Abstract
It has been well established over the last two decades that walking is not merely an automatic, motoric activity; it also utilizes executive function circuits, which play an increasingly important role in walking for older people and those with mobility and cognitive deficits. Dual-task walking, such as walking while performing a cognitive task, is a necessary skill for everyday functioning, and has been shown to activate prefrontal lobe areas in healthy older people. Another well-established point in healthy aging is the loss of grey matter, and in particular loss of frontal lobe grey matter volume. However, the relationship between increased frontal lobe activity during dual-task walking and loss of frontal grey matter in healthy aging remains unknown.
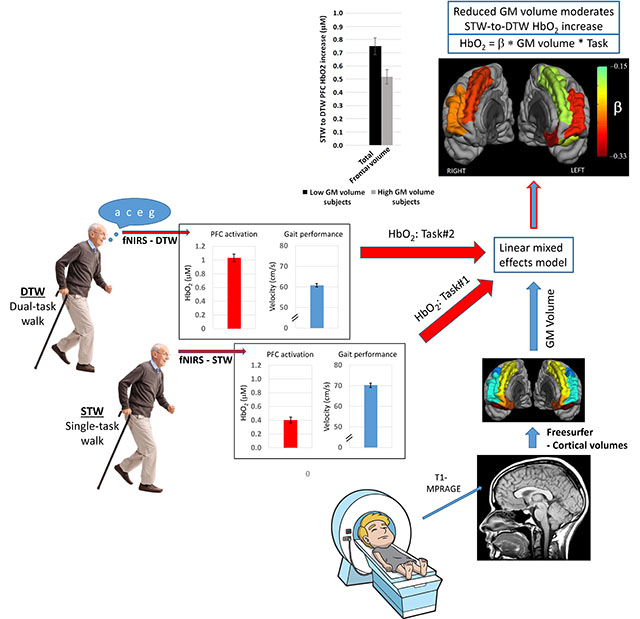
In the current study, we combined oxygenated hemoglobin (HbO2) data from functional near-infrared spectroscopy (fNIRS), taken during dual-task walking, with structural MRI volumetrics in a cohort of healthy older subjects to identify this relationship. We studied fifty-five relatively healthy, older participants (≥ 65 years) during two separate sessions: fNIRS to measure HbO2 changes between single-task (i.e., normal walking) and dual-task walking-while-talking, and high-resolution, structural MRI to measure frontal lobe grey matter volumes. Linear mixed effects modeling was utilized to determine the moderation effect of grey matter volume on the change in prefrontal oxygenated hemoglobin between the two walking tasks, while controlling for covariates including task performance. We found a highly significant interaction effect between frontal grey matter volume and task on HbO2 levels (p < 0.0001). Specifically, increased HbO2 levels during dual-task compared to single-task walking were associated with reduced frontal grey matter volume. Regional analysis identified bilateral superior and rostral middle gyri as the primary areas driving these results. The findings provide support for the concept of neural inefficiency: in the absence of behavioral gains, grey matter loss in relatively healthy, older individuals leads to over-activation of frontal lobe during a cognitively demanding walking task with established clinical and predictive utility.
Keywords: Brain Aging, Grey Matter Volume, fNIRS, Gait, Executive Function, Motor Activity
Graphical Abstract
1. Introduction
The ability to move about one’s environment without conscious effort is something many take for granted. However, when mobility becomes impaired, daily living can become much more difficult, impacting not only quality of life but overall health as well. Until recently, it was believed that locomotion required minimal involvement of higher-order cognitive processes. Over the last two decades, however, research has demonstrated that gait is not simply a motoric activity but critically dependent on cognition, and executive functions in particular, and involving multiple brain resources (Allali et al., 2017; Martin et al., 2013; Mirelman et al., 2014b; M. Montero-Odasso et al., 2012). Studies in healthy, older people have consistently shown strong associations of executive function with gait performance – typically evaluated via mean gait velocity (Holtzer et al., 2006; Holtzer et al., 2014c; Martin et al., 2013; Springer et al., 2006). Furthermore, as the complexity of the motor task increases or is combined with an unrelated cognitive task, executive functions are taxed more heavily in order to ensure successful completion of the task (Holtzer et al., 2012, 2014c).
Dual-tasking is a common method for assessing executive function, especially attention; attention is known to decline with age, and has been shown to be a critical component of gait function (Coppin et al., 2006; M. Montero-Odasso et al., 2012; Walshe et al., 2015; Yogev-Seligmann et al., 2008). In the context of gait, dual-tasking is the performance of another, typically cognitive, task while walking. Dual-task paradigms have demonstrated the non-automaticity of gait function, have been shown to tax executive function reserves, and can lead to decline in both the cognitive and walking task performance (Holtzer et al., 2012; Li et al., 2014; Lucas et al., 2018; Mirelman et al., 2017; M. Montero-Odasso et al., 2012; Springer et al., 2006; Yogev-Seligmann et al., 2008). Dual-task performance has been shown by a number of groups to be affected in older people (Holtzer et al., 2014b; Holtzer et al., 2014c; Springer et al., 2006), and to be a reliable predictor of falls, frailty and the transition to dementia (M. Montero-Odasso et al., 2012; M. M. Montero-Odasso et al., 2017; Verghese et al., 2012).
The frontal lobe, and in particular the prefrontal cortex (PFC), is an important brain region involved in executive function (Alvarez and Emory, 2006). The volume of the frontal lobes have been shown to decline with age; this is aligned with the “last-in, first-out” theory which posits that later developing brain areas are the first to deteriorate (Nyberg et al., 2010; Raz et al., 1997; Raz et al., 2005; Tisserand et al., 2002; Tisserand et al., 2004), even in the context of healthy aging. With respect to gait function, recent studies have shown associations between grey matter volumes and gait, both in the frontal lobe (Allali et al., 2018; Blumen et al., 2018; Callisaya et al., 2013; Callisaya et al., 2014a; Holtzer et al., 2014a; Rosenberg-Katz et al., 2016) and more globally (e.g. hippocampus (Allali et al., 2018; Beauchet et al., 2016)).
Imaging methods amenable to walking paradigms, such as functional Near-Infrared Spectroscopy (fNIRS) and electroencephalography (EEG), have been used to demonstrate the importance of cognitive function during dual-task walking (Beurskens et al., 2016; Chaparro et al., 2017; Chen et al., 2017; Hamacher et al., 2015; Holtzer et al., 2011; Maidan et al., 2016a; Metzger et al., 2017; Mirelman et al., 2014a; Mirelman et al., 2017; Pizzamiglio et al., 2017), and the effect of healthy aging on gait-related brain function (Holtzer et al., 2011; Mirelman et al., 2017). These studies have established the critical role of the frontal lobe in gait; for example, frontal activation increases when performing more challenging tasks – e.g. dual-task – compared to simple walking, significant increases in activation are seen in older people and individuals with gait disorders (Hamacher et al., 2016; Hamacher et al., 2015; Holtzer et al., 2011; Holtzer et al., 2016; Maidan et al., 2015; Maidan et al., 2016b) and gait disorders are associated with reduced frontal lobe volume (Beauchet et al., 2016; Rosenberg-Katz et al., 2013).
Unfortunately, the necessary constraints of the MRI environment used to assess brain structure have limited our ability to bridge the gap between structure and function needed to establish the relationship between gait-associated changes in brain activation and the structural changes in frontal lobe grey matter. A number of studies have used imagined walking functional MRI (Allali et al., 2018; Blumen et al., 2014; Hamacher et al., 2015; Holtzer et al., 2014a), but the extent to which the conclusions from these studies translate to structure-function relationships of real walking remains unknown; see, for example, (la Fougere et al., 2010). One recent paper was the first to explore the structure-function relationship between fNIRS-derived changes under dual-task walking with diffusion tensor imaging-derived white matter changes (Lucas et al., 2018). However, only association with whole brain white matter integrity were reported, without any specific focus on the PFC. To our knowledge, there has been only one study describing the relationship between changes in grey matter volume and fNIRS-derived brain activation (Iwashiro et al., 2016), and no studies which have explored these relationships vis-a-vis walking in particular. Without this information, we can only surmise a mechanistic explanation for any changes in brain activation seen with fNIRS. Thus, our primary objective was to combine data from high resolution structural MRI with dual-task walking fNIRS data in the same subjects to determine the relationship between frontal grey matter structure and fNIRS-derived brain activation changes during a dual-task paradigm: walking while talking. Based on a model of prefrontal neural inefficiency, (Daselaar et al., 2015; Rypma et al., 2002; Zarahn et al., 2007), we hypothesized that in healthy older subjects, prefrontal activation during Dual-Task-Walk (DTW) compared to Single-Task-Walk (STW) conditions, in the context of equivalent or worse gait velocity, is moderated by prefrontal grey matter volume (GMV) – higher fNIRS activation would be associated with smaller prefrontal GMV.
2. Methods
Data Sharing
All data used in this work will be made available upon request from the investigators. This data sharing arrangement is consistent with the requirements of the National Institutes of Health and with the policies of our institutional review board.
2.1. Study Population
Participants were all right-handed, community-dwelling older adults (> 65 years old), who were enrolled in a larger cohort study at our center; the study aims to identify cognitive and brain imaging predictors of mobility. Exclusion criteria included: inability to speak or understand English; visual or auditory loss; inability to walk independently; recent hospitalization for a condition affecting mobility; residence in a nursing home; diagnosis of a serious or acute illness, psychiatric condition, or neurodegenerative disease; and presence of a neurological gait disorder. MRI exclusion criteria included standard contraindications (e.g., claustrophobia, surgically implanted metal devices). All participants underwent a thorough review of past and present medical history, neurological examination, a comprehensive neurocognitive battery and assessment of functional status. Detailed study procedures have been described previously (Holtzer et al., 2014c). Established consensus case conference procedures, using all of these data, were implemented to determine cognitive status (Holtzer et al., 2008b). Specifically, of particular relevance to this study, vascular dementia was ruled out based on medical history, neurological examination and the neuropsychological (NP) testing battery that included measures of executive function and processing speed known to be sensitive to vascular dementia (Barbay et al., 2017). The HIPPA-compliant study was approved by the university’s Institutional Review Board, and written informed consent was obtained from all participants in person.
2.2. Measures
2.2.1. Walking Protocol
As described previously (Holtzer et al., 2015; Holtzer et al., 2017a), participants completed two walking tests in a quiet, well-lit room: Single-Task walking and Dual-Task walking. During each task, participants walked three consecutive, counterclockwise loops around an electronic walkway at their normal pace; the walkway consisted of a 4 × 20 foot electronic walkway, with demarcated start and end points (see Quantitative Gait Assessment section below). Tests were counterbalanced across participants. For DTW, participants recited alternate letters of the alphabet aloud while walking and were instructed to pay equal attention to walking and the verbal task. fNIRS sensors were attached to the forehead throughout the task and connected via a pivot-able gantry to avoid interference with walking. Participants wore comfortable footwear and did not use any assistive walking devices.
2.2.2. Quantitative Gait Assessment
ProKinetics Movement Analysis Software (PKMAS) was used to calculate gait velocity from footfalls measured on a 4 × 20 foot Zeno electronic walkway (Zenometrics, LLC: Peekskill, NY). Average gait velocity was calculated separately for each task condition as distance traveled divided by time elapsed, for each three loop trial. Split-half intra-class correlations (ICC) for stride velocity in STW and DTW were greater than 0.95 indicating excellent internal consistency (Holtzer et al., 2015).
Additional assessments of gait performance were performed independent of the fNIRS acquisitions, using a straight-track electronic walkway (GAITRite, CIR systems, Havertown, PA). Gait velocity, stride length, cadence and stride length variance (an established measure of gait variability) were collected and group means were compared to population norms (Oh-Park et al., 2010) to independently confirm the walking performance of the current study sample.
2.2.3. Magnetic Resonance Imaging
Magnetic resonance imaging was performed on a 3T Philips scanner (Achieva TX, Philips Medical Systems, Best, The Netherlands) with a 32-channel head coil. A single high-resolution, T1-weighted image (MPRAGE - TE/TR/TI=4.6/9.8/900 ms, voxel size 1 mm isotropic, SENSE acceleration factor 2.6) was used for all analyses in the paper. Additional scans were collected in all participants, including diffusion tensor imaging and either T2-FLAIR or T2-weighted images. FLAIR images were used for the quantification of white matter disease, evidenced by white matter hyperintensities (WMH). However, because these were only available in about half of our participants, we used the FLAIR WMH’s in this subset to validate the use of T1-hypointensities (available for all participants) as a surrogate measure; see below for WMH quantification details. DTI datasets were not considered in the current work.
2.2.4. Functional Near-Infrared Spectroscopy (fNIRS)
Acquisition procedures have been described previously (Holtzer et al., 2015; Holtzer et al., 2016). The fNIRS Imager 1100 was used (fNIRS Devices, LLC, Potomac, MD) to measure changes in PFC HbO2 levels during both walking tasks. HbO2, as opposed to deoxygenated hemoglobin, offers better signal-to-noise ratio, and thus better reliability and sensitivity to motor-related changes in cerebral blood flow (Leff et al., 2011). The fNIRS device utilizes four light emitting diodes at peak wavelengths of 730 and 850 nm, and 10 photoreceptors (light source and detectors are 2.5cm apart); this configuration results in 16 individual channels of data (Wang et al., 2017). The device was fixed to participants’ forehead with two elasticized bands, with placement based on landmarks from the international 10-20 system.
2.2.5. Demographic and Behavioral Measures
The following were included as potential confounding factors in the statistical models: age, sex, cognitive performance and global health status (GHS). Overall cognitive performance was measured with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Thaler et al., 2014) and GHS was the sum of dichotomous ratings (presence/absence) of ten health-related comorbid conditions: diabetes; chronic heart failure; arthritis; hypertension; depression; stroke; Parkinson’s disease; chronic obstructive pulmonary disease; angina; and myocardial infarction (Holtzer et al., 2008b).
2.3. Data Processing and Analysis
2.3.1. Image preprocessing
Volumetric segmentation was performed with the Freesurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (Dale et al., 1999; Fischl et al., 2002; Fischl et al., 2004; Salat et al., 2004). Briefly, this processing includes removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep grey matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles) (Fischl et al., 2002) intensity normalization (Sled et al., 1998), tessellation of the grey-white boundary, automated topology correction (Fischl et al., 2001), and surface deformation following intensity gradients to optimally identify tissue class transitions (Dale et al., 1999; Fischl and Dale, 2000). Once the cortical models are complete, further processing includes parcellation of the cerebral cortex into sub-units with respect to gyral and sulcal structure (Desikan et al., 2006; Fischl et al., 2004). The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. Freesurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and across field strengths (Reuter et al., 2012).
The automatic volume segmentation (“ASEG”) for each participant was manually checked in FSLview (Smith et al., 2004) by overlaying the participant’s cortical segmentation on their original T1 images to verify the quality of the segmentation. Only frontal cortical regions were checked and entered into our statistical models; our primary hypotheses were limited to associations between PFC activation and PFC GM volumetrics/atrophy. The frontal parcellations contain 12 frontal regions (six regions per side – caudal middle, lateral orbital, medial orbital, rostral middle, superior and frontal pole, see Figure 1). Cortical volumes were extracted with the Freesurfer “mri_segstats” command with the “--pv pvvol” flag in order to include partial volume effects. All participant results were collated with the “asegstats2table” command and entered into R for statistical analyses. Total, total right and total left frontal cortical volumes were calculated as the sum of these volumes. Prior to any statistical calculations, each volume was normalized to total intracranial volume and then standardized (i.e., zero mean and unit standard deviation) to allow for quantitative comparison of effect sizes across all cortical regions/measures. Thus, for example, the Volume-by-Task interaction terms quoted below are the change from DTW to STW in HbO2 per standardized cortical volume (μM/sV).
Figure 1:
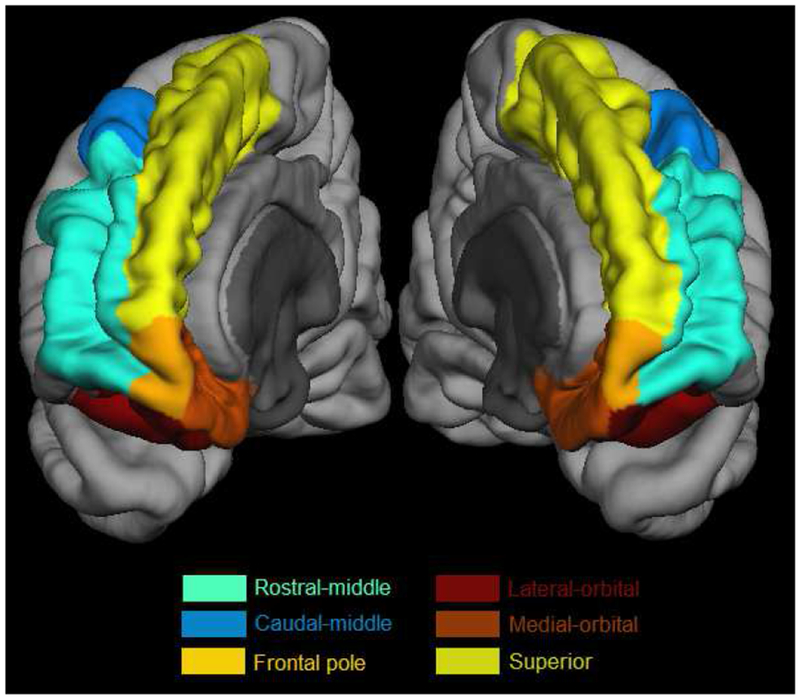
Regions of the prefrontal cortex considered in the linear mixed models, projected onto the “fsaverage” cortical surface from the Freesurfer package.
2.3.2. White matter abnormality quantification
The gold standard for quantification of white matter hyperintensities, which are expected to be present in a significant percentage of the age range of our cohort (de Leeuw et al., 2001), is T2-weighted fluid attenuated inversion recovery (T2-FLAIR). However, T2-FLAIR were not available for a substantial fraction of our participants (25/55). To overcome this shortcoming, we evaluated the Freesurfer estimated WM hypointensities (from T1-weighted images), which were available for all participants, as a potential surrogate marker of white matter disease. Initial, qualitative review of the Freesurfer based results indicated a significant overestimation of the load due to misidentification of partial volume effects at the white matter/CSF interface (i.e., periventricular signal). We thus attempted to correct this misidentification by dilating the ventricular CSF volume from Freesurfer (lateral, inferior lateral and third ventricle segmentations) with a 1.25 pixel 3D kernel (empirically determined), and masking the WM-hypointensity segmentation with this dilated volume. Both corrected and uncorrected WM-hypointensity loads (i.e. total volume in cc) were compared to FLAIR-based WMH load in the 30 participants with T2-FLAIR images. Corrected WM-hypo-intensity load was added to the linear mixed model to assess the potential effect of WM disease on the main study results (see below, §2.3.5).
2.3.3. fNIRS hemodynamic signal extraction
fNIRS data were preprocessed by an individual (M.I.) who was not involved in data collection. All data were visually inspected and artifactual data due to movement, saturation and high dark current levels were removed (Sweeney et al., 2011); high frequency interference such as respiration was removed by low-pass filtering at 0.14 Hz. The modified Beer-Lambert law was used to transform raw 730 and 850 nm signals into HbO2 signals for each of the 16 channels. Prior to each walking task, a 10 sec baseline HbO2 was taken with the participant standing still, counting silently and staring forward. HbO2 signals for each channel were then averaged across the entire task and used to quantify per-task and per-channel HbO2 changes from baseline. fNIRS and PKMAS gait data were synchronized with E-Prime 2.0 software (Psychology Software Tools, Inc.). Internal consistency of HbO2 measurements, determined by split-half intra-class correlations within each task, was excellent for both STW (0.830) and DTW (0.849) (Holtzer et al., 2015).
2.3.4. Data Visualization
In order to present visual representation of the regional moderation effects, the magnitude of the significant interaction terms from the linear mixed model were converted into a color scale and projected onto the fsaverage surface of the standard cortical surface provided with Freesurfer.
2.3.5. Statistical analysis
First, analyses of fNIRS data and MRI data were performed separately to 1) verify the expected change in HbO2 from STW to DTW, with and without correction for demographic and behavioral variables, and 2) to investigate potential relationships between GM volumes and demographic and behavioral variables. For HbO2 measures, linear mixed models (LMM) were used, while frontal volume measures used multiple linear regression. Paired t-test was used to test for changes in gait velocity from STW to DTW.
A fully adjusted LMM was used to test the primary hypothesis; namely, the presence or absence of a moderating effect of GMV on the change in PFC HbO2 during DTW compared to STW. The full model thus considered demographic and behavioral covariates, DTW velocity, and GM volume as fixed effects, participant and channel as random effects, Task as a two-level repeated within-subject factor, and a GMV-by-Task interaction term (see equation 1, showing the R format for the analyses).
| (1) |
All p-values were Bonferroni corrected for multiple comparisons; however, we considered total frontal calculations and regional frontal calculations as two levels of analysis, and thus adjusted p-values accordingly. Namely, total frontal p-values were uncorrected and regional p-values were corrected for multiple comparisons across the 12 regions considered (see §2.3.1). All statistical analyses were carried out in R (R3.4.2, https://www.R-project.org/). and the significant level was set at α = 0.05.
2.3.6. Sensitivity and exploratory analyses
There are a number of potential confounds not included in our primary models which could have an effect on our results. and thus were added one-by-one into the linear mixed model to explore potential associations with fNIRS-derived HbO2, and their potential effect on either the main Task effect or GMV-by-Task interaction effects. These included: stride length and variance, cadence, four key neuropsychological tests which assess processing speed, attention and executive function (Control Word Oral Associated Test, COWAT – letter (FAS) and category (CAT: fruit, vegetable, animals) fluency (Spreen, 1977); trail making tests forms A&B. TMT (Reitan. 1955); and Digit Symbol Substitution Test. DSST (Wechsler. 1981)), dual-task cost (i.e.. the change in gait velocity from STW to DTW), total WM-hypointensity load, and number of days between MRI and fNIRS. As noted above (§2.2.2). the additional gait parameters were measured outside the context of the fNIRS paradigms on a straight electronic walkway.
While our main hypotheses relate particularly to the prefrontal cortex, and we wanted to avoid the possibility of Type I errors which could occur with testing the entire Freesurfer atlas, we cannot ignore the possibility that regions outside of the PFC can also have a modulating effect on gait-related prefrontal activation. Thus, as a secondary, exploratory analysis, we tested the potential moderating effect of the remainder of the Freesurfer atlas, using a conservative, Bonferroni correction to control the familywise Type I error at α = 0.05. Similarly, it is possible that a number of our non-imaging measures have a moderating effect on gait-related prefrontal activation, and could even explain away any moderating effects of PFC GMV. Thus, we tested our neuropsychological testing measures, and gait performance measures as potential moderators of PFC activation through the same LMM models described above (e.g., with stride length substituted for GMV in Equation 1 above), with Bonferroni correction to control the familywise Type I error at α = 0.05. If a variable was found to have a significant moderation effect, it was then included in a double interaction LMM with this measure and total GMV; namely, a model testing for Task-by-GMV + Task-by-”other” effects.
3. Results
3.1. Participants
From an initial group of 73 participants with fNIRS and MRI data, a total of 66 participants had valid fNIRS data, as determined by the criteria listed in the §2.3.3. However, to preclude issues related to changes in neuroimaging parameters over time, we excluded 8 of these participants for whom the time between their MRI and fNIRS exam was > 1 year. Initial descriptive statistics of the data revealed three outliers who were also excluded from analyses, as follows: one with high variance in HbO2 measurements (> 15 * mean variance for all participants, across all channels and both tasks), one with extremely high gait velocity for both tasks, and one with unusually high leverage in the regression analysis (Cook’s distance > 0.5). Thus, we had a final sample of 55 participants with usable fNIRS and MRI data for analysis. Mean age for the group was 74.8 ± 5.0 years, with n = 27/28 male/female. The low mean disease comorbidity score (GHS=1.4 ±1.1) confirmed the relatively healthy nature of the group. The mean RBANS total score (92.5 ±11.4) indicates average cognitive function. Sample characteristics are presented in Table 1.
Table 1:
Descriptive statistics for all subjects in the study (n = 55). GHS: Global Health Scale, RBANS: Repeatable Battery for the Assessment of Neuropsychological Status, STW: Single-Task Walking, DTW: Dual-Task Walking.
| Parameter | mean | SD | range |
|---|---|---|---|
| Age (yrs) | 74.8 | 5.0 | (65 – 88) |
| Sex (M/F) | 27/28 | ||
| GHS (# comorbidities) | 1.36 | 1.04 | (0 – 4) |
| RBANS | 92.5 | 11.4 | (65 – 116) |
| STW Velocity (cm/s) | 70.3 | 15.1 | (41.7 – 105.5) |
| DTW Velocity (cm/s) | 60.8*** | 12.5 | (36.6 – 85.8) |
| STW HbO2 (μM) | 0.40 | 1.04 | (−7.74 – +5.55) |
| DTW HbO2 (μM) | 1.03*** | 1.58 | (−9.86 – +9.46) |
| Frontal Volume (cc) | 90.23 | 7.79 | (60.94 – 105.49) |
| Frontal Volume – L | 45.22 | 4.14 | (29.99 – 52.28) |
| Frontal Volume – R | 45.01 | 3.90 | (30.96 – 53.30) |
indicates p < 0.0001 compared to STW.
The GAITrite walking results are shown in Table 2. Mean GAITrite velocity was higher than both STW and DTW velocities, as expected, because the GAITrite data were collected on a straight walkway as compared to the fNIRS oval walkway. All of the GAITrite parameters – velocity, stride length, stride length variance and cadence – were within the expected norms for a healthy older cohort (Oh-Park et al., 2010). Neuropsychological testing results are also given in Table 2; these fall well within robust norms for this age group (Holtzer et al., 2008a). Taken together with the health and cognitive status results of Table 1, these confirm that the study population under investigation consisted of relatively healthy older individuals.
Table 2:
Descriptive statistics for straight-walkway (“GAITrite”) walking measures, and four key neuropsychological (NP) testing measures. All measures are well within expected norms from previous studies of these tests. Z-scores for the NP tests are taken with respect to the robust sample in (Holtzer et al., 2008a) with mean = 0, and SD = 1. TMT: Trail making test, DSST: Digit Symbol Substitution Test. Note: grip strength is reported for males and females separately because of the highly significant sex difference in this measure.
| Parameter | mean | SD | range |
|---|---|---|---|
| Gait velocity (cm/s) | 107.1 | 20.6 | (68.8 – 150.5) |
| Stride Length (cm) | 124.20 | 18.08 | (88.10 – 160.70) |
| Stride Length SD (cm) | 3.65 | 2.00 | (0.23 – 8.50) |
| Cadence (steps/min) | 103.70 | 11.44 | (81.10 – 133.30) |
| Letter fluency test (FAS) | 43.95 | 12.86 | (16 – 79) |
| FAS-Zscore | 0.42 | 1.05 | (−1.60 – +2.97) |
| Category fluency test (CAT) | 44.75 | 11.62 | (21– 73) |
| CAT – Zscore | 0.46 | 1.47 | (−2.74 – +4.43) |
| TMT-A | 46.89 | 25.44 | (23.72 – 161.00) |
| TMT-A Zscore | 0.30 | 1.52 | (−8.03 – +1.58) |
| TMT-B | 112.20 | 65.06 | (41.88 – 300.00) |
| TMT-B Zscore | 0.26 | 1.26 | (−2.83 – +1.93) |
| DSST | 12.00 | 2.79 | (5 – 18) |
| Grip Strength – M (kg) | 32.81 | 5.54 | (19.2 – 48.3) |
| Grip Strength – F (kg) | 20.65 | 8.15 | (12.3 – 36.9) |
3.2. White matter hyperintensity assessments
Almost all subjects were categorized as Fazekas scale 0 and 1; i.e., no white matter lesions or only small periventricular halo/small punctate lesions (Fazekas et al., 1987), while one subject was categorized as Fazekas scale 3 with large confluent areas of WM abnormalities and infiltration into the deep white matter (and T2-FLAIR load > 2* the next highest load). Both uncorrected and corrected WM-hypointensity volumes were highly correlated with T2-FLAIR WMH volume, with a clear overestimation of volume for participants with low WMH load.
Although there remained a discrepancy in the agreement even after the correction, our goal was not strict WM hyperintensity load quantification, but rather to establish the T1 hypointensity load as a surrogate measure of WM abnormality; the corrected data gave us confidence that WM-hypointensity was indeed a suitable measure for distinguishing relative WM disease (e.g. low vs. medium vs. high load, see Figure 2).
Figure 2:
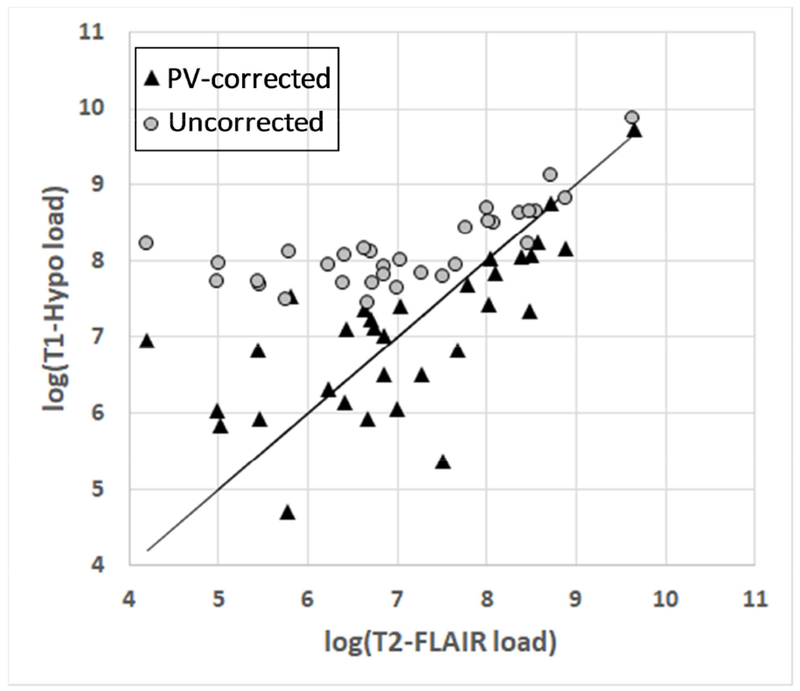
Relationship between WM hyperintensity load (i.e. total volume) as seen on T2-FLAIR and WM hypointensity as seen on T1-MPRAGE, with and without correction for periventricular signal
3.3. Main effects and covariates
Unadjusted LMM analysis showed a significant main Task effect, with PFC HbO2 increasing from STW to DTW: t55 = 11.3, β = 0.63 μM, 95% CI: 0.52 - 0.74, p < 0.0001. Adjusting for all covariates and DTW gait velocity showed no significant effect of these variables, with only a small effect on the main Task effect: t55 = 10.1, β = 0.57 μM, 95% CI: 0.46 - 0.68, p < 0.0001. Regression analysis to identify potential effects of covariates on total frontal cortex grey matter volumes did not reveal any significant associations (p = 0.79). Similarly, regression analysis with regional grey matter volumes, corrected for multiple comparisons, were not significant.
3.4. Fully adjusted LMM results.
The fully adjusted LMM analysis – to address the primary hypothesis that frontal GMV has a moderating effect on the HbO2 increase from STW to DTW conditions – revealed a significant interaction effect of total frontal GMV-by-Task on PFC HbO2 (t54 = −4.37, β = −0.24 μM/sV, 95% CI: −0.35 to −0.13, p = 1.29e-5). To better appreciate the magnitude of this effect, we can use the standard deviation in frontal volume across subjects (see Table 1) to convert back to non-normalized units: β = −0.022 μM per cc GMV change. Total left and right frontal volume interaction effects were similarly highly significant (see Table 3). With respect to regional volume measurements, we found significant volume-by-Task interactions in bilateral rostral middle and superior frontal gyri, and left lateral and medial orbital frontal gyri. The main Task effect was highly significant in all the models, and none of the covariates were significant in the linear models. It should be noted that model results were unchanged when co-varying for STW velocity as opposed to DTW velocity. All results from the linear mixed models can be found in Table 3; a pictorial illustration of the regional interaction effects is shown in Figure 3.
Table 3:
Linear mixed model GMV-by-Task moderation of PFC HbO2, over whole frontal cortex (upper portion) and sub-regions of the frontal cortex (lower portion). Units of interaction are μM per standardized volume per task; negative values indicate an increase in HbO2 from STW to DTW per unit decrease in standardized cortical volume. Significant interactions at p < 0.05, corrected, are shown in bold. Lower and upper bounds are 95% confidence intervals.
| Region | Interaction (μM/sV) | Lower Bound | Upper Bound | p-Value |
|---|---|---|---|---|
| Total Frontal Volume | −0.24 | −0.35 | −0.13 | 1.29e-05 |
| Frontal Volume — L | −0.26 | −0.36 | −0.15 | 3.33e-06 |
| Frontal Volume — R | −0.21 | −0.32 | −0.10 | 1.53e-04 |
| Caudal-middle — L | −0.16 | −0.32 | 0.0051 | 0.067 |
| Caudal-middle — R | −0.13 | −0.29 | 0.037 | 0.32 |
| Lateral-orbital — L | −0.18 | −0.34 | −0.019 | 0.016 |
| Lateral-orbital — R | 0.041 | −0.12 | 0.201 | 1.00 |
| Medial-orbital — L | −0.26 | −0.42 | −0.10 | 3.72e-05 |
| Medial-orbital — R | −0.017 | −0.18 | 0.143 | 1.00 |
| Rostral-middle — L | −0.26 | −0.42 | −0.098 | 4.96e-05 |
| Rostral-middle — R | −0.23 | −0.39 | −0.068 | 0.00057 |
| Superior — L | −0.17 | −0.33 | −0.0088 | 0.030 |
| Superior - R | −0.25 | −0.41 | −0.089 | 0.00011 |
| Pole — L | −0.11 | −0.28 | 0.053 | 0.61 |
| Pole — R | 0.15 | −0.013 | 0.31 | 0.10 |
Figure 3:
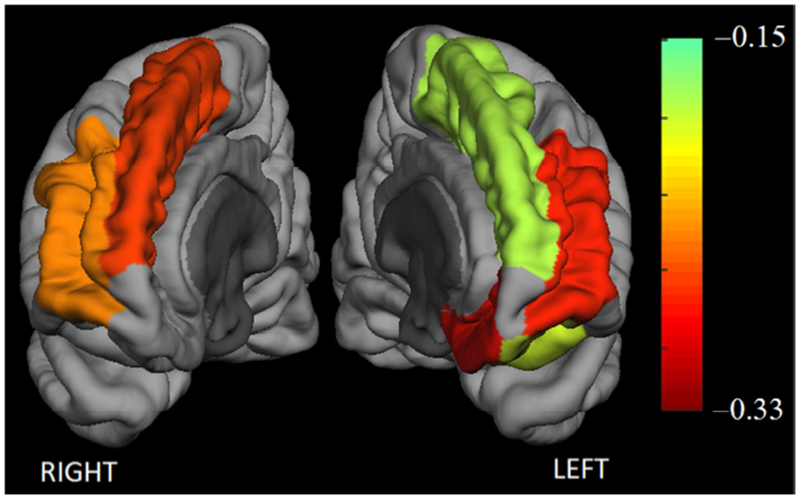
Moderation effect of grey matter volume on the change in prefrontal grey matter HbO2 from a simple to a complex walking task (single task walking → walking while talking). Color indicates strength of the GM volume-by-Task interaction term in the linear mixed model; only regions significant at p < 0.05, after Bonferroni correction, are shown.
3.5. Sensitivity analyses
To investigate the sensitivity of our models to potential confounders, the following parameters were added, one-by-one, to the LMM: dual-task cost, independently assessed gait performance measures (velocity, stride length and variance, and cadence), neuropsychological testing measures (FAS, CAT, TMT-A&B, DSST), WM hypo-intensity load and days between MRI and fNIRS. None of these parameters were found to have any effect on the either the main Task effect or the total GMV-by-Task interaction effect (i.e., all confounder p-values > 0.05 – the smallest p-value was 0.13 for stride length, but did not change the main Task or interaction terms). As noted above, one subject had a significant degree of WM abnormality, > 2* the next highest value. Excluding this subject alone from the main LMM did have an effect on the main Task effect and main interaction effect; the task effect size increased from 3.48 to 3.86 μM (p < 0.0001 both with and without this subject), and the magnitude of the total GMV-by-Task interaction effect similarly increased from −0.24 to −0.27 μM/sV, (p < 0.0001 both with and without this subject). Thus, it would appear that, if anything, the presence of WM disease may have had an attenuating effect on the main study outcomes.
Two sets of exploratory analyses were performed: testing for Task-by-Volume interaction effects for regions outside of the PFC, and testing for interaction effects with non-imaging measures. A number of regions demonstrated significant interaction effects, including bilateral cerebral white matter, bilateral caudate, and fusiform gyrus and corpus callosum, among others; the full list of significant regions is shown in Supplementary Table 1. All of the significant interaction effects were negative, i.e., decreased volumes being associated with an increased change in HbO2 from STW to DTW. Of the non-imaging measures, none of the gait-related measures showed significant effects, while all of the neuropsychological testing measures demonstrated highly significant interaction effects (FAS, CAT: p < 0.001, TMT-A&B, DSST: p < 0.0001; Bonferroni-corrected). For each measure, the interaction term was consistent with worse performance being associated with an increased change in HbO2 from STW to DTW. Finally, for each of these significant items a dual interaction model was investigated for total grey matter volume, i.e., total GMV-by-Task + NP test-by-Task. In all cases, both interactions remained significant.
4. Discussion
4.1. Results and interpretation.
In the current study, we aimed to bridge the structure-function gap in elucidating the neurophysiological underpinnings of gait, by combining data taken in a sample of relatively healthy older adults from both 1) high-resolution, structural MRI grey matter volumetrics and 2) functional Near-Infrared Spectroscopy-based measures of oxyhemoglobin changes from single-to dual-task walking. Extensive health status, neuropsychological and gait measures in the sample, and comparison to robust norms for this age group, clearly demonstrate that the group is a good representation of relatively healthy, community-residing older adults. Hence, we can be reasonably confident that the effects demonstrated are likely not a result of any neuropathological processes. A linear mixed model approach allowed us to account for potential random variability in fNIRS signals across both subjects and channels, while at the same time modeling for the expected fixed effects of task and GMV and controlling for the effects of covariates (e.g., participant age, disease comorbidities, gait velocity). The interaction term added to this model tested our main hypothesis that GMV moderates prefrontal brain activation, and in particular the increase in brain activation from dual- vs. single-task walking. Thus, we would predict a negative GMV-by-Task interaction indicating the association of higher DTW-to-STW HbO2 changes with smaller GMV’s and vice versa.
For all models considered, there was a highly significant and positive main effect of Task, indicating that the frontal lobe is more engaged in the complex dual task as compared to single-task walking; this effect has been established in numerous prior works (Fraser et al., 2016; Holtzer et al., 2014a; Holtzer et al., 2011; Mirelman et al., 2014a; Mirelman et al., 2017). Consistent with our predictions, we found a highly significant negative HbO2 total frontal GMV-by-Task interaction effect. To further explore the spatial distribution of this GMV moderation, we used the Freesurfer parcellations of the frontal lobe in individual LMM’s, using a conservative approach of Bonferroni correction to avoid Type 1 errors. The main contribution of the moderation effects were shown to involve bilateral superior and rostral-middle frontal gyri and left orbitofrontal gyrus (bilateral caudal middle gyri were significant at p < 0.05 uncorrected). It should be noted that all participants were right-handed, which may explain the inclusion of only left orbito-frontal involvement. We can interpret these results as indicative of an association between reduced GMV in the PFC and a greater increase in overall PFC activation from STW to DTW, and it is important to note that these results were upheld with appropriate adjustment for any differences in gait performance. It is also noteworthy that, on average, single task gait performance was comparable to established robust normative data indicating that these results are generalizable to ambulatory community residing older adults.
In exploratory analyses, LMM’s showed moderation effects of NP measures of processing speed, attention, and executive function, wherein poor neurocognitive performance was associated with increased brain activation during a complex walking task (walking-while-talking). These findings are not surprising, and indeed consistent with the directionality of the GMV interaction effects: it is well established that dual-tasks activate both executive function and attention networks (Holtzer et al., 2012; Mirelman et al., 2012; M. Montero-Odasso et al., 2012; Springer et al., 2006). Furthermore, our preliminary analyses using simultaneous modeling of GMV + NP moderation effects imply that GMV reductions and neurocognitive function loss independently moderate increased brain activation during dual-task walking.
4.2. Prior studies
In comparison to our study, previous studies of gait in healthy older people have focused on the relationship between behavioral outcome and either structural or functional brain changes, but rarely their combination. With respect to structural studies, the relationship of reduced grey and white matter density with worse gait performance has been well established by numerous groups (Callisaya et al., 2013; Callisaya et al., 2014a; Holtzer et al., 2014a; Persson et al., 2006); these results have generally focused on gait speed as a behavioral outcome, although relationships to gait variability and balance have also been shown. While most of these studies have used total grey matter volume as the structural outcome, a few investigations using voxel-based techniques have demonstrated the importance of the reduction in frontal lobe density in gait-related performance (Allali et al., 2018; Blumen et al., 2018; Callisaya et al., 2014a). White matter abnormalities (e.g., white matter hyperintensities, often indicative of small vessel disease) can also be predictive of poor functional gait outcome (Bhadelia et al., 2009; Bruijn et al., 2014; Callisaya et al., 2014a; Callisaya et al., 2014b; Siejka et al., 2018; Whitman et al., 2001).
With respect to functional studies, it is first important to point out that numerous studies have investigated structure-function relationships in healthy aging using MRI alone, typically assessing function through either task-based or resting-state functional MRI. A number of such studies have demonstrated a similar effect to that shown here; namely, increased functional activation in the presence of reduced structure (Di et al., 2014; Hakun et al., 2015; Sui et al., 2014). However, as mentioned above, the MRI environment has restricted these studies to nonmotor-related functions. With respect to gait-related studies, the majority of functional studies have utilized fNIRS because of its amenability to real-time gait testing. These have shown the importance of PFC activation in gait outcome, showing relationships of over-activation to reduced gait speed as well as changes in other gait-related outcomes (Chen et al., 2017; Hamacher et al., 2015; Holtzer et al., 2011; Holtzer et al., 2015; Maidan et al., 2016a; Metzger et al., 2017; Mirelman et al., 2014a; Mirelman et al., 2017). Of course, the focus in these studies on frontal lobe is in part a function of the limitations of fNIRS – activation within other brain areas are usually too distal to be visualized (Haeussinger et al., 2011) – rather than a limitation in the brain areas actually involved in the task. Other technologies, such as positron-emission tomography have been used to elucidate regions outside of frontal lobe involved in gait, such as parahippocampal and cerebellar regions (Hamacher et al., 2015; la Fougere et al., 2010). MRI studies have used imagined gait to show involvement of frontal, as well as cerebellar, supplementary motor and basal ganglia regions (Blumen et al., 2014; Hamacher et al., 2015). We believe our work, while limited to frontal brain activity, uniquely adds to this body of knowledge by combining data from structural and functional imaging in the same individual through linear mixed modeling to elucidate potential relationships between cortical atrophy/volume loss and hyper-activation.
4.3. Clinical relevance
The relationship between cortical over-activation and cortical volume loss is not merely academic; changes in cognitive function have been shown to be related to falls in older people, even in healthy aging with minimal comorbid disease (Allali et al., 2017; Ayers et al., 2014; Hausdorff and Yogev, 2006; Holtzer et al., 2007; Martin et al., 2013; Mirelman et al., 2012; M. Montero-Odasso et al., 2012; Muir-Hunter and Wittwer, 2016; Springer et al., 2006; Verghese et al., 2009). The risk of falls becomes a significant in older people, with an incidence of over 30% in people over 65 years of age, and is the leading cause of death from injury in older adults (Sattin, 1992; Tinetti, 2003; Tinetti et al., 1994). There is already a large body of literature independently linking cortical over-activation and cortical volume loss to incidence of falls in older people (Beauchet et al., 2017; Callisaya et al., 2015; Halliday et al., 2018; Srikanth et al., 2009; Verghese et al., 2017; Whitman et al., 2001). Thus, the results of the current study may have an important clinical relevance: Given the structure-function relationships identified, even in the absence of a clinically-relevant gait deficit, similar measures may be useful is predicting future gait decline and risk of falls in older people.
With respect to potential clinical implications, it is worthwhile to consider our results from the perspective of the neural inefficiency model. There are two contexts in which we can consider neural inefficiency in older people: one in which brain activity is increased in the context of poor gait performance; this has much evidence in the literature (Halliday et al., 2018; Hamacher et al., 2015; Hawkins et al., 2018; Holtzer et al., 2014a; Mirelman et al., 2017). In this context, inefficiency can be thought of as the over-use of brain resources with concurrent degradation in behavioral performance, and is most often demonstrated with the relationship between over-activation with under-performance (e.g., a negative correlation between activation and gait speed). On the other hand, brain over-activation can exist even in the context of similar behavioral performance. This context is more difficult to demonstrate because there is no behavioral deficit against which to measure the over-activation. One could, in fact, question the clinical relevance of cortical over-activation when there is no loss of functional outcome; however, the brain over-activation may foreshadow future clinical decline and thus can provide critical information for guiding therapeutic intervention to such decline. The data presented here - showing the moderation effect of reduced superior-medial frontal grey matter volume on increased PFC activation in the context of similar behavioral performance - lend support to this model and in particular to the notion of neural inefficiency and its relationship to grey matter loss, as well as the notion of “more firing, less wiring” in the aging brain (Daselaar et al., 2015). The independent findings of modulation effects of neurocognitive performance on prefrontal brain activation provide additional, converging, evidence for this model.
4.4. Exploratory analyses
As an exploratory aim, recognizing that the brain regions utilized during complex gait tasks is certainly not limited to the frontal cortex, we extended our LMM analyses to explore other brain regions which may be related to the (non-spatially localized) frontal brain activations seen with fNIRS. The regions identified in these analyses (Supplementary Table 1) are not surprising as potentially related to gait function: for example, cerebral white matter is a surrogate measure of brain atrophy, caudate has been shown to be related to gait performance (Macfarlane et al., 2015), and fusiform and pre-cuneus gyri have been shown to be related to gait and visuo-spatial orientation (Malouin et al., 2003; Sartor et al., 2017).
Furthermore, the LMM approach was applied to our non-imaging measures and identified the neuropsychological measures as independent moderators of gait-related brain activation. The directionality of these relationships provided additional support for the neural inefficiency model: poor neurocognitive performance was associated with increased, rather than decreased, pre-frontal brain activation during dual-task walking.
4.5. Study limitations and future direction
While the data presented demonstrate promising results in identifying gait-related structure-function relationships in the brain, there are a few limitations to the current study which should be noted. One limitation of the fNIRS technology, as implemented in this work, is the brain regions from which signal emanates, and has two important implications which should be noted. First, signal detected in any given channel can be a product of changes in blood flow from multiple cortical regions, e.g., the individual cortical regions identified by Freesurfer from the high resolution MRI data. At the same time, fNIRS has limited depth penetration, so that the signals are necessarily only a product of the nearby cortical regions, and thus mostly limited to frontal cortex. Because of these considerations, our primary analyses were limited to frontal cortex, and we intentionally decided to treat channel as a random variable in the linear mixed models rather than investigating spatially localized fNIRS effects. Thus, it is important to realize that all of the interaction effects seen, such as the localized interaction effects in specific regions of the frontal cortex are only localized with respect to changes in GMV as measured through the high resolution (and spatially resolved) MRI. fNIRS measures, on the other hand, are effectively integrated over the entire signal region. While there may be interesting localized fNIRS effects, we did not feel that this small cohort was appropriately to explore these effects; this will hopefully be the subject of future, larger prospective studies.
Structural analyses were restricted to frontal cortex, thus we cannot rule out the possibility that other brain regions are affected and related to gait activation (e.g., a number of studies have shown changes in parietal cortex). However, because our fNIRS device was limited to frontal cortical activation, we felt justified in limiting the primary structural analyses to frontal regions. Future studies will explore similar associations throughout other cortical and subcortical regions. The current fNIRS system offers significant advantages in terms of portability and capability to assess cortical activation during locomotion, but methodological consideration including depth of penetration, spatial resolution and possible effects of superficial layers should be acknowledged (Vitorio et al., 2017). By focusing on differences in HbO2 levels between single- and dual-task walking, the effect of these factors on the reported results were minimized. A number of studies have identified associations between grey matter volume and gait speed, which we did not find in our sample (Allali et al., 2018; Blumen et al., 2018; Callisaya et al., 2013; Callisaya et al., 2014a). However, our sample size was small (compared to ~ few 100 in these other studies) so we likely did not have the power to identify such relationships. Finally, we note that only the walking while talking was compared to single-task walking, which overlooks the possibility of increased HbO2 from the cognitive task alone. Previous research, however, has demonstrated increased PFC activation during DTW relative to both walking and cognitive single tasks (Holtzer et al., 2017a; Holtzer et al., 2017b). Given the focus on the brain substrate of gait in the current study and the small sample size, we restricted this investigation to walking conditions to the exclusion of a cognitive single-task condition (i.e. talking without walking).
The moderating effect of PFC grey matter volume on brain activation patterns of walking was established in a sample of non-demented and relatively healthy community residing older adults. However, because our study was limited to older adults, we cannot rule out the possibility that such moderating effects are not unique to older adults and either generalize or differ in younger subjects. Such possible age effects can be addressed in future studies. Furthermore, the generalizability of our findings to pathological samples including but not limited to older adults with neurodegenerative conditions should also be evaluated in future research.
4.6. Conclusions
In summary, the current study used the combination of structural cortical volume with brain activation measures to identify structure-function relationships involved in the neurophysiology of gait in older people. We found areas demonstrating a significant relationship between reduced cortical volume and overall prefrontal brain over-activation, with reduced volumes most notably in bilateral superior and rostral-middle frontal cortex. The results provide support for the notion of neural inefficiency in brain activation of older people and may have relevance toward identifying useful clinical biomarkers for predicting future decline in mobility and risk of falls.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institute on Aging grants (R01AG036921, R01AG044007).
Abbreviations:
- DTW:
Dual-task walking
- fNIRS:
Functional near-infrared spectroscopy
- GHS:
Global health status
- GMV:
Grey matter volume
- LMM:
Linear mixed model
- NP:
neuropsychological
- PFC:
Prefrontal cortex
- RBANS:
Repeatable Battery for the Assessment of Neuropsychological Status
- STW:
Single-task walking
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest: Dr. Izzetoglu has a very minor share in the company that manufactures the fNIRS device used in this study. All other authors have no conflicts of interest to report in relation to the current article.
References
- Allali G, Launay CP, Blumen HM, et al. (2017). Falls, Cognitive Impairment, and Gait Performance: Results From the GOOD Initiative. J Am Med Dir Assoc, 18(4), 335–340. doi: 10.1016/j.jamda.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allali G, Montembeault M, Brambati SM, et al. (2018). Brain Structure Covariance Associated with Gait Control in Aging. J Gerontol A Biol Sci Med Sci doi : 10.1093/gerona/gly123 [DOI] [PubMed] [Google Scholar]
- Alvarez JA, & Emory E (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev, 16(1), 17–42. doi: 10.1007/s11065-006-9002-x [DOI] [PubMed] [Google Scholar]
- Ayers EI, Tow AC, Holtzer R, et al. (2014). Walking while talking and falls in aging. Gerontology, 60(2), 108–113. doi: 10.1159/000355119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbay M, Taillia H, Nedelec-Ciceri C, et al. (2017). Vascular cognitive impairment: Advances and trends. Rev Neurol (Paris), 173(7-8), 473–480. doi: 10.1016/j.neurol.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Beauchet O, Allali G, Annweiler C, et al. (2016). Association of Motoric Cognitive Risk Syndrome With Brain Volumes: Results From the GAIT Study. J Gerontol A Biol Sci Med Sci, 71(8), 1081–1088. doi: 10.1093/gerona/glw012 [DOI] [PubMed] [Google Scholar]
- Beauchet O, Launay CP, Barden J, et al. (2017). Association Between Falls and Brain Subvolumes: Results from a Cross-Sectional Analysis in Healthy Older Adults. Brain Topogr, 30(2), 272–280. doi: 10.1007/s10548-016-0533-z [DOI] [PubMed] [Google Scholar]
- Beurskens R, Steinberg F, Antoniewicz F, et al. (2016). Neural Correlates of Dual-Task Walking: Effects of Cognitive versus Motor Interference in Young Adults. Neural Plast, 2016, 8032180. doi: 10.1155/2016/8032180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadelia RA, Price LL, Tedesco KL, et al. (2009). Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke, 40(12), 3816–3820. doi: 10.1161/STROKEAHA.109.564765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumen HM, Brown LL, Habeck C, et al. (2018). Gray matter volume covariance patterns associated with gait speed in older adults: a multi-cohort MRI study. Brain Imaging Behav. doi: 10.1007/s11682-018-9871-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumen HM, Holtzer R, Brown LL, et al. (2014). Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum Brain Mapp, 35(8), 4090–4104. doi: 10.1002/hbm.22461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn SM, Van Impe A, Duysens J, et al. (2014). White matter microstructural organization and gait stability in older adults. Front Aging Neurosci, 6, 104. doi : 10.3389/fnagi.2014.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Beare R, Phan T, et al. (2015). Progression of white matter hyperintensities of presumed vascular origin increases the risk of falls in older people. J Gerontol A Biol Sci Med Sci, 70(3), 360–366. doi: 10.1093/gerona/glu148 [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Beare R, Phan TG, et al. (2013). Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc, 61(7), 1074–1079. doi: 10.1111/jgs.12331 [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Beare R, Phan TG, et al. (2014a). Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people. PLoS One, 9(1), e84909. doi: 10.1371/journal.pone.0084909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Srikanth VK, Lord SR, et al. (2014b). Sub-cortical infarcts and the risk of falls in older people: combined results of TASCOG and Sydney MAS studies. Int J Stroke, 9 Suppl A100, 55–60. doi: 10.1111/ijs.12279 [DOI] [PubMed] [Google Scholar]
- Chaparro G, Balto JM, Sandroff BM, et al. (2017). Frontal brain activation changes due to dual-tasking under partial body weight support conditions in older adults with multiple sclerosis. J Neuroeng Rehabil, 14(1), 65. doi: 10.1186/s12984-017-0280-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Pillemer S, England S, et al. (2017). Neural correlates of obstacle negotiation in older adults: An fNIRS study. Gait Posture, 58, 130–135. doi : 10.1016/j.gaitpost.2017.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin AK, Shumway-Cook A, Saczynski JS, et al. (2006). Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing, 35(6), 619–624. doi: 10.1093/ageing/afl107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Iyengar V, Davis SW, et al. (2015). Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb Cortex, 25(4), 983–990. doi: 10.1093/cercor/bht289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Achten E, et al. (2001). Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry, 70(1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Di X, Rypma B, & Biswal BB (2014). Correspondence of executive function related functional and anatomical alterations in aging brain. Prog Neuropsychopharmacol Biol Psychiatry, 48, 41–50. doi: 10.1016/j.pnpbp.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, et al. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol, 149(2), 351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A, 97(20), 11050–11055. doi: 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, & Dale AM (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging, 20(1), 70–80. doi: 10.1109/42.906426 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, et al. (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fraser SA, Dupuy O, Pouliot P, et al. (2016). Comparable Cerebral Oxygenation Patterns in Younger and Older Adults during Dual-Task Walking with Increasing Load. Front Aging Neurosci, 8, 240. doi: 10.3389/fnagi.2016.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeussinger FB, Heinzel S, Hahn T, et al. (2011). Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One, 6(10), e26377. doi: 10.1371/journal.pone.0026377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakun JG, Zhu Z, Brown CA, et al. (2015). Longitudinal alterations to brain function, structure, and cognitive performance in healthy older adults: A fMRI-DTI study. Neuropsychologia, 71, 225–235. doi: 10.1016/j.neuropsychologia.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DWR, Hundza SR, Garcia-Barrera MA, et al. (2018). Comparing executive function, evoked hemodynamic response, and gait as predictors of variations in mobility for older adults. J Clin Exp Neuropsychol, 40(2), 151–160. doi: 10.1080/13803395.2017.1325453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher D, Hamacher D, Herold F, et al. (2016). Effect of dual tasks on gait variability in walking to auditory cues in older and young individuals. Exp Brain Res, 234(12), 3555–3563. doi: 10.1007/s00221-016-4754-x [DOI] [PubMed] [Google Scholar]
- Hamacher D, Herold F, Wiegel P, et al. (2015). Brain activity during walking: A systematic review. Neurosci Biobehav Rev, 57, 310–327. doi: 10.1016/j.neubiorev.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, & Yogev G (2006). Cognitive function may be important for fall injury prevention trials. J Am Geriatr Soc, 54(5), 865; author reply 865-866. doi: 10.1111/j.1532-5415.2006.00718.x [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Fox EJ, Daly JJ, et al. (2018). Prefrontal over-activation during walking in people with mobility deficits: Interpretation and functional implications. Hum Mov Sci, 59, 46–55. doi: 10.1016/j.humov.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Epstein N, Mahoney JR, et al. (2014a). Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci, <59(11), 1375–1388. doi: 10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Friedman R, Lipton RB, et al. (2007). The relationship between specific cognitive functions and falls in aging. Neuropsychology, 21(5), 540–548. doi: 10.1037/0894-4105.21.5.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Goldin Y, Zimmerman M, et al. (2008a). Robust norms for selected neuropsychological tests in older adults. Arch Clin Neuropsychol, 23(5), 531–541. doi: 10.1016/j.acn.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney J, & Verghese J (2014b). Intraindividual variability in executive functions but not speed of processing or conflict resolution predicts performance differences in gait speed in older adults. J Gerontol A Biol Sci Med Sci, 69(8), 980–986. doi: 10.1093/gerona/glt180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, et al. (2011). fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci, 66(8), 879–887. doi: 10.1093/gerona/glr068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, et al. (2015). Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage, 112, 152–159. doi: 10.1016/j.neuroimage.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Schoen C, Demetriou E, et al. (2017a). Stress and gender effects on prefrontal cortex oxygenation levels assessed during single and dual-task walking conditions. Eur J Neurosci, 45(5), 660–670. doi: 10.1111/ejn.13518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Allali G, et al. (2016). Neurological Gait Abnormalities Moderate the Functional Brain Signature of the Posture First Hypothesis. Brain Topogr, 29(2), 334–343. doi: 10.1007/s10548-015-0465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, et al. (2008b). Within-person across-neuropsychological test variability and incident dementia. JAMA, 300(7), 823–830. doi: 10.1001/jama.300.7.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, et al. (2006). Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology, 20(2), 215–223. doi: 10.1037/0894-4105.20.2.215 [DOI] [PubMed] [Google Scholar]
- Holtzer R, Wang C, & Verghese J (2012). The relationship between attention and gait in aging: facts and fallacies. Motor Control, 16(1), 64–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C, & Verghese J (2014c). Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordr), 36(1), 373–381. doi: 10.1007/s11357-013-9570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Yuan J, Verghese J, et al. (2017b). Interactions of Subjective and Objective Measures of Fatigue Defined in the Context of Brain Control of Locomotion. J Gerontol A Biol Sci Med Sci, 72(3), 417–423. doi: 10.1093/gerona/glw167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashiro N, Koike S, Satomura Y, et al. (2016). Association between impaired brain activity and volume at the sub-region of Broca’s area in ultra-high risk and first-episode schizophrenia: A multi-modal neuroimaging study. Schizophr Res, 172(1-3), 9–15. doi: 10.1016/j.schres.2016.02.005 [DOI] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, et al. (2010). Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage, 50(4), 1589–1598. doi: 10.1016/j.neuroimage.2009.12.060 [DOI] [PubMed] [Google Scholar]
- Leff DR, Orihuela-Espina F, Elwell CE, et al. (2011). Assessment of the cerebral cortex during motor task behaviours in adults: a systematic review of functional near infrared spectroscopy (fNIRS) studies. Neuroimage, 54(4), 2922–2936. doi: 10.1016/j.neuroimage.2010.10.058 [DOI] [PubMed] [Google Scholar]
- Li C, Verghese J, & Holtzer R (2014). A comparison of two walking while talking paradigms in aging. Gait Posture, 40(3), 415–419. doi: 10.1016/j.gaitpost.2014.05.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Wagshul ME, Izzetoglu M, et al. (2018). Moderating Effect of White Matter Integrity on Brain Activation During Dual-Task Walking in Older Adults. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/gly131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane MD, Looi JC, Walterfang M, et al. (2015). Shape abnormalities of the caudate nucleus correlate with poorer gait and balance: results from a subset of the LADIS study. Am JGeriatr Psychiatry, 23(1), 59–71 e51. doi: 10.1016/j.jagp.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidan I, Bernad-Elazari H, Gazit E, et al. (2015). Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures. J Neurol, 262(4), 899–908. doi: 10.1007/s00415-015-7650-6 [DOI] [PubMed] [Google Scholar]
- Maidan I, Nieuwhof F, Bernad-Elazari H, et al. (2016a). The Role of the Frontal Lobe in Complex Walking Among Patients With Parkinson’s Disease and Healthy Older Adults: An fNIRS Study. Neurorehabil Neural Repair, 30(10), 963–971. doi: 10.1177/1545968316650426 [DOI] [PubMed] [Google Scholar]
- Maidan I, Rosenberg-Katz K, Jacob Y, et al. (2016b). Altered brain activation in complex walking conditions in patients with Parkinson’s disease. Parkinsonism Relat Disord, 25, 91–96. doi: 10.1016/j.parkreldis.2016.01.025 [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, et al. (2003). Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp, 19(1), 47–62. doi : 10.1002/hbm.10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KL, Blizzard L, Wood AG, et al. (2013). Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol A Biol Sci Med Sci, 68(6), 726–732. doi: 10.1093/gerona/gls224 [DOI] [PubMed] [Google Scholar]
- Metzger FG, Ehlis AC, Haeussinger FB, et al. (2017). Functional brain imaging of walking while talking - An fNIRS study. Neuroscience, 343, 85–93. doi: 10.1016/j.neuroscience.2016.11.032 [DOI] [PubMed] [Google Scholar]
- Mirelman A, Herman T, Brozgol M, et al. (2012). Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One, 7(6), e40297. doi: 10.1371/journal.pone.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman A, Maidan I, Bernad-Elazari H, et al. (2014a). Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. Journal of Neuroengineering and Rehabilitation, 11(85), 11–85. doi: 10.1186/1743-0003-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman A, Maidan I, Bernad-Elazari H, et al. (2017). Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn, 115, 41–46. doi: 10.1016/j.bandc.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Mirelman A, Weiss A, Buchman AS, et al. (2014b). Association between performance on Timed Up and Go subtasks and mild cognitive impairment: further insights into the links between cognitive and motor function. J Am Geriatr Soc, 62(4), 673–678. doi: 10.1111/jgs.12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M, Verghese J, Beauchet O, et al. (2012). Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc, 60(11), 2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, et al. (2017). Association of Dual-Task Gait With Incident Dementia in Mild Cognitive Impairment: Results From the Gait and Brain Study. JAMA Neurol, 74(7), 857–865. doi: 10.1001/jamaneurol.2017.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Hunter SW, & Wittwer JE (2016). Dual-task testing to predict falls in communitydwelling older adults: a systematic review. Physiotherapy, 102(1), 29–40. doi: 10.1016/j.physio.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, et al. (2010). Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci U S A, 107(52), 22682–22686. doi: 10.1073/pnas.1012651108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Park M, Holtzer R, Xue X, et al. (2010). Conventional and robust quantitative gait norms in community-dwelling older adults. J Am Geriatr Soc, 58(8), 1512–1518. doi: 10.1111/j.1532-5415.2010.02962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, et al. (2006). Structure-function correlates of cognitive decline in aging. Cereb Cortex, 16(7), 907–915. doi: 10.1093/cercor/bhj036 [DOI] [PubMed] [Google Scholar]
- Pizzamiglio S, Naeem U, Abdalla H, et al. (2017). Neural Correlates of Single- and Dual-Task Walking in the Real World. Front Hum Neurosci, 11, 460. doi: 10.3389/fnhum.2017.00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, et al. (1997). Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex, 7(3), 268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, et al. (2005). Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex, 15(11), 1676–1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Reitan RM (1955). The relation of the trail making test to organic brain damage. J Consult Psychol, 19(5), 393–394. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, et al. (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage, 61(4), 1402–1418. doi : 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Katz K, Herman T, Jacob Y, et al. (2013). Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology, 50(16), 1476–1484. doi: 10.1212/WNL.0b013e31828cfaa4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Katz K, Herman T, Jacob Y, et al. (2016). Subcortical Volumes Differ in Parkinson’s Disease Motor Subtypes: New Insights into the Pathophysiology of Disparate Symptoms. Front Hum Neurosci, 10, 356. doi: 10.3389/fnhum.2016.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, & D’Esposito M (2002). The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci, 14(5), 721–731. doi: 10.1162/08989290260138627 [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, et al. (2004). Thinning of the cerebral cortex in aging. Cereb Cortex, 14(7), 721–730. doi: 10.1093/cercor/bhh032 [DOI] [PubMed] [Google Scholar]
- Sartor J, Bettecken K, Bernhard FP, et al. (2017). White Matter Changes-Related Gait and Executive Function Deficits: Associations with Age and Parkinson’s Disease. Front Aging Neurosci, 9, 213. doi: 10.3389/fnagi.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattin RW (1992). Falls among older persons: a public health perspective. Annu Rev Public Health, 13, 489–508. doi: 10.1146/annurev.pu.13.050192.002421 [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, et al. (2004). A hybrid approach to the skull stripping problem in MRI. Neuroimage, 22(3), 1060–1075. doi: 10.1016/j.neuroimage.2004.03.032 [DOI] [PubMed] [Google Scholar]
- Siejka TP, Srikanth VK, Hubbard RE, et al. (2018). Frailty and Cerebral Small Vessel Disease: A Cross-Sectional Analysis of the Tasmanian Study of Cognition and Gait (TASCOG). J Gerontol A Biol Sci Med Sci, 73(2), 255–260. doi: 10.1093/gerona/glx145 [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, & Evans AC (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging, 17(1), 87–97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23 Suppl 1, S208–219. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL . (1977). Neurosensory center comprehensive examination for aphasia: Manual of directions. Vicotria, BC, Canada: Neuropsychology Laboratory, University of Victoria. [Google Scholar]
- Springer S, Giladi N, Peretz C, et al. (2006). Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord, 21(7), 950–957. doi: 10.1002/mds.20848 [DOI] [PubMed] [Google Scholar]
- Srikanth V, Beare R, Blizzard L, et al. (2009). Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke, 40(1), 175–180. doi: 10.1161/STROKEAHA.108.524355 [DOI] [PubMed] [Google Scholar]
- Sui J, Huster R, Yu Q, et al. (2014). Function-structure associations of the brain: evidence from multimodal connectivity and covariance studies. Neuroimage, 102 Pt 1, 11–23. doi: 10.1016/j.neuroimage.2013.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney KT, Ayaz H, Ward TE, et al. (2011). A methodology for validating artifact removal techniques for fNIRS. Conf Proc IEEE Eng Med Biol Soc, 2011, 4943–4946. doi: 10.1109/IEMBS.2011.6091225 [DOI] [PubMed] [Google Scholar]
- Thaler NS, O’Rourke JJ, Scott JG, et al. (2014). Longitudinal stability of RBANS profiles in a geriatric community-dwelling sample. Clin Neuropsychol, 28(2), 269–280. doi: 10.1080/13854046.2014.884243 [DOI] [PubMed] [Google Scholar]
- Tinetti ME (2003). Clinical practice. Preventing falls in elderly persons. N Engl J Med, 348(1), 42–49. doi: 10.1056/NEJMcp020719 [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Baker DI, McAvay G, et al. (1994). A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med, 331(13), 821–827. doi: 10.1056/NEJM199409293311301 [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, et al. (2002). Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage, 17(2), 657–669. [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, et al. (2004). A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex, 14(9), 966–973. doi: 10.1093/cercor/bhh057 [DOI] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, et al. (2009). Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci, 64(8), 896–901. doi: 10.1093/gerona/glp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, et al. (2012). Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc, 60(10), 1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Ayers E, et al. (2017). Brain activation in high-functioning older adults and falls: Prospective cohort study. Neurology, 88(2), 191–197. doi: 10.1212/WNL.0000000000003421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorio R, Stuart S, Rochester L, et al. (2017). fNIRS response during walking - Artefact or cortical activity? A systematic review. Neurosci Biobehav Rev, 83, 160–172. doi : 10.1016/j.neubiorev.2017.10.002 [DOI] [PubMed] [Google Scholar]
- Walshe EA, Patterson MR, Commins S, et al. (2015). Dual-task and electrophysiological markers of executive cognitive processing in older adult gait and fall-risk. Front Hum Neurosci, 9, 200. doi: 10.3389/fnhum.2015.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ayaz H, Izzetoglu M, et al. (2017). Evaluation of light detector surface area for functional Near Infrared Spectroscopy. Comput Biol Med, 89, 68–75. doi : 10.1016/j.compbiomed.2017.07.019 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). Wechsler Adult Intelligence Scale-Revised. .New York: The Psychological Corporation. [Google Scholar]
- Whitman GT, Tang Y, Lin A, et al. (2001). A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology, 57(6), 990–994. [DOI] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, & Giladi N (2008). The role of executive function and attention in gait. Mov Disord, 23(3), 329–342; quiz 472. doi: 10.1002/mds.21720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, et al. (2007). Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging, 28(5), 784–798. doi : 10.1016/j.neurobiolaging.2006.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


