Abstract
Colorectal liver metastases (CLM) are not always resectable at the time of diagnosis. An insufficient future liver remnant is a factor excluding patients from curative intent resection. To deal with this issue, two-stage hepatectomy was introduced approximately 20 years ago. It is a sequential treatment strategy for bilateral CLM, which consists of preoperative chemotherapy, portal vein embolization, and planned first and second liver resections. This article reviews current evidences supporting use of two-stage hepatectomy.
Keywords: future liver remnant, portal vein embolization, two-stage hepatectomy, colorectal liver metastasis
1. Introduction
Approximately 15% of patients presenting with colorectal cancer have synchronous colorectal liver metastases (CLM), and approximately 30% have metachronous CLM.1 Liver resection has been proven to have a survival benefit over chemotherapy alone and provides 5-year overall survival (OS) rates that range from 40% to 58%.2–4 However, due to extent of disease, patients with CLM are not always candidates for curative intent resection at the time of diagnosis.5 Fortunately, preoperative chemotherapy downsizes CLM and can therefore increase the number of patients eligible for curative resection.6 Similarly, CLM patients can be excluded from curative intent resection due to an insufficient future liver remnant (FLR), as the low hepatic functional reserve of small FLR can lead to post-hepatectomy liver failure.7,8 Portal vein embolization (PVE) was first reported in the 1980s to deal with an insufficient FLR.9–11 Subsequently, two-stage hepatectomy for bilateral CLMs was reported in the early 2000s as the next technical advancement for improving resectability.12,13 This article reviews the historical background, safety, and oncological outcomes of two-stage hepatectomy to overcome an insufficient FLR.
2. Portal vein embolization
During PVE, the portal venous system draining to the affected liver planned for resection is embolized. The purpose of PVE is to induce hypertrophy of the non-embolized liver in order to reduce the risk of postoperative hepatic insufficiency. The rationale behind PVE is based on an animal experiment during which ligation of the portal vein caused atrophy of the PV-occluded liver and led to hypertrophy of the non-PV-occluded liver.14 In 1975, Honjo et al. reported the first clinical use of portal vein ligation for unresectable primary and metastatic liver cancers.15 In the 1980s, Makuuchi et al.,9 Matsuoka et al.,10 and Kinoshita et al.11 reported the use of percutaneous trans-hepatic PVE before liver resection. In 1994, Kawasaki et al. reported the use of PVE prior to liver resection for bilateral CLMs. Since that time, PVE has been adopted more commonly as a safe, image-guided procedure that increases the volume of FLR.16–20
3. Minimal requirement of future liver remnant volume
Minimal requirements of FLR volume are summarized in Table 1. However, interpretation of these minimal requirements must take various factors into account: (1) the definition of normal liver and injured liver vary, (2) total liver volume used to calculate percent minimal requirement of FLR vary. Generally, the minimal requirement of FLR in patients with normal liver ranges from 20 to 30% using CT-based non-tumor liver volume or formula-based liver volume.7,21–23 Studies reported that the FLR/standardized liver volume < 20–25% 7,23 or the FLR/total liver volume < 25–26.6%21,22 were associated with higher rate of major complication or hepatic dysfunction.
Table 1.
Minimal requirement of future liver remnant volume
| Region | Minimal requirement | Total liver volume | |
|---|---|---|---|
| Normal liver | |||
| Vauthey et al. 7 | USA | 25% | Formula-based liver volume* |
| Shoup et al. 21 | USA | 25% | CT-based non-tumor liver volume |
| Shindle et al. 22 | Europe | 26.6% | CT-based non-tumor liver volume |
| Kishi et al. 23 | USA | 20% | Formula-based liver volume† |
| Injured liver (chronic hepatitis/cirrhosis and chemotherapy) | |||
| Kubota et al. 8† | Asia | 40% or 50%‡ | CT-based non-tumor liver volume |
| Shirabe et al. 24 | Asia | 250mL/m2§ | NA |
| Azoulay et al.17 | Europe | 40%¶ | CT-based non-tumor liver volume |
| Shindoh et al. 25 | USA | 30%∥ | Formula-based liver volume† |
Abbreviations: CT, computed tomography; NA, not applicable.
(Standardized liver volume) = 706.2×(body surface area) + 2.4 by Urata K, et al. 93
(Standardized liver volume) = −794 + 1267.28×(body surface area) by Vauthey JN, et al.94
Based on indocyanine green retention rate at 15 minutes.
Categorized as injured liver based on cohort of patients with hepatocellular carcinoma.
Fibrosis or cirrhosis
Chemotherapy > 3 months
For patients with injured liver (chronic hepatitis or chemotherapy), safe limits of FLR after liver resection are reported to be 30–50% of FLR/CT-based non-tumor liver volume or formula-based liver volume.8,17,24,25 The safety of liver resection is also influenced by the degree of liver injury because FLR volume itself is not correlated with hepatic functional reserve. Therefore, it is difficult to generalize the safety limit of FLR in patients with injured liver. In primarily hepatocellular carcinoma patients, Kubota et al. stratified the minimal requirement of FLR/CT-based non-tumor liver volume using the indocyanine green retention rate at 15 minutes (ICG-R15). Patients with ICG-R15 < 10% tolerated 40% of FLR/CT-based non-tumor liver volume after liver resection and patients with ICG-R15 ≥ 10% tolerated 50% of FLR/CT-based non-tumor liver volume after liver resection. 8 Other groups have also used FLR volume and ICG-R15 for predicting liver dysfunction and/or postoperative mortality.26,27 Liver scintigraphy is another approach to estimate hepatic functional reserve.28 Dinant et al. reported that assessment of 99mTc-mebrofenin uptake in the FLR predicted better than measurement of liver volume. 28 For patients with injured liver, integration of FLR volume and functional assessment is ideal to evaluate “functional FLR” on an individual basis.
FLR volume has been used as a practical decision-making metric for PVE. Kinetic growth rate, defined as the degree of hypertrophy divided by the number of weeks elapsed after PVE, has also been reported to be a sensitive predictor of hepatic functional reserve.29 A kinetic growth rate of at least 2.0% per week is protective of hepatic insufficiency29 defined as a peak bilirubin level > 7.0 mg/dL postoperatively 30 (Figure 1).
Figure 1. Rates of hepatic insufficiency based on kinetic growth rate.
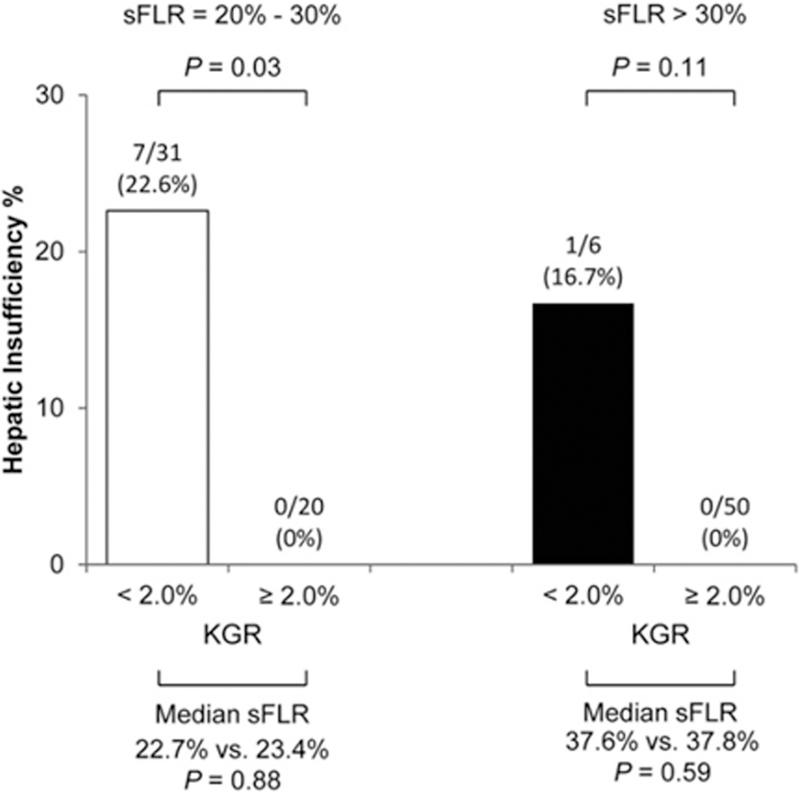
Kinetic growth rate < 2.0% is predictive of hepatic insufficiency irrespective of standardized future liver remnant. KGR, kinetic growth rate; sFLR, standardized future liver remnant. (J Am Coll Surg. 2013 Feb;216(2):201–9, with permission.)
4. Two-stage hepatectomy for patients with bilateral colorectal liver metastases
4.1. Development of two-stage hepatectomy
Although liver resection is the current standard of care for CLM, most patients are unresectable at the time of diagnosis.5 In most cases, the contraindication to curative intent resection is due to small FLR. In 1999, Lygidakis et al. first used the nomenclature, “two-stage hepatectomy” for sequential treatment strategy consisting of portal vein ligation in a first step, transarterial immunochemotherapy, and hemihepatectomy in a second step for treating advanced liver metastases. 31 This approach did not include liver resection in the first step. In 2000, Adam et al. proposed a strategy of two-stage hepatectomy in patients with bilateral CLM.12 The concept of this new strategy is that overall intention is curative, but a first-stage liver resection is not intended to remove all CLMs. A second-stage liver resection is performed after a while to allow hypertrophy of the FLR and to decrease the risk of postoperative liver failure. This approach of two-stage hepatectomy did not include use of PVE in all cases. In 2003, this strategy was further refined by Jaeck et al.13,32 Namely, the first stage hepatectomy aims to remove CLMs located in the hemi-liver with less tumor burden (most typically the left liver). PVE is then performed 2 to 5 weeks after the first stage, followed by a second stage hepatectomy (mostly right hepatectomy or extended right hepatectomy) performed 5 to 8 weeks after PVE. This treatment pathway is widely adopted as “two-stage hepatectomy” for bilateral CLMs to achieve safe and curative liver resection.
4.2. Portal vein embolization or ligation?
Two-stage hepatectomy for bilateral CLMs is a sequential treatment which consists of preoperative chemotherapy, clearance of one hemi-liver, PVE, and hemi-hepatectomy. For primary gastrointestinal tumors with bilateral liver metastases, a similar concept using a planned two-step approach was proposed as “two-step surgery” by Kianmanesh and Belgihiti et al. in 2003.33,34 The first step of this strategy consisted of resection of the primary tumor, clearance of metastases in left liver, and right portal vein ligation. The second step was to perform a right or extended right hepatectomy. Compared to portal vein ligation, PVE is minimally invasive, therefore it is preferred in patients who do not need a staged approach. However, for a two-stage hepatectomy, portal vein ligation may be a viable option because it can be performed in the operating room during the first stage liver resection. Thus, it remains controversial whether PVE or portal vein ligation is used for two-stage hepatectomy for bilateral CLMs.
Table 2 compares the current literature regarding changes in liver volume based on the use of PVE vs. portal vein ligation. Although background characteristics and procedures were heterogeneous in these studies, PVE was associated with better or similar volume increases compared to portal vein ligation.34–38 Specifically, van Lienden et al. demonstrated that collateral flow and reperfusion of the ligated portal venous system developed in patients who underwent portal vein ligation, causing smaller increases in FLR.38 Because the success of two-stage hepatectomy relies on liver hypertrophy of FLR between the first stage and the second stage, PVE is frequently selected based on the higher reported increases in liver volume.
Table 2.
Portal vein embolization vs. portal vein ligation
| PVE | PVL | Hypertrophy | |||||
|---|---|---|---|---|---|---|---|
| N | Volume increase | Morbidity | N | Volume increase | Morbidity | ||
| Broering et al. 35 | 17 | 188 mL | 5.8% | 17 | 123 mL | 5.8% | PVE > PVL |
| Aussilhou et al. 34 | 18 | 35% | NA | 17 | 38% | NA | PVE = PVL |
| Capusotti et al. 95 | 31 | 53.4% | 3.2% | 17 | 43.1% | 0% | PVE = PVL |
| Robles et al. 37 | 18 | 40% | NA | 20 | 30% | NA | PVE > PVL |
| Van Lienden et al. 38 | 14 | 41.6% | NA | 7 | 8.1% | NA | PVE > PVL |
Abbreviations: PVE, portal vein embolization; PVL, portal vein ligation; NA, not available.
4.3. Outcomes of two-stage hepatectomy
Short- and long-term outcomes of two-stage hepatectomy are shown in Table 3. The completion rates for planned first and second liver resections ranged from 60% to 90%.12,32,39–48 Postoperative morbidity rates were 20–60% and mortality rate was 0–15%. The rates of 3-year and 5-year overall survival in the reported series were 30–80% and 30–60%, respectively. The relatively low rates of morbidity and mortality and acceptable survival outcomes support the efficacy of two-stage hepatectomy for selected patients with initially unresectable bilateral CLMs. Among 91 patients who did not complete planned two-stage hepatectomy from 9 studies,39–47 the main reasons were tumor progression after first stage hepatectomy (80%), insufficient FLR (11%), poor physical status (5%), portal vein thrombosis (2%), and death after first stage hepatectomy (2%) (Figure 2). Clearly, it is crucial to protect against tumor progression after first-stage hepatectomy and PVE. Use of preoperative chemotherapy or targeted therapy with bevacizumab during liver hypertrophy was reported to be effective and did not affect growth of FLR.49,50 Recently, a fast-track two-stage hepatectomy pathway was reported, combining the first stage hepatectomy and PVE in a hybrid interventional radiology/operating suite. 51 This streamlined approach can reduce the time between first- and second-stage hepatectomy and allow early return to intended oncologic treatment.
Table 3.
Outcomes of two-stage hepatectomy for bilateral colorectal liver metastases
| Region | Number | Preoperative chemotherapy, % |
PVE, % |
PVL, % |
Completion rate, % |
Postoperative morbidity, % |
Postoperative mortality, % |
3-year OS, % |
5-year OS, % |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Adam et al. 12 | Europe* | 16 | 75 | 44 | 0 | 81 | 38 | 15 | 35 | NA |
| Jaeck et al. 13 | Europe† | 33 | 91 | 100 | 0 | 76 | 56 | 0 | 54 | NA |
| Tanaka et al. 39 | Asia | 24 | 64 | 73 | 0 | 100 | 23 | 0 | 33 | NA |
| Wicherts et al. 40 | Europe* | 59 | 97 | 78 | 0 | 69 | 59 | 7 | 60 | 42 |
| Homayounfar et al. 41 | Europe | 24 | 75 | 0 | 100 | 63 | 58 | 5 | NA | NA |
| Tsai et al. 42 | USA/Europe | 45 | 71 | 7 | 71 | 78 | 26 | 6 | 84 | NA |
| Brouquet et al. 43 | USA‡ | 65 | 100 | 70 | 0 | 72 | 49 | 6 | 84 | 64 |
| Tsim et al. 44 | Europe | 38 | 91 | 95 | 0 | 87 | 33 | 0 | 50§ | NA |
| Narita et al. 45 | Europe† | 80 | 84 | 86 | 4 | 76 | 54 | 0 | 59 | 32 |
| Muratore et al. 46 | Europe | 47 | 79 | 58 | 23 | 77 | 44 | 0 | 65 | NA |
| Turini et al. 47 | Europe | 48 | 100 | 100 | 0 | 71 | 20 | 6 | 59 | 35 |
| Passot et al. 48 | USA‡ | 109 | 100 | 73 | 0 | 82 | 27¶ | 6 | 68∥ | 49∥ |
Abbreviations: PVE, portal vein embolization; PVL, portal vein ligation; NA, not available.
Reports from the same institution.
Reports from the same institution.
Reports from the same institution.
Patients who underwent curative treatment.
Complications graded as the Clavien-Dindo classification ≥ 3
In 89 patients who underwent second-stage resection
Figure 2. Reasons for failure of two-stage hepatectomy.
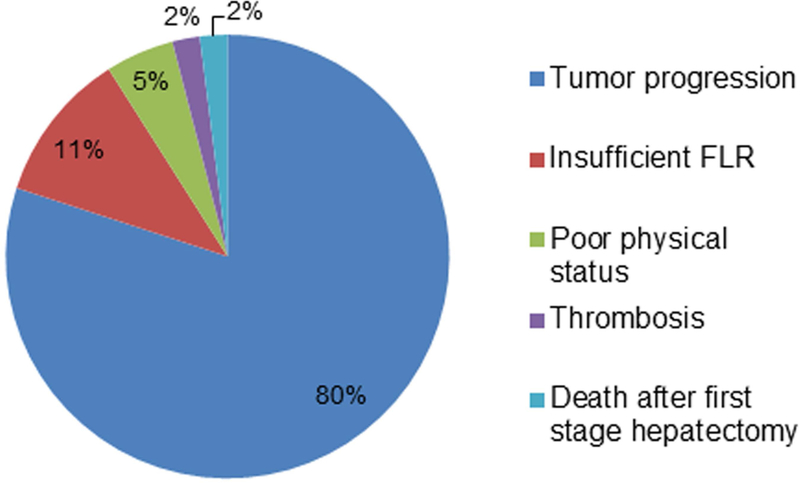
Summative 91 patients who did not complete planned second-stage hepatectomy in 9 studies39–48.
4.4. Technical refinements for Increasing FLR
Insufficient FLR before the second-stage hepatectomy is the second most common reason for non-completion of the two-stage hepatectomy approach. A number of technical tips have been shown to increase FLR. First, embolization using small spherical particles (tris-acryl microspheres) (Figure 3A) may contribute to better hypertrophy than embolization using large nonspherical particles (polyvinyl alcohol) (Figure 3B): increase of FLR, 69.0% ± 30.7% vs. 45.5% ± 40.9%, P = 0.001.52 It should be noted that there are many embolic agents for PVE, including n-butyl cyanoacrylate, ethiodized oil, fibrin glue, ethanol, and microparticles. Each embolic agent has unique technical demands, and no randomized controlled trials have been performed to compare them. Thus, the selection of embolic agents is based on cost, availability, and institutional expertise. Second, for patients undergoing extended right hepatectomy, embolization of segment 4 portal vein together with the right portal vein has been reported to be safe and useful for increasing the liver volume of the left lateral section (Figure 4).32,53,54 Kishi et al. demonstrated a 54% increase in left lateral section liver volume after combined PVE of the right portal vein and segment 4 portal vein compared to 26% increase after PVE of the right portal alone.54 The complication rate after PVE for the right portal vein and segment 4 portal vein was approximately 0–10%.53,54 Finally, sequential ipsilateral hepatic vein embolization after PVE has been reported to result in a greater FLR increase compared to PVE alone.55–59 According to the largest series including 12 patients, the mean proportion of FLR/total liver volume was 39.7 ± 0.6% 1–2 weeks after PVE, and 44.2 ± 1.1% 2 weeks after hepatic vein embolization. There was no report of embolization or dislodgement of coil in the heart or lung.55 However, more studies are needed to assess the benefit of sequential ipsilateral hepatic vein embolization after PVE.
Figure 3. Particles used for portal vein embolization.
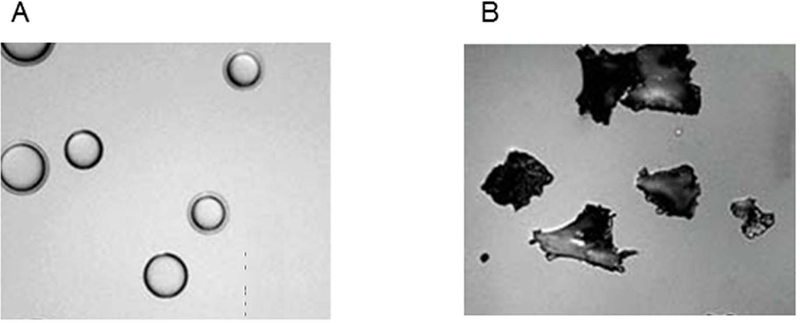
(A) Tris-acryl microspheres (B) Polyvinyl alcohol
Figure 4. Embolization to both the right portal vein and segment 4 portal vein.
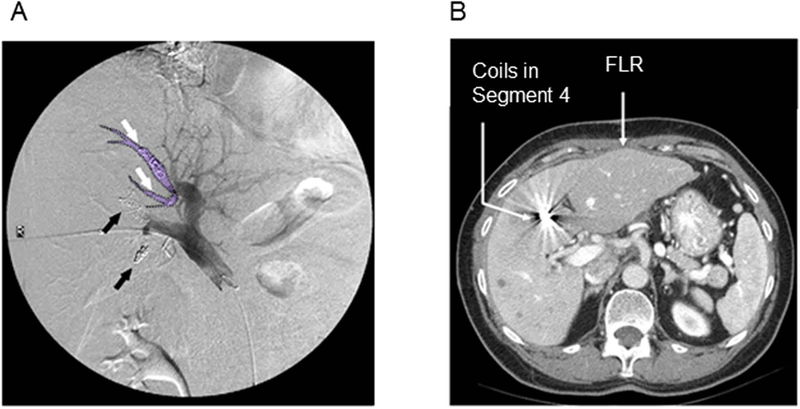
(A) Coils in portal vein 4 are marked with white arrows and the embolized portal vein 4 is outlined in purple. Black arrows indicate anterior and posterior branches of the right portal vein. (B) The right liver and segment 4 with portal vein embolization shows atrophy, whereas segments 2 and 3, the future liver remnant, results in hypertrophy.
4.5. Factors associated with failure of two-stage hepatectomy
Previous studies have reported that patients who undergo an incomplete two-stage hepatectomy have worse survival than patients able to successfully complete two-stage hepatectomy. 40,43,45,60 Thus, the selection of patients who are expected to complete two-stage hepatectomy is important to improve the outcomes for this procedure. Table 4 shows factors associated with failure of two-stage hepatectomy. Number of CLM, largest CLM diameter, longer duration of preoperative chemotherapy, and tumor progression were commonly reported factors. For patients who possess these risk factors, alternative treatment options need to be further investigated.
Table 4.
Factors associated with failure of two-stage hepatectomy
| Regions | N | Failure number | Factors | |
|---|---|---|---|---|
| Narita et al. 45 | Europe | 80 | 29 | 1. Number of CLM in FLR ≥ 3 |
| 2. Age > 70 yr | ||||
| Giuliante et al. 60 | Europe | 130 | 28 | 1. Tumor progression during chemotherapy* |
| Imai et al. 96 | Europe | 125 | 44 | 1. CEA > 30 ng/mL |
| 2. Largest CLM diameter > 4 cm | ||||
| 3. Preoperative chemotherapy > 12 cycles | ||||
| 4. Tumor progression during chemotherapy | ||||
| Passot et al. 48 | USA | 109 | 20 | 1. Number of CLM > 5 |
| 2. Largest CLM diameter > 5 cm | ||||
| 3. Preoperative chemotherapy > 6 cycles | ||||
| 4. PVE | ||||
| 5. Major complication after the first stage | ||||
Abbreviations: CLM, colorectal liver metastasis; FLR, future liver remnant; CEA, carcinoembryonic antigen; PVE, portal vein embolization.
Analyzed in 126 patients after excluding
4.6. Chemotherapy-associated liver injury
Because perioperative chemotherapy is part of the treatment pathway for two-stage hepatectomy, consideration of chemotherapy-associated liver injury is important. Types of liver injury are specific to regimen of chemotherapy. Oxaliplatin-based regimens are associated with sinusoidal obstruction syndrome. 61 Irinotecan-based regimens are associated with steatohepatitis. 62–64 Chemotherapy-associated liver injury and postoperative outcomes are summarized in Table 5. After preoperative chemotherapy, approximately 10% of patients developed sinusoidal obstruction syndrome and approximately 20% of patients developed steatosis, although chemotherapy regimens are different and patients with initially unresectable CLM often receive several regimens.63,64 Specifically, in patients who received oxaliplatin-based regimens, approximately 30–60 % of patients develop sinusoidal obstruction syndrome.65–67 Use of bevacizumab with oxaliplatin-based regimens was reported to have a protective effect on the incidence and severity of sinusoidal obstruction syndrome.68–70 Some studies have shown that patients with chemotherapy-associated liver injury had worse postoperative outcomes,63,67,71,72 and other studies demonstrate similar postoperative morbidity and mortality between patients who underwent chemotherapy and patients who did not. 64–66,73 With respect to the relationship between duration of chemotherapy and postoperative outcomes, evidence suggests prolonged preoperative chemotherapy is associated with a higher risk of postoperative morbidity. It should be noted that the definition of cut-off value for ‘prolonged’ preoperative chemotherapy is different by studies. Increased postoperative morbidity was reported in patients who received preoperative chemotherapy > 6, 9, or 12 cycles. 71,72,74,75
Table 5.
Chemotherapy-associated liver injury and postoperative outcomes
| n | Preoperative chemotherapy, % |
Irinotecan, % |
Oxaliplatin, % |
SOS, %*† |
Steatosis, % *‡ |
Findings | |
|---|---|---|---|---|---|---|---|
| Vauthey et al. 63 | 406 | 61 | 23 | 20 | 9 | 15 | Patients with steatohepatitis had an increased 90-day mortality. |
| Aloia et al. 71 | 92 | 82 | 0 | 57 | NA | 13 | Overall morbidity rates tended to be higher in patients undergoing preoperative chemotherapy. |
| Pawlik et al. 64 | 212 | 72 | 26 | 15 | 5 | 18 | Similar postoperative morbidity and mortality§ |
| Sahajpal et al. | 96 | 55 | 17 | 0 | NA | NA | Similar postoperative morbidity and mortality§ |
| Nakano et al. 72 | 90 | 100 | 22 | 69 | 41 | 38 | Patients with SOS has higher morbidity rate. Use of oxaliplatin > 6 cycles was associated with SOS. |
| Kandutsch et al. 65 | 63 | 79 | 0 | 100 | 43 | 22 | Similar postoperative morbidity and mortality§ |
| Komori et al. 66 | 27 | 56 | 0 | 100 | 33 | 0 | Similar postoperative morbidity and mortality§ |
| Takamoto et al. 97 | 136 | 40 | 47 | 95 | 16 | 11 | 2–4 weeks after chemotherapy cessation improves liver function. |
| Soubrane et al. 67 | 78 | 100 | 0 | 100 | 59 | 12 | High grade SOS was associated with more postoperative morbidity. |
Abbreviations: SOS, sinusoidal obstruction syndrome; VE, portal vein embolization; PVL, portal vein ligation; NA, not available.
Of patients who underwent chemotherapy
Grade 2/3 sinusoidal dilatation61
Steatosis ≥ 30%.
Between patients with and without preoperative chemotherapy.
4.7. The role of gene mutation in two-stage hepatectomy
Recently, molecular alterations in CLM have been a focus for identifying patients who may benefit from liver resection.76–79 Previous studies have shown that mutations in BRAF and KRAS are associated with a poor outcome after CLM resection.76,80–84 Passot et al. demonstrated the importance of RAS as a biologic marker to select patients with bilateral CLM for liver resection.48 In this series, the 5-year OS rate was 67 % in patients with RAS wild-type, compared to only 12% in patients with RAS mutation.
4.8. Two-stage hepatectomy using laparoscopic approach
Laparoscopic liver resection has been increasingly performed due to its advantages over open liver resection, in terms of better surgical and postoperative outcomes in selected patients.85,86 Some case series reported the feasibility of two-stage hepatectomy using laparoscopic approach.87–89 Each group employed different procedures such as a laparoscopic first-stage liver resection followed by an open second-stage hepatectomy, laparoscopic first- and second-stage hepatectomy, and etc. These studies emphasized that minimal adhesion during a second-stage hepatectomy is the benefit of laparoscopic approach. However, laparoscopic right hepatectomy or laparoscopic extended-right hepatectomy should be considered as having high surgical complexity.90 The use of laparoscopic two-stage hepatectomy should be limited to centers with advanced experiences in hepatobiliary open and laparoscopic surgery.
4.9. Repeat surgery for recurrence after two-stage hepatectomy
Recurrence after two-stage hepatectomy is frequent because this approach is typically employed for patients with multiple and bilateral CLMs.25, 27, 34–42 Two studies have focused on the prognosis of recurrence after two-stage hepatectomy.91,92 Imai et al reported that 38 patients (61%) underwent repeat surgery for recurrence after two-stage hepatectomy and patients who underwent repeat surgery had better overall survival than patients who did not (46% vs. 26%, P = 0.004). Lillemoe et al. reported that 31 patients (37%) underwent resection for recurrence.92 RAS mutation and first recurrence in multiple sites were associated with worse survival. Specifically, the 5-year OS rate in patients with RAS mutation who underwent repeat surgery for recurrence after two-stage hepatectomy was 38%, whereas the 5-year OS rate in patients with RAS wild-type who underwent repeat surgery was 86%.
5. Conclusion
Two-stage hepatectomy is an established treatment pathway which consists of perioperative chemotherapy, PVE, and two planned liver resections to deal with an insufficient FLR. This approach was originally designed to improve resectability of patients with bilateral CLMs and has been refined both in terms of the method for occluding the portal vein and advancements in chemotherapy over the past 20 years. Studies demonstrate that two-stage hepatectomy is safe for preserving sufficient FLR and is associated with better OS in patients who complete both planned liver resections than in patients who did not. Additionally, repeat surgery for patients who developed recurrence after two-stage hepatectomy has a survival benefit. Tumor progression between the first and second liver resections is the primary reason for failure of two-stage hepatectomy and remains a major limitation. This issue needs to be further investigated to increase the rate of completion of two-stage hepatectomy and to effectively use the two-stage hepatectomy approach.
Synopsis.
Colorectal liver metastases are not always resectable at the time of diagnosis. An insufficient future liver remnant is an issue to exclude patients from curative intent resection. Portal embolization and two-stage hepatectomy developed as safe and oncologically-effective methods to deal with insufficient future liver remnant.
Acknowledgement
The authors thank Ms. Ruth Haynes for administrative support in the preparation of this manuscript.
Grant Support: This research was supported in part by the National Institutes of Health (T32 CA 009599) and the MD Anderson Cancer Center Support Grant, CA016672.
ABBREVIATIONS
- CLM
colorectal liver metastases
- OS
overall survival
- FLR
future liver remnant
- PVE
portal vein embolization
- OS
overall survival
- FLR
future liver remnant
Footnotes
Disclosures: Nothing to disclose.
REFERENCES
- 1.Manfredi S, Lepage C, Hatem C et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choti MA, Sitzmann JV, Tiburi MF et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdalla EK, Vauthey J-N, Ellis LM et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez FG, Drebin JA, Linehan DC et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by Positron Emission Tomography with F-18 Fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheele J, Stang R, Altendorf-Hofmann A et al. Resection of colorectal liver metastases. World J Surg 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 6.Bismuth H, Adam R, Levi F et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vauthey JN, Chaoui A, Do KA et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512–519. [DOI] [PubMed] [Google Scholar]
- 8.Kubota K, Makuuchi M, Kusaka K et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176–1181. [DOI] [PubMed] [Google Scholar]
- 9.Makuuchi M, Takayasu K, Takuma T et al. Preoperative transcatheter embolization of the portal venous branch for patients receiving extended lobectomy due to the bile duct carcinoma. J Jpn Soc Clin Surg 1984;45:14–21. [Google Scholar]
- 10.Matsuoka T, Nakatsuka H, Kobayashi N et al. [Portal vein embolization for hepatoma with lipiodol-fibrin adhesive mixture]. Nihon Igaku Hoshasen Gakkai Zasshi 1984;44:1411–1413. [PubMed] [Google Scholar]
- 11.Kinoshita H, Sakai K, Hirohashi K et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803–808. [DOI] [PubMed] [Google Scholar]
- 12.Adam R, Laurent A, Azoulay D et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaeck D, Bachellier P, Nakano H et al. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg 2003;185:221–229. [DOI] [PubMed] [Google Scholar]
- 14.Rous P, Larimore LD. Relation of the portal blood to liver maintenance : A demonstration of liver atrophy conditional on compensation. J Exp Med 1920;31:609–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honjo I, Suzuki T, Ozawa K et al. Ligation of a branch of the portal vein for carcinoma of the liver. Am J Surg 1975;130:296–302. [DOI] [PubMed] [Google Scholar]
- 16.de Baere T, Roche A, Elias D et al. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology 1996;24:1386–1391. [DOI] [PubMed] [Google Scholar]
- 17.Azoulay D, Castaing D, Krissat J et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg 2000;232:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abulkhir A, Limongelli P, Healey AJ et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49–57. [DOI] [PubMed] [Google Scholar]
- 19.Giraudo G, Greget M, Oussoultzoglou E et al. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery 2008;143:476–482. [DOI] [PubMed] [Google Scholar]
- 20.Mueller L, Hillert C, Moller L et al. Major hepatectomy for colorectal metastases: is preoperative portal occlusion an oncological risk factor? Ann Surg Oncol 2008;15:1908–1917. [DOI] [PubMed] [Google Scholar]
- 21.Shoup M Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 2003;7:325–330. [DOI] [PubMed] [Google Scholar]
- 22.Schindl MJ, Redhead DN, Fearon KC et al. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005;54:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishi Y, Abdalla EK, Chun YS et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540–548. [DOI] [PubMed] [Google Scholar]
- 24.Shirabe K, Shimada M, Gion T et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999;188:304–309. [DOI] [PubMed] [Google Scholar]
- 25.Shindoh J, Tzeng CW, Aloia TA et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol 2013;20:2493–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamanaka N, Okamoto E, Oriyama T et al. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg 1994;219:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CF, Yu MC, Kuo LM et al. Using indocyanine green test to avoid post-hepatectomy liver dysfunction. Chang Gung Med J 2007;30:333–338. [PubMed] [Google Scholar]
- 28.Dinant S, de Graaf W, Verwer BJ et al. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med 2007;48:685–692. [DOI] [PubMed] [Google Scholar]
- 29.Shindoh J, Truty MJ, Aloia TA et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 2013;216:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullen JT, Ribero D, Reddy SK et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854–862. [DOI] [PubMed] [Google Scholar]
- 31.Lygidakis NJ, Vlachos L, Raptis S et al. New frontiers in liver surgery. Two-stage liver surgery for the management of advanced metastatic liver disease. Hepatogastroenterology 1999;46:2216–2228. [PubMed] [Google Scholar]
- 32.Jaeck D, Oussoultzoglou E, Rosso E et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg 2004;240:1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kianmanesh R, Farges O, Abdalla EK et al. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg 2003;197:164–170. [DOI] [PubMed] [Google Scholar]
- 34.Aussilhou B, Lesurtel M, Sauvanet A et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg 2008;12:297–303. [DOI] [PubMed] [Google Scholar]
- 35.Broering DC, Hillert C, Krupski G et al. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg 2002;6:905–913. [DOI] [PubMed] [Google Scholar]
- 36.Capussotti L, Muratore A, Ferrero A et al. Extension of right portal vein embolization to segment IV portal branches. Arch Surg 2005;140:1100–1103. [DOI] [PubMed] [Google Scholar]
- 37.Robles R, Marin C, Lopez-Conesa A et al. Comparative study of right portal vein ligation versus embolisation for induction of hypertrophy in two-stage hepatectomy for multiple bilateral colorectal liver metastases. Eur J Surg Oncol 2012;38:586–593. [DOI] [PubMed] [Google Scholar]
- 38.van Lienden KP, Hoekstra LT, Bennink RJ et al. Intrahepatic left to right portoportal venous collateral vascular formation in patients undergoing right portal vein ligation. Cardiovasc Intervent Radiol 2013;36:1572–1579. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka K, Shimada H, Matsuo K et al. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol 2007;33:329–335. [DOI] [PubMed] [Google Scholar]
- 40.Wicherts DA, Miller R, de Haas RJ et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg 2008;248:994–1005. [DOI] [PubMed] [Google Scholar]
- 41.Homayounfar K, Liersch T, Schuetze G et al. Two-stage hepatectomy (R0) with portal vein ligation--towards curing patients with extended bilobular colorectal liver metastases. Int J Colorectal Dis 2009;24:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai S, Marques HP, de Jong MC et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford) 2010;12:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brouquet A, Abdalla EK, Kopetz S et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsim N, Healey AJ, Frampton AE et al. Two-stage resection for bilobar colorectal liver metastases: R0 resection is the key. Ann Surg Oncol 2011;18:1939–1946. [DOI] [PubMed] [Google Scholar]
- 45.Narita M, Oussoultzoglou E, Jaeck D et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg 2011;98:1463–1475. [DOI] [PubMed] [Google Scholar]
- 46.Muratore A, Zimmitti G, Ribero D et al. Chemotherapy between the first and second stages of a two-stage hepatectomy for colorectal liver metastases: should we routinely recommend it? Ann Surg Oncol 2012;19:1310–1315. [DOI] [PubMed] [Google Scholar]
- 47.Turrini O, Ewald J, Viret F et al. Two-stage hepatectomy: who will not jump over the second hurdle? Eur J Surg Oncol 2012;38:266–273. [DOI] [PubMed] [Google Scholar]
- 48.Passot G, Chun YS, Kopetz SE et al. Predictors of safety and efficacy of 2-stage hepatectomy for bilateral colorectal liver metastases. J Am Coll Surg 2016;223:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribero D, Abdalla EK, Madoff DC et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386–1394. [DOI] [PubMed] [Google Scholar]
- 50.Zorzi D, Chun YS, Madoff DC et al. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann Surg Oncol 2008;15:2765–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odisio BC, Simoneau E, Holmes AA et al. Fast-track two-stage hepatectomy using a hybrid interventional radiology/operating suite as alternative option to associated liver partition and portal vein ligation for staged hepatectomy Procedure. J Am Coll Surg 2018;227:e5–e10. [DOI] [PubMed] [Google Scholar]
- 52.Madoff DC, Abdalla EK, Gupta S et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 2005;16:215–225. [DOI] [PubMed] [Google Scholar]
- 53.Nagino M, Kamiya J, Uesaka K et al. [Extended liver resection for hilar cholangiocarcinoma]. Nihon Geka Gakkai Zasshi 2000;101:408–412. [PubMed] [Google Scholar]
- 54.Kishi Y, Madoff DC, Abdalla EK et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery 2008;144:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang S, Lee SG, Ko GY et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg 2009;249:608–616. [DOI] [PubMed] [Google Scholar]
- 56.van Lienden KP, van den Esschert JW, Rietkerk M et al. Short-term effects of combined hepatic vein embolization and portal vein embolization for the induction of liver regeneration in a rabbit model. J Vasc Interv Radiol 2012;23:962–967. [DOI] [PubMed] [Google Scholar]
- 57.Hwang S, Ha TY, Ko GY et al. Preoperative sequential portal and hepatic vein embolization in patients with hepatobiliary malignancy. World J Surg 2015;39:2990–2998. [DOI] [PubMed] [Google Scholar]
- 58.Guiu B, Chevallier P, Denys A et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol 2016;26:4259–4267. [DOI] [PubMed] [Google Scholar]
- 59.Le Roy B, Perrey A, Fontarensky M et al. Combined preoperative portal and hepatic vein embolization (biembolization) to improve liver regeneration before major liver resection: A preliminary report. World J Surg 2017;41:1848–1856. [DOI] [PubMed] [Google Scholar]
- 60.Giuliante F, Ardito F, Ferrero A et al. Tumor progression during preoperative chemotherapy predicts failure to complete 2-stage hepatectomy for colorectal liver metastases: results of an Italian multicenter analysis of 130 patients. J Am Coll Surg 2014;219:285–294. [DOI] [PubMed] [Google Scholar]
- 61.Rubbia-Brandt L, Audard V, Sartoretti P et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004;15:460–466. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez FG, Ritter J, Goodwin JW et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg 2005;200:845–853. [DOI] [PubMed] [Google Scholar]
- 63.Vauthey JN, Pawlik TM, Ribero D et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065–2072. [DOI] [PubMed] [Google Scholar]
- 64.Pawlik TM, Olino K, Gleisner AL et al. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg 2007;11:860–868. [DOI] [PubMed] [Google Scholar]
- 65.Kandutsch S, Klinger M, Hacker S et al. Patterns of hepatotoxicity after chemotherapy for colorectal cancer liver metastases. Eur J Surg Oncol 2008;34:1231–1236. [DOI] [PubMed] [Google Scholar]
- 66.Komori H, Beppu T, Baba Y et al. Histological liver injury and surgical outcome after FOLFOX followed by a hepatectomy for colorectal liver metastases in Japanese patients. Int J Clin Oncol 2010;15:263–270. [DOI] [PubMed] [Google Scholar]
- 67.Soubrane O, Brouquet A, Zalinski S et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg 2010;251:454–460. [DOI] [PubMed] [Google Scholar]
- 68.van der Pool AE, Marsman HA, Verheij J et al. Effect of bevacizumab added preoperatively to oxaliplatin on liver injury and complications after resection of colorectal liver metastases. J Surg Oncol 2012;106:892–897. [DOI] [PubMed] [Google Scholar]
- 69.Ribero D, Wang H, Donadon M et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007;110:2761–2767. [DOI] [PubMed] [Google Scholar]
- 70.Rubbia-Brandt L, Lauwers GY, Wang H et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology 2010;56:430–439. [DOI] [PubMed] [Google Scholar]
- 71.Aloia T, Sebagh M, Plasse M et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol 2006;24:4983–4990. [DOI] [PubMed] [Google Scholar]
- 72.Nakano H, Oussoultzoglou E, Rosso E et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008;247:118–124. [DOI] [PubMed] [Google Scholar]
- 73.Sahajpal A, Vollmer CM Jr., Dixon E et al. Chemotherapy for colorectal cancer prior to liver resection for colorectal cancer hepatic metastases does not adversely affect peri-operative outcomes. J Surg Oncol 2007;95:22–27. [DOI] [PubMed] [Google Scholar]
- 74.Karoui M, Penna C, Amin-Hashem M et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 2006;243:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kishi Y, Zorzi D, Contreras CM et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 2010;17:2870–2876. [DOI] [PubMed] [Google Scholar]
- 76.Nash GM, Gimbel M, Shia J et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol 2010;17:572–578. [DOI] [PubMed] [Google Scholar]
- 77.Vauthey JN, Zimmitti G, Kopetz SE et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013;258:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karagkounis G, Torbenson MS, Daniel HD et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer 2013;119:4137–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vauthey JN, Kopetz SE. From multidisciplinary to personalized treatment of colorectal liver metastases: 4 reasons to consider RAS. Cancer 2013;119:4083–4085. [DOI] [PubMed] [Google Scholar]
- 80.Teng HW, Huang YC, Lin JK et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol 2012;106:123–129. [DOI] [PubMed] [Google Scholar]
- 81.Umeda Y, Nagasaka T, Mori Y et al. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J Hepatobiliary Pancreat Sci 2013;20:223–233. [DOI] [PubMed] [Google Scholar]
- 82.Yaeger R, Cercek A, Chou JF et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014;120:2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schirripa M, Bergamo F, Cremolini C et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer 2015;112:1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brudvik KW, Kopetz SE, Li L et al. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg 2015;102:1175–1183. [DOI] [PubMed] [Google Scholar]
- 85.Kawaguchi Y, Hasegawa K, Wakabayashi G et al. Survey results on daily practice in open and laparoscopic liver resections from 27 centers participating in the second International Consensus Conference. J Hepatobiliary Pancreat Sci 2016;23:283–288. [DOI] [PubMed] [Google Scholar]
- 86.Ciria R, Cherqui D, Geller DA et al. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 2016;263:761–777. [DOI] [PubMed] [Google Scholar]
- 87.Di Fabio F, Whistance R, Rahman S et al. Exploring the role of laparoscopic surgery in two-stage hepatectomy for bilobar colorectal liver metastases. J Laparoendosc Adv Surg Tech A 2012;22:647–650. [DOI] [PubMed] [Google Scholar]
- 88.Fuks D, Nomi T, Ogiso S et al. Laparoscopic two-stage hepatectomy for bilobar colorectal liver metastases. Br J Surg 2015;102:1684–1690. [DOI] [PubMed] [Google Scholar]
- 89.Kilburn DJ, Chiow AK, Lewin J et al. Laparoscopic approach to a planned two-stage hepatectomy for bilobar colorectal liver metastases. ANZ J Surg 2016;86:811–815. [DOI] [PubMed] [Google Scholar]
- 90.Kawaguchi Y, Fuks D, Kokudo N et al. Difficulty of laparoscopic liver resection: proposal for a new classification. Ann Surg 2018;267:13–17. [DOI] [PubMed] [Google Scholar]
- 91.Imai K, Benitez CC, Allard MA et al. Impact of surgical treatment for recurrence after 2-stage hepatectomy for colorectal liver metastases, on patient outcome. Ann Surg 2017; [DOI] [PubMed] [Google Scholar]
- 92.Lillemoe HA, Kawaguchi Y, Passot G et al. Surgical resection for recurrence after two-stage hepatectomy for colorectal liver metastases is feasible, is safe, and improves survival. J Gastrointest Surg 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]


