Abstract
Sulforaphane is a redox-active natural product present in cruciferous vegetables like broccoli. Broccoli sprout-derived products are promising agents for the prevention of oxidative stress-related diseases, but some have long been suspected of thyroidal toxicity. Recent findings also raise the possibility that long-term exposure to sulforaphane, or to other natural substances or drugs that modulate the activity of the transcription factor Nrf2 (NFE2-related factor 2) may lead to thyroid dysfunction or thyroid autoimmune disease, questioning the safety of trials with sulforaphane-containing products. Previous studies addressing possible effects of sulforaphane-related compounds from natural product extracts on the thyroid were quite short and/or inconsistent. To investigate whether long-term exposure to a beverage enriched with sulforaphane and its precursor glucoraphanin may affect thyroid function, we analyzed biochemical measures of thyroid function and thyroid autoimmunity in 45 female participants in a randomized clinical trial at baseline and after 84 days of beverage administration. Serum levels of thyroid-stimulating hormone, free thyroxine and thyroglobulin were not affected by the treatment, and neither was the thyroid autoimmunity status of participants. These results provide evidence in favor of the safety of chemoprevention strategies that target the activation of Nrf2 to protect against environmental exposures and other oxidative stress-related pathologies.
Keywords: Nrf2, thyroid, sulforaphane, glucoraphanin, broccoli sprout beverage, clinical trial
INTRODUCTION
Sulforaphane (1-Isothiocyanato-4-(methylsulfinyl)butane) is a redox-active natural substance that belongs to the isothiocyanate group and is present in foods and specifically in cruciferous vegetables like broccoli sprouts. These vegetables contain various glucosinolates including glucoraphanin (4-Methylsulfinylbutyl glucosinolate), the precursor of sulforaphane, which is converted to active sulforaphane via the catalytic activity of the plant enzyme myrosinase (Fahey et al., 2012). One of the best-characterized molecular targets of sulforaphane is the redox-sensitive transcription factor Nrf2 (NFE2-related factor 2). The activation of Nrf2 signaling by sulforaphane promotes binding of the transcription factor to DNA sequences called antioxidant response elements (AREs), with subsequent transcriptional upregulation of a battery of genes that function in antioxidant defense, cellular detoxification and other cell-protective mechanisms (Yamamoto et al., 2018). Broccoli sprout preparations are thus considered as promising natural products for the prevention of diseases related to oxidative stress, whereby the activation of proteostatic pathways by the administered compound(s) may prevent the onset or modify the natural course of disease pathogenesis (Yang et al., 2016). For example, a randomized clinical trial in China has shown that broccoli sprout beverage promotes the rapid and sustainable detoxification of airborne pollutants (Egner et al., 2014). Other trials with sulforaphane-rich broccoli sprout extracts demonstrated reductions of fasting blood glucose and glycated hemoglobin in obese patients with deregulated type 2 diabetes mellitus (Axelsson et al., 2017) as well as clinical improvement of symptoms related to autism spectrum disorder (Singh et al., 2014).
We have recently found that in cultured thyroid follicular cells, sulforaphane directly up-regulates the mRNA level and the protein abundance of thyroglobulin (TG), the precursor of thyroid hormones (Ziros et al., 2018). It does so by activating Nrf2, which in turn binds to two AREs in a conserved upstream enhancer of the gene encoding TG (Ziros et al., 2018). Moreover, it is known that when Nrf2-disrupted mice are bred into autoimmunity-permissive genetic backgrounds, they tend to develop an age-related systemic autoimmune syndrome, although it has not been reported whether the thyroid grand is also affected (Li et al., 2004; Ma et al., 2006; Yoh et al., 2001). These findings raise the possibility that long-term exposure to sulforaphane, or to other natural substances or drugs that modulate Nrf2 activity, may lead to thyroid dysfunction and cause hypothyroidism, hyperthyroidism or thyroid autoimmune disease, bringing into question the safety of interventions with sulforaphane or sulforaphane-containing products.
A small number of studies in humans and animals, reviewed by Felker et al. (Felker et al., 2016) and Latte et al. (Latte et al., 2011), have raised concerns about the possible effects of natural product extracts that contain glucosinolates and isothiocyanates on thyroid gland function. This is mostly based on the potential competitive inhibition of the sodium/iodide symporter by the thiocyanate ion (Tonacchera et al., 2004), which could lead to impaired iodide import and thus to reduced synthesis of thyroid hormones, resulting in hypothyroidism. However a decades-old study that addressed the effect of the addition of 8 mg thiocyanate in milk consumed daily for 12 weeks by human healthy volunteers showed no effect on thyroid hormone levels, with all subjects remaining euthyroid (Dahlberg et al., 1984). Another metabolite of glucosinolates with potential detrimental effects on thyroid function is goitrin (5-ethenyl-1,3-oxazolidine-2-thione). Goitrin synthesis from progoitrin, which can be detected in broccoli in trace amounts (Tian et al., 2005), is actually facilitated by myrosinase, the same enzyme that converts glucoraphanin into sulforaphane. Even though the circulating levels of goitrin after consumption of broccoli have not been directly measured in humans, it is known that administration of goitrin to humans can impair iodine uptake by the thyroid An old preliminary clinical trial on the effect of cooked Brussels sprouts administered to humans for 4 weeks showed no effects on thyroid function tests (McMillan et al., 1986); the authors speculated that this lack of activity of cooked Brussels sprouts is due to inactivation during cooking of myrosinase, the glucosinolate-metabolizing enzyme. This biochemical effect (later verified by other studies) is the rationale why the field trial in China within which the present study is nested used a sulforaphane-rich broccoli sprout beverage, in which myrosinase was used to catalyze the conversion of glucoraphanin into active sulforaphane (Egner et al., 2014). In addition, a myrosinase-like ß-thioglucosidase in the gut microflora also hydrolyzes glucoraphanin in the beverage to sulforaphane. A small phase I human clinical trial on the safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates (Shapiro et al., 2006) found no significant or consistent subjective or objective thyroidal toxicities; however, the study was very short (only 1 week) and small (only 3 subjects per group).
Regarding animal models, in decades-old studies the effect of goitrin on the thyroid has been investigated using rats (Faiman et al., 1967) and chicken (Akiba and Matsumoto, 1976); goitrin administration was shown to inhibit iodide uptake and to increase the weight of thyroid gland, causing goiter, hence the name of the substance. In another, more clinically relevant study, rats were administered over 4 weeks test diets with 15% or 30% of the dry food matter as uncooked or cooked Brussels sprouts and control diets with or without 0.2% potassium thiocyanate (a known thyroid function inhibitor) for comparison; it also administered diets with 0, 2.5, 5, 10 and 20% of the cooked vegetable, as well as diets with 0 and 20% of the cooked vegetable with extra iodine (de Groot et al., 1991). While thyroid-stimulating hormone (TSH, the most sensitive indicator of thyroid function) was not increased by feeding the vegetable, morphological activation of the thyroid (assessed by histology) was increased with 10% or more of the cooked vegetable (de Groot et al., 1991). These results suggest that the type and degree of preparation during cooking can affect the content of thyroid-active substances in diverse ways; hence, results may vary depending on the specific formulation and preparation method employed.
In summary, published studies on the thyroidal safety of plant-based compounds that activate Nrf2 are few in number, short in duration and/or inconsistent regarding the preparation of the plant extract and its possible effects on the thyroid gland. Therefore, to investigate whether long-term exposure to an orally administered sulforaphane-rich broccoli sprout beverage has an impact on thyroid function, we analyzed biochemical measures of thyroid function and thyroid autoimmunity in a subset of the participants in the aforementioned broccoli sprout clinical trial (Egner et al., 2014).
MATERIALS AND METHODS
Study design
The present work is a retrospective analysis of a subset of serum samples collected during a clinical trial that was conducted from mid-October 2011 to early January 2012 in the rural farming community of He-He Township, Qidong, Jiangsu Province, China (NCT number 01437501 in ClinicalTrials.gov). The structure of this clinical trial has been previously described in detail (Egner et al., 2014). Briefly, adults in good general health were randomized in a placebo-controlled trial designed to evaluate the pharmacokinetics and pharmacodynamics of a beverage containing 40 μmol sulforaphane and 600 μmol of its biogenic precursor glucoraphanin, both coming from 3-day old broccoli sprouts. Written informed consent was obtained from all participants; the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health, the Qidong Liver Cancer Institute and the University of Pittsburgh approved the protocols for the trial and for the biochemical analyses, respectively. 1205 individuals aged 21–65 years old were screened at local clinics by methods used in previous studies in the area (Kensler et al., 2012) and 539 were deemed eligible for the study. The initial 300 were randomized using a fix randomization scheme with a block size of 10; 79% were women and 21% men. Finally, 130 individuals completed the trial receiving placebo beverage and 137 receiving the broccoli sprout beverage for 84 consecutive days (12 weeks). Serum samples from a subset (5 randomization blocks) of participants were selected for the present study; only samples (n=45) from females were analyzed. Their baseline characteristics are shown in Table 1.
TABLE 1.
| group | placebo | broccoli sprout | p-value |
|---|---|---|---|
| number | 19 | 26 | N/A |
| female (n, %) | 19 (100%) | 26 (100%) | N/A |
| age (mean ±S.E.M.) | 48.1 ± 1.6 | 41.3 ±1.8 | <0.01 |
| TSH (mean ±S.E.M.) | 4.58 ± 0.47 | 3.8 ± 0.6 | 0.041 |
| fT4 (mean ±S.E.M.) | 1.51 ± 0.05 | 1.59 ± 0.04 | 0.15 |
| TG (mean ±S.E.M.) | 10.81 ± 1.71 | 7.47 ± 0.85 | 0.15 |
| subclinical hypothyroidism (n, %) | 5 (26%) | 6 (23%) | 0.99 |
| anti-TG and/or anti-TPO seropositivity (n, %) | 11 (58%) | 11 (42%) | 0.37 |
Characteristics of participants at baseline. N/A, not applicable; n, number; S.E.M., standard error of the mean; seropositivity, titer of anti-TG antibodies or anti-TPO antibodies (or both) above the respective reference range.
Preparation and quality control of broccoli sprout and placebo beverages
Broccoli sprouts were grown from specially selected BroccoSprouts seeds (cv. DM1999B) with technology licensed from Johns Hopkins University. The preparation of the broccoli sprouts extracts has been previously described in detail (Egner et al., 2014). Briefly, after 3 days of sprout growth, sprouts were allowed to boil for 30 minutes in deionized water. This aqueous extract contained about 5 mM glucoraphanin, the sulforaphane precursor, and was lyophilized to a glucoraphanin-rich powder that contained 329 μmol/g glucoraphanin as assessed by HPLC (Wade et al., 2007). The sulforaphane-rich powder was prepared by treating the aqueous broccoli sprout extract with myrosinase, an enzyme released from daikon (Raphanus sativus) sprouts, at 37°C for 4 hours to hydrolyze the glucosinolates to isothiocyanates. Total isothiocyanates levels were quantified by cyclocondensation analysis (Ye et al., 2002) and the sulforaphane levels were assessed by direct HPLC (Tang et al., 2006). This hydrolyzed aqueous extract was lyophilized to the sulforaphane-rich powder that contained 202 μmol/g sulforaphane, representing 91% of the total isothiocyanate content in the powder. To prepare the daily dose of broccoli sprout extract or placebo for the study participants, 360 g glucoraphanin- and 24 g sulforaphane-rich powder were dissolved in sterile water. Then, a mixture of 47:47:6 water: pineapple juice: lime juice was prepared, and 100 ml were distributed to each participant; the individual daily dose was 600 μmol of glucoraphanin and 40 μmol of sulforaphane (Egner et al., 2014). Placebo contained the same liquid components plus 1% molasses for color masking purposes. The placebo beverage contained no measurable glucoraphanin or sulforaphane and, unlike the broccoli sprout-derived beverage, showed negligible Nrf2 inducer activity in in vitro assays.
Serum analyses
Blood samples from day 0 and day 84 (last day) of the study were collected from all participants. In a subset of 45 female participants, serum TSH, free thyroxine (fT4), thyroglobulin (TG), anti-TG and anti-thyroid peroxidase (anti-TPO) antibodies were measured at the UPMC Presbyterian Automated Testing Laboratory and Immunoserology Laboratory (Pittsburgh, PA) using aliquoted serum samples that had been stored frozen (−80°C) since collection. TSH was measured using the immunoenzymatic Access TSH (3rd IS) assay (Beckman Coulter, Brea, CA) in a DXI 800 analyzer (Beckman Coulter); TSH reference range: 0.45–5.33 mIU/L. fT4 was measured using the fT4 ADVIA Centaur immunoassay (Siemens Healthcare Diagnostics) in an ADVIA Centaur analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY); fT4 reference range: 0.89–1.76 ng/dl. TG was measured using the immunoenzymatic Access Thyroglobulin assay (Beckman Coulter) in a DXI 800 analyzer. Anti-TG and anti-TPO levels were measured using solid-phase, enzyme-labeled, chemiluminescent sequential immunometric assays (Siemens Healthcare Diagnostics) in an Immulite 2000XPi analyzer (Siemens Healthcare Diagnostics); reference ranges: <20 IU/mL for anti-TG antibodies and <10 IU/ml for anti-TPO antibodies. Both the laboratory and the specific assays are certified for clinical use.
Statistical analyses
G*power software (Faul et al., 2009) was used to calculate the sample size needed for serum measurements of TSH, fT4 and TG. For two-way ANOVA repeated measures, to detect a medium effect size (f=0.25) with a power of 0.85 and α error set at 0.05, at least 19 samples per group are needed. As most of the participants in the clinical trial were women, we selected for analysis sera from a randomly pre-scrambled bloc of 50 study participants that comprised 19 women on placebo treatment and 26 women on broccoli sprout extract treatment with a mean age of about 48 and 41 years old respectively (as well as 5 men, not analyzed, who by chance had all received placebo). Groups were compared for characteristics at baseline (day 0) by Mann-Whitney test or chi-square test, as appropriate. To assess for effects of the broccoli sprout extract beverage, TSH, fT4 and TG levels were compared between the groups by two-way ANOVA with repeated measures using GraphPad Prism 7.00 (GraphPad Software, La Jolla, CA). Regarding thyroid autoimmunity status, the percentages of participants that were seropositive for either anti-TG or anti-TPO antibodies (or both) or seronegative for both anti-TG and anti-TPO antibodies were compared by chi-square test at days 0 and 84. Statistical significance was set at p<0.05.
RESULTS
Thyroid function
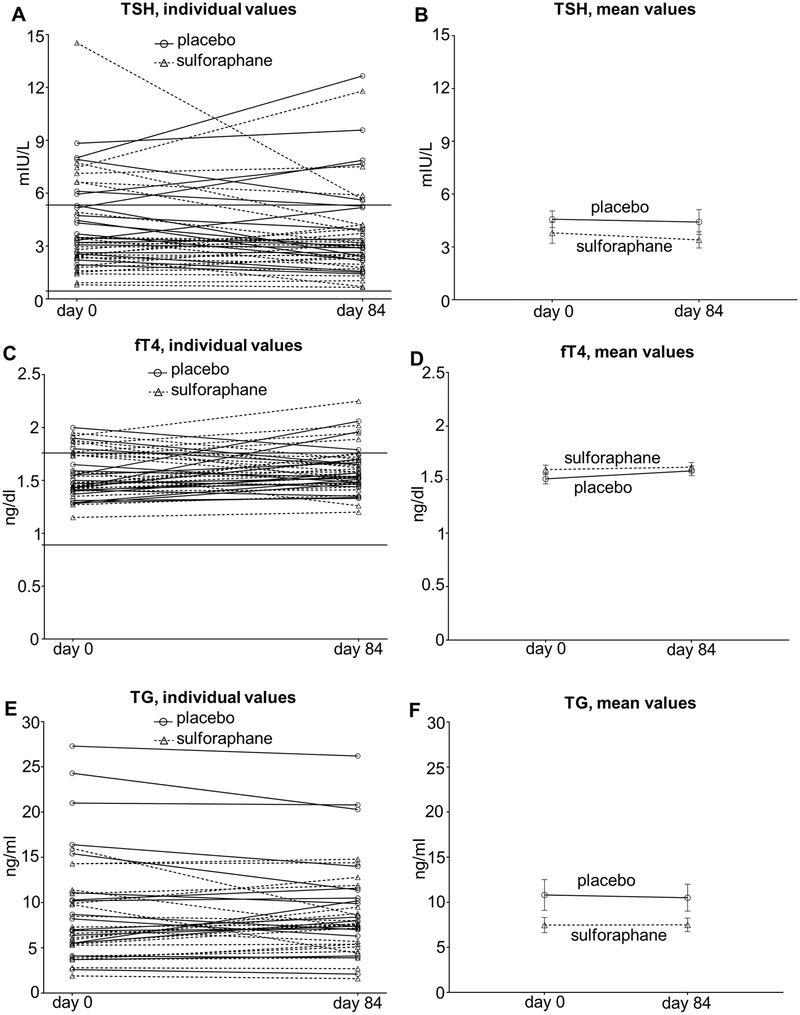
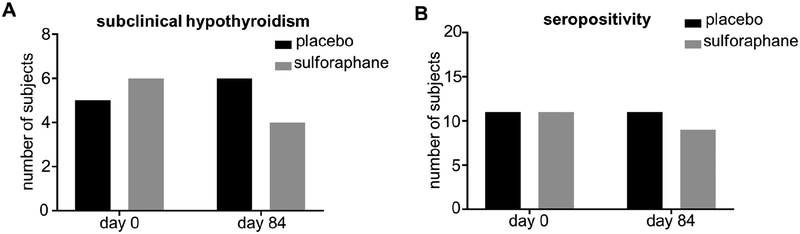
When comparing baseline characteristics between the placebo and treatment groups, there was a small but statistically significant difference in TSH levels (Table 1, comparison by Mann-Whitney test); nevertheless, mean values were within the reference range for both groups. Importantly, when the whole dataset (baseline and end-of-treatment) was analyzed by repeated measures two-way ANOVA, neither time nor treatment group allocation nor their interaction had a significant effect on TSH levels (p=0.39, p=0.23 and p=0.70, respectively) (Figure 1A, 1B). Similarly, neither time nor treatment group allocation nor their interaction had a significant effect on fT4 levels (p=0.12, p=0.29 and p=0.41, respectively), (Figure 1C, 1D). Moreover, the percentage of patients with subclinical hypothyroidism (elevated TSH with normal fT4) was not different between the two groups either before or after treatment (p=0.99 and p=0.2807, respectively) (Figure 2A). Taken together, these results indicate that thyroid function overall is unaffected by consuming the broccoli sprout extract beverage.
Figure 1:
Individual and mean (±S.E.M.) values of TSH (A and B), fT4 (C and D) and TG (E and F), respectively, at baseline and after 84 days of broccoli sprout beverage or placebo ingestion. The upper and lower limits of the reference range for TSH (0.45–5.33 mIU/L) and fT4 (0.89–1.76 ng/dl) are indicated by horizontal lines in panels A and C, respectively.
Figure 2:
(A) Number of subjects with subclinical hypothyroidism at baseline and after 84 days of broccoli sprout beverage or placebo ingestion. (B) Number of subjects positive for anti-TPO and/or anti-TG antibodies at baseline and after 84 days of broccoli sprout beverage or placebo ingestion.
Thyroglobulin
Because Nrf2 has been shown to control the expression of the TG gene, thyroglobulin levels were measured to assess for a possible impact of the broccoli sprout beverage. Given that anti-TG antibodies can interfere in the TG measurement assay, only subjects with anti-TG antibody titers within the reference range were retained in these analyses. Despite a near-significant difference among the two treatment allocation groups (p=0.06), neither time nor its interaction with treatment group allocation had a significant effect on TG levels (p=0.72 and p=0.67, respectively) (Fig. 1E, 1F). These results indicate that serum TG levels are unaffected by the broccoli sprout extract beverage.
Thyroid autoimmunity
The percentage of participants that were seropositive for either anti-TG or anti-TPO antibodies (or both) was not different between the placebo group and the broccoli sprout extract beverage group either before treatment (57.9% in placebo group vs. 42.3 % in treatment group, p=0.37) or after treatment (57.9 % in placebo group vs. 34.6 % in treatment group, p=0.14) (Figure 2B).
DISCUSSION
In the present study, thyroid function tests and measures of thyroid autoimmunity among participants in a randomized clinical trial of a broccoli sprout extract beverage versus placebo for 84 days showed no evidence for adverse effects of this treatment on the thyroid gland. Because this was a retrospective study nested within the original clinical trial, there are certain limitations that should be acknowledged. First, thyroidal parameters were assessed at only two time points. Since intermediate points were not included, temporary changes cannot be ruled out, but since measures were overall unchanged at 84 days, temporary changes would not be expected to have clinical relevance. Effects at longer durations of treatment can also not be excluded, but appear unlikely in view of the present results. Secondly, the participants were not selected for normal thyroid function at baseline, but were a randomly picked subset among the participants in the trial. The broccoli extract treatment and placebo groups analyzed here had a statistically significant difference in the age of participants (Table 1). It is known that serum TSH levels increase with age, likely as part of a physiological adaptation to aging. From a clinical perspective, the mean difference in age between the groups being small (6.84 years), it is unlikely to impact the results. In addition, in the two-way ANOVA repeated measures design employed to analyze the data, each participant serves as their own control. Lastly, the reference ranges employed are not specific for the Chinese population. However, the fact that no effects of the treatment were observed on TSH, fT4 and TG levels is independent of the absolute values of the respective reference ranges.
Notwithstanding these limitations, the data do provide strong evidence in favor of the safety of the specific regimen as far as the thyroid gland is concerned. One possible explanation is that, under the specific dosing regimen, the thyroid gland may not be exposed to substantial concentrations of sulforaphane; this is clearly not the case for other tissues, because a rigorous, sustained effect on air pollution biomarkers was observed in the same study (Egner et al., 2014). Given that the study analyzed the main biomarkers of thyroid function that can be measured non-invasively, there is no plausible way to confirm or refute this hypothesis using the materials available. Furthermore, in view of the data, the prospective use of invasive methods to directly address the question (e.g., measurement of sulforaphane or of mRNA levels of Nrf2 target genes in material obtained from thyroid fine-needle aspiration biopsy) is not justified either. If sulforaphane does reach the thyroid and activate Nrf2 in thyroid follicular cells, then the alternative hypothesis would be that the thyroid has mechanisms that can prevent such activation of Nrf2 from impacting the hormone synthesis machinery and ultimately the gland’s function. Auto-regulatory mechanisms are believed to exist in thyroid follicular cells that can compensate for reduced Nrf2 activity (Ziros et al., 2018), so it is conceivable that such mechanisms may also protect against increased Nrf2 activity, at least to a certain extent. Interestingly, chronic, potent activation of Nrf2 signaling due to inherited loss-of-function mutations in the KEAP1 gene that encodes the main inhibitor of Nrf2 have been associated with goiter in two unrelated Japanese families (Nishihara et al., 2016; Teshiba et al., 2013). Due to the retrospective nature of the present study, whose parent trial did not evaluate thyroid volume either by palpation or ultrasound, it is not possible to address this issue. Thus, these questions warrant further investigation in animal model studies. From a clinical perspective, the results of such studies could also help to address the question whether monitoring thyroid function is systematically warranted in clinical trials of Nrf2-activating compounds, or whether it can be generally assumed that all such compounds are safe for the thyroid gland (save for potential Nrf2-independent mechanisms). In the meantime, it may be prudent to evaluate the thyroidal safety of plant-based food supplements on a case-by-case basis, because the effects may vary depending on the plant used and the preparation of the extract, and therefore it may not be safe to draw conclusions from previous studies using different approaches.
In conclusion, the present study showed that daily ingestion of a broccoli sprout extract beverage over 84 days had no deleterious effect on thyroid function tests or measures of thyroid autoimmunity, providing evidence in favor of the safety of controlled chemoprevention strategies that target the activation of Nrf2 to protect against environmental exposures and other oxidative stress- and inflammation-related pathologies.
Supplementary Material
HIGHLIGHTS.
A broccoli sprout beverage was administered to healthy volunteers as a source of sulforaphane to activate Nrf2 signaling.
Daily consumption of the sulforaphane-rich beverage for 12 weeks did not affect the serum levels of fT4, TSH and TG.
The thyroid autoimmunity status of participants after 12 weeks on the broccoli sprout beverage was also unaffected.
Long-term ingestion of a sulforaphane-rich broccoli sprout beverage is safe for the thyroid gland.
Natural products activating Nrf2 should be individually evaluated for effects on thyroid hormonal and autoimmune status.
ACKNOWLEDGMENTS
The authors thank the staff of the He-He Public Health Station and the He-He Medical Clinic, the village doctors, and the residents of He-He for their participation in the parent trial; the clinical trial and biostatistics team at the Johns Hopkins Bloomberg School of Public Health; Mr. Jeff Tischler, MT (ASCP) and the medical technologists of the UPMC Presbyterian Automated Testing Laboratory and the Immunoserology laboratory for testing; and the laboratory and clinical staff of the Qidong Liver Cancer Institute for their support throughout the study. The work benefited from COST Action CA16112 (NutRedOx) supported by European Cooperation in Science and Technology (COST).
FUNDING INFORMATION
The clinical trial was funded by National Institutes of Health grant P01 ES006052 [to J.D.G.]. This work was supported by a Short Term Scientific Mission by European Cooperation in Science and Technology (COST) Action CA16112 “NutRedOx – Personalized nutrition in aging society: redox control of major age-related diseases” [to D.V.C. and G.P.S.]; National Institutes of Health grant R35 CA197222 [to T.W.K.]; Marie Curie PIOF-GA-2012–329442 “ADIPONRF2” (7th European Community Framework Programme) [to D.V.C.]; Swiss National Science Fund SNF-COST project IZCOZ0–177070 [to G.P.S.]; Swiss National Science Fund project 31003A_182105 [to G.P.S.]; a Swiss Society for Endocrinology-Diabetology 2014 Young Independent Investigator Award [to G.P.S.]); and a Leenaards Foundation 2016 Fellowship for Academic Promotion in Clinical Medicine [to G.P.S.].
Abbreviations
- fT4
free thyroxine
- Nrf2
NFE2-related factor 2
- TSH
thyroid-stimulating hormone
- TG
thyroglobulin
- TPO
thyroid peroxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akiba Y, Matsumoto J, 1976. Antithyroid activity of goitrin in chicks. Poult Sci 55, 716–719. [DOI] [PubMed] [Google Scholar]
- Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, Fahey JW, Derry JMJ, Wollheim CB, Wierup N, Haymond MW, Friend SH, Mulder H, Rosengren AH, 2017. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med 9. [DOI] [PubMed] [Google Scholar]
- Dahlberg PA, Bergmark A, Bjorck L, Bruce A, Hambraeus L, Claesson O, 1984. Intake of thiocyanate by way of milk and its possible effect on thyroid function. Am J Clin Nutr 39, 416–420. [DOI] [PubMed] [Google Scholar]
- de Groot AP, Willems MI, de Vos RH, 1991. Effects of high levels of brussels sprouts in the diet of rats. Food Chem Toxicol 29, 829–837. [DOI] [PubMed] [Google Scholar]
- Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, Kensler TW, 2014. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila) 7, 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P, 2012. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila) 5, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiman C, Ryan RJ, Eichel HJ, 1967. Effect of goitrin analogues and related compounds on the rat thyroid gland. Endocrinology 81, 88–92. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG, 2009. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Felker P, Bunch R, Leung AM, 2016. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr Rev 74, 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Munoz A, Egner PA, Chen JG, Qian GS, Chen TY, Fahey JW, Talalay P, Groopman JD, Yuan JM, Hecht SS, 2012. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis 33, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latte KP, Appel KE, Lampen A, 2011. Health benefits and possible risks of broccolian overview. Food Chem Toxicol 49, 3287–3309. [DOI] [PubMed] [Google Scholar]
- Li J, Stein TD, Johnson JA, 2004. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics 18, 261–272. [DOI] [PubMed] [Google Scholar]
- Ma Q, Battelli L, Hubbs AF, 2006. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am J Pathol 168, 1960–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan M, Spinks EA, Fenwick GR, 1986. Preliminary observations on the effect of dietary brussels sprouts on thyroid function. Hum Toxicol 5, 15–19. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Hishinuma A, Kogai T, Takada N, Hirokawa M, Fukata S, Ito M, Yabuta T, Nishikawa M, Nakamura H, Amino N, Miyauchi A, 2016. A Novel Germline Mutation of KEAP1 (R483H) Associated with a Non-Toxic Multinodular Goiter. Front Endocrinol (Lausanne) 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P, 2006. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer 55, 53–62. [DOI] [PubMed] [Google Scholar]
- Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, Zimmerman AW, 2014. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci U S A 111, 15550–15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Zhang Y, Jobson HE, Li J, Stephenson KK, Wade KL, Fahey JW, 2006. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Mol Cancer Ther 5, 935–944. [DOI] [PubMed] [Google Scholar]
- Teshiba R, Tajiri T, Sumitomo K, Masumoto K, Taguchi T, Yamamoto K, 2013. Identification of a KEAP1 germline mutation in a family with multinodular goitre. PLoS One 8, e65141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Rosselot RA, Schwartz SJ, 2005. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem 343, 93–99. [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, Santini F, Crump K, Gibbs J, 2004. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid 14, 1012–1019. [DOI] [PubMed] [Google Scholar]
- Wade KL, Garrard IJ, Fahey JW, 2007. Improved hydrophilic interaction chromatography method for the identification and quantification of glucosinolates. J Chromatogr A 1154, 469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kensler TW, Motohashi H, 2018. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev 98, 1169–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Palliyaguru DL, Kensler TW, 2016. Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin Oncol 43, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P, 2002. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta 316, 43–53. [DOI] [PubMed] [Google Scholar]
- Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S, 2001. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int 60, 1343–1353. [DOI] [PubMed] [Google Scholar]
- Ziros PG, Habeos IG, Chartoumpekis DV, Ntalampyra E, Somm E, Renaud CO, Bongiovanni M, Trougakos IP, Yamamoto M, Kensler TW, Santisteban P, Carrasco N, Ris-Stalpers C, Amendola E, Liao XH, Rossich L, Thomasz L, Juvenal GJ, Refetoff S, Sykiotis GP, 2018. NFE2-Related Transcription Factor 2 Coordinates Antioxidant Defense with Thyroglobulin Production and Iodination in the Thyroid Gland. Thyroid 28, 780–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.