterial blood pressure reference ranges, death and morbidity in very low birthweight infants during the first seven days of life. Early Hum. Dev 56, 151–165 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Roy CS & Sherrington CS On the regulation of the blood-supply of the brain. J. Physiol 11, 85–158 (1890). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamner JW, Tan CO, Lee K, Cohen MA & Taylor JA Sympathetic control of the cerebral vasculature in humans. Stroke 41, 102–109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saavedra JM & Nishimura Y Angiotensin and cerebral blood flow. Cell. Mol. Neurobiol 19, 553–573 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katusic ZS, Shepherd JT & Vanhoutte PM Vasopressin causes endothelium-dependent relaxations of the canine basilar artery. Circ. Res 55, 575–579 (1984). [DOI] [PubMed] [Google Scholar]
- 47.Yang S-P & Krasney JA Cerebral blood flow and metabolic responses to sustained hypercapnia in awake sheep. J. Cerebr. Blood F. Met 15, 115–123 (1995). [DOI] [PubMed] [Google Scholar]
- 48.Lee JK et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 40, 1820–1826 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Nusbaum D et al. Alteration in the lower limit of autoregulation with elevations in cephalic venous pressure. Neurol. Res 36, 1063–1071 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Brady KM et al. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth. Analg 108, 1278–1283 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Pesek M et al. The upper limit of cerebral blood flow autoregulation is decreased with elevations in intracranial pressure. Neurosurgery 75, 163–170 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Lee JK et al. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit. Care Med 39, 2337–2345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larson AC et al. Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J. Appl. Physiol 115, 1433–1442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armstead WM, Riley J & Vavilala MS TBI sex dependently upregulates ET-1 to impair autoregulation, which is aggravated by phenylephrine in males but is abrogated in females. J. Neurotraum 29, 1483–1490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstead WM, Riley J & Vavilala MS Sex and age differences in epinephrine mechanisms and outcomes after brain injury. J. Neurotraum 34, 1666–1675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Armstead WM, Riley J & Vavilala MS Preferential protection of cerebral autoregulation and reduction of hippocampal necrosis with norepinephrine after traumatic brain injury in female piglets. Pediatr. Crit. Care. Med 17, e130–e137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Armstead WM, Riley J & Vavilala MS Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatr. Crit. Care. Med 14, e103–e111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nichol J, Girling F, Jerrard W, Claxton E & Burton A Fundamental instability of the small blood vessels and critical closing pressures in vascular beds. Am. J. Physiol 164, 330–344 (1951). [DOI] [PubMed] [Google Scholar]
- 59.Jagersberg M et al. Simultaneous bedside assessment of global cerebral blood flow and effective cerebral perfusion pressure in patients with intracranial hypertension. Neurocrit. Care 12, 225–233 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Varsos GV et al. Critical closing pressure determined with a model of cerebrovascular impedance. J. Cereb. Blood Flow. Metab 33, 235–243 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhee CJ et al. Ontogeny of critical closing pressure. Pediatr. Res 78, 71–75 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Panerai R, Coughtrey H, Rennie J & Evans D A model of the instantaneous pressure-velocity relationships of the neonatal cerebral circulation. Physiol. Meas 14, 411–418 (1993). [DOI] [PubMed] [Google Scholar]
- 63.Panerai R, Kelsall A, Rennie J & Evans D Estimation of critical closing pressure in the cerebral circulation of newborns. Neuropediatrics 26, 168–173 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Kaiser JR, Gauss CH & Williams DK The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr. Res 58, 931–935 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaiser J, Gauss C & Williams D Tracheal suctioning is associated with prolonged disturbances of cerebral hemodynamics in very low birth weight infants. J. Perinatol 28, 34–41 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Kaiser J, Gauss C & Williams D The effects of closed tracheal suctioning plus volume guarantee on cerebral hemodynamics. J. Perinatol 31, 671–676 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaiser JR, Gauss CH & Williams DK Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very-low-birth-weight infants. J. Pediatr 144, 809–814 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Wyatt JS, Delpy DT, Cope M, Wray S & Reynolds EO Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 328, 1063–1066 (1986). [DOI] [PubMed] [Google Scholar]
- 69.Soul JS, Taylor GA, Wypij D, Duplessis AJ & Volpe JJ Noninvasive detection of changes in cerebral blood flow by near-infrared spectroscopy in a piglet model of hydrocephalus. Pediatr. Res 48, 445–449 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Massaro AN et al. Impaired cerebral autoregulation and brain injury in new-borns with hypoxic-ischemic encephalopathy treated with hypothermia. J. Neurophysiol 114, 818–824 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brady KM et al. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 39, 2531–2537 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aries MJH et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit. Care Med 40, 2456–2463 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK & Pickard JD Monitoring of cerebral autoregulation in head-injured patients. Stroke 27, 1829–1834 (1996). [DOI] [PubMed] [Google Scholar]
- 74.Blaine Easley R et al. Continuous cerebrovascular reactivity monitoring and autoregulation monitoring identify similar lower limits of autoregulation in patients undergoing cardiopulmonary bypass. Neurol. Res 35, 344–354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinmeier R et al. Slow rhythmic oscillations of blood pressure, intracranial pressure, microcirculation, and cerebral oxygenation: dynamic interrelation and time course in humans. Stroke 27, 2236–2243 (1996). [DOI] [PubMed] [Google Scholar]
- 76.Howlett JA et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr. Res 74, 525–535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tekes A et al. Apparent diffusion coefficient scalars correlate with near-infrared spectroscopy markers of cerebrovascular autoregulation in neonates cooled for perinatal hypoxic-ischemic injury. Am. J. Neuroradiol 36, 188–193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JK et al. Optimizing cerebral autoregulation may decrease neonatal regional hypoxic-ischemic brain injury. Dev. Neurosci 39, 248–256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burton VJ et al. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol 15, 209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carrasco M et al. Cerebral autoregulation and conventional and diffusion tensor imaging magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. Pediatr. Neurol 82, 36–43 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eriksen VR, Hahn GH & Greisen G Cerebral autoregulation in the preterm newborn using near-infrared spectroscopy: a comparison of time-domain and frequency-domain analyses. J. Biomed. Opt 20, 037009 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Vavilala MS et al. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr. Res 58, 574–578 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brady KM et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 38, 2818–2825 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steiner L et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med 30, 733–738 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Lee JK et al. A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation 85, 1387–1393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewis PM et al. Cerebrovascular pressure reactivity in children with traumatic brain injury. Pediatr. Crit. Care. Med 16, 739–749 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Giller CA & Mueller M Linearity and non-linearity in cerebral hemodynamics. Med. Eng. Phys 25, 633–646 (2003). [DOI] [PubMed] [Google Scholar]
- 88.Hahn GH, Heiring C, Pryds O & Greisen G Applicability of near-infrared spectroscopy to measure cerebral autoregulation noninvasively in neonates: a validation study in piglets. Pediatr. Res 70, 166–170 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Chalak L & Zhang R New wavelet neurovascular bundle for bedside evaluation of cerebral autoregulation and neurovascular coupling in newborns with hypoxic-ischemic encephalopathy. Dev. Neurosci 39, 89–96 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tian F, Tarumi T, Liu H, Zhang R & Chalak L Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic–ischemic encephalopathy. Neuroimag Clin 11, 124–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitra S et al. Heart rate passivity of cerebral tissue oxygenation is associated with predictors of poor outcome in preterm infants. Acta Paediatr 103, e374–e382 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Kusaka T, Okubo K, Nagano K, Isobe K & Itoh S Cerebral distribution of cardiac output in newborn infants. Arch. Dis. Child. Fetal Neonatal Ed 90, F77–F78 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bouma GJ & Muizelaar JP Relationship between cardiac output and cerebral blood flow in patients with intact and with impaired autoregulation. J. Neurosurg 73, 368–374 (1990). [DOI] [PubMed] [Google Scholar]
- 94.da Costa CS et al. Monitoring of cerebrovascular reactivity for determination of optimal blood pressure in preterm infants. J. Pediatr 167, 86–91 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Soul JS et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr. Res 61, 467–473 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Wong F et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Riera J, Cabanas F, Serrano JJ, Madero R & Pellicer A New developments in cerebral blood flow autoregulation analysis in preterm infants: a mechanistic approach. Pediatr. Res 79, 460–465 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Tsuji M et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106, 625–632 (2000). [DOI] [PubMed] [Google Scholar]
- 99.O’Leary H et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics 124, 302–309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alderliesten T et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr 162, 698–704.e692 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Wong FY, Silas R, Hew S, Samarasinghe T & Walker AM Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS ONE 7, e43165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eriksen VR, Hahn GH & Greisen G Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatr 103, 1221–1226 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Bassan H et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr. Res 57, 35–41 (2005). [DOI] [PubMed] [Google Scholar]
- 104.Brady KM et al. Monitoring Cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke 41, 1957–1962 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith B et al. Does hypothermia impair cerebrovascular autoregulation in neonates during cardiopulmonary bypass? Pediatr. Anesth 27, 905–910 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Votava-Smith JK et al. Impaired cerebral autoregulation in preoperative newborn infants with congenital heart disease. J. Thorac. Cardiovasc. Surg 154, 1038–1044 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Zamora CA et al. Resistive index variability in anterior cerebral artery measurements during daily transcranial duplex sonography. J. Ultras Med 35, 2459–2465 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Foley LM et al. Enduring disturbances in regional cerebral blood flow and brain oxygenation at 24h after asphyxial cardiac arrest in developing rats. Pediatr. Res 81, 94–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Good M et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am. J. Physiol. Gastrointest. Liver Physiol 306, G1021–G1032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lear CA et al. Antenatal dexamethasone before asphyxia promotes cystic neural injury in preterm fetal sheep by inducing hyperglycemia. J. Cereb. Blood Flow. Metab 38, 706–718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.SAGE Publications, van Beek AH, Claasen JA, Rikkert MG & Jansen RW Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J. Cereb. Blood Flow. Metab 28, 1071–1085 (2008). [DOI] [PubMed] [Google Scholar]
- 112.American Physiological Society, Larson AC et al. Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J. Appl. Physiol 115, 1433–1442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolters Kuwer, Lee JK et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 40, 1820–1826 (2009). [DOI] [PubMed] [Google Scholar]


