Abstract
Cardiovascular disease remains the single largest cause of natural death in the United States, with a significant cause of mortality associated with cardiac arrhythmias. Presently, options for treating and preventing myocardial electrical dysfunction, including sudden cardiac death, are limited. Recent studies have indicated that conduction of electrical activation in the heart may have an ephaptic component, wherein intercellular coupling occurs via electrochemical signaling across narrow extracellular clefts between cardiomyocytes. The perinexus is a 100-200nm-wide stretch of closely apposed membrane directly adjacent to connexin 43 gap junctions. Electron and super-resolution microscopy studies, as well as biochemical analyses, have provided evidence that perinexal nanodomains may be candidate structures for facilitating ephaptic coupling. This work has included characterization of the perinexus as a region of close inter-membrane contact between cardiomyocytes (< 30 nm) containing dense clusters of voltage-gated sodium channels. Here, we review what is known about perinexal structure and function and the potential that the perinexus may have novel and pivotal roles in disorders of cardiac conduction. Of particular interest is the prospect that cell adhesion mediated by the cardiac sodium channel β subunit (Scn1b) may be a novel anti-arrhythmic target.
Keywords: gap junctions, perinexus, heart, conduction, arrhythmia, ephaptic, NaV1.5/Scn5a, β1/Scn1b
Introduction
Cardiovascular disease remains the single largest cause of natural death in the United States and projections suggest that this unfortunate statistic is unlikely to change in the foreseeable future (World Health Organization, 2017). A significant cause of sudden cardiac death is arrhythmias associated with aberrant cardiac conduction (Chugh et al., 2008). Cardiac conduction is a term used to describe the process by which action potential (AP) is propagated throughout the heart, ensuring synchronous contraction of the myocardium. Conduction is triggered by waves of activating impulses, or action potentials (APs), originating at the sinoatrial node, which are spread to the ventricles via the atrioventricular node and the His-Purkinje system. At the cellular level, APs are produced and propagated by a series of membrane ion cascades coordinated by ion channels in the heart.
The study of cardiac electrical activity dates as far back as the 19th century, but it was not until the early 1900s that conduction velocity (CV) in the heart was first measured (Lewis et al., 1914). CV remains a primary metric for the spread of electrical activation in the myocardium by measuring the time it takes an AP wave front to traverse a known distance of tissue. In 1959, Hodgkin and Horowicz made the discovery that excitability in cardiac tissue had parallels to the excitability of neuronal tissue, specifically as it relates to sodium entry into the cell (Hodgkin and Horowicz, 1959). These workers were able to demonstrate that sodium ion penetrance was low when cells were electrically dormant, but increased upon depolarization of the membrane associated with the onset of AP. This is now recognized to largely result from the opening of the alpha subunit of the cardiac voltage-gated sodium channel (VGSC) known as NaV1.5. The density of NaV1.5-formed channels is now widely accepted as the main determinant of cellular excitability in cardiovascular tissue and is the primary contributor of recorded sodium currents (INa) in the heart (Shaw and Rudy, 1997). Disruption of cardiac AP propagation (CV slowing/block) can lead to potentially deadly spontaneous arrhythmias (Kleber and Rudy, 2004).
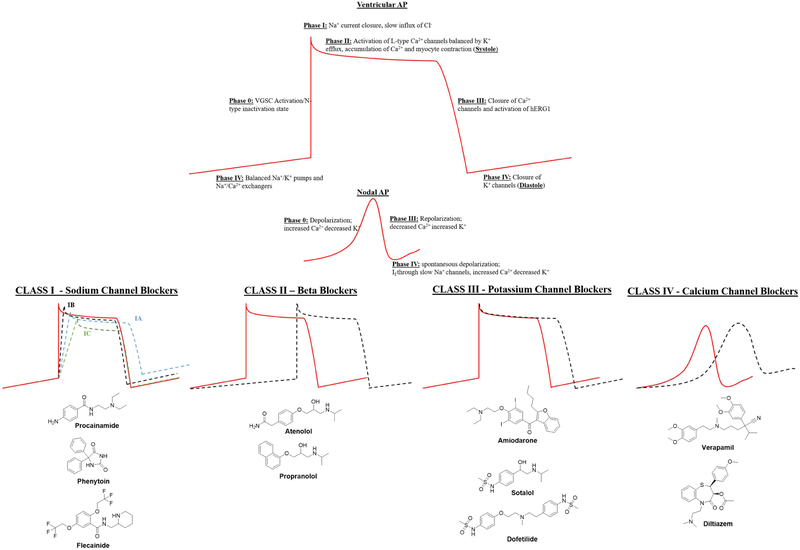
Traditionally, AP morphology is viewed as a product of five phases; phase 0-IV, each characterized by unique patterns of ion current activation and downstream effects. Existing anti-arrhythmic drugs (AADs) act on these various phases to modulate CV by regulating the kinetics of the AP curve. Summarized in Figure 1, there are four major classes of AADs that are effective in acting on individual aspects of cardiac AP, but many have arrhythmic propensity and suffer from poor efficacy (Fragakis and Vassilikos, 2016). The Cardiac Arrhythmia Suppression Trial (CAST) and Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) studies laid bare the lack of benefit, in terms of mortality, of many current AADs and highlighted the need for new approaches to the alleviation of deadly arrhythmias (Corley et al., 2004; Echt et al., 1991; Wyse et al., 2002). With the high costs involved in developing new therapeutics, combined with the perceived risk in targeting cardiac electrical activity, the drug discovery pipeline for safe and effective anti-arrhythmics remains challenging to replenish.
Figure 1:
Representative molecules from the four primary classes of existing anti-arrhythmic drugs (AADs).
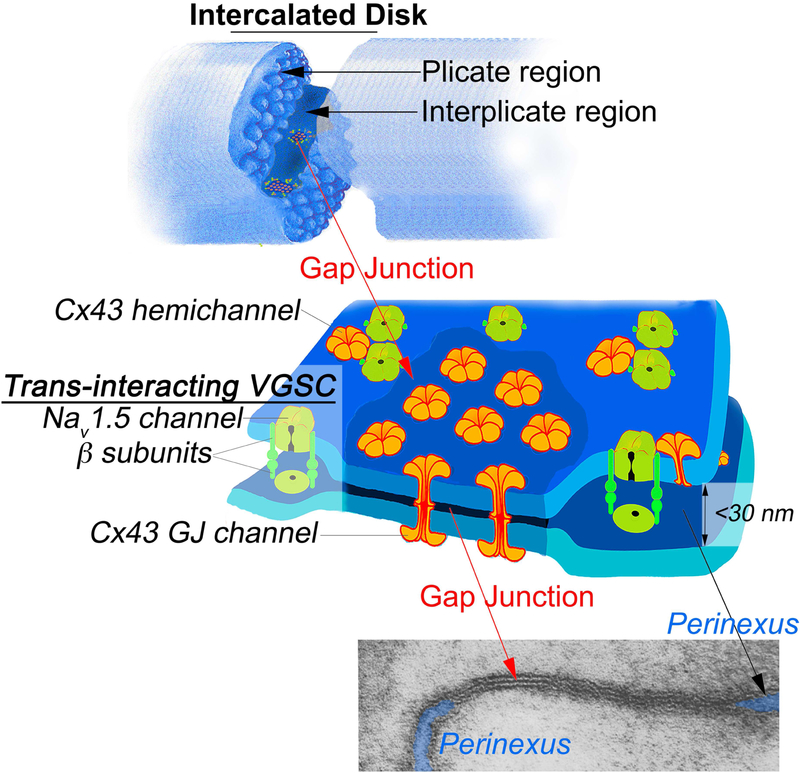
Extensive mathematical modeling and computational biology work over the years have outlined theoretical components required for a mechanism of cardiac AP known as ephaptic coupling (see Table 1). Mori et al 2008 described a ‘cardiac ephapse’ using in silico models and established these requisite criteria: a tightly regulated extracellular space (ES) between two apposed myocytes (10-30nm) and a high density of current-generating Na+ channels located at these points of close apposition. Recent work from the Kucera lab has shown the clustering of NaV1.5 on opposite sides of a narrow intercellular cleft potentiates ephaptic interactions and AP transmission from cell to cell (Hichri et al., 2018). Work in the Gourdie lab has demonstrated high concentrations of NaV1.5 and its β1 subunit in a nanodomain adjacent gap junctions composed of connexin 43 (Cx43) known as the perinexus (Rhett et al., 2011; 2012b; Veeraraghavan et al., 2015a; 2015b; 2018; Veerarghavan and Gourdie, 2016) This review focuses on the recent findings that support the claim that such a region could emerge as a non-canonical target for novel AADs.
Table 1.
Contributions supporting or relevant to the cardiac ephaptic conduction hypothesis since 1959.
| Citation | Year | Major Finding |
|---|---|---|
| Sperelakis et al | 1959 | Evidence for non-syncytial (i.e., ephaptic) conduction in frog hearts. |
| Pertsov and Medvinskii | 1976 | A simple two cell mathematical model suggests ephaptic coupling possible |
| Spach et al. | 1981 | Demonstration of discontinuous nature of cardiac conduction in vivo. |
| Gutstein et al | 2001 | Cx43 knockout mouse hearts show conduction in absence of gap junctions (GJs). |
| Kucera et al | 2002 | Modeling suggests Nav1.5 voltage gated sodium channels (VGSCs) at intercalated discs modulate cardiac conduction. |
| Mori et al | 2008 | Modeling suggests ephaptic conduction requires inter-membrane spacings <30 nm, concentrated VGSCs and that it can self-attenuate*. |
| Lin and Keener | 2010 | Models of ephaptic conduction incorporating irregular geometry of myocytes and VGSC localization at intercalated disks reported. |
| Rhett et al | 2011 | Identification of the perinexus – the peri-junctional zone surrounding Cx43 GJs - as a specialized region of cell-to-cell interaction. |
| Rhett et al | 2012a | Demonstration of concentrations of Nav1.5 VGSCs at the perinexus. |
| Veeraraghavan et al | 2015a; 2015a | Width (10-30 nm) of perinexal inter-membrane spacing shown to be modulatable and correlated with conduction velocity/arrhythmia. |
| George et al | 2016 | Ephaptic self-attenuation shown to be based on perinexal inter-membrane spacing. |
| Greer-Short et al | 2017 | Reduced/increased perinexal inter-membrane spacing shown to mask/unmask arrhythmia risk in LQT syndrome. |
| Hichri et al | 2018 | High-resolution computer models point to significance of trans-apposed** Nav1.5 VGSCs clusters in ephaptic conduction. |
| Raisch et al | 2018 | Increased perinexal inter-membrane spacing found in patients with atrial arrhythmia. |
| Veeraraghavan et al | 2016;2018 | Trans-membrane adhesion mediated by VGSC β1 subunit shown to regulate perinexal inter-membrane spacing and cardiac conduction. |
Self attenuation is when sodium current diminishes due to reduced inter-membrane spacing between cells. When inter-membrane spacing is sufficiently narrow, the extracellular cleft is predicted not contain enough Na+ ions to drive current across the membrane.
Transapposed VGSCs can be envisaged as the alignment of sodium channel clusters from apposing cells, such that the pores of the sodium channels directly face each other across the narrow extracellular gap (e.g. Figure 2)
Generation of the Cardiac Action Potential
Resting membrane potentials are around −85-95mV in human myocardial tissue before Phase 0 activation of voltage gated sodium channels (VGSC) leads to a rapid influx of Na+ and a sharp depolarization to +20−25mV. Concurrently, these channels close their inactivation gates and enter the N-type inactivation state until reaching resting potential. Phase I is defined as the initial deflection of AP and is regulated by closing of the Na+ current and the slow intake of extracellular Cl− ions, balanced by the efflux of K+. Activation of the L-type Ca2+ channels initiate the onset of Phase II, causing the plateau of the AP curve. KCNQ and KCNH K+ efflux balance this, leading to an accumulation of Ca+ ions, which in conjunction with ATP, leads to myocyte contraction or systole. Phase III begins the repolarization of the membrane and is marked by inactivation of Ca2+ channels. This phase is progressively accelerated by the activation of the K+ channel KCNH2 (hERG1), a pore thought responsible for many of the off-target effects of pharmaceutical agents used to treat aberrant cardiac electrophysiological activity. Upon achieving full repolarization, K+ channels close and the AP reaches Phase IV or resting state. Resting state or diastole, has balanced Na+/K+ pumps, Na+/Ca2+ exchangers and inward K+ channels to accumulate cytoplasmic ions, generating a negative membrane potential (−85-95mV), until the activation of VGSC signals the re-initiation of the next AP.
Cardiac Conduction – Gap Junction Coupling
Cardiomyocytes associate in highly ordered anisotropic arrangements and are coupled by a region specialized for electromechanical interaction called the intercalated disc (ID). IDs are composed of three distinct intercellular junctional structures not found between mature skeletal muscle cells; adherens junctions, desmosomes, and gap junctions (GJ) (Angst et al., 1997). These junctional structures have roles in mechanically connecting and electrically coupling myocytes. Adherens junctions provide extended domains of cell-cell adhesion and a platform for actin filament insertion, aiding contractile force generation by cardiac muscle cells. Desmosomes also contribute to intercellular mechanical strength and there is evidence that in the adult mammalian myocardium, adherens and desmosomal junctional components mix to form composite adhesive structures (Franke, 2009). GJs directly connect the cytoplasm of two cells allowing for small molecule (<1000 daltons) and ion exchange. Two hexameric assemblies of connexon hemichannels form each GJ channel. There are three main isoforms of connexin proteins expressed by mammalian myocardial tissue (Connexins 40,43, and 45) named for the full length molecular weight of the protein predicted by their gene (Beyer et al., 1987; Kanter et al., 1992; Gourdie et al., 1993; Coppen et al., 1998; Palatinus et al., 2012; Severs et al., 1989). However, in the working adult myocardium the predominant isoform is thought to be Cx43. For example, in mouse 99.6% of all connexin protein in this tissue is Cx43 (Bao et al., 2011).
The propagation of AP in the ventricles has long thought to result from direct cytoplasmic coupling mediated by Cx43 GJs, which are primarily located in IDs (Gourdie et al., 1991; Kleber and Rudy, 2004; Palatinus et al., 2012; Delmar and Liang, 2012). Furthermore, there is considerable evidence that cardiac injury results in GJ remodeling, marked by loss of GJs, which in turn is associated with decreases in CV and increased susceptibility to arrhythmias (Smith et al., 1991; Wit and Peters, 2012; Peters et al., 1995; Severs et al., 2008). Early investigators used a macroscopic approach to study Purkinje fibers, which have cable-like properties leading to the initial postulation of a cable theory for cardiac conduction (Weidmann, 1952). This theory hinges on modeling the coupling of myocardial tissue as a resistive pathway, and envisages that current would flow through the path of least resistance. In the late 1940s, the intercellular structure thought to underpin this pathway was identified between cardiac myocytes; initially termed “the nexus”, and eventually more commonly known as the gap junction - the GJ ( Sjostrand and Andersson, 1954; Barr et al., 1965; Dewey and Barr, 1962). Gap junctional conductance is often incorporated into intracellular resistance (ri) but assumes that the cytoplasms of myocytes are electrically contiguous (i.e. offer extremely low resistance), not accounting for what appears to be discontinuous or delayed CV noted previously by Spach and colleagues (Spach et al., 2000; Weidmann, 1966; Weidmann, 1970).
Limitations of GJ Coupling in Explaining Cardiac Conduction
Cable theory has long been used to describe the myocardium as a syncytium with flow of electrical current moving between low resistance pathways identified as GJs. However, ongoing theoretical and experimental studies have suggested potential incompatibilities in applying the macroscopic view of cable theory to the microscale couplings mediated by Cx43 GJs. Cx43 remodeling is a hallmark of many cardiovascular diseases and has been shown to have significant effects on cardiac AP (Gutstein et al., 2001; Spach et al., 2000). Slowed conduction is a well-established risk underlying arrhythmia, but there has been debate on the contribution of gap junctional uncoupling to CV changes. Pharmacological uncoupling of Cx43 GJs, such as that mediated by carbenoxolone, can slow conduction at certain doses (Veeraraghavan et al., 2012). However, this treatment does not appear to demonstrate any type of dose-dependent correlation between uncoupling and conduction. Moreover, it has been demonstrated that CV slowing occurs before changes in Cx43 takes place, suggesting that channel availability may not be the sole determinant of altered conduction propagation (Akar et al., 2007).
Further questions on the relationship between cardiac GJs and conduction have been raised by studies of Cx43/Gja1 knock-out mice. Cardiac-restricted knockout of Cx43 (CKO) was reported to circumvent the perinatal lethality of global knockout Cx43, and these mice demonstrated normal heart structure and function, albeit GJs were undetectable at IDs (Gutstein et al., 2001). However, CKO mice did not succumb to sudden cardiac death due to spontaneous ventricular arrhythmias until the 2nd to 4th postnatal weeks, relatively far along in the maturational growth of mice. These data suggested that Cx43 was not required for beat-to-beat maintenance of cardiac contractility during this period following birth.
Several researchers using heterozygous global Cx43 knockout mouse (Cx43+/−) have uncovered paradoxical findings. Different groups observed varying consequences of loss of Cx43-based coupling on conduction. While some researchers reported a significant decrease in CV with a 50% reduction in Cx43 protein levels in heterozygous null mouse, whereas others could ascertain no difference between animals expressing null and wildtype Cx43/Gja1 alleles (Eloff et al., 2001; Morley et al., 1999; Rohr et al., 1998; Stein et al., 2009; Stein et al., 2011; Thomas et al., 2003). The work of Poelzing and his group provided some resolution of this controversy, demonstrating that CV in Cx43 −/+ mice could be modulated via altering the ionic composition of perfusion fluids (George et al., 2016; George and Poelzing, 2016; George et al., 2015). Specifically increasing Na+ ions accelerated CV in Cx43+/− mice, while increasing K+ resulted in the opposite effect. These studies also reported correlations between myocyte inter-membrane spacing within the ID and conduction velocity that occurred in association with adjustments to perfusate composition, which we will return to later in this review.
Basis of Ephaptic Coupling in the Heart
Theoretical studies have long-raised the possibility of non-GJ-mediated or ephaptic coupling between cardiomyocytes. Table 1 provides a summary of progress on the cardiac ephaptic conduction hypothesis over the last 60 years. Ephaptic conduction involves cell-to-cell transfer of electrical activation via electric fields, or ion transients, within a confined extracellular space between cells (Lin and Keener, 2010; Mori et al., 2008; Rhett et al. 2013; Veeraraghavan et al., 2014b). The possibility of ephaptic conduction in the heart was first identified by Sperelakis and co-workers from the late 1950s and into the 1970s - a minority viewpoint that coincided with the emergence of GJ-based intercellular coupling as the dominant mechanistic hypothesis for explaining electrical coupling between heart muscle cells (Mann and Sperelakis, 1979). Recent experimental evidence has provided support for ephaptic coupling as an alternative means of AP propagation (see Table 1).
The Gourdie lab’s first step in this direction occurred when it was determined that sodium channels were localized in close proximity to cardiomyocyte Cx43 GJs (Rhett et al., 2012a). Ephaptic coupling requires a high density of active VGSCs opening into a narrow (< 30 nm) inter-membrane space or cleft between cells (Lin and Keener, 2010; Mori et al., 2008). According to Ohm’s Law, rapid current change in this space results in negative extracellular potential. This, in turn, will prompt membrane depolarization on the opposite side of the cleft, leading to the activation of VGSCs on that membrane (Rhett et al., 2013; Veeraraghavan et al., 2014b; 2014c). This non-syncytial contribution to cardiac conduction envisions electrical excitation jumping across a tiny gap in extracellular space between myocytes in a manner not wholly dissimilar to how AP propagates between neurons at the synaptic cleft, although electro-chemical transmission is used instead of neurochemical signaling. Ephaptic coupling has remained controversial in the cardiovascular field, largely due to a lack of tangible experimental evidence to support it and identification of the cellular nano-structures that might facilitating such coupling.
Sodium Channels
VGSCs are comprised of a pore-forming α-subunit and two β-subunits and are responsible for the rapid depolarization of membrane potential characterizing the initial upstroke of the AP. Early work on the structure of this channel complex in the 1980s was assisted by the use of covalent protein labeling with a photoactive analog of scorpion toxin (Beneski and Catterall, 1980). Other significant advances aiding structural elucidation was the sequencing of the full human genome and the X-ray crystallization of analogous bacterial channels at different functional states (McCusker et al., 2012; Payandeh et al., 2012; Payandeh et al., 2011). The latter revealed, in three dimensions, structural components of the channel that have significantly aided development of channel-specific drugs to treat cardiac arrhythmias, epilepsy, and pain. To date, nine VGSCs have been successfully cloned (NaV1-9) and a tenth partial sequence has been reported (NaV2) (Watanabe et al., 2000). The large single-chain polypeptide (260kDa) α-subunit is responsible for ion selectivity, pore opening and closing, and voltage sensitivity. The primary sequence of the α-subunit predicts a series of four repeated domains (I-IV) comprised of six α-helical transmembrane domains (S1-6) that form the channel. Segments S1-4 from each domain form the regulatory voltage-sensing domain that is responsible for channel opening in response to the initial membrane depolarization. The hallmark of this structure is the distribution of positively charged arginine and lysine residues every three amino acids, which allow for significant shifts in the secondary structure of this domain in response to changes in transmembrane potential. Upon depolarization, these residues move towards the membrane surface causing a conformational change revealing a central aqueous channel known as the pore domain, formed by S5 and S6. This movement simultaneously inactivates the channel until repolarization, enabling the S4 segment residues to return to their initial positions. The pore domain contains a narrow selectivity filter that allows for the preferential permissibility of Na+ compared to K+ ions (Heinemann et al., 1992). Salt bridges between charged residues in domains 1-3 form this filter known as the DEKA region, corresponding to the single letter amino acid contribution from each domain. The final structural components of the α-subunit are the in/activation gates that regulate channel kinetics during their respective phases of the AP.
Cardiac Voltage Gated Sodium Channels
There are numerous mutations in the gene encoding the α-subunit of NaV1.5, SCN5A, associated with cardiovascular pathophysiology, many of which directly affect cardiac AP (Antzelevitch et al., 2014). The majority of gain-of-function mutations influence fast sodium channel inactivation that occurs directly after the inward rectifying current in Phase 0, leading to a persistent inward flow of Na+ and an untoward prolongation of the AP. Loss-of-function mutations of SCN5A often produce truncated proteins, non-functional channel activity or deficient trafficking to the cell membrane. In each case, altered AP occurs and can lead to ventricular arrhythmias and potentially death. Class I antiarrhythmic drugs such as lidocaine, mexilentine, flecandide, quinidine, and procainamide act as frequency dependent inhibitors of sodium current. This pharmacological block of channel activity results in a decrease in the rate of AP generation and an overall reduction in heart excitability, which can help quell tachyarrhythmia to restore normal rhythm (Antzelevitch et al., 2014). However, these agents promote arrhythmia in otherwise healthy patients. Newer agents have been developed with higher degrees of selectivity for the NaV1.5 isoform with effects on late sodium current and the sodium/calcium exchanger, lowering the ischemic burden and cardiac glycoside-induced calcium overload (Zablocki et al., 2016). Poor clinical trial results in recent years, such as those unveiled in the AFFIRM trials, have left the field of anti-arrhythmic drug discovery with a limited repertoire of novel pharmacological classes to meet the increasing burden of electrophysiological dysfunction associated cardiovascular disease.
VGSC Beta Subunits
The α-subunit is flanked by one or two β-subunits. There are five isoforms of β-subunits in humans encoded by the four genes: SCN1B (β1), SCN2B (β2), SCN3B (β3), and SCN4B (β4). β1-4 subunits are single transmembrane glycoproteins that contain a single extracellular immunoglobulin (Ig) loop, while β1B is a splice variant of SCN1B, which is a secreted variant comprising the N-terminal and Ig loop sequence, only untethered to the cell membrane. β-subunits can interact covalently or non-covalently with α-subunits, with β1/ β3 interacting through electrostatic interactions, whereas β2/ β4 have an unpaired cysteine residue to form a disulfide bond with an α-subunit. This structural difference is interesting due to the additional intramolecular disulfide bond found in β1/3, which further stabilizes the Ig loop and provides a potentially distinguishing feature in the morphology of covalently and non-covalently associating subtypes. β-subunit utility is often dictated by a series of post-translational modifications that can effect function, localization and expression levels (Calhoun and Isom, 2014). The β1 subunit is intracellularly phosphorylated at Y181 and heavily glycosylated along the extracellular Ig loop, affecting cell surface expression and channel modulation (Brackenbury et al., 2008; Johnson et al., 2004). The N and C-terminal tails of β-subunits can be cleaved by BACE1 and γ-secretase to release physiologically relevant polypeptides that can have effects on α-subunit transcription (Kim et al., 2007).
β1, in particular, has been shown to influence INa in the presence of physiological amounts of NaV1.5, as well as, cause a depolarizing shift in steady-state inactivation, suggesting that the subunit can allow the voltage-sensing domains to recover more rapidly to resting state (Mishra et al., 2011; Qu et al., 1995). These effects vary depending on the model system used, indicating that there may be more factors involved than simple stoichiometry or localization between α/β-subunits. In Xenopus oocytes, INa increased proportionally to increases in β1 mRNA, as well as a depolarizing shift in the steady state inactivation of NaV1.5, suggesting that β1 allows the channel to return to a resting state more quickly, expediting frequency activation (Qu et al., 1995; Zhu et al., 2017). Antisense silencing Scn1b mutations in rat cardiomyocytes altered α-subunit mRNA and protein levels and led to a decreased INa (Baroni et al., 2014). However in Scn1b null cardiomyocytes, there was an increase in both mRNA and protein levels of the α-subunit and a corresponding increase in the INa , which led to prolonged QT in mice (Lopez-Santiago et al., 2007).
It has been suggested that β1 may play a role in facilitating interactions between NaV1.5 dimers (Mercier et al., 2012), a function that could theoretically increase current-generating channels in a small subcellular area, such as a domain required for ephaptic coupling. NaV1.5 dimers display coupled gating properties, mediated via 14-3-3 proteins (Allouis et al., 2006; Clatot et al., 2017). 14-3-3-deficient mutations have highly pro-arrhythmic effects on myocardial electrical properties, suggesting high densities of VGSC clusters to be important for normal conduction. In addition to sodium channel interactions, β1 is capable of modulating the biophysical properties of other ion channels such as voltage-gated potassium (Kv) channels, a family of channels responsible for generating outward rectifying current (Ito). Most notably β1 influences Kv4.3 voltage dependence and channel kinetics (Deschenes and Tomaselli, 2002) and increases Kv4.2 surface expression resulting in higher current densities compared to Kv4.2 alone (Marionneau et al., 2012). β1 has also been shown to influence the Kv1 family (Kv1.1, 1.2, 1.3, 1.6) and Kv7.2 (Nguyen et al., 2012), and removing β1 from heterologous systems simultaneously reduces INa and Ito , indicating that β1 may facilitate VGSC and Kv interactions (Deschenes et al., 2008). These multifaceted aspects of VGSC β subunit function suggests its promise as a clinically viable therapeutic target in the manipulation of cardiac sodium channel activity.
The primary role of β-subunits is thought to be as a cell adhesion molecule and all are members of the Ig superfamily. β1 facilitates homo and heterophillic interactions and has noted ability to modulate sodium channel electrophysiology and trafficking (Chen et al., 2002; Dhar Malhotra et al., 2001; Lopez-Santiago et al., 2007; Zhu et al., 2017). Cell adhesion molecules facilitate cell-cell communication and signaling cascades, but can also be crucial for the regulation of physical domains. For example, other Ig superfamily members, desmoglein-2 and N-cadherin, whose extracellular domains share homologies with β1, are key structural members of the ID (Schlipp et al., 2014; Williams et al., 2002). Both β1 and β2 recruit AnkG, a protein important in regulating the perinexus (Delmar and Liang, 2012), via trans-homophillic adhesion (Malhotra et al., 2000). This process occurs independent of α-subunit availability in vitro, suggesting that β-subunits exert unique regulatory control over the VGSC, aiding in subcellular localization of the channel complex. β1 has been shown by super resolution microscopy to be co-localized near Cx43 GJs between ventricular myocytes, as well as highly associated with NaV1.5 (Veeraraghavan et al., 2016), suggesting that the β1 could have assignments in promoting high-density accumulation of current-generating channels such as VGSCs.
Perinexus Structure
The nexus is an alternative name for the GJ, thus the peripheral region of the GJ has been dubbed the perinexus (Figure 2). Accumulating evidence suggests that the perinexus may facilitate ephaptic conduction (see Table 1). The perinexus is currently defined as a narrow (< 200nm) stretch of closely apposed membranes (15-30nm) located immediately adjacent to GJs (Rhett et al., 2011; 2012c; 2013). Electron microscopy (EM) has allowed for high spatial resolution images of GJs and adjacent structures in the heart and has aided in identifying proteins that play integral roles in the putative ephaptic capabilities of the perinexus. Interaction between the scaffolding protein Zonula occludens-1 (ZO-1) and Cx43 governs the rate at which undocked connexon channels are recruited at the GJ edge, thereby regulating the rate at which Cx43 GJ plaques increase in size (Barker et al., 2002; Hunter et al., 2005; Zhu et al., 2005; Rhett et al., 2011). Ankyrin G (AnkG) has been found to localize in, or near, perinexal nano-domains, and thus may be available for binding to VGSCs, which together with the complex of interactions involving Nav1.5, Cx43, N-cadherin, ZO-1 and actin may form a framework for these specialized regions of membrane (Agullo-Pascual et al., 2014; Leo-Macias et al., 2016). Confocal and super resolution microscopy has demonstrated that active NaV1.5 channels accumulate within this region, with over 50% of all ID NaV1.5 signal found within 200nm of GJ plaques (Veeraraghavan and Gourdie, 2016; Veeraraghavan et al., 2015a; 2015b). Subsequent super resolution analysis of this region found an even higher enrichment of β1 subunits at Cx43 GJ edges (Veeraraghavan et al., 2016; Veeraraghavan et al., 2018), thus providing a further key structural element in the template that dictates the proper scaffolding, ion current density and enclosed extracellular space required for ephaptic conduction.
Figure 2:
Schematic model illustrating the gap junction and perinexus. Top over view of two myocytes interacting act an intercalated disk. Note that gap junctions are prominently found in the interplicate regions of the disk. Middle of gap junction and surrounding perinexus. Note the depiction of clusters of the trans-interacting sodium channel complexes in the perinexus. The electron micrograph of a gap junction and its adjacent peri-junctional regions now dubbed the perinexus is reproduced from Dewey and Barr, 1965. The cleft of extracellular space bounded by perinexal membranes is indicated by blue highlights.
The closest point of contact between any two cells is the 2nm gap between two connexon hemichannels docked as a GJ channel. The next closest point of contact between cardiomyocytes is directly adjacent to the GJ in the perinexus. Perfusion of an ex vivo preparation of hearts with the osmolyte mannitol was reported to be correlated with an increase in intercellular spacing between myocytes and conduction slowing and spontaneous arrhythmia (Veeraraghavan et al., 2012). This result was directly counter-intuitive to traditional cable theory predictions, in that increased extracellular space should increase cardiac AP due to decreased membrane resistance. The finding however fits mathematical models of the proposed extracellular space requirements of a cardiac ephapse outlined by Mori et al in 2008. Further collaborative ultrastructural studies by the Gourdie and Poelzing labs showed that osmolyte-induced swelling was localized in the extracellular space of the perinexus (Veeraraghavan et al., 2015a; 2015b). As the space between apposed membranes swelled beyond 30 nm, the limit described as a requisite for ephaptic coupling to occur, the noted CV decrease was observed. The perinexus has since been discovered to be malleable to a host of osmotic and ionic pressures, and these manipulations are capable of unmasking or blunting conduction defects and arrhythmic propensities (George and Poelzing, 2016; Greer-Short et al., 2017). Recently, a correlation has been demonstrated between perinexal widening and arrhythmia severity in patients with atrial conduction disturbance (Raisch et al., 2018). Importantly, this observation links the dynamic change in inter-membrane spacing in the perinexus to the most common arrhythmic disease seen in the clinic. It is the hope of many researchers that further studies in the composition of reperfusion solutions could make significant strides in aiding patients with conduction malignancies tied to dysregulated ephaptic coupling. Moreover, the dehiscence (i.e., de-adherence) of perinexal membranes observed in response to mannitol indicates the potential for specific trans-adhesive interactions within this nanodomain. As noted earlier, the β subunit of the sodium channel is a cell adhesion molecule and localized at high levels at GJ edges and thus it is of considerable interest to test the hypothesis that this cell adhesion molecule may be directly involved in maintaining structural integrity of inter-cellular adhesion at the perinexus.
Future Drug Discovery Targeting the β1 Subunit and the Perinexus
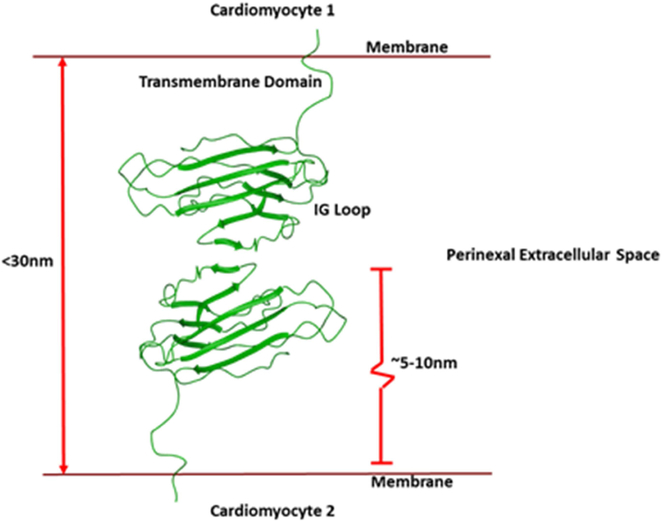
Recent computational biology work has explored two potential ephaptic modulation phenomenon that could be in part regulated by VGSC β subunits. The first finding being that a high concentration of sodium channels in small clusters at the ID allowed for greater peak INa to be generated, relative to the same amount broadly distributed (Hichri et al., 2018). This fits with our super resolution localization observations of dense pockets of NaV1.5 within the perinexus (Veeraraghavan and Gourdie, 2016; Veeraraghavan et al., 2015a). Similarly, high levels of β1 signal, and previous studies demonstrating a role for β1 in NaV1.5 α-subunit localization, implies a possible mechanism to aid in this clusterization. The second finding was that a narrow cleft was required for ephaptic conduction to occur, (Greer-Short et al., 2017; Veeraraghavan et al., 2015a), similar to results presented by Mori et al previously (Mori et al., 2008). Again, these insights correlate well with TEM analysis of the perinexal width and the predicted length of β1 subunits on apposed membranes in this narrow span of extracellular space. Our own homology modeling of β1 based on the structure resolved for the β3 extracellular domain , as well as structural analysis of E. electricus, (Yan et al., 2017), suggest that the length of each β1 subunit to be approximately 5-10 nm between either axis, with flexibility just outside their transmembrane domain (Figure 3). This means that two subunits, adhered Ig domain-to-Ig domain, across apposing membranes at the perinexus could tether an extracellular space of around 10-30 nm, within the theoretical requirements of ephaptic coupling (Mori et al., 2008; Greer-Short et al., 2017). These data suggest that stabilizing or enhancing the adhesive properties of the β1 subunit may be a route to novel therapeutics for treating diseases of cardiac conduction, including lethal ventricular arrhythmias. Such strategies would seek to keep perinexal inter-membrane spacing in ranges that would maintain ephaptic coupling i.e., < 30 nm. While the task of generating compounds that stabilize and/or agonize trans-adhesive interactions mediated by Ig domain-containing CAMs is difficult, agonists have been developed by two independent labs for N-cadherin and desmoglein-2, Ig-domain cell adhesion molecules that share homologies with β1 (Veeraraghavan et al., 2014a; Schlipp et al., 2014; Williams et al., 2002).
Figure 3:
Schematic of the spacing afforded by trans-adhesion of two β1 subunits (green) on apposed membranes at the GJ perinexus. The distance of two trans-interacting β1 subunits would maintain an inter-membrane spacing within the limit specified by Mori and co-workers (2008) for the theoretical cardiac ephapse.
Targeting protein-protein interactions is a difficult task. Specificity, and ultimately the potency, required to out-compete protein complexes with small molecules is daunting, which is why peptides and biologics tend to dominate the field. On-going work is focused on optimizing a β1 adhesion antagonist peptide as a first step towards development of an agonist. The extracellular space of the ID excludes molecules larger than 3kDa, so keeping the molecular mass of any peptide agent as low as possible is critical to allowing this molecule to reach its site of action. Furthermore, efforts will be taken to fasten the ideal linker length between the adhesion interfaces and the type of cyclization. Initial efforts might start with disulfide bonds between the optimized sequences, and expand to test peptide stapling techniques such as; stapling between olefin side chains, covalent linkage of the polypeptide N and C terminals, and branched peptides (Fairlie and de Araujo, 2016; Spokoyny et al., 2013; Verdine and Hilinski, 2012; Walensky and Bird, 2014). With the development of new lead molecules, finer-grained parameters, such as target specificity and residency time, can be evaluated to further aid the study of the biological ramifications of disruption/promotion of perinexal adhesion.
In addition to targeting β1 adhesion, the possibility remains to target other aspects of perinexus structure and function. Most specifically, perinexal clusterization of ion channels such as NaV1.5 and the ability of these membrane pores at the GJ edge to generate currents are of interest. Various ion modulators of the NaV1.5 channel exist, and their effects on conduction in circumstances that favor ephaptic conduction have begun to be examined (Veeraraghavan et al., 2015a). Such investigations will shed light on the potential contribution of ion currents, which will in turn begin to demonstrate the relative importance of the phenomenon in a host of physiological states. Another way to alter ion channel distribution would be via manipulations of protein trafficking. β1 plays a role in facilitating interactions between NaV1.5 dimers (Mercier et al., 2012), and targeting this activity could, in theory, increase the INa in the confined extracellular space of the perinexus. NaV1.5 are also clustered via 14-3-3 proteins (Allouis et al., 2006; Clatot et al., 2017), resulting in gated dimers. A lack of 14-3-3 has pro-arrhythmic effects on myocardial electrical properties suggesting that stabilizing, or otherwise modulating this protein-protein interaction, could result in higher densities of VGSC clusters with potentially beneficial effects on the rate and stability of AP conduction in the heart. This emerging field of cardiovascular research could also be useful in developing patient-specific therapies, for example, providing new ways to treat disorders that have been identified to be associated with mutations to the β1 Ig domain, such as Brugada syndrome and epilepsy (Patino et al., 2011; Wallace et al., 1998; Watanabe et al., 2009; Watanabe et al., 2008). Further explorations into the role ephaptics play in the total cardiac AP or the discovery of circumstances that would bolster its importance (i.e rapid elevation of heart rate) could lead to expanded uses of novel β1 acting AADs.
Conclusions
Cardiac ephaptic coupling has long been a provocative topic, but has recently gained renewed interest. This is in part due to the work and results of a small, but growing group of researchers, who find that GJ coupling may not tell the whole story of how electrical impulse is propagated between myocardial cells. With the recent identification of a perinexal swelling response in patients with atrial arrhythmia (Raisch et al., 2018), the work of linking ephaptics to cardiac conduction disease in humans is underway. No doubt, researchers will now be looking carefully at changes in this GJ-associated nanodomain in a spectrum of other arrhythmic pathologies. The long-term implications of characterizing the role of this putative and non-canonical intercellular coupling pathway, and how it may contribute to cardiac AP, could open up a new approach to the development of novel anti-arrhythmic drugs. The ability to rescue faulty cardiac conduction due to disrupted perinexal adhesion could be an alternative to riskier rhythm control therapies that rely on direct blockade of ion channel activity and would certainly aid in β1-deficient pathological disease states mentioned previously. Additionally, a new mechanism based approach could provide relief for those patients whose suffering would currently be exacerbated by existing therapeutics. There is a paucity of drugs that safely and effectively treat arrhythmic disease and prevent sudden cardiac death. Targeting the organization and dynamics of the GJ perinexus may provide a new path to such novel and necessary therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agullo-Pascual E, Cerrone M and Delmar M, 2014. Arrhythmogenic cardiomyopathy and Brugada syndrome: diseases of the connexome, FEBS Lett 588, 1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA and Tomaselli GF, 2007. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure, Am J Physiol Heart Circ Physiol 293, H1223–30. [DOI] [PubMed] [Google Scholar]
- Allouis M, Le Bouffant F, Wilders R, Peroz D, Schott JJ, Noireaud J, Le Marec H, Merot J, Escande D and Baro I, 2006. 14–3-3 is a regulator of the cardiac voltage-gated sodium channel Nav1.5, Circ Res 98, 1538–46. [DOI] [PubMed] [Google Scholar]
- Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI and Gourdie RG, 1997. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium, Circulation research. 80, 88–94. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Nesterenko V, Shryock JC, Rajamani S, Song Y and Belardinelli L, 2014. The role of late I Na in development of cardiac arrhythmias, Handb Exp Pharmacol. 221, 137–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M, Kanter EM, Huang RY, Maxeiner S, Frank M, Zhang Y, Schuessler RB, Smith TW, Townsend RR, Rohrs HW, Berthoud VM, Willecke K, Laing JG and Yamada KA, 2011. Residual Cx45 and its relationship to Cx43 in murine ventricular myocardium, Channels (Austin) 5, 489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni D, Picco C, Barbieri R and Moran O, 2014. Antisense-mediated post-transcriptional silencing of SCN1B gene modulates sodium channel functional expression, Biology of the Cell. 106, 13–29. [DOI] [PubMed] [Google Scholar]
- Barr L, Dewey MM and Berger W, 1965. PROPAGATION OF ACTION POTENTIALS AND THE STRUCTURE OF THE NEXUS IN CARDIAC MUSCLE, J Gen Physiol 48, 797–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneski DA and Catterall WA, 1980. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin, Proceedings of the National Academy of Sciences of the United States of America. 77, 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Paul DL and Goodenough DA, 1987. Connexin43: a protein from rat heart homologous to a gap junction protein from liver, J Cell Biol 105, 2621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, Dickendesher TL, Ranscht B and Isom LL, 2008. Voltage-Gated Na+ Channel β1 Subunit-Mediated Neurite Outgrowth Requires Fyn Kinase and Contributes to Postnatal CNS Development In Vivo, The Journal of Neuroscience. 28, 3246–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun JD and Isom LL, 2014. The Role of Non-pore-Forming β Subunits in Physiology and Pathophysiology of Voltage-Gated Sodium Channels, in: Ruben CP (Ed.), Voltage Gated Sodium Channels. Springer Berlin; Heidelberg, Berlin, Heidelberg, pp. 51–89. [DOI] [PubMed] [Google Scholar]
- Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, Kazen-Gillespie KA, Kazarinova-Noyes K, Shrager P, Saunders TL, Macdonald RL, Ransom BR, Scheuer T, Catterall WA and Isom LL, 2002. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel β2-subunits, Proceedings of the National Academy of Sciences. 99, 17072–17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K and Jui J, 2008. Epidemiology of sudden cardiac death: clinical and research implications, Prog Cardiovasc Dis 51, 213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatot J, Hoshi M, Wan X, Liu H, Jain A, Shinlapawittayatorn K, Marionneau C, Ficker E, Ha T and Deschênes I, 2017. Voltage-gated sodium channels assemble and gate as dimers, Nature Communications. 8, 2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copene ED and Keener JP, 2008. Ephaptic coupling of cardiac cells through the junctional electric potential, J Math Biol 57, 265–84. [DOI] [PubMed] [Google Scholar]
- Coppen SR, Dupont E, Rothery S, Severs NJ, 1998. Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circulation Research. 82, 232–43. [DOI] [PubMed] [Google Scholar]
- Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL and Wyse DG, 2004. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study, Circulation. 109, 1509–13. [DOI] [PubMed] [Google Scholar]
- Delmar M and Liang F-X, 2012. Connexin43, and the regulation of intercalated disc function, Heart Rhythm. 9, 835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes I, Armoundas AA, Jones SP and Tomaselli GF, 2008. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents, J Mol Cell Cardiol 45, 336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes I and Tomaselli GF, 2002. Modulation of Kv4.3 current by accessory subunits, FEBS Lett 528, 183–8. [DOI] [PubMed] [Google Scholar]
- Dewey MM and Barr L, 1962. Intercellular Connection between Smooth Muscle Cells: the Nexus, Science. 137, 670–2. [DOI] [PubMed] [Google Scholar]
- Dhar Malhotra J, Chen C, Rivolta I, Abriel H, Malhotra R, Mattei LN, Brosius FC, Kass RS and Isom LL, 2001. Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes, Circulation. 103, 1303–10. [DOI] [PubMed] [Google Scholar]
- E. Mann J and Sperelakis N, 1979. Further development of a model for electrical transmission between myocardial cells not connected by low-resistance pathways. [DOI] [PubMed] [Google Scholar]
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW and Investigators* t.C., 1991. Mortality and Morbidity in Patients Receiving Encainide, Flecainide, or Placebo, New England Journal of Medicine. 324, 781–788. [DOI] [PubMed] [Google Scholar]
- Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE and Rosenbaum DS, 2001. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice, Cardiovascular Research. 51, 681–690. [DOI] [PubMed] [Google Scholar]
- Entz M, George SA, Zeitz MJ, Raisch T, Smyth JW and Poelzing S, 2016. Heart Rate and Extracellular Sodium and Potassium Modulation of Gap Junction Mediated Conduction in Guinea Pigs, Frontiers in Physiology. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairlie DP and de Araujo AD, 2016. Stapling peptides using cysteine crosslinking, Peptide Science. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Fragakis N and Vassilikos VP, 2016. New antiarrhythmic drugs for atrial fibrillation, Continuing Cardiology Education. 2, 151–157. [Google Scholar]
- Franke WW, 2009. Discovering the molecular components of intercellular junctions--a historical view, Cold Spring Harb Perspect Biol 1, a003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Bonakdar M, Zeitz M, Davalos RV, Smyth JW and Poelzing S, 2016. Extracellular sodium dependence of the conduction velocity-calcium relationship: evidence of ephaptic self-attenuation, American Journal of Physiology - Heart and Circulatory Physiology. 310, H1129–H1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA and Poelzing S, 2016. Cardiac conduction in isolated hearts of genetically modified mice – Connexin43 and salts, Progress in Biophysics and Molecular Biology. 120, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Sciuto KJ, Lin J, Salama ME, Keener JP, Gourdie RG and Poelzing S, 2015. Extracellular sodium and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene, Pflügers Archiv - European Journal of Physiology. 467, 2287–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdie RG, Green CR, Severs NJ, 1991. Gap junction distribution in adult mammalian myocardium revealed by an anti-peptide antibody and laser scanning confocal microscopy. Journal of Cell Science 99, 41–55. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Severs NJ, Green CR, Rothery S, Germroth P, Thompson RP, 1993. The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system. Journal of Cell Science. 105, 985–91. [DOI] [PubMed] [Google Scholar]
- Greer-Short A, George SA, Poelzing S and Weinberg SH, 2017. Revealing the Concealed Nature of Long-QT Type 3 Syndrome, Circ Arrhythm Electrophysiol 10, e004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H and Fishman GI, 2001. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43, Circ Res 88, 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann SH, Terlau H, Stuhmer W, Imoto K and Numa S, 1992. Calcium channel characteristics conferred on the sodium channel by single mutations, Nature. 356, 441–3. [DOI] [PubMed] [Google Scholar]
- Hichri E, Abriel H and Kucera JP, 2018. Distribution of cardiac sodium channels in clusters potentiates ephaptic interactions in the intercalated disc, J Physiol 596, 563–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL and Horowicz P, 1959. Movements of Na and K in single muscle fibres, The Journal of Physiology. 145, 405–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko T, Sperelakis N and Berne RM, 1959. Evidence for Non-Syncytial Nature of Cardiac Muscle from Impedance Measurements, Proceedings of the Society for Experimental Biology and Medicine. 101, 602–604. [DOI] [PubMed] [Google Scholar]
- Johnson D, Montpetit ML, Stocker PJ and Bennett ES, 2004. The Sialic Acid Component of the β1 Subunit Modulates Voltage-gated Sodium Channel Function, Journal of Biological Chemistry. 279, 44303–44310. [DOI] [PubMed] [Google Scholar]
- Kanter HL, Saffitz JE and Beyer EC, 1992. Cardiac myocytes express multiple gap junction proteins, Circ Res 70, 438–44. [DOI] [PubMed] [Google Scholar]
- Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM, Woolf CJ and Kovacs DM, 2007. BACE1 regulates voltage-gated sodium channels and neuronal activity, Nat Cell Biol 9, 755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber AG and Rudy Y, 2004. Basic mechanisms of cardiac impulse propagation and associated arrhythmias, Physiol Rev 84, 431–88. [DOI] [PubMed] [Google Scholar]
- Kucera JP, Rohr S and Rudy Y, 2002. Localization of Sodium Channels in Intercalated Disks Modulates Cardiac Conduction, Circulation research. 91, 1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo-Macias A, Agullo-Pascual E, Sanchez-Alonso JL, Keegan S, Lin X, Arcos T, Feng Xia L, Korchev YE, Gorelik J, Fenyo D, Rothenberg E, Rothenberg E and Delmar M, 2016. Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc, Nat Commun 7, 10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T, Meakins J and White PD, 1914. The Excitatory Process in the Dog's Heart. Part I. The Auricles, Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character. 205, 375–420. [Google Scholar]
- Lin J and Keener JP, 2010. Modeling electrical activity of myocardial cells incorporating the effects of ephaptic coupling, Proceedings of the National Academy of Sciences. 107, 20935–20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J and Keener JP, 2013. Ephaptic coupling in cardiac myocytes, IEEE Trans Biomed Eng 60, 576–82. [DOI] [PubMed] [Google Scholar]
- Lin J and Keener James P., 2014. Microdomain Effects on Transverse Cardiac Propagation, Biophysical Journal. 106, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Meadows LS, Ernst SJ, Chen C, Malhotra JD, McEwen DP, Speelman A, Noebels JL, Maier SKG, Lopatin AN and Isom LL, 2007. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals, Journal of molecular and cellular cardiology. 43, 636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kazen-Gillespie K, Hortsch M and Isom LL, 2000. Sodium Channel β Subunits Mediate Homophilic Cell Adhesion and Recruit Ankyrin to Points of Cell-Cell Contact, Journal of Biological Chemistry. 275, 11383–11388. [DOI] [PubMed] [Google Scholar]
- Marionneau C, Carrasquillo Y, Norris AJ, Townsend RR, Isom LL, Link AJ and Nerbonne JM, 2012. The Sodium Channel Accessory Subunit Navβ1 Regulates Neuronal Excitability through Modulation of Repolarizing Voltage-Gated K(+) Channels, The Journal of Neuroscience. 32, 5716–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker EC, Bagneris C, Naylor CE, Cole AR, D'Avanzo N, Nichols CG and Wallace BA, 2012. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing, Nat Commun 3, 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier A, Clément R, Harnois T, Bourmeyster N, Faivre J-F, Findlay I, Chahine M, Bois P and Chatelier A, 2012. The β1-Subunit of Nav1.5 Cardiac Sodium Channel Is Required for a Dominant Negative Effect through α-α Interaction, PLOS ONE. 7, e48690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Undrovinas NA, Maltsev VA, Reznikov V, Sabbah HN and Undrovinas A, 2011. Post-transcriptional silencing of SCN1B and SCN2B genes modulates late sodium current in cardiac myocytes from normal dogs and dogs with chronic heart failure, American Journal of Physiology - Heart and Circulatory Physiology. 301, H1596–H1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Fishman GI and Peskin CS, 2008. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion, Proceedings of the National Academy of Sciences. 105, 6463–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley GE, Vaidya D, Samie FH, Lo C, Delmar M and Jalife J, 1999. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping, J Cardiovasc Electrophysiol 10, 1361–75. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Miyazaki H, Hoshi N, Smith BJ, Nukina N, Goldin AL and Chandy KG, 2012. Modulation of voltage-gated K+ channels by the sodium channel β1 subunit, Proceedings of the National Academy of Sciences. 109, 18577–18582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2017. Global Status Report On Noncommunicable Diseases 2014 Geneva, Switzerland: World Health Organization; 2014, in: Editor (Ed.), Book Global Status Report On Noncommunicable Diseases 2014. Geneva, Switzerland: World Health Organization; 2014. City. [Google Scholar]
- Palatinus JA, Rhett JM and Gourdie RG, 2012. The Connexin43 Carboxyl Terminus and Cardiac Gap Junction Organization, Biochimica et Biophysica Acta. 1818, 1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Brackenbury WJ, Bao Y, Lopez-Santiago LF, O'Malley HA, Chen C, Calhoun JD, Lafreniere RG, Cossette P, Rouleau GA and Isom LL, 2011. Voltage-gated Na+ channel beta1B: a secreted cell adhesion molecule involved in human epilepsy, J Neurosci 31, 14577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N and Catterall WA, 2012. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states, Nature. 486, 135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N and Catterall WA, 2011. The crystal structure of a voltage-gated sodium channel, Nature. 475, 353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertsov AM and Medvinskii AB, 1976. [Electric coupling in cells without highly permeable cell contacts], Biofizika. 21, 698–70. [PubMed] [Google Scholar]
- Peters NS, Green CR, Poole-Wilson PA and Severs NJ, 1995. Cardiac arrhythmogenesis and the gap junction, Journal of molecular and cellular cardiology. 27, 37–44. [DOI] [PubMed] [Google Scholar]
- Poelzing S, Greer-Short A, Jessup DK and Weinberg SH Ephaptic Self-Attenuation Conceals Early Afterdepolarizations Associated with Long QT-3 Syndrome, Biophysical Journal. 110, 275a. [Google Scholar]
- Qu Y, Isom LL, Westenbroek RE, Rogers JC, Tanada TN, McCormick KA, Scheuer T and Catterall WA, 1995. Modulation of Cardiac Na+ Channel Expression in Xenopus Oocytes by β1 Subunits, Journal of Biological Chemistry. 270, 25696–25701. [DOI] [PubMed] [Google Scholar]
- Raisch TB, Yanoff MS, Larsen TR, Farooqui MA, King DR, Veeraraghavan R, Gourdie RG, Baker JW, Arnold WS, AlMahameed ST and Poelzing S, 2018. Intercalated Disk Extracellular Nanodomain Expansion in Patients With Atrial Fibrillation, Frontiers in Physiology. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Jourdan J and Gourdie RG, 2011. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1, Mol Biol Cell. 22, 1516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Ongstad EL, Jourdan J and Gourdie RG, 2012a. Cx43 Associates with Na(v)1.5 in the Cardiomyocyte Perinexus, The Journal of membrane biology. 245, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM and Gourdie RG, 2012b. The Perinexus: A New Feature of Cx43 Gap Junction Organization, Heart Rhythm. 9, 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Poelzing S, Price RL and Gourdie RG, 2012c. Ultrastructural Analysis of the Cardiomyocyte Perinexus Points to the Potential for Conduction by Extracellular Electrodiffusion, Circulation. 126, A10030–A10030. [Google Scholar]
- Rhett JM, Veeraraghavan R, Poelzing S and Gourdie RG, 2013. The perinexus: Sign-post on the path to a new model of cardiac conduction?, Trends in Cardiovascular Medicine. 23, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S, Kucera JP and Kleber AG, 1998. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction, Circ Res 83, 781–94. [DOI] [PubMed] [Google Scholar]
- Schlipp A, Schinner C, Spindler V, Vielmuth F, Gehmlich K, Syrris P, McKenna WJ, Dendorfer A, Hartlieb E and Waschke J, 2014. Desmoglein-2 interaction is crucial for cardiomyocyte cohesion and function, Cardiovasc Res 104, 245–57. [DOI] [PubMed] [Google Scholar]
- Scott DE, Bayly AR, Abell C and Skidmore J, 2016. Small molecules, big targets: drug discovery faces the protein–protein interaction challenge, Nature Reviews Drug Discovery. 15, 533. [DOI] [PubMed] [Google Scholar]
- Severs NJ, Bruce AF, Dupont E and Rothery S, 2008. Remodelling of gap junctions and connexin expression in diseased myocardium, Cardiovasc Res 80, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs NJ, Shovel KS, Slade AM, Powell T, Twist VW and Green CR, 1989. Fate of gap junctions in isolated adult mammalian cardiomyocytes, Circ Res 65, 22–42. [DOI] [PubMed] [Google Scholar]
- Shaw RM and Rudy Y, 1997. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling, Circ Res 81, 727–41. [DOI] [PubMed] [Google Scholar]
- Sjostrand FS, Andersson E, 1954. Electron microscopy of the intercalated discs of cardiac muscle tissue. Experientia 10, 369–70. [DOI] [PubMed] [Google Scholar]
- Smith JH, Green CR, Peters NS, Rotary S, Severs NJ, 1991. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol 139, 801–21. [PMC free article] [PubMed] [Google Scholar]
- Spach MS, Heidlage JF, Dolber PC and Barr RC, 2000. Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth, Circ Res 86, 302–11. [DOI] [PubMed] [Google Scholar]
- Spach MS, Miller WT 3rd, Geselowitz DB, Barr RC, Kootsey JM and Johnson EA, 1981. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents, Circ Res 48, 39–54. [DOI] [PubMed] [Google Scholar]
- Sperelakis N, Hoshiko T, Berne RM, 1959. Evidence for non-syncytial nature of cardiac muscle from impedance measurements. Proc Soc Exp Med Biol 101, 602–604. [DOI] [PubMed] [Google Scholar]
- Spokoyny AM, Zou Y, Ling JJ, Yu H, Lin Y-S and Pentelute BL, 2013. A Perfluoroaryl-Cysteine SNAr Chemistry Approach to Unprotected Peptide Stapling, Journal of the American Chemical Society. 135, 5946–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, van Veen TA, Remme CA, Boulaksil M, Noorman M, van Stuijvenberg L, van der Nagel R, Bezzina CR, Hauer RN, de Bakker JM and van Rijen HV, 2009. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction, Cardiovasc Res 83, 52–60. [DOI] [PubMed] [Google Scholar]
- Stein M, van Veen TAB, Hauer RNW, de Bakker JMT and van Rijen HVM, 2011. A 50% Reduction of Excitability but Not of Intercellular Coupling Affects Conduction Velocity Restitution and Activation Delay in the Mouse Heart, PLOS ONE. 6, e20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SP, Kucera JP, Bircher-Lehmann L, Rudy Y, Saffitz JE and Kleber AG, 2003. Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43, Circ Res 92, 1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R and Gourdie R, 2016. Stochastic Optical Reconstruction Microscopy-based Relative Localization Analysis (STORM-RLA) for Quantitative Nanoscale Assessment of Spatial Protein Organization, Molecular Biology of the Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Hoeker GS, Poelzing S and Gourdie RG, 2016. Acute Inhibition of Sodium Channel Beta Subunit (β1) -mediated Adhesion is Highly Proarrhythmic, Circulation. 134, A13129–A13129. [Google Scholar]
- Veeraraghavan R, Lin J, Hoeker GS, Keener JP, Gourdie RG and Poelzing S, 2015a. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study, Pflugers Arch 467, 2093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Poelzing S and Gourdie RG, 2014a. Novel ligands for zipping and unzipping the intercalated disk: today's experimental tools, tomorrow's therapies?, Cardiovascular Research. 104, 229–230. [DOI] [PubMed] [Google Scholar]
- Veeraraghavan R, Lin J,. Keener JP, Poelzing S, Gourdie RG (2015b). Superresolution Microscopy Reveals Sodium Channel Localization within Intercalated Disk Microdomains: Implications for Ephaptic Coupling. Biophysical Journal 108, 572–573. [Google Scholar]
- Veeraraghavan R, Poelzing S and Gourdie RG, 2014b. Old Cogs, New Tricks: A Scaffolding Role For Connexin43 And A Junctional Role For Sodium Channels?, FEBS letters. 588, 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Gourdie RG, Poelzing S 2014c. Mechanisms of cardiac conduction: a history of revisions. Am J Physiol Heart Circ Physiol 306, H619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Salama ME and Poelzing S, 2012. Interstitial volume modulates the conduction velocity-gap junction relationship, American Journal of Physiology - Heart and Circulatory Physiology. 302, H278–H286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdine GL and Hilinski GJ, 2012. All-hydrocarbon stapled peptides as Synthetic Cell-Accessible Mini-Proteins, Drug Discovery Today: Technologies. 9, e41–e47. [DOI] [PubMed] [Google Scholar]
- Walensky LD and Bird GH, 2014. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress, Journal of Medicinal Chemistry. 57, 6275–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Wang DW, Singh R, Scheffer IE, George AL Jr., Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF and Mulley JC, 1998. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B, Nat Genet 19, 366–70. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Fujikawa A, Matsunaga H, Yasoshima Y, Sako N, Yamamoto T, Saegusa C and Noda M, 2000. Nav2/NaG channel is involved in control of salt-intake behavior in the CNS, J Neurosci 20, 7743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Darbar D, Kaiser DW, Jiramongkolchai K, Chopra S, Donahue BS, Kannankeril PJ and Roden DM, 2009. Mutations in sodium channel beta1- and beta2-subunits associated with atrial fibrillation, Circ Arrhythm Electrophysiol 2, 268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, Demolombe S, Probst V, Anselme F, Escande D, Wiesfeld AC, Pfeufer A, Kaab S, Wichmann HE, Hasdemir C, Aizawa Y, Wilde AA, Roden DM and Bezzina CR, 2008. Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans, J Clin Invest. 118, 2260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S, 1952. The electrical constants of Purkinje fibres, The Journal of Physiology. 118, 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S, 1966. The diffusion of radiopotassium across intercalated disks of mammalian cardiac muscle, J Physiol 187, 323–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S, 1970. Electrical constants of trabecular muscle from mammalian heart, J Physiol 210, 1041–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G, Williams E-J and Doherty P, 2002. Dimeric Versions of Two Short N-cadherin Binding Motifs (HAVDI and INPISG) Function as N-cadherin Agonists, Journal of Biological Chemistry. 277, 4361–4367. [DOI] [PubMed] [Google Scholar]
- Wit AL and Peters NS, 2012. The role of gap junctions in the arrhythmias of ischemia and infarction, Heart rhythm. 9, 308–311. [DOI] [PubMed] [Google Scholar]
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE and Corley SD, 2002. A comparison of rate control and rhythm control in patients with atrial fibrillation, N Engl J Med 347, 1825–33. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhou Q, Wang L, Wu J, Zhao Y, Huang G, Peng W, Shen H, Lei J and Yan N, 2017. Structure of the Nav1.4-β1 Complex from Electric Eel, Cell. 170, 470–482.e11. [DOI] [PubMed] [Google Scholar]
- Zablocki JA, Elzein E, Li X, Koltun DO, Parkhill EQ, Kobayashi T, Martinez R, Corkey B, Jiang H, Perry T, Kalla R, Notte GT, Saunders O, Graupe M, Lu Y, Venkataramani C, Guerrero J, Perry J, Osier M, Strickley R, Liu G, Wang W-Q, Hu L, Li X-J, El-Bizri N, Hirakawa R, Kahlig K, Xie C, Li CH, Dhalla AK, Rajamani S, Mollova N, Soohoo D, Lepist E-I, Murray B, Rhodes G, Belardinelli L and Desai MC, 2016. Discovery of Dihydrobenzoxazepinone (GS-6615) Late Sodium Current Inhibitor (Late INai), a Phase II Agent with Demonstrated Preclinical Anti-Ischemic and Antiarrhythmic Properties, Journal of Medicinal Chemistry. 59, 9005–9017. [DOI] [PubMed] [Google Scholar]
- Zhu W, Voelker TL, Varga Z, Schubert AR, Nerbonne JM and Silva JR, 2017. Mechanisms of noncovalent β subunit regulation of Na(V) channel gating, The Journal of General Physiology. 149, 813–831. [DOI] [PMC free article] [PubMed] [Google Scholar]