Abstract
Most people have or will experience traumatic stress at some time over the lifespan, but only a subset of traumatized individuals develop post-traumatic stress disorder (PTSD). Clinical research supports high rates of traumatic brain injury (TBI)-PTSD comorbidity and demonstrates TBI as a significant predictor of the development of PTSD. Biological factors impacted following brain injury that may contribute to increased PTSD risk are unknown. Heightened stress reactivity and dysregulated hypothalamic-pituitary-adrenal (HPA) axis function are common to both TBI and PTSD, and affect amygdalar structure and function, which is implicated in PTSD. In this review, we summarize a growing body of literature that shows HPA axis dysregulation, as well as enhanced fear and amygdalar function after TBI. We present the hypothesis that altered stress reactivity as a result of brain injury impacts the amygdala and defense systems to be vulnerable to increased fear and PTSD development from traumatic stress. Identifying biological mechanisms that underlie this vulnerability, such as dysregulated HPA axis function, may lead to better targeted treatments and preventive measures to support psychological health after TBI.
Keywords: Traumatic brain injury, PTSD, stress reactivity, HPA axis, amygdala
Introduction
Stress is a multidimensional construct that is typically used to define an organism’s response to a threat. The stress response is highly conserved to indicate that the stress process successfully guides survival in many organisms. For example, soldiers exposed to combat stress are often confronted by true and anticipated threats to survival, including injuries such as traumatic brain injury (TBI). TBI or a history of TBI can affect subsequent response to stress that may lead to neuroendocrine abnormalities as well as neuropsychiatric comorbidities. In the class of trauma and stressor-related disorders, post-traumatic stress disorder (PTSD) is a debilitating and complex disorder that sometimes manifests following a traumatic event (American Psychiatric Association 2013). TBI and PTSD are often comorbid although PTSD is not a requisite sequela of TBI (Molaie and Maguire 2018).
PTSD is a complex condition that develops after a traumatic stressor. In addition to stressor exposure, PTSD symptoms are defined in clusters that include: (1) intrusion symptoms (e.g., intrusive re-experiencing of the event in the form of nightmares and flashbacks, with an exaggerated response to trauma-related reminders and cues); (2) avoidance (e.g., avoidance of stimuli or thoughts associated with the trauma); (3) negative alterations in cognition and mood; and (4) changes in arousal or reactivity (e.g., exaggerated startle response, increased physiological arousal, and sustained preparedness for an instant alarm response); (American Psychiatric Association 2013). One study reported that approximately 20% to 30% of individuals exposed to traumatic stressors will develop PTSD (Breslau et al. 1991), with a lifetime prevalence of about 7% in the general population (Fairbank et al. 1995, Kessler et al. 2005). This risk is significantly greater in those that have sustained a TBI. In one study, a military survey of soldiers returning from Iraq reported 44% of troops who had sustained mild TBIs with loss of consciousness screened positive for PTSD compared to 16% who sustained only bodily injury (Hoge et al. 2008). Moreover, a recent study of veterans returning from Iraq or Afghanistan found significant main effects of mild TBI on self-report questionnaires measuring PTSD symptoms (Undurti et al. 2018). We still do not have a clear biological basis for understanding the full complexity of PTSD. The discordance between populations of people exposed to a traumatic event that develop PTSD and those that do not, suggests differences in vulnerability and/or resilience, with TBI as a strong predictor of PTSD development.
Research studies suggest that PTSD is related to a complex dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and its end product cortisol (humans) or corticosterone (rodents; Steudte-Schmiedgen et al. 2015). A general hypocortisolism in PTSD patients has been reported in most studies (Meewisse et al. 2007, Morris et al. 2012). Research on cortisol stress reactivity has also revealed mixed results: while some studies found PTSD patients to exhibit an exaggerated cortisol response to a variety of acute stressors (Bremner et al. 2003, Elzinga et al. 2003), others have not replicated this association (Simeon et al. 2007). In addition to studies suggesting HPA dysfunctions specifically related to PTSD, there is increasing evidence that trauma exposure per se might be related to altered HPA function. Specifically, traumatized individuals exhibit lower basal cortisol levels (Morris et al. 2012) and diminished cortisol stress reactivity to psychosocial stress (Elzinga et al. 2008, Lovallo et al. 2012).
In this review, we discuss neuroendocrine stress reactivity in the context of TBI and PTSD. In the literature, there are patterns of alterations in neuroendocrine function and stress reactivity in both TBI and PTSD that may have overlapping neural substrates. Here we introduce the HPA axis and review clinical and preclinical findings that demonstrate HPA alterations after TBI and in PTSD patients. We then discuss the amygdala as a candidate limbic structure that is sensitive to stress, TBI, and implicated in fear and PTSD. We propose the hypothesis that TBI may lead to a vulnerability for subsequent PTSD development by way of dysregulated neuroendocrine and amygdalar activity.
Stress and the HPA axis
The HPA axis is activated in response to stressful systemic and /or psychogenic stimuli that potentially disrupt homeostasis. It is well established (Herman et al. 2003) that the HPA response to a stressor involves an immediate surge of adrenocorticotropic hormone (ACTH) release from the pituitary, and the subsequent secretion of corticosteroids from the adrenal cortex that, in turn, generate a shut-off negative feedback signal. The ACTH surge is initiated by hypophysiotropic neurons in the medial parvocellullar division of the paraventricular hypothalamic nucleus (PVN) that produce corticotropic releasing hormone (CRH), among other ACTH-releasing factors. The medial PVN receives synaptic innervation via neurons projecting from various central nervous system (CNS) structures (e.g., the limbic brain regions and brainstem), which evoke rapid activation of the HPA axis. Termination of the HPA stress response is mediated by glucocorticoid negative feedback. Glucocorticoid (and mineralocorticoid) receptors are abundantly expressed in the forebrain (i.e. hippocampus, prefrontal cortex, and amgydala; Diorio et al. 1993, Herman 1993, Akana et al. 2001).
Forebrain glucocorticoid receptors are essential for negative feedback regulation of the HPA axis, in particular glucocorticoid feedback inhibition of acute psychogenic stress responses (Furay et al. 2008). The rapid action of glucocorticoids is triggered by the activation of membrane-associated receptors and non-genomic signaling mechanisms (de Kloet 2000, Tasker et al. 2006, Haller et al. 2008). Evidence indicates that glucocorticoids activate divergent G-protein signaling pathways that act in a synapse-specific manner to suppress excitatory synaptic glutamate inputs and facilitate inhibitory synaptic GABA inputs to PVN neurons(Miklos and Kovacs 2002, Di et al. 2009). Local GABAergic PVN-projecting neurons can be either activated or inhibited by glutamatergic or GABAergic afferent innervation from upstream limbic or cortical regions that are stress responsive and regulate the HPA axis (e.g., ventral subiculum, medial prefrontal cortex, amygdaloid nuclei, and lateral septum; Cullinan et al. 2008).
Stress Reactivity in TBI
Altered activity of the HPA axis after TBI has been demonstrated both clinically (Cernak et al. 1999, Benvenga et al. 2000, Cohan et al. 2005, Agha et al. 2007) and experimentally (Shohami et al. 1995, Roe et al. 1998, Grundy et al. 2001, Gottesfeld et al. 2002, McCullers et al. 2002). These reports all point to baseline neuroendocrine dysfunction after TBI. A more recent study demonstrated heightened stress responsiveness during the first two weeks after mild fluid percussion injury (FPI) in rats (Griesbach et al. 2011). Another recent study, in male and female mice at 7–10 days after mild blast-induced TBI (mbTBI), showed that whereas the HPA response to stress was affected in both sexes, the profile of reactivity differed in the first week after injury (Russell et al. 2018). Males exposed to mbTBI had increased restraint-induced serum CORT but attenuated restraint-induced CRH immunoreactivity in the PVN, while females showed an opposite response, with attenuated restraint-induced CORT and enhanced restraint-induced CRH immunoreactivity in the PVN. The investigators concluded that mbTBI appears to disrupt limbic pathways involved in HPA axis stress reactivity in males, while producing a sex-dependent link to stress dysregulation of pre-autonomic neurons in females (Russell et al. 2018).
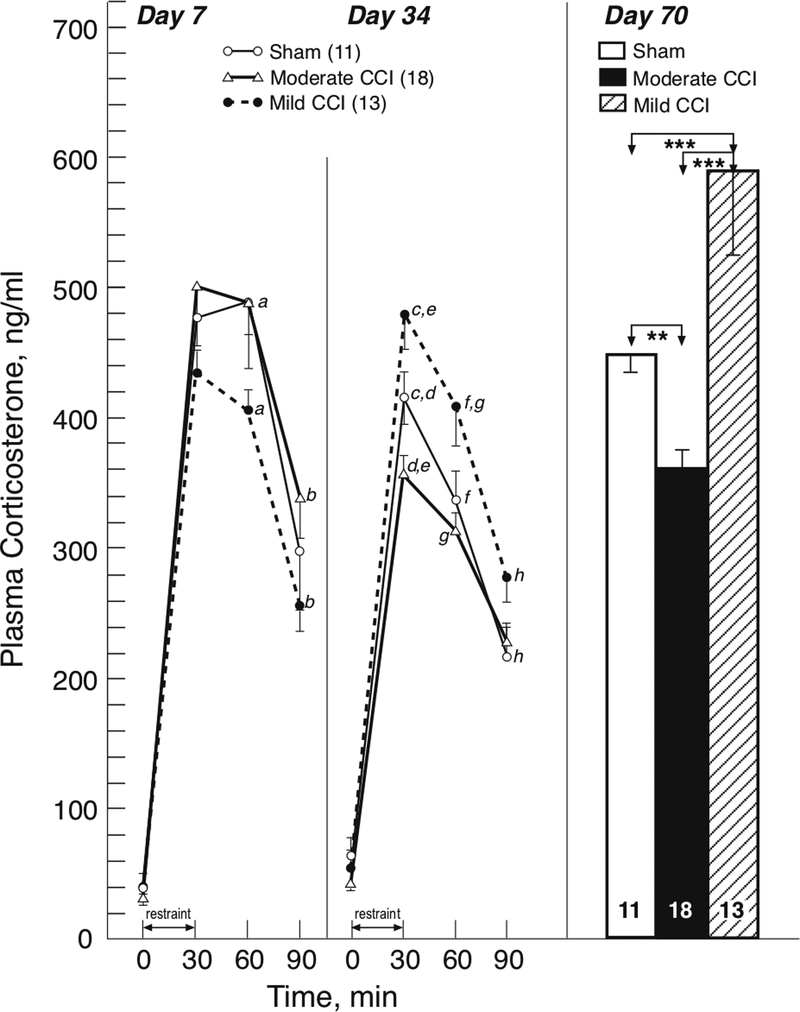
In contrast to the earlier reports of baseline neuroendocrine dysfunction after TBI cited above, we have demonstrated that TBI, produced by mild or moderate lateral controlled cortical impact (CCI) in rodents, causes long-term dysregulation of the neuroendocrine stress response (Taylor et al. 2006, 2008). The patterns of the neuroendocrine dysregulation differed depending upon the severity of the CCI: the initially blunted CORT response to restraint stress at 7 days after mild CCI transitioned to an enhanced response at 34 and 70 days, while at these later time points after moderate CCI the restraint-induced CORT response was blunted (Fig. 1, Taylor et al. 2008). We further demonstrated that the attenuated HPA stress response at 28 days after moderate CCI is mediated by enhanced glucocorticoid (i.e. dexamethasone) negative feedback control of the HPA axis (Taylor et al. 2010). Subsequently we showed that attenuation of the stress-induced CORT response after moderate CCI is mediated by the inhibitory actions of both glucocorticoid receptors and GABA, with a loss of inhibitory neurons within brain regions with neural pathways affecting limbic stress-integrative pathways (Taylor et al. 2013). Our long-term findings after moderate CCI have been confirmed in another rat model at 2 months after diffuse TBI induced by midline fluid percussion injury: resting plasma CORT levels were decreased and the CORT response to restraint stress was blunted (Rowe et al. 2016). It was also reported in the same study that there were no concomitant changes in testosterone levels but that there was altered complexity of neuron processes in the PVN without any neuropathology or astrocytosis. Taken together, work from our lab and others has supported baseline and stress induced neuroendocrine dysfunction in various models of experimental TBI that may be severity-dependent.
Figure 1.
Effect of mild and moderate CCI and sham injury on HPA responsiveness to 30-min restraint stress on days 7, 34, and 70 post-injury. On days 7 and 34, tail vein samples were collected for plasma corticosterone (mean ± SEM) at baseline (time=0) and at 30, 60 and 90 min after stress onset. On day 70, trunk blood was obtained at the end of the 30-min stress period. a, p<0.01; b, p<0.05; c, p<0.05; d, p<0.05; e, p<.0001; f, p<0.05; g, p<0.01; h, p<0.05, for groups with similar letters.**, p<0.01; ***, p<0.001, for the bracketed groups. Adapted from (Taylor et al. 2008)
Stress Reactivity in PTSD
Clinical studies have supported alterations in stress induced HPA axis function in PTSD populations. A recent clinical study found that attenuated cortisol secretion is a risk marker for subsequent development of PTSD symptomatology in response to trauma during military deployment (Steudte-Schmiedgen et al. 2015). Briefly, male soldiers were examined before deployment to Afghanistan and at a 12-month post-deployment follow-up, using hair cortisol concentrations (HCC) for baseline activity and salivary cortisol for stress reactivity, measured by the Trier Social Stress Test (TSST). The results showed that lower HCC and lower cortisol stress reactivity were predictive of a greater increase in PTSD symptomatology in soldiers who had experienced new-onset traumatic events. These findings suggest a two-stage process of endocrine alterations in response to traumatic stress: first, trauma exposure may result in a long-term dose-dependent cortisol attenuation, which, upon exposure to additional traumatic events may predispose to development of PTSD (Steudte-Schmiedgen et al. 2015). Interestingly, another study found that despite an increase in subjective stress perception during the TSST, a cohort of female PTSD patients had a blunted cortisol response compared to healthy controls (Zaba et al. 2015). Hypo-responsive TSST cortisol was replicated in a separate study of female PTSD patients (Wichmann et al. 2017). Other studies have shown enhanced cortisol negative feedback inhibition in clinical PTSD (Yehuda et al. 1995, 1996, 2004). Additional biological factors, such as genetic variation, have been shown to contribute to attenuated HPA activity and may also mediate the risk of developing PTSD symptoms in response to trauma (Kolassa and Elbert 2007, Bomyea et al. 2012, Zoladz and Diamond 2013).
Stress reactivity and the amygdala
Of particular relevance to implications for neuroendocrine dysfunction in PTSD following TBI is the amygdala, a limbic structure that shows increased patterns of plasticity in response to stress. The amygdala is a phylogenetically old limbic brain structure, that is involved in the encoding and processing of emotionally salient information (LeDoux 2003), and HPA axis excitation (Herman et al. 2005). While the hippocampus and medial prefrontal cortex (mPFC) display dendritic atrophy following stressor exposure (Watanabe et al. 1992, Magarinos and McEwen 1995, 2001, Wellman 2001, Cook and Wellman 2004, Brown et al. 2005, Izquierdo et al. 2006), repeated stress causes dendritic hypertrophy within the amygdala (Vyas et al. 2002, Vyas et al. 2004), with extended durations of stress showing enhanced synaptic connectivity (Vyas et al. 2006). Chronic stress also induces neurophysiological changes in amygdalar principal neurons, including hyperexcitability in the lateral amygdala (Rosenkranz et al. 2010). These stress-induced structural and physiological changes correspond to changes in emotionally-laden behavior including increases in anxiety-like behaviors (Vyas et al. 2002), facilitated acquisition of fear learning (Conrad et al. 1999, Hoffman et al. 2010), and resistance to fear extinction (Izquierdo et al. 2006, Hoffman et al. 2014), and reconsolidation (Hoffman et al. 2015), all of which are phenotypic of PTSD. In contrast to the dynamic nature of structural changes observed within hippocampus and mPFC, the dendritic hypertrophy within the amygdala tends to be persistent and does not recover within the same timeframe (21d; Vyas et al. 2004). Furthermore, it has been shown that animals given time to recover (7d) following chronic stress show enhanced fear memories compared to nonstressed controls (McGuire et al. 2010). These consequences could be portrayed as maladaptive plasticity when considering the role of these observed changes within the amygdala in the development of cognitive and emotional psychiatric conditions including PTSD.
Recent research using animal models of TBI are beginning to reveal structural and functional changes in the amygdala as a result of injury. We recently showed dendritic hypertrophy in excitatory neurons within the basolateral amygdala (BLA) following a single midline FPI (Hoffman et al. 2017). This BLA dendritic hypertrophy was observed within one day following FPI that persisted for at least 28 days. It is unknown whether these effects were due to TBI enhanced stress reactivity and glucocorticoid release to indirectly impact amygdalar structural plasticity, or by another mechanism as a result of TBI. Another study assessed functional alterations in the BLA after CCI in rats (Almeida-Suhett et al. 2014). CCI led to a reduced GABAergic inhibition, and increased BLA excitability that was associated with increased anxiety-like behavior (Almeida-Suhett et al. 2014). These studies demonstrate increased amygdalar plasticity after TBI that may reflect vulnerability for enhanced fear following subsequent stressor exposure.
The amygdala is sensitive to stress and it is well known that the BLA complex is a key locus of plasticity for the formation of fear memories (Fanselow and LeDoux 1999), which is thought to be the basis for traumatic memory formation in PTSD. The lateral subdivision (lateral amygdala, LA), which receives direct cortical and thalamic sensory input, is known as the sensory interface of the amygdala and is required for auditory fear conditioning (LeDoux et al. 1990). The basal amygdala (BA) receives inputs from the LA as well as the hippocampus and PFC to integrate contextual and higher order information (Orsini et al. 2011). The BLA then projects to the output center of the amygdala, the central amygdala (CEA), which sends inhibitory projections to the hypothalamus, locus coeruleus, and periaqueductal gray (PAG) to coordinate HPA axis activation, sympathetic activation, and defensive behavioral responding (freezing), respectively, in response to threat detection (LeDoux et al. 1988, LeDoux 2000, 2007). While the amygdala has been relatively understudied in TBI research, recent data from our lab and others have begun to show TBI-induced changes in amygdalar structure and function towards enhanced excitatory processes and increased plasticity (Reger et al. 2012, Almeida-Suhett et al. 2014, Zuckerman et al. 2016, Hoffman et al. 2017). Specifically, we have reported that 48h after FPI, rats displayed increased fear learning to both contextual and discrete cues (Reger et al. 2012). These enhanced fear memories were associated with increased N-methyl-D-aspartate (NMDA) receptor expression in the BLA (Reger et al. 2012). NMDA receptors in the BLA are necessary for fear conditioning (Miserendino et al. 1990, Fanselow and Kim 1994). The neural circuits necessary for encoding adaptive auditory fear memories are vulnerable to disruption after TBI and may lead to increases in fear learning and expression.
Fear conditioning as a model to study PTSD
Pavlovian fear conditioning is widely used to study fear learning and memory in basic neuroscience research. In widely used rodent models of fear conditioning, an innocuous stimulus (conditional stimulus, CS), such as a novel environment (context) and/or an auditory cue is paired with an aversive stimulus (unconditional stimulus, US), such as a footshock, which promotes natural defensive responses. Following paired CS-US presentation, both the context and CS alone will elicit a learned, conditional response (CR, freezing). Fear conditioning has proven to be a useful tool to study the mechanisms of basic associative learning and memory processes. In addition, fear conditioning is a useful model to study disordered function of highly conserved defense systems such as in anxiety and stress related disorders like PTSD by teasing out differences in adaptive and maladaptive fear responses. For example, increases in expression of learned fear may reflect a maladaptive response if it is disproportionate to the severity of a threat (Rau et al. 2005, Poulos et al. 2014, 2015, Perusini et al. 2016). These inappropriate fear responses can interfere with behaviors that serve other adaptive functions that promote survival (e.g. feeding, mating, etc., Fanselow 1994). Furthermore, fear that generalizes outside of the context of the initial trauma or to novel stimuli is commonly seen in clinical PTSD and in rodent models of PTSD (Hoffman et al. 2014, Dunsmoor and Paz 2015, Dymond et al. 2015). Analogous to exposure therapy in humans, a common PTSD treatment approach, fear extinction occurs with repeated unreinforced CS presentations that result in a new, inhibitory memory trace, or a CS-no US association. One challenge with PTSD populations is the relapse of symptoms between extinction sessions, i.e., fear responding recovers between exposure therapy sessions and outside the therapy context (discussed in Hamner et al. 2004). Enhanced fear learning, fear generalization, and deficits in fear extinction can be used to model these distinct aspects and analogs of clinical PTSD and may be useful to determine in prospective preclinical research to identify potential risk factors for comorbid TBI and PTSD.
Fear conditioning has also been employed in human research settings. In healthy and clinical populations alike, human fear conditioning research has helped our understanding of emotion and defense processing as well as extinction (Sehlmeyer et al. 2009, Hartley and Phelps 2010). Furthermore, when used with modern neuroimaging or peripheral endocrine manipulations, we gain understanding of the biological mechanisms that underlie threat processing and human fear and anxiety. In humans, CSs tend to be visual stimuli paired with US noxious stimuli such as shock, loud noise, or air blast (Sehlmeyer et al. 2009). Fear conditioning training itself may serve as the stressor exposure in rodent models of PTSD that aim to explore and test potential mechanisms and treatments. However, in clinical PTSD populations, controlled fear conditioning studies in humans can shed light on changes in subjectively learned and physiological threat processing, extinction and generalization differences, and/or HPA function such as negative feedback via the dexamethasone suppression test (Jovanovic et al. 2011, Michopoulos et al. 2017).
Neuroendocrine basis of exaggerated fear in PTSD
HPA axis function has been postulated to underlie the etiology of PTSD following trauma exposure. Furthermore, homeostatic regulators such as glucocorticoids have been proposed as promising biomarkers for stress susceptibility and resilience (Daskalakis et al. 2016). In animal models, prior stressor exposure leads to enhanced or sensitized subsequent fear learning (Rau et al. 2005, Hoffman et al. 2014), supportive of maladaptive exaggerated fear, a PTSD-like phenotype. One recent study demonstrated that exaggerated fear to a single trial of fear conditioning is dependent on stress-induced CORT release during a prior traumatic experience (Perusini et al. 2016). In that study when metyrapone, a CORT synthesis blocker, was given prior to traumatic stressor exposure (15 random footshocks), there was a dose-dependent reduction in newly learned fear to a mild stressor (1 footshock). When CORT was co-administered with metyrapone prior to the trauma, the enhanced fear phenotype was rescued, indicating the requirement of trauma-induced glucocorticoid release to influence subsequent exaggerated fear. More specifically, CORT action on BLA glucocorticoid receptors (GRs) is shown to be required for the induction of PTSD-phenotype. In another experiment, mifepristone, a GR antagonist was delivered directly into the BLA via cannula infusion prior to the 15-footshock trauma. Mifepristone-treated rats displayed normal levels of fear learning to the single shock mild stressor (Perusini et al. 2016). Additionally, another study showed a life-long upregulation of BLA GRs when the 15-shock trauma was administered in early life (Poulos et al. 2014). Recent clinical studies also support changes in cortisol suppression during fear conditioning and extinction with the dexamethasone suppression test in PTSD patients (Jovanovic et al. 2011, Michopoulos et al. 2017). These studies showed that HPA suppression via dexamethasone both reduced exaggerated fear potentiated startle (Jovanovic et al. 2011), and facilitated fear extinction (Michopoulos et al. 2017) in PTSD patients. Taken together, these studies demonstrate the dependence and influence of neuroendocrine HPA axis function on the amygdala in the development of exaggerated fear phenotypes observed in rodent models and in clinical PTSD.
Stress reactivity in TBI, implications for cormorbid PTSD
The current review synthesizes an emerging body of literature identifying a potential link between neuroendocrine disruption commonly observed after TBI and its potential influence on promoting a vulnerability to development of PTSD. Both TBI and stressor exposure via glucocorticoids increase amygdalar structural and functional plasticity. Stress sensitivity in emotional centers in the brain, such as the amygdala, promote exaggerated fear and defensive behaviors. Neuroendocrine effects after TBI tend to mimic those that are observed in PTSD patient populations. The integration of these findings leads us to hypothesize that TBI effects on neuroendocrine and amygdalar function may underlie a vulnerability to development of PTSD following subsequent trauma or stressor exposure. Future studies designed to systematically test the causal link between TBI neuroendocrine dysfunction on amygdala-dependent fear memories will shed light on this relationship.
Most people have experienced or will experience traumatic stress at some time over the lifespan, but only a subset of traumatized individuals develop PTSD. Clinical research supports high rates of TBI-PTSD comorbidity and demonstrate TBI as a significant predictor of PTSD development. Identifying biological mechanisms that underlie this vulnerability, such as dysregulated HPA axis function, may lead to better targeted treatments and preventive measures to support psychological health after TBI.
Acknowledgements:
This work was supported in part by F32NS098694 (AH); Staglin Music Festival Center for Brain and Behavioral Health.
References
- Agha A, Phillips J and Thompson CJ (2007). Hypopituitarism following traumatic brain injury (TBI). Br. J. Neurosurg 21: 210–216. [DOI] [PubMed] [Google Scholar]
- Akana SF, Chu A, Soriano L and Dallman MF (2001). Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J. Neuroendocrinol 13: 625–637. [DOI] [PubMed] [Google Scholar]
- Almeida-Suhett CP, Prager EM, Pidoplichko V, Figueiredo TH, Marini AM, Li Z, Eiden LE, et al. (2014). Reduced GABAergic inhibition in the basolateral amygdala and the development of anxiety-like behaviors after mild traumatic brain injury. PloS one 9: e102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A. P.(2013). Diagnostic and statistical manual of mental disorders Arlington, VA, American Psychiatric Publishing. [Google Scholar]
- Benvenga S, Campenni A, Ruggeri RM and Trimarchi F (2000). Clinical review 113: Hypopituitarism secondary to head trauma. J. Clin. Endocrinol. Metab 85: 1353–1361. [DOI] [PubMed] [Google Scholar]
- Bomyea J, Risbrough V and Lang AJ (2012). A consideration of select pre-trauma factors as key vulnerabilities in PTSD. Clin. Psychol. Rev 32: 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, et al. (2003). Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology 28: 733–750. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P and Peterson E (1991). Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch. Gen. Psychiatry 48: 216–222. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S and Wellman CL (2005). Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb. Cortex 15: 1714–1722. [DOI] [PubMed] [Google Scholar]
- Cernak I, Savic VJ, Lazarov A, Joksimovic M and Markovic S (1999). Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Inj. 13: 1005–1015. [DOI] [PubMed] [Google Scholar]
- Cohan P, Wang C, McArthur DL, Cook SW, Dusick JR, Armin B, Swerdloff R, et al. (2005). Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit. Care Med 33: 2358–2366. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM and McEwen BS (1999). Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci 113: 902–913. [DOI] [PubMed] [Google Scholar]
- Cook SC and Wellman CL (2004). Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol 60: 236–248. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR and Herman JP (2008). Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct 213: 63–72. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Cohen H, Nievergelt CM, Baker DG, Buxbaum JD, Russo SJ and Yehuda R (2016). New translational perspectives for blood-based biomarkers of PTSD: From glucocorticoid to immune mediators of stress susceptibility. Exp. Neurol 284: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER (2000). Stress in the brain. Eur. J. Pharmacol 405: 187–198. [DOI] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A and Tasker JG (2009). Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J. Neurosci 29: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V and Meaney MJ (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci 13: 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE and Paz R (2015). Fear Generalization and Anxiety: Behavioral and Neural Mechanisms. Biol. Psychiatry 78: 336–343. [DOI] [PubMed] [Google Scholar]
- Dymond S, Dunsmoor JE, Vervliet B, Roche B and Hermans D (2015). Fear Generalization in Humans: Systematic Review and Implications for Anxiety Disorder Research. Behav. Ther 46: 561–582. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R and Bremner JD (2003). Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology 28: 1656–1665. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J and Spinhoven P (2008). Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology 33: 227–237. [DOI] [PubMed] [Google Scholar]
- Fairbank JA, Schlenger WE, Saigh PA and Davidson JRT (1995). An epidememiologic profile of post-traumatic stress disorder: prevalence, comorbidity, and risk factors Neurobiological and clinical consequences of stress: from normal adaptation to PTSD. Charney DS, Friedman MJ and Y DA. Philadelphia, PA, Lippincott-Raven: 417–427. [Google Scholar]
- Fanselow MS (1994). Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev 1: 429–438. [DOI] [PubMed] [Google Scholar]
- Fanselow MS and Kim JJ (1994). Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav. Neurosci 108: 210–212. [DOI] [PubMed] [Google Scholar]
- Fanselow MS and LeDoux JE (1999). Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23: 229–232. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE and Herman JP (2008). The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 149: 5482–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld Z, Moore AN and Dash PK (2002). Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J. Neurotrauma 19: 317–326. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Tio DL and Taylor AN (2011). Heightening of the stress response during the first weeks after a mild traumatic brain injury. Neuroscience 178: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy PL, Harbuz MS, Jessop DS, Lightman SL and Sharples PM (2001). The hypothalamo-pituitary-adrenal axis response to experimental traumatic brain injury. J. Neurotrauma 18: 1373–1381. [DOI] [PubMed] [Google Scholar]
- Haller J, Mikics E and Makara GB (2008). The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front. Neuroendocrinol 29: 273–291. [DOI] [PubMed] [Google Scholar]
- Hamner MB, Robert S and Frueh BC (2004). Treatment-resistant posttraumatic stress disorder: strategies for intervention. CNS Spectr. 9: 740–752. [DOI] [PubMed] [Google Scholar]
- Hartley CA and Phelps EA (2010). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharm 35: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP (1993). Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell. Mol. Neurobiol 13: 349–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC and Cullinan WE (2003). Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol 24: 151–180. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK and Figueiredo H (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 29: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Armstrong CE, Hanna JJ and Conrad CD (2010). Chronic stress, cyclic 17beta-estradiol, and daily handling influences on fear conditioning in the female rat. Neurobiol Learn Mem 94: 422–433. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Lorson NG, Sanabria F, Foster Olive M and Conrad CD (2014). Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiol. Learn. Mem 112: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AN, Parga A, Paode PR, Watterson LR, Nikulina EM, Hammer RP Jr. and Conrad CD (2015). Chronic stress enhanced fear memories are associated with increased amygdala zif268 mRNA expression and are resistant to reconsolidation. Neurobiol. Learn. Mem 120: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AN, Paode PR, May HG, Ortiz JB, Kemmou S, Lifshitz J, Conrad CD, et al. (2017). Early and Persistent Dendritic Hypertrophy in the Basolateral Amygdala following Experimental Diffuse Traumatic Brain Injury. J. Neurotrauma 34: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC and Castro CA (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med 358: 453–463. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL and Holmes A (2006). Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci 26: 5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Phifer JE, Sicking K, Weiss T, Norrholm SD, Bradley B and Ressler KJ (2011). Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder. Psychoneuroendocrinology 36: 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR and Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62: 593–602. [DOI] [PubMed] [Google Scholar]
- Kolassa IT and Elbert T (2007). Structural and functional neuroplasticity in relation to traumatic stress. Curr. Dir. Psychol. Sci 16: 321–325. [Google Scholar]
- LeDoux J (2000). Emotion circuits in the brain. Annu. Rev. Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux J (2003). The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol 23: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2007). The amygdala. Curr. Biol 17: R868–874. [DOI] [PubMed] [Google Scholar]
- LeDoux J, Iwata J, Cicchetti P and Reis DJ (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci 8: 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, Cicchetti P, Xagoraris A and Romanski LM (1990). The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience 10: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ and Vincent AS (2012). Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biol. Psychiatry 71: 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM and McEwen BS (1995). Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience 69: 83–88. [DOI] [PubMed] [Google Scholar]
- McCullers DL, Sullivan PG, Scheff SW and Herman JP (2002). Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 947: 41–49. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2001). Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci 933: 265–277. [DOI] [PubMed] [Google Scholar]
- McGuire J, Herman JP, Horn PS, Sallee FR and Sah R (2010). Enhanced fear recall and emotional arousal in rats recovering from chronic variable stress. Physiol. Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP and Olff M (2007). Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry 191: 387–392. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Norrholm SD, Stevens JS, Glover EM, Rothbaum BO, Gillespie CF, Schwartz AC, et al. (2017). Dexamethasone facilitates fear extinction and safety discrimination in PTSD: A placebo-controlled, double-blind study. Psychoneuroendocrinology 83: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos IH and Kovacs KJ (2002). GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience 113: 581–592. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR and Davis M (1990). Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature 345: 716–718. [DOI] [PubMed] [Google Scholar]
- Molaie AM and Maguire J (2018). Neuroendocrine Abnormalities Following Traumatic Brain Injury: An Important Contributor to Neuropsychiatric Sequelae. Front. Endocrinol 9: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Compas BE and Garber J (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin. Psychol. Rev 32: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E and Maren S (2011). Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J. Neurosci 31: 17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini JN, Meyer EM, Long VA, Rau V, Nocera N, Avershal J, Maksymetz J, et al. (2016). Induction and Expression of Fear Sensitization Caused by Acute Traumatic Stress. Neuropsychopharmacology 41: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Reger M, Mehta N, Zhuravka I, Sterlace SS, Gannam C, Hovda DA, et al. (2014). Amnesia for early life stress does not preclude the adult development of posttraumatic stress disorder symptoms in rats. Biol. Psychiatry 76: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Zhuravka I, Long V, Gannam C and Fanselow M (2015). Sensitization of fear learning to mild unconditional stimuli in male and female rats. Behav. Neurosci 129: 62–67. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP and Fanselow MS (2005). Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev 29: 1207–1223. [DOI] [PubMed] [Google Scholar]
- Reger ML, Poulos AM, Buen F, Giza CC, Hovda DA and Fanselow MS (2012). Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biological psychiatry 71: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe SY, McGowan EM and Rothwell NJ (1998). Evidence for the involvement of corticotrophin-releasing hormone in the pathogenesis of traumatic brain injury. Eur. J. Neurosci 10: 553–559. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER and Padival M (2010). Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry 67: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RK, Rumney BM, May HG, Permana P, Adelson PD, Harman SM, Lifshitz J, et al. (2016). Diffuse traumatic brain injury affects chronic corticosterone function in the rat. Endocr. Connect 5: 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AL, Richardson MR, Bauman BM, Hernandez IM, Saperstein S, Handa RJ and Wu TJ (2018). Differential Responses of the HPA Axis to Mild Blast Traumatic Brain Injury in Male and Female Mice. Endocrinology 159: 2363–2375. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V and Konrad C (2009). Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One 4: e5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E, Bass R, Trembovler V and Weidenfeld J (1995). The effect of the adrenocortical axis upon recovery from closed head injury. Journal of neurotrauma 12: 1069–1077. [DOI] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J and Smith LM (2007). Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biol. Psychiatry 61: 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Stalder T, Schonfeld S, Wittchen HU, Trautmann S, Alexander N, Miller R, et al. (2015). Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology 59: 123–133. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S and Malcher-Lopes R (2006). Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology 147: 5549–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AN, Rahman SU, Tio DL, Sanders MJ, Bando JK, Truong AH and Prolo P (2006). Lasting neuroendocrine-immune effects of traumatic brain injury in rats. J. Neurotrauma 23: 1802–1813. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Rahman SU, Sanders NC, Tio DL, Prolo P and Sutton RL (2008). Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J. Neurotrauma 25: 311–323. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Rahman SU, Tio DL, Gardner SM, Kim CJ and Sutton RL (2010). Injury severity differentially alters sensitivity to dexamethasone after traumatic brain injury. J. Neurotrauma 27: 1081–1089. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tio DL and Sutton RL (2013). Restoration of neuroendocrine stress response by glucocorticoid receptor or GABA(A) receptor antagonists after experimental traumatic brain injury. J. Neurotrauma 30: 1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undurti A, Colasurdo EA, Sikkema CL, Schultz JS, Peskind ER, Pagulayan KF and Wilkinson CW (2018). Chronic Hypopituitarism Associated with Increased Postconcussive Symptoms Is Prevalent after Blast-Induced Mild Traumatic Brain Injury. Frontiers in neurology 9: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS and Chattarji S (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG and Chattarji S (2004). Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 128: 667–673. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S and Chattarji S (2006). Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 143: 387–393. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E and McEwen BS (1992). Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 588: 341–345. [DOI] [PubMed] [Google Scholar]
- Wellman CL (2001). Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol 49: 245–253. [DOI] [PubMed] [Google Scholar]
- Wichmann S, Kirschbaum C, Bohme C and Petrowski K (2017). Cortisol stress response in post-traumatic stress disorder, panic disorder, and major depressive disorder patients. Psychoneuroendocrinology 83: 135–141. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT and Giller EL Jr. (1995). Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch. Gen. Psychiatry 52: 583–593. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA and Siever LJ (1996). Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatry 40: 79–88. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Halligan SL, Meaney M and Bierer LM (2004). The ACTH response to dexamethasone in PTSD. Am. J. Psychiatry 161: 1397–1403. [DOI] [PubMed] [Google Scholar]
- Zaba M, Kirmeier T, Ionescu IA, Wollweber B, Buell DR, Gall-Kleebach DJ, Schubert CF, et al. (2015). Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology 55: 102–115. [DOI] [PubMed] [Google Scholar]
- Zoladz PR and Diamond DM (2013). Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neurosci. Biobehav. Rev 37: 860–895. [DOI] [PubMed] [Google Scholar]
- Zuckerman A, Ram O, Ifergane G, Matar MA, Sagi R, Ostfeld I, Hoffman JR, et al. (2016). Controlled Low-Pressure Blast-Wave Exposure Causes Distinct Behavioral and Morphological Responses Modelling Mild Traumatic Brain Injury, Post-Traumatic Stress Disorder, and Comorbid Mild Traumatic Brain Injury-Post-Traumatic Stress Disorder. J. Neurotrauma [DOI] [PubMed] [Google Scholar]