Abstract
Adopted guidelines reflect a harmonised European approach to a specific scientific issue and should reflect the most recent scientific knowledge. However, whilst EU regulations are mandatory for all member states and EU directives must be followed by national laws in line with the directive, EMA guidelines do not have legal force and alternative approaches may be taken, but these obviously require more justification. This new series of the BJCP, developed in collaboration with the EMA, aims to address this issue by providing an annotated version of some relevant EMA guidelines and regulatory documents by experts. Hopefully, this will help in promoting their diffusion and in opening a forum for discussion with our readers.
BACKGROUND
For a medicine to be authorised, clinical efficacy and safety should be established, usually through robust and relevant clinical trial data. The conduct of paediatric clinical trials is, however, fraught with hurdles related to operational practicalities, regional differences in standards of care, lack of standard of care, cultural expectations, and ethical challenges. Additional complexities relate to the rarity of the diseases and gaps in knowledge about the pathophysiology and epidemiology of diseases across paediatric age subsets, particularly in neonates and children less than 2 years of age. These challenges lead to concern internationally that despite the implementation of the legal framework in the United States and from the first 10 years of the Paediatric Regulation in the EU, depending on the disease and age of the child, 50% to 80% of children are still treated off‐label.1, 2, 3
Often, drug development proceeds in adults first, and once approved in adults, medicines are prescribed off‐label to children, out of need, long before an evidence base establishes efficacy, safety, and appropriate dosing. This is particularly of relevance for the youngest age ranges, as typically, for these groups, dosages are more difficult to predict, and both the disease and the response to the drug may differ, to at least some extent. For the last decades, when a medicine is not authorised for children, paediatric clinical practice will rely on experience and judgement to determine dosing, using data from adults, non‐clinical studies, case reports, publications, and clinical practice as standard sources of information. In Figure 1, this situation is represented as “implicit extrapolation.” “Implicit extrapolation” is inherently subjective and is not informed by an evidence base that could lead to a therapeutic indication in paediatrics in a Summary of Product Characteristics (SmPC). Acknowledging the need to generate robust evidence on the safety and efficacy of medicines in children, the practical limitations of conducting clinical studies, and the potential for data generated in one population (eg, adults) to be useful for making predictions of drug effects in another population (eg, children), a systematic and quantitative approach to using available evidence leading towards evidence‐based or “explicit” extrapolation has the potential to be an important component of the development of medicines in children.
Figure 1.
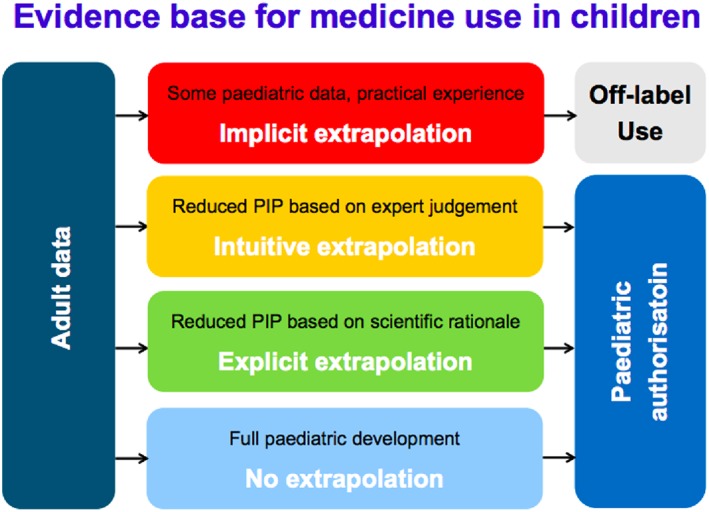
Extrapolation and paediatric indication, C. Male EMA extrapolation workshop4 May 2016
Paediatric extrapolation has been described in FDA and international guidance to industry at International Council for Harmonisation (ICH) level over the past decades.5, 6 The FDA published in 1994 an algorithm, revised7 in 2014 which provided an assumptions‐based framework for the extrapolation of efficacy from adults to the paediatric population. Based on US law, the paediatric study decision tree allows extrapolation if there is sufficient similarity of both (i) disease progression and (ii) response to intervention between source and target population.8, 9
With knowledge and experience in paediatric development increasing over the years, the EMA identified in its Extrapolation Concept Paper published10 in 2012 the benefit of going beyond the FDA algorithm and its set of rules. The concept paper discussed the possibility to develop an expanded and refined algorithm for extrapolation allowing for a more refined approach towards paediatric development, relying on three main areas: pharmacology, disease manifestation and progression, and clinical response to treatment. Such an approach is moving away from the prerequisite that the disease between adult and children should be entirely similar to use extrapolation; instead, differences, uncertainties, and gaps in knowledge are addressed by evidence generated within an extrapolation exercise. This aligns the use of extrapolation with the new era of precision medicine, where medical practice will be based on detailed genetic and other molecular understanding about a patient and its disease.
The EMA framework for the use of extrapolation approaches in paediatric programmes promotes context‐dependent approaches for how different types of prior knowledge, quantitatively synthesised, could be used to support assumptions or make predictions for treatment effects in a paediatric target population.11 The framework promotes a multidisciplinary approach to integrate the available evidence in order to determine the studies that should be conducted in the target population. The concept paper was followed by a DRAFT Reflection Paper in 2017, which was revised following a public consultation held between12 October 2017 and January 2018. The framework was developed with the input from many stakeholders (EMA committees and working parties, medicine developers, academia, patients, and international regulatory partners).
Historically, the evidence base generated on the use of a medicine in children has been insufficient to support a regulatory assessment of positive risk‐benefit and inadequate to properly inform prescribers. Despite an increase in “intuitive extrapolation,” it is recognised that paediatric development programmes planned in recent years have continued to be suboptimal. Whilst the difficulties of conducting clinical trials in children remain, improved understanding of diseases and pharmacology gives the possibility to move towards “explicit extrapolation” based on scientific rationale. This should represent a paradigm shift whereby a high‐quality evidence base for the use of medicines in children can be generated, leading to paediatric indications in section 4.1 of the SmPC13 to reflect in which disease and target population the benefit/risk balance is established to be positive.
The use of paediatric extrapolation is now not only a strategy to increase the efficiency of paediatric medicines development but also an ethical imperative and part of a new paradigm in global paediatric drug development.14, 15
This commentary aims to put the Reflection Paper into perspective by guiding the reader of the regulatory document through its chapters and expanding on how the Reflection Paper might impact practical aspects of paediatric development. The section headings in this commentary provide a direct hyperlink to the corresponding section of the Reflection Paper under discussion in a copy of the Reflection Paper that is available as Supporting Information to this article.
1. INTRODUCTION
The introduction to the Reflection Paper provides a motivation, stating that the aims of the Paediatric Regulation,16 which is to ensure that medicines for use in children are of high quality, are ethically researched and are authorised appropriately. The primary rationale for using extrapolation approaches is to avoid unnecessary studies in the paediatric population for ethical reasons, for efficiency, and to allocate resources to areas where studies are the most needed. The Reflection Paper encourages applicants to use integrated approaches for the planning of extrapolation prospectively and early in the drug development. It promotes the need for designing and conducting study(ies) (in adults and/or in older children) with an intention to inform a paediatric development based on extrapolation. The complexity of this integrated approach requires careful scientific scrutiny, documentation, and collaboration to ensure that children only participate in clinical trials that further the scientific understanding of a medicinal product for use in children and address the requirements for regulatory decision‐making.
2. SCOPE
The final version of the Reflection Paper aims to assist medicine developers, regulators, patients and health care professionals, and investigators and stakeholders, using extrapolation approaches with the purpose to inform the authorisation and use of compounds in the paediatric population. The team of experts should be supported by the use of modern tools, such as clinical biomarkers, pharmacometrics, and statistical models, including physiologically based pharmacokinetic modelling (PBPK). Appropriate use of such tools has the potential to enable a faster progress of paediatric development and hence a timely access for paediatric patients to an authorised medicinal product. On the other hand, the possibility to use extrapolation rather than to conduct stand‐alone clinical efficacy and safety studies in the target population might be found attractive simply because of potential savings in time and cost to the developer. The use of extrapolation should always be scientifically justifiable, and faster progress should not come at the cost of increased risk to children. Hence, a thorough understanding of the value and limitations of the various tools/methods/technologies and appropriate use of the data derived from these should ultimately promote scientifically justified studies in the target population, a robust clinical evidence base and a better use of resources. The focus of the Reflection Paper is to provide a framework for extrapolation as an approach to generate evidence on one or more specific research questions (cross refer to Section 4) to support regulatory assessment of marketing authorisation application in a target paediatric population.
3. LEGAL BASIS AND RELEVANT GUIDELINES
This Reflection Paper should be read in conjunction with the introduction and general principles of the Annex I to Directive 2001/83/EC as amended,17 which apply to the request for authorisation of a clinical trial to competent authorities. Applications for marketing authorisation submitted to the European Medicines Agency (EMA) that concern medicines not authorised in the European Union (EU) on 26 July 2008 must include the results of studies carried out as part of an agreed paediatric investigation plan (PIP) or information on a PIP deferral or waiver, except for products authorised under Articles 10, 10a, 13 to 16, or 16a to 16i of Directive 2001/83/EC as amended. The Reflection Paper refers to a long list of EU and ICH guidelines relating to paediatric development, which should be considered when planning and designing paediatric development.
4. GENERAL CONSIDERATIONS
A fundamental principle underpinning a well‐justified extrapolation exercise under the new EMA framework is that evidence to support regulatory decision‐making is not compromised. An extrapolation exercise with or without (limited) confirmatory data from trials seeks to generate the same strength of evidence as would be derived from clinical trials measuring treatment effects in the target population.
Extrapolation exercises will be constructed for specific research questions within a development programme to support marketing authorisation; it will usually be necessary to address research questions related to pharmacokinetics, pharmacodynamics, efficacy, and safety. For example, depending on the therapeutic area (see section 5.5) and pharmacological parameters of a compound, evidence of efficacy might comprise demonstration of short‐term effects, maintenance of effect, and/or effects on long‐term clinical outcomes. Thus, for each development programme, multiple research questions can be identified. Extrapolation exercises are more likely to be employed in addressing research questions related to pharmacokinetics, dose‐response, and clinical efficacy than questions of clinical safety or where response to treatment will be impacted by maturation. Extrapolation of exposure, efficacy, and safety data should be addressed separately for each age group and/or disease subsets. Safety information from a source population (eg, the same paediatric population for another disease or from other drugs with the same of mode of action) may be used to predict short‐term risks related to the mode of action of the drug and related to dose. However, considering that long‐term risks related to growth and maturation cannot be extrapolated from adults, generation of new safety data is needed in the target population to address unexpected (age‐specific) risks; thus, to rely only on extrapolation for understanding of safety will not usually be possible, certainly for treatments intended to be dosed chronically. This indicates that a full paediatric development programme can be a combination of extrapolation exercises and generation of stand‐alone clinical data.
By using relevant and reliable information generated in a source population and the synthesis (preferably quantitative, guided by the extrapolation framework table) of available information characterising similarities, differences, and gaps in knowledge between source and target populations, this evidence might be derived using fewer paediatric patients in clinical trials.
The EMA framework introduces, for each specific research question to be addressed using extrapolation, an extrapolation concept, an extrapolation plan, and the mitigation of uncertainties. The extrapolation concept, based on the synthesis of available information, is a statement of the effects of treatment in the target population that might be predicted if specified assumptions hold true and gaps in knowledge can be addressed. These assumptions and gaps in knowledge are addressed in prospective studies that are detailed in the extrapolation plan. Once completed, the data generated lead to the extrapolation concept being validated or, if needed, revised. Studies detailed in the extrapolation plan might be continued to provide more precise estimates of treatment effect and additional long‐term data on efficacy or safety or otherwise to mitigate uncertainties in the extrapolation exercise.
Extrapolation exercises can take different forms based on the therapeutic setting and the available knowledge which might support an extrapolation plan aiming to establish a dose level that will give rise to similar exposures in the source and the target populations; a plan aiming to demonstrate a similar PK/PD relationship; or a plan aiming to generate some efficacy and safety data in the target population that is to be compared with, or pooled with, similar data from the target population.
In most cases, developing an extrapolation concept should include a detailed analysis of the mechanism of action of the drug (drug disposition and pharmacodynamic effects), disease characteristics, and manifestations of the source and target populations. These are to be synthesised using multidisciplinary techniques in order to make the best possible predictions for the expected clinical response to treatment for efficacy and safety in the target population and, critically, to identify uncertainties that need to be addressed to make the extrapolation concept valid. Factors that might modify the effects of treatment between source and target populations should be identified. For factors where reliable and informative data are not available, these gaps in knowledge need to be investigated in the extrapolation plan. It is of utmost importance that the best possible predictions about treatment effects in the target population are made (extrapolation concept) and then the appropriate data collected in the studies covered in the extrapolation plan that can confirm, amend, or refute the validity of the predictions.
Importantly, as knowledge increases along the course of the development, the assumptions made initially may need to be revisited (iterations) and potentially revised based on newly generated data. For example, if not supported in a CHMP guideline, the implications of assumptions introduced into the extrapolation concept such as a proposal ruling out any differences between age groups and disease subsets and the required data to evaluate the assumptions in the extrapolation plan could be based on a literature review supported by M&S. However, if the outcome of the literature review and M&S cannot confirm the original assumptions and the impact of the remaining gaps in knowledge cannot be characterised, it will be necessary to investigate differences between age groups and disease subsets as part of the clinical studies. Conversely, if differences are being ruled out, the clinical studies might particularly focus on those age subsets or disease subsets where gaps in knowledge are greatest (eg, infants and neonates). The need for incorporating emerging data can be outlined as interim steps in the extrapolation plan.
Indeed, a major emphasis in the Reflection Paper is on the importance of pre‐planning, considering how paediatric development might be conducted as early as the exploratory phase of development in adults (where that is the initial target population). A development programme in adults that generates data to support a future extrapolation exercise might eventually focus the objectives and reduce the size of clinical studies in different paediatric age subsets. A full understanding of the disease and its epidemiology will also help in characterising the paediatric population: It might be that features other than chronological age better distinguish parts of the paediatric population, eg, genotype or phenotype of disease. In addition, if a valid extrapolation concept in an extreme of the paediatric population can be substantiated, interpolation to other parts of the paediatric population might be justified, again reducing the need for clinical studies in paediatric patients without compromising the evidence base to support licensing.
5. PROPOSED FRAMEWORK
Whereas Section 4 outlines the framework in general terms, Section 5 goes into further details and presents some methodological considerations. Whilst some of the methods referenced are not novel, experience with their use in extrapolation exercises is limited and research into methodology to support extrapolation is continuing. Because regulatory guidance documents should not limit development of methodology, it is not in the scope of the Reflection Paper to dictate a preferred methodological approach.
5.1. Extrapolation concept: synthesising evidence to identify gaps in knowledge and to derive expectations for effects in the target population
Following the public consultation, section 5.1 of the Reflection Paper has been revised in order to better delineate the steps required not only to build and structure the extrapolation concept but also to facilitate the use and development of model‐informed strategies. The evaluation of the extrapolation concept submitted will relate to uncertainties in understanding the compound, the characteristics of disease in the source and target populations, and the anticipated clinical response to the treatment, including related to physiological uncertainties.
Ultimately, the outcome of the extrapolation concept exercise should provide a scientific rationale which, once gaps in knowledge and uncertainties are identified and addressed, provide knowledge of treatment effects without a complete set of prospective studies, or indicate that additional clinical information is needed.
5.1.1. Existing knowledge and data sources to develop the extrapolation concept, 5.1.2 Evidence synthesis leading to expectations for drug effects in the target population, and 5.1.3 Factors that could limit extrapolation
The development of explicit qualitative and quantitative hypothesis on the expected difference in response to the medicine between the target population and the source population is a complex matter. These sections outline points to consider in this respect.
The challenge of an extrapolation approach in initial planning of paediatric drug development is facing the scarcity of available “source data” (eg, typically, minimal adult data are available). Whilst the concept and the plan can develop as knowledge accumulates, it is of utmost importance to ensure that the selected data sources gathered are to the highest possible standards in terms of quality, completeness, and relevance from the source population. Additionally, the Reflection Paper promotes as a crucial step the use of quantitative methods for evidence synthesis to identify the potential similarities and potential differences between source and target population. A specific paragraph states how assumptions and uncertainties should be structured around the main areas of pharmacology (drug disposition and effect), disease manifestation and progression, and clinical response to treatment (efficacy and safety). The assumptions and uncertainties in these areas should establish a line of reasoning about the relation between dose, exposure, pharmacodynamic effect(s), and clinical response(s) for each target age group or disease subset. As per the Addendum to ICH E11, in paediatric development, chronological age alone may not in all cases serve as an adequate categorical determinant to define developmental subgroups in paediatric studies. The arbitrary division of paediatric subgroups by chronological age for some conditions may have no scientific basis and could unnecessarily delay development of medicines for children by limiting the population for study. Therefore, as per illustrated in Table 1, physiological development and maturity of organs, pathophysiology and natural history of the disease or condition, available treatment options, and the pharmacology of the investigational product are factors to be considered in determining the subgroups in paediatric studies. It is important to note that Table 1 represents one summary of available information, considering that the knowledge will accumulate and change over time and others' approach might be justified. Particularly for modelling and simulation, the inclusion of factors related to ontogeny or organ maturity is essential to ensure the accuracy of developed models. The relevance of factors and therefore their inclusion in the model should be based on the pharmacology of the drug, the age and physiology of the target population, and the dose and route of administration of the drug.20
Table 1.
Potential factors related to ontogeny or organ maturity to be considered in paediatric development
| Preterm and term newborn (0‐27 d) infants18 | Infants and toddlers (28 d‐23 mo) | Children (2‐11 y) | Adolescents (12‐18 y)19 | ||
|---|---|---|---|---|---|
| Factors associated to changes in organ function20, 21 | Factors associated to changes in growth bodyweight and size, organ weight, tissue composition (plasma proteins, water, and fat composition) | ||||
| GI system | Attention must be paid to some increased or decreased enzyme activity or secretion such as enzyme activity increased such as lactase or CYP1A1 that increases over time to adult levels, but also to gastrointestinal (GI) pH, GI volume, GI transit times | ||||
| Lung | Alveolar proliferation, microvascular maturation, alveolar (expansion), normal lung growth period: until at least 8 y of age | Interpolation from data generated in studies in adults may be possible if specific studies have been carried out in children less than 12 y of age.22 | |||
| Renal system | Glomerular filtration rate (GFR), tubular secretion, tubular reabsorption key stage of maturation | Renal capacity can approach adult levels. | |||
| Liver | Phase I enzymes, phase II enzymes, and bile flow | Hepatic enzyme expression can approach adult levels. | |||
| Brain | Neural tube differentiation, blood brain barrier, proliferation and organisation of synapses, neurotransmitter system maturation (NMDA receptors), subcortical grey matter, myelination, brain size, cortical grey matter, amygdala and hippocampus, white matter maturation, prefrontal cortex maturation, cholinergic and serotonergic systems: up to at least 18 y of age | ||||
| Extrapolation parameters | Disease | Many diseases in the preterm and term newborn infant are unique or have unique manifestations | Physiological development and maturity of organs, pathophysiology and natural history of the disease or condition, available treatment options, and the pharmacology of the investigational product are factors to be considered in determining the subgroups in paediatric studies.6 | Pharmacokinetics in adolescent patients are often similar to the pharmacokinetics in adults but weight and clearance should be considered. | |
| Pharmacology | Physiology changes dramatically with both gestational age (ie, from 22 to 42 wk) and post‐natal age affecting medicinal product disposition and organ/tissue responsiveness. | Adolescent doses may be able to be derived from adult data using bodyweight‐based allometric scaling. | |||
| Clinical response | The prediction and measurement of a medicinal product's PK (exposure) and PD (response) is essential to the clinical pharmacology assessment. | Confirmatory pharmacokinetic data may then be obtained through sparse sampling during pivotal efficacy and safety trials. | |||
| Extrapolation challenges | Factors such as physiological development including physical growth, organs maturation, transporters and enzymes ontogeny create size and age‐dependent variability in PK parameter.23 Therefore the response to intervention may be undefined or dissimilar to that in adults or older children. | Neurodevelopmental and behavioural disorders, monitoring the onset of puberty | |||
| Extrapolation opportunities | Extrapolation unlikely possible particularly for preterm neonates, but general modelling and simulation principles may be applied for rational interpretation of the available evidence in the context of information from other sources to guide study design and proposed dosages | Modelling and simulation to support study design and proposed dosages | Modelling and simulation (and interpolation where possible) to support study design and proposed dosages | Number of adolescents could be considered to be studied with the adult studies in appropriate paediatric setting, modelling and simulation can be used to support study design and proposed dosages | |
With regard to neonates, there are some specific points to consider, because many diseases in the preterm and term newborn infant are unique or have unique manifestations precluding extrapolation of efficacy from older paediatric patients and rather call for novel methods of outcome assessment18 than the use extrapolation.
When qualitative evidence from expert judgement is used, section 5.1.1 refers to (semi) quantitative methods. Although as of today there is not much experience with these methods at regulatory level, there are examples in the literature, for example, in Hampson et al24 using Bayesian methods based on “expert opinion” to incorporate pathophysiological or pharmacological assumptions with the response parameter. Such approach may be an option for situations where reference data and/or availability of prior knowledge are limited. But it is appropriate to acknowledge that expert judgement might be a weaker source of information than empirical evidence from a relevant source population and hence the extent of data generated in the extrapolation plan might need to be greater to address the increase uncertainties.
The Reflection Paper also states in section 5.2.2 that for evidence synthesis, when more empirical approaches are used, appropriate statistical methods can be applied. Examples of such approaches are well described and discussed by Weber et al,25 particularly aspects that need to be considered in the context of paediatric development when a large body of evidence is available in the adult setting and would eventually be combined with limited paediatric information (for example, for drugs already licensed in adult). In such setting, an alternative approach might be to argue for a content‐wise reduction of the adult information (eg, by simply taking the information from young adults for extrapolation to the adolescent population instead of using the full trial information).
It is important to note from section 5.1.3 that the use of the extrapolation framework will not systematically lead to an agreement by regulatory authorities to use extrapolation approaches for paediatric development. The aim of the section is to give a framework so that medicines developers and regulators can discuss in a structured way the strengths, limitations, gaps in knowledge, and uncertainties. The section may allow to anticipate challenges as early as possible with the emphasis that planning for the paediatric programme should not be an isolated aspect of the drug development programme but, rather, should be considered an integral part in the overall planning.
Extrapolation should always have a scientific basis and should not be proposed solely because of feasibility restrictions (ie, where demonstration of efficacy and safety to usual standards is not possible, eg, because of the rarity of the condition). However, the principles of the extrapolation framework may be applied in such cases, to highlight gaps in knowledge and uncertainties and for rational interpretation of the limited evidence in the target population in the context of data from other sources. The developer and the regulator should clearly identify whether a scientific basis for extrapolation is available or whether the framework is being used simply to provide some structure and insight into uncertainties in decision‐making where data from a source population is not directly relevant, but generating adequate clinical efficacy and safety data is not feasible. The latter might be an acceptable basis for evidence generation where feasibility is compromised but is not an extrapolation exercise and should not be referred to as such.
5.2. Extrapolation plan
The section builds on the previous sections related to the extrapolation concept, discussing the approach to conducting the studies that will address the assumptions and gaps in knowledge that have been identified in the extrapolation concept. The section expands on general considerations for planning and designing paediatric studies which include extrapolation approaches. In this context, model‐informed drug discovery and development (MID3)26 methodology is likely to prove useful; hence, the section encourages the use of MID3 not only for the purpose of structuring an extrapolation exercise but also for study design optimisation. To complement this, mathematical optimisation algorithms can be applied to define how to structure data collection to answer focused research questions. These techniques can be used to determine an optimal sample size, optimal sample times, and the number of samples required for pharmacokinetic and pharmacodynamic studies. Risk, value, and uncertainties related to the use of these methods should be thoroughly described in context of the indented purpose use and according to the impact on regulatory decision: to replace the usual evidence base (high impact), to justify the evidence base (medium impact), or to describe the evidence base (low impact).27
This section acknowledges the risks and challenges that are inherent to paediatric development and the factors that contribute to variability in drug disposition and response. These risks and challenges are related to physiological development and include physical growth, organs maturation, transporters and enzymes ontogeny. Such factors create size and age‐dependent variability in PK parameter; but also play a role in genetic differences (polymorphisms). If these parameters are not appropriately reflected, studies can be inappropriately designed leading to the failure of the paediatric development. The understanding of the factors that can change the PK and/or PD and thus the dose‐response relationship are the key for an adequate paediatric pharmacotherapy across age and disease subgroups. These factors that contribute to variability in drug disposition and response justify the need for paediatric‐specific endpoints; however, the selection of appropriate endpoints is a critical aspect of trial design and it is important to select to the extent possible biomarkers and clinical and surrogate endpoints appropriate to both adults and paediatric subsets to minimise differences in clinical outcomes between source and target populations and to avoid increasing the complexity to set expectations, make predictions, or integrate available clinical data.
5.2.1. Design of studies in the extrapolation plan (5.2.1.1 Pharmacokinetic studies and pharmacokinetic/pharmacodynamic studies in the extrapolation plan, and 5.2.1.2 therapeutic studies in the extrapolation plan), and 5.2.2 Regulatory confirmation of the extrapolation concept
Sections 5.2.2.1 and 5.2.2.2 provide general recommendations on the design of paediatric studies when extrapolation strategies are considered. Having clarity in the trial objectives in terms of the precise treatment effect or exposure‐response relationship to be estimated is vital, for the paediatric trial itself and how it relates to any estimated treatment effect that is being used from the source population.28 It is also very important to justify and predefine criteria to evaluate the success of the study(ies).
Whilst in the past sparse exposure‐response data have been collected in paediatric development, in the context of explicit extrapolation, pharmacokinetic/pharmacodynamic studies are important in understanding the dose‐concentration‐effect relationship and hence in developing dosing recommendations in children. The section on pharmacokinetic/pharmacodynamic studies does not introduce any specific outline for guidance on PK/PD investigations because reference is made to the “Guideline on the Role of Pharmacokinetics in the Development of Medicinal Products in the Paediatric Population.” However; there are many aspects that have to be considered when designing PK/PD studies, such as including determination of the appropriate number of subjects that must be recruited, “optimal” sample times, and how to handle rich vs sparse data. Answering these questions requires previous knowledge of the pharmacokinetics of the drug under investigation.29 The understanding of clinical pharmacology and of the dose‐exposure‐response relationship of the medicinal product usually is established early in development and in adult healthy volunteers and adult patients, respectively. As such, dose‐ranging studies are often not considered to be required in children. It is emphasised here that it might be beneficial to test more dose levels/regimens in children, particularly if the exposure‐response relationship in adults is not known or cannot be assumed to be the same between adults and children of all ages.
Additionally, population PK or PK/PD model‐based analyses can be used to guide the dosing regimen in the paediatric population. The justification for such approach might be a comparison between PK metrics (eg, plasma concentration) in the adult and paediatric populations, so‐called PK similarity or PK matching. For such comparison to be valid, it is of importance that all relevant covariates including size and maturational changes are captured in the paediatric PK models and that justification is given why these covariate models will have high predictive value in the relevant age groups. Furthermore, when PK comparison between age groups is to be performed, it is expected that the choice of PK metric(s) is justified based on the exposure‐efficacy and safety relationships in the reference population and that the decision criteria are pre‐specified. Body size relationship can be described by different scaling approaches including allometric scaling, and it is often useful to use theoretical values of the exponents (clearance = 0.75 and volume of distribution = 1).30, 31 There is ongoing research in the area, and other allometric scaling values can be justified.32, 33, 34 Another alternative is to use physiologically based pharmacokinetic (PBPK) models for dose predictions across age. PBPK models allow for incorporation of multiple levels of information such as in vitro assay, in silico preclinical PK data, and clinical data including known information about physiological processes.
Replacement of PK or PK/PD studies with model predictions (simulations) is only acceptable if the model assumptions and simulation properties are well understood and thoroughly described in context of the intended use of the model. Several approaches can be employed; population PK(/PD) models and/or PBPK models can be accepted if adequately justified. Early scientific advice is recommended to discuss such cases.
Therapeutic studies might be required as part of the extrapolation plan. The relevant section outlines some of the options available to those conducting an extrapolation plan to generate efficacy data, whilst acknowledging that the standard hurdle of P < 0.05 may not be appropriate. The purpose of the study depends on the question to be answered and the quantity, quality, and scope of the source data and its relevance to the target population. In some cases, the purpose would be to rule out any large differences between the predicted efficacy and the actual observed efficacy data. In others, a realistic prediction may not be available, but the source data could still be considered to have some relevance to the exercise and could justify lowering the regulatory hurdle without lowering the regulatory standard. Such approaches could be to use a scientifically justified larger level of alpha, the type 1 error rate, to widen the non‐inferiority margin, or to formally include the a priori knowledge into the analysis through a Bayesian paradigm.35, 36 The primary purpose of the study should always be to generate the data required to draw robust conclusions on efficacy.
The regulatory confirmation of the extrapolation concept section clarifies the importance of having justified and predefined criteria to evaluate the success of a study. This approach is directly linked to the regulatory decision‐making and is a plea for structure and clarity in the applications using extrapolation approaches submitted to EMA, such as, where possible, a sensitivity analysis (tabulation with varying assumptions and statistical parameters and the resulting sample sizes).
5.3. Mitigation of uncertainty
This section of the Reflection Paper acknowledges the benefits of continuing to address residual scientific uncertainties in the target population even once efficacy and a positive risk‐benefit having been established. Given the potential long‐term exposure of patients to these drugs, it is essential to consider whether new approaches are needed to better understand the safety of long‐term use of these drugs.37 Examples might be where long‐term follow‐up studies are required to address uncertainties related to growth and maturation and specific uncertainties related to the understanding of therapeutic efficacy and/or safety that have implications for understanding the benefit‐risk of a medicine with the potential to inform better use of the medicine in clinical practice. Data addressing the identified uncertainties in dosing, efficacy, or safety can be reflected in revisions to the SmPC.
The revised Reflection Paper specifies that data sources other than clinical trials can be used in addressing uncertainties. Indeed, the value and importance of data generated in routine clinical practice data are increasing. In the future, such data have the potential to track the developing child via assessment of longitudinal within‐patient data, which remains a frontier at the moment. Advance planning in addressing uncertainties facilitates such post‐marketing data proving complementary to well‐designed, longitudinal studies in tracking the standard of care. Using available data from multiple sources to address gaps in knowledge, may result in the SmPC being updated to provide, for example, dosing guidance. Routine clinical practice data may ultimately also provide a source for data associated with clinically relevant response to therapy that may complement mechanistic biomarkers and surrogate markers as the basis for drug approval.11 Additionally, the Reflection Paper mentions particularly the use of registries in the context of extrapolation. Whilst for regulators, the geographical spread of a registry network is a key factor for understanding treatment practices and outcomes, data need to be of appropriate quality.38 The qualification of the European Cystic Fibrosis Society Patient Registry (ECFSPR) as suitable for performing pharmacoepidemiological studies, ie, post‐authorisation safety surveillance (PASS) and efficacy (PAES) studies to support regulatory decision‐making in medicines for the treatment of cystic fibrosis,39 is a step forward towards the use of other sources than clinical trials in regulatory setting.
5.4. Decision process for extrapolation and 5.5 Examples of the decision process for extrapolation (5.5.1. Where PK can be used as a basis of extrapolation, 5.5.2. Well‐studied pharmacological classes, 5.5.3. Partial similarity in disease manifestations between populations, and 5.5.4. Examples where extrapolation is not recommended)
Section 5.4 summarises the thought process for constructing an extrapolation concept and plan whilst illustrating what are the consecutive steps to be followed in order to develop an extrapolation approach. Additionally, it acknowledges that there is a wide spectrum of approaches and study designs that may be acceptable; hence, section 5.5 rehearses some scenarios where regulators already have some experience and the types of extrapolation concept/exercise that might be constructed. As explained in Section 5, it is not in the scope of the Reflection Paper to dictate a preferred methodological approach; hence, the decision process does not provide guidance as to whether a specific approach can be acceptable or not. Each of the step in the process relates to the relevant sections of the Reflection Paper and hence the extrapolation framework.
As stated above, there are different approaches to extrapolation, which will be determined by the therapeutic setting and the extent of available knowledge. There are considerable differences between therapeutic areas particularly in terms of the knowledge and characterisation of the paediatric pathophysiology and the pharmacology of a compound under study. For example, in some areas, increasing experience of extrapolation approaches over time has led to agreed development programmes that do not require large and well‐controlled trials in children to establish efficacy, whilst in other areas, some paediatric conditions highlight the challenges to acceptability and use of paediatric extrapolation, for example, in Gaucher disease, pulmonary arterial hypertension (PAH), or diseases where there are differences in terms of neurodevelopment stages, including growth, sexual, and cognitive development that will impact on both efficacy and safety endpoints such as for the treatment of autism spectrum disorder (ASD). The examples used in the Reflection Paper cover a range of scenarios and include positive (HIV, juvenile idiopathic arthritis, and Gaucher disease) and negative outcomes of the extrapolation concept (autistic spectrum disorder). The description includes the extrapolation concept, the results of the studies covered by the extrapolation plan, and the regulatory outcome. These examples are for illustration purposes and should be planned and agreed prospectively with regulatory authorities at an early stage.
6. SUBMISSION AND REPORTING OF THE EXTRAPOLATION CONCEPT AND PLAN
The plea for clarity in the applications submitted to EMA extends to the reporting of the extrapolation concept and plan. Submissions using extrapolation approaches as part of a paediatric investigation plan or a scientific advice should follow the procedural guidance available. However, clearly describing an extrapolation exercise can be expected to be more complex than a usual paediatric development programme, and there is the need to ensure appropriate communication between all the different parties involved alongside of the product life cycle, including post‐licensing for health technology assessment (HTA) and payers. Principles of evidence‐based medicine should be followed including for modelling and simulation, especially with respect to a systematic approach, such as completeness of data, assessment and consideration of bias, and transparency of reporting. During the development stages of the medicinal product, to facilitate the understanding and rationale behind the development of the extrapolation concept and its plan outside of the sponsor team, a summary integrating all results from the source population and emerging studies in the target population can be provided.
In line with the principles of extrapolation that is based on extending information and conclusion from studies and population, the use of extrapolation in paediatric development may lead to indications that can be wider compared with the population studied provided that the extrapolated data support a positive benefit‐risk balance in not studied populations. In this regard, HTAs and payers who have contributed to the public consultation of the Reflection Paper40, 41 reiterated the need to increase transparency. Therefore, it is to the utmost importance to provide details of the extrapolation concept and the results of the extrapolation studies as a scientific basis for the reasoning behind the indication proposed. To facilitate a better understanding of extrapolation outcome to all stakeholders, this will be included after marketing authorisation application in the European public assessment report (EPAR).
EXTRAPOLATION FRAMEWORK TABLE
The extrapolation framework table presents a summary of tools and methods for studies that can be used at the different steps of the framework for dose finding/confirmation, for characterising disease progression, and evaluating clinical response in the target population.
COMPETING INTERESTS
At the time of writing and submission of the manuscript, C.O. was an employee of the European Medicines Agency and R.H. was an employee of the Medicines and Healthcare products Regulatory Agency.
Supporting information
Data S1. Supporting information
ACKNOWLEDGEMENTS
C. Male, A. Koch, M. Posch, J. Brogren, F. Musuamba Tshinanu, I. Skottheim Rusten, L. Brassart, C. Espinasse, J. Karres, C. de Vries, S. Vamvakas, M. Berntgen, K. Dunder, D. Brasseur, D. Mentzer, J. Raine, T. Salmonson.
Ollivier C, Thomson A, Manolis E, et al. Commentary on the EMA Reflection Paper on the use of extrapolation in the development of medicines for paediatrics. Br J Clin Pharmacol. 2019;85:659–668. 10.1111/bcp.13883
REFERENCES
- 1. Kimland E, Nydert P, Odlind V, et al. Paediatric drug use with focus on off‐label prescriptions at Swedish hospitals—a nationwide study. Acta Paediatr. 2012;101:7‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COMMITTEE ON DRUGS, MMM . Off‐label use of drugs in children. Pediatrics. 2014;133(3):563‐567. [DOI] [PubMed] [Google Scholar]
- 3. Avant D. Pediatric information in drug product labeling. JAMA. 2012;307(18):1914. [DOI] [PubMed] [Google Scholar]
- 4. Public workshop on extrapolation of efficacy and safety in medicine development across age groups. Outcome of a multi‐stakeholder meeting with experts and regulators held at EMA on Tuesday 17 May and Wednesday 18 May 2016 [Internet]. Available from: https://www.ema.europa.eu/documents/other/ema‐public‐workshop‐extrapolation‐efficacy‐safety‐medicine‐development‐outcome‐multi‐stakeholder_en.pdf
- 5. ICH guideline—clinical investigation of medicinal products in the pediatric population E11 [Internet]. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_Guideline.pdf
- 6. Addendum to ICH E11: clinical investigation of medicinal products in the pediatric population E11(R1) [Internet]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/E11‐R1EWG_Step4_Addendum_2017_0818.pdf [PubMed]
- 7. General clinical pharmacology considerations for pediatric studies for drugs and biological products [Internet]. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm425885.pdf
- 8. Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug‐development programs. Pediatrics. 2011;128(5):e1242‐e1249. [DOI] [PubMed] [Google Scholar]
- 9. Mulugeta Y, Barrett JS, Nelson R, et al. Exposure matching for extrapolation of efficacy in pediatric drug development: extrapolation of efficacy in pediatric drug development. J Clin Pharmacol. 2016;56(11):1326‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Concept paper on extrapolation of efficacy and safety in medicine development [Internet]. Available from: https://www.ema.europa.eu/documents/scientific‐guideline/concept‐paper‐extrapolation‐efficacy‐safety‐medicine‐development_en.pdf
- 11. Barrett JS, Bishai R, Bucci‐Rechtweg C, et al. Challenges and opportunities in the development of medical therapies for pediatric populations and the role of extrapolation. Clin Pharmacol Ther. 2018;103(3):419‐433. [DOI] [PubMed] [Google Scholar]
- 12. EMA Reflection paper on extrapolation of efficacy and safety in paediatric medicine development [Internet]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/10/WC500236640.pdf
- 13. Notice to applicants: a guideline on summary of product characteristics (SmPC) [Internet]. Available from: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol‐2/c/smpc_guideline_rev2_en.pdf
- 14. Ollivier C, Mulugeta YL, Ruggieri L, Saint‐Raymond A, Yao L. Paediatric extrapolation: A necessary paradigm shift. Br J Clin Pharmacol. 2019;85(4):675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. New strategies for the conduct of clinical trials in paediatric pulmonary arterial hypertension (PAH): outcome of a multi‐stakeholder meeting with patients, academia, industry and regulators held at the European Medicines Agency on Monday 12th June 2017. [DOI] [PMC free article] [PubMed]
- 16. Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004 [Internet]. Available from: https://eur‐lex.europa.eu/legal‐content/EN/TXT/PDF/?uri=CELEX:32006R1901&from=GA
- 17. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use [Internet]. Available from: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol‐1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf
- 18. Ward RM, Benjamin D, Barrett JS, et al. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr Res. 2016;81:692. [DOI] [PubMed] [Google Scholar]
- 19. Momper JD, Mulugeta Y, Green DJ, et al. Adolescent dosing and labeling since the Food and Drug Administration Amendments Act of 2007. JAMA Pediatr. 2013;167(10):926‐932. [DOI] [PubMed] [Google Scholar]
- 20. EMA modelling and simulation: questions and answers [Internet]. Available from: https://www.ema.europa.eu/en/human‐regulatory/research‐development/scientific‐guidelines/clinical‐pharmacology‐pharmacokinetics/modelling‐simulation‐questions‐answers
- 21. Karres J. Organ maturation tables, possibly identifying maturation related adverse drug reactions [Internet]. Available from: https://www.ema.europa.eu/documents/presentation/presentation‐organ‐maturation‐tables‐janina‐karres_en.pdf
- 22. CHMP guideline on the requirements for clinical documentation for orally inhaled products (OIP) including the requirements for demonstration of therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease (COPD) in adults and for use in the treatment of asthma in children and adolescents [Internet]. 2009. Available from: https://www.ema.europa.eu/documents/scientific‐guideline/guideline‐requirements‐clinical‐documentation‐orally‐inhaled‐products‐oip‐including‐requirements_en.pdf
- 23. Barker CIS, Standing JF, Kelly LE, et al. Pharmacokinetic studies in children: recommendations for practice and research. Arch Dis Child. 2018;103(7):695‐702. 10.1136/archdischild-2017-314506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hampson LV, Whitehead J, Eleftheriou D, Brogan P. Bayesian methods for the design and interpretation of clinical trials in very rare diseases. Stat Med. 2014;33(24):4186‐4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weber K, Hemmings R, Koch A. How to use prior knowledge and still give new data a chance? Pharm Stat. 2018. Jul;17(4):329‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. EFPIA MID3 Workgroup , Marshall S, Burghaus R, et al. Good practices in model‐informed drug discovery and development: practice, application, and documentation. CPT Pharmacomet Syst Pharmacol. 2016;5(3):93‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manolis E, Rohou S, Hemmings R, Salmonson T, Karlsson M, Milligan PA. The role of modeling and simulation in development and registration of medicinal products: output from the EFPIA/EMA Modeling and Simulation Workshop. CPT Pharmacomet Syst Pharmacol. 2013;2(2):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials [Internet]. Available from: https://www.ema.europa.eu/documents/scientific‐guideline/draft‐ich‐e9‐r1‐addendum‐estimands‐sensitivity‐analysis‐clinical‐trials‐guideline‐statistical_en.pdf
- 29. Roberts JK, Stockmann C, Balch A, et al. Optimal design in pediatric pharmacokinetic and pharmacodynamic clinical studies. Anderson B, editor. Pediatr Anesth. 2015;25(3):222‐230. [DOI] [PubMed] [Google Scholar]
- 30. Holford N, Heo Y‐A, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941‐2952. [DOI] [PubMed] [Google Scholar]
- 31. Calvier EAM, Krekels EHJ, Välitalo PAJ, et al. Allometric scaling of clearance in paediatric patients: when does the magic of 0.75 fade? Clin Pharmacokinet. 2017;56(3):273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang C, Peeters MYM, Allegaert K, et al. A bodyweight‐dependent allometric exponent for scaling clearance across the human life‐span. Pharm Res. 2012;29(6):1570‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calvier EAM, Krekels EHJ, Yu H, et al. Drugs being eliminated via the same pathway will not always require similar pediatric dose adjustments. CPT Pharmacomet Syst Pharmacol. 2018;7(3):175‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krekels EHJ, Calvier EAM, van der Graaf PH, Knibbe CAJ. Children Are Not Small Adults, but Can We Treat Them As Such? CPT Pharmacometrics Syst Pharmacol. 2019; 10.1002/psp4.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hlavin G, Koenig F, Male C, Posch M, Bauer P. Evidence, eminence and extrapolation. Stat Med. 2016;35(13):2117‐2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gamalo‐Siebers M, Savic J, Basu C, et al. Statistical modeling for Bayesian extrapolation of adult clinical trial information in pediatric drug evaluation: extrapolation in pediatric drug evaluation through Bayesian methods. Pharm Stat. 2017;16(4):232‐249. [DOI] [PubMed] [Google Scholar]
- 37. Zimmerman KO, Smith PB, McMahon AW, et al. Duration of Pediatric Clinical Trials Submitted to the US Food and Drug Administration. JAMA Pediatr. 2019;173(1):60‐67. 10.1001/jamapediatrics.2018.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. EMA Patient Registries Workshop, 28 October 2016—observations and recommendations arising from the workshop [Internet]. Available from: https://www.ema.europa.eu/documents/report/report‐patient‐registries‐workshop_en.pdf
- 39. CHMP Qualification procedure [Internet] . Available from: https://www.ema.europa.eu/documents/regulatory‐procedural‐guideline/qualification‐opinion‐european‐cystic‐fibrosis‐society‐patient‐registry‐ecfspr‐cf‐pharmaco_en.pdf
- 40. Overview of comments received on Reflection paper on the use of extrapolation in the development of medicines for paediatrics' [Internet]. Available from: https://www.ema.europa.eu/documents/scientific‐guideline/overview‐comments‐received‐reflection‐paper‐use‐extrapolation‐development‐medicines‐paediatrics‐ema/189724/2018‐revision‐1_en.pdf
- 41. Minutes EMA—Payer Community meeting 19 September 2017 [Internet]. Available from: https://www.ema.europa.eu/documents/minutes/minutes‐european‐medicines‐agency‐payer‐community‐meeting_en.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information


