Chronic kidney disease (CKD) affects 30–50% of all patients with heart failure (HF) and is a potent risk factor for cardiovascular death and HF hospitalization.1 The correlation of inflammatory markers, such as C-reactive protein (CRP) and interleukin-6 (IL-6), to the severity of both kidney2 and heart3 dysfunction suggests that inflammation may contribute to the pathogenesis of CKD in HF and provides rationale for identifying potential mediators of cardio-renal inflammation. Based upon experimental animal and cellular models, we postulated that interleukin-1 (IL-1), a master regulator of the inflammatory response, could be one such mediator.4
In this post-hoc analysis of the REDHART study (N=52),5 we compared the anti-inflammatory effects of 2 weeks of anakinra 100 mg daily or placebo within the subgroup who had estimated glomerular filtration rate (eGFR) between 30 and 60 mL/min/1.73 m2 (n=22 [42%]). In brief, REDHART was a 2:1 randomized, double-blind, placebo-controlled clinical trial that compared placebo against 2 or 12 weeks of anakinra, recombinant human IL-1 receptor antagonist, in patients with systolic heart failure and C-reactive protein (CRP) ≥2 mg/L who were enrolled within 2 weeks of discharge from heart failure hospitalization. We measured systemic inflammation using CRP, an established surrogate of IL-1 activity, myeloperoxidase activity and neutrophil count. Continuous and categorical variables were summarized as median (interquartile range) and number (percentage), respectively. Between-group differences in baseline characteristics were compared with the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. All analyses were performed in the on-treatment population of patients who completed 2 weeks of blinded treatment. Within-group treatment response changes were tested for statistical significance with the Wilcoxon signed-rank test and between-group changes with an analysis of covariance model adjusted for baseline value. P<0.05 was considered statistically significant.
The median ages in the anakinra (n=15) and placebo (n=7) groups were 57 (55–69) and 64 (59–70) years. The majority of patients were African American men in both arms (Table S1). The median eGFR was 50 (36–55) mL/min/1.73 m2 and 49 (45–53) mL/min/1.73 m2 in the anakinra and placebo groups, respectively.
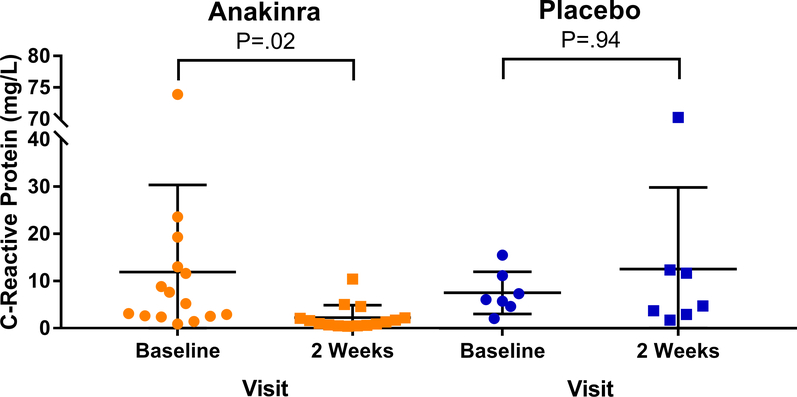
At baseline, serum CRP level was 5.2 (2.6–12.3) mg/L in the anakinra arm and 6.0 (5.1–9.2) mg/L in the placebo arm (Table S2). At 2 weeks, anakinra had reduced CRP significantly by 84% (−92 to −2%) to 1.3 (0.7–2.2) mg/L (P=0.02) whereas placebo had no effect (−22% [−30 to 26%]; P=0.94) (Table S2). With adjustment for baseline level, the between-group absolute change in CRP was significantly greater in the anakinra arm than placebo (P=.004) (Figure).
Figure. Effects of Anakinra on C-Reactive Protein in Patients with Heart Failure and Chronic Kidney Disease.
Anakinra reduced C-reactive protein in patients with heart failure and chronic kidney disease compared to placebo.
A larger proportion of anakinra patients than placebo patients achieved a CRP level within normal limits of <2 mg/L at 2 weeks, although this did not reach statistical significance (10 [67%] vs. 1 [14%], respectively; P=0.06).
Anakinra significantly reduced plasma myeloperoxidase activity from 386 (315–511) pmol/L at baseline to 335 (275–387) pmol/L at 2-weeks (−18% [−26 to −13%]; P=.03) (Figure). There was no change in myeloperoxidase activity in the placebo group (394 [308–470] pmol/L at baseline and 413 [337–428] pmol/L at 2-weeks; within-group change, 3% [−11 to 8%]; P=.94). With adjustment for baseline MPO activity, the between-group change in MPO level strongly trended towards greater reduction in the anakinra arm compared to placebo (P=.050) (Table S2).
In the anakinra arm, absolute neutrophil count decreased from 4.6 (3.8–5.6) x103 cells/mm3 at baseline to 2.8 (2.1–4.2) x103 cells/mm3 at 2 weeks (P=.005 for within-group change). In the placebo arm, absolute neutrophil count was also numerically lower at 2 weeks compared to baseline, although the difference did not reach statistical significance (3.4 [3.0–4.1] x103 cells/mm3 at baseline vs. 2.5 [2.5–3.5] x103 cells/mm3 at 2-weeks; P=0.07 for within-group change). The between-group change in absolute neutrophil count was not statistically different between the anakinra and placebo arms (P=.09).
Serum creatinine did not significantly change at 2 weeks in either the anakinra (1.70 [1.35–1.85] mg/dL at baseline vs. 1.60 [1.40–1.90] mg/dL at 2 weeks; P=.67) or placebo (1.40 [1.35–1.65] mg/dL at baseline vs. 1.40 [1.15–1.55] mg/dL at 2 weeks; P=.09) arms. Similarly, blood urea nitrogen did not change in the anakinra (25 [22–37] mg/dL at baseline vs. 27 [23–33] mg/dL at 2 weeks; P=.90) or placebo (24 [24–41] mg/dL at baseline vs. 26 [20–32] mg/dL at 2 weeks; P=0.22) arms.
In anakinra-treated patients (n=9) who were randomized to 12-weeks of treatment, there were trends for greater reductions in myeloperoxidase activity (P=.06) and neutrophil count (P=.07) compared to placebo at 12-weeks (Table S2). Changes in CRP, serum creatinine and blood urea nitrogen at 12-weeks were not significantly different between the anakinra 12-weeks and placebo arms (Table S2).
In this post-hoc analysis, we found that 2 weeks of treatment with anakinra reduced CRP and myeloperoxidase activity, with a trend towards a reduction in neutrophil count, in patients with HF and moderate CKD. Our observations of anakinra’s potent anti-inflammatory effects in patients with HF and moderate CKD are consistent with results from a randomized study of a soluble IL-1 decoy protein, which lowered CRP by 68% in patients with moderate CKD.6 In 98 patients with decompensated HF, Hanberg et al. found a significant association between urinary level of IL-6, a downstream effector of IL-1, and the odds of eGFR <60 mL/min/m2.7
Over this short-period of follow-up, we did not find evidence of a beneficial effect of anakinra on serum creatinine or blood urea nitrogen. Any effects of anakinra on kidney structure or function likely require several months to manifest. The use of novel kidney biomarkers may improve sensitivity for detecting changes in tubular structure. Limitations of the study include the lack of stratification according to CKD status at randomization and the small sample size, which may explain the lack of significant anti-inflammatory effects at 12-weeks.
Our findings implicate IL-1 as a potentially key mediator of inflammation in patients with HF and CKD.
Supplementary Material
Acknowledgments
Funding: REDHART was supported by the National Heart, Lung, and Blood Institute (1R34HL117026) to Drs. Abbate and Van Tassell and by the National Center for Research Resources Clinical Translational Science Award (UL1TR000058) to Virginia Commonwealth University. The active drug (anakinra) and placebo were provided by Swedish Orphan Biovitrum (Stockholm, Sweden).
Footnotes
Conflict of Interest: None.
References
- 1.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 2015;36:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 2005;68:237–245. [DOI] [PubMed] [Google Scholar]
- 3.Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: A clinical update. Eur Heart J 2018;39:2063–2069. [DOI] [PubMed] [Google Scholar]
- 4.Anders H-J. Of Inflammasomes and Alarmins: IL-1 and IL-1 in Kidney Disease. J Am Soc Nephrol 2016;27:2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tassell BW Van, Canada J, Carbone S, Trankle C, Buckley L, Erdle CO, Abouzaki NA, Dixon D, Kadariya D, Christopher S, Schatz A, Regan J, Viscusi M, Buono M Del, Melchior R, Mankad P, Lu J, Sculthorpe R, Biondi-Zoccai G, Lesnefsky E,. Arena R, Abbate A. Interleukin-1 blockade in recently decompensated systolic heart failure: Results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Hear Fail 2017;10:e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak KL, Hung A, Ikizler TA, Farmer-Bailey H, Salas-Cruz N, Sarkar S, Hoofnagle A, You Z, Chonchol M. Interleukin-1 inhibition, chronic kidney disease-mineral and bone disorder, and physical function. Clin Nephrol 2017;88:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanberg JS, Rao VS, Ahmad T, Chunara Z, Mahoney D, Jackson K, Jacoby D, Chen M, Wilson FP, Tang WHW, Kakkar R, Testani JM. Inflammation and cardio-renal interactions in heart failure: a potential role for interleukin-6. Eur J Heart Fail 2018;20:933–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.