Abstract
Background:
Cytotoxic cancer treatments, such as irradiation, can cause permanent sterility in male mammals due to the loss of spermatogonial stem cells (SSCs). In animal models, spermatogenesis could be restored from transplanted SSCs. Previously we showed that transient suppression of FSH, LH, and testosterone in the recipient with a gonadotropin-releasing hormone antagonist (GnRH-ant), given immediately after irradiation, enhanced spermatogenesis from transplanted SSCs in mice and monkeys.
Objectives:
To explore improvements in the preparation of the recipient for efficient and reliable spermatogenic recovery from SSC transplantation, so that it can be used effectively in clinical practice.
Materials and methods:
In mouse recipients we evaluated the effects of hormone suppression given after germ cell depletion was complete, which is a more clinically relevant model, and also the importance of total androgen ablation and maintenance of FSH levels. Three regimens: GnRH-ant, GnRH-ant plus flutamide (androgen receptor antagonist), or GnRH-ant plus FSH, were administered prior to and around the time of transplantation of testis cells from immature mice or from prepubertal monkeys.
Results:
Treatment with GnRH-ant resulted in a 4-fold increase in spermatogenic recovery from GFP-marked transplanted mouse cells. Total androgen ablation with the addition of flutamide, started 2 weeks before transplantation, did not further enhance recovery. Surprisingly, FSH supplementation, started around the time of transplantation actually reduced spermatogenic recovery from transplanted spermatogonial stem cells in GnRH-ant-treated mice. When prepubertal monkey testicular cells were transplanted into nude mice that were given the same hormone treatments, the numbers of donor-derived colonies were independent of hormone treatment.
Discussion and Conclusion:
The enhancements in spermatogenic recovery may only occur when syngeneic or closely related donor-recipient pairs are used. These results are useful in further investigations in choosing a hormone suppression regimen in combination with spermatogonial transplantation as a treatment to restore fertility in primates after cytotoxic therapy.
Keywords: Spermatogonia, Transplantation, irradiation, GnRH-antagonist
INTRODUCTION
Male infertility is one of the adverse consequences of cytotoxic exposures during cancer therapy. High doses of radiation or chemotherapy can produce permanent azoospermia by killing all of the stem spermatogonia. Although postpubertal males can preserve their fertility by semen cryopreservation, the only option for prepubertal boys involves cryopreservation of testicular tissue containing spermatogonial stem cells (SSC) for possible later transplantation (Brinster, 2007), a promising method for the preservation of fertility (Orwig & Schlatt, 2005).
Autologous intratesticular transplantation of cryopreserved testicular cells has been well documented to restore fertility in rodent models and some farm animals (Honaramooz & Yang, 2011). Although it has not been successfully demonstrated in humans, there are reports of recovery of spermatogenesis from transplanted germ cell suspensions into irradiated (Jahnukainen et al., 2011; Schlatt et al., 2002) and busulfan-treated monkeys (Hermann et al., 2012). However, improvements in the efficiency and reliability of this transplantation technique are needed before it can be used effectively in clinical practice.
One approach to improve transplantation efficiency is based on our demonstration that transient suppression of the gonadotropins and testosterone (T) with GnRH-analogs actually stimulates endogenous recovery of spermatogenesis after exposure to cytotoxic agents (Meistrich & Kangasniemi, 1997). Enhancement of testis colonization and differentiation of transplanted spermatogonia by suppressing gonadotropins and intratesticular T has also been demonstrated in busulfan-treated (Ogawa et al., 1999; Zhang et al., 2003) and irradiated (Zhang et al., 2007) recipient rats and in busulfan-treated (Dobrinski et al., 2001; Ogawa et al., 1998; Ohmura et al., 2003) and irradiated (Wang et al., 2010) mice. Furthermore, we showed that GnRH-ant given prior to transplantation enhanced the recovery of spermatogenesis from donor cells in irradiated monkey testes (Shetty et al., 2013). Still particularly in primates, the efficiency of spermatogenic recovery from transplanted SSCs is low and further optimization of the hormonal conditions around the time of transplantation should be tested.
Although GnRH-ant reduces serum T levels in rats or monkeys to near baseline levels (<0.1 ng/ml), the levels of T in the testis were only reduced to about 2–10 ng/gm-tissue (Shetty et al., 2013; Shetty et al., 2006b; Shetty et al., 2000). Combined treatment with GnRH-ant and the androgen receptor antagonist, flutamide, to produce total androgen ablation has been shown to more rapidly suppress spermatogenesis in normal rats (Kangasniemi et al., 1995) and further enhance the recovery of spermatogenesis in irradiated rats above the level seen with GnRH-ant alone (Porter et al., 2009; Shetty et al., 2000).
In addition, we reasoned that the suppression of FSH by the GnRH-ant might be detrimental to the recovery of spermatogenesis by transplanted SSC, since FSH has been shown to be important for maintenance of spermatogonial numbers (Meachem et al., 1999) and especially for the recovery of spermatogonial numbers in rats after gonadotropin suppression (Meachem et al., 1998). Since GnRH-ant is given as bolus injections, the decay of the drug concentration is gradual and the levels of FSH at the time of transplant would likely still be low; FSH supplementation at this time is expected to be beneficial.
In this study, we assessed the efficacy of specifically further suppressing the action of T and supplementation with FSH levels on colonization and development of mouse or monkey germ cells transplanted into irradiated mouse recipients. The goal was to identify optimum conditions, for application in subsequent studies with non-human primate hosts and eventual clinical applications.
MATERIALS AND METHODS
Animals
Adult C57BL/6Law (B6) and nude (Swiss nu-nu/Ncr) male mice at 7–9 weeks of age, bred at The University of Texas, M. D. Anderson Cancer Center, were used as transplantation recipients. Donor mice were obtained by breeding C57BL/6-Tg (CAG-EGFP) 1Osb/J mice (obtained from Jackson Laboratory), ubiquitously expressing green fluorescent protein, with B6 mice. The animals were maintained on a 12-h light 12-h dark cycle and were allowed food and water ad libitum. All animal procedures were approved by The University of Texas M. D. Anderson Cancer Center Animal Care and Use Committee.
Irradiation
Unanesthetized mice were irradiated by a 137Cs gamma-ray unit with a 3-cm diameter field covering only the lower abdominal and scrotal area (Wang et al., 2010). A total radiation dose of 13.5 Gy was delivered as an initial 1.5-Gy dose and followed by a second dose of 12 Gy one day later.
Hormone modulation
The schedules of treatments administered after irradiation to modulate hormone levels are given in Fig. 1. The GnRH antagonist, acyline (kindly provided by Contraceptive Development Program, National Institute of Child Health and Human Development, Rockville, MD), was prepared in sterile water and injected sc at 4 weeks after irradiation at an initial dose of 20 mg/kg body weight and followed by a maintenance dose of 10 mg/kg body weight given 2 weeks later (Shetty et al., 2006b). Such a treatment in mice suppressed the intratesticular testosterone levels by 97–98% to 3 ng/g-tissue in mice (Shetty et al., 2006b). These levels are below the basal levels of seminiferous tubule testosterone levels of 13 ng/ml, shown to be required for maintenance of spermatogenesis in rats (Zirkin et al., 1989). Sham-treated controls were given injection of sterile water. The androgen receptor antagonist, flutamide, was delivered by implanting two 2-cm Silastic silicone capsules filled with the drug at 6 weeks after irradiation and removing them 18 days later, which was 4 days after transplantation. FSH (recombinant human FSH, National Hormone & Peptide Program) was delivered using Alzet 2002 osmotic pumps at a dose of 5 IU/day (Baines et al., 2008) starting 1 day before transplantation and continued for 2 weeks.
FIG. 1.
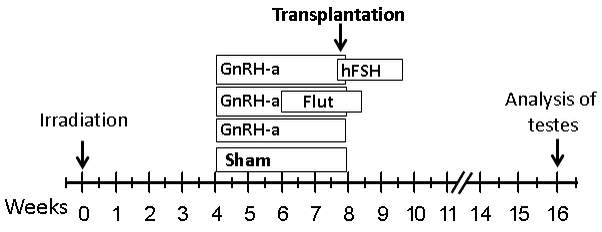
Schematic of the protocol used in the syngeneic and xenotransplantation experiments. Mice were irradiated at week 0 with total doses of 13.5 Gy. Hormonal suppression treatment was started at 4 weeks after irradiation. Transplantation was performed at 8 weeks after irradiation. Mice were euthanized at 8 weeks after transplantation for analysis.
Transplantation
Syngeneic transplantation employed hemizygous EGFP mice as donors and B6 mice as recipients. Testicular cells were prepared from immature 14–17 day old EGFP mice with sequential collagenase, hyaluronidase, and trypsin digestion as previously reported (Wang et al., 2010), except that a 40 μm screen was used to filter the suspension.
For xenotransplantation, donor cells from testes of four prepubertal rhesus monkeys, cryopreserved at Magee Womens Research Institute, Pittsburgh, were used. The cells were transported to Houston in liquid nitrogen. The cryopreserved cells were thawed, washed and suspended at approximately 20–40 × 106 cells/ml in MEMα containing FBS, Trypan blue, antibiotic-antimycotic mixture, DNase I, and Trypan blue. The prepubertal status of the donor monkeys at the time of harvesting testicular tissues was confirmed by the absence of mature spermatids in the cell suspension. The overall viability of the cells was 72%. Irradiated nude mice were used as recipients. Each suspension was used for at least three of the different treatment groups; furthermore, no significant differences were observed in the colonizing efficiencies of the four donors.
Mice were used as recipients for spermatogonial transplantation at 4–5 weeks after irradiation. Transplantation was performed by injection through the efferent ducts as described previously (Wang et al., 2010; Zhang et al., 2006). For syngeneic transplantations, the average injection per testis was 6 μl containing 1.65× 105 total cells; for xenotransplantations, the respective values were 7 μl and 3.4 × 105 cells. The different numbers of cells injected in these two experiments depended on the number of viable cells that we obtained from the GFP pup testes (for syngeneic) and from the cryopreserved monkey testes cells after thawing (xenogeneic), and also the volume of the cell suspension that could be injected each time.
The experiments were repeated 4–5 times to have enough number of successfully transplanted testes in each experimental group. Each time all the experimental groups were uniformly represented to avoid inter-experiment bias.
The syngeneic transplantation assay is widely used as an assay for mouse SSCs (Brinster, 2007; Dobrinski et al., 2001). In support of this we have previously shown, in a model very similar to the current one, that the colonies and sperm production are sustained for 43 weeks after transplantation (Wang et al., 2010). Furthermore, the colonies that we observe at 8 weeks contain spermatocytes and spermatogonia, indicating that the stem cell activity is continuing.
Although primate spermatogonia do not regenerate complete spermatogenesis in mouse seminiferous tubules, there are several criteria that provide support for the identification of these colonies as being derived from SSCs. The colonizing events by nonhuman primate and human testis cells appear very similar to early mouse SSC colonies two weeks after transplantation (Nagano et al., 1999; Nagano et al., 2002). For example, they are preferentially produced when cells sorted for stem cell markers are used. The cells migrate to the basement membrane of recipient seminiferous tubules, they proliferate to produce characteristic chains and networks of spermatogonia, and persist for periods of time ranging from several months to more than one year. Although the ultimate proof that these colonies are stem cells-derived awaits the ability to obtain differentiation, based on these criteria, xenotransplantation to mouse seminiferous tubules is considered by many authors as an assay for primate SSCs (Hermann et al., 2007; Izadyar et al., 2011; Nagano et al., 2001; Nagano et al., 2002; Sadri-Ardekani et al., 2011; Wu et al., 2009).
Evaluation of syngeneic donor-derived spermatogenesis
For evaluation of spermatogenic recovery after syngeneic transplantation, testes were harvested 8 weeks after transplantation. The 8-week time point for analysis was selected since that is typically used to evaluate the results of spermatogonial transplantation in mice (Tanaka et al., 2016). Testes were fixed in 4% paraformaldehyde at 4°C for up to 24 h, embedded in paraffin and sectioned. After deparaffinization, rehydration, antigen retrieval, and nonspecific antibody-binding blocking, the sections were then incubated with rabbit monoclonal anti-GFP (Cell Signaling, Danvers, MA) at 1:300 dilution at 4°C overnight, followed by a biotinylated anti-rabbit immunoglobulin G and an avidin-biotin-peroxidase complex reagent (Vectastain Elite kit, Vector Laboratories, Burlingame, CA). The immunoreactivity was visualized by incubation with the peroxidase substrate diaminobenzidine (Vector Laboratories). The slides were counterstained with hematoxylin.
Spermatogenic recovery was evaluated as previously described (Shetty et al., 2001) by the tubule differentiation index (TDI), which is defined as the percentage of tubules that contain three or more differentiating germ cells at the B spermatogonial stage or beyond. GFP staining was used to differentiate whether the germ cells originated from the donor cells (GFP positive) or from the endogenous spermatogonia (GFP negative). The percentage of tubule cross-sections with donor-derived differentiating cells give a measure proportional to the number of colonizing donor SSCs and the length of the donor colonies. The TDI for donor cells was corrected for injection of different cell numbers by normalization to the average cell numbers injected in all syngeneic transplants.
Evaluation of xenogeneic donor-derived colonization
For evaluation of colonization of mouse testes by prepubertal rhesus monkey SSC, testes were harvested from nude mice 8 weeks after transplantation and seminiferous tubules were dispersed with collagenase and DNase, and fixed in paraformaldehyde. Tubules were processed for immunostaining with a rabbit anti-rhesus antibody as described (Hermann et al., 2007). The rhesus testis-cell antibody was used at a 1:800 dilution and detected with Alexa Fluor 488 conjugated goat anti-rabbit IgG (1:300 dilution; Invitrogen, Carlsbad, CA) (Shetty et al., 2013). In addition, the tubules were stained with a goat-anti-VASA antibody (1:200, AF2030, R&D, Systems, Minneapolis, MN), which was detected using a donkey-anti-goat Alexa Fluor 594 conjugated second antibody (1:300). The monkey donor cells were identified by their green fluorescence in whole-mounted tubules. Samples were mounted with Vectashield medium containing DAPI (Vector Laboratories, Burlingame, CA) on slides with raised coverslips and visualized by fluorescence microscopy.
Donor colonies were identified containing at least four cells exhibiting spermatogonial morphology and topology (<100 μm between cells), located on the basement membrane of the recipient seminiferous tubule, and exhibiting VASA staining (Hermann et al., 2009), criteria consistent with their formation by stem cells. The number of colonies in the whole testicular preparation and the number of cells in each colony were counted.
Statistical analysis
The TDI and colony counts were represented as arithmetic mean ± SEM. When only two groups were being compared (transplanted and untransplanted testes within a given treatment group), the significance of differences between different treatments was evaluated by the t-test and non-parametric Mann-Whitney U test. When multiple treatment groups were being compared, a one-way analysis of variance (ANOVA) test was performed to test whether there were significant differences between the groups (ANOVA P < 0.05) and then individual groups were compared using a post-hoc-Tukey test. All analyses were performed with the IBM SPSS (version 23) statistical package.
RESULTS
With the objective of optimizing hormone treatment to enhance colonization and later spermatogenesis, we manipulated gonadotropin and androgen levels/actions in irradiated adult B6 mice that received testicular germ cell transplants from immature immunocompatible EGFP-labeled mice. Three treatment regimens were tested (Fig. 1): treatment for 4 weeks prior to the transplantation with GnRH-ant only, GnRH-ant plus flutamide for the last 2 weeks before transplant, and GnRH-ant plus FSH for 1 week after transplantation, and compared with sham hormone treatments. Significant increases in testes weights were observed in all the transplanted groups compared to the respective untransplanted group, confirming successful transplantation (Table 1).
Table 1.
Irradiated (1.5 Gy + 12 Gy) C57BL6 mice that received different hormone-suppression treatments were transplanted with cells from syngeneic mouse pups expressing EGFP; resulting recipient testis weights at 8 weeks after transplantation. Values given as mean ± SE (number of testes). (*P<0.05; comparison of transplanted vs untransplanted, t test).
| Hormone Treatment |
Cells injected (× 105) |
Testis weight (mg) | |
|---|---|---|---|
| Not transplanted (sham) |
Transplanted | ||
| None (Sham) | 1.77 ± 0.19 | 22.8 ± 0.6 (6) | 30.8 ± 1.0 (10)* |
| GnRH-ant | 1.66 ± 0.10 | 24.7 ± 0.6 (3) | 30.9 ± 1.0 (11)* |
| GnRH-ant+Flutamide | 1.46 ± 0.17 | 22.0 ± 0.9 (10) | 27.0 ± 1.2 (12)* |
| GnRH-ant+FSH | 1.77 ± 0.15 | 22.0 ± 0.3 (5) | 26.8 ± 0.9 (13)* |
Initially we tested whether the dose of radiation (13.5 Gy) given to the mice was sufficient to cause extensive depletion of the germ cells as required in the current model. Indeed, at 4 weeks after irradiation, at the time of initiation of hormone modulations, 99.5±0.5 (n=5) of the tubular cross-sections of the testis were depleted of germ cells and at 8 weeks after irradiation, at the time of transplantation, 97.4±0.3 (n=4) of tubules were still depleted (Fig. 2).
FIG. 2.
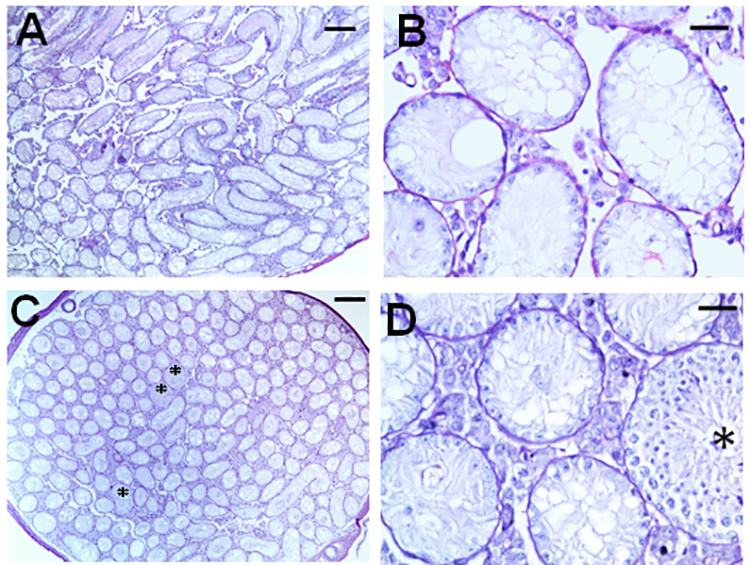
Representative histology of the testis in B6 mice at 4 weeks (A&B) and 8 weeks (C&D) after testicular irradiation with total doses of 13.5 Gy. B and D are the magnified views of areas in A and C, respectively. Asterisks in C &D represent the tubules showing differentiated germ cells derived from a few endogenous radioresistant A spermatogonia. Note that at 4 weeks, the tubules with differentiated germ cells are almost absent. The bars indicate 200 μm in A&C and 30μm in B&D.
In testes harvested at 8 weeks after transplantation, we observed spermatogenesis from both endogenous GFP-negative and donor-derived GFP-positive spermatogonial stem cells (Fig. 3A-C). The GnRH-ant treatment given from 4 to 8 weeks after irradiation, with or without the addition of flutamide or FSH restoration, did not produce any significant increases in endogenous spermatogenic recovery (Fig 3E). However, the percentages of the tubules that showed differentiated germ cells that originated from donor spermatogonia, which was just 1.6% in irradiated-only mice, was significantly increased 4-fold to 6.5% by the GnRH-ant treatment (Fig. 3D). The GnRH-ant treatment suppresses FSH and LH and the LH suppression results in lower basal intratesticular testosterone levels (Shetty et al., 2006b), and this was sufficient to achieve maximal recovery. The additional blockade of the residual basal testosterone action with flutamide treatment resulted in donor-derived spermatogenic cells in only 5.7% of tubules. Thus, the total androgen ablation with the addition of flutamide did not have any further promotional effect. Furthermore, the addition of FSH supplementation around the time of transplantation to GnRH-ant treatment actually resulted in a decrease in the percentage of tubules with differentiated donor-derived germ cells to 3.3%, which was significantly different from the value for GnRH-ant only using non-parametric statistics. This result indicates that the suppression of FSH by the GnRH-ant treatment may actually be beneficial to the recovery of spermatogenesis from the transplanted cells.
FIG. 3.
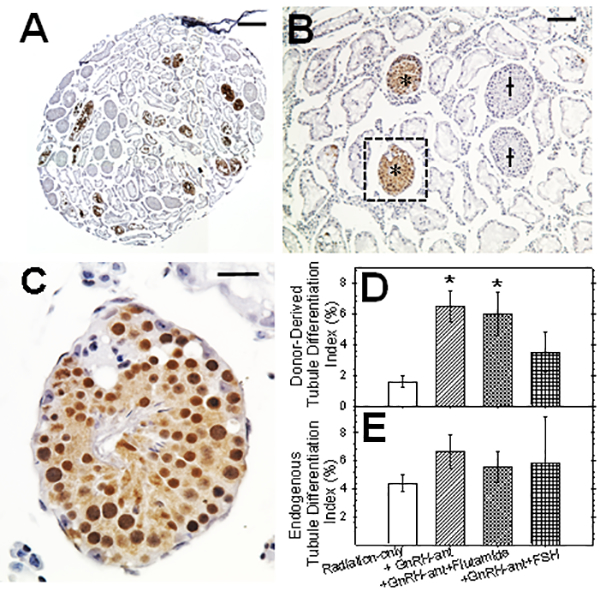
Testicular histology (A-C) and analysis of tubules with spermatogenesis (D-E) after syngeneic (B6 GFP mouse to B6 mouse) transplantation. (A) Testicular section 8 weeks after transplantation. Donor colonies were immunolocalized with anti-GFP followed by DAB staining and then counterstained with hematoxylin. (B) Another section at higher magnification with tubules showing endogenous (†) and donor-derived (*) spermatogenesis. (C) Higher magnification of tubule with donor derived spermatogenesis showing the presence of spermatid and sperm. Percentages of tubules with (D) donor-derived and (E) endogenous spermatogenesis after transplantation to recipients with different hormone manipulations. The number of recipient testes analyzed in each group were 10–12. Significant differences in the tubule differentiation indices between different groups and irradiated-only mice by ANOVA and post-hoc Tukey’s test are indicated (* = P<0.05). In addition, the differentiation of donor cells in the group receiving GnRH-ant plus FSH was significantly lower than in the group only receiving GnRH-ant by non-parametric tests. The bars indicate 350 μm in A, 100 μm in B and 25μm in C.
Next, we tested the effect of hormone manipulation on the colonization of prepubertal monkey testicular cells that were transplanted to nude mice. When the tissues were harvested 8 weeks after transplantation, the weights of the transplanted testes tended to be greater than the untransplanted testes but the difference was only significant for one treatment group (Table 2).
Table 2.
Irradiated (1.5 Gy + 12 Gy) nude mice that received different hormone-suppression treatments were transplanted with cryopreserved prepubertal monkey testicular cells; resulting recipient testes weights at 8 weeks after transplantation are shown. Values given as mean ± SE (number of testes). (*P<0.05; comparison of transplanted vs untransplanted, t test).
| Hormone Treatment |
Cells injected (× 105) | Testis weight (mg) | |
|---|---|---|---|
| Not transplanted (sham) |
Transplanted | ||
| None (Sham) | 4.1 ± 0.4 | 30.5 ± 2.8 (5) | 41.5 ± 3.6 (6) |
| GnRH-ant | 3.4 ± 0.6 | 30.9 ± 1.1 (11) | 35.1 ± 2.2 (11) |
| GnRH-ant+Flutamide | 3.6 ± 0.3 | 28.6 ± 1.2 (8) | 35.9 ± 2.5 (13) |
| GnRH-ant+FSH | 3.0 ± 0.3 | 27.9 ± 1.8 (11) | 36.4 ± 2.3 (9)* |
At this time, colonies formed from the monkey donor cells could be visualized by the staining for nHP and germ-cell specific VASA antibodies (Fig. 4A-C). The staining intensities of these colonies for VASA was variable, and it appeared that some donor-cell colonies were VASA-negative. Since cross-sections of control rhesus monkey testes that showed strong immunostaining for VASA in the pachytene spermatocytes and spermatids, only showed weak or no staining of the cells in the basal layer (Fig. 5A), we thought it necessary to evaluate the use of the criterion of VASA immunostaining for identification of rhesus monkey spermatogonial colonies. In the normal monkey testis cross-sections, we identified type A spermatogonia by their basal location, relatively large size, and lack of heterochromatin (Fig. 5B). In this tubule, three such cells were identified (Fig. 5C, D, E) and all showed clear cytoplasmic VASA staining (Fig. 5C’, D’, E’). Analysis of three other tubules in different stages of the seminiferous epithelial cycle also showed that all type A spermatogonia displayed VASA immunofluorescence, which we estimate from the photomicrographs to be about 50% of the intensity of the spermatocyte or round spermatid fluorescence intensities. In addition, the dark areas in the basal layer corresponded to Sertoli cells, which showed no VASA immunofluorescence and preleptotene and leptotene spermatocytes were weakly fluorescent. Hence, these results support the use of VASA immunofluorescence for identifying donor monkey spermatogonial colonies in whole-mounted tubules after transplantation.
FIG. 4.
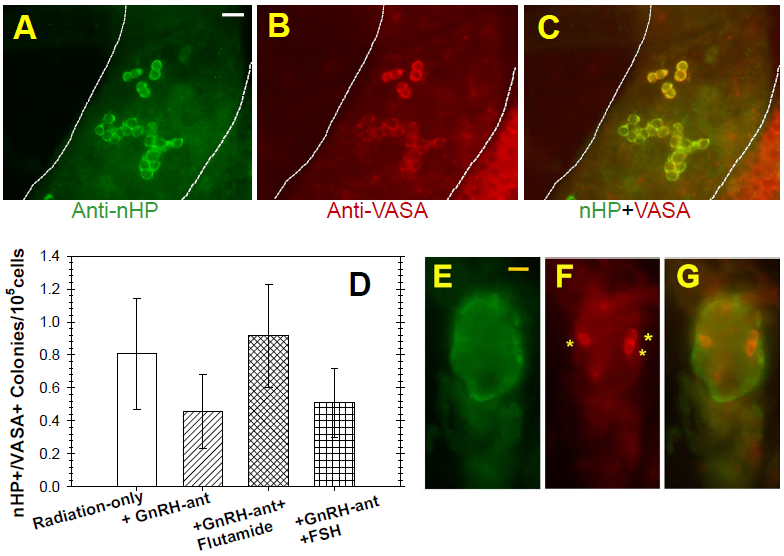
Whole mounts of seminiferous tubules from xenotransplantation recipients and their analyses. Representative whole mounts of seminiferous tubules from immunostained with (A) anti-nHP and (B) anti-VASA showing the donor-derived colonies. (C) Overlay of nHP and VASA immunofluorescence. Note the variability in the intensity of VASA staining. (D) Donor-derived nHP-positive, VASA-positive colonies in xenotransplanted recipients with different hormone manipulations. The numbers of recipient testes analyzed in each group were between 5 to 10. There were no significant differences in the numbers of colonies in the different groups. (E) Example of ovoid-shaped, primate donor-derived multicellular structure, stained for nHP-antigen (green), within mouse seminiferous tubules. (F) Three donor-derived (nHP-antigen+) germ cells (VASA+) within multicellular structure. (G) Overlay of A and B. Bars represent 25μm.
FIG. 5.
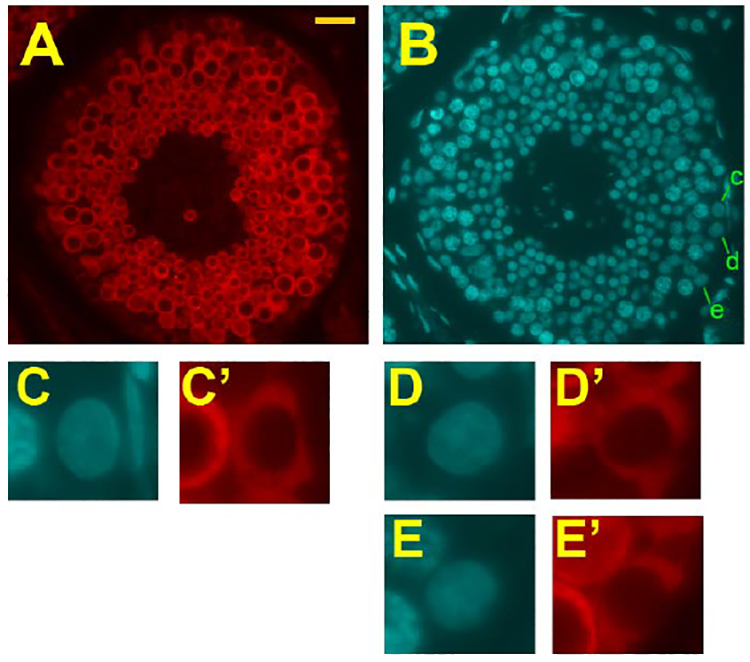
Sections of normal adult rhesus monkey testis, demonstrating VASA staining of spermatogonia. (A) VASA-stained seminiferous tubule at about Stage VI. (B) DAPI fluorescence with 3 spermatogonia identified (c, d, e). (C, D, E) Enlarged images of the 3 spermatogonia identified in B. (C’, D’, E’) VASA staining of spermatogonia shown in C, D, and E. The bar in A represents 25 μm.
It should be noted that the monkey spermatogonia merely survive and proliferate along the basal lamina but fail to produce differentiated cells (Fig. 4A-C). In the radiation-only recipients, we observed an average of 0.80 nHP- and VASA- double positive colonies per 105 donor cells (Fig. 4D), similar to result observed in busulfan-treated mouse recipients (Hermann et al., 2009). The respective number of colonies per 105 cells did not change significantly with GnRH-antagonist treatment before the transplantation (0.46 colonies), or with additional flutamide (0.92 colonies) or rFSH treatment (0.51 colonies). Furthermore, the sizes of the colonies, which overall had a median of 10 cells, did not differ between the treatment regimens. Thus, none of the hormone suppression treatments enhanced the colonization of the transplanted monkey cells in the mouse testes.
In addition to the colonies of spermatogenic cells, other clusters of donor cells were observed in seminiferous tubules (Fig. 4E-G) and probably accounted for the increased testis weights of the transplanted testes. These ovoid multicellular clusters were mainly of VASA-negative somatic cells, with some VASA-positive germ cells. It is possible that the somatic cells are donor Sertoli cells surviving in the lumen of recipients tubules, as observed in rat testes transplanted with immature rat testis cells (Zhang et al., 2009).
DISCUSSION
The recovery of spermatogenesis after transplantation is dependent on the ability of the transplanted stem cells to first home to the available stem cell niches and then colonize and survive, proliferate to increase their numbers, and finally to differentiate to produce the full sequence of germ cells. The process can be divided into the colonization step (homing the niche and functionally surviving), which occur within the first week after transplantation, and the post-colonization phase (proliferation and differentiation), which begins after 1 week (Nagano, 2003). Hormone suppression could modulate all of these phases. It should be noted that, since depot injections of GnRH analogs are used for hormone suppression, the effects of hormone suppressive treatments before transplantation, can extend for several weeks after transplant. Various studies in the literature have shown that both using hormone suppression before transplantation (Dobrinski et al., 2001; Kanatsu-Shinohara et al., 2004; Ogawa et al., 1998; Shetty et al., 2013; Wang et al., 2010), and hormone suppression after colonization (Dobrinski et al., 2001; Ogawa et al., 1999; Ohmura et al., 2003; Tanaka et al., 2016; Wang et al., 2010; Zhang et al., 2007) stimulate recovery of spermatogenesis from transplanted cells. Because the spermatogonia lack gonadal steroid and gonadotropin receptors, hormonal effects on the success of transplantation are dependent on the action of hormones on the somatic environment, not the transplanted germ cells.
Factors involved in the homing step appear to be the ability of the SSCs to attach to Sertoli cells and the integrity of the tight junctions of the Sertoli cell barrier, which can impede the migration of cells to the niches in the basal layer. Specific interactions between spermatogonia and Sertoli cells, involving β1-integrin expressed on Sertoli cells, are necessary for this homing step (Kanatsu-Shinohara et al., 2008). The maintenance of the Sertoli cell barrier in rodents is dependent on androgen (Haverfield et al., 2014; Meng et al., 2005), which acts by regulating tight junction proteins, occludin and several claudins (Chakraborty et al., 2014). Loss of the barrier function appears to be an important mechanism by which hormone suppression facilitates donor SSC colonization.
For colonization, after arrival at the niche, the SSC must functionally attach to the basal lamina for survival (Kanatsu-Shinohara et al., 2008; Nagano, 2003). Integrin β1 on spermatogonia is necessary for attachment to the laminins present there. However, there is no indication that hormone suppression increases laminin gene expression in germ cell depleted testes (Zhou et al., 2010a; Zhou et al., 2010b).
A proliferative phase, involving self-renewal of SSCs, starts about 1 week after transplantation. GDNF appears to be the most important factor stimulating the self-renewal of SSCs within the niche (Meng et al., 2000). Several reports suggest that GDNF is positively regulated by FSH in Sertoli cells (Ding et al., 2011; Tadokoro et al., 2002) and by testosterone in peritubular myoid cells (Chen et al., 2014), but others find no effect of FSH (Kanatsu-Shinohara et al., 2012; Tanaka et al., 2016). However, in irradiated rats, we observed an increase in Sertoli cell GDNF protein after suppression of testosterone and FSH (Albuquerque et al., 2013). WNT proteins also increase numbers of undifferentiated type A spermatogonia by enhancing proliferation (Yeh et al., 2011). Wnt5a levels can be increased by suppression of testosterone (Tanaka et al., 2016; Zhou et al., 2010b) and also by increasing FSH (Zhou et al., 2010a). Furthermore, CSF1 (colony-stimulating factor 1) has been reported to accelerate self-renewal (Oatley et al., 2009) but its levels do not appear to be modulated by hormone suppression or FSH addition (Zhou et al., 2010a; Zhou et al., 2010b).
After one or more self-renewing divisions, differentiation needs to be initiated. Fibroblast growth factors, such as FGF2, are capable of increasing the numbers of Aaligned spermatogonia that are preparing for differentiation (Sakai et al., 2018). In addition, WNT proteins, such as WNT3A and WNT6, act on the Apaired and Aaligned spermatogonia to enhance proliferation and inhibit apoptosis (Takase & Nusse, 2016; Yeh et al., 2012). Furthermore, retinoic acid and stem cell factor (Kit-ligand) are essential for the transition of Aaligned to A1 spermatogonia and further steps in the differentiation process (Endo et al., 2017). Although in rats hormone suppression decreases expression of alcohol dehydrogenases 1 and 4, which are involved in the biosynthesis of retinoic acid, and Kit-ligand, in mice, elimination of the Sertoli cell androgen receptor in mice increases expression of the Adh1 genes (Zhou et al., 2010a; Zhou et al., 2010b). Hence, no consistent effects of hormone suppression on spermatogonial differentiation are expected.
In the current study, we investigated the efficacy of several modifications of the hormone suppression strategy. Since previous studies employed hormone suppression started immediately after irradiation (Wang et al., 2010) or busulfan (Dobrinski et al., 2001), here we delayed the hormone suppression for 4 weeks so that the endogenous spermatogenesis will have been completely depleted. This is more relevant to the eventual clinical application in which the transplantation will likely be done years after stem cell harvest and radiation or chemotherapy. The stimulation of donor-derived spermatogenic recovery by delayed hormone suppression, about 4-fold (Fig. 3), was similar to that observed previously with hormone suppression started immediately after irradiation (Wang et al., 2010).
Next, we investigated whether total androgen ablation by combining GnRH-antagonist treatment with the androgen receptor antagonist, flutamide, would enhance the colonization and/or developmental ability of syngeneic spermatogonial stem cells (SSCs) in transplanted mouse recipients. The suppression was given before the transplantation, so that the homing step would be affected. Also, we have shown that the regimen of acyline at 20 mg/kg followed by a maintenance dose of 10 mg/kg depresses intratesticular testosterone levels for a least 6 weeks, or 2 weeks after the transplant (Shetty et al., 2006b); hence, effects of suppressing testosterone will last through the colonization and proliferation phase. Thus, androgen suppression likely acts by enhancing the homing step by downregulating tight junction proteins and perhaps also during the proliferation step by upregulating Wnt5a expression. In any case, whatever the mechanism of stimulation by androgen suppression, the reduction of intratesticular testosterone to 2 ng/gm/tissue achieved by the GnRH-antagonist, acyline, is sufficient for a maximal stimulation of transplantation effectiveness, and the additional blockade with flutamide is unnecessary. This is important for possible future clinical application because flutamide and other more widely used androgen receptor antagonists have side effects such as liver toxicity and seizures.
Based on reports that FSH might stimulate GDNF and WNT5A expression, we tested addition of FSH during a 1-week period after transplantation, which corresponds to the colonization and start of the proliferation phase. Our results show that FSH supplementation actually decreased the recovery of spermatogenesis from transplanted cells in mice, as we had observed in rats with recovery from endogenous surviving stem cells (Shetty et al., 2006a). The mechanism of this detrimental effect of FSH is not known.
Finally, the failure of any of these hormone treatments to enhance colonization of monkey testicular cells xenotransplanted into mouse testes was disappointing. This was surprising, because not only does hormone suppression enhance the efficiency of allogeneic transplantation in mouse testes, it also enhances the efficiency of allogeneic transplantation in monkey testes (Shetty et al., 2013). It should be noted that nearly all of the previous studies of stimulation of colonization and spermatogenic recovery using hormone suppression have been with allogeneic, rather than xenogeneic transplantation. The only xenogeneic transplantation studies (Ogawa et al., 1999; Zhang et al., 2003) that indicated stimulation of spermatogenic recovery by hormone suppression was after mouse to rat transplantation. However, those studies used closely related species in which the recipient testis supports donor spermatogenesis of the other species. The failure of increased colonization of monkey cells transplanted into mouse testes, suggests that more than merely the opening of the Sertoli cell tight junctions, the mechanisms of binding to Sertoli cells and chemotaxis to niche are also necessary for colonization. In addition, actions of survival and proliferation factors that are enhanced by hormone suppression, may only act in a species-specific manner. Also, stimulation of recovery by enhanced differentiation, which might be occurring in the allogeneic models, is not relevant to the xenogeneic model, since the donor cells can only colonize and proliferate.
In conclusion, we confirm the stimulation of recovery of spermatogenesis from transplanted cells by suppression of hormones with GnRH-ant, after decline of endogenous spermatogenesis and up to and just after syngeneic transplantation. Additional treatment with androgen receptor antagonists or FSH were not beneficial. Further investigation of the mechanisms involved in the stimulation of colonization and spermatogenic recovery are important for further optimization of transplantation.
ACKNOWLEDGEMENTS
This work was supported by research grants P01 HD075795 from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH) to KEO and Cancer Center Support Grant P30 CA 016672 from NCI/NIH to The University of Texas MD Anderson Cancer Center. We sincerely thank Dr. Min S. Lee, Contraceptive Development Program, National Institute for Child Health and Human Development, for providing the Acyline.
FUNDING INFORMATION
This work was supported by research grants P01 HD075795 from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH) to KEO and Cancer Center Support Grant P30 CA 016672 from NCI/NIH to The University of Texas MD Anderson Cancer Center.
Footnotes
DISCLOSURES
The authors have no conflicting financial interests.
REFERENCES
- Albuquerque AV, Almeida FR, Weng CC, Shetty G, Meistrich ML & Chiarini-Garcia H. (2013) Spermatogonial behavior in rats during radiation-induced arrest and recovery after hormone suppression. Reproduction 146, 363–376. [DOI] [PubMed] [Google Scholar]
- Baines H, Nwagwu MO, Hastie GR, Wiles RA, Mayhew TM & Ebling FJ. (2008) Effects of estradiol and FSH on maturation of the testis in the hypogonadal (hpg) mouse. Reprod Biol Endocrinol 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL. (2007) Male germline stem cells: from mice to men. Science 316, 404–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P, William Buaas F, Sharma M, Smith BE, Greenlee AR, Eacker SM & Braun RE. (2014) Androgen-dependent sertoli cell tight junction remodeling is mediated by multiple tight junction components. Mol Endocrinol 28, 1055–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Brown PR, Willis WB & Eddy EM. (2014) Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance. Endocrinology 155, 4964–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding LJ, Yan GJ, Ge QY, Yu F, Zhao X, Diao ZY, Wang ZQ, Yang ZZ, Sun HX & Hu YL. (2011) FSH acts on the proliferation of type A spermatogonia via Nur77 that increases GDNF expression in the Sertoli cells. FEBS Lett 585, 2437–2444. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Ogawa T, Avarbock MR & Brinster RL. (2001) Effect of the GnRH-agonist leuprolide on colonization of recipient testes by donor spermatogonial stem cells after transplantation in mice. Tissue Cell 33, 200–207. [DOI] [PubMed] [Google Scholar]
- Endo T, Freinkman E, de Rooij DG & Page DC. (2017) Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc Natl Acad Sci U S A 114, E10132–E10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverfield JT, Meachem SJ, Nicholls PK, Rainczuk KE, Simpson ER & Stanton PG. (2014) Differential permeability of the blood-testis barrier during reinitiation of spermatogenesis in adult male rats. Endocrinology 155, 1131–1144. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, et al. (2007) Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells 25, 2330–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM & Orwig KE. (2009) Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in Rhesus macaques. Hum Reprod 24, 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, et al. (2012) Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 11, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A & Yang Y. (2011) Recent advances in application of male germ cell transplantation in farm animals. In: Vet Med Int, Vol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, Howerton K, Yuen C, Greilach S, Zhao HH, Chow M, et al. (2011) Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod 26, 1296–1306. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M & Schlatt S. (2011) Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod 26, 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Takashima S, Takehashi M, Ogonuki N, Morimoto H, Nagasawa T, Ogura A & Shinohara T. (2012) Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell 11, 567–578. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Morimoto T, Toyokuni S & Shinohara T. (2004) Regulation of mouse spermatogonial stem cell self-renewing division by the pituitary gland. Biol Reprod 70, 1731–1737. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fassler R & Shinohara T. (2008) Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell 3, 533–542. [DOI] [PubMed] [Google Scholar]
- Kangasniemi M, Wilson G, Parchuri N, Huhtaniemi I & Meistrich ML. (1995) Rapid protection of rat spermatogenic stem cells against procarbazine by treatment with a gonadotropin-releasing hormone antagonist (Nal-Glu) and an antiandrogen (flutamide). Endocrinology 136, 2881–2888. [DOI] [PubMed] [Google Scholar]
- Meachem SJ, McLachlan RI, Stanton PG, Robertson DM & Wreford NG. (1999) FSH immunoneutralization acutely impairs spermatogonial development in normal adult rats. J Androl 20, 756–762. [PubMed] [Google Scholar]
- Meachem SJ, Wreford NG, Stanton PG, Robertson DM & McLachlan RI. (1998) Follicle-stimulating hormone is required for the initial phase of spermatogenic restoration in adult rats following gonadotropin suppression. J Androl 19, 725–735. [PubMed] [Google Scholar]
- Meistrich ML & Kangasniemi M. (1997) Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J Androl 18, 80–87. [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD & Braun RE. (2005) Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A 102, 16696–16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, et al. (2000) Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489–1493. [DOI] [PubMed] [Google Scholar]
- Nagano M, Avarbock MR & Brinster RL. (1999) Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod 60, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, McCarrey JR & Brinster RL. (2001) Primate spermatogonial stem cells colonize mouse testes. Biol Reprod 64, 1409–1416. [DOI] [PubMed] [Google Scholar]
- Nagano M, Patrizio P & Brinster RL. (2002) Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril 78, 1225–1233. [DOI] [PubMed] [Google Scholar]
- Nagano MC. (2003) Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod 69, 701–707. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Oatley MJ, Avarbock MR, Tobias JW & Brinster RL. (2009) Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 136, 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR & Brinster RL. (1998) Leuprolide, a gonadotropin-releasing hormone agonist, enhances colonization after spermatogonial transplantation into mouse testes. Tissue Cell 30, 583–588. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I & Brinster RL. (1999) Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell 31, 461–472. [DOI] [PubMed] [Google Scholar]
- Ohmura M, Ogawa T, Ono M, Dezawa M, Hosaka M, Kubota Y & Sawada H. (2003) Increment of murine spermatogonial cell number by gonadotropin-releasing hormone analogue is independent of stem cell factor c-kit signal. Biol Reprod 68, 2304–2313. [DOI] [PubMed] [Google Scholar]
- Orwig KE & Schlatt S. (2005) Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr 34, 51–56. [DOI] [PubMed] [Google Scholar]
- Porter KL, Shetty G, Shuttlesworth G, Weng CY, Huhtaniemi I, Pakarinen P & Meistrich ML. (2009) Estrogen enhances recovery from radiation-induced spermatogonial arrest in rat testes. J Androl 30, 440–451. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S & van Pelt AM. (2011) In vitro propagation of human prepubertal spermatogonial stem cells. JAMA 305, 2416–2418. [DOI] [PubMed] [Google Scholar]
- Sakai M, Masaki K, Aiba S, Tone M & Takashima S. (2018) Expression dynamics of self-renewal factors for spermatogonial stem cells in the mouse testis. J Reprod Dev 64, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatt S, Foppiani L, Rolf C, Weinbauer GF & Nieschlag E. (2002) Germ cell transplantation into X-irradiated monkey testes. Hum Reprod 17, 55–62. [DOI] [PubMed] [Google Scholar]
- Shetty G, Uthamanthil RK, Zhou W, Shao SH, Weng CC, Tailor RC, Hermann BP, Orwig KE & Meistrich ML. (2013) Hormone suppression with GnRH antagonist promotes spermatogenic recovery from transplanted spermatogonial stem cells in irradiated cynomolgus monkeys. Andrology 1, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty G, Weng CC, Meachem SJ, Bolden-Tiller OU, Zhang Z, Pakarinen P, Huhtaniemi I & Meistrich ML. (2006a) Both testosterone and FSH independently inhibit spermatogonial differentiation in irradiated rats. Endocrinology 147, 472–482. [DOI] [PubMed] [Google Scholar]
- Shetty G, Weng CC, Porter KL, Zhang Z, Pakarinen P, Kumar TR & Meistrich ML. (2006b) Spermatogonial differentiation in juvenile spermatogonial depletion (jsd) mice with androgen receptor or follicle stimulating hormone mutations. Endocrinology 147, 3563–3570. [DOI] [PubMed] [Google Scholar]
- Shetty G, Wilson G, Huhtaniemi I, Boettger-Tong H & Meistrich ML. (2001) Testosterone inhibits spermatogonial differentiation in juvenile spermatogonial depletion mice. Endocrinology 142, 2789–2795. [DOI] [PubMed] [Google Scholar]
- Shetty G, Wilson G, Huhtaniemi I, Shuttlesworth GA, Reissmann T & Meistrich ML. (2000) Gonadotropin-releasing hormone analogs stimulate and testosterone inhibits the recovery of spermatogenesis in irradiated rats. Endocrinology 141, 1735–1745. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A & Nishimune Y. (2002) Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 113, 29–39. [DOI] [PubMed] [Google Scholar]
- Takase HM & Nusse R. (2016) Paracrine Wnt/beta-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc Natl Acad Sci U S A 113, E1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kanatsu-Shinohara M, Lei Z, Rao CV & Shinohara T. (2016) The luteinizing hormone-testosterone pathway regulates mouse spermatogonial stem cell self-renewal by suppressing WNT5A expression in Sertoli cells. Stem Cell Reports 7, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Shao SH, Weng CC, Wei C & Meistrich ML. (2010) Hormonal suppression restores fertility in irradiated mice from both endogenous and donor-derived stem spermatogonia. Toxicol Sci 117, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Schmidt JA, Avarbock MR, Tobias JW, Carlson CA, Kolon TF, Ginsberg JP & Brinster RL. (2009) Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A 106, 21672–21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JR, Zhang X & Nagano MC. (2011) Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci 124, 2357–2366. [DOI] [PubMed] [Google Scholar]
- Yeh JR, Zhang X & Nagano MC. (2012) Indirect effects of Wnt3a/beta-catenin signalling support mouse spermatogonial stem cells in vitro. PLoS One 7, e40002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Renfree MB & Short RV. (2003) Successful intra- and interspecific male germ cell transplantation in the rat. Biol Reprod 68, 961–967. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao S & Meistrich M. (2007) The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol 211, 149–158. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao S & Meistrich ML. (2006) Irradiated mouse testes efficiently support spermatogenesis derived from donor germ cells of mice and rats. J Androl 27, 365–375. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao S, Shetty G & Meistrich M. (2009) Donor Sertoli cells transplanted into irradiated rat testes stimulate partial recovery of endogenous spermatogenesis. Reproduction 137, 497–508. [DOI] [PubMed] [Google Scholar]
- Zhou W, Bolden-Tiller OU, Shetty G, Shao SH, Weng CC, Pakarinen P, Liu Z, Stivers DN & Meistrich ML. (2010a) Changes in gene expression in somatic cells of rat testes resulting from hormonal modulation and radiation-induced germ cell depletion. Biol Reprod 82, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang G, Small CL, Liu Z, Weng CC, Yang L, Griswold MD & Meistrich ML. (2010b) Gene expression alterations by conditional knockout of androgen receptor in adult sertoli cells of Utp14b(jsd/jsd) (jsd) mice. Biol Reprod 83, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkin BR, Santulli R, Awoniyi CA & Ewing LL. (1989) Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124, 3043–3049. [DOI] [PubMed] [Google Scholar]


