Abstract
Background:
Despite promising outcomes of antiretroviral therapy (ART), challenges to improving adherence among youth living with HIV (YLWH) exist. Mobile games are popular among youth and may improve skills related to resilience, coping, and ART adherence. This study examines the preliminary effects of an iPhone game/app on ART adherence, viral load, and relevant knowledge and attitudes among YLWH in Jackson, MS.
Methods:
A RCT with 61 YLWH tested the impact of BattleViro, an ART-related iPhone game, over 16 weeks. Participants, ages 14–26, were recruited from HIV clinics and randomly assigned to receive BattleViro or a non-HIV-related game. All participants received a medication monitoring device. Chi-square and t-test analyses examined baseline differences between conditions. Continuous outcomes were examined using analyses of covariance (ANCOVAs) controlling for baseline scores. Cohen’s d effect size differences (ESD) between groups were calculated.
Results:
The sample was 79% male, 97% Black, and 74% non-heterosexual, with a mean age of 22 years. A third had started ART in the past 3 months. There were no demographic differences between conditions. Examination of ESDs revealed that BattleViro demonstrated promising, but nonsignificant, improvements in HIV knowledge (ESD=0.50), ART knowledge (ESD=0.42) and social support (ESD=0.62). Exploratory moderation analyses revealed interactions between BattleViro and newly starting ART. Those newly starting ART in the BattleViro condition, compared to those in the control, experienced a 0.96 log greater decrease in viral load (ESD=−2.21, F=4.33, p=0.04), better adherence (71% vs 48%; ESD=1.15, F=3.90, p=0.05), more HIV knowledge (ESD=0.90), and more ART knowledge (ESD=0.72).
Conclusion:
BattleViro showed promising improvements in HIV knowledge, ART knowledge and social support. Also, there was improved adherence and viral load outcomes specifically among those newly starting ART. ART initiation may be an opportunity to empower and motivate YLWH to build healthy skills.
Keywords: Adolescents, Adherence, Antiretrovirals, Gaming
Introduction
Antiretroviral medications can allow youth living with HIV (YLWH) to manage their infection as a chronic, rather than an imminently life-threatening disease (Kim, Gerver, Fidler, & Ward, 2014; Patel et al., 2008; Resino et al., 2006). Adherence to antiretroviral treatment (ART) leads to decreased incidence of opportunistic infections, improved growth and development, decreased morbidity and mortality, decreased hospitalizations, and reduced risk of HIV transmission (Bain-Brickley, Butler, Kennedy, & Rutherford, 2011; Evans, et al., 2013). However, these favorable outcomes can only be achieved when youth (i.e. those ages 15 to 24) take their medications as prescribed and maintain consistent medical care (Bain-Brickley et al., 2011). Unfortunately, YLWH have poor rates of adherence to antiretroviral treatment (ART), are at great risk for being lost to follow-up, and are less likely to have suppressed viral loads compared to adults (Chandwani et al., 2012; MacDonell, Naar-King, Huszti, & Belzer, 2013). YLWH often have life stressors that may interfere with their ability to adhere to medical care such as poverty, stigmatization, and limited social support (Brown, Lourie, & Pao, 2000). Furthermore, adolescence and young adulthood is an age-related period characterized by mood instability, impulsivity, decreased motivation for planning, and less parental monitoring (Belzer, Fuchs, Luftman, & Tucker, 1999; Reisner et al., 2009).
There is an urgent need for innovative and rigorously tested interventions to improve adherence for YLWH. Reviews of ART adherence interventions for YLWH have identified studies with methodological issues such as a lack of theory or not having a comparison group (Navarra et al., 2017; Reisner et al., 2009). For example, a Cochrane Review of interventions to improve adherence to ART among YLWH identified only four interventions (all face-to-face) and the results of which were inconsistent (Bain-Brickley et al., 2011). There is no definitive, scalable, evidence-based intervention to improve adherence for YLWH.
Interventions to improve adherence to care for youth living with other illnesses, such as asthma, cancer and diabetes have shown that improving motivation and behavioral skills can bolster resilience, decrease distress, and improve adherence (Kato, Cole, Bradlyn, & Pollock, 2008; Stokes, 2005). Resilience, broadly defined as the capacity and skills to confront life challenges, can be a target for prevention and intervention programs for youth. Interventions can focus on resilience factors that can be measured and improved, such as self-efficacy, motivation, and support seeking (Johnson et al., 2003; Furniss, Barber, Lyons, Eliasson, & Blandford, 2014). This project developed a multilevel gaming intervention that uses cutting-edge and appealing mobile technology to empower youth to improve adherence by increasing information, motivation, behavioral skills.
Mobile and gaming interventions are particularly compelling for use with youth (Hightow-Weidman, Muessig, Bauermeister, LeGrand, & Fiellin, 2017; Pew Research Center, 2015). Youth have high rates of mobile phone use with more than 90% of 15–24 year olds owning a mobile phone (Hightow-Weidman et al., 2017; Pew Research Center, 2015). Technologies, such as the smart phone, play an increasingly significant role in the lives of adolescents and young adults as devices communicate information, reinforce cultural norms, and influence personal identity and behaviors. Additionally, gaming is popular among youth. In the United States, 99% of teenage males and 94% of teenage females play video games, and 46% of all video gaming occurs on mobile phones or portable devices (Pew Research Center, 2015; Raney, Smith, & Baker, 2006). The widespread appeal of gaming among youth creates a unique opportunity to deliver interventions to YLWH during leisure time and outside of the clinic (Lee & Peng, 2006; Lieberman, 2006).
Unfortunately, there is a lack of controlled trials related to interventions for YLWH. A review specifically examining gaming interventions for YLWH from NIH Reporter did identify five digital games to improve ART adherence that are in development or testing (including our intervention described here) (Hightow-Weidman, et al., 2017). However, there are no published, controlled trial outcomes utilizing mobile gaming interventions to improve adherence to ART for YLWH that we can find. There is a published paper which describes the development of “Epic Allies,” a game to improve ART uptake and adherence among young men who have sex with men (YMSM) and transgender women who have sex with men. In this game, players can interact with each other, earn tokens for taking medications, and read health related articles (LeGrand et al., 2016).
Mobile and video game interventions have been tested in youth with diabetes, cancer, and asthma. These interventions have been most successful in influencing health behavior when they involved the use of story and fantasy and if they utilized theories of behavior change (Cole et al., 2006; Homer et al., 2000; Kato et al., 2008; Shames et al., 2004). Building on this knowledge and the need for innovative adherence interventions, we developed a multi-level gaming intervention for youth living with HIV guided by the Information Motivation and Behavioral Skills (IMB). This gaming intervention, using an asset model, also promoted self-mastery and social support for adherence.
This study reports the results of a small, 16 week, randomized controlled trial that tested BattleViro (an iPhone gaming adherence intervention with game related text messages) among 61 YLWH. The project assessed the impact on participants’ medication adherence and viral load, as well as, ART-related knowledge, motivation, social support, and self-efficacy. It was hypothesized that participants receiving BattleViro, compared to those receiving a non-HIV-related game, would have and greater improvements in adherence, viral load, knowledge, motivation, social support and self-efficacy.
Methods
Intervention Development
Intervention development was guided by qualitative interviews with a diverse group of 30 YLWH (20 from Rhode Island; 10 from Mississippi) and the details on intervention development and initial acceptability are reported elsewhere (Whiteley, Brown, Curtis, & Heck, 2014; Whiteley, Brown, Lally, Heck, & van den Berg, In press). YLWH were queried about how information, motivation and behavioral skills influenced their adherence to medication and medical appointments (Fisher et al., 2006). Interviews revealed a number of themes related to empowerment and resilience to stressors of living with HIV. The motivational themes included the desire for the game and messages to enhance future orientation, improve perceived social support, and increase personal relevance of HIV care. Participants wanted the intervention to reinforce positive influences from doctors, partners, and friends. Behavioral skills themes centered around self-efficacy for medical adherence and strategies for reinforcing routines. Empowerment themes included the desire for mastery by “fighting” or “destroying” HIV in the body with weapons. Youth also wanted to earn points for “taking” or “swallowing” pills. YLWH also desired empowering game-related text messages with gaming graphics to engage gamers in play. Participants wanted congratulatory messages if they were > 90% adherent as per Wisepill measurement. If participants were less than 90% adherent, as per Wisepill, they desired text messages to encourage and remind them to take their medication. The majority of participants also wanted text messages to last 2 months (or 8 weeks), stating that texts beyond this time would lose their appeal or even become annoying (See Figure 1). Participants suggested that gender and frequency of playing games might be related to the impact of BattleViro. Also, they suggested that adherence-related text messages be sent twice weekly. YLWH from both Rhode Island and Mississippi were in general agreement with the desired content and game play.
Figure 1.
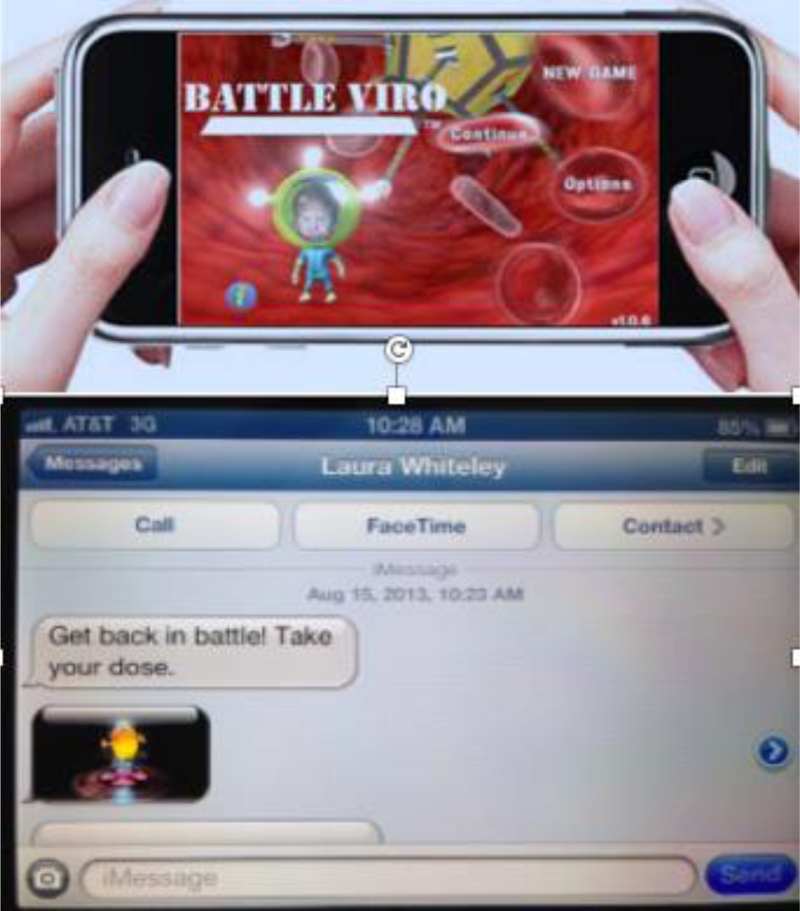
BattleViro and Sample Adherence Guided Text Message
Using data from these interviews, the iPhone game/app, named BattleViro, was developed by the investigators and a software development company (Mission Critical Studios, 2012). In BattleViro, YLWH battle HIV, engage with healthcare providers, and take medication. While building skills, they move to new, distinctive levels (arterial system, lungs, kidneys, liver, eyes, and brain) (See Figure 2a). Messages from the doctors, nurses and friends encourage and provide clues during difficult twists and turns in the battle. Answering quiz questions from clinician avatars allows players to earn strength and points; wrong answers are corrected and explained (See Figure 2b). In the development phase of this project, participants rated the game using standardized scales (Whiteley et al., 2014; Whiteley et al., 2018). They found the game enjoyable and useful. For example, 82% felt they would recommend the intervention to a friend and 95% felt it was relevant to their lives.
Figure 2a.
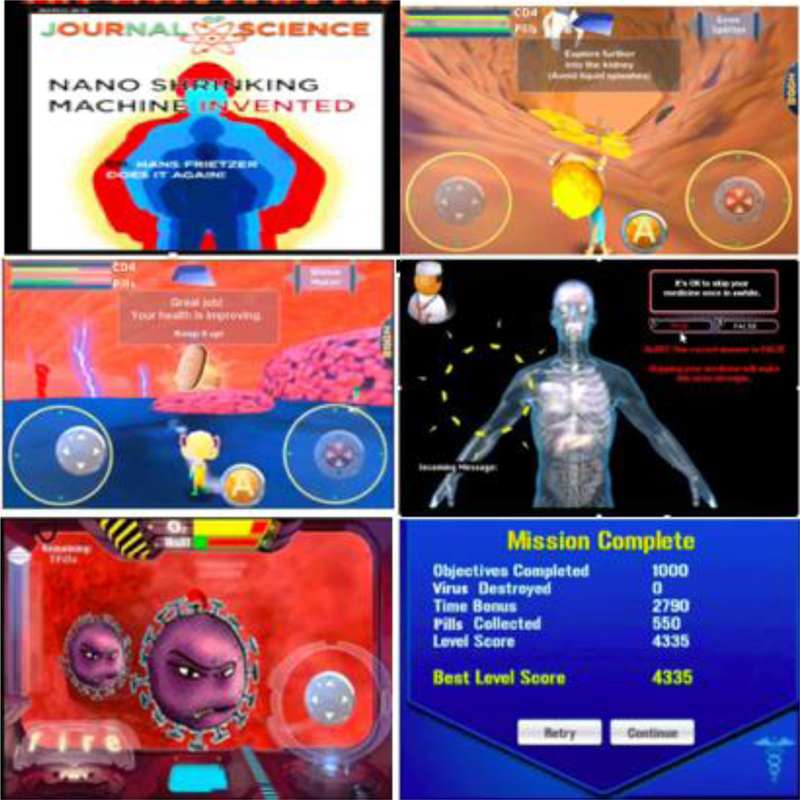
Examples of BattleViro Levels and Activities
Figure 2b.
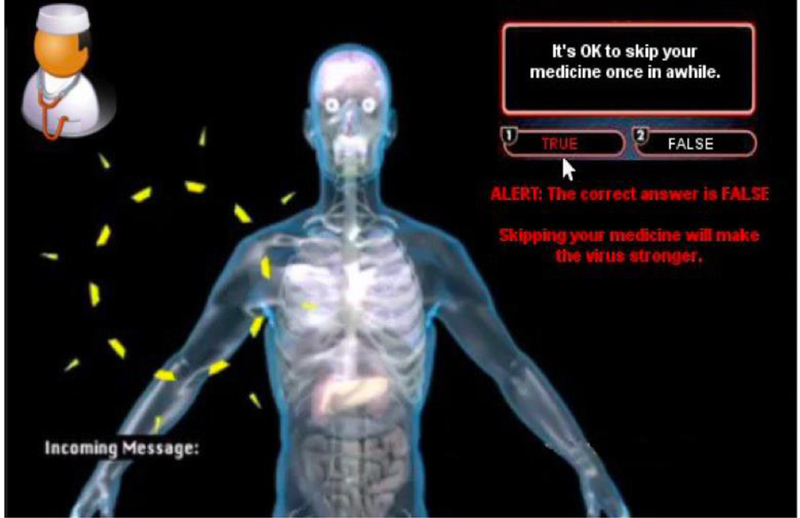
Example of a quiz question from a clinician with a correction to a wrong answer displayed
Procedures
All project procedures and materials were approved by institutional review boards at Rhode Island Hospital and University of Mississippi Medical Center (UMMC). Participants were recruited from HIV clinics in the greater Jackson, MS area. To be eligible, study participants needed to be: 1) 14–26 years of age, inclusive; 2) in medical care for HIV and receiving or starting ART; 3) aware of their HIV status as per clinician and clinical record; 4) have a detectable viral load at study entry as measured by blood testing within a month of screening; 5) understand written and spoken English; and 6) able to give consent/assent. Participants were excluded from the study if it was determined that the participant was impaired by cognitive or medical limitations as per clinical assessment. Research staff obtained consent from participants over the age of 18 and obtained parental consent and adolescent assent for those under 18 years of age.
Participants in the intervention arm received BattleViro on a smart phone provided by the study, an electronic pill monitoring device, and twice weekly game-related text messages guided by monitoring device data. Participants with perfect adherence were sent texts of congratulations (e.g. “Great job in Battle. You are winning!”) and others were sent motivational texts (e.g. “Get back in battle! Take your dose!) (See Figure 1). Throughout the intervention, participants in both conditions continued clinical care visits in their HIV clinic. Participants in treatment as usual plus (TAU+) condition received a non-HIV related mobile game on a smart phone provided by the study and an electronic pill monitoring device. The non-HIV-related game (Dr. Nano X: Incredible Voyage Inside the Body) had been developed by Mission Critical Studios and was stylistically similar to BattleViro. TAU+ was designed to have many similarities (access to a non-tailored game + electronic pill monitoring) but did not contain the crucial and defining elements of BattleViro (e.g. HIV-related game, integration between the electronic pill monitoring device and adherence-related text messages).
Randomization and 16 week study timeline
At the completion of the baseline questionnaire, participants were randomized to intervention condition via REDCap (Harris et al., 2009) using stratification based on gender and frequency of gaming (daily gamer versus not) as suggested by our developmental interviews. All participants received the electronic pill monitoring device after the assessment in order to establish their baseline medication adherence rate. After two weeks, participants returned to receive their study phones, which had a cell plan for the study period, and their assigned intervention game (BattleViro or non-HIV-related game). Participants in both conditions continued to have their phones, assigned games, and pill monitoring device for the next 14 weeks. Participants in BattleViro received adherence-related text messages for the first eight weeks after receiving their game while TAU+ did not. After the eight-week BattleViro texting period, the adherence text messages stopped, and all participants had four additional weeks of use of study phones and their assigned game.
Measures
Participants completed audio computer-assisted self-interview (ACASI) assessments at baseline and at study end (week 16). Study data were collected and managed using REDCap, a HIPAA compliant, secure, web-based application designed to support data capture for research studies (Harris et al., 2009).
Demographics:
Participants reported demographic information including age, gender, race, ethnicity, educational level, sexual orientation, current housing situation and stability of housing, gaming history (daily or almost daily vs. not every day), time since starting ART (three months or less vs. more than 3 months), and health literacy (“how often do you need help understanding materials from a doctor or pharmacy?”).
Biological Outcome:
HIV-1 Viral Load.
Viral load (HIV-1 RNA PCR) was measured by the medical clinic laboratory at study entry and within a month of the final assessment.
Behavioral Outcomes:
Self-report ART adherence.
Youth reported the number of doses they were prescribed per day and estimated the number of doses they had missed in the past seven days. Past 7-day adherence at baseline and at week 16 was calculated and is reported as a proportion.
Adherence measured by electronic pill monitoring device.
Participants were given a Wisepill monitoring device two weeks before they received their study phone and game, in order to assess their baseline adherence levels prior to using the game and phone. Each opening of the Wisepill dispenser is wirelessly relayed the data to Wisepill’s secure network. The past 7-day Wisepill adherence was calculated based on the total number of prescribed doses and the total number of missed doses and is reported as a proportion. Adherence is reported for the pre-game period, as well as four and eight weeks after receiving the study phones and games. These time points correspond to the middle and end of the adherence-related text period for the BattleViro condition.
Measures of related knowledge, attitudes and behaviors:
HIV and ART Knowledge scales.
The HIV Treatment Knowledge Scale assessed knowledge about complex treatment issues such as adherence, side effects, and drug resistance, using items with “true,” “false,” or “do not know.” Items included True/False statements such as “One can get infected with a drug-resistant type of HIV.” The original 21-item scale’s alpha is reported as 0.90 among adults (Balfour et al., 2007). Five items were selected for this project to represent distinct domains of knowledge about HIV. The alpha for this sample is 0.37. This low alpha was not unexpected due to the selection of only a few items each with a distinct and different content area.
ART treatment knowledge was assessed using three Likert-style items with five response options ranging from “Strongly Disagree” to “Strongly Agree” Items included “I know what the possible side effects of each of my HIV medications are” and “I understand how each of my HIV medications works to fight HIV in my body.” Higher scores on both scales indicate greater knowledge (Fisher et al., 2006; LifeWindows Project Team, 2006). The reported alpha among adults living with HIV is 0.65 (Konkle-Parker et.al, 2014) and in our sample the alpha is 0.69.
ART Motivation.
Personal and social motivations for ART were measured by a four Likert-style items (Fisher et al., 2006; LifeWindows Project Team, 2006). Items included “I am worried that other people might realize that I am HIV infected if they see me taking my HIV medications” and “It upsets me that the HIV medications that I have been prescribed can cause side effects”. Response options ranged from “Strongly Disagree” to “Strongly Agree”. Higher scores indicate greater motivation for ART. The reported alpha among adults living with HIV is 0.79 (Konkle-Parker et.al, 2014) and in our sample the alpha is 0.68.
Self-Efficacy for ART Use.
Five Likert-style items assessed perception of the ability to perform the necessary ART skills (Fisher et al., 2006; LifeWindows Project Team, 2006). Items included “How hard or easy is it for you to take your HIV medications when your usual routine changes?” and “How hard or easy is it for you to take your HIV medications when you do NOT feel good physically?” Higher scores indicate greater perceived ability. The reported alpha among adults living with HIV is 0.87 (Konkle-Parker et.al, 2014) and in our sample the alpha is 0.84.
Social Support.
Six Likert-style items measured social support for taking medications, going to medical appointments and other tasks related to adherence using four-point scales. The reported alpha among adults is 0.92 (Cutrona and Russel 1987) and in our sample the alpha is 0.77.
Psychological Distress.
Psychological distress was assessed by the Global Symptom Index of the 18-item Brief Symptom Inventory (BSI-18) (Derogatis & Spencer, 1983). Participants reported the severity of 18 symptoms in the past seven days. The reliability, validity, and utility of the BSI instrument have been tested in more than 400 research studies in adolescents and adults. The reported alpha among youth living with HIV is 0.85 (Brown et.al, 2015) and in our sample the alpha is 0.94.
Sexual Activity.
The Risk Behavior Assessment is a reliable and valid computer-assisted structured interview assessing self-reported sexual behaviors (Donenberg, Emerson, Bryant, Wilson, & Weber-Shifrin, 2001). It assesses type of sexual behavior (i.e., anal, oral, vaginal) in the past 3 months, frequency of sex, and number and gender of partners. This assessment has been shown to be sensitive to the impact of interventions and developmental progression. (Brown et. al., 2014)
Data Analysis
With a projected enrolment of 65 YLWH and an estimated 10% attrition, power was .80 to detect large treatment effect sizes (0.72 or greater) between intervention conditions in Analyses of Covariance (ANCOVA), controlling for pretest scores (p<0.05). YLWH who completed both baseline and the follow-up assessment (n=61) were compared to those who completed baseline only (n=5). Independent F-tests and Chi-square analyses were conducted to examine whether differences existed between conditions on baseline. Scale outcome scores were analyzed using ANCOVA, adjusting for baseline scores and any demographics found significant at baseline. As this was an exploratory intervention with a modest sample, Cohen’s d was calculated to estimate intervention effect sizes (using the recommended interpretations of d ≥ 0.2 representing a ‘small’ effect size, d ≥ 0.5 representing a ‘medium’ effect size and d ≥ 0.8 a ‘large’ effect size) (Kelley, 2007). As this was an exploratory intervention study, demographic factors that could be associated with the impact of the iPhone gaming intervention (gender, age, gaming frequency, time since starting ART) were examined in interaction terms between the factors and intervention condition in ANCOVAs, controlling for baseline scores. Data were analyzed using SPSS 22.0.
Results
See the CONSORT diagram (Figure 3) for details of recruitment, enrollment and retention. Sixty-six participants were enrolled and completed baseline assessment. Of this group, five either withdrew or did not participate in study activities. There were no differences in demographic or outcome variables between the five nonparticipants and the 61 participants, who form the sample for these per protocol analyses (data not shown). Thirty-two participants were randomized to BattleViro and 29 to TAU+. No differences in demographic variables were found between intervention groups at baseline (See Table 1). Ten participants were missing viral load data at the follow-up because of not attending a clinic visit during the study period. Participants missing follow-up viral load data were more likely to be in the BattleViro intervention (23% vs. 10%, p=0.06) but otherwise there were no demographic or differences in outcome variables at baseline between conditions. Non-imputed viral load data is presented in tables and figures. Because 16% of the follow-up viral load data was missing, imputation was performed for significance testing using the “last value carried forward,” (i.e. the participant’s baseline viral load). This procedure is the most conservative imputation method and is least likely to over-estimate the impact of BattleViro in this study, since there were more cases missing in the BattleViro condition.
Figure 3.
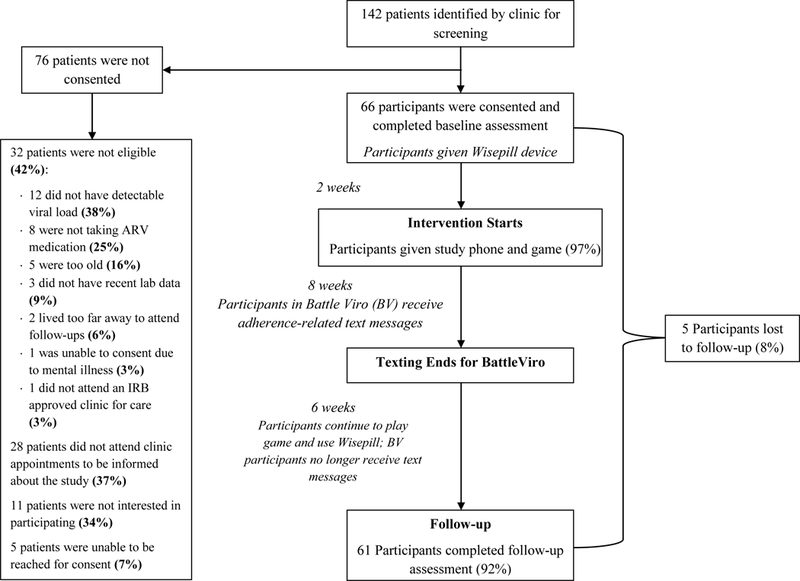
Consort Sheet
Table 1.
Characteristics at baseline of 61 youth living with HIV by Intervention Condition
| Variable | Total Sample (n=61) |
Intervention (n=32) |
Control (n=29) |
Test Statistic (t, F or χ2) |
p-value |
|---|---|---|---|---|---|
| Age (years, M(SD)) | 22.4 (2.5) | 22.5 (2.5) | 22.3 (2.5) | 0.34 | 0.73 |
| Gender(% ,n) | 0.01 | 0.91 | |||
| Male | 78.7 (48) | 78.1 (25) | 79.3 (23) | ||
| Female | 21.3 (13) | 21.9 (7) | 20.7 (6) | ||
| Race (% ,n) | 2.28 | 0.32 | |||
| Black, African American, or Haitian | 96.7 (59) | 96.9 (31) | 93.1 (28) | ||
| Other | 1.6 (1) | 0 (0) | 3.4 (1) | ||
| Multiple | 1.6 (1) | 0 (0) | 3.4 (1) | ||
| Ethnicity | |||||
| Non-Hispanic/Latino | 100 (61) | 100 (32) | 100 (29) | - | - |
| Hispanic/Latino | 0 (0) | 0 (0) | 0 (0) | ||
| Most Recent Grade Completed (% ,n) | 2.99 | 0.57 | |||
| 10th | 3.3 (2) | 3.1 (1) | 3.4 (1) | ||
| 11th | 6.6 (4) | 6.3 (2) | 6.9 (2) | ||
| 12th | 36.1 (22) | 40.6 (13) | 31.0 (9) | ||
| Some College | 45.9 (28) | 37.5 (12) | 55.2 (16) | ||
| Graduated College | 8.2 (5) | 12.5 (4) | 3.4 (1) | ||
| Started ARV in past 3 months (% ,n) | 0.08 | 0.77 | |||
| No | 63.9 (39) | 65.6 (21) | 62.1 (18) | ||
| Yes | 36.1 (22) | 34.4 (11) | 37.9 (11) | ||
| Gaming Status (% ,n) | 0.68 | 0.41 | |||
| Daily or almost daily | 36.1 (22) | 31.3 (10) | 41.4 (12) | ||
| Not everyday | 63.9 (39) | 68.8 (21) | 58.6 (17) | ||
| Health Literacy (Need help with materials from doctor or pharmacy)*(% ,n) | 1.09 | 0.30 | |||
| Never/Rarely | 98.3 (59) | 100.0 (31) | 96.6 (28) | ||
| Sometimes or More | 1.7 (1) | 0 (0) | 3.4 (1) | ||
|
Current Living Situation
(% ,n) |
0.14 | 0.93 | |||
| Own house or apartment | 26.2 (16) | 25.0 (8) | 27.6 (8) | ||
| Parent’s house or apartment | 47.5 (29) | 46.9 (15) | 48.3 (14) | ||
| Other | 26.2 (16) | 28.1 (9) | 24.1 (7) | ||
|
Stable of Living Situation
(% ,n) |
0.83 | 0.36 | |||
| No | 13.1 (8) | 9.4 (3) | 17.2 (5) | ||
| Yes | 86.9 (53) | 90.6 (29) | 82.8 (24) | ||
| Sexual Orientation (% ,n) | 1.75 | 0.63 | |||
| Heterosexual | 26.2 (16) | 28.1 (9) | 24.1 (7) | ||
| Homosexual | 57.4 (35) | 53.1 (17) | 62.1 (18) | ||
| Bisexual | 11.5 (7) | 15.6 (5) | 6.9 (2) | ||
| Undecided | 4.9 (3) | 3.1 (1) | 6.9 (2) | ||
| Global Symptom Index (M(SD)) | 0.9 (0.9) | 1.0 (1.1) | 0.9 (0.8) | 0.04 | 0.97 |
n<61
Age of participants ranged from 15–25 years (mean of 22 years). The sample was 79% male, 97% Black, and 74% non-heterosexual. More than one-third of the sample (36%) was newly starting ART (≤ 3 months). All participants had detectable viral loads (mean 3.82 log viral load) at baseline. Eighty one percent reported oral, anal, or vaginal sex in the past three months and 88% reported condom use at last sex. Participants reported an average level of distress (mean= 0.9, T score of 50) on the Global Symptom Index of the BSI. Participants self-reported high rates of past 7-day ART adherence (mean of 89% of adherence) at baseline. In the week before participants received their study phone and game, mean Wisepill adherence was 63% (SD: 0.35).
Intervention outcomes.
Examination of effect size differences between groups indicated medium effects for BattleViro on improvements in HIV knowledge (d=0.5), ART knowledge (d=0.42), and social support (d=0.62) but there were no statistically significant differences at follow-up between conditions (see Table 2). Contrary to expectations, there was more improvement in ART motivation in the control condition (d=−0.41), although nonsignificant. The following outcomes were observed in the entire sample (intervention plus control groups combined). The entire sample had a significant reduction in viral load at posttest (3.78 to 1.67, F= 4.3, p= 0.04); self-reported adherence remained high (mean of 89% at pre and posttest, F=0.56, p =0.46) and Wisepill-rated adherence decreased significantly over time (mean of 63% to 42%, F=9.17, p < 0.01).
Table 2.
Intervention outcomes at 16-weeks post baseline among 61 youth living with HIV
| Battle Viro Intervention | Control | Effect Size Difference (di –dc) c |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretest | Post a | Pretest | Post a | ||||||
| di b | dc b | F d | p | ||||||
|
Log Viral Load (M (SD)) |
3.62 (1.20) | 1.48 (2.06) | −0.99 | 3.96 (1.18) | 1.82 (2.14) | −1.21 | 0.22 | 0.97 | 0.62 |
|
Wisepill 7–day Adherencee
(M (SD)) |
0.62 (0.36) | 0.43 (0.38) | −0.51 | 0.63 (0.35) | 0.32 (0.36) | −0.65 | 0.14 | 0.23 | 0.63 |
|
Self-Reported 7-day Adherence (M (SD)) |
0.86 (0.25) | 0.88 (0.23) | 0.08 | 0.93 (0.20) | 0.91 (0.23) | −0.09 | 0.17 | 0.56 | 0.46 |
|
HIV Knowledge (M (SD)) |
2.44 (1.16) | 3.09 (1.42) | 0.50 | 3.00 (1.04) | 3.00 (1.28) | 0 | 0.50 | 1.59 | 0.33 |
|
ARV Treatment Knowledge (M (SD)) |
11.36 (3.85) | 12.34 (3.03) | 0.28 | 12.11 (2.06) | 11.65 (4.26) | −0.14 | 0.42 | 0.73 | 0.40 |
|
IMB-Motivation
(M (SD)) |
12.81 (3.81) | 13.10 (4.00) | −0.01 | 10.82 (4.29) | 12.38 (4.45) | 0.40 | −0.41 | 0.06 | 0.81 |
| Self-Efficacy for ARV Use (M (SD)) | 17.94 (4.02) | 19.83(4.68) | 0.43 | 18.21 (5.53) | 19.48 (5.12) | 0.24 | 0.19 | 0.21 | 0.65 |
|
Social Support
(M (SD)) |
26.13 (3.96) | 26.97 (5.39) | 0.18 | 26.38 (2.72) | 24.45 (5.52) | −0.44 | 0.62 | 3.34 | 0.07 |
: 16 weeks post-baseline
: Cohen’s d
: Cohen’s d effect size difference (Battle Viro – Control)
: Fisher’s exact statistic for ANCOVA analyses, controlling for baseline
. Wisepill adherence assessed in week prior to receiving game/phone and 12 weeks later at the end of BattleViro adherence texts
Potential moderators of intervention impact.
We explored the impact of gender, age, newly starting ART, and gaming frequency in the interaction between the factors and intervention conditions in separate ANCOVAs, controlling for baseline scores. Only time on ART showed interaction effects at follow-up. See Table 3 for outcomes among those starting ART within the past three months and significance testing for the interaction between newly starting ART and intervention condition. Those in BattleViro, who had newly started ART, experienced a 0.96 log greater decrease in viral load (effect size difference of −2.21) compared to those newly starting ART who were in the control group (F=4.33, p=0.04 for the interaction effect in ANCOVA with the entire sample). Similarly, those newly starting ART in BattleViro had better adherence at post-test, as measured by Wisepill, than those in the control who were newly starting ART (71% vs. 48% adherence at posttest; effect size difference of 1.18, F=3.20, p=0.05 for the interaction effect in ANCOVA the entire sample) (See Figures 4 and 5). Among those newly starting ART, Battle Viro also demonstrated an impact on HIV-related knowledge (effect size difference of 0.90), ART-related knowledge (effect size difference of 0.72). Also, ART motivation increased more in the control condition newly starting ART (effect size difference of −0.71), although the interaction was nonsignificant.
Table 3.
Outcomes by intervention condition among those starting ART in past 3 months
| Battle Viro Intervention | Control | Effect Size Difference (di –dc)c |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretest | Post a | Pretest | Posta | ||||||
| d b | d b | F d | p | ||||||
| Log Viral Load (M (SD)) | 3.63 (1.11) | 0.93 (1.63) | −2.74 | 3.94 (0.90) | 1.53 (2.49) | −0.53 | −2.21 | 4.33 | 0.04 |
|
Wisepill 7-day Adherence
(M (SD)) |
0.70 (0.35) | 0.71 (0.32) | 0.03 | 0.86 (0.17) | 0.48 (0.44) | −1.12 | 1.15 | 3.90 | 0.05 |
|
Self-Report 7-day Adherence (M (SD)) |
0.96 (0.09) | 0.88 (0.31) | −0.35 | 0.97 (0.06) | 0.91 (0.30) | −0.27 | −0.08 | 0.05 | 0.82 |
| HIV Knowledge (M (SD)) | 2.27 (0.79) | 2.91 (1.45) | 0.55 | 2.90 (0.94) | 2.55 (1.13) | −0.35 | 0.90 | 0.52 | 0.47 |
|
ART Treatment Knowledge (M (SD)) |
11.82 (3.46) | 13.18 (1.94) | 0.47 | 12.45 (1.81) | 11.63 (4.23) | −0.25 | 0.72 | 0.42 | 0.52 |
|
Motivation for ART
(M (SD)) |
13.18 (3.22) | 12.00 (4.12) | −0.32 | 10.70 (4.19) | 12.20 (3.52) | 0.39 | −0.71 | 0.39 | 0.53 |
|
Self-Efficacy for ART Skills (M (SD)) |
19.18 (3.71) | 19.09 (5.65) | −0.02 | 18.36 (5.51) | 19.36 (3.64) | 0.21 | −0.23 | 0.56 | 0.46 |
|
Social Support
(M (SD)) |
27.64 (3.32) | 27.64 (5.18) | 0 | 25.45 (2.94) | 25.00 (4.02) | −0.13 | 0.13 | 0.03 | 0.87 |
: 16-weeks post-baseline
: Cohen’s d
: Cohen’s d effect size difference (Battle Viro - Control)
: ANCOVA interaction statistic between intervention condition and newly starting ARV in past 3 months in entire sample, controlling for baseline
Figure 4.
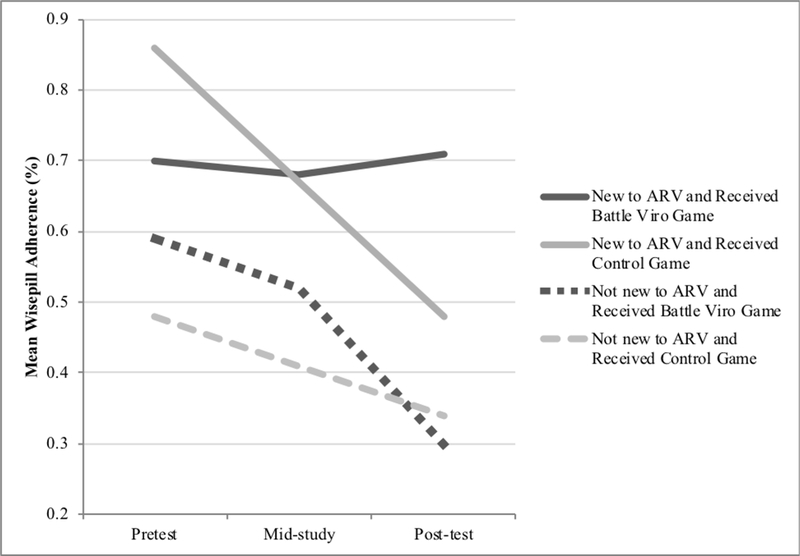
Wisepill Adherence (past 7-day) by Intervention Condition by Starting ARV in past 3 months
Figure 5.
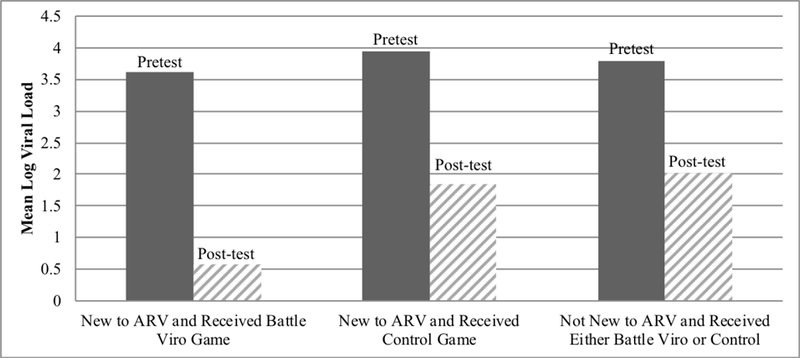
Log Viral Load by Intervention Condition by Newly Starting ARV
Discussion
Findings from this small trial suggest that the gaming intervention was most effective with YLWH who were new to ART and had a positive influence on reducing viral load, improving medication adherence, HIV knowledge, ART knowledge, and social support. The clinical period of ART initiation is a “teachable moment” and an opportunity to empower and motivate YLWH to engage in healthy behaviors during a natural transition stage (McBride, Emmons, & Lipkus, 2003; Naar-King et al., 2013). Building motivation and skills for self-care at the point of treatment initiation can promote resilience against the challenges of living with HIV and adhering to medical care. Taking advantage of this naturally occurring clinical transition point could increase the effectiveness of interventions for YLWH (Bangsberg, 2011; Lima et al., 2010; Naar-King et al., 2013; Rosenblum, Deeks, Van Der Laan, & Bangsberg, 2009). Although this time period is critical, there is a dearth of interventions for YLWH who are newly starting ART (Naar-King et al., 2013).
In our study, there were no differences in demographic characteristics between those newly starting ART compared to those who had been on ART for longer periods of time (data not reported in paper). However, challenges to ART adherence for those who have lived with HIV for longer periods of time could be different and unique. Living with chronic HIV and frequently co-morbid illnesses for many years (e.g. depression and substance use) will influence engagement in treatment and relationships with healthcare providers. Improving self-care may be especially challenging for patients who live in chaotic circumstances and do not have a pattern of self-care in other health areas. The first few months after receiving a HIV diagnosis could be a window of opportunity in which there is inherent flexibility. The ART initiation period may be a particularly good time to improve relationships with clinicians and supportive friends, as well as build resilience and feelings of empowerment. Additionally, those newly starting ART may be more open to the presentation of knowledge and skills through a fun, non-stigmatizing game, while those who have been living with HIV for longer may find this method less helpful, or even superficial. Life-long feelings of stigma, as well as mental health issues that stem from living with a chronic disease for many years may not be adequately addressed in a brief gaming intervention.
Participants newly starting ART who received BattleViro had significantly decreased viral load and maintained their adherence. In addition, their perception of social support and HIV and ART related knowledge improved. However, self-efficacy was unchanged, and motivation improved more in the control group. These findings were surprising. It is possible that greater knowledge and social support, rather than self-efficacy and motivation, were the primary factors in improving ART adherence. Also, factors not measured by this study may have been improved by BattleViro such as empowerment, perceived health, or acceptance of HIV diagnosis and treatment. In addition, the motivation and self-efficacy scales may not have accurately captured the constructs. Participants rated their ART-related behavioral ability as very high at baseline, so there was little room for improvement on this measure. There could have been comprehension difficulties with the motivation scale, as many of the items expressed a lack of motivation, so participants had to “Disagree” with the statement in order express motivation for ART. If this was a systematic misunderstanding, then the effect size difference between groups might reflect better, rather than less, motivation in the BattleViro group. Inquiry into scale comprehension and further scale development is needed for ART motivation and self-efficacy.
Interestingly, this iPhone game/app was not influenced by gender, age, or extent that participants engaged in playing other games or past experience of “gaming.” This finding may demonstrate BattleViro’s widespread appeal. Our sample included participants who were 15–25 years of age; however, the game could be successful in improving adherence for those newly starting ART who are older. Data from the Pew Research Center Survey on technology use shows that gaming is extremely popular among older adults. Six-in-ten Americans ages 18 to 29 and 53% of those ages 30 to 49 say they play video games often, and roughly a quarter of Americans ages 65 and older also say they play video games at least sometimes.
Mobile games have the potential to engage diverse groups of participants in interventions that otherwise may not be willing or able to participate in prevention programs. Mobile interventions have the potential to reinforce skills learned in the clinic and require fewer resources to deliver patient-centered, evidence-based interventions (Lee & Peng, 2006). Gaming and mobile apps also have the potential to advance the delivery of information and promote healthy decision making in disproportionately affected populations, including disadvantaged urban and minority youth that often have less access to medical care and support (Pew Research Center, 2008). National data indicates that younger, ethnic and racial minority populations use smart phones frequently and some data show that African American youth are more likely to be mobile phone users than their Caucasian peers (Pew Research Center, 2008; Pew Research Center, 2015). The adolescents and young adults in this study repeatedly expressed having access to, and familiarity with, iPhones. This wide-spread use of iPhones facilitates the uptake of gaming apps in clinical populations. Therefore, mobile technologies, like smart phone games and apps, have a great potential to enhance medical care for populations who are disproportionately affected with HIV.
Limitations
Findings should be interpreted in light of study limitations. This study had a small sample and limited power, so findings will need to be replicated. Our participants in this RCT were recruited from Jackson, MS. Therefore, this sample is not representative of all HIV clinics in the United States or internationally and the generalizability of the data collected to inform the development of the app is unknown and may be limited. However, the importance of intervention among those living in the southern United States should be underscored as eight of the ten states with the highest rates of new HIV diagnoses are in the South. There is no perfect measure of adherence and the assessments by self-report and Wisepill differed at all time points. Self-report of adherence remained high for all 16 weeks of the study but systematically declined as measured by Wisepill. Some participants reported that they grew tired of using Wisepill and others enjoyed the fact that its use led to personalized text messages in BattleViro. This discrepancy between self-report and Wisepill measurement is not unexpected as previous studies have shown that self-report of medication adherence tends to overestimate adherence behavior compared with other assessment methods. (Stirratt et al., 2015) The lack of a perfect measure of adherence limits our ability to examine the impact of purported intervention mediators (e.g. knowledge, social support). This study also focused on adolescent and young adult patient perspectives. It may be equally important to integrate caregiver perspectives into the game. Perspectives of family and friends could also lead to a more robust understanding of barriers and facilitators to adherence to medication and treatment for YLWH. Therefore, future research could examine the utility of integrating feedback from caregivers, and friends into the gaming/app. Finally, this app was developed for the iPhone. Development of the app for Android devices could allow for greater availability of the game and could be a forthcoming step in the future phases of research.
Conclusions
Despite these limitations, this study is a significant step in working toward app/gaming intervention to promote adherence to ART for youth and young adults living with HIV. The long-term goal of this research program is to test our mobile game, Battle Viro in a larger multi-site randomized trial, and if effective, prepare of intervention dissemination. There are many advantages to using newer interactive technology to improve adherence, rather than traditional face-to-face counseling, including scalability, efficiency, and cost effectiveness. Since electronic games are highly appealing to adolescents and young adults (Lieberman, 1997) they are a natural opportunity to deliver health education during leisure time and outside of the clinic (Hightow-Weidman et al., 2017; Lieberman, 1997; Lieberman, 2006; Raney et al., 2006; Thompson et al., 2010). We are not aware of other adherence interventions that integrate medication adherence monitoring technology, text messaging, and a theoretically informed game to improve information, motivation, and behavioral skills for ART adherence. An intervention with these components may engage and empower HIV infected adolescents and young adults, build resilience, and result in improvements in health.
Acknowledgements:
This work was supported by grants from the National Institutes of Health (R01 HD074846, PI: Brown, and 2T32 MH078788, PI: Brown) and the Providence/Boston Center for AIDS Research (P30 AI042853, PI: Cu-Uvin)
References:
- Bain-Brickley D, Butler LM, Kennedy GE, & Rutherford GW (2011). Interventions to improve adherence to antiretroviral therapy in children with HIV infection. The Cochrane Database of Systemic Reviews, 12, CD009513. doi: 10.1002/14651858.CD009513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour L, Kowal J, Tasca GA, Cooper CL, Angel JB, Macpherson PA, … Cameron DW (2007). Development and psychometric validation of the HIV treatment knowledge scale. AIDS Care, 19, 11141–11148. doi: 10.1080/09540120701352241 [DOI] [PubMed] [Google Scholar]
- Bandura A Social foundations of thought and action: A social cognitive theory. (1986). Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Bangsberg DR (2011). Perspectives on adherence and resistance to ART. Poster session presented at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA. [Google Scholar]
- Baranowski T, Buday R, Thompson DI, & Baranowski J (2008). Playing for real: video games and stories for health-related behavior change. American Journal of Preventive Medicine, 34, 74–82. doi: 10.1016/j.amepre.2007.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzer ME, Fuchs DN, Luftman GS, & Tucker DJ (1999). Antiretroviral adherence issues among HIV-positive adolescents and young adults. Journal of Adolescent Health, 25(5), 316–319. [DOI] [PubMed] [Google Scholar]
- Brown LK Lourie KJ, Pao M (2000). Children and adolescents living with HIV and AIDS: a review. Journal of Child Psychology and Psychiatry, 41(1), 81–96. [PubMed] [Google Scholar]
- Brown L, Hadley W, Donenberg G, Lescano C, Diclemente R, Barker D, Lang D & Oster D (2014). Project STYLE: A multisite HIV prevention randomized controlled trial for youth in mental health treatment. Psychiatric Services, 65:338–344. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LK, Whiteley L, Harper GW, Nichols S, & Nieves A (2015). Psychological Symptoms Among 2032 Youth Living with HIV: A Multisite Study. AIDS Patient Care and STDs, 29(4), 212–219. 10.1089/apc.2014.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandwani S, Koenig LJ, Sill AM, Abramowitz S, Conner LC, & D’Angelo L (2012). Predictors of antiretroviral medication adherence among a diverse cohort of adolescents with HIV. The Journal of Adolescent Health, 51, 242–251. doi: 10.1016/j.jadohealth.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Cole SW, Kato PM, Marin-Bowling VM, Dahl GV, & Pollock BH (2006). Clinical trial of Re-Mission: a video game for young people with cancer. Cyberpsychology & Behavior, 9, 665–666. [Google Scholar]
- Cutrona CE, & Russell DW (1987). The provisions of social relationships and adaptation to stress. Advances in personal relationships, 1(1), 37–67. [Google Scholar]
- Derogatis LR, & Spencer MS (1983).The Brief Symptoms Inventory (BSI): Administration, scoring, and procedures manual. Baltimore, MD: Johns Hopkins University School of Medicine, Clinical Psychometrics Unit. [Google Scholar]
- Donenberg GR, Emerson E, Bryant FB, Wilson H, & Weber-Shifrin E (2001). Understanding AIDS-risk behavior among adolescents in psychiatric care: links to psychopathology and peer relationships. Journal of the American Academy of Child and Adolescent Psychiatry, 40, 642–653. doi: 10.1097/00004583-200106000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D, Menezes C, Mahomed K, Macdonald P, Untiedt S, Levin L, … Maskew M (2013). Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpu-malanga, South Africa. AIDS Research and Human Retroviruses, 29, 892–900. doi: 10.1089/aid.2012.0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA, Amico KR, & Harman JJ (2006). An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology, 25, 462–473. doi: 10.1037/0278-6133.25.4.462 [DOI] [PubMed] [Google Scholar]
- Furniss D, Barber N, Lyons I, Eliasson L, & Blandford A (2014). Unintentional non-adherence: can a spoon full of resilience help the medicine go down? BMJ Quality and Safety, 23, 95–98. doi: 10.1136/bmjqs-2013-002276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightow-Weidman LB, Muessig KE, Bauermeister JA, LeGrand S, & Fiellin LE (2017). The future of digital games for HIV prevention and care. Current Opinion in HIV and AIDS, 12, 501–507. doi: 10.1097/COH.0000000000000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer C, Susskind O, Alpert HR, Owus MS, Schneider L, Rappaport LA, & Rubin DH (2000). An evaluation of an innovative multimedia educational software program for asthma management: report of a randomized, controlled trial. Pediatrics, 106(1 Pt 2), 210–215. [PubMed] [Google Scholar]
- Hugen PW, Langebeek N, Burger DM, Zomer B, van Leusen R, Schuurman R, … Hekster YA (2002). Assessment of adherence to HIV protease inhibitors: comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. Journal of Acquired Immune Deficiency Syndromes, 30(3), 324–334. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Catz SL, Remien RH, Rotheram-Borus MJ, Morin SF, Charlebois E, …Chesney MA (2003). Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the healthy living project. AIDS Patient Care & STDs, 17, 645–656. doi: 10.1089/108729103771928708 [DOI] [PubMed] [Google Scholar]
- Kato PM, Cole SW, Bradlyn AS, & Pollock BH (2008). A video game improves behavioral outcomes in adolescents and young adults with cancer: A randomized trial. Pediatrics, 122, e305–e317. doi: 10.1542/peds.2007-3134 [DOI] [PubMed] [Google Scholar]
- Kelley K (2007). Confidence intervals for standardized effect sizes: Theory, application, and implementation. Journal of Statistical Software, 20 (8): 1–24. [Google Scholar]
- Kim SH, Gerver SM, Fidler S, & Ward H (2014). Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS, 28, 1945–1956. doi: 10.1097/QAD.0000000000000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle-Parker DJ, Amico KR, & McKinney VE (2014). Effects of an Intervention Addressing Information, Motivation, and Behavioral Skills on HIV Care Adherence in a Southern Clinic Cohort. AIDS Care, 26(6), 674–683. 10.1080/09540121.2013.845283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, & Peng W (2006). What do we know about social and psychological effects of computer games? A comprehensive review of the current literature In: Vorderer P & Bryant J (Eds.), Playing video games: motives, responses and consequences (325–346). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- LeGrand S, Muessig KE, McNulty T, Soni K, Knudtson K, Lemann A,… Hightow-Weidman LB (2016). Epic Allies: development of a gaming app to improve antiretroviral therapy adherence among young HIV-positive men who have sex with men. Journal of Medical Internet Research Serious Games, 4, e6. doi: 10.2196/games.5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DA (1997). Interactive video games for health promotion: effects on knowledge, self-efficacy, social support, and health In: Street RL, Gold WR, & Manning T (Eds). Health promotion and interactive technology: theoretical applications and future directions (103–120). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Lieberman DA. (2006). What can we learn from playing interactive games? In: Vorderer P & Bryant J (Eds.), Playing video games: motives, responses and consequences (325–346). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- LifeWindows Project Team. (2006). The LifeWindows Information Motivation Behavioral Skills ART Adherence Questionnaire (LW-IMB-AAQ). Storrs, CT: Center for Health, Intervention, and Prevention, University of Connecticut. [Google Scholar]
- Lima VD, Bangsberg DR, Harrigan PR, Deeks SG, Yip B, Hogg RS, & Montaner JSG (2010). Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. Journal of Acquired Immune Deficiency Syndromes, 55, 460–465. doi: 10.1097/QAI.0b013e3181f2ac87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonell K, Naar-King S, Huszti H, & Belzer M (2013). Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS and Behavior, 17, 86–93. doi: 10.1007/s10461-012-0364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Harper G, Carleton RA, Hosek S, Bojan K, Clum G,& Ellen J (2012). The impact of stigma on medication adherence among HIV-positive adolescent and young adult females and the moderating effects of coping and satisfaction with health care. AIDS Patient Care and STDs, 26, 108–115. doi: 10.1089/apc.2011.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Emmons KM, & Lipkus IM (2003). Understanding the potential of teachable moments: the case of smoking cessation. Health Education Research, 18(2), 156–170. [DOI] [PubMed] [Google Scholar]
- Mission Critical Studios. (2012). Dr. Nano X: Incredible Voyage. Available from http://www.missioncriticalstudios.com.
- Murphy DA, Lu MC, Martin D, Hoffman D, & Marelich WD (2002). Results of a pilot intervention trial to improve antiretroviral adherence among HIV-positive patients. The Journal of the Association of Nurses in AIDS Care, 13, 57–69. doi: 10.1177/1055329002238026 [DOI] [PubMed] [Google Scholar]
- Naar-King S, Outlaw AY, Sarr M, Parsons JT, Belzer M, Macdonell K, … Ondersma SJ (2013). Motivational enhancement system for adherence (MESA): pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment. Journal of Pediatric Psychology, 38, 638–648. doi: 10.1093/jpepsy/jss132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra AD, Gwadz MV, Whittemore R, Bakken SR, Cleland CM, Burleson W, Jacobs SK, & Melkus GD (2017). Health technology-enabled interventions for adherence support and retention in care among US HIV-infected adolescents and young adults: an integrative review. AIDS and Behavior, 21(11), 3154–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, & Seage GR 3rd. (2008). Long- term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clinical Infectious Diseases, 46, 507–515. doi: 10.1086/526524 [DOI] [PubMed] [Google Scholar]
- Pew Research Center (2008). Teens, videogames, and civics. Washington, DC: Lenhart, A., Kahne, J., Middaugh, E., Macgill, A.R., Evans, C., & Vitak, J
- Pew Research Center. (2015).Technology device ownership: 2015. Washington, DC: Anderson, M. [Google Scholar]
- Raney AA, Smith JK, & Baker K (2006). Adolescents and the appeal of video games In: Vorderer P, & Bryant J (Eds). Playing video games: motives, responses and consequences (165–180). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, & Safren SA (2009). A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Topics in HIV Medicine, 17(1), 14–25. [PMC free article] [PubMed] [Google Scholar]
- Resino S, Resino R, Bellón JM, Micheloud D, Gutiérrez MDG, de José MI, … Muñoz-Fernández MA (2006). Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV type-1-infected children. Clinical Infectious Diseases, 43, 243–252. doi: 10.1086/505213 [DOI] [PubMed] [Google Scholar]
- Rosenblum M, Deeks SG, Van Der Laan M, & Bangsberg DR (2009). The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One, 4, e7196. doi: 10.1371/journal.pone.0007196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Pearson CR, Pantalone DW, Marks G, & Crepaz N (2006). Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load: A meta-analytic review of randomized controlled trials. Journal of Acquired Immune Deficiency Syndromes, 43, S23–S35. doi: 10.1097/01.qai.0000248342.05438.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames RS, Sharek P, Mayer M, Robinson TN, Hoyte EG, Gonzalez-Hensley F, … Umetsu DT (2004). Effectiveness of a multicomponent self-management program in at-risk, school-aged children with asthma. Annals of Allergy, Asthma & Immunology, 92, 611–618. doi: 10.1016/S1081-1206(10)61426-3 [DOI] [PubMed] [Google Scholar]
- Starace F, Massa A, Amico R, & Fisher JD (2006). Adherence to antiretroviral therapy: an empirical test of the information-motivation-behavioral skills model. Health Psychology, 25, 153–162. doi: 10.1037/0278-6133.25.2.153 [DOI] [PubMed] [Google Scholar]
- Stokes B (2005). Video games have changed: Time to consider “serious games”? Development Education Journal, 11(3), 12–14. [Google Scholar]
- Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, … Nilsen WJ (2015). Self-report measures of medication adherence behavior: recommendations on optimal use. Translational Behavioral Medicine, 5(4), 470–482. 10.1007/s13142-015-0315-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Baranowski T, Buday R, Baranowski J, Thompson V, Jago R & Griffith MJ (2010). Serious video games for health: how behavioral science guided the development of a serious video game. Simulation & Gaming, 41, 587–606. doi: 10.1177/1046878108328087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley L, Brown LK, Curtis V, & Heck N (2014, June). An iPhone app/game to improve ART adherence. Poster session presented at the 9th International Conference on HIV Treatment and Prevention Adherence, Miami, FL. [Google Scholar]
- Whiteley L, Brown LK, Lally M, Heck N, & Van den burg JJ (In press). The development of a mobile gaming intervention to increase motivation and adherence to antiretroviral treatment for youth living with HIV. Journal of Medical Internet Research mHealth and uHealth. [DOI] [PMC free article] [PubMed] [Google Scholar]


