Abstract
The technology of transcranial focused ultrasound (FUS) enables a novel approach to neuromodulation, a tool for selective manipulation of brain function to be used in neurobiology research and with potential applications in clinical treatment. The method uses transcranial focused ultrasound to non-invasively open the blood-brain barrier (BBB) in a localized region such that a systemically injected neurotransmitter chemical can be delivered to the targeted brain site. The approach modulates the chemical signaling that occurs in and between neurons, making it complimentary to most other neuromodulation techniques that affect the electrical properties of neuronal activity. Here, we report delivering the inhibitory neurotransmitter GABA to the right somatosensory cortex of the rat brain during bilateral hind paw electrical stimulation and measure the inhibition of activation using functional MRI (fMRI). In a 2 × 2 factorial design, we evaluated conditions of BBB Closed vs BBB Open and No GABA vs GABA. Results from fMRI measurements of the blood oxygen level-dependent (BOLD) signal show: 1) intravenous GABA injection without FUS-mediated BBB opening does not have an effect on the BOLD response; 2) FUS-mediated BBB opening alone significantly alters the BOLD signal response to the stimulus, both in amplitude and shape of the time course; 3) the combination of FUS-mediated BBB opening and GABA injection further reduces the peak amplitude and spatial extent of the BOLD signal response to the stimulus. The data support the thesis that FUS-mediated opening of the BBB can be used to achieve non-invasive delivery of neuroactive substances for targeted manipulation of brain function.
Keywords: Blood-brain barrier, neuromodulation, Focused Ultrasound, Drug delivery, Functional MRI, brain, brain networks
Introduction
Neuromodulation refers to the selective activation or suppression of neuronal function in targeted brain regions and represents an important tool for both basic science research and clinical applications. A longstanding goal of neuroscience research has been to develop a neuromodulation approach that achieves spatial or cellular specificity for precise targeting of a particular brain region, but is still minimally invasive enough such that the procedure does not affect other brain regions and can be translated to human clinical application. Many approaches to neuromodulation have been developed, each with their own advantages and limitations (Luan et al., 2014). Direct injection of neuroactive agents and direct electrical stimulation have long been used in animal neuroscience research but are too invasive for most human applications. One exception is deep brain stimulation (DBS), which has found clinical utility as a last resort treatment of neurological disorders such as Parkinson’s disease, essential tremor, and major depressive disorder (Perlmutter and Mink, 2006). Transcranial magnetic stimulation (TMS) is another neuromodulation approach that is in clinical use (Hallett, 2000; Rossi et al., 2009), but its low spatial resolution limits its ability to achieve precise targeting. Most recently, optogenetics (Boyden et al., 2005; Deisseroth, 2011; Lee et al., 2010) and chemogenetics (Armbruster et al., 2007; Roth, 2016) have achieved great success as a way to induce cellular specific excitation or inhibition of action potential firing. However, these techniques both require genetic manipulation of cells in the targeted area which poses significant complications for human use.
Recently our group demonstrated a novel approach to neuromodulation based on targeted delivery of neurotransmitters to the brain via focused ultrasound (FUS) induced disruption of the blood-brain barrier (BBB) (McDannold et al., 2015). FUS disruption of the BBB works by combining the FUS pressure wave with intravenously injected microbubbles (Hynynen et al., 2001). The large pressure changes at the FUS focus cause the microbubbles to undergo a rapidly oscillating expansion and contraction in size known as stable cavitation which exerts forces on vessel walls. The exact mechanism responsible for opening the BBB is unknown, but studies have implicated both the presence of widened tight junctions between the endothelial cells and increases in active transport mechanisms (Aryal et al., 2014; Cho et al., 2016; Zhao et al., 2018). The BBB remains permeable for several hours such that systemically injected agents will leak out of the blood stream into the brain parenchyma at the targeted site. The FUS energy can be delivered through the intact skull, focused to a volume encompassing only few cubic millimeters in extent, and targeted to both cortical and subcortical regions. FUS-mediated BBB disruption has been used to deliver a variety of agents including antibodies, chemotherapeutic agents, liposomes carrying plasmid DNA, and neural stem cells (Aryal et al., 2014; Meairs, 2015). The neuromodulation work from our group demonstrated that delivery of the neurotransmitter γ-Aminobutyric acid (GABA) to the rat somatosensory cortex could suppress the response to sciatic nerve stimulation as measured by electrophysiology (McDannold et al., 2015). GABA is the primary inhibitory neurotransmitter in the brain and has been shown to not cross the BBB in rats (van Gelder and Elliott, 1958).This novel approach to neuromodulation has advantages and limitations that are complimentary to existing techniques.
This study uses fMRI measurements to demonstrate the effect that FUS-mediated delivery of GABA has on brain activity in the primary somatosensory cortex. FUS-mediated BBB opening was targeted to the hind limb region of the somatosensory cortex in the right hemisphere of Sprague-Dawley rats. Intravenous injections of GABA were given during fMRI sessions with simultaneous bilateral hind paw electrical stimulation. GABA is a naturally occurring neurotransmitter widely present in the brain and its inhibitory effects are well documented (McCormick, 1989). We hypothesized that the combination of FUS BBB opening and intravenous GABA injection would reduce the activation response to the hind limb stimulus in the right cortex only as measured by the blood oxygenation level-dependent (BOLD) signal. A 2 × 2 factorial design was used to test conditions of BBB Closed vs BBB Open and No GABA vs GABA. Effects due to BBB opening alone, GABA delivery alone, and combined BBB opening plus GABA delivery were compared against the baseline condition.
Methods
Study Design (see Figure 1)
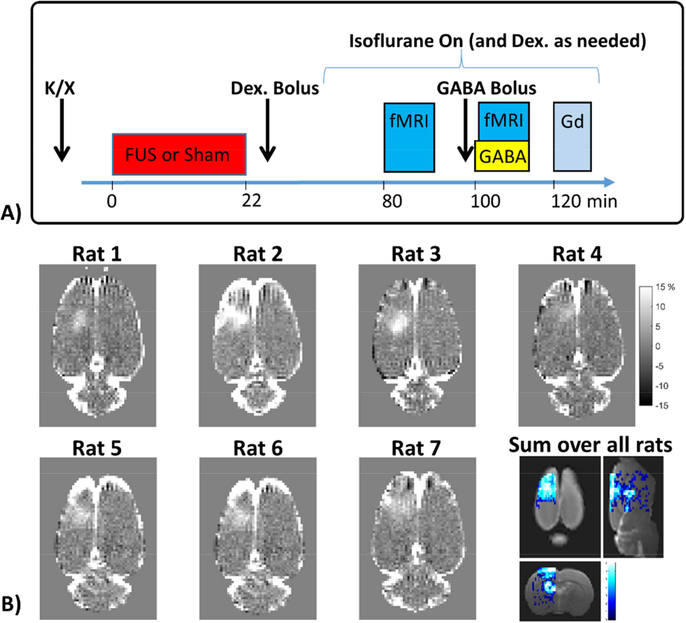
Figure 1: Experiment timing and BBB opening.
A) Timing of an experimental session, which consists of ketamine/xylazine injection, FUS sonications (for BBB Open cases) or Sham FUS (for BBB Closed cases), injection of the Dexdomitor bolus and a waiting period to transfer the rat to the fMRI imaging set up and allow for physiological stabilization, the fMRI runs without and with GABA injection, and finally gadolinium contrast imaging to assess BBB opening. B) T1-weighted contrast images (converted to percent difference) showing the BBB opening for each individual rat and a map of the sum of binary opening images over all rats overlaid on a T2w anatomical image.
The study was carried out in a 2 × 2 factorial design paradigm. The factors were BBB opening (BBB Open vs BBB Closed) and GABA delivery (No GABA vs GABA), giving four experimental conditions under which identical functional imaging tests were performed. Cases of BBB Open vs BBB Closed where done on separate days, separated by at least a week. Cases of No GABA vs GABA were done within the same session. An experimental session consisted of FUS sonications (for BBB Open) or sham FUS (for BBB Closed), fMRI data acquisition without GABA delivery, fMRI data acquisition with GABA delivery, and T1-weighted contrast imaging (for BBB Open cases only). The timing is shown schematically in Figure 1A. Bilateral hindpaw stimulation was used to produce activation in both the left and right S1 hind limb cortical areas. FUS BBB opening was targeted to the right hemisphere only, thereby allowing the left S1 region to act as an internal control. A total of 17 experimental sessions were conducted on nine rats (9 with BBB Closed, 8 with BBB Open). Data from three experimental sessions were discarded due to unstable physiological conditions (see Discussion for further explanation), leaving N=7 data sets for each of the four conditions. Five of the rats underwent both BBB Open and BBB Closed experiment days, two rats underwent only BBB Open experiments, and two rats underwent only BBB Closed experiments. All imaging experiments were performed in a 7 Tesla Bruker BioSpec small animal MRI scanner (Bruker Corp., Billerica, MA, USA).
Animal Preparation
All experiments were done in accordance with procedures approved by the Brigham and Women’s Hospital Institutional Animal Care and Use Committee. The animals were housed, fed, and watered according to the Office of Laboratory Animal Welfare and the Association for Assessment and Accreditation of Laboratory Care regulations. Male Sprague Dawley rats were used for all experiments (257 – 384 g). At the start of each experiment, the rats were anesthetized with one dose of ketamine and xylazine (80 mg/kg / 10 mg/kg). The head of the rat was shaved and treated with depilatory cream to remove all fur for optimal ultrasound coupling. A tail vein catheter was placed for administration of the GABA and MRI contrast agent.
FUS Blood-Brain Barrier Opening
The rat’s head was fixed in a custom made holder that slots into the bed of an in-house made MRI-compatible FUS system. The FUS system consists of a 690 kHz single element focused ultrasound transducer (3.0 cm diameter, 3.5 cm radius of curvature), a single element passive cavitation detector, a three-axis manual positioning system, and a transmit/receive MRI receive coil. The rat was coupled to the ultrasound transducer by placing it upside down with its head in a bath of degassed and deionized water. The FUS sonications were applied using a function generator (33220A, Agilent), amplifier (240L, E&I) and custom Matlab user interface.
Targeting of FUS energy to the right hindlimb somatosensory cortex was done in two steps. First, a ten second FUS sonication was applied to a silicone gel phantom, creating a hotspot detectable by MRI temperature-sensitive imaging which provided the location of the FUS focal point in the MRI coordinate system. Then, the rat was placed on the system and structural imaging was performed to allow visual identification of the right S1 in MRI coordinates. The FUS transducer was moved such that the center of the focus would lie at the location of the right S1.
To achieve BBB opening, repeated bursts of FUS sonications were applied immediately after injection of microbubbles (200 μL/kg bolus injection of Optison, GE Healthcare). Sonication parameters were 10 ms bursts, 1 Hz repetition frequency, and 120 repeats. Five sets of sonications were applied over a 2 × 2 mm square (4 corners and one in the middle) to ensure coverage of the right S1 hind limb region. Applied FUS power levels were fixed for each rat at either 0.32 or 0.34 MPa (peak negative pressure calibrated in water). A warming blanket was used throughout the BBB opening process to maintain body temperature and the rat’s breathing was continuously monitored. T1-weighted contrast images in Figure 1B show the extent and location of BBB opening in each individual rat and a map showing the spatial overlap in BBB opening over all rats.
At the power levels used here, the BBB re-closes on the order of a few hours (Park et al., 2012) and any changes to the local tissue are fully resolved in a day or two. We therefore varied the order in which the BBB Closed vs BBB Open experimental days were done. Four of the rats underwent BBB Closed experimental sessions first, and three of the rats underwent BBB Open experimental sessions first, followed by a BBB Closed experimental session a week or more later. The order of the fMRI runs (No GABA vs GABA) was not similarly permuted as it was not known how long the GABA would remain in the system, and therefore the No GABA runs were always done first.
Functional MRI
After BBB opening, the rat was removed from the FUS system and set up in the conventional Bruker animal holder. The rat was laid prone and its nose was inserted into a nose cone for head immobilization and delivery of isoflurane and oxygen. A line was inserted into the rat’s abdomen for subcutaneous injection of Dexdomitor (Dexmedetomidine; Orion, Espoo, Finland) (Adamczak et al., 2010) and a 2 cm diameter surface coil was placed over the rat’s head. A warming blanket was used to maintain body temperature and breathing rate was monitored throughout. Once the rat was set up, a bolus injection of Dexdomitor was given (0.025 mg/kg) and delivery of 0.25% isoflurane in 60% oxygen was started. This low level of isoflurane was kept constant throughout the remainder of the experiment, and periodic infusions of Dexdomitor were given as needed to keep the rat in a stable physiological state. This combination of medetomidine and light isoflurane has been shown to be beneficial for functional imaging studies of sedated animals (Brynildsen et al., 2017).
Bilateral activation of the hind limb region of the somatosensory cortex was achieved by electrical stimulation of the rat’s hind paws. Pairs of 30 gauge needles were inserted into the pads of the second and fourth digits on each hind paw. Electrical pulses of 300 ms duration were delivered at 6 Hz using a TENS unit (TU 7000, Tensunits.com, Largo, Florida) connected to an in-house built circuit that synchronized the stimulus to the MR data acquisition. The voltages in each branch of the circuit going to the two hind paws were measured on an oscilloscope and adjusted to have equal values of 700 mV, corresponding to approximately 2 mA of current to each hind paw. The stimuli were repeated in 1 minute blocks of 40.5 second off, 19.5 second on.
All fMRI data were acquired with a 2D single-shot gradient echo echo-planar imaging (EPI) sequence. Sequence parameters were: 3.2 × 3.2 cm field of view; 64 × 64 × 18 imaging matrix; 0.5 × 0.5 × 1.2 mm resolution; 18 1.0 mm slices with 0.2 mm slice gap; TR = 1500 ms; TE = 18 ms; four dummy scans prior to data acquisition; 300 image volumes acquired in 7 minutes and 30 seconds. For the first fMRI run, no GABA was injected. For the second fMRI run, a total of 100 mg/kg of GABA was delivered intravenously: a bolus of GABA was injected immediately prior to imaging (50 mg/kg GABA in 0.2 ml saline solution) and a continuous infusion of GABA was injected throughout the duration of scanning (50 mg/kg in 0.2 ml volume infused over 8 minutes). The scan was started shortly after the onset of the GABA infusion. Prior to the fMRI runs, main field homogeneity was optimized using the Bruker MAPSHIM protocol and an anatomical image was acquired with a T2-weighted RARE sequence (0.3 × 0.3 × 0.5 mm resolution; 60 slices with no slice gap; TR = 6500 ms; TE = 50 ms). At the conclusion of the fMRI runs, the extent of BBB open was assessed by contrast images acquired with a T1-weighted RARE sequence (0.3 × 0.3 × 0.6 mm resolution; 18 slices with 0.4 mm slice gap; TR = 609 ms; TE = 18 ms) before and after injection of gadolinium (Magnevist, 0.25 mL/kg). The contrast images were converted to percent signal change and co-registered to the anatomical image and normalized into template space (as described below).
Data Analysis
All fMRI images were pre-processed using SPM12 (SPM12, 2014) and custom Matlab scripts. The T2-weighted anatomical image was segmented in SPM12 using the template of Valdes-Hernández et al (Valdés-Hernández et al., 2011). The EPI images were realigned, co-registered to the anatomical image, normalized to the template space, and spatially smoothed with a Gaussian filter of 0.8 × 0.8 × 0.8 mm FWHM. To assess the location and extent of stimulus induced activation, the data were analyzed in terms of percent change in the BOLD signal.
To extract the percent change in the BOLD signal, the data sets were further processed with a temporal highpass filter (0.008 Hz) and both the motion traces and the average time signal from a white matter mask were regressed out. This ensured removal of signal changes that may be due to head motion instead of activation. The signal was converted into percent change from the non-stimulus baseline and averaged over stimulation blocks to produce an average signal over the one minute on/off stimulation block.
Two metrics were calculated to assess the extent of activation in the left and right S1 hind limb regions. The first metric calculated the average BOLD signal percent change over a spatial region of interest (ROI) at each time point in the stimulation block. An ellipsoidal cylinder shape was used for the ROI with dimensions, 1.75 × 2.5 mm in the cortical plane and 2.25 mm through the cortex. The location in the through-cortical plan direction was fixed to cover the entire depth of the cortex. The location in the cortical plane was determined using each individual rat’s peak of activation, as defined by the center-of-mass of the BOLD signal change 8 seconds into the stimulation block. Locations of the individual ROIs did not vary more than [+/− 0.5 mm, +/− 0.5 mm] over all data sets. The data was spatially averaged over these ROIs and the plots are presented as the mean +/−standard error over the seven rats.
The second metric used a larger ROI (3.5 × 4.5 × 3.25 mm) to cover the entire extent of any possible activation in the S1 region and counted the number of voxels within that ROI that exceed a 0.5% signal change at each time point over the one minute stimulation block. These results are also plotted as the mean +/− standard error over the seven rats. To determine the presence of significant differences in activation between two particular experimental conditions, single tailed t-tests were done for each metric at all time points.
Histology
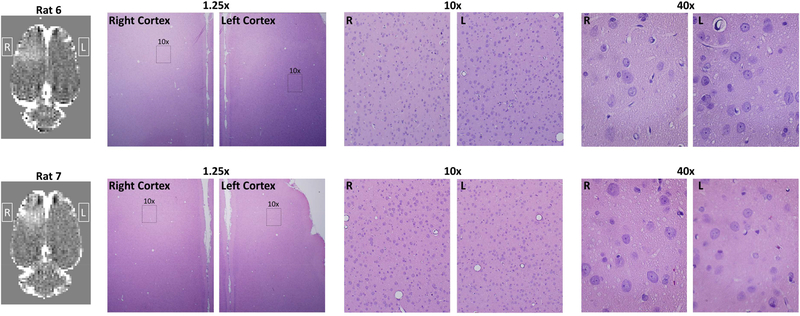
Histology was performed on two of the rats that underwent BBB opening to assess if the FUS sonications induced any damage to neuronal cells or other local tissue. One rat was sacrificed one week after FUS sonications and one rat was sacrificed 24 hours after FUS sonications. The brains of both rats were perfused with saline solution (100 mL, 0.9% NaCl) and 10% buffered formalin (100 mL), fixed in 10% buffered formalin phosphate, and cut into axial blocks embedded in paraffin. The blocks were sectioned into 5 μm thick slices and stained with Haematoxylin and Eosin (H&E).
Results
Summary
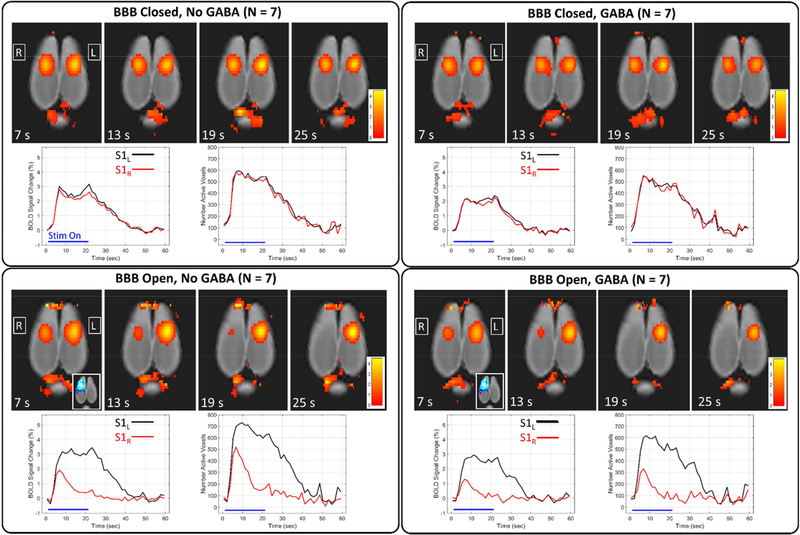
A summary of results over the four conditions is shown in Figure 2. Maps of percent change in the BOLD signal are shown at four time points of the stimulation block along with plots of the BOLD signal change and the number of active voxels in the left S1 (black) and right S1 (red). For the two cases when the BBB is closed, the activations in the left and right S1 are almost identical throughout the stimulation block. For the cases when the BBB is open, there is an obvious decrease in activation in the right S1 region, which was targeted for BBB opening. The decrease in activation is most pronounced in the second half of the time period that the stimulus is on. Activation in the left S1 region, which was not targeted for BBB opening, does not appear to be affected.
Figure 2: Summary of results over the four experimental conditions.
Maps of BOLD signal percent change are shown at four different time points for each of the four experimental conditions. Corresponding plots of BOLD signal change and number of active voxels are shown for the left and right S1 regions (mean over all rats).
GABA-only Effects
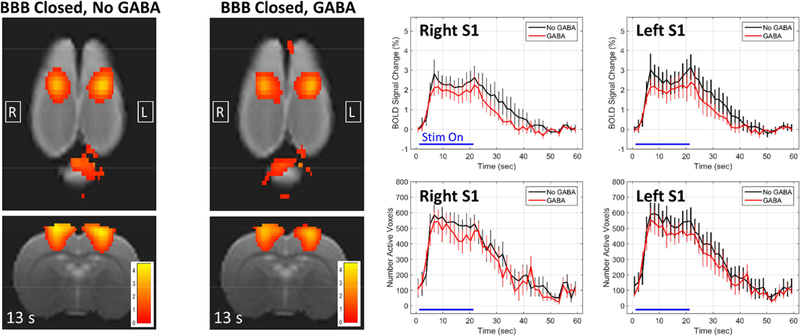
The effects of GABA being delivered when the BBB is closed are shown in Figure 3. Here, the plots are comparing the BBB Closed, No GABA condition (black) against the BBB Closed, GABA condition (red), with the right and left S1 regions shown in different subfigures. The mean BOLD signal change appears to be slightly systemically lower throughout the stimulation block for the condition when GABA is delivered. The difference in the number of activated voxels is not as evident. However, there were no significant differences between the No GABA case and the GABA case at any time point along the stimulation block, either for the percent change in the BOLD signal or the number of active voxels. This was true for both the left and right S1 regions. When the BBB is closed, the total dose of 100 mg/kg of GABA delivered in these experiments does not appear to have a significant effect on activation in response to the stimulus.
Figure 3: Effects of GABA when the BBB is closed.
Maps of BOLD signal change at 13 seconds into the stimulation block are shown for the two conditions of BBB Closed, No GABA and BBB Closed, GABA. The plots compare the BBB Closed, No GABA condition (black) against the BBB Closed, GABA condition (red) for the right and left S1 regions (mean +/− standard error). No significant differences exist at any time point for either the BOLD change or the number of active voxels in either the right or left S1.
BBB Open-only Effects
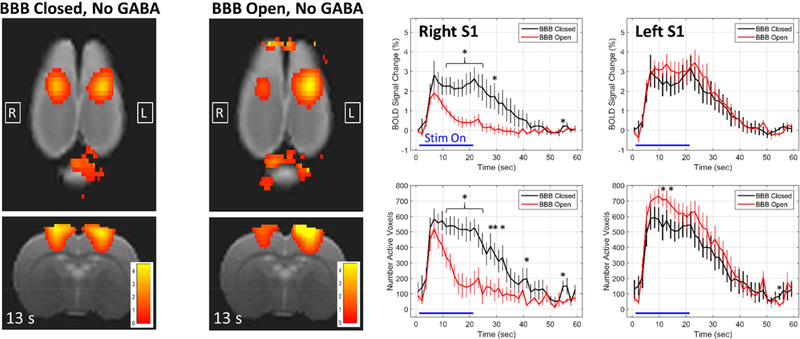
Figure 4 assesses the impact on the BOLD signal response to activation when the BBB has been opened but no GABA has been delivered. BOLD signal change maps and plots are shown in Figure 4, this time comparing the BBB Closed, No GABA condition (black) against the BBB Open, No GABA condition (red). The right and left S1 regions are again plotted in different subfigures. There is an obvious decrease in activation seen in the right S1 when the BBB is open. The BOLD signal change rises at the same rate for the two conditions, but the BBB open condition has a lower peak and returns towards baseline even while the stimulus is still on. The differences in the BOLD signal change at the peak of activation around 7 seconds are not quite significantly different, but the values in the latter half of the stimulus on time period are clearly significantly different. Interestingly, the BOLD change in the left S1 region appears to be slightly larger for the BBB Open case, but only rises to the level of significance at two time points for the metric measuring the number of active voxels. FUS-mediated opening of the BBB, even without delivering GABA, clearly has an effect on the BOLD signal response to stimulation.
Figure 4: Changes in the BOLD signal response due to BBB opening alone.
Maps of BOLD signal change at 13 seconds into the stimulation block are shown for the two conditions of BBB Closed, No GABA and BBB Open, No GABA. The plots compare the BBB Closed, No GABA condition (black) against the BBB Open, No GABA condition (red) for the right and left S1 regions (mean +/− standard error). * indicates time points at which the two conditions are significantly different (p < 0.05).
Histological Evaluation
In order to evaluate potential damage to the brain tissue, histological evaluation of the relevant brain regions was performed. Histology results from the H&E stained brain slices of two rats are shown in Figure 5. The only abnormality found in either of the two rats was one instance of what was most likely a hemosiderin particle. No instances of red blood cell extravasation were seen, which can occur when higher power FUS sonications rupture capillary blood vessels, and no damage to neuronal cells was seen. It does not appear that damage to local tissue is the cause of the reduced BOLD signal changes seen in the BBB Open, No GABA case.
Figure 5: Histology after BBB opening.
The two row shows the T1w contrast difference image and hematoxylin and eosin staining from two different rats. Regions from the right and left somatosensory cortex are shown at 1.25x, 10x, and 40x magnification. The 10x and 40x images of the right cortex where taken from a region that displayed hyperintensity on the T1w contrast difference images. No signs of damage to neuronal cells were seen in either rat.
Another indication that no lasting damage was done to the neurons comes from comparing the BBB Closed experiments done without any previous BBB opening (N=4) against those done after a previous BBB Open experiment (N=3). While the number of data sets is not sufficient for reliable statistics, the two metrics of BOLD signal change and number of active voxels in the right S1 do not show any clear differences between the two cases.
Effects of Combined BBB Opening and GABA Delivery
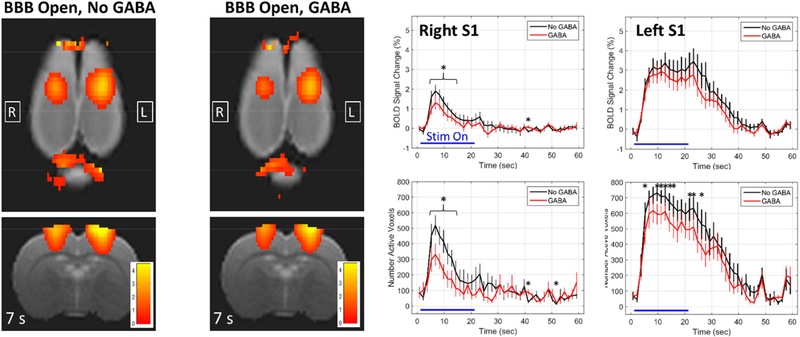
The effects of combining BBB opening with GABA delivery are shown in Figure 6. The BOLD change maps are shown at 7 seconds into the stimulation block, near the peak of activation. The plots are comparing the BBB Open, No GABA condition (black) against the BBB Open, GABA condition (red), for the right and left S1 regions. In the right S1, where the BBB was opened, there is a significant reduction in the peak of activation when GABA is delivered. The significant differences in both the BOLD signal change and number of active voxels are seen towards the beginning of the stimulus, when the BOLD change is reaching its peak. No differences are seen later in the stimulation block as the signal returns towards baseline. For the left S1, where BBB opening was not targeted, there also appears to be a slight reduction in the level of activation. Although, it is only significant in terms of the number of active voxels. These results indicate that FUS-mediated opening of the BBB alters the BOLD response to stimulation, and delivery of GABA further reduces the activation.
Figure 6: Effects of BBB opening combined with GABA delivery.
Maps of BOLD signal change at 7 seconds into the stimulation block are shown for the two conditions of BBB Open, No GABA and BBB Open, GABA. The plots compare the BBB Open, No GABA condition (black) against the BBB Open, GABA condition (red) for the right and left S1 regions (mean +/− standard error). * indicates time points at which the two conditions are significantly different (p < 0.05).
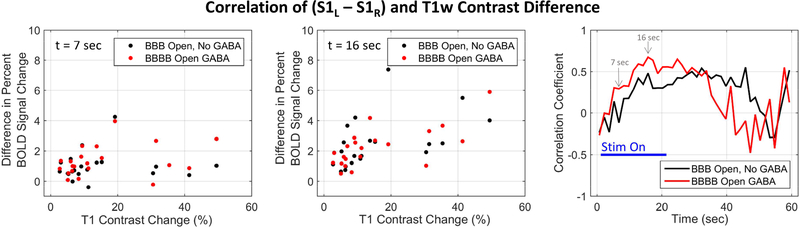
As can be seen in Figure 1, the extent of BBB opening achieved in the different rats varied quite a bit. Results shown in Figure 7 assesses the extent to which the level of BBB opening affected the changes in the BOLD response shown above. The effect on the BOLD response was measured as the difference in the BOLD signal change between the left and right S1 regions (S1L – S1R) in each individual rat. When the BBB was closed, this difference was essentially zero, as shown in Figure 2. The extent of BBB opening was measured as the mean T1-weigthed contrast image percent change over the same spatial ROI used for calculating the BOLD signal change. A correlation coefficient was calculated over the 7 rats between these two values at each time point of the stimulation block, and the results are plotted in Figure 7. Each ROI was split into three layers at different cortical depths to better capture the variation in contrast difference in that direction, resulting in 21 total data points for the correlation. The positive correlation values seen indicate that a larger difference in BOLD signal change between the left and right S1 regions is correlated with more extensive BBB opening as measured by the contrast imaging. The correlations are strongest during the latter half of the stimulation on period, and slightly stronger for the case where GABA is delivered.
Figure 7.
Correlation of GABA effects with extent of BBB opening. The difference in BOLD change values between the left S1 and right S1 regions were calculated for each individual rat. These values were correlated with the extent of BBB opening seen in each individual rat, as measured by the mean gadolinium percent change in the right S1 ROI. The first two panels show scatter plots of the data at two different time points (t = 7 seconds and t = 16 seconds). The third panel shows a plot of correlation values for each time point in the stimulation block. Strong positive correlation indicates that lower activation seen in the right S1 compared to the left S1 was associated with stronger BBB opening.
Discussion
This study presents further demonstration and validation of a novel approach to neuromodulation. Unlike most neuromodulation techniques that affect the electrical properties of neuronal cells to induce or inhibit action potential firing, our approach targets the chemical signaling that occurs at synaptic junctions between neurons. This is achieved by delivering additional amounts of neurotransmitter chemicals that are naturally present in the brain using FUS-mediated disruption of the BBB. The first paper on this approach demonstrated that GABA delivered to the rat cortex suppressed somatosensory-evoked potentials in response to electrical stimulation of the sciatic nerve as measured by electrophysiology recordings (McDannold et al., 2015). Here we used fMRI to measure the BOLD response to hind paw electrical stimulation under a baseline condition and conditions of GABA injection alone, BBB opening alone, and combined BBB opening and GABA injection.
FUS-mediated opening of the BBB had an effect on the BOLD signal by itself, as previously reported by Chu et al. (Chu et al., 2015). In their study, they did not see the effect at the FUS power level of 0.2 MPa (0.3 mechanical index); at the higher power of 0.35 MPa (0.55 mechanical index), there was a significant decrease in BOLD signal at one hour post-sonication that returned to baseline levels by 24 hours, but no significant change in the somatosensory evoked potential P1 amplitude. The FUS power levels used in this study were 0.32 MPa and 0.34 MPa (0.39 and 0.41 mechanical index). Based on the Chu et al. study, these FUS power levels would be expected to alter the BOLD signal but not neuronal activity as measured by evoked potentials. In our study, the effects of FUS BBB opening on the BOLD signal are characterized by a slightly lower peak amplitude (although not statistically significant) and a very prominent return towards baseline that occurs about 7 seconds after the stimulus onset and while the stimulus is still on. The underlying cause of the effect is not known, but the histology results indicate that damage to local tissue can be ruled out. It could be any one of the numerous factors that influence the BOLD signal, including neuronal activity, oxygen consumption, neurovascular coupling signaling, blood flow dynamics, and local blood volume. FUS BBB opening has been shown to cause vascular constriction (Raymond et al., 2007), however the measured effect only lasts a few minutes after the FUS sonications and the BOLD data acquired for this study was taken more than an hour after sonication. We hypothesize that the stress of FUS BBB opening on the vessels reduces their ability to react to the signaling mechanisms of neurovascular coupling which alters the regular blood flow response to a stimulus. Further studies will be carried out to fully understand the underlying causes.
The strategy of combing FUS BBB opening with intravenous injection of GABA was successful in further reducing the BOLD response to the external stimulus. Because the BBB has been opened, the BOLD signal time course exhibits the same rapid return to baseline soon after the onset of the stimulus as was seen in the BBB Open, No GABA case. However, there is a statistically significant reduction in the BOLD peak amplitude when the GABA is delivered compared to when it is not. The effect is primarily confined to the right S1HL region where the BBB opening was targeted. No additional areas of activation were seen outside of the left and right S1 regions, however there is some evidence that the left S1HL may be effected. The percent change in the BOLD signal in the left S1HL was slightly lower than the BBB Open, No GABA case, but the difference was not statistically significant. The extent of activation in the left S1HL, as measured by the number of active voxels, was decreased by the GABA delivery to a degree that reached statistical significance during the second half of the stimulus on period. It is unlikely that the GABA diffused across hemispheres to have a direct effect in the left S1HL region. It is possible that it entered the subarachnoid space or ventricular system and was circulated to the other hemisphere along with the cerebral spinal fluid. If the effect is real, another possibility is that it originates from the known functional connectivity that exists between the two regions.
The GABA was delivered in a total of 0.4 ml of saline solution, representing about 2% of the rat’s total blood volume. To make sure that the GABA delivery effect was not due to this change in the total blood volume, we performed an additional fMRI run on four of the rats where the same volume of saline-only solution was injected. The BOLD signal time courses for the saline-only runs do not show any clear differences compared to the No GABA runs. In particular, when the BBB is open there is no decrease in the BOLD peak amplitude in the right S1HL when saline-only is delivered.
Neuronal activity in the cortex is primarily mediated by a balance between excitatory glutamatergic neurons and inhibitory GABAergic interneurons. A number of different studies have shown that changes in GABA concentration in the cortex has an effect on the functional response to a stimulus, as measured by fMRI. The intrinsic level of GABA concentration in the cortex has been shown to be negatively correlated with the amplitude of the BOLD signal change in humans (Donahue et al., 2010; Muthukumaraswamy et al., 2009). It has also been shown in both rats and humans that BOLD signal responses can be manipulated by drugs such Lorazepam, Vigabatrin or gabaculine that are known to act as GABA receptor agonists or antagonists (Chen et al., 2005; Northoff et al., 2002).
One possible confounding factor is that vessels contain GABA receptors and GABA agonists and antagonists have been shown to be able to modulate vessel tone. For example, GABA and the GABAA receptor agonist, muscimol, elicited vasodilation in an in vitro preparation of rat microvessels (Fergus and Lee, 1997) and GABA and muscimol produced increases in cerebral blood flow in unanesthetized goat experiments (Alborch et al., 1984). An increase in baseline blood flow should not have had an effect on the evoked potential measurements that were performed in the original wok on delivering GABA for neuromodulation (McDannold et al., 2105). However, changes in baseline flow can effect stimulus-evoked fMRI experiments in complicated ways. A minor dilation should not affect additional changes in blood flow elicited by the stimulus. However if the dilation due to GABA is too great, then it is possible that the vessel will have reached a limit beyond which it cannot dilate further and then the hemodynamic response to a stimulus will be diminished.
The approach of FUS-mediated disruption of the BBB for targeted delivery of neurotransmitters has several qualities that it make an attractive option to use as a neuromodulation technique in neurobiology research. The non-invasive opening on the BBB leaves the skull intact and does not damage brain tissue, either at the targeted site or in intervening regions. This is an advantage over direct microinjections of neurotransmitters. The spatial specificity achievable with the method is on the order of a few millimeters, which is moderate. It is better than what is currently achievable with a non-invasive technique such as TMS, but not as good as invasive techniques like optogenetics or direct electrical stimulation. In a rat brain the spatial specificity is small enough to confine the effects to one functional region of the cortex but not small enough to pick out particular sub-cortical structures. In non-human primates and humans, this spatial specificity would be small enough to confine the effects to single cortical and many sub-cortical regions. Another advantage of the method is the flexibility of the targeting. FUS BBB opening can target anywhere in the brain and can target small, large, or multiple areas. The method also offers some cellular specificity in that the response will be confined to cells that express receptors for the particular neurotransmitter delivered. Finally, the method can be used together with other existing neuromodulation techniques as the effect of altering the level of local neurotransmitter concentration is complimentary to most neuromodulation techniques that act on the electrical properties of neuronal firing.
There are also several limitations to the technique, some that are inherent and some that could be improved with future development. The slow time dynamics of the neurotransmitter effects is inherent to the method. The onset and duration of the neuromodulation will be determined by the wash in and wash out characteristics of the neurotransmitter being delivered. The initial arrival time is only seconds after injection, but it is not clear how the concentration of the neurotransmitter at the targeted site changes over time and how long it takes before returning to normal levels. This will depend on efflux mechanisms, reuptake systems, and diffusion properties. Another challenge is delivering a consistent amount of the neurotransmitter into the brain. This will depend primarily on the level of BBB disruption achieved during the FUS sonications, which is known to vary considerably from animal to animal even when fixed FUS parameters are used (Wu et al., 2016). Methods for more consistent dosing are currently under investigation (Sun et al., 2017). MRI was used to achieve the best targeting of the FUS focus in this study, which may not be available or cost effective for all studies. It is possible to perform targeting without MRI, using only external anatomical landmarks, a stereotactic frame, and a brain atlas.
One potential limitation that requires more investigation is related to the appropriate level of GABA dosing. During three of the 17 experimental sessions, the administration of GABA caused the onset of very rapid breathing in the rat, from a baseline of 40 to 60 breathes per minute to as high as 120 breathes per minute. These were the data sets that were excluded from the analysis. The breathing rate eventually returned to baseline after the level of Isoflurane was increased and several minutes passed. This GABA-induced onset of rapid breathing occurred twice in rats where the BBB was closed and once in a rat where the BBB was open. It is therefore not thought to be related to the opening of the BBB. Prior to this study, 51 experimental sessions were carried out as part of refining the GABA delivery technique. These were done with varying levels of FUS power, anesthetic regimes, GABA levels, and stimulus types. Of these 51 sessions, 9 had occurrences of a moderate GABA-induced increase in breathing rate on the order of 10 breathes per minute and 2 had occurrences of severe GABA-induced increase in breathing rate similar to the discarded experimental sessions from this study. The amount of GABA delivered during these session varied from 25 mg/kg to 200 mg/kg. It is not clear if the effect is GABA dose related, anesthesia-state related, or due to some other cause.
In addition to basic science research applications, the ability to non-invasively deliver neurotransmitter chemical to a targeted location in the brain could be used for treatment of certain neurological diseases. A transcranial FUS medical device currently has regulatory approval in the United States, Canada, Europe, and Asia (InSightec LTD, Tirat Carmel, Israel) and FUS-mediated disruption of the BBB is currently being used in a human clinical trial at Sunnybrook Health Sciences Centre (Toronto, Canada) to deliver chemotherapeutic drugs to brain tumor patients. The problem of transmitting FUS energy through the human skull has been solved practically (Hynynen and Jolesz, 1998) and the low levels of FUS energy required for BBB disruption (as opposed to ablation) make targeting almost anywhere in the brain feasible and safe (Downs et al., 2015; Kobus et al., 2016; McDannold et al., 2012). Neurological disorders such as chronic pain or depression that are characterized by changes in neuronal function and reorganization of functional networks would be candidates for treatment. Similar to TMS treatments for depression, the targeted delivery of a neurotransmitter to the right region of the brain could help to drive the diseased state back towards normalized function. The biggest challenge will be adequate dosing as it is not clear how many FUS BBB opening sessions would be required for the treatment to have an effect. One solution would be to use encapsulated delivery of the agent such that it is delivered in one session and released over an extended period of time, as has been done with other treatment applications (Timbie et al., 2015).
Acknowledgements
The research was supported by NIH grants R25CA089017–13, K01EB023983 and P01CA17464501.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare that they have no financial or non-financial competing interests related to this work.
References
- Adamczak JM, Farr TD, Seehafer JU, Kalthoff D, Hoehn M, 2010. High field BOLD response to forepaw stimulation in the mouse. Neuroimage 51, 704–712. 10.1016/j.neuroimage.2010.02.083 [DOI] [PubMed] [Google Scholar]
- Alborch E, Torregrosa G, Terrasa JC, Estrada C, 1984. GABA receptors mediate cerebral vasodilation in the unanesthetized goat. Brain Res. 321, 103–110. 10.1016/0006-8993(84)90685-1 [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, 2007. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci 104, 5163–5168. 10.1073/pnas.0700293104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal M, Arvanitis CD, Alexander PM, McDannold N, 2014. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev 72, 94–109. 10.1016/j.addr.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K, 2005. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci 8, 1263–8. 10.1038/nn1525 [DOI] [PubMed] [Google Scholar]
- Brynildsen JK, Hsu LM, Ross TJ, Stein EA, Yang Y, Lu H, 2017. Physiological characterization of a robust survival rodent fMRI method. Magn. Reson. Imaging 35, 54–60. 10.1016/j.mri.2016.08.010 [DOI] [PubMed] [Google Scholar]
- Chen Z, Silva AC, Yang J, Shen J, 2005. Elevated endogenous GABA level correlates with decreased fMRI signals in the rat brain during acute inhibition of GABA transaminase. J. Neurosci. Res 79, 383–391. 10.1002/jnr.20364 [DOI] [PubMed] [Google Scholar]
- Cho HS, Lee HY, Han M, Choi JR, Ahn S, Lee T, Chang Y, Park J, 2016. Localized Down-regulation of P-glycoprotein by Focused Ultrasound and Microbubbles induced Blood-Brain Barrier Disruption in Rat Brain. Sci. Rep 6 10.1038/srep31201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu P-C, Liu H-L, Lai H-Y, Lin C-Y, Tsai H-C, Pei Y-C, 2015. Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening. Sci. Rep 5, 15477 10.1038/srep15477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, 2011. Optogenetics. Nat. Methods 8, 26–29. 10.1038/nmeth.f.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Near J, Blicher JU, Jezzard P, 2010. Baseline GABA concentration and fMRI response. Neuroimage 53, 392–398. 10.1016/j.neuroimage.2010.07.017 [DOI] [PubMed] [Google Scholar]
- Downs ME, Buch A, Karakatsani ME, Konofagou EE, Ferrera VP, 2015. Blood-Brain Barrier Opening in Behaving Non-Human Primates via Focused Ultrasound with Systemically Administered Microbubbles. Sci. Rep 5, 15076 10.1038/srep15076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergus A, Lee KS, 1997. GABAergic regulation of cerebral microvascular tone in the rat. J. Cereb. Blood Flow Metab 17, 992–1003. 10.1097/00004647-199709000-00009 [DOI] [PubMed] [Google Scholar]
- Hallett M, 2000. Transcranial magnetic stimulation and the human brain. Nature 406, 147–150. 10.1038/35018000 [DOI] [PubMed] [Google Scholar]
- Hynynen K, Jolesz FA, 1998. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol 24, 275–283. 10.1016/S0301-5629(97)00269-X [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA, 2001. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220, 640–6. 10.1148/radiol.2202001804 [DOI] [PubMed] [Google Scholar]
- Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, McDannold N, 2016. Safety Validation of Repeated Blood-Brain Barrier Disruption Using Focused Ultrasound. Ultrasound Med. Biol 42, 481–492. 10.1016/j.ultrasmedbio.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim D-S, Fenno LE, Ramakrishnan C, Deisseroth K, 2010. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792. 10.1038/nature09108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S, Williams I, Nikolic K, Constandinou TG, 2014. Neuromodulation : present and emerging methods. Front. Neuroengineering 7, 1–9. 10.3389/fneng.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, 1989. GABA as an inhibitory neurotransmitter in human cerebral cortex. J. Neurophysiol 62, 1018–1027. [DOI] [PubMed] [Google Scholar]
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS, 2012. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res. 72, 3652–3663. 10.1158/0008-5472.CAN-12-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Zhang Y, Power C, Arvanitis CD, Vykhodtseva N, Livingstone M, 2015. Targeted, noninvasive blockade of cortical neuronal activity. Sci. Rep 5, 16253 10.1038/srep16253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meairs S, 2015. Facilitation of Drug Transport across the Blood-Brain Barrier with Ultrasound and Microbubbles. Pharmaceutics 7, 275–93. 10.3390/pharmaceutics7030275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD, 2009. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci 106, 8356–8361. 10.1073/pnas.0900728106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Witze T, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, Kaulisch T, Tempelmann C, Heinzel A, Kötter R, Hagner T, Barge B, Hinrichs H, Bogerts B, Scheich H, Heinze H-J, 2002. GABA-ergic Modulation of Prefrontal Spatio-temporal Activation Pattern during Emotional Processing: A Combined fMRI/MEG Study with Placebo and Lorazepam. J. Cogn. Neurosci 14, 348–370. 10.1162/089892902317361895 [DOI] [PubMed] [Google Scholar]
- Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ, 2012. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J. Control. Release 162, 134–42. 10.1016/j.jconrel.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW, 2006. DEEP BRAIN STIMULATION. Annu. Rev. Neurosci 29, 229–257. 10.1146/annurev.neuro.29.051605.112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SB, Skoch J, Hynynen K, Bacskai BJ, 2007. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J. Cereb. Blood Flow Metab 27, 393–403. 10.1038/sj.jcbfm.9600336 [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Avanzini G, Bestmann S, Berardelli A, Brewer C, Canli T, Cantello R, Chen R, Classen J, Demitrack M, Di Lazzaro V, Epstein CM, George MS, Fregni F, Ilmoniemi R, Jalinous R, Karp B, Lefaucheur JP, Lisanby S, Meunier S, Miniussi C, Miranda P, Padberg F, Paulus W, Peterchev A, Porteri C, Provost M, Quartarone A, Rotenberg A, Rothwell J, Ruohonen J, Siebner H, Thut G, Valls-Solè J, Walsh V, Ugawa Y, Zangen A, Ziemann U, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, 2016. DREADDs for Neuroscientists. Neuron 89, 683–694. 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPM12, 2014. SPM12 Framework [WWW Document].
- Sun T, Zhang Y, Power C, Alexander PM, Sutton JT, Aryal M, Vykhodtseva N, Miller EL, McDannold NJ, 2017. Closed-loop control of targeted ultrasound drug delivery across the blood–brain/tumor barriers in a rat glioma model. Proc. Natl. Acad. Sci 114, E10281–E10290. 10.1073/pnas.1713328114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbie KF, Mead BP, Price RJ, 2015. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J. Control. Release 219, 61–75. 10.1016/j.jconrel.2015.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Hernández PA, Sumiyoshi A, Nonaka H, Haga R, Aubert-Vásquez E, Ogawa T, Iturria-Medina Y, Riera JJ, Kawashima R, 2011. An in vivo MRI Template Set for Morphometry, Tissue Segmentation, and fMRI Localization in Rats. Front. Neuroinform 5, 26 10.3389/fninf.2011.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder NM, Elliott KAC, 1958. DISPOSITION OF γ-AMINOBUTYRIC ACID ADMINISTERED TO MAMMALS. J. Neurochem 3, 139–143. 10.1111/j.1471-4159.1958.tb12620.x [DOI] [PubMed] [Google Scholar]
- Wu SY, Sanchez CS, Samiotaki G, Buch A, Ferrera VP, Konofagou EE, 2016. Characterizing Focused-Ultrasound Mediated Drug Delivery to the Heterogeneous Primate Brain in Vivo with Acoustic Monitoring. Sci. Rep 6 10.1038/srep37094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Chen Y, Liu J, Zhang L, Wang J, Yang Y, Lv Q, Xie M, 2018. Blood-brain barrier disruption induced by diagnostic ultrasound combined with microbubbles in mice. Oncotarget 9, 4897–4914. 10.18632/oncotarget.23527 [DOI] [PMC free article] [PubMed] [Google Scholar]