Abstract
Background:
Moderate intensity exercise is associated with a decreased incidence of atrial fibrillation (AF). However, extensive training in competitive athletes is associated with an increased AF risk. We evaluated the effects of 24-months of high intensity exercise training on left atrial (LA) mechanical and electrical remodeling in sedentary, healthy middle-aged adults.
Methods:
Sixty-one participants (53±5 years) were randomized to 10 months of exercise training followed by 14 months of maintenance exercise or stretching/balance control. 14 Masters athletes were added for comparison. 3D echocardiographic assessment of LA and left ventricular (LV) volumes, and signal-averaged ECGs for filtered P-wave duration (FPD) and atrial late potentials (RMS20) was completed at 0, 10, and 24 months. Extended ambulatory monitoring was performed at 0 & 24 months. Within and between group differences from baseline were compared using mixed-effects model repeated-measures analysis.
Results:
Fifty-three participants completed the study (25 control, 28 exercise) with 88±11% adherence to assigned exercise sessions. In the exercise group, both LA and LV end diastolic volumes (LV EDV) increased proportionately (19% and 17%, respectively) after 10 months of training (peak training load). However, only LA volumes continued to increase with an additional 14 months of exercise training (LA volumes 55%; LV EDV 15% at 24 months vs baseline; p<0.0001 for all). The LA:LV EDV ratio did not change from baseline to 10 months, but increased 31% from baseline in the Ex group (p<0.0001) at 24 months, without a change in controls. There were no between group differences in the LA ejection fraction, FPD, RMS20, and PAC burden at 24 months and no AF was detected. Compared with Masters athletes, the exercise group demonstrated lower absolute LA and LV volumes, but had a similar LA:LV ratio after 24 months of training.
Conclusions:
24 months of high intensity exercise training resulted in LA greater than LV mechanical remodeling with no observed electrical remodeling. Together, these data suggest different thresholds for electrophysiologic and mechanical changes may exist in response to exercise training, and provide evidence supporting a potential mechanism by which high intensity exercise training leads to AF.
Keywords: Exercise Training, Atrial fibrillation, Left Atrium, Athletes, Atrial Fibrillation, Exercise
Introduction
Atrial fibrillation (AF) is the most common clinical arrhythmia and is associated with a large and increasing global health burden.1 Exercise training modulates the risk of AF. Improvements in cardiorespiratory fitness have been associated with a reduction in incident AF in several population-based cohorts.2–4 More recently, randomized controlled trials have demonstrated low physical activity levels are a modifiable risk factor for AF, with a 9% decrease in AF recurrence for every increase in 1 metabolic equivalent (MET).5 Conversely, high levels of long-term physical exercise are associated with an increased risk of incident AF.6–8 In a recent meta-analysis of 6 contemporary studies, high-intensity trained athletes demonstrated a 5-fold greater odds of incident AF when compared to controls.9 These data support a U-shaped association between physical activity and AF risk.10 However, the theoretical upper limit of exercise training duration and intensity necessary to increase the risk of incident AF are unknown. Further, the mechanisms by which high levels of exercise may mediate an increased risk of incident AF remain largely unknown.
Recently, our group demonstrated that 24 months of exercise training decreased cardiac stiffness in the primary analysis of this group of healthy, previously sedentary middle-aged adults.11 In a further analysis, we demonstrated 10 months of exercise training (the peak training load in these subjects) led to a balanced increase in left atrial (LA) and left ventricular (LV) volumes.12 However, even 10 months of intense exercise training may not reflect the changes seen in competitive athletes. In our current analyses, we explored the impact of prolonged high-intensity exercise training on LA electrical and mechanical function in these previously sedentary middle-aged adults over a 24-month period. We hypothesized that vigorous exercise training would result in LA mechanical remodeling reflected by a progressive increase in LA volume (primary outcome of this sub-analysis), increased left atrial ejection fraction (LA EF), increased active LA filling percentage, and electrical remodeling evidenced by slower impulse propagation, increased premature atrial contraction burden, and greater incident AF when compared to controls.
METHODS
Subjects:
Two hundred and sixty-two individuals underwent initial screening for medical comorbidities. A complete medical history was obtained and a study physician performed a physical examination. Subjects were excluded if any of the following conditions were documented by a primary care physician or were self-reported, unless otherwise specified: hypertension (use of antihypertensive medications or ambulatory systolic blood pressure>135 mm Hg), measured body mass index ≥ 30 kg/m2, untreated hypo- or hyperthyroidism, the presence of obstructive sleep apnea or use of a home continuous positive airway pressure machine, chronic obstructive pulmonary disease, tobacco use during the past 10 years, coronary artery disease, or structural heart disease, as previously reported.11 A 12-lead electrocardiogram (ECG) was obtained for each participant and interpreted for arrhythmias, conduction abnormalities, or evidence of ischemia. All participants were fitted with a 24-hour ambulatory blood pressure (BP) monitor (SunTech Oscar 2, USA) for baseline readings and circadian variability.13 Participants with a self-reported exercise regimen of more than 90 minutes per week or a self-reported history of AF were excluded.
Sixty-one healthy, middle-aged, sedentary adults aged 45 to 64 years (29 men, 32 women; average age 52.1 + 5 years) met these criteria, agreed to participate, and underwent block randomization to either an aerobic exercise interval training program (exercise) or an active control group of balance and flexibility (control). Baseline anthropomorphic data including height and weight were recorded and used to calculate the BMI. Subject screening and recruitment details have been reviewed in previously published manuscripts.11 All participants signed an informed consent form. The institutional review board of the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas approved the study. These analyses are a sub-study of the NCT02039154 trial registered on ClinicalTrials.gov, with a detailed description of the subject population and sample size calculation provided in a previous publication.11 The trial was overseen by an independent data and safety monitoring board. All authors had full access to the study data and take responsibility for its integrity and the data analysis.
All participants (both exercise and control) were assigned an exercise physiologist who provided close follow-up and monitored training at least twice per month at our institute or at their place of training. Participants kept logs of their training activities and wore heart rate (HR) monitors (Polar, Kempele, Finland) to record exercise duration, training HR, and to document adherence to the exercise prescription. Baseline (time 0), 10 month, and 24 month testing was performed including body composition, exercise testing, echocardiography, and P-wave signal average electrocardiogram (P-SAECG). Extended ambulatory telemetry monitoring was conducted at time 0 and 24 months. An exercise log and heart rate monitor (Polar) were used to monitor training compliance. The mean monthly training load has been reported.11
Exercise Training
The exercise training regimen consisted of two distinct phases: an initial 10-months of progressive exercise training to peak load, followed by 14-months of maintenance exercise training. Full details have been previously published and are available in the Appendix.11 Briefly, the initial phase was comprised of 6-months of progressive training, during which an increase in frequency, duration, and intensity of exercise (including 2 high intensity aerobic interval sessions/week) were prescribed to peak training load. Peak training load included 5-6 hours of exercise per week including 2 interval sessions, at least one 1 hour long session, and two 30 minute sessions. Peak training load was then sustained for 4 months, after which 10-month measurements were recorded. Following this phase, a 14-month period of maintenance exercise was completed, where the frequency of high intensity intervals was reduced to once/week plus continuous training (24-month time point). During the maintenance phase, participants performed a total of approximately 3 hours/week of aerobic exercise.
Balance & Flexibility Training (Control)
The balance and flexibility group was prescribed a combination of one-hour balance, strength, or relaxation training at least 3 times per week for 24 months. Participants performed the activities in groups, stretching classes in the community, or completed an instructional video at home with the goal of providing a similar level of interaction with research staff between both groups, as previously published.11
Endurance Athletes
A separate, non-randomized group of competitive endurance athletes was added as a cross-sectional comparator group for the effects of long-term endurance exercise on cardiac structure and function in the type of athletes who have been reported to have an increased risk of AF. Fourteen representative age-matched competitive athletes in sinus rhythm with a minimum training duration of 10 years were enrolled (11 men and 3 women; average age 52 ± 4 years). Four athletes reported a history of paroxysmal AF, but all were in sinus rhythm at the time of the study. Athletes with AF were excluded only if they had a history of catheter ablation or were taking anti-arrhythmic drugs at the time of enrollment. Inclusion criteria for the endurance athletes were (i) participation in competitive swimming, cycling, marathon running, or triathlons for a minimum of 10 years, (ii) participation in at least 2 competitive events per year, (iii) and current active training for a competitive event. As in the longitudinal study, body composition, exercise testing, echocardiography, and extended ambulatory telemetry monitoring were performed on all participants. Because 2 years of exercise training is still less than that typically observed in competitive Masters athletes and of those athletes at elevated risk of developing AF, this group was added to provide important context and comparison for individuals with prolonged training regimens.
Echocardiography
Assessment of LA and LV structure and function were obtained using 3-dimensional (3D) echocardiography (IE33 Phillips Medical Systems, Andover, USA) at baseline, 10 months, and 24 months. Left ventricular end-systolic (LV ESV), LV end-diastolic volumes (LV EDV), LA end-systolic (LA ESV) and LA end-diastolic volumes (LA EDV) were measured in the 2- and 4 -chamber views with 5 points at the apex and mitral annulus. A LA:LV volume ratio was calculated by dividing the 3D LA EDV by LV EDV.14 LA ejection fraction (LA EF) was calculated using a standard approach, as described in the Appendix. We refer to LA EDV as LA volume throughout the manuscript, unless otherwise specified.
Pulsed wave Doppler at the mitral inflow was used to assess LV diastolic filling patterns, where early and diastasis filling (E+D wave) and late filling (A wave) velocity time intervals (VTI) were measured.15, 16 As described in previous publications, an active emptying percentage of the total LA emptying was calculated as follows: LA Active Emptying % = [AVTI/ (EVTI + DVTI + AVTI)]*100.12, 17 A trained analyst manually adjusted all computer-generated markings offline and was blinded to participant identifiers. All images were subsequently verified and further modified as needed by a board-certified cardiologist to ensure high quality markings.
P-wave Signal Average Electrocardiogram (P-SAECG) and Telemetry Monitoring
P-SAECG and extended telemetry monitoring were employed to non-invasively analyze cardiac electrical remodeling. Two P-SAECG variables were calculated: the filtered P-wave duration (FPD) and the root mean square voltage of the last 20 milliseconds (RMS20), which reflects atrial late potentials. Both variables have previously been used to identify an elevated risk of AF and predict AF recurrence.18–21 Continuous supine 12-lead ECGs were obtained using a Mortara XScribe system (Mortara Instruments, USA) at a 1 kHz sample rate. Digital ECG files were analyzed using MATLAB custom software (Mathworks, Natick, MA). QRS detection was performed by template matching algorithm of median QRS complexes generated from a 10-second segment for each of the ECG leads. A segment of the ECG, free of artifact and with stable resting heart rate, was manually selected for P-wave analysis. An initial average P-wave was calculated for each lead based on QRS triggering. This averaged P-wave was then cross-correlated with each raw P-wave for temporal alignment. A total of at least 200 P-waves per participant were examined. Individual P-waves more than two standard deviations from the mean P-wave were excluded in the final calculation of the averaged P-wave. Filtered signal-averaged P-wave analysis was done by an orthogonal lead ECG approximation of the averaged 12-lead P-wave derived using the inverse Dower transformation.22 The XYZ signals were then high pass filtered at 40 Hz then combined into a vector magnitude, ((x2 + y2 + z2)1/2). An operator blinded to the participant’s identity and group randomization performed all markings.
At both baseline and 24 months, subjects underwent additional extended telemetry monitoring for up to 14 days at baseline via a 5” × 2” bipolar electrode patch (ZioPatch, iRhythm technologies, CA, USA),23 which provided a qualitative and quantitative burden of atrial arrhythmias (premature atrial contractions [PACs] and AF). AF was defined a priori as an irregularly irregular atrial activity lasting > 30 seconds with the absence of P-waves; all tracings in which AF was considered were reviewed by a board-certified electrophysiologist.
Total Blood Volume, Body Composition, and Right Heart Catheterization
Right heart catheterization was performed and wedge position was confirmed under fluoroscopic guidance and the presence of typical waveforms, as described by our group previously.11 Mean pulmonary capillary wedge pressure and right atrial pressures were determined at end expiration. Total blood volume and body composition were calculated at baseline and 24 months. Total blood volume was measured using the carbon monoxide rebreathing method and has been reported in detail previously by our group.24 Body density and composition were determined by underwater weighing with correction for residual lung volume.25 Cardiac index was determined by the foreign gas rebreathing technique with acetylene as the soluble gas and helium as the insoluble gas.11
Statistical Analysis
Continuous variables are expressed as mean and standard deviation or least square means and 95% confidence intervals (CIs), and categorical variables are expressed as n (%). The primary analysis included all participants who completed the 2-year follow-up. Our a-priori primary outcome of interest was the change in LA volumes. Continuous end points were compared between groups using mixed-effects model repeated-measures analysis. The repeated-measures models included the intervention group factor (control versus exercise), a repeated factor for study visits, and a group × visit interaction term; the study participant was modeled as a random effect. The difference in response between control and exercise groups was assessed via the group × visit interaction effect. Within-group and between-group pairwise comparisons were made using the least square means derived from these mixed-effects models only if the respective interaction term was significant. The covariance structure for mixed-effects models was selected based on Akaike Information Criteria and model parsimony. PACs are reported as median PAC per hour (25th percentile and 75th percentile) and mixed-effects modeling was not used owing to their skewed distribution. For the PAC data, 24 month between-group analyses used Wilcoxon Rank Sum testing. For all analyses, a 2-sided P value of <0.05 was considered statistically significant. The analysis was performed using SAS version 9.4, SAS Institute (Cary, NC). The data and study materials will not routinely be made available to other researchers for purposes of reproducing the results or replicating the procedure, though we are happy to provide additional details regarding the analytic methods to interested investigators. Specific requests from individual researchers will be considered on a case-by-case basis.
Results
Participant Demographics and Baseline Cardiovascular Risk Factors
Out of the 61 randomized participants, 28 in the exercise group and 25 in the control group completed the training regimen at 24 months and were included in the final analyses. Table 1 illustrates the baseline participant characteristics. Participants in the exercise group maintained excellent compliance with the two-year exercise intervention (mean 88±11%).
Table 1:
Baseline Characteristics
| Variable | Control Group (n=25) | Exercise Group (n=28) | Athletes (n=14) |
|---|---|---|---|
| Age (years) | 52 (50-54) | 54 (52-55) | 52 (50-54) |
| Male Sex n (%) | 15 (60) | 15 (54) | 11 (79) |
| Race n (%) | |||
| White | 20 (80) | 22 (79) | 14 (100) |
| Asian | 3 (12) | 3 (10) | 0 |
| Other | 2 (8) | 3 (10) | 0 |
| Weight (kg) | 76 (71-82) | 74 (67-80) | 75 (69-81) |
| Height (cm) | 168 (164-172) | 165 (153-177) | 175 (171-179) |
| BMI (kg/m2) | 26.5 (25.2-27.8) | 25.6 (24.5-26.7) | 24.4 (23.0-25.8) |
| SBP (mmHg) | 123 (120-126) | 120 (118-123) | 126 (121-131) |
| DBP (mmHg) | 74 (71-76) | 72 (70-74) | 72 (69-76) |
| Heart Rate (bpm) | 74 (71-78) | 73 (70-76) | 58 (54-63) |
| VO2 max (mL/kg/min) | 29 (27-31) | 29 (27-31) | 45 (41-49) |
Variables are expressed as mean (95% confidence intervals) or n (%). BMI indicates body mass index; SBP, mean 24-hour ambulatory systolic blood pressure; DBP, mean 24-hour ambulatory diastolic blood pressure; VO2 max, maximal oxygen uptake; bpm, beats per minute.
Effect of Exercise Intervention
These data are reported elsewhere and are reproduced here for convenience.11 The exercise intervention resulted in a 5.3 mL/kg/min [95% CI, 4.15-6.40] increase in VO2 max in the exercise group and no change in the control group VO2 max (−1.0 mL/kg/min [−1.3 – 0.7] at twenty-four months when compared to baseline. Twenty-four months of exercise training led to a reduction in the heart rate (P interaction < 0.001) and prevented weight gain in the exercise group (P interaction = 0.03). No changes in resting hemodynamics (right atrial pressure, PCWP or cardiac index) or body composition (weight, body fat, or fat-free mass) were observed (Table 2).
Table 2:
The Effect of Exercise Training on Cardiovascular Hemodynamics and Cardiovascular Function
| Control | Exercise | ||||
|---|---|---|---|---|---|
| Variable | Pre | Post | Pre | Post | Group × Time |
| Heart rate, bpm | 64 (60-67) | 64 (61-67) | 63 (60-67) | 58 (55-61)* | 0.001 |
| Systolic blood pressure, mmHg | 109 (106-113) | 107 (103-110) | 107 (104-110) | 104 (102-107) | 0.953 |
| Diastolic blood pressure, mmHg | 69 (67-72) | 70 (67-73) | 67 (65 - 69) | 68 (65-71) | 0.953 |
| Cardiac index, L/min2 | 2.5 (2.4-2.7) | 2.6 (2.4-2.7) | 2.5 (2.4-2.7) | 2.5 (2.3-2.6) | 0.417 |
| Weight, kg | 75.8 (70.0-81.6) | 77.1 (71.4-82.8) | 74.3 (68.9-79.6) | 73.6 (68.1-79.0) | 0.029 |
| Body fat, % | 32.9 (30.0-35.7) | 35.5 (33.1-37.9) | 32.3 (30.3-34.2) | 33.0 (31.0-35.1) | 0.058 |
| Fat-free mass, kg | 50.9 (46.4-55.3) | 49.9 (45.4-54.4) | 50.4 (46.3-54.5) | 49.3 (45.2-53.5) | 0.083 |
| Hemoglobin, g/dL | 13.3 (12.9-13.7) | 14.0 (13.5-14.6)* | 13.1 (12.6-13.5) | 13.6 (13.1-14.0)* | 0.310 |
| Plasma volume, mL | 3302 (3058-3546) | 3122 (2891-3354)* | 3337 (3061-3614) | 3290 (3024-3555) | 0.164 |
| Total blood volume, mL | 5245 (4822-5668) | 5081 (4657-5504) | 5247 (4787-5707) | 5263 (4797-5729) | 0.148 |
| RAP, mmHg | 8.8 (8.2-9.4) | 8.7 (8.1-9.4) | 8.8 (8.2-9.3) | 8.6 (8.0-9.3) | 0.905 |
| PCWP, mmHg | 12.0 (11.2 - 12.8) | 11.8 (10.9-12.6) | 11.6 (11.1-12.2) | 11.8 (11.2-12.5) | 0.473 |
Variables are expressed as least square means (95% confidence intervals). Group x time represents the interaction term and * p<0.05 represents within group comparison from baseline to 24 months derived from mixed effects repeated measure analysis. bpm indicates beats per minute; RAP, right atrial pressure; PCWP, pulmonary capillary wedge pressure. These data are reported elsewhere and are reproduced here for convenience.
Mechanical Changes in 3-D Left Atrial Volume and Function
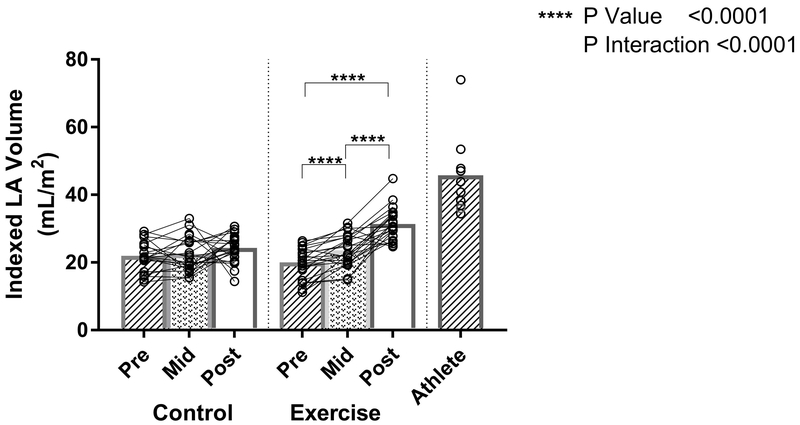
Although both LA and LV volumes increased proportionately from baseline to peak training (10 months), only LA volumes of the exercise group increased further with additional exercise training. Mixed effects modeling showed a differential treatment effect in the exercise versus control groups (P interaction<0.0001). LA volumes increased 19% from baseline to peak training load (mean difference 3.7mL/m2 ([95% CI 2.2-5.2], p<0.0001) and 55% from baseline to study completion (mean difference 11mL/m2 [95% CI 8.2-13], p<0.0001) (Figure 1). In contrast, there was only a small increase in LA volumes in the control group over 24 months of follow-up (Figure 1). Overall, the 24-month net estimated treatment difference between the two groups resulted in an LA volume difference of 8.2mL/m2 ([95% CI 4.8-12], p<0.0001). Although small differences in baseline left atrial volumes were present between sexes (data not shown), the response to exercise did not differ between the two sexes (group x visit x male, P-interaction = 0.85), with a net mean difference in LA volumes of 8.8mL/m2 for females versus 7.3 mL/m2 for males between the two groups. While there were significant increases in the LA volume in the exercise group, the resultant volumes were less than that of the comparative Masters athlete cohort (Figure 1).
Figure 1: The Effect of Exercise Training on Left Atrial Volume.
Individual Exercise, Control, and Athlete data points (open circles) with mean values (bar graphs). P-interaction reflects the mixed-effects modeling overall group × visit interaction between Exercise and Control groups. P-values indicate within-group pairwise comparisons between Exercise and Control groups. LA indicates left atrium; BSA, body surface area.
Mixed effects modeling showed a trend toward a differential treatment effect in LA EF in the exercise versus control groups (P interaction=0.06). Although the overall interaction did not meet statistical significance, exploratory analyses investigating the effect of exercise on the LA EF demonstrated a significant interaction from baseline to 10 months (mean between group difference 8.0% ([95% CI 2.7-12], p=0.002)), that was not observed at 24 months (mean between group difference 3.4% ([95% CI −3.0-9.8], p=0.29)) (Figure 2). The mean LA EF in the control group did not change from baseline over 24 months of follow-up (Figure 2). The LA EF of the exercise group approximated the mean LA EF of the Masters athlete group at the conclusion of the 24-month training regimen (Figure 2).
Figure 2: The Effect of Exercise Training on Left Atrial Ejection Fraction.
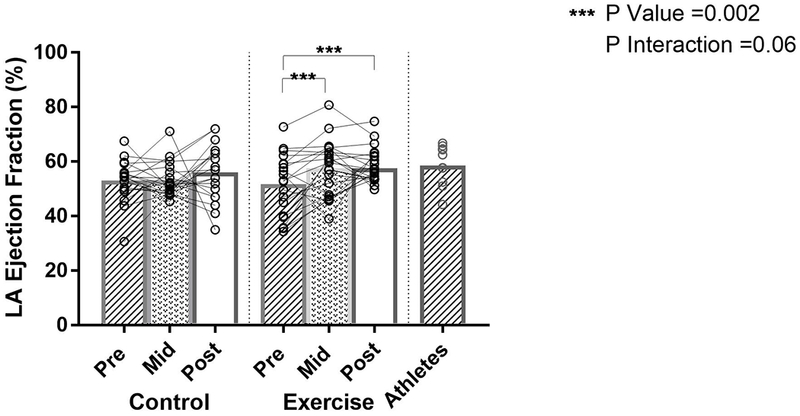
Individual Exercise, Control, and Athlete data points (open circles) with mean values (bar graphs). P-interaction reflects the mixed-effects modeling overall group × visit interaction between Exercise and Control groups. P-values indicate within-group pairwise comparisons between Exercise and Control groups. LA indicates left atrium.
There was no significant difference in treatment effect of LA active emptying percentage for the exercise and control groups (P interaction=0.18). Baseline LA active emptying percentage for the exercise and control groups were 41% [95% CI 38-44] and 41% [39-44], respectively. Over the 24 month course of the study, the mean LA active emptying percentage decreased in both groups (exercise −11% ([95% CI −14-−7.7], p<0.0001) and control −8.4% ([−12−−4.9], p<0.0001)), when compared to baseline. This resulted in no net estimated treatment difference in LA active emptying percentage (mean between-group difference −2.6% [95% CI −7.5-2.3], p=0.29) at 24 months.
Changes in 3-Dimensional Left Ventricular Volume
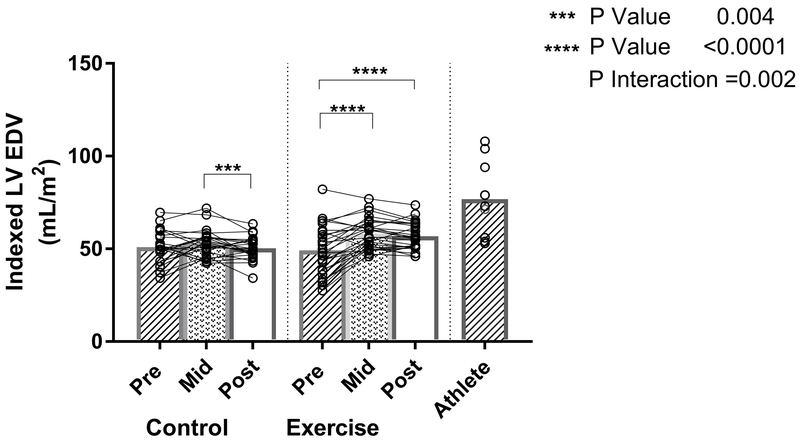
Mixed effects modeling demonstrated a differential treatment effect in the exercise versus control groups (P interaction=0.002). In subjects randomized to exercise training, the LV EDV increased 17% (mean within group difference 8.5mL/m2 [95% CI 5.9-11], p<0.0001) from baseline with 10 months of progressive exercise. However, no further increase in LV volume was observed during the maintenance exercise phase (mean within group difference from baseline 7.6mL/m2 [95% CI 4.8-10], p<0.0001), as previously reported (Figure 3). In those randomized to control, the indexed LV EDV did not change from baseline to 24 months (Figure 3). At 24 months, a net estimated between-group difference in indexed LV EDV of 7.6mL/m2 ([95% CI 3.6-12], p=0.0004) was observed. The resultant mean LV EDV in the exercise and control groups were lower than those in the Masters athlete cohort.
Figure 3: The Effect of Exercise Training on Left Ventricular End Diastolic Volume.
Individual Exercise, Control, and Athlete data points (open circles) with mean values (bar graphs). P-interaction reflects the mixed-effects modeling overall group × visit interaction between Exercise and Control groups. P-values indicate within-group pairwise comparisons between Exercise and Control groups. LVEDV indicates left ventricular end diastolic volume; BSA, body surface area.
Changes in Left Atrial:Left Ventricular End Diastolic Volume Ratio
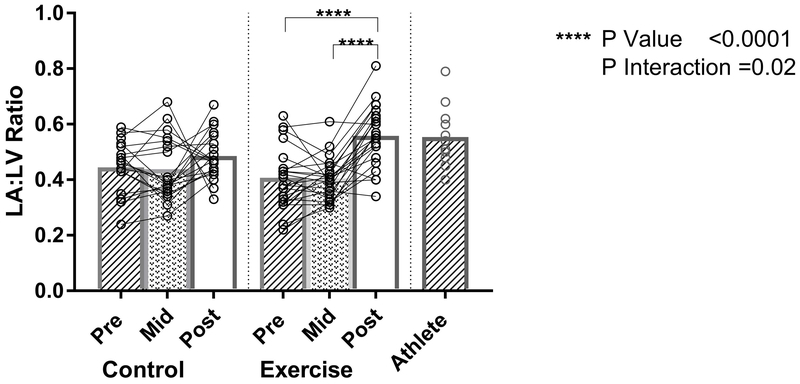
Mixed effect modeling demonstrated a differential treatment effect in the exercise versus control groups (P interaction=0.02). In the exercise group, there was no change in the LA:LV ratio during the initial 10 months of progressive high intensity exercise (Figure 4). However, the exercise group demonstrated a 32% increase (mean difference 0.13 [95% CI 0.08-0.18], p<0.0001) in the LA:LV ratio when compared to baseline (Figure 4). There was a small change in the LA:LV ratio from baseline to 24 months in the control group (mean within group difference 0.05 [95% CI −0.0006-0.10], p=0.051). The LA:LV ratio of the subjects following 24 months of exercise training approximated the ratio of the Masters athletes (Figure 4).
Figure 4: Ratio of Left Atrial to Left Ventricular End Diastolic Volume.
Individual Exercise, Control, and Athlete data points (open circles) with mean values (bar graphs). P-interaction reflects the mixed-effects modeling overall group × visit interaction between Exercise and Control groups. P-values indicate within-group pairwise comparisons between Exercise and Control groups. LA indicates left atrial; LV, left ventricle.
Electrical
Electrophysiologic Remodeling Using P-SAECG and Extended Telemetry Monitoring
Mixed effect modeling did not show a differential treatment effect in FPD between the exercise and control groups (P interaction=0.18). The FPD did not change in either the exercise group (p=0.13) or the control group (p=0.51) from baseline to 24 months (Table 3). No difference in the treatment effect of exercise on RMS20 was seen between the two groups (P interaction=0.73). The RMS20 of both groups did not change and there was no net estimated treatment difference in RMS20 between the two groups (0.07μV [95% CI −0.15-0.30], p=0.52) at 24 months (Table 3).
Table 3.
The Effect of Exercise Training on Electrophysiologic Remodeling Using P-SAECG
| P-Wave Duration | RMS20 | |||||
|---|---|---|---|---|---|---|
| Group | Baseline | Change from Baseline to 24 months | P-value | Baseline | Change from Baseline to 24 months | P-value |
| Control | 129msec [119-139] | −4.2msec [−17.0-8.6] | 0.51 | 0.61μV [0.47-0.75] | −0.02μV [−0.18-0.15] | 0.84 |
| Exercise | 130msec [120-141] | 9.5msec [−2.7-12.7] | 0.13 | 0.64μV [0.51-0.78] | 0.06μV [−0.10-0.21] | 0.47 |
P-wave duration and RMS20 variables are expressed as least square means [95% confidence intervals]. Change from baseline and P values represent the within-group change from baseline to 24-month values derived from mixed effects repeated measure analysis. RMS20 indicates the root mean square voltage of the last 20 milliseconds of the averaged P wave.
On telemetry monitoring, baseline testing revealed a median PACs/HR burden of 9.4 PACs/hour [25th percentile – 75th percentile (5.2-22)] in the exercise group and 10 PACs/hour [5.8-28] in the control group. No difference in PAC burden between treatment groups was observed at 24 months (median [25th percentile – 75th percentile] 13 PACs/hour [7.9-22] versus 14 PACs/hour [7.0-24], p=0.71). We identified no episodes of AF in either the exercise or control groups on extended monitoring at 24 months with a median follow-up of 234 hours [25th percentile – 75th percentile (185-306)] and 304 hours (150-324), respectively.
Discussion
The primary results of this randomized, prolonged exercise training study in sedentary, healthy, middle aged adults are: 1) both LA volume and LV EDV experienced balanced remodeling during 10 months of progressive high intensity exercise, but only LA volume continued to increase with additional exercise training; 2) 24 months of exercise training increased the LA:LV EDV ratio 32% from baseline, approaching values similar to the long term athletic comparator group, with no change in the control group; and 3) no qualitative or quantitative electrical changes were observed following 24 months of exercise training and no AF was identified. Together, these data suggest there may be different thresholds for electrophysiologic and mechanical changes in response to 24 months of exercise training and provide a potential mechanism by which high intensity exercise training leads to AF.
The effect of exercise on LA mechanical and electrical remodeling is of significant physiologic and clinical importance. A U-shaped association exists between physical exercise and the risk of AF where low rates of physical activity are associated with an increased risk of incident AF, while moderate physical activity is associated with reduced rates of AF.2 However, there is growing epidemiologic evidence that high doses of exercise are also associated with an increased risk of incident AF in the general population26–28 and a 2 to 7-fold increase in the risk of incident AF in high intensity endurance athletes.29, 30 This relationship represents an important paradox in AF – despite an overall reduction in cardiovascular disease,31 high doses of exercise may, in fact, increase the risk of AF. However, the mechanism by which extreme exercise increases the risk for incident AF remains largely unknown.
During endurance exercise, cardiac output increases 4-fold from rest in untrained individuals and nearly 8-fold in highly trained athletes, proportionate to the increase in metabolic demand (VO2).32 This increased flow provides a volume challenge affecting all four cardiac chambers. At 10 months, exercise training led to a balanced remodeling of the LA and LV evidenced by a proportional increase in both the LA and LV volumes. This remodeling is congruent with previous studies highlighting the physiologic four-chamber dilation in response to exercise, a hallmark of an athlete’s heart.33 At 24 months, however, we demonstrate that a prolonged high intensity training regimen results in LA and LV EDV increases that were not proportional. Indeed, the LA:LV EDV ratio increased more than 30% in the exercise group when compared to baseline and was similar to that of the endurance Masters athlete comparator group.
To explain the differential effects on the two left-sided chambers, it is necessary to consider the effects of exercise on the cardiac cycle. During exercise, the proportion of time spent in systole increases, resulting in mitral valve closure for a greater proportion of the cardiac cycle. Due to this altered timing, the LA, as the upstream chamber, experiences increased pressure during exercise.34 The downstream consequences may disproportionately impact the LA over the LV due to the “dam wall” effect proposed by Andre La Gerche.35 Due to its thin walled structure, the LA may more quickly dilate in response to the stress of exercise than the left ventricle. In our study, LA volumes progressively increased with additional high-intensity exercise training (from baseline to 10 months and 10 months to 24 months). Yet, the LV volumes appeared to plateau after 10 months of exercise. Whether these LA structural changes represent physiologic hypertrophy or pathologic remodeling in response to prolonged and intense exercise training have yet to be determined. Our data support the physiology behind the dam wall effect during exercise, which may underlie the differential mechanical remodeling of the LA and LV.
This disproportionate increase in LA volume likely has important clinical consequences. Population-based studies have identified LA size as strongly associated with cardiovascular disease outcomes, including incident AF.36–38 Endurance athletes have larger LA volumes and an increased PAC burden when compared to controls.39 But whether the association between high doses of exercise and AF is mediated by structural changes, electrical remodeling, or both remains unknown. Endurance athletes with a high cumulative training “dose” have prolonged FPD and more frequent PACs in addition to larger LA volumes.40 Yet, it is unclear whether LA structural changes preceded the aforementioned electrical remodeling.
Contrary to our hypothesis, our data suggest two years of high intensity exercise training does not affect non-invasive markers of electrical remodeling, despite significant mechanical LA remodeling. Furthermore, previous analyses from this randomized trial demonstrated no differences in plasma volume, total blood volume, cardiac index, or PCWP, further supporting the theory that these mechanical changes may be due to the “dam wall” effect.11 The concept that mechanical changes may precede, or perhaps occur independently of prolonged FPD, increased atrial late potentials, and a greater PAC burden, is novel. The previous theory that athletes have an increased susceptibility to atrial arrhythmias due to concomitant mechanical and electrical changes may need to be updated.41 The susceptibility to AF may be a sequential process in which the electrical remodeling requires a very prolonged exercise exposure or is a downstream consequence of the atrial mechanical remodeling.
Several theories may explain the profound LA mechanical changes but lack of electrical remodeling observed in our study. First, the duration of exercise prescribed may have been insufficient to result in qualitative or quantitative electrical changes. Cardiac electrical remodeling is a complex process that has been attributed in large part to modulation of the autonomic nervous system.42, 43 Autonomic nervous system control mediated by increased vagal tone has been associated with prolongation of the PR interval and more frequent PACs.44 It has been hypothesized that vagally-mediated electrical changes play a significant role in the development of AF in athletes. These autonomic changes typically occur early after starting an exercise regimen;45 however no increase in FPD nor PAC burden was observed in our population. Thus, it is unlikely the autonomic nervous system alone leads to the electrical changes seen in athletes.
Previous work has shown electrical remodeling including bradycardia in response to exercise is present even after complete autonomic blockade, indicating mechanisms beyond vagal tone play a substantial role.46 Fibrosis of the cardiac conduction system due to mechanical stress has also been proposed as a potential mechanism for the prolonged FPD and greater atrial late potentials observed in extreme exercise training.47 This timing of cardiac fibrosis remains unclear and our study was not designed to directly test this hypothesis. Alternatively, downregulation of specific ion channels (ex. funny channel HCN4) in response to exercise has been demonstrated in rodent models with similar mechanisms proposed in humans.48 However, the intensity and duration of exercise necessary for regulation of these mechanisms in humans remains unknown.
Some authors have argued that cumulative lifetime training hours are critical to LA remodeling.40 In our population of sedentary middle aged adults, the prescribed exercise regimen may have not overcome a lifetime of low training hours and thus may have been insufficient to reach the lifetime threshold necessary to result in qualitative or quantitative electrical changes. It is unlikely the intensity of the exercise prescribed was below the threshold resulting in electrical remodeling. Our prescribed regimen is based on prior literature supporting high intensity training effects on cardiorespiratory fitness, and our data demonstrate an objective, substantial improvement in VO2 with excellent maintenance of the prescribed exercise regimen.11, 49 It is more likely that the duration as opposed to the intensity of exercise training explains this difference in electrical and mechanical remodeling. Performing a longer exercise training duration may ultimately result in the prolonged FPD with an increased PAC burden observed in athletes. However, the logistics of conducting such a very prolonged longitudinal study may prove to be prohibitive to directly test this hypothesis.
Strengths and Limitations
Our study had several strengths that merit highlighting. First, participant compliance with the prescribed high intensity regimen was excellent, with an 88±11% adherence to exercise sessions. Second, objective measurement of qualitative electrical remodeling and LA and LV volumes were determined by highly trained readers blinded to the study participants to limit the risk of introducing bias and to maintain the internal validity of our findings. Third, to our knowledge this study represents the longest duration and most comprehensive exercise training regimen to assess mechanical and electrical LA along with invasive measurement of LA pressure in otherwise healthy adults. Lastly, although this study was not powered for safety endpoints, no participants developed major adverse events nor developed AF during the follow-up period. This outcome suggests the long-term effect of high-interval training has a reasonable safety profile in this demographic with demonstrable beneficial effects on the cardiovascular system. This design provides a unique, comprehensive assessment to help expand the knowledge base of a clinically important topic.
Several limitations of our study are notable. This was a single-centered design and participants were screened to be a healthy and sedentary population (participants were excluded if they exercised >90 minutes per week). While this thorough screening approach allowed for isolation of the specific variable being tested (exercise training), it selected a motivated cohort of subjects. Generalizing these findings to the broader population should be done with caution, though it should be noted that this particular characteristic is also typical of competitive Masters athletes. Second, we employed non-invasive testing to evaluate P-wave impulse propagation and atrial late potentials in our participants. While P-SAECG may not be sensitive enough to detect subtle changes, the non-invasive testing used was designed to provide a relevant electrical remodeling phenotype with available non-invasive testing. Third, it is important to highlight that our sample size provided sufficient power to detect structural differences in LA volumes and clinically meaningful changes in FPD, though we may be underpowered to detect small differences in the this variable. Fourth, AF ascertainment was performed using two weeks of extended telemetry monitoring. Since our participants were at low absolute risk for AF (with a mean age of 52±5 years and free from cardiovascular risk factors), the expected rates of detecting AF were low. Future studies may consider incorporating longitudinal data from implantable devices, including implantable loop recorders, and mobile health technology to lengthen the follow-up period and increase detection rates.50, 51
Conclusions
In conclusion, we demonstrated that 24 months of high intensity exercise training resulted in LA greater than LV mechanical remodeling highlighted by increased LA volumes and an increased LA:LV ratio with no observed electrical remodeling. Together, these data suggest there may be different thresholds for electrophysiologic and mechanical changes in response to 24 months of exercise training and provides evidence supporting a potential mechanism by which high intensity exercise training may lead to AF.
Supplementary Material
Clinical Perspective.
What is New?
Extensive training in competitive athletes has been associated with an increased risk of atrial fibrillation, but the duration of exercise necessary to develop atrial fibrillation and its underlying mechanisms remain incompletely understood.
Our study demonstrates twenty-four months of high intensity exercise training led to a disproportionate dilation of the left atrium compared to the left ventricle; no electrical remodeling was seen.
Compared with Masters athletes, those participants randomized to exercise training demonstrated lower absolute left atrial and left ventricular volumes, but a similar left atrial to left ventricular ratio after 24 months of exercise training.
What Are The Clinial Implications?
Different thresholds may exist for mechanical and electrophysiologic changes to occur in response to prolonged exercise training.
Our findings support a potential mechanism by which prolonged endurance exercise training leads to atrial fibrillation.
Acknowledgments
Funding sources:
This study was supported by the National Institutes of Health grants 3R01AG017479 and 3R01AG017479-11S1. Dr. McNamara is supported by the National Heart, Lung, and Blood Institute (T32-HL125247). Drs. Levine, Sarma, and Hieda were supported in part by the American Heart Association Strategically Focused Research Network (14SFRN20600009-03).
Footnotes
Disclosures and Relationship to Industry:
None
CLINICAL TRIAL REGISTRATION:
URL: https://clinicaltrials.gov. Unique identifier: NCT02039154.
References:
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Furberg CD, Psaty BM and Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY and Alonso A. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities study. Circ Arrhythm Electrophysiol. 2014;7:620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA and Al-Mallah MH. Cardiorespiratory Fitness and Risk of Incident Atrial Fibrillation: Results From the Henry Ford Exercise Testing (FIT) Project. Circulation. 2015;131:1827–34. [DOI] [PubMed] [Google Scholar]
- 5.Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Hendriks JM, Twomey D, Kalman JM, Abhayaratna WP, Lau DH and Sanders P. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol. 2015;66:985–96. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GS and Rhodes EC. A review of blood lactate and ventilatory methods of detecting transition thresholds. Sports Med. 1989;8:43–55. [DOI] [PubMed] [Google Scholar]
- 7.Myrstad M, Lochen ML, Graff-Iversen S, Gulsvik AK, Thelle DS, Stigum H and Ranhoff AH. Increased risk of atrial fibrillation among elderly Norwegian men with a history of long-term endurance sport practice. Scand J Med Sci Sports. 2014;24:e238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, Vidal B, Scalise A, Tejeira A, Berruezo A, Brugada J and Investigators G. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;10:15–20. [DOI] [PubMed] [Google Scholar]
- 9.Abdulla J and Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–9. [DOI] [PubMed] [Google Scholar]
- 10.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr., Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr., Tomaselli GF and American College of Cardiology/American Heart Association Task Force on Practice G. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- 11.Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, Urey MA, Adams-Huet B and Levine BD. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation. 2018;137:1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opondo MA, Aiad N, Cain MA, Sarma S, Howden E, Stoller DA, Ng J, van Rijckevorsel P, Hieda M, Tarumi T, Palmer MD and Levine BD. Does High-Intensity Endurance Training Increase the Risk of Atrial Fibrillation? A Longitudinal Study of Left Atrial Structure and Function. Circ Arrhythm Electrophysiol. 2018;11:e005598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering TG, Shimbo D and Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–74. [DOI] [PubMed] [Google Scholar]
- 14.Spevack DM, Blum L, Malhotra D, Nazari R, Ostfeld RJ, Doddamani S, Bello R, Cohen HW and Sonnenblick EH. Ratio of left atrial to left ventricular size: an anatomical marker of the diastolic left ventricular pressure-volume relationship. Echocardiography. 2008;25:366–73. [DOI] [PubMed] [Google Scholar]
- 15.Cohen GI, Pietrolungo JF, Thomas JD and Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol. 1996;27:1753–60. [DOI] [PubMed] [Google Scholar]
- 16.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD and Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol. 2007;99:1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 18.Fukunami M, Yamada T, Ohmori M, Kumagai K, Umemoto K, Sakai A, Kondoh N, Minamino T and Hoki N. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram. Circulation. 1991;83:162–9. [DOI] [PubMed] [Google Scholar]
- 19.Guidera SA and Steinberg JS. The signal-averaged P wave duration: a rapid and noninvasive marker of risk of atrial fibrillation. J Am Coll Cardiol. 1993;21:1645–51. [DOI] [PubMed] [Google Scholar]
- 20.Opolski G, Scislo P, Stanislawska J, Gorecki A, Steckiewicz R and Torbicki A. Detection of patients at risk for recurrence of atrial fibrillation after successful electrical cardioversion by signal-averaged P-wave ECG. Int J Cardiol. 1997;60:181–5. [DOI] [PubMed] [Google Scholar]
- 21.Aytemir K, Aksoyek S, Yildirir A, Ozer N and Oto A. Prediction of atrial fibrillation recurrence after cardioversion by P wave signal-averaged electrocardiography. Int J Cardiol. 1999;70:15–21. [DOI] [PubMed] [Google Scholar]
- 22.Edenbrandt L and Pahlm O. Vectorcardiogram synthesized from a 12-lead ECG: superiority of the inverse Dower matrix. J Electrocardiol. 1988;21:361–7. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg MA, Samuel M, Thosani A and Zimetbaum PJ. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol. 2013;36:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gore CJ, Rodriguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J and Levine BD. Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000-5,500 m). J Appl Physiol (1985). 2006;101:1386–93. [DOI] [PubMed] [Google Scholar]
- 25.Wilmore JH and Behnke AR. An anthropometric estimation of body density and lean body weight in young men. J Appl Physiol. 1969;27:25–31. [DOI] [PubMed] [Google Scholar]
- 26.Morseth B, Graff-Iversen S, Jacobsen BK, Jorgensen L, Nyrnes A, Thelle DS, Vestergaard P and Lochen ML. Physical activity, resting heart rate, and atrial fibrillation: the Tromso Study. Eur Heart J. 2016;37:2307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE and Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103:1572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C, Trivedi C, Gokoglan Y, L DIB and Natale A. Differential Association of Exercise Intensity With Risk of Atrial Fibrillation in Men and Women: Evidence from a Meta-Analysis. J Cardiovasc Electrophysiol. 2016;27:1021–9. [DOI] [PubMed] [Google Scholar]
- 29.Andersen K, Farahmand B, Ahlbom A, Held C, Ljunghall S, Michaelsson K and Sundstrom J. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J. 2013;34:3624–31. [DOI] [PubMed] [Google Scholar]
- 30.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, Rebato C and Elosua R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008;10:618–23. [DOI] [PubMed] [Google Scholar]
- 31.Wasfy MM and Baggish AL. Exercise Dose in Clinical Practice. Circulation. 2016;133:2297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyner MJ and Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015;95:549–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paterick TE, Gordon T and Spiegel D. Echocardiography: profiling of the athlete’s heart. J Am Soc Echocardiogr. 2014;27:940–8. [DOI] [PubMed] [Google Scholar]
- 34.Reeves JT, Groves BM, Cymerman A, Sutton JR, Wagner PD, Turkevich D and Houston CS. Operation Everest II: cardiac filling pressures during cycle exercise at sea level. Respir Physiol. 1990;80:147–54. [DOI] [PubMed] [Google Scholar]
- 35.La Gerche A and Claessen G. Increased Flow, Dam Walls, and Upstream Pressure: The Physiological Challenges and Atrial Consequences of Intense Exercise. JACC Cardiovasc Imaging. 2016;9:1389–1391. [DOI] [PubMed] [Google Scholar]
- 36.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS and Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–23. [DOI] [PubMed] [Google Scholar]
- 37.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW and Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–75. [DOI] [PubMed] [Google Scholar]
- 38.Vaziri SM, Larson MG, Benjamin EJ and Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–30. [DOI] [PubMed] [Google Scholar]
- 39.Iskandar A, Mujtaba MT and Thompson PD. Left Atrium Size in Elite Athletes. JACC Cardiovasc Imaging. 2015;8:753–62. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelm M, Roten L, Tanner H, Wilhelm I, Schmid JP and Saner H. Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am J Cardiol. 2011;108:580–5. [DOI] [PubMed] [Google Scholar]
- 41.Mont L. Arrhythmias and sport practice. Heart. 2010;96:398–405. [DOI] [PubMed] [Google Scholar]
- 42.Maron BJ and Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114:1633–44. [DOI] [PubMed] [Google Scholar]
- 43.al-Ani M, Munir SM, White M, Townend J and Coote JH. Changes in R-R variability before and after endurance training measured by power spectral analysis and by the effect of isometric muscle contraction. Eur J Appl Physiol Occup Physiol. 1996;74:397–403. [DOI] [PubMed] [Google Scholar]
- 44.Lok NS and Lau CP. Abnormal vasovagal reaction, autonomic function, and heart rate variability in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1998;21:386–95. [DOI] [PubMed] [Google Scholar]
- 45.Iwasaki K, Zhang R, Zuckerman JH and Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol (1985). 2003;95:1575–83. [DOI] [PubMed] [Google Scholar]
- 46.Lewis SF, Nylander E, Gad P and Areskog NH. Non-autonomic component in bradycardia of endurance trained men at rest and during exercise. Acta Physiol Scand. 1980;109:297–305. [DOI] [PubMed] [Google Scholar]
- 47.Chang Y, Yu T, Yang H and Peng Z. Exhaustive exercise-induced cardiac conduction system injury and changes of cTnT and Cx43. Int J Sports Med. 2015;36:1–8. [DOI] [PubMed] [Google Scholar]
- 48.Boyett MR, D’Souza A, Zhang H, Morris GM, Dobrzynski H and Monfredi O. Viewpoint: is the resting bradycardia in athletes the result of remodeling of the sinoatrial node rather than high vagal tone? J Appl Physiol (1985). 2013;114:1351–5. [DOI] [PubMed] [Google Scholar]
- 49.Simonsen T, Helgesen C, Hjorth N, Bach R and Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665671. [DOI] [PubMed] [Google Scholar]
- 50.Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D, Dokainish H, Philippon F, Barake W, McIntyre WF, Simek K, Hill MD, Mehta SR, Carlson M, Smeele F, Pandey AS, Connolly SJ and Investigators A-I. Subclinical Atrial Fibrillation in Older Patients. Circulation. 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 51.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D and Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.