Abstract
Background:
Testosterone (T)/Nestorone (NES) combination gel is a potential transdermal male contraceptive that suppresses gonadotropins and spermatogenesis. Transfer of transdermal T from men to women can be prevented by washing or covering application sites with clothing.
Objectives:
We hypothesized that showering or wearing a shirt over gel application sites would prevent secondary exposure of T and NES to a woman after close skin contact.
Materials and Methods:
12 healthy male and 12 healthy female participants were recruited. Men applied T/NES 62mg/8mg gel to their shoulders and upper arms. 2h after application, female partners rubbed the application site for 15 min. Exposure in the female partner was assessed under 3 conditions: a shirt covered the application site; the man showered prior to skin contact; or without intervention to reduce transfer. Serum T and NES concentrations were measured by LC-MS/MS in serial blood samples for 24h after gel exposure.
Main Outcomes:
Change in female serum T and NES levels as measured by average concentration over 24h (Cavg).
Results:
Median female serum T Cavg was 23.9 ng/dl (interquartile range, 19.3, 33.9) with the shirt barrier and 26.7 ng/dl (20.7, 33.9) after showering, which was higher than baseline 20.9 ng/dl (16.7, 25.0), both p < 0.03) but lower than without intervention (58.2 ng/dl [30.9, 89.1], both p<0.01). Female serum NES Cavg and maximum concentration were below the lower limit of quantification with the shirt barrier and after showering, but increased without intervention in 6 out of 12 women (maximum concentration <60 pg/ml). Men had lower average serum NES levels after showering (47 pg/ml [20,94] compared to no intervention (153.3 pg/ml [51, 241], p<0.02).
Conclusion:
Secondary transfer of T and NES occurs after intensive skin contact with the gel application site. Secondary transfer is decreased by a shirt barrier or showering before contact.
Keywords: male contraceptive, androgen, progestin, skin transfer, transdermal gels, Nestorone
Introduction
It has been over 50 years since the approval of the use of hormonal contraception for women. However, there is no effective, reversible, and safe contraception option for men. Hormonal male contraception inhibits the hypothalamic-pituitary-gonadal axis via exogenous sex hormones to suppress endogenous testosterone (T) production, intratesticular T concentrations, and spermatogenesis while providing sufficient systemic androgens to preserve normal functions (Chao, et al., 2014; Wang, et al., 2016). With T alone, prior studies demonstrated that spermatogenesis suppression is not uniform in all men; some men did not fully respond to the dose used or escaped from suppression, despite the use of supraphysiologic doses of T (Gu, et al., 2009; World Health Organization Task Force on Methods for the Regulation of Male Fertility, 1990; World Health Organization Task Force on the Regulation of Male Fertility, 1996). Progestin coadministration with T has been shown to suppress sperm production more rapidly and to a greater extent than T alone, and permits lower doses of T (Liu, et al., 2008; Meriggiola, et al., 2003; Wang & Swerdloff, 2004).
Nestorone (NES, 16-methylene-17α-acetoxy-19-norpregn-4-ene-3, 20-dione) is a synthetic 19-norprogesterone derivative, also known as ST-1435 or segesterone acetate (SA). It is a progestin that does not have androgen or estrogen receptor binding activity and is 2.9-fold more potent than progesterone in relative receptor binding affinity assays (Kumar, et al., 2000). Administration of NES combined with T gel transdermally has been shown to be safe and effective at suppressing gonadotropins (Mahabadi, et al., 2009) and spermatogenesis in men (Ilani, et al., 2012). Use of T+NES steroidal gels applied daily was reported to be acceptable by over 56% men and 50% would recommend the method to others (Roth, et al., 2014). However, use of a topical steroid carries the risk of transfer to others at close skin contact. Testosterone gel may transfer to women and children after significant skin contact with the application site of male users; this secondary transference may increase serum T levels 86–185% above baseline (De Ronde, 2009; Stahlman, et al., 2012a). Transference is reduced by wearing clothing over the application site or washing the site prior to contact (Stahlman, et al., 2012a; Stahlman, et al., 2012b). Because of the possible transfer of T applied on the skin to another person, regulatory agencies recommend men wash their hands after applying the gel and cover the exposed skin with clothing or take a shower prior to physical contact with another person.
It is unknown to what extent NES might transfer to women after skin contact with the T/NES gel application site and whether secondary exposure to NES will affect female reproductive function. We hypothesized that the T/NES gel would transfer similarly to the T gel and that showering or wearing a shirt over gel application sites would prevent secondary exposure of T and NES to a woman after close skin contact.
Research Participants and Methods
This was a Phase Ib multicenter, open label study of T/NES (62mg/8mg) combined gel conducted in healthy male and female paired volunteers. The study consisted of 3 single applications (on days 1, 8, 15) of the T/NES gel on the shoulders/upper arms of the male participant followed 2 hours later by 15 min of supervised skin contact with the non-dosed female partner. Three scenarios were studied: shirt barrier, shower, and no intervention to reduce transfer. The primary endpoint was to quantify the T/NES secondary exposure in the female participants in each of the above scenarios by measuring her serum T and NES levels at baseline and serially for 24 hours after 15 min of supervised skin contact with the male participant who had applied transdermal T/NES gel. Secondary endpoints were to compare the pharmacokinetic parameters (PK) of T and NES in male participants after application of T/NES gel in each of the above scenarios, as well as evaluating the safety and tolerability of the T/NES gel through monitoring of adverse events and safety labs.
Research Participants
Healthy men between 18–50 years and healthy women between 18–40 years were recruited. All participants were screened by a study physician. In brief, they were required to have normal medical history and physical examination; normal laboratory tests including blood count, chemistry panel, liver enzymes, lipid panel and electrocardiogram (EKG). Men were excluded if they had used hormonal therapy in the last 6 months, had elevated PSA levels (≥ 4 ng/ml), significant lower urinary obstructive symptoms (IPSS > 19), or if their partner was known to be pregnant. Women were excluded if they were pregnant or breastfeeding, used anabolic steroids including androgens, had serum T outside normal reference ranges as measured by the local laboratory, or had evidence of hirsutism (modified Ferriman-Gallwey score >8). (See Supplementary materials for full inclusion and exclusion criteria.) This trial was registered at www.clinicaltrials.gov, National Clinical Trial Number NCT02994602.
The study was performed at two sites, Los Angeles Biomedical Research Institute at the Harbor-UCLA Medical Center (LABioMed) in Torrance, California and the University of Washington in Seattle, Washington. The study protocol received approval by the institutional review boards for both participating institutions. All participants signed a written informed consent before any study procedures. The safety laboratory tests drawn during the study were reviewed by an investigator within 48 h after results became available. Clinically significant changes and all adverse events were discussed with the medical monitor every week.
Study Medications
The combined T/NES gel was manufactured by Particle Science International (PSI) (Bethlehem PA) and supplied through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). NES was provided by the Population Council (New York, NY). The amount of gel applied by the male to both shoulders and upper arms was 5 ml in volume containing 62.5 mg of T and 8.3 mg of NES. Approximately 10% (range 9 to 14%) of the T applied to the skin was absorbed demonstrated by prior studies (Swerdloff, et al., 2000; Wang, et al., 2000). Similarly, by comparing the area under the serum NES curve after intravenous (IV) and transdermal application in women, it was estimated that transdermal NES absorption was about 10 to 12% of the IV dose (Brache, et al., 2015). Thus approximately 10% of the NES (Zitzmann, et al., 2017) in the gel applied was available to the body in men. The estimated amount of hormone absorbed was 6mg T and 0.8 mg NES per dose per day.
Study Design
Following screening, twelve healthy male and twelve healthy female volunteers were enrolled with 6 males and 6 females per site. Baseline 24-hour pharmacokinetics (PK) were obtained for each subject with serum samples collected at 1, 2, 4, 8, 12, 16, 20, and 24 hours (h). Upon completion of the baseline PK visit, the couple returned for three more 24-hour inpatient visits, designated as day 1 (shirt scenario), 8 (shower scenario), and 15 (no intervention scenario). For all inpatient visits, baseline vital signs were taken (the average of blood pressures measured 3 times at 5-minute intervals, weight, respiratory rate, and pulse) and the participants were asked about concomitant medications, adverse events, and alcohol or recreational drug use. For the female participant, her current contraception method and date of last menstrual period were recorded, and a urine pregnancy test was administered and confirmed negative prior to T/NES gel application by the male.
For the male participant, serum samples for NES and T levels were collected −15 and −5 minutes (min) before the gel application. At about 08:00 (± 30 min), the male participant applied 2.5 ml of the T/NES gel on one shoulder and upper arm above the elbow, repeated application on the other side, and then washed his hands with soap and water. Serial blood draws for NES and T levels were collected from the male at 1, 2, 4, 8, 12, 16, 20, and 24 h (± 15 min) after gel application. After the blood draw 2 h post application to the male participant (± 15 min), the female participant used her hands, wrists, and arms to rub vigorously up and down the arms and shoulders of the male for a contact period of 15 min under direct supervision of the study staff. The female participant then washed her hands with soap and water and refrained from showering for 24 h. For the female participant, serial blood draws for NES and testosterone were collected at 2, 3, 4, 5, 6, 8, 10, 14, 18, 22, 26, and 50 h after the male participant’s gel application. Additionally, on day 16 blood samples for safety laboratory tests were collected from both male and female participants; these included a complete blood count, comprehensive metabolic panel, and liver enzymes. Female participants were examined for acne and hirsutism on days 3, 10, and 17. The end-of-study visit occurred approximately 2 weeks after the last gel exposure. Male participants completed an end-of-study visit via telephone call to query regarding potential adverse events. Female participants were examined for acne qualitatively and for hirsutism using the Ferriman-Gallwey score. Both participants were asked about concomitant medications including alcohol and drugs and any adverse events.
The three different types of contact between female and male participants were as follows: On day 1, the male wore a cotton T-shirt (supplied by the study) over the application area prior to contact with the female. On day 8, the male showered approximately 1 h and 45 min after gel application, prior to contact with the female. On day 15, there was no intervention to reduce transfer before skin contact with the female. Additionally, on days 8 and 15, at 90 and 150 min after gel application, adhesive D-squame strips 22mm in diameter (Cuderm Corporation, Dallas, TX, USA) were applied to the gel application site on the male to sample the site, 10 strips at 90 min and 10 strips at 150 min. These strips were then analyzed for T and NES concentrations.
Analytical methods
Safety laboratory tests were performed at each study site’s local certified laboratory. All hormones, except for the screening T, were measured by the licensed Endocrine and Metabolic Research Laboratory at LA Biomed by liquid chromatography tandem mass spectrometry (LC-MS/MS). We have developed and validated a sensitive and accurate LC-MS/MS assay for the measurement of NES in human serum for clinical research purposes. Serum samples with NES (m/z=371.4/253.1) and internal standard (13C3-nestorone, m/z=374.4/253.1) were prepared by solid phase extraction using DPx Hybrid 50mg tips (DPX Technologies, Columbia, SC) and separated on a Kinetex C18 column (100×3.0mm, 1.7mcm, Phenomenex, Torrance, CA) within 5 min with a gradient profile (acidified mobile phase of Methanol (MeOH) and HPLC water with 0.1% formic acid, from 45% to 100% MeOH) at the flow rate of 0.6 ml/min. The retention time of both NES and NES internal standard was at 3.5 min. The triple quadruple mass spectrometer was operated in the positive mode using multiple reaction monitoring (MRM) with the transitions (m/z): 371.4/253.1 and 371.4/269.2 for Nestorone and Nestorone quantifier, and 374.4/253.1 for the internal standard. No metabolites of Nestorone were studied in this assay. The lower limit of quantification (LLOQ) for serum NES was 10pg/ml (27pmol/L, conversion factor 1pg/ml=2.7pmol/l). The intra-assay and inter-assay precision expressed as coefficient of variation (CV) were less than 10%; the assay accuracy was from 88.0% to 104.7%, spanning the range of the clinically relevant range for NES (30–2000 pg/ml). Serum T concentrations were measured using LC-MS/MS as described in detail previously (Shiraishi, et al., 2008; Wang, et al., 2008). Briefly, the samples were extracted with ethylacetate:Hexane (3:2 volume:volume), and separated in a Thermo Hypersil GOLD column (100mm × 1mm, 3mcm, Waltham, MA) column with a gradient profile (acidified mobile phase of MeOH and HPLC water with 0.1% formic acid, from 45% to 100% MeOH) at a flow rate of 0.12 ml/min. The triple quadruple mass spectrometer was operated in the positive mode using multiple reaction monitoring (MRM) with the transitions (m/z): 289.2/109.0 (289.2/97.2 verification) for T and m/z 291.2/110.9 for the internal standard d2-T. The LLOQ was 2 ng/dL for T. Intra- and inter-assay CVs were <11% for T; assay accuracy was 93.5 to 107.0% spanning 2 to 2000 ng/dl. The reference range for healthy adult men is 265 – 972ng/dl (9.2 to 33.7 nmol/l, conversion factor 1ng/dl =0.0347nmol/l) and for adult pre-menopausal women is 9.5 – 58.2 ng/dl (0.033 to 0.20 nmol/l). There was no cross reactivity of T in the NES assay and NES did not cross react in the T assay.
For the analyses of the NES and T contained on the D-squame strips, the strips were placed and extracted in 5mL of 100% MeOH. The recovery of NES and T were close to 100% as the strips were completely dissolved in the methanol. 20 mcl of the supernatant was transferred into 1980 mcl MeOH. An aliquot of the extracted sample was then further diluted with the reconstituting solution (10mcl in 990 mcl of 40%MeOH H2O with 0.1% formic acid) for LC-MS/MS analysis.
Statistical analyses
Based on the published data assessing testosterone transference, a sample size of 12 female participants provides an 80% power to detect a change from baseline in serum testosterone of non-dosed females for a mean difference of 9 ng/dl with a standard deviation of 10 ng/dl (Stahlman, et al., 2012a; Stahlman, et al., 2012b).
The PK parameters for each full sampling day for T and NES were determined by non-compartmental methods and were primarily assessed using the area under the curve (AUC) from 0–24 hours (AUC0–24) of serum T and NES (10 time points for males, 11 for females) for each gel application day and computed using the trapezoid method. Other PK parameters assessed for testosterone and NES included Cavg (average concentration over 24 h), Cmax (maximum concentration over 24 h), and Cmin (minimum concentration over 24 h).
Medians and interquartile ranges (IQR) were calculated for each PK parameter. The PK and strip data did not follow a normal distribution, therefore the nonparametric method of the Wilcoxon Signed Rank test was used for comparisons across groups. For each subject, the change in the PK parameters for NES and T from the shirt barrier and shower scenarios compared to the no intervention was calculated. The change in the PK parameters of T from the shirt barrier, shower, and no intervention scenarios compared to baseline was calculated as well. All calculations were performed using SAS Version 9.4.
Results
Study participants
There were a total of 12 couples enrolled at the two study sites, 6 couples at each site. 23 couples were screened and 13 couples enrolled; 12 paired male and female participants completed the study. One couple had to terminate the study early due to discovery of ineligibility. Eleven couples were not eligible due to withdrawal of consent, abnormal lab tests, or elevated BMI. Of the subjects enrolled, 10 were Hispanic and 14 were not Hispanic. Age, baseline weight, height, BMI of the volunteers are detailed in Table 1.
Table 1.
Participant Characteristics (Mean ± SD)
| Parameters | Female (N = 12) | Male (N = 12) |
|---|---|---|
| Age (years) | 25.8 ± 4.53 | 27.8 ± 5.36 |
| Ethnicity, n(%) | ||
| Hispanic | 5 (41.7%) | 5 (41.7%) |
| Not Hispanic | 7 (58.3%) | 7 (58.3%) |
| Race, n(%) | ||
| Asian | 1 (8.3%) | 1 (8.3%) |
| Black or African American | 1 (8.3%) | 2 (16.7%) |
| American Indian or Native | 0 | 1 (8.3%) |
| Alaskan | ||
| Native Hawaiian or Pacific | 0 | 0 |
| Islander | ||
| White | 6 (50.0%) | 6 (50.0%) |
| Multiple | 3 (25.0%) | 0 |
| Other | 1 (8.3%) | 2 (16.7%) |
| Weight at Baseline (kg) | 66.1 ± 7.0 | 78.6 ± 12.9 |
| Body Mass Index BMI | 25.4 ± 3.5 | 25.1 ± 3.3 |
| (kg/m2) | ||
| Baseline BMI | ||
| Underweight (< 18.5) | 0 | 0 |
| Normal (18.5 – 24.9) | 7 (58.3%) | 6 (50.0%) |
| Overweight (25.0 – 29.9) | 5 (41.7%) | 5 (41.7%) |
| Obese (≥ 30.0) | 0 | 1 (8.3%) |
Serum T and NES Pharmacokinetics
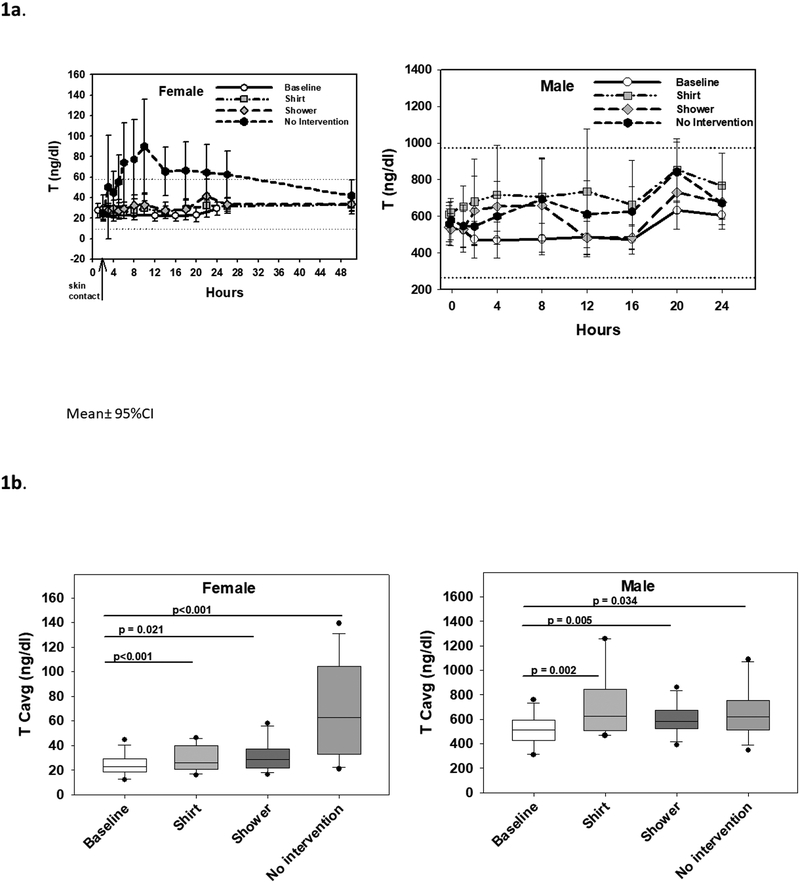
Female serum T concentrations over 24 hours were similar to baseline in the shirt barrier and shower scenarios, but in the no intervention scenario, T rose above the reference range and peaked at 10 hours after gel administration (median, IQR 8, 20 hours); this is 8 hours after skin contact. Serum T remained above the female reference range for 24 hours after skin contact, but returned to the normal reference range by 48 hours (Fig. 1a). Median female serum T Cavg was 23.9 ng/dl (IQR 19.3, 33.9) with shirt barrier and 26.7 ng/dl (20.7, 33.9) with showering which was significantly higher than baseline (20.9 ng/dl [16.7, 25.0], p<0.01 and p<0.03 respectively) but lower than without intervention (58.2 ng/dl (30.9, 89.1), both p<0.01) and within the normal reference range for women. Similarly, female serum T Cmax was 35.1 ng/dl (28.8, 49.2) in the shirt scenario and 41.3 ng/dl (31.6, 59.0) ng/dl with showering, significantly higher than baseline (28.1 ng/dl [23.4, 37.5], both p<0.01) but significantly lower than without intervention (88.8 ng/dl [59.9, 173], p<0.01 and p<0.02 respectively) (Table 2a, Fig. 1b). With the shirt barrier, one female participant out of 12 had a transient elevation of T above the reference range; with showering, one female participant had a transient elevation of T and two had elevated T Cavg; and with no intervention, nine female participants had elevated T Cavg.
Fig. 1.
A. Serum testosterone (T) concentrations over 24 hours in female participants after secondary exposure to T/NES gel (left panel) and in male participants after T/NES gel application (right panels). Mean ± 95% confidence intervals are displayed. Dotted lines show the normal reference range of T in women and men. B. Average serum T concentrations (Cavg) over 24 hours in female participants (left panel) and male participants (right panel). Medians (line in box), interquartile ranges (box), 5th and 95th percentile ranges (whiskers), and outliers (closed circles) are displayed.
Table 2a:
Serum Testosterone and Nestorone Concentrations for Female Participants (Median and 25%,75%)
| Baseline | Shirt | Showering | No Intervention | ||
|---|---|---|---|---|---|
| Testosterone | Cmin (ng/dl) | 19.5 (14.4, 21.6) | 19.6 (15.5, 25.9) | 19.1 (17.6, 28.9) | 20.7 (16.9, 29.4) |
| Cmax (ng/dl) | 28.1 (23.4, 37.5) | 35.1 (28.8, 49.2)a2b2 | 41.3 (31.6, 59.0)a2b1 | 88.8 (59.9,173.0)a3 | |
| Cavg (ng/dl) | 20.9 (16.7, 25.0) | 23.9 (19.3, 33.9)a3b2 | 26.7 (20.7, 33.9)a1b2 | 58.2 (30.9, 89.1)a3 | |
| AUC (h*ng/dl) | 501 (400, 599) | 621 (501, 876)a3b2 | 693 (538, 871)a2b2 | 1509 (800, 2310)a3 | |
| Nestorone | Cmin (pg/ml) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | |
| Cmax (pg/ml) | 0.0 (0.0, 0.0)b1 | 0.0 (0.0, 0.0)b1 | 7.1 (0.0, 57.7) | ||
| Cavg (pg/ml) | 0.0 (0.0, 0.0)b1 | 0.0 (0.0, 0.0)b1 | 1.1 (0.0, 17.0) | ||
| AUC (h*pg/ml) | 0.0 (0.0, 0.0)b1 | 0.0 (0.0, 0.0)b1 | 27.5 (0.0, 441.8) |
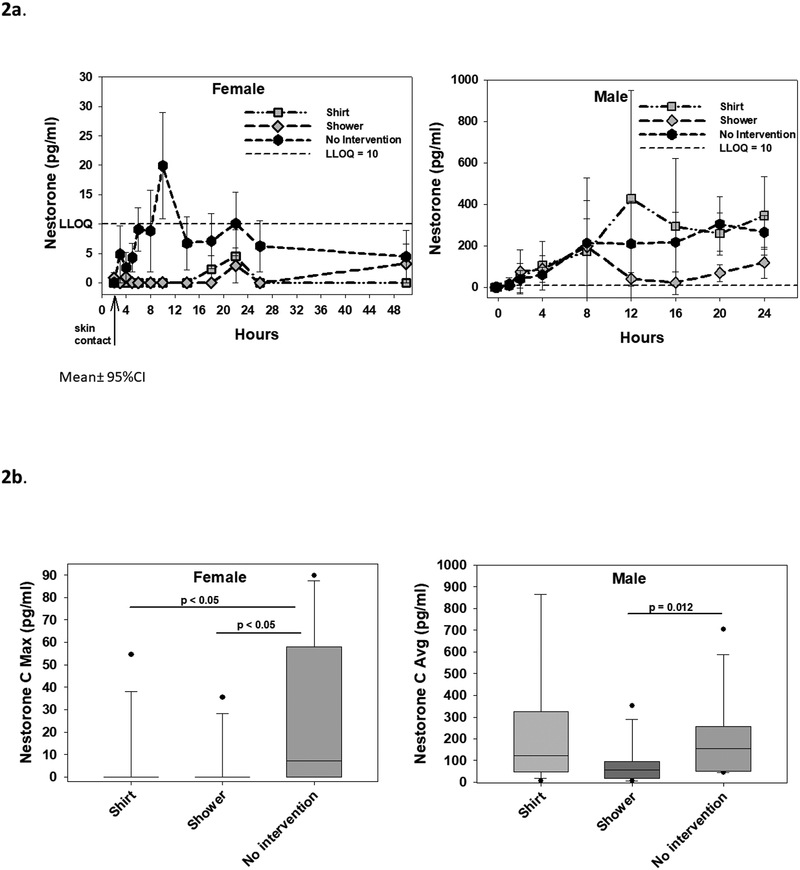
Female serum NES concentrations over 24 hours were mostly below the LLOQ of 10 pg/ml in the shirt barrier and shower scenarios. NES showed a peak at 10 hours after gel administration (8 hours after skin contact), declined, then rose again at 22 hours after gel administration (20 hours after skin contact) (Fig. 2a). With no intervention, serum NES concentrations were above LLOQ in at least two time points over 24 hours in 6 out of 12 female participants. The median female serum NES Cavg was below LLOQ for the shirt barrier and shower scenarios, and both significantly lower than in the no intervention scenario (1.1 pg/ml [0, 17.0], both p<0.05). The median female serum NES Cmax was below LLOQ for both with the shirt and showering, both significantly lower than with no intervention (7.1 pg/ml [0, 57.7], both p<0.05) (Table 2a, Fig. 2b). With the shirt barrier, 1 female participant had serum NES concentrations above LLOQ. With showering, 2 female participants had NES concentrations above LLOQ, and one female participant had NES concentrations above LLOQ 48 hours after skin contact. With no intervention, 6 female participants had NES concentrations above LLOQ.
Fig. 2.
A. Serum Nestorone (NES) concentrations over 24 hours in female participants after secondary exposure to T/NES gel (left panel) and in male participants after T/NES application (right panel). Mean±95% confidence intervals are displayed. The LLOQ of NES (10 pg/ml) is represented by the dotted line. B. Maximum serum NES concentrations (Cmax) over 24 hours in female participants (left panel) and average serum NES concentrations (Cavg) over 24 hours in male participants (right panel). Average serum NES concentrations over 24 hours (Cavg) in females were below LLOQ for all three scenarios and not shown. Medians (line in box), interquartile ranges (box), 5th and 95th percentile ranges (whiskers), and outliers (closed circles) are displayed.
In male participants, after T/NES gel application, serum T levels rapidly increased within 2 hours, plateaued between 4–8 hours, and then increased to peak levels between 20 to 24 hours (Tmax 20 hours median) after application, in the shirt and no intervention scenarios. In the shower scenario, serum T levels decreased between 8 and 16 hours before increasing again at 20 to 24 hours (Fig. 1a). Male serum T Cavg increased significantly from baseline T of 490 ng/dl (428, 557) after T/NES gel application in all 3 scenarios: serum T Cavg was 597 ng/dl (492, 783) with a shirt barrier (p<0.01), 561 ng/dl (507, 646) with shower (p<0.01), and 601 ng/dl (501, 708) with no intervention (p<0.05) compared to baseline. In contrast to serum T levels, serum NES levels in male increased gradually after gel application in the shirt and no intervention scenarios with a median Tmax at 20 hours (14, 24). Showering at 1 h 45 min after gel application reduced serum T levels between 12 – 24 hours (Fig. 2a). Men had lower average serum Cavg NES levels after showering compared to no intervention (47.0 pg/ml [19.9, 93.8] compared to 153 pg/ml [50.7, 241], p<0.02) (Table 2b, Fig. 2b).
Table 2b:
Testosterone and Nestorone Results for Male Participants (Median and 25%,75%)
| Baseline | Shirt | Showering | No Intervention | ||
|---|---|---|---|---|---|
| Testosterone | Cmin (ng/dl) | 393 (282, 456) | 479 (345, 605)a1 | 400 (348, 466) | 447 (388, 527)a1 |
| Cmax (ng/dl) | 687 (582, 762) | 1040 (852, 1305)a3b2 | 811 (690, 1130)a1 | 846 (646, 1055)a1 | |
| Cavg (ng/dl) | 490 (428, 557) | 597 (492, 783)a2 | 561 (507, 646)a2 | 601 (501, 708)a1 | |
| AUC (h*ng/dl) | 11750 (10262, 13362) | 14320 (11768, 18742)a2 | 13440 (12180, 15498)a2 | 14390 (11999, 16935)a1 | |
| Nestorone | Cmin (pg/ml) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 7.0) | |
| Cmax (pg/ml) | 403 (152, 774) | 229 (58.9, 297) | 364 (192, 480) | ||
| Cavg (pg/ml) | 124 (51.5, 324) | 47.0 (19.9, 93.8)b1 | 153 (50.7, 240.9) | ||
| AUC (h*pg/ml) | 2966 (1235, 7731) | 1409 (478, 2242)b1 | 3665 (1214, 5764) |
P<0.05 from baseline a1
P<0.01 from baseline a2
P<0.001 from baseline a3
P<0.05 from no intervention b1
P<0.01 from no intervention b2
P<0.001 from no intervention b3
Concentration of NES below LLOQ (10 pg/ml) was represented as 0
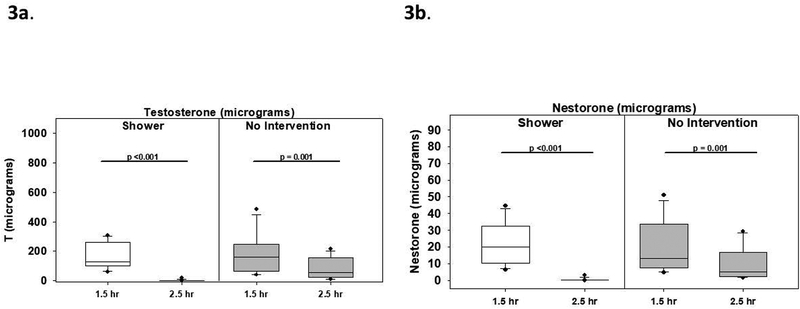
The skin D-squame strip data showed that in the shower scenario, skin T levels per subject as measured by the adhesive strips declined markedly from 127 mcg (99, 252) to 0.7 mcg (0.5, 2.3), and NES levels declined from 16.3 mcg (10.4, 29.4) to 0.15 mcg (0.1, 0.2) (both p<0.001). After skin contact alone, T levels also declined from 159 mcg (66, 234) to 55 mcg (23, 145), and NES levels declined from 13.2 mcg (7.9, 32.0) to 5.2 mcg (2.6, 16.3) (both p<0.01) (Fig 3). The difference in the change in measured T after showering compared to no intervention was −68.7mcg (−193.4, 24.9) but due to the large variation this was not statistically significant (p = 0.11); on the other hand, the difference in the decrease in measured NES after showering compared to no intervention was −7.6 mcg (−17.0, −1.5) which was significant (p<0.01).
Fig 3.
A. Adhesive D-squame strips were applied and stripped from the gel application site at 1.5 h and 2.5 h after T/NES gel application. The left panel shows T concentrations on the skin per participant before and after shower and skin contact. The right panel shows T concentrations on the skin before and after skin contact alone without intervention. B. The left panel shows NES concentrations on the skin before and after shower and skin contact; the right panel shows NES concentrations on the skin before and after skin contact alone with no intervention. Medians (line in box), interquartile ranges (box), 5th and 95th percentile ranges (whiskers), and outliers (closed circles) are displayed. T/NES was applied at 0h, the male participant showered at 1.75h (1 h 45 min), and skin contact occurred at 2h in the shower scenario. T/NES was applied at 0 h and skin contact occurred at 2h in the no intervention scenario.
Safety
Neither female nor male participants had any serious adverse events and very few T/NES gel related adverse events. One female participant with a history of anxiety noted worsening of her anxiety; this same subject had worsening of her baseline acne that developed after the day 8 (shower) visit and lasted for 7 weeks in duration. Another female participant had baseline pre-existing mild acne that worsened slightly after the day 15 (no intervention) visit, noted by the investigators and the patient; it resolved 2 days later. One participant with a history of prior menorrhagia developed menorrhagia. Her menses began the morning of day 1 (shirt barrier visit) prior to any drug exposure and persisted for 18 days. These adverse events were considered mild and possibly related to gel application. In all participants, there were no significant changes in serum chemistry panels, liver function tests, lipid profiles or hematocrit. In male participants, one male participant noted increased irritability after the day 1 (shirt barrier) visit. Another male participant with a history of mild depression and anxiety noted increased depression and anxiety on his second screening visit, prior to any drug exposure. Another male participant noted mild worsening of acne.
Discussion
In this study, we assessed the transfer of T and NES to women after direct skin contact following application of the combined T/NES gel by men. Direct skin included vigorous supervised rubbing of the gel application area by the female participants under direct supervision of the study team. We demonstrated transfer of T as well as NES to the female participants that was mitigated by either a clothing barrier or a shower. There are a few case reports of secondary transfer of testosterone from men prescribed testosterone gels to women and children (De Ronde, 2009; Nelson, et al., 2013). We used an experimental paradigm similar to prior studies with Androgel 1.62%® and our data on transfer of T to female participants are consistent with prior reports (De Ronde, 2009; Stahlman, et al., 2012a).
Use of a hormonal patch over a gel would limit transfer, but testosterone patches are associated with dermal irritation; 56% of participants in one trial developed local skin irritation (Arver, et al., 1997). The T skin patch is not used widely because of this reason.
We demonstrated that wearing a shirt as a barrier or washing the gel application site almost completely eliminates hormone transfer, similar to the results noted by Stahlman (Stahlman, et al., 2012a; Stahlman, et al., 2012b). Using adhesive strips, we also demonstrated that showering effectively removes T and NES from the skin of male participants.
Showering and wearing a shirt barrier greatly reduced the transference of NES and T. Although T and NES transfer was not completely eliminated, the small increases in serum T in females after secondary exposure to T in the shirt barrier and shower scenarios, though statistically significant, was minor, and average T levels remained within the normal reference range for pre-menopausal women. Adverse events were mild and possibly related to gel application. The female participant with mild worsening of acne after the shower scenario had a history of baseline acne. One participant with a history of prior menorrhagia, previously on oral contraceptives, developed menorrhagia, but menses starting prior to any exposure to the gel. A female participant developed mild worsening of acne after the no intervention scenario, which underlines the importance of using a shirt barrier or showering prior to skin contact. A placebo arm was not included as the duration of exposure is short, thus significant side effects were not anticipated. For the PK measurements, the baseline 24-hour PK visit served as a control arm. Overall, in our small sample of female subjects, a shirt barrier or shower was effective at reducing T transfer.
The average serum T levels in male participants after T/NES gel application were about 22% higher than the concentration at baseline in our healthy male participants. The increase in serum T was observed in our prior study when T gel 1 % were applied to the skin on the upper arms and shoulders and NES was applied to the abdomen (Ilani, et al., 2012; Mahabadi, et al., 2009). The gel did not cause irritation to the skin. Two male participants reported mood changes; however, in one participant mood changes occurred during the second screening visit, prior to any drug exposure. The use of T with a progestin in hormonal contraceptive trials in healthy men has been reported to affect mood when compared to placebo (Mommers, et al., 2008) and is an important parameter to monitor during any hormonal contraceptive trial.
Similar to T, NES was also transferred from the T/NES gel application site in half of the female participants (resulting in levels of NES above the LLOQ) when there was no intervention to prevent transference. Using a shirt barrier or washing the application site in the male participant greatly reduced NES transfer; NES was only detectable in 1/12 of female participants in the scenario with a shirt barrier and 3/12 of female participants with showering. Some women appeared to have more NES transfer than other women; in the no intervention scenario NES was above LLOQ in 6/12 women. The median maximum NES concentration was 7 pg/ml in the no intervention scenario, with an interquartile range between 0 and 57.7 pg/ml. suggesting some women may have NES nearing but below the level required to inhibit ovulation in the female [NES (250pmol/l; ~90 pg/ml)] (Brache, et al., 2015; Brache, et al., 2001; Jensen, et al., 2018). NES Cmax in the shirt and shower scenarios were negligible. Thus the transfer of NES to the women is unlikely to have significant effects on her reproductive function even if a shirt barrier or shower is not used to decrease transfer.
The serum NES in men did not reach a peak until at least 8 hours after T/NES gel application. Showering at 2 h after T/NES gel application markedly reduced average serum NES levels (Cavg) in the men to less than 50% of the levels seen in the shirt and no intervention scenarios. This was verified by the adhesive strip studies which demonstrated negligible NES and T levels on the skin after showering. NES/T gel has been shown to suppress gonadotropins and sperm concentration in prior studies, where subjects were instructed to shower at least 2 hours after application or wear protective clothing before close contact with another person (Ilani, et al., 2012). Our current study shows that showering prematurely may affect serum NES levels. This observation has importance in the clinical design of future contraceptive clinical trials with T/NES gel. To maintain a more consistent level of NES, in our ongoing contraceptive efficacy trial men must not shower for at least 4 hours after application of the NES/T gel to allow adequate absorption. As with testosterone gel, one method to limit transfer may be for men to shower prior to applying the gel, then cover the area with a shirt during the day and wash the area prior to close contact with other persons. Of note, the scenarios studied used single dose applications of the gel, and thus the pharmacokinetics after repeated dosing/after reaching steady state were not examined in this study.
For serum T, the rise at 20–24 hours is likely related to the first application of the gel which has been previously shown by us (Wang, et al., 2000). However, for NES, levels also rose at 20–24 hours. This is consistent with prior data in women (Fraser, et al., 2007). The steroids may accumulate in the stratum corneum of the skin (“reservoir effect”) and release slowly into the circulation. It is also possible that the NES is distributed into the fat and brain, then released back into the circulation later – NES is known to have a large volume of distribution and accumulate in the extravascular space (Sitruk-Ware, et al., 2003).
Our study limitations include the small sample size of men and women, and study conditions that may not be representative of realistic contact situations. In the “real world” situation, contact of the application site may occur with the female chest, abdomen and/or back and secondary exposure to steroids may be greater or less than intensive rubbing with hands and arms as in our experimental condition.
In conclusion, our study demonstrates that secondary transfer of T and NES occurs after intensive skin contact at the gel application site. This exposure was decreased by a shirt barrier or showering before skin contact. In all scenarios tested, the average serum T concentrations in female participants were within the normal range for pre-menopausal women. Based on these results, we recommend that men using transdermal gels containing T+NES or other androgens/progestins should wash before intensive physical contact with a woman or a child, or wear clothing over the application site to minimize secondary transfer. To maintain sustained concentrations of NES after T/NES gel application, the male should not shower for a length of time, at least after 4 hours after gel application to allow full absorption of NES from the application site.
Supplementary Material
Acknowledgements
The LA Biomed center was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) Contraceptive Clinical Trial Network Contract HHSN27520130024I, Task Orders HHSN27500001, 27500005 and the UCLA Clinical and Translational Science Institute (UL1TR000124) at Harbor-UCLA/LA BioMed. The University of Washington center was supported by NICHD Contraceptive Clinical Trial Network Contract HHSN275201300025I, Task Orders HHSN27500005, the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423, and the National Institute of Diabetes and Digestive and Kidney Diseases training grant 5T32 DK007247-39-40. STP is also supported by the Robert McMillen Professorship in Lipid Research. PYL is supported in part by K24 HL138632. We thank our research coordinators Xiao-Dan Han, Elizabeth Ruiz, Lauryn Raj, Kathy Winter, and Kathryn Torrez-Duncan for their assistance with the study. We thank the staff of the Endocrine and Metabolic Research Laboratory at LA Biomed and the University of Washington Center for Research in Reproduction and Contraception and most importantly our research participants.
Funding Sources:
The Los Angeles Biomedical Research Institute (LA Biomed) center was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Contraceptive Clinical Trial Network Contract HHSN27520130024I Task Orders HHSN 27500001, 27500005 and the National Center for Advancing Translational Sciences of the National Institutes of Health award to the UCLA Clinical and Translational Science Institute (UL1TR000124) at Harbor-UCLA/LA BioMed. The University of Washington center was supported by NICHD Contraceptive Clinical Trial Network Contract HHSN275201300025I, Task Orders HHSN27500005, the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423, and the National Institute of Diabetes and Digestive and Kidney Diseases training grant 5T32 DK007247-39-40. STP is also supported by the Robert McMillen Professorship in Lipid Research. PYL was supported in part by the National Heart, Lung and Blood Institute K24 HL138632.
Footnotes
Disclosure Summary:
Fiona Yuen, Sherry Wu, Arthi Thirumalai, Peter Y. Liu, Feng Bai, Laura Hull, William J. Bremner, and Bradley D. Anawalt do not have any disclosures. Ronald Swerdloff is a consultant for Clarus Therapeutics and receives research support from Clarus Therapeutics and Testosterone Replacement Therapy Manufacturers Consortium. Stephanie T. Page has served as a consultant for Clarus Therapeutics. Clint Dart and Hongsheng Wu are employees of Health Decisions. Diana L. Blithe and Jill Long are employees of the US Government. Regine Sitruk-Ware is an employee of the Population Council, a not for profit research organization developing the NES/T gel. Christina Wang receives research support from Clarus Therapeutics, Antares, TesoRX, and Testosterone Replacement Therapy Manufacturers Consortium.
References
- Arver S, Dobs AS, Meikle AW, Caramelli KE, Rajaram L, Sanders SW & Mazer NA. (1997) Long-term efficacy and safety of a permeation-enhanced testosterone transdermal system in hypogonadal men. Clin. Endocrinol. (Oxf) 47, 727–737. [DOI] [PubMed] [Google Scholar]
- Brache V, Merkatz R, Kumar N, Jesam C, Sussman H, Hoskin E, Roberts K, Alami M, Taylor D, Jorge A, Croxatto H, Lorange E, Mishell DR & Sitruk-Ware R. (2015) A dose-finding, cross-over study to evaluate the effect of a Nestorone(R)/Estradiol transdermal gel delivery on ovulation suppression in normal ovulating women. Contraception 92, 289–297. [DOI] [PubMed] [Google Scholar]
- Brache V, Mishell DR, Lahteenmaki P, Alvarez F, Elomaa K, Jackanicz T & Faundes A. (2001) Ovarian function during use of vaginal rings delivering three different doses of Nestorone. Contraception 63, 257–261. [DOI] [PubMed] [Google Scholar]
- Chao J, Page ST & Anderson RA. (2014) Male contraception. Best Pract Res Clin Obstet Gynaecol 28, 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ronde W (2009) Hyperandrogenism after transfer of topical testosterone gel: case report and review of published and unpublished studies. Hum. Reprod 24, 425–428. [DOI] [PubMed] [Google Scholar]
- Fraser IS, Weisberg E, Kumar N, Kumar S, Humberstone AJ, McCrossin L, Shaw D, Tsong YY & Sitruk-Ware R. (2007) An initial pharmacokinetic study with a Metered Dose Transdermal Systemfor delivery of the progestogen Nestorone as a possible future contraceptive. Contraception 76, 432–438. [DOI] [PubMed] [Google Scholar]
- Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, Bo L, Xiong C, Wang X, Liu X, Peng L & Yao K. (2009) Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J. Clin. Endocrinol. Metab 94, 1910–1915. [DOI] [PubMed] [Google Scholar]
- Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, Page ST, Bremner WJ, Sitruk-Ware R, Kumar N, Blithe DL & Wang C. (2012) A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J. Clin. Endocrinol. Metab 97, 3476–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JT, Edelman AB, Chen BA, Archer DF, Barnhart KT, Thomas MA, Burke AE, Westhoff CL, Wan LS, Sitruk-Ware R, Kumar N, Variano B & Blithe DL. (2018) Continuous dosing of a novel contraceptive vaginal ring releasing Nestorone(R) and estradiol: pharmacokinetics from a dose-finding study. Contraception 97, 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Koide SS, Tsong Y & Sundaram K. (2000) Nestorone: a progestin with a unique pharmacological profile. Steroids 65, 629–636. [DOI] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, Gu YQ, Kersemaekers WM, McLachlan RI, Meriggiola MC, Nieschlag E, Sitruk-Ware R, Vogelsong K, Wang XH, Wu FC, Zitzmann M, Handelsman DJ & Wang C. (2008) Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: An integrated analysis. J. Clin. Endocrinol. Metab 93, 1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabadi V, Amory JK, Swerdloff RS, Bremner WJ, Page ST, Sitruk-Ware R, Christensen PD, Kumar N, Tsong YY, Blithe D & Wang C. (2009) Combined transdermal testosterone gel and the progestin nestorone suppresses serum gonadotropins in men. J. Clin. Endocrinol. Metab 94, 2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriggiola MC, Farley TM & Mbizvo MT. (2003) A review of androgen-progestin regimens for male contraception. J. Androl 24, 466–483. [DOI] [PubMed] [Google Scholar]
- Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, Behre HM, Beynon J, Bouloux PM, Costantino A, Gerbershagen HP, Gronlund L, Heger-Mahn D, Huhtaniemi I, Koldewijn EL, Lange C, Lindenberg S, Meriggiola MC, Meuleman E, Mulders PF, Nieschlag E, Perheentupa A, Solomon A, Vaisala L, Wu FC & Zitzmann M. (2008) Male hormonal contraception: a double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab 93, 2572–2580. [DOI] [PubMed] [Google Scholar]
- Nelson D, Ho J, Pacaud D & Stephure D. (2013) Virilization in two pre-pubertal children exposed to topical androgen. J. Pediatr. Endocrinol. Metab 26, 981–985. [DOI] [PubMed] [Google Scholar]
- Roth MY, Shih G, Ilani N, Wang C, Page ST, Bremner WJ, Swerdloff RS, Sitruk-Ware R, Blithe DL & Amory JK. (2014) Acceptability of a transdermal gel-based male hormonal contraceptive in a randomized controlled trial. Contraception 90, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS & Wang C. (2008) Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clin. Chem 54, 1855–1863. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R, Small M, Kumar N, Tsong YY, Sundaram K & Jackanicz T. (2003) Nestorone: clinical applications for contraception and HRT. Steroids 68, 907–913. [DOI] [PubMed] [Google Scholar]
- Stahlman J, Britto M, Fitzpatrick S, McWhirter C, Testino SA, Brennan JJ & Zumbrunnen TL. (2012a) Serum testosterone levels in non-dosed females after secondary exposure to 1.62% testosterone gel: effects of clothing barrier on testosterone absorption. Curr. Med. Res. Opin 28, 291–301. [DOI] [PubMed] [Google Scholar]
- Stahlman J, Britto M, Fitzpatrick S, McWhirter C, Testino SA, Brennan JJ & Zumbrunnen TL. (2012b) Effect of application site, clothing barrier, and application site washing on testosterone transfer with a 1.62% testosterone gel. Curr. Med. Res. Opin 28, 281–290. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J & Berman N. (2000) Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J. Clin. Endocrinol. Metab 85, 4500–4510. [DOI] [PubMed] [Google Scholar]
- Wang C, Berman N, Longstreth JA, Chuapoco B, Hull L, Steiner B, Faulkner S, Dudley RE & Swerdloff RS. (2000) Pharmacokinetics of transdermal testosterone gel in hypogonadal men: application of gel at one site versus four sites: a General Clinical Research Center Study. J. Clin. Endocrinol. Metab 85, 964–969. [DOI] [PubMed] [Google Scholar]
- Wang C, Festin MP & Swerdloff RS. (2016) Male Hormonal Contraception: Where Are We Now? Current obstetrics and gynecology reports 5, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shiraishi S, Leung A, Baravarian S, Hull L, Goh V, Lee PW & Swerdloff RS. (2008) Validation of a testosterone and dihydrotestosterone liquid chromatography tandem mass spectrometry assay: Interference and comparison with established methods. Steroids 73, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C & Swerdloff RS. (2004) Male hormonal contraception. Am. J. Obstet. Gynecol 190, S60–S68. [DOI] [PubMed] [Google Scholar]
- World Health Organization Task Force on Methods for the Regulation of Male Fertility. (1990) Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet 336, 955–959. [PubMed] [Google Scholar]
- World Health Organization Task Force on the Regulation of Male Fertility. (1996) Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil. Steril 65, 821–829. [PubMed] [Google Scholar]
- Zitzmann M, Rohayem J, Raidt J, Kliesch S, Kumar N, Sitruk-Ware R & Nieschlag E. (2017) Impact of various progestins with or without transdermal testosterone on gonadotropin levels for non-invasive hormonal male contraception: a randomized clinical trial. Andrology 5, 516–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.